Abstract
A distinct association between airway eosinophilia and chronic cough is well documented. Eosinophil granule-derived cationic proteins, such as major basic protein (MBP), have been shown to activate and enhance the excitability of bronchopulmonary C-fiber sensory nerves, which may then lead to an increase in cough sensitivity. This study was carried out to determine whether cough responses to inhaled irritant gases were altered by delivery of MBP into the airways. An awake mouse moved freely in a recording chamber that was ventilated with a constant flow of air or irritant gas mixture. Cough responses to separate inhalation challenges of sulfur dioxide (SO2; 300 and 600 ppm) and ammonia (NH3; 0.1 and 0.2%), each for 5-min duration, were measured daily for 3 days before and for up to 8 days after MBP (10–20 µg) instillation into the trachea. During control, inhalations of SO2 and NH3 consistently elicited cough responses in a dose-dependent manner. After MBP treatment, cough responses to both SO2 and NH3 increased significantly and progressively and reached peaks 2–3 days after the treatment before returning to control level in 3–7 days. In sharp contrast, cough responses to these irritant gases were not affected by the treatment with the vehicle of MBP. These results suggest that the MBP-induced lingering elevation of cough responsiveness may be a contributing factor in the pathogenesis of chronic cough associated with eosinophilic infiltration of the airways.
Keywords: airway, cationic protein, C-fiber, reflex, vagus
INTRODUCTION
A distinct association between eosinophilic infiltration of the airway and chronic cough has been extensively documented (4), but the underlying pathophysiology is not fully understood. Degranulation of eosinophils leads to the release of five types of low-molecular-weight cationic proteins, and major basic protein (MBP) is the dominant type among them (9, 21). These cationic proteins are known to contribute to the airway mucosal injury and bronchial hyperresponsiveness caused by eosinophilic infiltration (8, 9, 12, 13, 19, 23).
Vagal C-fiber sensory nerves innervating the airways and lung play an important role in eliciting the cough reflex, an essential function for protecting the lung against inhaled irritants and expelling excessive airway secretion or aspirated substances. These sensory nerves are also believed to be involved in the manifestation of cough hypersensitivity associated with airway inflammatory diseases (6, 18). The direct evidence of a stimulatory effect of eosinophil granule-derived cation proteins on bronchopulmonary C-fibers was first reported by Lee et al. (15) in rats. In addition, cationic proteins could also enhance the excitability of these sensory nerves and result in airway hypersensitivity (10). Follow-up studies using the patch-clamp electrophysiological recording technique in isolated vagal pulmonary sensory neurons further demonstrated that the sensitizing effect of cationic proteins on these neurons was mediated primarily through an inhibitory effect on the voltage-gated K+ current (11). Furthermore, MBP is known to cause airway mucosa injury and inflammation (8, 19), which may also lead to the development of sustaining hypersensitivity of these sensory nerves.
In light of this background information, we postulated that these cationic proteins altered the cough response to inhaled irritants, and this study was carried out to test this hypothesis in awake mice. Sulfur dioxide (SO2) and ammonia (NH3) were selected for this study, because both are irritant gases existing in the environment and can cause lung injury when inhaled at high concentrations or during prolonged exposure (1, 2).
METHODS
The procedures described below were approved by the University of Kentucky Institutional Animal Care and Use Committee.
Measurement of coughs in mouse.
Methods for measuring cough responses in mice have been described in detail in our recent study (25). Briefly, awake mice (C57BL6/J) moved freely in a Plexiglas recording chamber (volume 160 ml) during the experiment. To prevent CO2 and heat accumulation, room air or irritant gas mixtures were drawn through the chamber at a constant flow rate (300 ml/min) by negative pressure. CO2 concentration and temperature of the outlet air were continuously monitored and reached steady levels of ~0.5% and ~23°C, respectively. The pressure in the chamber (Pcham) was recorded continuously with a pressure transducer (Biopac model TSD160A); both audio and video signals of mouse movements were also recorded simultaneously with a microphone and a video camera, respectively. To further verify the accuracy of cough analysis, intrapleural pressure (Pip) was measured directly by implanting the tip of a telemetry sensor (DSI model PA-C10) surgically into the intrapleural space under anesthesia (inhalation of 5% isoflurane) 2–3 wk before the experiment. Cough frequency was determined by analyzing all the signals recorded (Pcham, Pip, audio, and video) by two individuals independently, and their data were then averaged.
Inhalation challenges of irritant gases.
During the inhalation challenge, a gas mixture of SO2 or NH3 was drawn into the chamber (replacing room air) at the same flow rate (300 ml/min) for 5 min. Two concentrations of SO2 (300 and 600 ppm) and NH3 (0.1 and 0.2%) gas mixtures balanced in air were tested in each animal; the sequences of SO2 and NH3 as well as the high/low concentrations of each gas were alternated between days in each animal to achieve a balanced design. A mouse was placed in the recording chamber for a 30-min adaptation period before the beginning of experiment. These inhalation challenges were not repeated in the same animals on the same day, and at least 30 min elapsed between two challenges.
Intratracheal instillation of cationic proteins.
The mouse was lightly anesthetized with inhalation of isoflurane (5%) and tilted head-up at an angle of ~30°, and MBP (0.2 mg/ml, 50–100 µl) or its vehicle (Veh; acidic acetate buffer diluted in isotonic saline, 50–100 µl) was instilled into the trachea via a blunted, angled 24-gauge needle inserted through the larynx. This dose of MBP was determined based on our previous studies in rats (15) and also the results obtained from our preliminary experiments in mice. The success of this procedure had been confirmed in our preliminary experiments by using fluorescent dye and histological examination of tissue samples to verify that the delivery of solution was limited to the respiratory tract. This procedure took ~5 min, and the mouse began to move around in the cage 10 min later. The first post-MBP cough responses to inhalation challenges were tested 24 h later. In our preliminary trials, experiments were also carried out 30–60 min after the MBP delivery, but the cough responses were not reproducible and were apparently suppressed by the lingering effect of the anesthesia. Therefore, the effect immediately after MBP was not studied.
MBP was purified as described previously (20). Briefly, human eosinophils were obtained by cytapheresis of patients with marked blood eosinophilia. After cell lysis and granule isolation, granules were solubilized in 0.01 M HCl (pH 2.0) by vigorous suspension with a Pasteur pipette. After centrifugation (13,600 g, 5 min), the supernatant was fractionated on a Sephadex G-50 column equilibrated with 0.025 M sodium acetate (pH 4.3) containing 0.15 M NaCl. Acetate buffer was harvested for vehicle control. Individual protein peaks were pooled, and protein concentrations were determined by absorbance at 280 nm. MBP used in these studies gave a single band at the expected migration (size) by polyacrylamide gel electrophoresis (20).
Experimental protocols.
Experiments were initiated after the mouse had acclimated to the recording chamber and experimental protocols daily for a week. Cough responses to SO2 and NH3 inhalation challenges during control (before MBP or Veh treatment) were studied on 3 consecutive days in each animal, and the data obtained from these 3 days were averaged as control responses. Identical protocols were then carried out daily on days 1, 2, 3, and 7 following the MBP or Veh treatment. The same experiments were also repeated on other days (e.g., days 4, 5, or 8 after treatment) in some but not all mice when additional testing was warranted.
In each experiment, the cough frequency was analyzed and averaged for the 5-min durations of “baseline” (immediately before SO2 or NH3 inhalation challenge), “during challenge” and “recovery” (immediately after the termination of challenge), as shown in Fig. 1, in each animal. The data were then pooled for all animals in the same group.
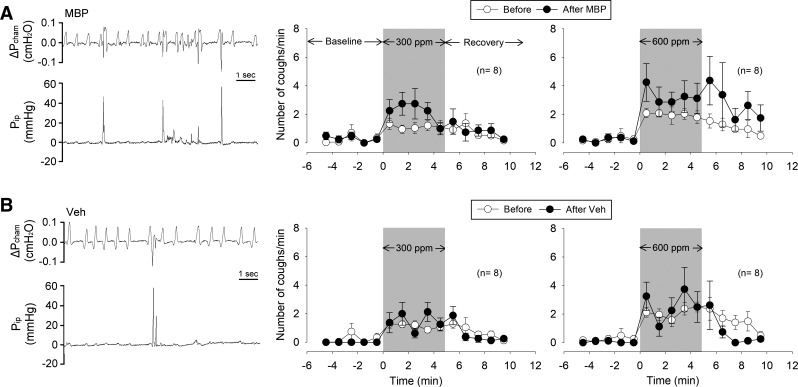
Fig. 1.
Effects of major basic protein (MBP; A) and vehicle (Veh; B) treatments on cough response to SO2 inhalation challenge in awake mice. Left: representative experimental records of coughs evoked during the SO2 (600 ppm) inhalation challenge. Pcham, pressure inside the recording chamber; Pip, intrapleural pressure recorded by telemetry. Middle and right: group data of cough responses to SO2 inhalation challenges (300 and 600 ppm, respectively, marked by shaded areas) before (average of 3 consecutive days) and on the second day after MBP or Veh treatment. Data are means ± SE (n = 8 in each group).
Statistical analysis.
Data were analyzed with a two-way repeated-measures ANOVA; one factor was the treatment effect of MBP or Veh, and the other factor was the effect of SO2 or NH3 inhalation challenge. When ANOVA showed a significant positive interaction, pairwise comparisons were made with a post hoc analysis (Fisher’s least significant difference test). A value of P < 0.05 was considered significant. Data are reported as means ± SE.
RESULTS
In awake, free-moving mice, breathing was usually unsteady and irregular (e.g., Fig. 1, left). In each breath, the change in Pcham represented the volume change in the lung. During inspiration, air inhaled into the lung expanded its volume due to its increase in temperature (from chamber temperature to body temperature), causing the Pcham to increase, which reached the peak at the end of inspiration, and Pcham decreased during expiration. The respiratory actions were more precisely distinguished by the changes of Pip (measured directly by the telemetry sensor), which was synchronized with the changes in Pcham (e.g., Fig. 1, left).
Cough was identified by the three distinct but tightly connected actions generated by an initial inspiratory effort followed by a chest compression and a forced expiration (Fig. 1, left), as described in detail in a recent report (25). During cough, Pip rose sharply to >40 mmHg during the chest compression phase. The forced expiration coincided with a weak but detectable coughing sound and a jerking movement of the head, which were identified by playing back the audio and video recordings of the experiment.
Cough was seldom detected in mice before inhalation challenge (Fig. 1). However, during SO2 inhalation challenge, cough frequency increased rapidly, approached a relatively steady level, and then gradually returned to baseline after resuming room air breathing during the recovery period (Fig. 1, middle and right). Cough responses to SO2 exhibited a concentration-dependent pattern (Figs. 1 and 2).
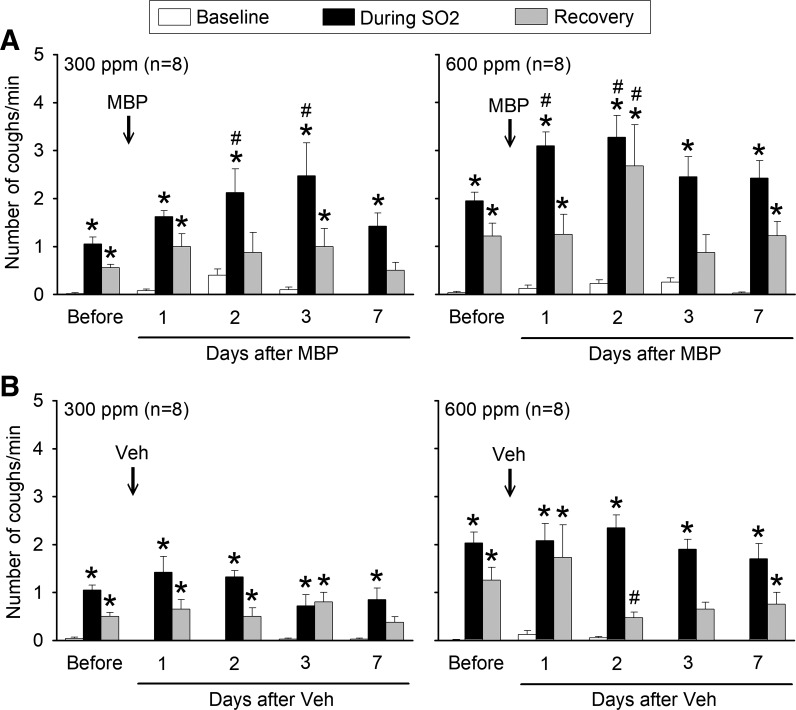
Fig. 2.
Group data illustrating effects of major basic protein (MBP; A) and vehicle (Veh; B) treatments on the cough response to SO2 inhalation challenges before (average of 3 days) and on different days after treatment. In each animal, cough frequencies were averaged over 5-min durations of baseline, during SO2, and recovery, as shown in Fig. 1. Data are means ± SE (n = 8 in each group). *Significantly (P < 0.05) different from its baseline (immediately before SO2 inhalation challenge); #significantly (P < 0.05) different from corresponding control data (before treatment of MBP or Veh).
Intratracheal instillation of MBP caused pronounced increases in cough responses to SO2 inhalation challenges at both low and high concentrations (Figs. 1 and 2). This increase in cough response to SO2 began to appear on the first day after the MBP treatment. It continued to increase, reached a peak between days 2 and 3, and then returned toward control in 3–7 days (Fig. 2A). For example, on the second day after the MBP treatment, cough frequencies during the SO2 inhalation challenges of 300 and 600 ppm rose to 197 ± 38% (P < 0.05, n = 8) and 185 ± 37% (P < 0.05, n = 8) of their control responses (before MBP), respectively (Fig. 2A).
In contrast, intratracheal instillation of Veh following the identical protocol did not cause any significant increase in cough responses to inhalation challenge of SO2 at the concentration of either 300 ppm (P > 0.05, n = 8) or 600 ppm (P > 0.05, n = 8; Fig. 2B).
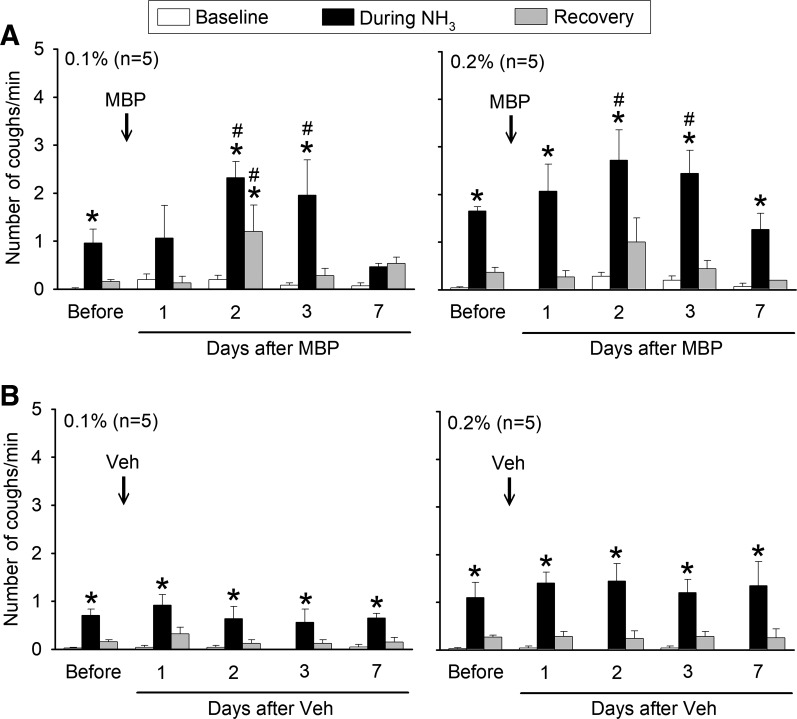
Inhalation of NH3 also evoked coughs in a concentration-dependent manner during control. Intratracheal instillation of MBP markedly elevated cough responses to NH3 inhalation challenges at both low and high concentrations (Fig. 3A). In a similar pattern and time course as that of SO2, the increase in cough response to NH3 emerged on the first day after the MBP treatment, continued to increase, and reached a peak on the second day. Cough responses then declined and returned toward control between days 3 and 7 (Fig. 3A). The Veh treatment did not induce any significant change in cough responses to NH3 (Fig. 3B).
Fig. 3.
Group data illustrating effects of major basic protein (MBP; A) and vehicle (Veh; B) treatments on the cough response to NH3 inhalation challenges before (average of 3 days) and on different days after treatment. In each animal, cough frequencies were averaged over 5-min durations of baseline, during NH3, and recovery as shown in Fig. 1. Data are means ± SE (n = 5 in each group). *Significantly (P < 0.05) different from its baseline (immediately before NH3 inhalation challenge); #significantly (P < 0.05) different from corresponding control data (before treatment of MBP or Veh).
DISCUSSION
In this study, we have clearly demonstrated that a single administration of MBP into the trachea significantly elevated cough responses to inhaled irritant gases; the cough responses to these inhalation challenges increased progressively and reached peaks 2–3 days after the MBP treatment and returned to controls after 3–7 days. In sharp contrast, cough responses to these irritant gases were not altered by the vehicle treatment. On the basis of these results, we suggest that the MBP-induced lingering elevation of cough responsiveness may play a part in the pathogenesis of chronic cough associated with eosinophilic infiltration of the airways.
Eosinophila is generally recognized as a reliable biomarker and plays a vital role in the pathogenesis of allergic airway inflammatory diseases (3, 9). A distinct association between an increase in the sputum eosinophil count and chronic cough has been extensively documented in patients with nonasthmatic eosinophilic bronchitis (4), but the pathophysiological mechanism involved is still not fully understood. One possible cause is the degranulation of eosinophils, which leads to the release of five low-molecular-weight cationic proteins: MBP, eosinophil cationic protein (ECP), eosinophil-derived neurotoxin, eosinophil peroxidase, and the MBP homolog MBP2 (9, 21). These cationic proteins are believed to be primarily responsible for the eosinophilic infiltration-induced airway mucosal injury and bronchial hyperresponsiveness (8, 12, 13, 19, 23). Furthermore, the plasma protein extravasation in the lung and airways induced by cationic proteins was mediated through the release of tachykinins (7), implying a possible involvement of C-fiber afferents innervating the respiratory tract (16).
Inhalation of selective stimulants of vagal bronchopulmonary C-fiber sensory nerves (e.g., capsaicin) consistently and reproducibly evokes coughs in humans and various animal species (5, 17, 18). Indeed, these afferents are believed to play an important role in cough hypersensitivity associated with airway inflammatory diseases (6, 18). A recent study has demonstrated that the cough response to inhaled SO2 was mediated almost exclusively through the activation of bronchopulmonary C-fiber sensory nerves in mice (14), further suggesting the involvement of these sensory nerves in the enhanced cough responsiveness induced by MBP.
In a series of studies, Lee and coworkers established the direct evidence that an intratracheal instillation of MBP, ECP, or synthetic cationic proteins triggered a sporadic but intense discharge of bronchopulmonary C-fibers (15) and also significantly enhanced the sensitivities of these afferents to capsaicin and lung inflation in anesthetized rats (10, 15). In addition, these eosinophil-derived cationic proteins induced a direct and sustained (~60 min) sensitizing effect on the responses of isolated rat pulmonary sensory neurons to chemical and electrical stimulations (11). Furthermore, a subsequent study revealed that MBP produced a significant inhibition of the sustained delayed-rectifier voltage-gated K+ current and the A-type, fast-inactivating K+ current, which were primarily responsible for the marked increase in excitability of these neurons (11). Thus, these studies have demonstrated a direct sensitizing effect of eosinophil granule cationic proteins on bronchopulmonary C-fiber sensory neurons. Taken together, it seems plausible that this direct effect of MBP on the C-fiber endings was responsible for the enhanced cough responsiveness observed in this study. However, a challenge to this premise should also be considered in view of the conspicuous difference in the time course between these two effects induced by cationic proteins. The direct electrophysiological effect of MBP on the sensory neurons exhibited a rapid onset and lasted only for a relatively short duration (<90 min) (10, 11, 15), whereas its augmenting effect on cough responsiveness was slow developing and was sustained for a substantially longer duration (>3 days; Figs. 2 and 3).
Another possible contributing factor to the MBP-induced cough hyperresponsiveness merits further consideration. MBP and other eosinophil granule cation proteins are known to possess potent cytotoxicity, which causes airway mucosal injury and inflammation in a relatively slow and sustained process (8, 19, 23). Immunohistochemical studies have shown that many of these C-fiber sensory endings are located superficially in the airway mucosa, immediately beneath or between airway epithelial cells (24). As such, the airway mucosal injury and epithelial shedding resulting from the action of MBP can expose these endings and render them more susceptible to the inhaled irritant gases and thereby heighten the cough sensitivity. A parallel can be drawn from cutaneous nociceptors, the counterpart of bronchopulmonary C-fiber endings in the skin. When skin surface (epidermis and dermis) is damaged by chemical, physical, or thermal assaults, the nociceptive endings are exposed and/or sensitized, which leads to the development of hyperalgesia (22).
In addition, eosinophil cationic proteins are also known to induce inflammatory processes in the airways, which develops progressively following the initial assault. For example, these cationic proteins can cause the release of histamine from mast cells and basophils, activate neutrophils and alveolar macrophages, and release other potent inflammatory mediators and cytokines (13). Many of these endogenous chemical mediators (e.g., prostaglandins, histamine, etc.) and cytokines (e.g., tumor necrosis factor-α; interleukin-1β, etc.) are known to exert a potent stimulatory and/or sensitizing effect on the C-fiber sensory endings (16). Therefore, these indirect effects of cationic proteins on airway sensory nerves may also contribute to the heightened cough responses to inhaled irritants.
Perspectives and Significance
Both SO2 and NH3 are common irritant gases to human lung. At high concentrations, or during sustained exposure in the case of chemical accident or unregulated occupational conditions, they can cause acute lung injury and other harmful systemic effects (1, 2). When eosinophilic infiltration of the respiratory tract occurs during allergic airway inflammation, an escalated cough sensitivity can more effectively expel and reduce the inhalation of these harmful gases, and thereby protects the lung from further injury.
GRANTS
This study was supported in part by National Institutes of Health Grants AI-123832, HL-96914, and UL1 TR-001998.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.-H.L., A.A., G.J.G., and L.-Y.L. conceived and designed research; A.-H.L., A.A., G.J.G., and L.-Y.L. performed experiments; A.-H.L., A.A., and L.-Y.L. analyzed data; A.-H.L., A.A., G.J.G., and L.-Y.L. interpreted results of experiments; A.-H.L., A.A., and L.-Y.L. prepared figures; A.-H.L., G.J.G., and L.-Y.L. drafted manuscript; A.-H.L., G.J.G., and L.-Y.L. edited and revised manuscript; A.-H.L., A.A., G.J.G., and L.-Y.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Ruei-Lung Lin, Tanya Seward, and Melissa Hollifield for technical assistances.
REFERENCES
- 1.Agency for Toxic Substances and Disease Registry (ATSDR), Division of Toxicology Public Health Statement: Sulfur Dioxide (CAS#: 7446-09-5). https://www.atsdr.cdc.gov/ToxProfiles/TP.asp?id=253&tid=46. 1998.
- 2.Agency for Toxic Substances and Disease Registry (ATSDR), Division of Toxicology Public Health Statement: Ammonia (CAS#: 7664-41-7). https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=11&tid=2. 2004.
- 3.Berry A, Busse WW. Biomarkers in asthmatic patients: has their time come to direct treatment? J Allergy Clin Immunol 137: 1317–1324, 2016. doi: 10.1016/j.jaci.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Brightling CE, Ward R, Goh KL, Wardlaw AJ, Pavord ID. Eosinophilic bronchitis is an important cause of chronic cough. Am J Respir Crit Care Med 160: 406–410, 1999. doi: 10.1164/ajrccm.160.2.9810100. [DOI] [PubMed] [Google Scholar]
- 5.Canning BJ, Chang AB, Bolser DC, Smith JA, Mazzone SB, McGarvey L; CHEST Expert Cough Panel . Anatomy and neurophysiology of cough: CHEST Guideline and Expert Panel report. Chest 146: 1633–1648, 2014. doi: 10.1378/chest.14-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung KF. Approach to chronic cough: the neuropathic basis for cough hypersensitivity syndrome. J Thorac Dis 6, Suppl 7: S699–S707, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coyle AJ, Uchida D, Ackerman SJ, Mitzner W, Irvin CG. Role of cationic proteins in the airway. Hyperresponsiveness due to airway inflammation. Am J Respir Crit Care Med 150: S63–S71, 1994. doi: 10.1164/ajrccm/150.5_Pt_2.S63. [DOI] [PubMed] [Google Scholar]
- 8.Frigas E, Loegering DA, Gleich GJ. Cytotoxic effects of the guinea pig eosinophil major basic protein on tracheal epithelium. Lab Invest 42: 35–43, 1980. [PubMed] [Google Scholar]
- 9.Gleich GJ, Adolphson CR. The eosinophilic leukocyte: structure and function. Adv Immunol 39: 177–253, 1986. doi: 10.1016/S0065-2776(08)60351-X. [DOI] [PubMed] [Google Scholar]
- 10.Gu Q, Lee LY. Hypersensitivity of pulmonary C fibre afferents induced by cationic proteins in the rat. J Physiol 537: 887–897, 2001. doi: 10.1113/jphysiol.2001.012819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu Q, Lim ME, Gleich GJ, Lee LY. Mechanisms of eosinophil major basic protein-induced hyperexcitability of vagal pulmonary chemosensitive neurons. Am J Physiol Lung Cell Mol Physiol 296: L453–L461, 2009. doi: 10.1152/ajplung.90467.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gundel RH, Letts LG, Gleich GJ. Human eosinophil major basic protein induces airway constriction and airway hyperresponsiveness in primates. J Clin Invest 87: 1470–1473, 1991. doi: 10.1172/JCI115155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamann KJ, Gleich GJ, Gundel RH, White AR. Interaction between respiratory epithelium and eosinophil granule in asthma: the eosinophil hypothesis. In: The Airway Epithelium: Physiology, Pathophysiology, and Pharmacology. Lung Biology in Health and Disease Series, edited by Farmer SG, Hay DWP. New York: Dekker, 1991, p. 255–300. [Google Scholar]
- 14.Lin RL, Zhang C, Khosravi M, Lin AH, Athukorala A, Lee LY. . Cough response to sulfur dioxide inhalation challenge is enhanced by tumor necrosis factor alpha: a primary role of vagal bronchopulmonary C-fibers. FASEB J 32: 913.2, 2018. [Google Scholar]
- 15.Lee LY, Gu Q, Gleich GJ. Effects of human eosinophil granule-derived cationic proteins on C-fiber afferents in the rat lung. J Appl Physiol (1985) 91: 1318–1326, 2001. doi: 10.1152/jappl.2001.91.3.1318. [DOI] [PubMed] [Google Scholar]
- 16.Lee LY, Yu J. Sensory nerves in lung and airways. Compr Physiol 4: 287–324, 2014. doi: 10.1002/cphy.c130020. [DOI] [PubMed] [Google Scholar]
- 17.Mazzone SB, Undem BJ. Cough sensors. V. Pharmacological modulation of cough sensors. Handb Exp Pharmacol 187: 99–127, 2009. doi: 10.1007/978-3-540-79842-2_6. [DOI] [PubMed] [Google Scholar]
- 18.Morice AH, Millqvist E, Belvisi MG, Bieksiene K, Birring SS, Chung KF, Dal Negro RW, Dicpinigaitis P, Kantar A, McGarvey LP, Pacheco A, Sakalauskas R, Smith JA. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur Respir J 44: 1132–1148, 2014. doi: 10.1183/09031936.00218613. [DOI] [PubMed] [Google Scholar]
- 19.Motojima S, Frigas E, Loegering DA, Gleich GJ. Toxicity of eosinophil cationic proteins for guinea pig tracheal epithelium in vitro. Am Rev Respir Dis 139: 801–805, 1989. doi: 10.1164/ajrccm/139.3.801. [DOI] [PubMed] [Google Scholar]
- 20.Ohnuki LE, Wagner LA, Georgelas A, Loegering DA, Checkel JL, Plager DA, Gleich GJ. Differential extraction of eosinophil granule proteins. J Immunol Methods 307: 54–61, 2005. doi: 10.1016/j.jim.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Plager DA, Loegering DA, Weiler DA, Checkel JL, Wagner JM, Clarke NJ, Naylor S, Page SM, Thomas LL, Akerblom I, Cocks B, Stuart S, Gleich GJ. A novel and highly divergent homolog of human eosinophil granule major basic protein. J Biol Chem 274: 14464–14473, 1999. doi: 10.1074/jbc.274.20.14464. [DOI] [PubMed] [Google Scholar]
- 22.Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev 89: 707–758, 2009. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- 23.Uchida DA, Ackerman SJ, Coyle AJ, Larsen GL, Weller PF, Freed J, Irvin CG. The effect of human eosinophil granule major basic protein on airway responsiveness in the rat in vivo. A comparison with polycations. Am Rev Respir Dis 147: 982–988, 1993. doi: 10.1164/ajrccm/147.4.982. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe N, Horie S, Michael GJ, Keir S, Spina D, Page CP, Priestley JV. Immunohistochemical co-localization of transient receptor potential vanilloid (TRPV)1 and sensory neuropeptides in the guinea-pig respiratory system. Neuroscience 141: 1533–1543, 2006. doi: 10.1016/j.neuroscience.2006.04.073. [DOI] [PubMed] [Google Scholar]
- 25.Zhang C, Lin RL, Hong J, Khosravi M, Lee LY. Cough and expiration reflexes elicited by inhaled irritant gases are intensified in ovalbumin-sensitized mice. Am J Physiol Regul Integr Comp Physiol 312: R718–R726, 2017. doi: 10.1152/ajpregu.00444.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]