Abstract
Background
KIF1C (Kinesin Family Member 1C) variants have been associated with hereditary spastic paraplegia and spastic ataxia.
Case report
We report fraternal twins presenting with cerebellar ataxia and dystonic tremor. Their brain MRI showed a hypomyelinating leukoencephalopathy. Whole exome sequencing identified a homozygous KIF1C variant in both patients.
Discussion
KIF1C variants can manifest as a complex movement disorder with cerebellar ataxia and dystonic tremor. KIF1C variants may also cause a hypomyelinating leukoencephalopathy.
Keywords: KIF1C, dystonic tremor, cerebellar ataxia, hypomyelinating leukoencephalopathy
Introduction
KIF1C (Kinesin Family Member 1C MIM 603060) variants have been associated with hereditary spastic paraplegia (HSP) and spastic ataxia-2 (SPAX2 MIM 611302).1–4 Patients bearing KIF1C variants were first reported in two consanguineous Palestinian and Moroccan families with early-onset cerebellar ataxia followed by pyramidal symptoms,1 at a locus previously associated with autosomal recessive spastic ataxia-2 (SPAX2 MIM 611302).5 Subsequently, Novarino et al. identified a homozygous deletion of exon 14–18 in the original SPAX2 family, confirming that KIF1C was the SPAX2 gene.2 The disease was then designated as spastic paraplegia type 58 (SPG58).3
Thanks to whole exome sequencing (WES), the phenotype of genes known to be associated with HSP keeps expanding and the overlap between HSPs and other neurological diseases suggests common cellular mechanisms.6 Hereinafter, we report two fraternal twins carrying a homozygous KIF1C variant detected by WES and presenting with cerebellar ataxia, dystonic tremor, and a hypomyelinating leukoencephalopathy.
Case description
Seventeen-year-old fraternal twins (a female and a male) of Moroccan descent were referred to a neurometabolic reference center for early-onset cerebellar ataxia associated with white matter abnormalities on brain MRI. Their asymptomatic parents were first cousins, and medical family history was unremarkable. Pregnancy, delivery (at 37 gestational weeks), and early development were normal for both patients. They started walking at around 1 year of age, but frequent falls occurred during infancy, complicated by writing difficulties and dysarthria at primary school. Gait and speech worsened during adolescence, but both patients obtained their bachelor’s degree. At 17-year-old, the boy presented with cerebellar ataxia with a SARA (Scale for the Assessment and Rating of Ataxia) score of 12/40, associated with dystonic tremor. He displayed increased deep tendon reflexes in the lower extremities and a right Babinski sign. Vibration sense was mildly reduced in the lower extremities. Oculomotor examination showed a saccadic pursuit with dysmetric saccades and a bilateral ptosis. The girl displayed a similar phenotype with cerebellar ataxia – SARA score of 10/40 – associated with a dystonic tremor predominantly in the neck, which appeared at 12-year-old. Vibration sense was reduced in the lower extremities but without any pyramidal sign. Oculomotor examination showed a saccadic pursuit with dysmetric saccades. Over 3 years of observation, cerebellar ataxia remained stable in both patients, but dystonic tremor became more prominent in the upper limbs and executive functions declined. A formal neuropsychological evaluation with WAIS-IV scale was performed at 19-year-old. Both patients showed heterogeneous scores. The boy obtained a verbal comprehension index (VCI) of 84, a perceptual organization index (POI) of 72, a working memory index (WMI) of 68, and a processing speed index (PSI) of 55. The girl obtained a VCI of 83, a POI of 84, a WMI of 80, and a PSI of 66. Furthermore, the boy presented with increasing spasticity in the lower limbs and the girl had an episode of intestinal occlusion (later diagnosed as Crohn’s disease) that worsened her tremor and dystonic features. Patients’ neurological examination is shown at 20-year-old (Video 1).
Video 1.
Clinical examination in both fraternal twins. They both present with cerebellar gait and dysmetria as well as distal postural and action tremor of the upper limbs. In addition, the boy displays mild spastic gait and the girl dystonic tremor of the neck (barely noticeable on the video due to regular botulinum toxin injections) as well as dystonic postures of the upper limbs.
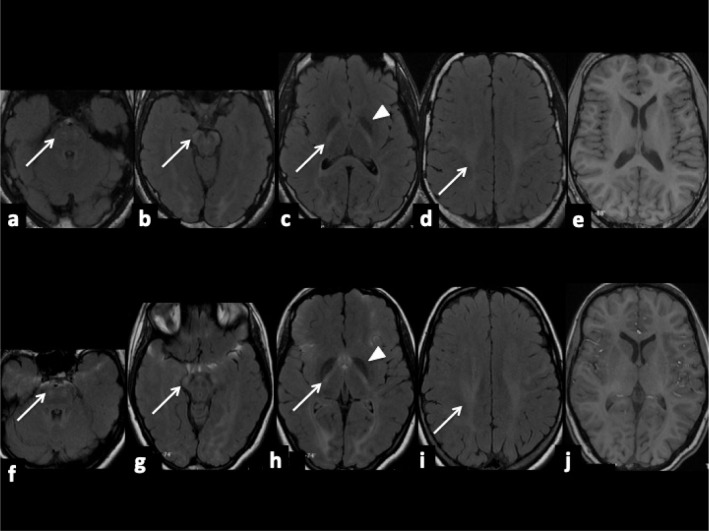
Polymyographic recording found postural and action tremor of the upper limbs in both twins and dystonic tremor of the neck in the girl (frequency of 5.5 Hz), without myoclonus. Brain MRI showed diffuse but mild T2- and FLAIR-hyperintensities, with T1-isointensity, of the internal capsules, optic radiations and cortico-spinal tracts, associated with mild T2-hypointensities of the globus pallidi, suggesting a hypomyelinating leukoencephalopathy (Figure 1).7 DaTscan and nerve conduction studies were normal. Visual, brainstem auditory, somatosensory, and motor evoked potentials displayed diffuse conduction anomalies, compatible with a hypomyelinating disorder. So far, the demyelinating neuropathy has remained asymptomatic with normal visual campimetry, visual acuity, and optical coherence tomography in both patients. A targeted panel of 45 genes associated with leukodystrophies and leukoencephalopathies was performed, but failed to reveal the cause of the disease. The probands and their unaffected parents consented to WES. A homozygous variant in KIF1C (NM_006612.5:c.1019+1dup) was detected in both twins and confirmed by Sanger sequencing. Both parents were heterozygous carriers of the variant. The c.1019+1dup variant (class 5, pathogenic) occurs at one of the splicing donor sites and alters the splice site recognition, is not present in the Exome Variant Server (EVS), in the Exome Aggregation Consortium (ExAC) nor in GnomAD databases.
Figure 1.
Brain MRI of fraternal twins (boy: a-b-c-d-e and girl: f-g-h-i-j). Axial FLAIR-weighted images (a-b-c-d/f-g-h-i) showing diffuse but mild hyperintensities (arrows) of the cortico-spinal tracts with T1-isointensity (e and j), associated with mild hypointensities of the globus pallidi (arrowheads), suggesting a hypomyelinating leukoencephalopathy.
Discussion
Our patients show that biallelic KIF1C variants can cause a predominant movement disorder characterized by cerebellar ataxia and dystonic tremor. Brain MRI may provide additional diagnostic clues in the presence of a hypomyelinating leukoencephalopathy.
KIF1C variants have been associated mostly with complicated forms of HSP, initially called SPAX25 and then HSP58.3 Cerebellar ataxia may occur in infancy, later complicated by spastic paraplegia.4 Adult-onset forms of spastic ataxia have also been reported.3 Of note, heterozygous mutation carriers may present with a mild phenotype such as demyelinating sensory-motor neuropathy or mild spasticity.3 This intriguing mode of inheritance, with a severe phenotype in recessive forms and a milder one in heterozygous carriers, has been previously described for other forms of HSP, such as SPG7.8 Dystonia has been previously reported in three patients 1,3,4 (two at the cervical level and one affecting upper extremities), otherwise presenting with a complicated HSP phenotype. As observed in other recessive ataxias (i.e., ataxia-telangiectasia variants or ataxia with vitamin E deficiency), our patients exhibited a predominant movement disorder. This observation is in line with recent research highlighting a putative role of the cerebellum in the pathophysiology of dystonia.9 White matter abnormalities of the cortico-spinal tracts have been observed by several groups in young adults with KIF1C variants.1,3,4 However, these studies did not specify the T1 appearance of patients’ FLAIR and T2 hyperintense lesions, which would help discriminating between demyelinating and hypomyelinating lesions.7 Here, we provide clear evidence for a pattern of hypomyelinating leukoencephalopathy in our KIF1C-mutated patients, consistent with the common onset of the disease in infancy.
KIF1C (Kinesin Family Member 1C MIM 603060) encodes a microtubule-based motor protein belonging to the kinesin family.10 Two other members of this family (KIF5A and KIF1A) have been associated with pure and complicated forms of HSP, SPG10, and SPG30, respectively.11,12 KIF1C is localized at the pericentrosome and was initially thought to participate in protein transport from the Golgi to the endoplasmic reticulum.13
More recently, the putative role of KIF1C on the structure of the Golgi complex14 and in membrane dynamics in podosome turnover15–17 have been demonstrated. Several model systems have been proposed to explore the role of KIF1C in neuronal development. KIF1C knock-out mice did not show any neurological phenotype, presumably because of the redundant expression of other kinesins in brain.18 Instead, a KIF1C-mutated cattle has been proposed as the first efficient animal model to explore KIF1C function.19 KIF1C-deficient cattle display a progressive form of ataxia named ataxia of Charolais, mimicking spastic ataxia in humans. Authors hypothesized that KIF1C loss of function alters membrane trafficking and membrane wrapping in oligodendrocytes along axons, highlighting the role of KIF1C protein in preserving the structural integrity and function of myelin.19 The observation of a hypomyelinating leukoencephalopathy in our patients, supporting the early involvement of oligodendrocytes in the disease process, is of particular relevance in this context.
Acknowledgments
We are grateful to the patients for their participation.
Footnotes
Citation: Marchionni E, Méneret A, Keren B, Melki J, Denier C, Durr A, et al. KIF1C Variants Are Associated with Hypomyelination, Ataxia, Tremor and Dystonia in Fraternal Twins. Tremor Other Hyperkinet Mov. 2019: 9. doi: 10.7916/tohm.v0.641
Editor: Elan D. Louis, Yale University, USA
Funding: None.
Financial Disclosures: None.
Conflicts of Interest: The authors report no conflicts of interest.
Ethics Statements: This study was performed in accordance with the ethical standards detailed in the Declaration of Helsinki. We have received a written patient consent form from the participants in the study. The consent is kept on file with the patients’ case notes.
References
- 1.Dor T, Cinnamon Y, Raymond L, et al. KIF1C mutations in two families with hereditary spastic paraparesis and cerebellar dysfunction. J Med Genet 2014;51(2):137–142. doi: 10.1136/jmedgenet-2013-102012 [DOI] [PubMed] [Google Scholar]
- 2.Novarino G, Fenstermaker AG, Zaki MS, et al. Exome sequencing links corticospinal motor neuron disease to common neurodegenerative disorders. Science 2014;343(6170):506–511. doi: 10.1126/science.1247363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caballero Oteyza A, Battaloğlu E, Ocek L, et al. Motor protein mutations cause a new form of hereditary spastic paraplegia. Neurology 2014;82(22):2007–2016. doi: 10.1212/WNL.0000000000000479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yücel-Yılmaz D, Yücesan E, Yalnızog˘lu D, et al. Clinical phenotype of hereditary spastic paraplegia due to KIF1C gene mutations across life span. Brain Dev 2018;40(6):458–464. doi: 10.1016/j.braindev.2018.02.013 [DOI] [PubMed] [Google Scholar]
- 5.Bouslam N, Bouhouche A, Benomar A, et al. A novel locus for autosomal recessive spastic ataxia on chromosome 17p. Hum Genet 2007;121(3–4):413–420. doi: 10.1007/s00439-007-0328-0 [DOI] [PubMed] [Google Scholar]
- 6.Parodi L, Coarelli G, Stevanin G, Brice A, Durr A. Hereditary ataxias and paraparesias: clinical and genetic update. Curr Opin Neurol 2018;31(4):462–471. doi: 10.1097/WCO.0000000000000585 [DOI] [PubMed] [Google Scholar]
- 7.Schiffmann R, van der Knaap MS. Invited article: an MRI-based approach to the diagnosis of white matter disorders. Neurology 2009;72(8):750–759. doi: 10.1212/01.wnl.0000343049.00540.c8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klebe S, Depienne C, Gerber S, et al. Spastic paraplegia gene 7 in patients with spasticity and/or optic neuropathy. Brain 2012;135(10):2980–2993. doi: 10.1093/brain/aws240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shakkottai VG, Batla A, Bhatia K, et al. Current opinions and areas of consensus on the role of the cerebellum in Dystonia. Cerebellum 2017;16(2):577–594. doi: 10.1007/s12311-016-0825-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol 2009;10(10):682–696. doi: 10.1038/nrm2774 [DOI] [PubMed] [Google Scholar]
- 11.Reid E, Kloos M, Ashley-Koch A, et al. A kinesin heavy chain (KIF5A) mutation in hereditary spastic paraplegia (SPG10). Am J Hum Genet 2002;71(5):1189–1194. doi: 10.1086/344210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klebe S, Lossos A, Azzedine H, et al. KIF1A missense mutations in SPG30, an autosomal recessive spastic paraplegia: distinct phenotypes according to the nature of the mutations. Eur J Hum Genet 2012;20(6):645–649. doi: 10.1038/ejhg.2011.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorner C, Ciossek T, Müller S, Møller PH, Ullrich A, Lammers R. Characterization of KIF1C, a new kinesin-like protein involved in vesicle transport from the Golgi apparatus to the endoplasmic reticulum. J Biol Chem 1998;273(32):20267–20275. doi: 10.1074/jbc.273.32.20267 [DOI] [PubMed] [Google Scholar]
- 14.Lee PL, Ohlson MB, Pfeffer SR. The Rab6-regulated KIF1C kinesin motor domain contributes to Golgi organization. Elife 2015;4:e06029. doi: 10.7554/eLife.06029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopp P, Lammers R, Aepfelbacher M, et al. The kinesin KIF1C and microtubule plus ends regulate podosome dynamics in macrophages. Mol Biol Cell 2006;17(6):2811–2823. doi: 10.1091/mbc.e05-11-1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhuwania R, Castro-Castro A, Linder S. Microtubule acetylation regulates dynamics of KIF1C-powered vesicles and contact of microtubule plus ends with podosomes. Eur J Cell Biol 2014;93(10–12):424–437. doi: 10.1016/j.ejcb.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 17.Zhu X, Efimova N, Arnette C, Hanks SK, Kaverina I. Podosome dynamics and location in vascular smooth muscle cells require CLASP-dependent microtubule bending. Cytoskeleton(Hoboken) 2016;73(6):300–315. doi: 10.1002/cm.21302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakajima K, Takei Y, Tanaka Y, et al. Molecular motor KIF1C is not essential for mouse survival and motor-dependent retrograde Golgi apparatus-to-endoplasmic reticulum transport. Mol Cell Biol 2002;22(3):866–873. doi: 10.1128/MCB.22.3.866-873.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duchesne A, Vaiman A, Frah M, et al. Progressive ataxia of Charolais cattle highlights a role of KIF1C in sustainable myelination. PLoS Genet 2018;14(8):e1007550. doi: 10.1371/journal.pgen.1007550 [DOI] [PMC free article] [PubMed] [Google Scholar]