Abstract
β-Cell dysfunction is central to the pathogenesis of impaired glucose tolerance (IGT) and type 2 diabetes. Compared with adults, youth have hyperresponsive β-cells and their decline in β-cell function appears to be more rapid. However, there are no direct comparisons of β-cell responses to pharmacological intervention between the two age-groups. The Restoring Insulin Secretion (RISE) Adult Medication Study and the RISE Pediatric Medication Study compared interventions to improve or preserve β-cell function. Obese youth (n = 91) and adults (n = 132) with IGT or recently diagnosed type 2 diabetes were randomized to 3 months of insulin glargine followed by 9 months of metformin, or 12 months of metformin. Hyperglycemic clamps conducted at baseline, after 12 months of medication, and 3 months after medication withdrawal assessed β-cell function as steady-state and maximal C-peptide responses adjusted for insulin sensitivity. Temporal changes in β-cell function were distinctly different. In youth, β-cell function deteriorated during treatment and after treatment withdrawal, with no differences between treatment groups. In adults, β-cell function improved during treatment, but this was not sustained after treatment withdrawal. The difference in β-cell function outcomes in response to medications in youth versus adults supports a more adverse trajectory of β-cell deterioration in youth.
Introduction
The incidence and prevalence of type 2 diabetes have increased and reached alarming levels (1,2). These increases are not limited to the adult population, as type 2 diabetes is becoming more common in youth during their second decade of life (1), with an incidence in the U.S. that is projected to triple over the next 40 years (2). The pathogenesis of type 2 diabetes in youth is poorly understood, and how it differs from that seen in adults is not known.
The Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study revealed that β-cell function in youth-onset type 2 diabetes declined at a rate of 20–35% per year, considerably accelerated compared with adult diabetes (3,4). This rapid decline resulted in progressive worsening of glycemic control even with good adherence to metformin and/or rosiglitazone (5). The TODAY Study established β-cell dysfunction as a critical factor on the path to the development of youth-onset type 2 diabetes.
The Restoring Insulin Secretion (RISE) Consortium was established by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to test approaches to preserve β-cell function in youth and adults with impaired glucose tolerance (IGT) or recently diagnosed type 2 diabetes. The RISE Pediatric Medication Study and RISE Adult Medication Study were designed in tandem to allow for a direct comparison of the impact of pharmacological treatment on β-cell function, comparing metformin alone with insulin glargine followed by metformin (the only medications approved by the U.S. Food and Drug Administration for use in type 2 diabetes in youth). An identical hyperglycemic clamp protocol performed in both studies allowed for direct comparison of these treatments between youth and adults (6).
We have previously reported a number of important and revealing differences between the youth and adult RISE study cohorts at baseline (7). Despite similar levels of obesity and dysglycemia, obese youth with IGT or recently diagnosed type 2 diabetes 1) were markedly more insulin resistant than adults, 2) exhibited markedly augmented β-cell responses to intravenously administered glucose and to the nonglucose secretagogue arginine, and 3) exhibited augmented β-cell responses even after accounting for insulin sensitivity, suggesting that the workload on their β-cells is greater than that observed in adults. Further, we have reported that the RISE treatments failed to halt progressive β-cell failure over time in youth (8). These fundamental metabolic differences described between youth and adults at baseline, together with the poor response to both metformin alone or insulin glargine followed by metformin in youth, suggest potential differences between youth and adults in the pathogenesis and/or time course of β-cell failure.
Here we test the hypothesis that youth and adults respond differently to identical pharmacological treatments targeting β-cell function. To address this hypothesis, the following key questions were posed: 1) Do the effects of glargine followed by metformin or metformin alone on β-cell function differ between youth and adults? and 2) Are changes in β-cell function following 12 months of active treatment more durable in adults compared with youth when assessed 3 months after treatment withdrawal?
Research Design and Methods
Study Protocols
The RISE Pediatric Medication Study and RISE Adult Medication Study were randomized, partially blinded clinical trials. The rationale and methods have been described in detail previously (6,9) and the study protocol is available online at https://rise.bsc.gwu.edu/web/rise/collaborators. Each participating center’s institutional review board (IRB) approved the appropriate protocol. Consistent with the Declaration of Helsinki and each center’s IRB guidelines, written informed consent or assent was obtained from each participant.
Participants
Screening and Eligibility: Youth
Youth aged 10–19 years with pubertal development at or beyond Tanner stage II at high risk for IGT or with recently diagnosed type 2 diabetes were screened with a medical history, physical examination, and additional laboratory tests for purposes of inclusion/exclusion (8). A BMI in the 85th percentile or greater for age and sex with a maximum BMI ≤50 kg/m2 was required. A screening 75-g oral glucose tolerance test (OGTT) and hemoglobin A1c (HbA1c) were obtained. If drug naive, an OGTT with a fasting plasma glucose ≥5 mmol/L, 2-h glucose ≥7.8 mmol/L, and HbA1c ≤8.0% (≤64 mmol/mol) were required. The HbA1c criteria for youth with type 2 diabetes already taking metformin were ≤7.5% (≤58 mmol/mol) if on metformin for <3 months and ≤7.0% (≤53 mmol/mol) if on metformin for 3–6 months. Testing negative for GAD and IA2 autoantibodies was also required.
Screening and Eligibility: Adults
Adults were similarly screened with a medical history, physical examination, and laboratory tests for inclusion/exclusion (10). Eligibility criteria included age 20–65 years and BMI ≥25 kg/m2 (≥23 kg/m2 for Asian Americans) but <50 kg/m2. A screening 75-g OGTT and HbA1c were obtained. Eligibility required a fasting glucose 5.3–6.9 mmol/L, OGTT 2-h glucose ≥7.8 mmol/L, and HbA1c ≤7.0% (≤53 mmol/mol). Adults with known IGT or diabetes for <1 year were eligible if they had never received glucose-lowering medications and otherwise qualified.
For both groups, final eligibility for the study required ≥80% compliance with 3 weeks of oral placebo medication and placebo injection. Eligible participants underwent a baseline 3-h OGTT and two-step hyperglycemic clamp, followed by random treatment assignment stratified by study site. Pediatric and adult participants were randomized to glargine for 3 months followed by metformin for 9 months, or to metformin alone for 12 months. Additional adults were also randomized to two other arms that were not part of the study in youth and therefore are not included in this report.
Interventions
Adult participants randomized to metformin alone received double-blinded tablet assignment, while metformin was unmasked in youth. Titration of medication took place over 4 weeks, starting at a dose of 500 mg once daily, to a maximum dose of 1,000 mg twice daily. Participants unable to tolerate a given dose increment were reverted to the highest previously tolerated dose.
The insulin glargine followed by metformin group received 3 months of insulin glargine titrated at least twice weekly by a preset algorithm (8,10) to achieve a fasting glucose of 4.4–5.0 mmol/L on the basis of daily self-monitored blood glucose. This was followed immediately by 9 months of metformin (titrated to 1,000 mg twice daily). Youth already taking metformin and randomized to insulin glargine therapy discontinued active metformin at the time of initiation of insulin glargine treatment.
Participants took their assigned intervention for 12 months, after which it was withdrawn, and follow-up continued while off study medication for 3 months to determine the 15-month coprimary outcomes.
Participants returned to the clinic every 3 months for determination of medication adherence and assessment of adverse events. Metformin adherence was determined by the pill count in the returned medication bottle, and insulin glargine adherence was based on the residual volume of fluid in the pen. If HbA1c safety thresholds were exceeded at any visit, outcome assessments were performed whenever possible before instituting rescue therapy. Safety was monitored by an independent data and safety monitoring board.
Procedures and Calculations
Anthropometrics
Anthropometric measurements were obtained with participants wearing light clothing without shoes. Waist circumference, height, and weight were measured with standard methods as previously described (8).
Hyperglycemic Clamp
After a 10-h overnight fast, a two-step hyperglycemic clamp was performed with goal glucose levels of 11.1 mmol/L followed by ≥25 mmol/L, the latter representing maximal glycemic potentiation and at which the nonglucose secretagogue arginine was administered (7). Briefly, target glucose levels were achieved using boluses and a variable rate intravenous infusion of 20% dextrose, the rate based on a computerized algorithm developed by the Consortium, combined with bedside glucose monitoring. Arterialized blood samples were obtained prior to and at 2, 4, 6, 8, 10, 30, 60, 90, 100, 110, and 120 min after starting the glucose infusion; the final three samples were used to define the steady-state interval for the first step (11.1 mmol/L) target. For the second step, after a minimum of an additional 15 min with glucose levels >25 mmol/L, the response to hyperglycemia plus arginine was determined using blood samples drawn 5 and 1 min prior to and 2, 3, 4 and 5 min after bolus injection of arginine (5 g).
Hyperglycemic Clamp–Derived Measures
Insulin Sensitivity.
Insulin sensitivity (M/I) was quantified as the mean of the glucose infusion rate at 100, 110, and 120 min of the clamp, expressed as per kilogram of body weight and corrected for urinary glucose loss (M), divided by the mean steady-state plasma insulin concentration (I) at these same time points. Urinary glucose loss was determined as the product of the measured urinary glucose concentration and urinary volume (7).
C-Peptide Responses.
The acute (first-phase) C-peptide response to glucose (ACPRg) was calculated as the mean incremental response above baseline (average of −10 and −5 min) from samples drawn at 2, 4, 6, 8, and 10 min following glucose administration (7).
Steady-state (second-phase) C-peptide levels were calculated as the mean C-peptide concentrations at 100, 110, and 120 min of the hyperglycemic clamp (7).
The acute C-peptide response to arginine at maximal glycemic potentiation (ACPRmax; >25 mmol/L) was calculated as the mean concentrations in samples drawn 2, 3, 4, and 5 min after arginine injection minus the average of concentration of the samples obtained 1 and 5 min prior to arginine administration (7).
Assays
After sampling, all blood samples were immediately placed on ice, separated, frozen, and stored at −80°C. Frozen samples were shipped overnight to the central biochemistry laboratory at the University of Washington for assay of glucose, insulin, and C-peptide concentrations using methods previously described (7).
Statistical Analyses
Data were collected centrally, and analyses were performed according to a prespecified analysis plan. For these proof-of-principle trials, we chose two measures of β-cell function as coprimary outcomes: clamp-derived steady-state C-peptide and ACPRmax at 15 months, both evaluated jointly with M/I (6,8,10). Clamp-derived ACPRg was evaluated as a secondary outcome.
The prespecified primary analysis was the comparison of β-cell responses paired with M/I between treatment groups at month 15 of the study (i.e., 3 months after treatment withdrawal), adjusted for baseline β-cell response and M/I. This provides a treatment group comparison of the durability effect after treatment withdrawal. Major secondary analyses compared treatment groups within each study at the end of active intervention, 12 months. Joint models for β-cell response and M/I were fit simultaneously using seemingly unrelated regression techniques (11–13), which provide a 2-DF χ2 test of the treatment arm difference in the joint values of β-cell response and M/I between treatment groups within study. All models used natural logarithmically transformed insulin sensitivity (M/I) and β-cell response variables due to the skewed distribution of these data. Prior to taking logs, a constant of 1.06 was added to the ACPRg because of negative values in this β-cell response variable. Two youth participants randomized to metformin had HbA1c levels that required treatment during the active intervention. These participants successfully completed the 12-month visit before being withdrawn from the study. Their data are included through 12 months. Imputing worst-case values at 15-months for these participants did not appreciably alter the results; these results are not presented further.
The Hotelling T2 method was used to simultaneously test changes in β-cell responses and M/I within each treatment group over time (13). Glucose and C-peptide concentrations across study and treatment groups throughout the hyperglycemic clamp were compared using t tests. Percent changes from baseline across study within treatment arm in β-cell responses and M/I were compared using the nonparametric Kruskal-Wallis test due to their skewed distributions. Changes from baseline in BMI and HbA1c were compared across study within each treatment arm at specific time points using t tests.
Results
Baseline Demographic, Physical, and Metabolic Characteristics of Youth and Adults by Treatment Groups
Adults assigned to insulin glargine plus metformin were similar in age to those assigned to metformin alone, whereas youth assigned to the metformin alone group were slightly younger than those assigned to insulin glargine followed by metformin, but similar in Tanner stage (Table 1). Notably, the pediatric study included a larger proportion of females and of nonwhite participants than the adult study. In both treatment groups, youth and adults had similar body weight and BMI, whereas the waist-to-hip ratio was higher in adults compared with youth in both treatment arms. Systolic and diastolic blood pressures were higher in adults as was the use of blood pressure–lowering medication. HbA1c and the proportion of participants with IGT were similar among adults and youth and across treatment arms.
Table 1.
Baseline characteristics by treatment group
| Glargine followed by metformin |
Metformin alone |
||||
|---|---|---|---|---|---|
| Adult |
Youth | Adult | Youth | ||
| N = 67 | N = 44 | N = 65 | N = 47 | P value | |
| Anthropometrics | |||||
| Age (years) | 53.5 ± 9.3 | 14.9 ± 2.0 | 55.2 ± 8.2 | 13.9 ± 2.1 | <0.001 |
| Female | 23 (34.3) | 27 (61.4) | 37 (56.9) | 38 (80.9) | <0.001 |
| Race/ethnicity | <0.001 | ||||
| White | 37 (55.2) | 13 (29.5) | 34 (52.3) | 12 (25.5) | |
| Black | 21 (31.3) | 14 (31.8) | 19 (29.2) | 9 (19.1) | |
| Hispanic (any) | 5 (7.5) | 14 (31.8) | 6 (9.2) | 20 (42.6) | |
| Other | 4 (6.0) | 3 (6.8) | 6 (9.2) | 6 (12.8) | |
| Weight (kg) | 104.4 ± 20.0 | 102.0 ± 25.7 | 98.1 ± 18.6 | 97.7 ± 23.3 | 0.274 |
| BMI (kg/m2) | 35.0 ± 5.9 | 36.5 ± 6.4 | 35.0 ± 5.1 | 36.9 ± 6.4 | 0.201 |
| BMI percentile | 98.4 ± 2.5 | 98.8 ± 1.3 | 0.297 | ||
| Waist-to-hip ratio | 0.97 ± 0.07 | 0.93 ± 0.08 | 0.95 ± 0.08 | 0.94 ± 0.07 | 0.029 |
| Systolic BP (mmHg) | 127.7 ± 12.0 | 120.7 ± 7.8 | 127.1 ± 13.3 | 119.5 ± 8.7 | <0.001 |
| Diastolic BP (mmHg) | 78.7 ± 9.5 | 67.6 ± 7.7 | 77.8 ± 11.1 | 70.1 ± 7.9 | <0.001 |
| BP-lowering medication use (yes) | 34 (50.7) | 1 (2.3) | 35 (53.8) | 2 (4.3) | <0.001 |
| Metabolic phenotype | |||||
| HbA1c (%) | 5.80 ± 0.33 | 5.73 ± 0.60 | 5.77 ± 0.40 | 5.68 ± 0.57 | 0.561 |
| HbA1c (mmol/L) | 39.9 ± 3.6 | 39.2 ± 6.5 | 39.5 ± 4.3 | 38.6 ± 6.3 | 0.561 |
| IGT | 50 (74.6) | 26 (59.1) | 49 (75.4) | 28 (59.6) | 0.105 |
| Lipids | |||||
| Total cholesterol (mmol/L) | 4.21 ± 0.96 | 3.86 ± 0.91 | 4.51 ± 0.94 | 3.72 ± 0.65 | <0.001 |
| LDL cholesterol (mmol/L) | 2.41 ± 0.77 | 2.25 ± 0.80 | 2.68 ± 0.81 | 2.08 ± 0.63 | <0.001 |
| Triglycerides (mmol/L) | 1.36 [0.58, 3.20] | 1.11 [0.39, 3.14] | 1.28 [0.50, 3.28] | 1.17 [0.45, 3.06] | 0.314 |
| HDL cholesterol (mmol/L) | 1.12 ± 0.24 | 1.02 ± 0.25 | 1.17 ± 0.30 | 1.04 ± 0.21 | 0.007 |
| Lipid-lowering medication use (yes) | 31 (46.3) | 1 (2.3) | 20 (30.8) | 0 (0.0) | <0.001 |
Data are n (%), mean ± SD, or geometric mean [95% CI]. P values for nonnormally distributed data based on log-transformed values. P value for ANOVA Type III F test. “Other” for race/ethnicity includes mixed, Asian, American Indian, and other. BP, blood pressure.
Although fasting glucose concentrations were similar in adults and youth across treatment arms, fasting insulin and C-peptide concentrations were significantly higher in youth (P < 0.001) (Table 2). Notably, all β-cell response measures were significantly higher in youth in both treatment arms, reflecting their profound insulin resistance, as quantified by M/I, and β-cell hyperresponsiveness. Insulin clearance, defined as the ratio of fasting C-peptide to fasting insulin (6), was significantly lower in youth compared with adults (P < 0.001).
Table 2.
Metabolic phenotypes and clamp-derived measures of β-cell function and insulin sensitivity at baseline by treatment group
| Glargine followed by metformin |
Metformin alone |
||||
|---|---|---|---|---|---|
| Adult |
Youth | Adult | Youth | ||
| N = 67 | N = 44 | N = 65 | N = 47 | P value | |
| Fasting glucose (mmol/L) | 6.1 ± 0.5 | 6.0 ± 0.9 | 6.1 ± 0.6 | 6.1 ± 1.1 | 0.311 |
| Fasting C-peptide (nmol/L) | 1.242 ± 0.471 | 1.627 ± 0.552 | 1.206 ± 0.430 | 1.820 ± 0.581 | <0.001 |
| Fasting insulin (pmol/L) | 109.2 [40.2, 296.8] | 211.1 [54.8, 813.5] | 102.9 [36.8, 287.8] | 247.4 [74.1, 825.4] | <0.001 |
| Steady-state (second-phase) C-peptide response (nmol/L) | 4.0 [1.9, 8.4] | 5.2 [2.4, 11.2] | 3.9 [2.0, 7.6] | 5.1 [2.3, 11.2] | <0.001 |
| ACPRmax (nmol/L) | 4.8 [1.8, 12.5] | 7.3 [3.3, 16.2] | 4.8 [2.0, 11.7] | 8.1 [3.4, 9.3] | <0.001 |
| ACPRg (nmol/L) | 1.7 [1.0, 3.2] | 2.4 [0.9, 6.4] | 1.8 [1.0, 3.2] | 2.3 [0.8, 6.4] | <0.001 |
| Glucose disposal rate (M; mmol/kg/min) | 0.021 ± 0.010 | 0.025 ± 0.013 | 0.022 ± 0.009 | 0.023 ± 0.010 | 0.209 |
| M/I (mmol/kg/min per pmol/L) | 2.8 [0.7, 12.1] | 1.6 [0.3, 7.4] | 3.3 [0.8, 13.1] | 1.5 [0.4, 6.8] | <0.001 |
| Fasting C-peptide/fasting insulin (×10 nmol/pmol) | 1.06 [0.62, 1.81] | 0.73 [0.29, 1.87] | 1.10 [0.59, 2.06] | 0.70 [0.28, 1.77] | <0.001 |
Data are mean ± SD or geometric mean [95% CI]. P values for nonnormally distributed data based on log-transformed values. P value for ANOVA Type III F test.
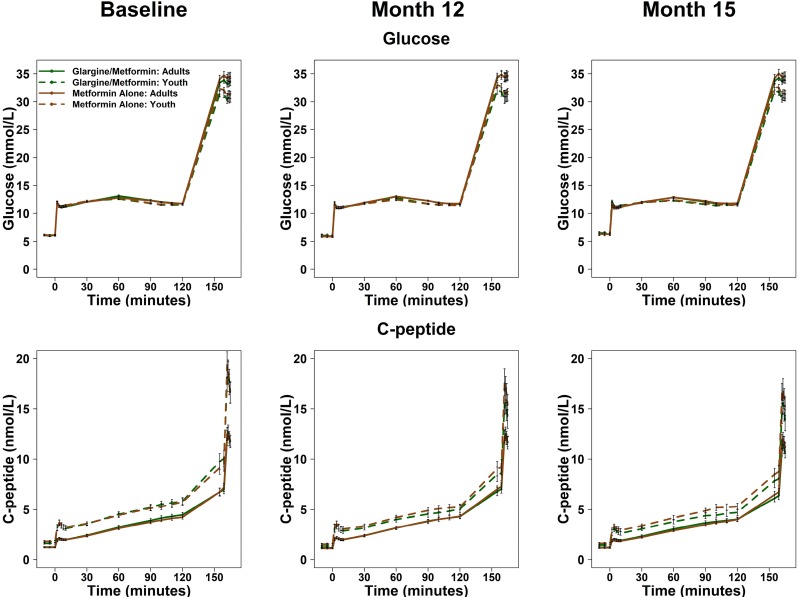
Plasma Glucose and C-Peptide Concentrations During the Hyperglycemic Clamp in Youth and Adults by Treatment Arm
Plasma glucose and C-peptide concentrations during the two-step hyperglycemic clamp are illustrated at baseline, 12 months (on treatment), and 15 months (3 months after withdrawal of treatment) for each treatment arm in youth and adults (Fig. 1). Of note, identical target glucose concentrations of 11.1 and >25 mmol/L (i.e., stimuli driving the β-cell response) were achieved between treatment groups, over time, and between youth and adults. Despite these matched clamped glucose levels and with treatment interventions, at all time points during the clamp, C-peptide concentrations were higher in youth than adults (all P < 0.001). Within each age-group, there were no significant treatment group differences on either glucose or C-peptide.
Figure 1.
Glucose and C-peptide concentrations during the hyperglycemic clamp. Glucose and C-peptide concentrations from the hyperglycemic clamps at baseline, after 12 months of treatment (Month 12), and 3 months after discontinuing the intervention (Month 15). The goal steady-state glucose targets were 11.1 mmol/L between 90 and 120 min and >25 mmol/L at 150 min. Data are displayed as mean ± SEM.
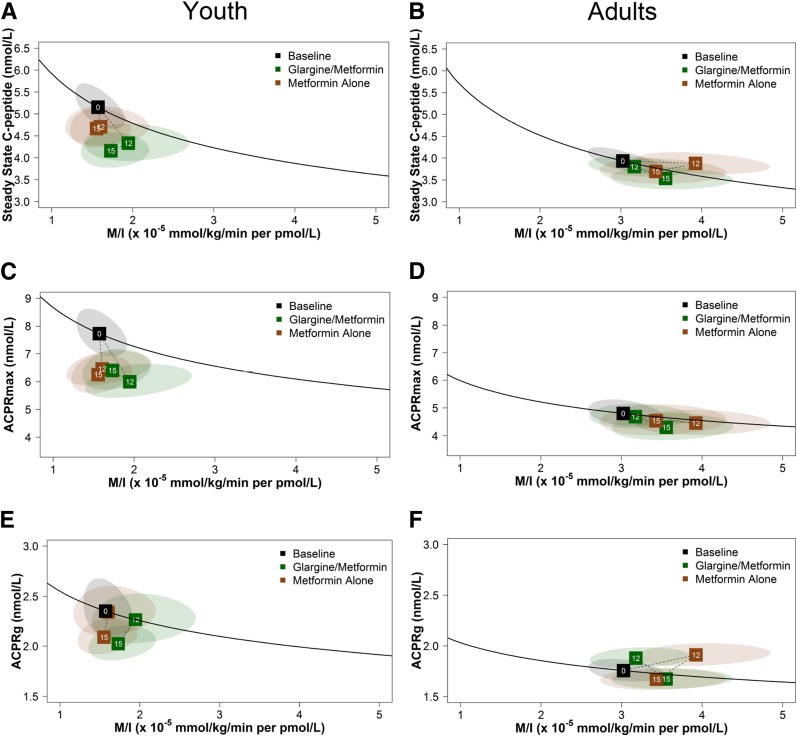
Treatment Effects on Hyperglycemic Clamp–Derived Insulin Sensitivity and β-Cell Responses From Baseline to 12 and 15 Months
We used vector plots to better illustrate concurrent changes in the hyperglycemic clamp–derived β-cell responses (steady-state C-peptide, ACPRmax, and ACPRg) expressed jointly with M/I. In Fig. 2, for each age-group, the mean value at baseline is indicated by the black box with a 0 (i.e., month 0). The dashed lines from the boxes at months 12 and 15 indicate the trajectory of mean values from baseline to 12 months of intervention and then to 3 months after discontinuation of the intervention (15 months) for insulin glargine followed by metformin (green) and metformin alone (brown). Values above the black line represent improved β-cell function, and values below the line represent a decline in β-cell function. The ellipses depict the 95% confidence bands around the boxes (group mean values) at months 12 and 15. There are a number of major differences between youth and adults, not only at baseline but notably in the temporal changes in β-cell responses during the interventions.
Figure 2.
Vector plots illustrating the treatment effects on concurrent model-based changes from baseline to 12 and 15 months in hyperglycemic clamp–derived insulin sensitivity and β-cell responses in youth and adults. The figures depict the relationships in youth (A, C, and E) and adults (B, D, and F) of the two coprimary outcomes and secondary outcome: hyperglycemic clamp–derived β-cell responses (steady-state C-peptide, ACPRmax, and ACPRg), each paired with M/I, at baseline, 12 months, and 15 months, in green for the insulin glargine followed by metformin and in brown for metformin alone. The black line depicts the joint relationship between β-cell response and M/I at baseline for the full cohort within each study, with the mean value at baseline for the full cohort indicated by the black box with a 0. The dotted lines to boxes at months 12 and 15 show the trajectory of values from baseline to 12 months of intervention and then to 3 months after discontinuation of the intervention (15 months). Values above the black line represent improved β-cell function; values below the line represent poorer β-cell function. The ellipses depict the 95% confidence bands around the points at months 12 and 15.
In the pediatric group, as previously reported (8), no significant differences were found between treatment groups at 12 or 15 months in either primary measure of β-cell function (steady-state C-peptide or ACPRmax, each paired with M/I) or in the secondary measure of β-cell function (ACPRg paired with M/I) (Fig. 2A, C, and E). Further, β-cell responses declined from baseline to 12 months and from 12 to 15 months in both treatment groups. These changes indicate a failure of both interventions to improve or halt the rapid deterioration of β-cell function in youth.
In contrast, as recently reported (10), in adults after 12 months of treatment, ACPRg significantly improved in both treatment groups, M/I increased slightly in the metformin group, and no significant changes were observed in steady-state C-peptide or ACPRmax in either group (Fig. 2B, D, and F). The changes in ACPRg combined with M/I represent improved β-cell function during active treatment. At 15 months, 3 months after treatment withdrawal, all response measures combined with M/I were not different from the baseline curve. Further, no β-cell response measure paired with M/I differed between treatment groups at 12 or 15 months. Importantly, the marked progressive deterioration seen in β-cell function in youth during treatment and after discontinuing treatment was not observed in adults.
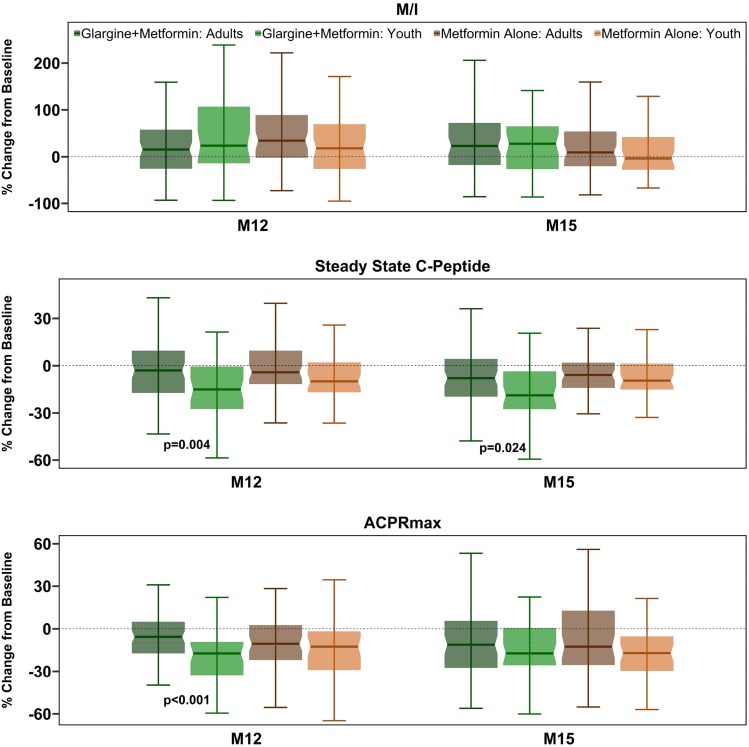
Youth Versus Adults Comparisons of Percent Changes in Hyperglycemic Clamp–Derived Insulin Sensitivity and β-Cell Responses Over Time
Figure 3 compares the percent changes in youth and adults in hyperglycemic clamp–derived insulin sensitivity and the two primary β-cell response measures by treatment arm from baseline to 12 and 15 months.
Figure 3.
Comparison of percent changes from baseline to 12 and 15 months in hyperglycemic clamp–derived insulin sensitivity and β-cell measures in youth vs. adults. Shown in shades of green are data from the insulin glargine followed by metformin arm (dark green, adults; light green, youth). Shades of brown are data from the metformin alone arm (dark brown, adults; light brown, youth). The bars indicate 95% CI.
M/I
Although modest improvements were seen in insulin sensitivity in both youth and adults in each treatment arm, the percent change in M/I in adults and youth was not significantly different in either treatment arm at both 12 and 15 months.
Steady-State C-Peptide
In the insulin glargine followed by metformin group, the percent decrease in steady-state C-peptide from baseline to 12 months and to 15 months was significantly greater in youth than in adults (P = 0.004 and P = 0.024, respectively). In the metformin alone group, the percent decline in steady-state C-peptide at both 12 and 15 months was not significantly different between youth and adults.
ACPRmax
The values for ACPRmax were numerically lower at 12 and 15 months in both treatment arms in each age-group. However, the percent reduction was only significantly greater in youth than adults at 12 months in the insulin glargine followed by metformin arm (P < 0.001).
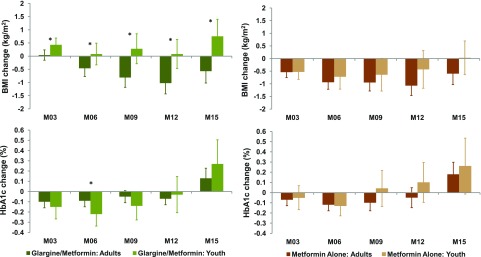
Youth Versus Adults Comparisons of Percent Changes in BMI and HbA1c Over Time
Figure 4 illustrates percent change in BMI and HbA1c from baseline to months 3, 6, 9, 12, and 15. The change in BMI was significantly different between youth and adults in the insulin glargine followed by metformin arm, with BMI decreasing starting at month 6 in adults but not youth (P < 0.05 at all time points in adults vs. youth). In the metformin alone arm, BMI decreased at all times in adults and in youth through month 12, with no significant differences between youth and adults.
Figure 4.
Comparison in the temporal changes in BMI and HbA1c from baseline in youth vs. adults. Shown in shades of green are data from the insulin glargine followed by metformin arm (dark green, adults; light green, youth). Shades of brown are data from the metformin alone arm (dark brown, adults; light brown, youth). The bars indicate 95% CI. The asterisks represent visits where changes were significantly different in youth vs. adults (P < 0.05).
In the insulin glargine followed by metformin arm, the relative decline in HbA1c from baseline to month 6 was significantly greater in youth than adults (P = 0.046), with HbA1c change from baseline to all other time points (months 3, 9, 12, and 15) not significantly different between youth and adults (P > 0.1 at all time points). There were no significant differences in the relative change in HbA1c from baseline between youth and adults in the metformin alone arm.
Discussion
These analyses of the RISE medication studies provide the first direct comparisons in youth and adults of the effect of medication interventions targeting improved β-cell function. The effects of metformin alone or insulin glargine followed by metformin on insulin sensitivity and their ability to preserve or restore β-cell function were quantified at the end of 12 months of treatment and again at 15 months, the latter time point at which interventions had been withheld for 3 months. The hyperglycemic clamp provided an assessment of β-cell responses to glucose as well as arginine, a nonglucose secretagogue, in relation to insulin sensitivity. Within each age-group, the two interventions did not differ in their effects on β-cell function. Importantly however, unlike adults, youth did not have improved or preserved β-cell function at 12 months (during active treatment). Furthermore, following withdrawal of the study treatments, there was a steep and progressive decline in β-cell function in the pediatric group indicated by the downward shift in their vector plot relative to the baseline curvilinear relationship between insulin sensitivity and C-peptide responses.
Although a decline in β-cell function also occurs in adults with type 2 diabetes, this process is accelerated in youth (3,4). The TODAY Study showed that the rate of loss of glycemic control on metformin monotherapy approximated 50%, suggesting that β-cell failure may be more rapid in youth than in adults (5,14). Until now this comparison has been merely speculative as no prior study has performed a head-to-head comparison between youth and adults with the same levels of dysglycemia and duration of disease studied under similar prospective conditions.
Contrary to our original hypothesis, in RISE we have found that early intervention with insulin glargine was not associated with any improvement in β-cell function in youth with IGT or early diagnosed type 2 diabetes (8). Further, while the decline in β-cell function was greater in youth than adults, in RISE we failed to replicate the previously reported effect of <2 weeks of intensive insulin therapy in recently diagnosed Chinese patients with type 2 diabetes to improve β-cell function 1 year later (15). The reason(s) for these differences across the two age-groups remain unclear, but they do suggest that even resting the β-cell for 3 months in youth does not slow the rate of decline in β-cell function to render it similar to that in adults. It is important to note the difference in insulin interventions used by Weng et al. (15) from that employed in RISE. Weng et al. provided intensive insulin therapy either as a continuous subcutaneous insulin infusion or multiple daily insulin injections, while in RISE insulin was administered as a once-daily injection based on the fasting glucose level.
The current data from RISE not only confirm the poor efficacy of metformin as a treatment for hyperglycemia in obese adolescents (5) but also demonstrate that when compared with adults, youth have a greater decline in β-cell function over time both while on treatment as well as after metformin withdrawal. Interestingly, after 12 months of metformin, adults demonstrated improvement or maintenance in the acute and steady-state responses to glucose, while youth did not. This improvement in β-cell function in adults while receiving metformin is in keeping with that observed with oral glucose administration in adults being studied for the effects of the medication to prevent diabetes or produce durable glycemic control in recently diagnosed type 2 diabetes (4,16).
The C-peptide response to arginine at maximal glycemic potentiation (ACPRmax) also declined in youth with both treatments; the same changes were not exhibited in adults. This measure, an estimate of β-cell secretory capacity (17), has previously been suggested to provide an estimate of β-cell mass in animal models and in humans after islet autotransplantation (18). However, it should be kept in mind that this measure can also change fairly rapidly in a time interval that would not be compatible with a change in β-cell mass (19,20). Whether the decline in β-cell secretory capacity in RISE youth reflects a reduction in β-cell mass or an alteration in molecular aspects of cellular function that is more severe than observed in adults is not certain and warrants further investigation. Understanding such would be important, as it will likely have an impact on the selection of future approaches to preserve β-cell function in youth and possibly in adults.
In the current analysis, each clamp-derived β-cell response measure was expressed as a function of insulin sensitivity, as depicted in Fig. 2. Notably, the location of the baseline curve was distinctly different in the two age-groups, as were the responses to the interventions and their subsequent withdrawal. The combined measure at baseline in youth was leftward and upward shifted compared with adults (Fig. 2A, C, and E in youth vs. Fig. 2B, D, and F in adults). It is conceivable that the initial position along this curve may have influenced the effects of the medications used in this study to affect the progression of β-cell failure. The precise mechanisms that underlie the profound differences in insulin sensitivity and responsiveness of the β-cell observed between youth and adults during and after treatment are not clearly evident.
It is plausible that the pathophysiology of dysglycemia in youth is driven by more severe hepatic and adipose tissue insulin resistance, both of which are potentially related to the obesity and the co-occurrence of fatty liver (21,22). Studies from the Arslanian group (23,24) showed that obese youth with normal glucose tolerance have 32–45% higher fasting glycerol turnover compared with that reported in obese adults. We have not assessed hepatic and adipose tissue insulin sensitivity, and therefore further studies are needed to determine their potential role as a modulator of β-cell function in youth with IGT and recently diagnosed type 2 diabetes. Future studies should evaluate lipid metabolism between phenotypically matched adults and youth along the spectrum of glucose tolerance. Puberty is another potential factor in the profound reduction in insulin sensitivity seen in RISE youth. While >60% of participants were already Tanner stage V (8), puberty is known to be characterized by an ∼30% reduction in whole-body insulin sensitivity and increased β-cell function in nonoverweight adolescents (25,26), which is exaggerated by the presence of obesity (27). When this insulin resistance of puberty wanes is not well understood.
Strengths in RISE are the robust approach to quantification of insulin sensitivity and β-cell responses to both glucose and the nonglucose secretagogue arginine in both youth and adults, thus providing mechanistic insights into how the tested interventions affected two key metabolic defects of type 2 diabetes: insulin sensitivity and β-cell responses. The enrollment of the pediatric group and use of both the same interventions and the same study paradigm has allowed, for the first time, comparative analysis of multiple outcomes across the life span. There are also some limitations to this study. First, we did not use the hyperinsulinemic-euglycemic clamp coupled with stable isotope measures of hepatic glucose production and insulin suppressive effects of lipolysis; this would have added additional information regarding the tissue-specific responses to insulin and of potential differences between youth and adults. Second, the waist-to-hip ratio differed marginally between age-groups, but we did not directly quantify body fat distribution or hepatic fat content to evaluate possible contributions to the greater insulin resistance in youth. Third, in some instances we clearly observed differences between youth and adults, although these did not reach statistical significance because of the relatively small sample sizes.
In conclusion, we describe the underlying pathophysiological mechanisms explaining the lack of effects of either metformin alone or insulin glargine followed by metformin on improving the relationship between insulin sensitivity and β-cell function (i.e., reducing β-cell secretory demand) in youth compared with adults with similar degrees of obesity and dysglycemia. The inability of these two treatments to reduce β-cell secretory demand contrasts with that in adults in whom a modest improvement in β-cell function occurred with metformin. These findings reinforce a need for mechanistic studies to further explore the pathophysiology and identify other interventions or medications that alone or in combination could successfully combat the insulin resistance and progressive loss of β-cell function that leads to IGT and type 2 diabetes.
Appendix
Writing Group. Sonia Caprio (chair), David A. Ehrmann (co-chair), Sharon L. Edelstein, Kieren J. Mather, Silva A. Arslanian, Mark T. Tripputi, Kristen J. Nadeau, Ellen W. Leschek, Thomas A. Buchanan, and Steven E. Kahn.
Supplementary Material
Article Information
Acknowledgments. The RISE Consortium acknowledges the support and input of the RISE Data and Safety Monitoring Board; Barbara Linder, the NIDDK program official for RISE; and Peter Savage, who served as the scientific officer for RISE prior to his retirement. The Consortium is also grateful to the participants who, by volunteering, are furthering our ability to reduce the burden of diabetes.
Funding. RISE was supported by grants from the National Institutes of Health (NIH) (NIDDK U01DK-094406, U01DK-094430, U01DK-094431, U01DK-094438, U01DK-094467, P30DK-017047, P30DK-020595, P30DK-045735, and P30DK-097512 and National Center for Advancing Translational Sciences UL1TR-000430, UL1TR-001082, UL1TR-001108, UL1TR-001855, UL1TR-001857, UL1TR-001858, and UL1TR-001863), the Department of Veterans Affairs, and Kaiser Permanente Southern California. Additional financial and material support from the American Diabetes Association, Allergan Corporation, Apollo Endosurgery, Abbott Laboratories, and Novo Nordisk A/S is gratefully acknowledged.
Duality of Interest. S.E.K. and S.A.A. serve as paid consultants on advisory boards for Novo Nordisk. S.E.K. is a member of a steering committee for a Novo Nordisk–sponsored clinical trial. K.J.M. held an investigator-initiated research grant from Novo Nordisk during the performance of this study. S.A.A. is an investigator in a Novo Nordisk–sponsored clinical trial. T.A.B. has received research support from Allergan Corporation and Apollo Endosurgery. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. The steering committee (principal investigator at each site, the data coordinating center, and the NIDDK project scientist) designed and implemented the study. All writing group members performed the research. S.L.E. and M.T.T. performed all data analysis. S.C. wrote the first draft, and all others contributed to the discussion and edited the manuscript. S.C., S.L.E., and S.E.K. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Data and Resource Availability. In accordance with the NIH Public Access Policy, we continue to provide all manuscripts to PubMed Central including this manuscript. RISE has provided the protocols to the public through its public website (https://www.risestudy.org). The RISE Consortium abides by the NIDDK data sharing policy and implementation guidance as required by the NIH/NIDDK (https://www.niddkrepository.org/studies/rise/).
Prior Presentation. Parts of this study were presented in abstract form at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
Footnotes
Clinical trial reg. nos. NCT01779362 and NCT01779375, clinicaltrials.gov
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-0299/-/DC1.
A complete list of the RISE Consortium Investigators can be found in the Supplementary Data online.
Contributor Information
Collaborators: RISE Consortium Investigators, David A. Ehrmann, Karla A. Temple, Abby Rue, Elena Barengolts, Babak Mokhlesi, Eve Van Cauter, Susan Sam, M. Annette Miller, Steven E. Kahn, Karen M. Atkinson, Jerry P. Palmer, Kristina M. Utzschneider, Tsige Gebremedhin, Abigail Kernan-Schloss, Alexandra Kozedub, Brenda K. Montgomery, Emily J. Morse, Kieren J. Mather, Tammy Garrett, Tamara S. Hannon, Amale Lteif, Aniket Patel, Robin Chisholm, Karen Moore, Vivian Pirics, Linda Pratt, Kristen J. Nadeau, Susan Gross, Philip S. Zeitler, Jayne Williams, Melanie Cree-Green, Yesenia Garcia Reyes, Krista Vissat, Silva A. Arslanian, Kathleen Brown, Nancy Guerra, Kristin Porter, Sonia Caprio, Mary Savoye, Bridget Pierpont, Thomas A. Buchanan, Anny H. Xiang, Enrique Trigo, Elizabeth Beale, Ting Chow, Fadi N. Hendee, Namir Katkhouda, Krishan Nayak, Mayra Martinez, Cortney Montgomery, Xinhui Wang, Jun Wu, Sharon L. Edelstein, John M. Lachin, Ashley Hogan Tjaden, Mark T. Tripputi, Santica Marcovina, Jessica Harting, John Albers, Dave Hill, Peter J. Savage, and Ellen W. Leschek
References
- 1.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al.; SEARCH for Diabetes in Youth Study . SEARCH for Diabetes in Youth Study: incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med 2017;376:1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imperatore G, Boyle JP, Thompson TJ, et al.; SEARCH for Diabetes in Youth Study Group . Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care 2012;35:2515–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.TODAY Study Group Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care 2013;36:1749–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn SE, Lachin JM, Zinman B, et al.; ADOPT Study Group . Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT. Diabetes 2011;60:1552–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.TODAY Study Group A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.RISE Consortium Restoring Insulin Secretion (RISE): design of studies of β-cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes Care 2014;37:780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.RISE Consortium Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. Observations using the hyperglycemic clamp. Diabetes Care 2018;41:1696–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.RISE Consortium Impact of insulin and metformin versus metformin alone on β-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 2018;41:1717–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannon TS, Kahn SE, Utzschneider KM, et al.; RISE Consortium . Review of methods for measuring β-cell function: design considerations from the Restoring Insulin Secretion (RISE) Consortium. Diabetes Obes Metab 2018;20:14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.RISE Consortium Lack of durable improvements in β-cell function following withdrawal of pharmacological interventions in adults with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 9 June 2019. [Epub ahead of print]. DOI: 10.2337/dc19-0556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zellner A. An efficient method of estimating seemingly unrelated regression equations and test for aggregation bias. J Am Stat Assoc 1962;57:348–368 [Google Scholar]
- 12.Henningsen A, Hamann J. Systemfit: a package for estimating systems of simultaneous equations in R. J Stat Softw 2007;23:1–40 [Google Scholar]
- 13.Hotelling H. The generalization of Student’s ratio. Ann Math Stat 1931;2:360–378 [Google Scholar]
- 14.Kahn SE, Haffner SM, Heise MA, et al.; ADOPT Study Group . Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 15.Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 2008;371:1753–1760 [DOI] [PubMed] [Google Scholar]
- 16.Diabetes Prevention Program Research Group Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the Diabetes Prevention Program: effects of lifestyle intervention and metformin. Diabetes 2005;54:2404–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D Jr. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest 1984;74:1318–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson RP. Estimation of β-cell mass by metabolic tests: necessary, but how sufficient? Diabetes 2007;56:2420–2424 [DOI] [PubMed] [Google Scholar]
- 19.Kahn SE, Beard JC, Schwartz MW, et al. Increased β-cell secretory capacity as mechanism for islet adaptation to nicotinic acid-induced insulin resistance. Diabetes 1989;38:562–568 [DOI] [PubMed] [Google Scholar]
- 20.Kahn SE, Klaff LJ, Schwartz MW, et al. Treatment with a somatostatin analog decreases pancreatic B-cell and whole body sensitivity to glucose. J Clin Endocrinol Metab 1990;71:994–1002 [DOI] [PubMed] [Google Scholar]
- 21.Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest 2016;126:12–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samuel VT, Shulman GI. Nonalcoholic fatty liver disease as a nexus of metabolic and hepatic diseases. Cell Metab 2018;27:22–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JY, Bacha F, Tfayli H, Michaliszyn SF, Yousuf S, Arslanian S. Adipose tissue insulin resistance in youth on the spectrum from normal weight to obese and from normal glucose tolerance to impaired glucose tolerance to type 2 diabetes. Diabetes Care 2019;42:265–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JY, Nasr A, Tfayli H, Bacha F, Michaliszyn SF, Arslanian S. Increased lipolysis, diminished adipose tissue insulin sensitivity, and impaired β-cell function relative to adipose tissue insulin sensitivity in obese youth with impaired glucose tolerance. Diabetes 2017;66:3085–3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caprio S, Plewe G, Diamond MP, et al. Increased insulin secretion in puberty: a compensatory response to reductions in insulin sensitivity. J Pediatr 1989;114:963–967 [DOI] [PubMed] [Google Scholar]
- 26.Amiel SA, Caprio S, Sherwin RS, Plewe G, Haymond MW, Tamborlane WV. Insulin resistance of puberty: a defect restricted to peripheral glucose metabolism. J Clin Endocrinol Metab 1991;72:277–282 [DOI] [PubMed] [Google Scholar]
- 27.Caprio S, Hyman LD, Limb C, et al. Central adiposity and its metabolic correlates in obese adolescent girls. Am J Physiol 1995;269:E118–E126 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.