Abstract
The storage and transport of frozen cells underpin the emerging/existing cell-based therapies and are used in every biomedical research lab globally. The current gold-standard cryoprotectant dimethyl sulfoxide (DMSO) does not give quantitative cell recovery in suspension or in two-dimensional (2D) or three-dimensional (3D) cell models, and the solvent and cell debris must be removed prior to application/transfusion. There is a real need to improve this 50-year-old method to underpin emerging regenerative and cell-based therapies. Here, we introduce a potent and synthetically scalable polymeric cryopreservation enhancer which is easily obtained in a single step from a low cost and biocompatible precursor, poly(methyl vinyl ether-alt-maleic anhydride). This poly(ampholyte) enables post-thaw recoveries of up to 88% for a 2D cell monolayer model compared to just 24% using conventional DMSO cryopreservation. The poly(ampholyte) also enables reduction of [DMSO] from 10 wt % to just 2.5 wt % in suspension cryopreservation, which can reduce the negative side effects and speed up post-thaw processing. After thawing, the cells have reduced membrane damage and faster growth rates compared to those without the polymer. The polymer appears to function by a unique extracellular mechanism by stabilization of the cell membrane, rather than by modulation of ice formation and growth. This new macromolecular cryoprotectant will find applications across basic and translational biomedical science and may improve the cold chain for cell-based therapies.
Introduction
Cryopreservation is an essential process for the long-term storage of cells and tissues. Red blood cells (RBCs) are the most frequently transfused blood product, with 12–16 million units transfused per year in the United States alone,1 but difficult processing has shifted the use of cryopreserved RBCs to settings only where their availability is limited.2 Leukemia therapy is underpinned by the transfusion of dimethyl sulfoxide (DMSO)-cryopreserved hematopoietic stem cells, and mammalian cells are widely used in the biopharmaceutical industry for the production of recombinant therapeutic proteins (biologics).3 However, it is not currently possible or practical to supply this material reproducibly because of phenotypic changes of growing cultures, and therefore banking of these materials is essential.4 Additionally, the ability to preserve cells as monolayers, which can be readily used and do not need to be propagated forward, would be revolutionary in providing identical starting materials, for instance, ensuring precisely engineered reporter lines do not experience any sort of propagated “phenotypic drift” which would alter their precise reporting mechanisms.5 Furthermore, there is evidence that the core response of cells to cryopreservation is different if the cells are part of a network, and the scale-up of procedures from a microscopic cellular level to a macroscopic tissue scale will introduce new modes of injury specific to tissue freezing.6 Our monolayer results could pave the way to improve organ-on-a-chip preservation outcomes7 or perhaps even be the key to successful tissue preservation.
The gold standard protocol for the (suspension) cryopreservation of mammalian cells is vial freezing in a solution containing 5–10% of the cryoprotective agent, DMSO, which is able to enter the cells and partly reduce injury by moderating the increase of solute concentration during freezing.8−10 Additionally, the cells frozen in vials must be propagated forward through several passages before they are stable enough to be used for reproducible assays. While vial freezing in DMSO works for most cell lines, many types are highly sensitive to DMSO.11 The ability to reliably store all cells and employ lower concentrations of DMSO would aid multiple fields by increasing post-thaw viability and reducing processing. Compared to freezing in solution, DMSO does not work well for cell monolayers,12 typically resulting in only around 20–35% cell recovery.13,14 Clearly, there exists a real need to transform our approach to cryopreservation in order to boost cell recovery and function and reduce the processing challenges.
Despite the above cryopreservation challenges, extremophiles have evolved in nature to allow survival at sub-zero conditions by both promoting and/or inhibiting the formation and growth of ice, presenting major opportunities for biomedicine. In addition to these strategies, extremophiles use the production of osmotic protectants such as trehalose,15−17 proline,18−21 and sucrose22 as well as macromolecules such as antifreeze proteins23,24 or late embryogenesis abundant proteins.25 Synthetic polymers, which mimic antifreeze proteins’ ice recrystallization inhibition (IRI) properties, have emerged in recent years as promising cryoprotectants.26−28 Ice recrystallization during thawing is a major cause of cell death, and IRI-active polymers such as poly(vinyl alcohol) (PVA) can lead to increases in the post-thaw yield.29 Poly(proline), which displays moderate IRI, has been shown to improve post-thaw recovery of cell monolayers potentially by interacting with cell membranes.30 These examples show that macromolecular cryoprotectants can be designed to modulate membrane stability as well as ice growth, presenting an exciting opportunity. Carboxylated poly(lysine) and other synthetic poly(ampholyte)s bearing mixed positive and negative charges can interact with cell membranes and have been evaluated in cryovial vitrification,31 monolayer slow-vitrification,32 or slow-freezing in cryovials33 but never slow-freezing of monolayers. These result in good post-thaw recoveries, but require, for example, 6.5 M ethylene glycol solutions and often very fast cooling rates;32 also there is the issue of removing such a high concentration of solvent from the cells before toxicity occurs. They have also not been synthesized on a large scale, necessary for a step-change in how cryopreservation is conducted, and hence they have not been widely adopted.
Despite the above progress, the actual cell yields obtained with poly(ampholyte)s can still be low and often require vitrification (formation of an ice-free state using very high solvent loads), which is not typically practical for most cellular cryopreservation settings. An ideal polymeric cryoprotectant would increase the cell yield post thaw, be easily removed, available on a commercial scale for wide use, enable monolayer as well as suspension cell cryopreservation, and enhance the cryopreservation of all cell types with minimal reformulation requirements.
Here, we report a poly(ampholyte) cryopreservative that significantly enhances the post-thaw cell yield of a broad range of cell types, including nucleated and anucleated cells in suspension and monolayers under slow-freezing conditions. The polymer is easily obtained from a clinically used, low cost precursor, which ensures scalability. Additionally, it appears that the polymer externally protects cell membranes during freezing as its main mode of action, rather than ice growth modulation, and since it does not enter the cells, it is easily removed. We also demonstrate that the DMSO concentration required can be reduced to as low as 2.5 wt % through the use of a higher concentration of polymer.
Methods and Materials
Materials
Phosphate-buffered saline (PBS) solutions were prepared using pre-formulated tablets (Sigma-Aldrich) in 200 mL of Milli-Q water (>18.2 Ω mean resistivity) to give [NaCl] = 0.138 M, [KCl] = 0.0027 M, and pH 7.4. Poly(methyl vinyl ether-alt-maleic anhydride) Mn 80 000 and 311 000 g.mol–1, dimethylaminoethanol, and N-[4-(aminomethyl)benzyl]rhodamine 6G-amide bis(trifluoroacetate) were purchased from Sigma-Aldrich. Poly(methyl vinyl ether-alt-maleic anhydride) average Mn 20 000 g mol–1 was purchased from Scientific Polymer Products; the dispersity of polymer P2 from the manufacturer is 2.7 and that of P3 is 3.47; the supplier of P1 did not list the Mw value, only the Mn. Sodium chloride was purchased from Fisher Scientific. All solvents were purchased from VWR or Sigma-Aldrich, and the reagents were used without further purification unless indicated.
Note on the Choice of Polymer
Dimethylamino functionality was selected for the cationic component. This was driven by our previous observations on the role of hydrophobicity on ice crystal growth that ethyl and propyl modifications gave lower activity.34 Second, there were solubility reasons; when more hydrophobic pendant groups are installed (diethyl and diisopropyl), dissolution becomes an issue, with these samples having limited solubility at concentrations greater than 20 mg mL–1. For these polymers to be practical in a lab, concentration stocks need to be made, meaning that concentrations of at least 2× what is required for cryopreservation must be achieved. The dimethyl amino functionality gave this balance.
Synthesis of Poly(ampholyte)s
As a representative example, poly(methyl vinyl ether-alt-maleic anhydride), average Mn ≈ 80 000 Da (1 g), was dissolved in tetrahydrofuran (50 mL) and heated to 50 °C with stirring. After dissolution, dimethylamino ethanol (2 g) was added in excess, forming a pink waxy solid, which was allowed to stir for 30 min. Water (50 mL) was added, and the reaction was left to stir overnight followed by purification in dialysis tubing (Spectra/Por, 12–14 kDa MWCO) for 48 h with seven water changes. The resulting solution was freeze-dried to evolve a white solid.
1H NMR (DMSO): δ 2.30–2.80 (CH2CH(OCH3)CH2, br), 2.42 ((CH3)2NCH2, s, 6H), 2.50 (CH2CH(OCH3)CH2, s, 3H), 2.66 (NCH2CH2COO, t, 2H), 3.02–3.37 (CH2CH(OCH3)CH2), 3.41–4.48 (CH(OCH3)CH(COOH)CH(COOC), CH(OCH3)CH(COOH)CH(COOC), br), 3.56 (NCH2CH2COO, t, 2H). 13C NMR (DMSO): δ 42 ((CH3)2NCH2), 55 (NCH2CH2COO), 58 (NCH2CH2COO). IR: 1220 cm–1 (C–O), 1342 (C–N), 1560 (O=C–O– carboxylate), 1724 (C=O), 2364 (Me2N–H+ ammonium).
Synthesis of Fluorescently Labelled Poly(ampholyte)s
As a representative example, poly(methyl vinyl ether-alt-maleic anhydride), average Mn ≈ 80 000 Da (300 mg), was dissolved in tetrahydrofuran (50 mL) and heated to 50 °C with stirring. After dissolution, N-[4-(aminomethyl)benzyl]rhodamine 6G-amide bis(trifluoroacetate) (3 mg) and triethylamine (10 mg) were added and left for 20 min before dimethylamino ethanol (2 g) was added in excess, forming a pink waxy solid, which was allowed to stir for 30 min. Water (50 mL) was added, and the reaction was left to stir overnight followed by purification in dialysis tubing (Spectra/Por, 12–14 kDa MWCO) for 48 h with seven water changes. The resulting solution was freeze-dried to evolve a white solid.
Physical and Analytical Methods
1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on a Bruker AVANCE III HD 300 MHz, HD 400 MHz, or HD 500 MHz spectrometer using deuterated solvents obtained from Sigma-Aldrich. Chemical shifts are reported relative to the residual nondeuterated solvent.
IRI Assay
Ice wafers were annealed on a Linkam Biological cryostage BCS196 system with a T95-Linkpad system controller equipped with a LNP95-liquid nitrogen cooling pump, using liquid nitrogen as the coolant (Linkam Scientific Instruments UK, Surrey, UK). An Olympus CX41 microscope equipped with a UIS-2 20×/0.45/∞/0-2/FN22 lens (Olympus Ltd., Southend on Sea, UK) and a Canon EOS 500D SLR digital camera was used to obtain all images. Image processing was conducted using ImageJ, which is freely available from http://imagej.nih.gov/ij/. log P was calculated from the hydrophobicity of the pendant group using ChemDraw Professional 16.0.
IRI Evaluation
A 10 μL droplet of polymer in PBS solution was dropped from 1.4 m onto a glass microscope coverslip on top of an aluminium plate cooled to −78 °C using dry ice. The droplet froze instantly upon impact with the plate, spreading out and forming a thin wafer of ice. This wafer was then placed on a liquid nitrogen-cooled cryostage held at −8 °C. The wafer was then left to anneal for 30 min at −8 °C. The number of crystals in the image were counted, again using ImageJ, and the area of the field of view divided by the number of crystals gives the average crystal size per wafer and reported as a percentage of area compared to the PBS control.
Differential Scanning Calorimetry
Samples were prepared by weighing standard 40 μL aluminium pans and lids (Mettler Toledo, Leicestershire, UK) and adding 20 μL of solution before hermetically sealing and reweighing in order to quantify the exact mass of the sample. Each sample was then transferred to a liquid nitrogen-cooled differential scanning calorimeter (DSC 1 STAR system, Mettler Toledo). The mass of the aluminium pan and the sample mass was input into the complimentary STARe thermal analysis software to retain a digital record and aid the analysis.
Freezing and Thawing of DSC Samples and Evaluation of DSC Spectra
Each differential scanning calorimetry (DSC) sample was individually cooled from +25 to −150 °C at a rate of 10 °C·min–1 while concurrently monitoring the heat flow (mW) of the system to detect any endothermic or exothermic transitions. When samples reached −150 °C, each sample was held for 10 min and then warmed at a rate of 10 °C·min–1 from −150 to +25 °C.
Blood Testing Protocol
Sheep blood (10 mL) in Alsever’s solution was added to a 15 mL centrifuge tube and centrifuged at 2000 rpm for 5 min to concentrate the solution; 7 mL of the supernatant was removed and replaced with 7 mL of PBS solution. Polymer solutions were made at 2× the required concentration to ensure the correct final cryoprotectant concentration. Blood solution (0.5 mL) was added to 0.5 mL of the polymer solutions in 2 mL cryovials. These were then incubated in the fridge for 30 min before freezing in liquid nitrogen vapor. After 1 h, the samples were thawed in a water bath at 45 °C for 10 min, after which they were transferred to Eppendorf tubes and centrifuged at 2000 rpm for 5 min. The supernatant (40 μL) was removed and added to 750 μL of Alkaline Haematin D-575 solution. After vortexing, the samples were pipetted into a 96 well-plate in triplicate (3 × 200 μL per sample), and the absorbance was recorded at 580 nm in a BioTek plate reader. The samples were compared against unfrozen PBS and lysis buffer samples as the 0 and 100% lysis samples, respectively.
Cell Culture
Human Caucasian lung carcinoma cells (A549) were obtained from the European Collection of Authenticated Cell Cultures (ECACC) (Salisbury, UK) and grown in 175 cm2 cell culture Nunc flasks (Corning Incorporated, Corning, NY). The standard cell culture medium was composed of Ham’s F-12K (Kaighn’s) Medium (F-12K) (Gibco, Paisley, UK) supplemented with 10% USA-origin fetal bovine serum (FBS) purchased from Sigma-Aldrich (Dorset, UK), 100 units·mL–1 penicillin, 100 μg·mL–1 streptomycin, and 250 ng·mL–1 amphotericin B (PSA) (Hyclone, Cramlington, UK). Mouse calvarial osteoblastic cells (MC-3T3) were obtained from ECACC and grown in 75 cm2 cell culture Nunc flasks. The standard cell culture medium was composed of minimum essential medium α (Gibco) supplemented with 10% USA-origin FBS, 100 units·mL–1 penicillin, 100 μg·mL–1 streptomycin, and 250 ng·mL–1 amphotericin B (PSA). Mouse brain neuroblastoma cells (Neuro-2a) were obtained from the American Tissue Culture Collection (ATCC) (Middlesex, UK) and grown in 75 cm2 cell culture Nunc flasks. The standard cell culture medium was composed of Eagle’s minimum essential media (Gibco) supplemented with 10% USA-origin FBS, 100 units·mL–1 penicillin, 100 μg·mL–1 streptomycin, and 250 ng·mL–1 amphotericin B (PSA). All cells were maintained in a humidified atmosphere of 5% CO2 and 95% air at 37 °C, and the culture medium was renewed every 3–4 days. The cells were subcultured every 7 days or before reaching 90% confluency. To subculture, the cells were dissociated using 0.25% trypsin plus 1 mM ethylenediaminetetraacetic acid (EDTA) in balanced salt solution (Gibco). A549 cells were reseeded at 1.87 × 105 cells per 175 cm2 cell culture flasks. MC-3T3 cells were reseeded at 1.87 × 105 cells per 75 cm2 cell culture flasks. Neuro-2a cells were reseeded at 7.5 × 104 per 75 cm2 cell culture flasks.
Cell Solution Preparation
Solutions for cell incubation experiments were prepared by dissolving the individual compounds in the base cell media supplemented with 10% FBS and 1× PSA (solutions used as freezing buffers did not contain PSA) and sterile-filtered prior to use.
Cytotoxicity Screening
Poly(ampholyte) Cytotoxicity
A549 cells were seeded at 4 × 104 cells per well in 200 μL of cell culture medium with indicated concentrations of poly(ampholyte) in 96-well plates (Thermo Fisher). The cells were incubated with the polymer for 10 min and exchanged against completed cell media or incubated with the polymer for 24 h in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. Following the incubation period, resazurin sodium salt (Sigma-Aldrich) was dissolved in PBS (Sigma-Aldrich) and added to the wells in an amount of 1/10th of the initial well volume. Readings were taken using the Synergy HTX Multi-Mode Reader (BioTek, Swindon, UK) at 570/600 nm absorbance every 30 min until the control cells reached ∼70% reduction.
DMSO Cytotoxicity
A549 cells were seeded at 1 × 104 cells per well in 100 μL of cell culture medium with indicated concentrations of DMSO in 96-well plates. The cells were incubated for 30 min in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. After 30 min, the media was exchanged for fresh cell media, and a resazurin sodium salt assay was performed as described previously. The values were normalized by dividing the experimental values by control values.
Membrane Kinetics
Methods adapted from Su.35 A549 cells were dissociated and incubated with 1 μM of calcein-AM (BD Biosciences, Wokingham, UK) in completed cell media for 30 min at 37 °C with frequent mixing. The cells were then centrifuged and rinsed twice with media. The cells were plated at a density of 4 × 105 cells per well in 100 μL in 96-U-well suspension plates (Sarstedt Ltd., Leicester, UK) and centrifuged at 500 rpm for 2 min. Experimental solutions of 50 μL were added at 4×, and 50 μL of 0.32% trypan blue was added for a final concentration of 0.08%. The plate was placed in the BioTek plate reader at 37 °C and read every 10 min at 494/517 nm for 4 h. The plate was then imaged with a CKX41 microscope with light-emitting diode (LED) illumination and a XC30 camera and processed using cellSens software.
Live/Dead Staining of Poly(ampholyte) Incubation
A549 cells were seeded at 4 × 105 cells per well in 500 μL of cell culture medium in 24-well plates. The cells were incubated with the polymer for 24 h in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. Following the 24 h incubation, the cells were incubated with 0.3 μM calcein (Thermo Fisher) and 10 μM ethidium homodimer-1 (Thermo Fisher) in PBS. The cells were incubated at room temperature for 45 min. The stain solution was removed, and the wells were washed twice with PBS. The plate was read using a BioTex plate reader at 494/517 nm and 528/617 nm. The plate was then imaged with a CKX41 microscope with pE-300-W LED illumination and a XC30 camera and processed using cellSens software.
Cryopreservation of Cell Suspensions
A549 cells for suspension freezing were removed from the adherent culture by treatment with 0.25% trypsin plus 1 mM EDTA in balanced salt solution for 5 min in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. The number of viable cells was determined by counting with a hemocytometer (Sigma-Aldrich) at room temperature after 1:1 dilution of the sample with 0.4% trypan blue solution (Sigma-Aldrich). The cell density was adjusted to obtain a cell suspension of 1 × 105 cells·mL–1. Solutions containing the poly(ampholyte) were prepared at 2× the final concentration in F12-K media containing 20% FBS and 2× the indicated concentration of DMSO. The cell suspension (500 μL) and 500 μL of the freezing solution were added to individual cryovials (2 mL, Nalgene, Thermo Scientific, NY) and mixed three times with a 1000 μL pipette. The final concentration of FBS in freezing solutions was 10%. Cryovials were placed in a CoolCell LX freezing container (BioCision, LLC, Larkspur, CA) and transferred to a −80 °C freezer where they were frozen at a rate of 1 °C·min–1. After 2 h at −80 °C, the vials were transferred to the vapor phase of a liquid nitrogen dewar at −196 °C for 24 h. After 24 h at −196 °C, the cells were rapidly thawed by placing the vials in a foam support in a water bath set to 37 °C. The contents of each vial were added to 9 mL of complete F-12K media and centrifuged at 2g for 5 min to pellet the cells. The supernatant was discarded, and the cell pellet was resuspended in 500 μL of complete cell media and then transferred to individual wells of a 24-well plate (Corning Incorporated, Corning, NY). The cells were placed in a humidified atmosphere for 24 h and then dissociated using 0.25% trypsin plus 1 mM EDTA in balanced salt solution. The number of viable cells was then determined by counting with a hemocytometer at room temperature after 1:1 dilution of the sample with 0.4% trypan blue solution. The initial cell medium was discarded such that any nonattached cells were not included in the assessment. The percentage of recovered cells was calculated by dividing the number of cells with intact membranes after freezing and thawing by the number of cells initially frozen and multiplied by 100.
Cryopreservation of Cell Monolayers
Methods adapted from Bailey.14 A549 cells to be frozen in the monolayer format were seeded 4 × 105 cells per well in 500 μL of cell culture medium in 24-well plates. MC-3T3 and Neuro-2a cells to be frozen in the monolayer format were seeded 5 × 105 cells per well in 500 μL of cell culture medium in 24-well plates (Neuro-2a cells were frozen on collagen coated plates). The plates had a total available volume of 3.4 mL with an approximate growth area of 1.9 cm2; no coverslips were used; and the plates were used with the accompanying lid. The cells were allowed to attach to the entire free surface of the bottom of the well and formed a confluent layer not greater in height than one cell. Before the experimental treatments, the cells were allowed to attach for 2 h to the plates in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. The medium was exchanged against fresh medium to remove all unattached cells and then incubated for 24 h in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. Following the incubation period, the culture medium was removed, and the cells were exposed for 10 min at room temperature to different concentrations of solutes dissolved in base media supplemented with 10% FBS and the indicated concentrations of DMSO. After 10 min, the freezing solutions were removed, and the plate was placed inside a CoolCell MP plate (BioCision), transferred to a −80 °C freezer, and frozen at a rate of 1 °C·min–1. After 24 h at −80 °C, the cells were rapidly thawed by the addition of 500 μL of cell culture medium warmed to 37 °C. The cells were placed in a humidified atmosphere for 24 h and then dissociated using 0.25% trypsin plus 1 mM EDTA in balanced salt solution. The number of viable cells was then determined by counting with a hemocytometer at room temperature after 1:1 dilution of the sample with 0.4% trypan blue solution. The initial cell medium was discarded such that any nonattached cells were not included in the assessment. The percentage of recovered cells was calculated by dividing the number of cells with intact membranes after freezing and thawing by the number of cells present prior to freezing (i.e., after application of pre-treatments) and then multiplied by 100.
Collagen Plate Coating
Collagen I from rat tail (Sigma-Aldrich) was diluted to 50 μg·mL–1 in 200 mM acetic acid (Sigma) and added to each well of the cell culture plate at 5 μg collagen·cm–2. The plates were incubated with the dissolved collagen for 1 h, and after this incubation period, the collagen solution was removed, and the plates were rinsed three times with 200 μL of PBS to remove any residual acetic acid solution. The collagen-treated plates were allowed to dry for 1 h in a laminar flow hood and stored for less than 1 week at 4 °C prior to use.
Post-freeze Viability
A549 cells were cryopreserved as previously indicated. After dissociation and counting, the cells were plated at a density of 1.25 × 104 per well in a 6-well plate (Thermo Fisher). For low-yield recovery, multiple freezing wells were combined to provide an adequate number of cells. Control cells were plated at the same density with no prior manipulation. The cells were incubated in a humidified atmosphere of 5% CO2 and 95% air at 37 °C for the indicated number of days and then dissociated using 0.25% trypsin plus 1 mM EDTA in balanced salt solution for counting. The cell media was replaced on day four with fresh media. Growth rates were calculated by dividing the number of cells present by the initial plating density.
Post-freeze Membrane Permeability
A549 cells were cryopreserved as previously indicated. Following the 24 h post-thaw incubation, the cells were incubated with 0.3 μM calcein and 10 μM ethidium homodimer-1 in PBS. The cells were incubated at room temperature for 45 min. The stain solution was removed, and the wells were washed twice with PBS. The plate was read using the BioTex plate reader at 494/517 nm and 528/617 nm. The plate was then imaged with a CKX41 microscope with pE-300-W LED illumination and a XC30 camera and processed using cellSens software.
Statistical Analyses
Data were analyzed with a one-way analysis of variance on ranks followed by comparison of experimental groups with the appropriate control group, (Holm–Sidak method) followed by Tukey’s post hoc test. Excel 2013 (Microsoft, Redmond, WA) and R (R Foundation for Statistical Computing, Vienna, Austria) were used for the analyses and graphs. Data sets are presented as mean ± standard error of the mean (SEM).
Results
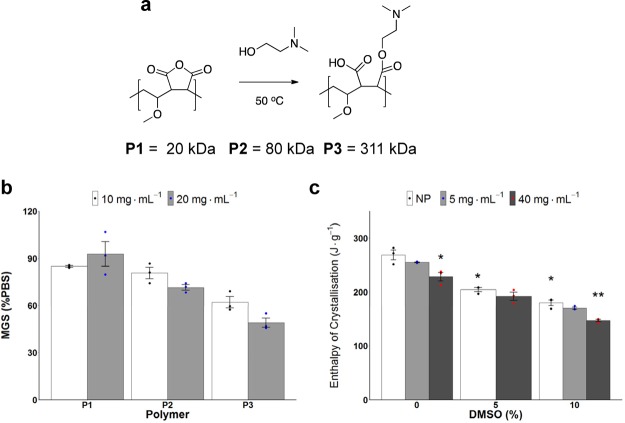
Poly(methyl vinyl ether-alt-maleic anhydride) Mn = 20 kDa (P1), 80 kDa (P2), and 311 kDa (P3) was selected as the precursor to a poly(ampholyte) as it is produced on multiton scales and is safe as both a food additive and a bioadhesive.36 The anhydride was ring-opened by the addition of dimethylamino ethanol to give a poly(ampholyte) of appropriate hydrophobicity and solubility34 (Figure 1a, characterization data shown in Figure S1). Other more hydrophobic amine substituents result in less soluble materials, and the dimethyl was found in screening to provide the best balance. By using an anhydride precursor, we guarantee the 1:1 ratio of cationic/anionic groups and regioregularity, not obtainable by other copolymerization methods. IRI assays37 (where smaller ice crystals indicate more activity) showed that P1–P3 had only weak, molecular weight-dependent IRI activity (Figure 1b), below those of other poly(ampholyte)s.34,38 For reference, type I antifreeze protein, used as a positive control, leads to <20% mean grain size (MGS) at <1 mg mL–1 concentrations (Figure S2), and hence the poly(ampholyte)s can be considered to be very weakly IRI active when discussing the mechanisms of cryopreservation (below). DSC confirmed ice crystallization in the presence of P2, thus ruling out noncolligative effects and showing vitrification (an entirely different cryopreservation approach where a glass is formed instead of ice31,32) is not occurring (Figure 1c). The small changes in the enthalpy of crystallization are what we would expect due to the solute concentration effects on freezing point depression.39 Full DSC traces and onset melting temperatures are shown in Figures S3 and S4, respectively. As there is only weak IRI activity, any emergent cryoprotectant properties (vide infra) can be correlated with unique cellular interactions, such as with the cell membrane.40
Figure 1.
Polymer synthesis and ice activity. (a) Synthesis of poly(ampholyte)s used. (b) IRI assay of P1–P3. MGS compared to a PBS control after 30 min of annealing at −8 °C. Error bars represent ±SEM of three repeats. (c) Enthalpy of crystallization (proportional to ice volume) for cryopreservation solutions containing P2 (NP = no polymer) via DSC at a cooling rate of 1 °C·min–1. (N = 24, P = 0.0000000004, *P < 0.001 from NP w/0% DMSO, **P < 0.001 from NP w/10% DMSO). Error bars represent ±SEM of three repeats.
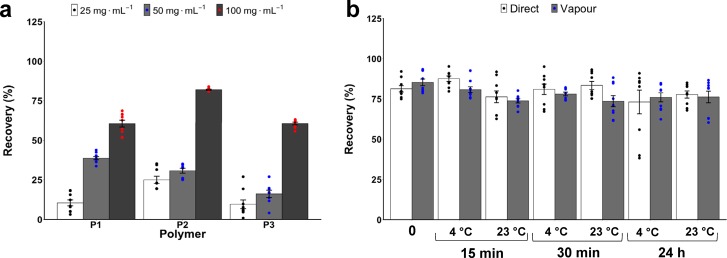
Erythrocyte cryopreservation was first used as a simple cellular screening model for membrane damage/stabilization.41Figure 2a shows that 100 mg mL–1 (∼10 wt %) of all molecular weights of poly(ampholyte)s gave >60% recovery, higher than what is achieved for IRI-active polymers42 alone (such as PVA, Figure S5), showing that this unique material does not function (primarily) by ice growth modulation. Higher concentrations could not be tested because of the solubility limits of the polymer, which is prepared at 2-fold concentration. The recovery levels here are comparable to those using 20–40 wt % glycerol, which also requires extensive post-thaw washing, resulting in a significant cell loss.28,43,44P2 was found to be the most potent molecular weight of the cryoprotectant, giving >80% recovery compared to longer/shorter polymers, and erythrocytes frozen in P2 also showed slightly less osmotic fragility than unfrozen controls (Figure S6). The post-thaw recovery values utilizing 100 mg mL–1P2 were also independent of pre-freeze incubation time/temperature (Figure 2b), in stark contrast to glycerol, which needs carefully controlled addition/removal to reduce toxicity and maximize recovery and introduces processing challenges, which are overcome in this polymeric system.45,46 It should be noted that the addition of uncharged water-soluble macromolecules often reduces post-thaw hemolysis for erythrocytes [such as glycerol and polyvinylpyrrolidone (Figure S5)], and the key aim here was to ensure that the material was suitable for cryopreservation; these results were utilized for primary screening to ensure compatibility before progressing to nucleated cells (below).
Figure 2.
RBC post-thaw recovery [defined as (1 – haemolysis (×100))] with cryoprotective polymers. (a) RBC frozen rapidly to −196 °C/thaw 45 °C. (b) Effect of prefreezing incubation time and temperature on post-thaw recovery with 100 mg mL–1P2. Incubation time (0, 15, 30 min, 24 h), incubation temperature (4 or 23 °C), and freezing conditions [directly submerged (direct) or in vapor (vapor) of liq. N2]. All error bars represent ±SEM from a minimum of three repeats.
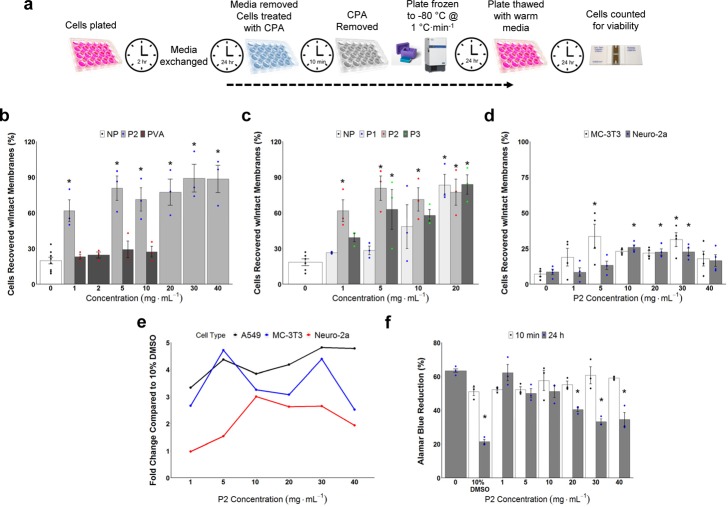
Mammalian cell monolayer freezing is significantly more challenging than suspension freezing13 but is important for regenerative medicine, biotechnology, and basic research applications and was hence chosen as a robust challenge.47 Confluent monolayers of A549 (epithelial lung carcinoma) cells were selected to screen for cryopreservation, using the polymer as a supplement to DMSO to increase recovery, which is already low (see data below).
A549 cells were cryopreserved to −80 °C at 1 °C·min–1, following a 10 min incubation with 10 wt % DMSO containing a concentration gradient of P2 or PVA. PVA was used as it is a potent IRI and has been reported to enhance cell recovery by inhibiting ice recrystallization and hence enables us to compare the utility of this new material and also mechanisms of action, as P2 is not a potent IRI.48 The cells were subsequently thawed, allowed to recover for 24 h, and the total cell yield determined (Figure 3b). [Note: it is crucial to allow 24 h post-thaw incubation as shorter periods give false positive results and over-estimate the cell recovery.13 The addition of 40 mg mL–1P2 resulted in >4-fold increase in recovered cells (89%) compared to DMSO alone (20%). Lower concentrations of P2 also gave significant cell recovery with just 1 mg mL–1 giving a 2.5-fold increase, which is remarkable for such a dilute additive, and outperformed other cryopreservative polymers.30−32 PVA, which has been shown to protect cells in solution during cryopreservation,29 was less effective in the preservation of monolayers. Cells frozen in cryovials are in a large quantity of solution, which may make IRI activity the most important damaging factor, whereas cells frozen as monolayers exist in only a small liquid layer, and therefore the benefit of IRI may not be sufficient for the protection of monolayers. These results support our hypothesis that P2 is a unique polymer cryoprotectant which surpasses the performance of previously reported materials and may function by a unique mechanism. Molecular weight effects of the polymer were also screened, with P2 found to outperform P1 and P3 in this cryopreservation system, but all can be considered very potent cryoprotectants (Figure 3c).
Figure 3.
Cell monolayer cryopreservation and cytotoxicity. Recovered cells were counted by trypan blue exclusion assay relative to unfrozen controls. All cells were cryopreserved for 24 h at −80 °C and then 24 h post-thaw incubation. (a) Schematic of the cryopreservation/thawing protocol; (b) Post-thaw cell recovery (as % of unfrozen controls) of A549 cells cryopreserved with 10% DMSO plus variable concentrations of P2 or PVA (NP = no polymer) (N = 39, P = 0.000000002, *P < 0.001 from NP w/10% DMSO). The data represent the mean ± SEM of three independent experiments with two nested replicates each. (c) Effect of polymer molecular weight (P1–P3, NP = no polymer) and concentration on A549 cell recovery (N = 42, P = 0.000006, *P < 0.001 from NP w/10% DMSO). The data represent the mean ± SEM of three independent experiments with two nested replicates each. (d) Post-thaw recovery of Neuro-2a and MC-3T3 cells after cryopreservation with 10% DMSO plus variable concentrations of P2. The data represent the mean ± SEM of four independent experiments with two nested replicates each (Neuro-2a: N = 28, P = 0.0005, *P < 0.001 from Neuro-2a w/0 mg mL–1P2; MC-3T3: N = 28, P = 0.02, *P < 0.01 from MC-3T3 w/0 mg mL–1P2). (e) Fold change (relative to 10% DMSO) post-thaw recovery for all cell lines as a function of P2 concentration. (f) Cytotoxicity of P2 against A549 cells; incubation time is 10 min or 24 h (N = 48, P = 0.0000000001, *P < 0.001 from 0 mg mL–1P2). The data represent the mean ± SEM of three independent experiments.
Two additional mammalian cell lines, Neuro-2a and MC-3T3, were also explored. DMSO cryopreservation alone gave <20% cell recovery, as these cells appear more delicate than the (robust) A549 line and avenues to fine tune the recoveries of these cells exist, as it may simply be that the osmolarity for these formulations is too high since hyperosmotic stress has been implicated in cell cycle arrest, DNA damage, oxidative stress, inhibition of transcription and translation, and mitochondrial depolarization49 and is a known consequence of cryopreservation.50 However, supplementing P2 gave a 3-fold enhancement in the post-thaw cell recovery in both cases (Figure 3d), showing that more fragile cells will also respond well to this new cryoprotectant. We summarize the fold-increase in post-thaw recovery compared to 10% DMSO alone for all cell lines in Figure 3e, demonstrating the universal applicability of our new material.
Cytotoxicity testing of P2 revealed no decrease in cell viability for short exposure times, which is used in our cryopreservation protocol (as excess solution is removed before freezing) and is all less cytotoxic than DMSO (Figure 3f). A 24 h incubation, as a means of extreme stress outside our protocol, was conducted to exaggerate any effects and to provide a mechanistic insight. Above 20 mg mL–1, the 24 h incubation reduced the cell metabolism, and microscopy suggested membrane perturbations for long exposure times (Supporting Information, Figure S7), which guided further investigations into how these polymers function (vide infra).
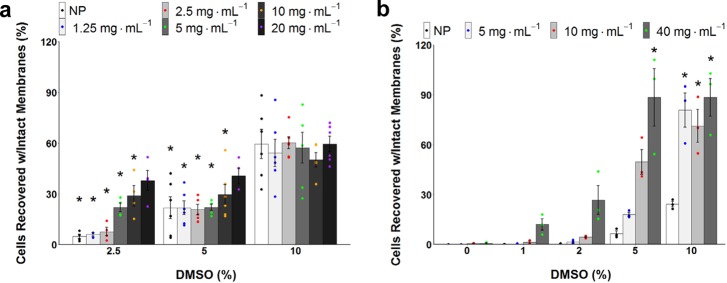
In addition to the (major) challenge of cell monolayer freezing, suspension cryopreservation is also challenging for cells that cannot tolerate the high (10 wt %) concentrations of DMSO, which has been shown to cause downstream toxicity after just 30 min of exposure (Figure S8). We therefore explored if P2 could rescue post-thaw cell recovery when used at DMSO concentrations which are normally too low to enable cryopreservation. A549 suspension freezing showed that supplementing P2 with 10 wt % DMSO did not enhance the recovery of suspension cells, suggesting a ceiling for total recovery and distinct to the monolayer results (highlighting the challenge of cryopreservation). However, adding 20 mg mL–1P2 allowed DMSO reduction down to 5 wt % and even 2.5 wt %, with cell recovery levels being comparable to 10 wt % DMSO with no polymer (Figure 4a). The ability to lower DMSO to 2.5%, with the addition of P2, and retain recoveries similar to 10 wt % DMSO is a remarkable finding and will permit the cryopreservation of cells incapable of tolerating high concentrations of DMSO. Additionally, the ability to drastically lower DMSO could permit higher recoveries and allow, for example, a larger amount of human peripheral blood progenitor cells to be infused to patients.51 For A549 cells frozen as monolayers, we found that 40 mg mL–1P2 provided significant cell recovery at 5 wt % DMSO, demonstrating the potent cryopreservative properties of this macromolecular polymer. It was also observed that a reduction in DMSO to 5 wt %, combined with 5 mg mL–1P2, enabled recovery similar to polymer-free 10% DMSO and also comparable to even lower DMSO at 2 wt % with 40 mg mL–1P2 (Figure 4b). The importance of this cannot be understated as lowering DMSO (whilst retaining cell yield) will dramatically increase the availability of cells for regenerative medicine, reduce downstream toxicology challenges, and enable the storage of cells which cannot tolerate higher concentrations of DMSO. For comparison, other poly(ampholyte)s reported for cryopreservation required a complex, high organic solvent content solution of 6.5 M ethylene glycol (∼40 wt %), 0.5 M sucrose (∼15 wt %), and 10 wt % polymer to obtain a similar result32 or only gave cell yields below that of DMSO alone,52 highlighting the unique potency of the present system.
Figure 4.
A549 variable DMSO/P2 suspension and monolayer cryopreservation. Recovered cells were counted by trypan blue exclusion assay relative to unfrozen controls. Suspension cells were cryopreserved for 24 h at −196 °C, monolayer cells were cryopreserved for 24 h at −80 °C, and both methods incubated 24 h post-thaw. (a) Impact on suspension post-thaw recovery of varying DMSO and P2 concentrations (NP = no polymer) (N = 90, P = 0.0000000001, *P < 0.001 from NP w/10% DMSO). The data represent the mean ± SEM of at least three independent experiments. (b) Impact on monolayer post-thaw recovery of varying DMSO and P2 concentrations (NP = no polymer) (N = 60, P = 0.0000000001, *P < 0.001 from NP w/10% DMSO). The data represent the mean ± SEM of three independent experiments with two nested replicates each.
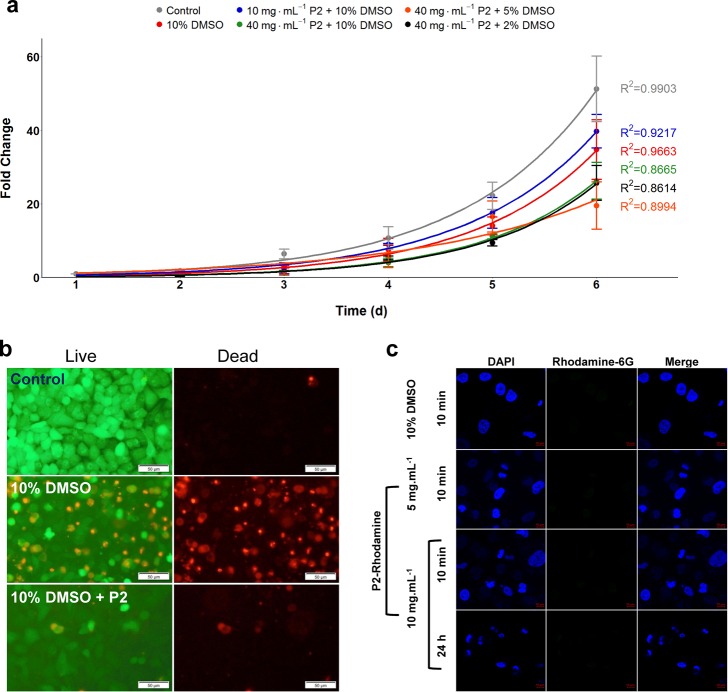
While membrane integrity post-thaw is a general measure of cell survival, it does not provide any insight into long-term damage. Thus, a 6-day growth assay was conducted to evaluate the cell function. Post-thaw proliferation rates of A549 cells, which had been cryopreserved in a monolayer format with/without P2, were measured and compared to unfrozen control cells seeded at an identical density (Figure 5a). The cells cryopreserved with 10 mg mL–1P2 recovered at equal or faster rates than those in 10% DMSO alone, but 40 mg mL–1 gave slower growth rates than 10 mg mL–1. Therefore, 10 mg mL–1 can be considered the optimum formulation for this format and cell line to ensure high yield and retention of function. As membrane interaction and stabilization are a potential mechanism of action, supported by the erythrocyte observations (Supporting Information, Figure S6), membrane permeation post-thaw was also assessed. Ethidium homodimer-1 (EhtD-1)/calcein-AM stains (LIVE/DEAD assay) were used. Healthy cells internalize calcein-AM and convert it to calcein, which then fluoresces green; EthD-1 passes through damaged membranes to the nuclei where it brightly fluoresces red. Cells previously cryopreserved in 10% DMSO had significant numbers of compromised membranes, but in contrast, addition of P2 lead to far fewer red nuclei, supporting the hypothesis that P2 stabilizes the cell membranes during freeze/thaw cycles (Figure 5b); graphical values are shown in Figure S9. Poly(ampholyte)s are known to interact with model phospholipid membranes to a greater extent than other zwitterions such as poly(sulfobetaines),40 and we have shown that poly(ampholyte)s do not offer any stabilization to prokaryotes which have markedly different phospholipid compositions, supporting a specific membrane interaction.53
Figure 5.
Post-thaw growth and polymer interaction. (a) Post-thaw cell proliferation for 6 d; cells were seeded at 1.25 × 104 cells per well. (b) LIVE/DEAD staining of A549 cells; 24 h post-thaw after cryopreservation using indicated cryoprotectants. The scale bar is 50 μm. (c) Confocal fluorescence microscopy of A549 cells incubated with 10% DMSO or P2 (with no DMSO) for the indicated time and concentration. Nuclei are stained with 4′,6-diamidino-2-phenylindole) (DAPI) and incubated with P2 tagged with rhodamine 6G, which is green fluorescent. The scale bar is 10 μm.
To visualize the uptake or cell binding, a rhodamine 6G-tagged poly(ampholyte) (confirmed in Figure S10) was synthesized and exposed to A549 cells for both 10 min and 24 h. Confocal microscopy revealed no polymer-associated intracellular- or extracellular-bound fluorescence (Figure 5c) after a single washing step, and hence the unique mode of action of this polymer is purely extracellular, although not through ice interactions, unlike previous polymer cryoprotectants.28,48 It is extremely beneficial that the polymer appears to be removed by simple washing, rather than multiple washings and/or equilibrations required for DMSO or glycerol (for RBCs), which is a major issue in the logistics of cell-based therapies.
To further probe the impact of the polymers on membrane integrity, a membrane flux assay was modified from Su et al.35 A549 cells were loaded with calcein-AM, and the quencher trypan blue was exogenously added, allowing membrane flux to be continuously monitored by a reduction in calcein-associated fluorescence (Figure 6a). DMSO at 20 wt % (above cryopreservation levels as a positive control) led to significantly more calcein loss compared to other solutions, whereas DMSO at 10 wt % showed no reduction in flux, supporting the hypothesis that 10 wt % thins membranes while 20 wt % promotes pore formation,54 yet it still remains that 10 wt % DMSO reduces cell metabolism after only 30 min at room temperature (Figure S8). However, all other formulations did not dramatically change the rate of flux, and P2 even lead to a small reduction in the flux rate at higher concentrations, supporting the membrane stabilization hypothesis. Under identical conditions, the LIVE/DEAD stain was again applied to A549 monolayers (not frozen). After 24 h with P2, there were no significant changes in the red nuclei at 5 mg mL–1 and only a small increase at 10 mg mL–1 (Figure 6c), but these time periods are far longer than our actual exposure time of 10 min (which is used in the pre-cryopreservation incubation protocol). Taken together, this data supports a mechanism of external polymer membrane integrity stabilization during the freezing process that enables cells to recover and proliferate faster.
Figure 6.
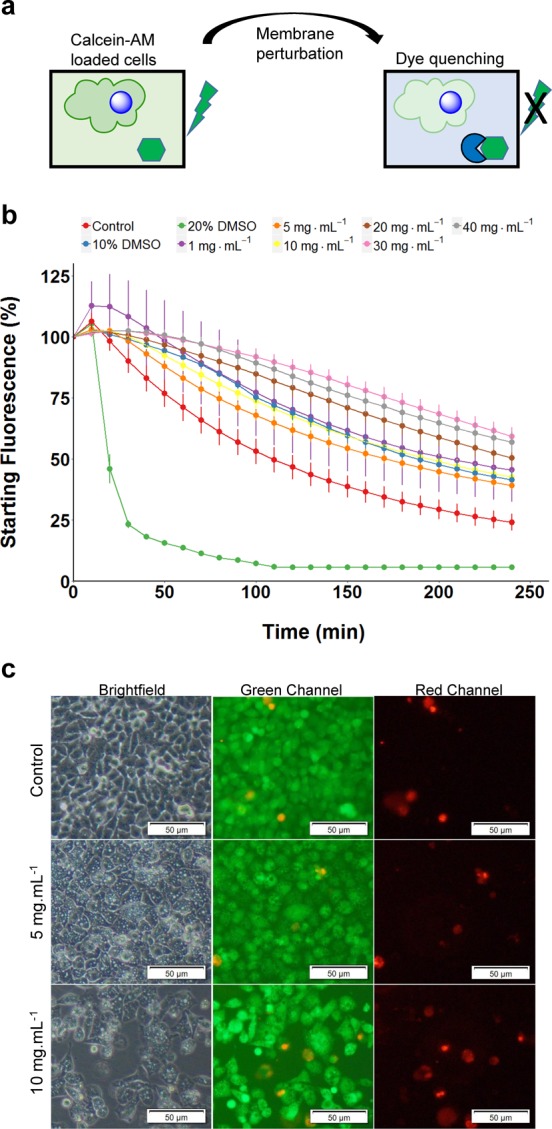
Impact of polymer on the membrane flux and permeability. (a) Graphical depiction of dye leakage assay. (b) Continuous live cell dye leakage assay from calcein-AM loaded A549 cells treated with varying concentrations of P2. Decreasing fluorescence corresponds to an increased membrane flux. (c) LIVE/DEAD staining of A549 cells after 24 h of incubation with P2. The scale bar is 50 μm.
Discussion
The cryopreservation of cells is an incredibly complex process where biochemical as well as mechanical and physical factors govern both the total cell recovery and the retention of function/viability. The recovery of large number of cells, with retention of function, is essential in cell-based therapies (to ensure therapeutic outcome) and in basic biomedical research (where cell studies guide new treatments and understanding). Here, we introduce a unique macromolecular cryopreservative, which enables cells to be frozen in a monolayer format with high recovery, allows a reduction in the concentration of DMSO required for suspension and monolayer cell cryopreservation, increases the viability of recovered cells, and reduces the extent of membrane damage. This macromolecular cryopreservative, a poly(ampholyte), was obtained in a single step from a clinically used commodity bulk polymer in a quantitative yield. Previous reports of polymer cryopreservatives have focused on materials which can mimic antifreeze proteins55,56 by controlling the ice crystal formation and/or growth, which benefits cell cryopreservation.28 The poly(ampholyte) we introduce here had very moderate effects on ice crystal growth, but led to remarkable increases in post-thaw viability of over 400%, compared to DMSO alone for cell monolayers, and allowed [DMSO] to be reduced to just 2% in some cases. It was found that an 80 kDa polymer weight was optimal (P2), although all molecular weights were extremely potent. Mechanistic studies showed that this polymer does not enter the cells and appears to function at the membrane, stabilizing it during freezing but without promoting pore formation or affecting the intrinsic membrane flux. This is supported by erythrocyte (RBC) studies, which is a useful model for membrane damage/stabilization as well as a crucial component of modern medicine. Utilizing the optimal polymer, >80% post-thaw recovery of RBCs was possible using the polymer alone, with no additional solvents, comparable to the gold standard of glycerol44 but with the advantage of less processing challenges and easy removal, as our polymer is not cell permeable. Taken together, this data supports a mechanism where this new macromolecular cryoprotectant appears to stabilize cell membranes against cooling without affecting the flux inside to outside the cell, enabling faster post-thaw recovery with more intact membranes and fast proliferation rates. Increasing cell yields will speed up biomedical research (by reducing thaw to assay time) as well as decrease the number of donor cells required for cell-based therapies by ensuring higher recovery from the critical step of cryopreservation. Simpler and/or more effective cryopreservatives may also improve the cold chain, leading to reductions in the cost associated with cell-based therapies and regenerative medicine.
Conclusions
We have shown that our potent and easily scalable regio-regular poly(ampholyte) significantly improves post-thaw cell recoveries of up to 88% for adherent cell monolayers compared to just 24% using conventional DMSO cryopreservation. The poly(ampholyte) also enables reduction of [DMSO] from 10 wt % to just 2.5 wt % in suspension cryopreservation with comparable outcomes, which can enable the preservation of DMSO-intolerant cell lines. We have also shown that this polymer leads to cells with reduced membrane damage and faster post-thaw growth rates and can additionally cryopreserve RBCs. Our poly(ampholyte) appears to function extracellularly and is thus nonpenetrating and easily removed. This new polyampholytic cryoprotectant will improve applications across basic and translational biomedical science and may drastically enhance the cold-chain processes for biological materials.
Acknowledgments
This work was supported by the ERC (638661 and 789182 to MIG). Studentship for R.M.F.T is supported by the EPSRC Centre for Doctoral Training in Molecular Analytical Science, (EP/L015307/1RT). We thank the support from a seed grant through the Wellcome Warwick Quantitative Biomedicine Programme (Institutional Strategic Support Fund: 105627/Z/14/Z). The Wellcome Trust-Warwick QBP program is thanked for the financial support. UoW Advanced BioImaging RTP is supported by BBSRC ALERT14 Award BB/M01228X/1. TLB thanks M. Menze for providing the Biocision CoolCell to enable controlled rate freezing.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.biomac.9b00681.
Osmotic fragility, nile red staining, confocal poly(ampholyte) uptake, neutral red staining, polymer characterization, example splat images, DSC traces, onset melting temperatures, blood freezing with other protective compounds, mammalian cell phenotype, DMSO toxicity, graphical representation of live/dead staining post-freeze, and confirmation of rhodamine-6G tagged P2 (PDF)
Author Contributions
T.L.B. conducted mammalian cell experiments. C.S. undertook polymer synthesis. C.S. and L.O. undertook erythrocyte screening data. K.M. conducted suspension mammalian cell freezing and DMSO toxicity. R.M.F.T conducted confocal microscopy. M.I.G. devised experiments alongside other authors and directed the research. All authors contributed to writing the manuscript.
The authors declare the following competing financial interest(s): CS, TB and MIG are named on a patent application relating to this work.
Supplementary Material
References
- Wald M.Blood Industry Shrinks as Transfusions Decline; New York Times; 2014.p A1. [Google Scholar]
- García-Roa M.; Del Carmen Vicente-Ayuso M.; Bobes A. M.; Pedraza A. C.; González-Fernández A.; Martín M. P.; Sáez I.; Seghatchian J.; Gutiérrez L. Red Blood Cell Storage Time & Transfusion: Current Practice, Concerns & Future Perspectives. Blood Transfus. 2017, 15, 222–231. 10.2450/2017.0345-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh G. Biopharmaceutical Benchmarks 2006. Nat. Biotechnol. 2006, 24, 769–776. 10.1038/nbt0706-769. [DOI] [PubMed] [Google Scholar]
- Seth G. Freezing Mammalian Cells for Production of Biopharmaceuticals. Methods 2012, 56, 424–431. 10.1016/j.ymeth.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Kang J.; Hsu C.-H.; Wu Q.; Liu S.; Coster A. D.; Posner B. A.; Altschuler S. J.; Wu L. F. Improving Drug Discovery with High-Content Phenotypic Screens by Systematic Selection of Reporter Cell Lines. Nat. Biotechnol. 2016, 34, 70–77. 10.1038/nbt.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J. O. M.; Toner M. Long-Term Storage of Tissues by Cryopreservation: Critical Issues. Biomaterials 1996, 17, 243–256. 10.1016/0142-9612(96)85562-1. [DOI] [PubMed] [Google Scholar]
- Wang S.; Elliott G. D. Synergistic Development of Biochips and Cell Preservation Methodologies: A Tale of Converging Technologies. Curr. Stem Cell Rep. 2017, 3, 45–53. 10.1007/s40778-017-0074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stéphenne X.; Najimi M.; Sokal E. M. Hepatocyte Cryopreservation: Is It Time to Change the Strategy?. World J. Gastroenterol. 2010, 16, 1–14. 10.3748/wjg.v16.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur P. Cryobiology: The Freezing of Biological Systems. Science 1970, 168, 939–949. 10.1126/science.168.3934.939. [DOI] [PubMed] [Google Scholar]
- Mazur P.; Farrant J.; Leibo S. P.; Chu E. H. Y. Survival of hamster tissue culture cells after freezing and thawing. Cryobiology 1969, 6, 1–9. 10.1016/s0011-2240(69)80002-7. [DOI] [PubMed] [Google Scholar]
- Timm M.; Saaby L.; Moesby L.; Hansen E. W. Considerations Regarding Use of Solvents in in Vitro Cell Based Assays. Cytotechnology 2013, 65, 887–894. 10.1007/s10616-012-9530-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.; Cowley S.; Flaim C. J.; James W.; Seymour L.; Cui Z. The Roles of Apoptotic Pathways in the Low Recovery Rate after Cryopreservation of Dissociated Human Embryonic Stem Cells. Biotechnol. Prog. 2010, 26, 827–837. 10.1002/btpr.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng B. C.; Ye C. P.; Liu H.; Toh W. S.; Rufaihah A. J.; Yang Z.; Bay B. H.; Ge Z.; Ouyang H. W.; Lee E. H.; Cao T. Loss of Viability during Freeze-Thaw of Intact and Adherent Human Embryonic Stem Cells with Conventional Slow-Cooling Protocols Is Predominantly Due to Apoptosis Rather than Cellular Necrosis. J. Biomed. Sci. 2006, 13, 433–445. 10.1007/s11373-005-9051-9. [DOI] [PubMed] [Google Scholar]
- Bailey T. L.; Wang M.; Solocinski J.; Nathan B. P.; Chakraborty N.; Menze M. A. Protective Effects of Osmolytes in Cryopreserving Adherent Neuroblastoma (Neuro-2a) Cells. Cryobiology 2015, 71, 472–480. 10.1016/j.cryobiol.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Clark M. S.; Thorne M. A.; Purać J.; Burns G.; Hillyard G.; Popović Ž. D.; Grubor-Lajšić G.; Worland M. R. Surviving the Cold: Molecular Analyses of Insect Cryoprotective Dehydration in the Arctic Springtail Megaphorura Arctica (Tullberg). BMC Genomics 2009, 10, 328. 10.1186/1471-2164-10-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. S. Metabolic Studies of Crytobiosis in Encysted Embryos of Artemia Salina. Comp. Biochem. Physiol. 1967, 20, 801–809. 10.1016/0010-406x(67)90054-0. [DOI] [Google Scholar]
- Erkut C.; Penkov S.; Fahmy K.; Kurzchalia T. V. How worms survive desiccation. Worm 2012, 1, 61–65. 10.4161/worm.19040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M. B. Molecular Basis of Osmotic Regulation. Am. J. Physiol. 1995, 268, F983–F996. 10.1152/ajprenal.1995.268.6.f983. [DOI] [PubMed] [Google Scholar]
- Armstrong D. A.; Strange K.; Crowe J.; Knight A.; Simmons M. High Salinity Acclimation by the Prawnmacrobrachium Rosenbergiiuptake of Exogenous Ammonia and Changes in Endogenous Nitrogen Compounds. Biol. Bull. 1981, 160, 349–365. 10.2307/1540844. [DOI] [Google Scholar]
- Takagi H.; Sakai K.; Morida K.; Nakamori S. Proline accumulation by mutation or disruption of the proline oxidase gene improves resistance to freezing and desiccation stresses inSaccharomyces cerevisiae. FEMS Microbiol. Lett. 2000, 184, 103–108. 10.1016/s0378-1097(00)00023-9. [DOI] [PubMed] [Google Scholar]
- Grothe S.; Krogsrud R. L.; McClellan D. J.; Milner J. L.; Wood J. M. Proline Transport and Osmotic Stress Response in Escherichia Coli K-12. J. Bacteriol. 1986, 166, 253–259. 10.1128/jb.166.1.253-259.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrenko Y. A.; Jones D. R. E.; Petrenko A. Y. Cryopreservation of Human Fetal Liver Hematopoietic Stem/Progenitor Cells Using Sucrose as an Additive to the Cryoprotective Medium. Cryobiology 2008, 57, 195–200. 10.1016/j.cryobiol.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Capicciotti C. J.; Malay D.; Ben R. N.. Ice Recrystallization Inhibitors: From Biological Antifreeze to Small Molecules. Recent Developments in the Study of Recrystallization, 2013; Vol. 177–224.
- Griffith M.; Yaish M. W. F. Antifreeze Proteins in Overwintering Plants: A Tale of Two Activities. Trends Plant Sci. 2004, 9, 399–405. 10.1016/j.tplants.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Moore D. S.; Hand S. C. Cryopreservation of Lipid Bilayers by LEA Proteins from Artemia Franciscana and Trehalose. Cryobiology 2016, 73, 240–247. 10.1016/j.cryobiol.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Congdon T.; Notman R.; Gibson M. I. Antifreeze (Glyco)Protein Mimetic Behavior of Poly(Vinyl Alcohol): Detailed Structure Ice Recrystallization Inhibition Activity Study. Biomacromolecules 2013, 14, 1578–1586. 10.1021/bm400217j. [DOI] [PubMed] [Google Scholar]
- Holt C.B. The effect of antifreeze proteins and poly(vinyl alcohol) on the nucleation of ice: a preliminary study. CryoLetters 2003, 24, 323–330. [PubMed] [Google Scholar]
- Deller R. C.; Vatish M.; Mitchell D. A.; Gibson M. I. Synthetic Polymers Enable Non-Vitreous Cellular Cryopreservation by Reducing Ice Crystal Growth during Thawing. Nat. Commun. 2014, 5, 3244. 10.1038/ncomms4244. [DOI] [PubMed] [Google Scholar]
- Deller R. C.; Vatish M.; Mitchell D. A.; Gibson M. I. Synthetic Polymers Enable Non-Vitreous Cellular Cryopreservation by Reducing Ice Crystal Growth during Thawing. Nat. Commun. 2014, 5, 3244. 10.1038/ncomms4244. [DOI] [PubMed] [Google Scholar]
- Graham B.; Bailey T. L.; Healey J. R. J.; Marcellini M.; Deville S.; Gibson M. I. Polyproline as a Minimal Antifreeze Protein Mimic That Enhances the Cryopreservation of Cell Monolayers. Angew. Chem., Int. Ed. Engl. 2017, 56, 15941–15944. 10.1002/anie.201706703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K.; Bae J. Y.; Kim H. H.; Hyon S. H. Effective vitrification of human induced pluripotent stem cells using carboxylated ε-poly-l-lysine. Cryobiology 2011, 63, 76–83. 10.1016/j.cryobiol.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Matsumura K.; Kawamoto K.; Takeuchi M.; Yoshimura S.; Tanaka D.; Hyon S.-H. Cryopreservation of a Two-Dimensional Monolayer Using a Slow Vitrification Method with Polyampholyte to Inhibit Ice Crystal Formation. ACS Biomater. Sci. Eng. 2016, 2, 1023–1029. 10.1021/acsbiomaterials.6b00150. [DOI] [PubMed] [Google Scholar]
- Matsumura K.; Hyon S.-H. Polyampholytes as Low Toxic Efficient Cryoprotective Agents with Antifreeze Protein Properties. Biomaterials 2009, 30, 4842–4849. 10.1016/j.biomaterials.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Stubbs C.; Lipecki J.; Gibson M. I. Regioregular Alternating Polyampholytes Have Enhanced Biomimetic Ice Recrystallization Activity Compared to Random Copolymers and the Role of Side Chain versus Main Chain Hydrophobicity. Biomacromolecules 2017, 18, 295–302. 10.1021/acs.biomac.6b01691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M.; He C.; West C. A.; Mentzer S. J. Cytolytic Peptides Induce Biphasic Permeability Changes in Mammalian Cell Membranes. J. Immunol. Methods 2001, 252, 63–71. 10.1016/s0022-1759(01)00334-9. [DOI] [PubMed] [Google Scholar]
- Scientific Opinion on the Safety of “Methyl Vinyl Ether-Maleic Anhydride Copolymer” (Chewing Gum Base Ingredient) as a Novel Food Ingredient. EFSA J. 2013, 11, 1–17. 10.2903/j.efsa.2013.3423. [DOI] [Google Scholar]
- Knight C. A.; Hallett J.; DeVries A. L. Solute Effects on Ice Recrystallization: An Assessment Technique. Cryobiology 1988, 25, 55–60. 10.1016/0011-2240(88)90020-x. [DOI] [PubMed] [Google Scholar]
- Mitchell D. E.; Lilliman M.; Spain S. G.; Gibson M. I. Quantitative Study on the Antifreeze Protein Mimetic Ice Growth Inhibition Properties of Poly(Ampholytes) Derived from Vinyl-Based Polymers. Biomater. Sci. 2014, 2, 1787–1795. 10.1039/c4bm00153b. [DOI] [PubMed] [Google Scholar]
- Fullerton G. D.; Keener C. R.; Cameron I. L. Correction for Solute/Solvent Interaction Extends Accurate Freezing Point Depression Theory to High Concentration Range. J. Biochem. Biophys. Methods 1994, 29, 217–235. 10.1016/0165-022x(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Rajan R.; Hayashi F.; Nagashima T.; Matsumura K. Toward a Molecular Understanding of the Mechanism of Cryopreservation by Polyampholytes: Cell Membrane Interactions and Hydrophobicity. Biomacromolecules 2016, 17, 1882–1893. 10.1021/acs.biomac.6b00343. [DOI] [PubMed] [Google Scholar]
- Mohandas N.; Gallagher P. G. Red Cell Membrane: Past, Present, and Future. Blood 2008, 112, 3939–3948. 10.1182/blood-2008-07-161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs C. I.; Stubbs C.; Graham B.; Fayter A. E. R.; Hasan M.; Gibson M. I. Mimicking the Ice Recrystallization Activity of Biological Antifreezes. When Is a New Polymer “Active”?. Macromol. Biosci. 2019, 10.1002/mabi.201900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeri C. R.; Ragno G.; Pivacek L. E.; Cassidy G. P.; Srey R.; Hansson-Wicher M.; Leavy M. E. An Experiment with Glycerol-Frozen Red Blood Cells Stored at -80°C for up to 37 Years. Vox Sang. 2003, 79, 168–174. 10.1046/j.1423-0410.2000.7930168.x. [DOI] [PubMed] [Google Scholar]
- Briard J. G.; Poisson J. S.; Turner T. R.; Capicciotti C. J.; Acker J. P.; Ben R. N. Small Molecule Ice Recrystallization Inhibitors Mitigate Red Blood Cell Lysis during Freezing, Transient Warming and Thawing. Sci. Rep. 2016, 6, 23619. 10.1038/srep23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy G. M. The relevance of cryoprotectant ″toxicity″ to cryobiology. Cryobiology 1986, 23, 1–13. 10.1016/0011-2240(86)90013-1. [DOI] [PubMed] [Google Scholar]
- Meryman H. T.; Hornblower M. A Method for Freezing and Washing Red Blood Cells Using a High Glycerol Concentration. Transfusion 1972, 12, 145–156. 10.1111/j.1537-2995.1972.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Fowler A.; Toner M. Cryo-Injury and Biopreservation. Ann. N.Y. Acad. Sci. 2005, 1066, 119–135. 10.1196/annals.1363.010. [DOI] [PubMed] [Google Scholar]
- Deller R. C.; Pessin J. E.; Vatish M.; Mitchell D. A.; Gibson M. I. Enhanced Non-Vitreous Cryopreservation of Immortalized and Primary Cells by Ice-Growth Inhibiting Polymers. Biomater. Sci. 2016, 4, 1079–1084. 10.1039/c6bm00129g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M. B.; Ferraris J. D.; Dmitrieva N. I. Cellular Response to Hyperosmotic Stresses. Physiol. Rev. 2007, 87, 1441–1474. 10.1152/physrev.00056.2006. [DOI] [PubMed] [Google Scholar]
- Mazur P.; Leibo S. P.; Chu E. H. Y. A two-factor hypothesis of freezing injury. Exp. Cell Res. 1972, 71, 345–355. 10.1016/0014-4827(72)90303-5. [DOI] [PubMed] [Google Scholar]
- Abrahamsen J. F.; Bakken A. M.; Bruserud Ø. Cryopreserving human peripheral blood progenitor cells with 5-percent rather than 10-percent DMSO results in less apoptosis and necrosis in CD34+ cells. Transfusion 2002, 42, 1573–1580. 10.1046/j.1537-2995.2002.00242.x. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Johnson M. A.; Fisher R.; Burke N. A. D.; Stöver H. D. H. Synthetic Polyampholytes as Macromolecular Cryoprotective Agents. Langmuir 2019, 35, 1807–1817. 10.1021/acs.langmuir.8b01602. [DOI] [PubMed] [Google Scholar]
- Hasan M.; Fayter A. E. R.; Gibson M. I. Ice Recrystallization Inhibiting Polymers Enable Glycerol-Free Cryopreservation of Microorganisms. Biomacromolecules 2018, 19, 3371–3376. 10.1021/acs.biomac.8b00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtovenko A. A.; Anwar J. Modulating the Structure and Properties of Cell Membranes: The Molecular Mechanism of Action of Dimethyl Sulfoxide. J. Phys. Chem. B 2007, 111, 10453–10460. 10.1021/jp073113e. [DOI] [PubMed] [Google Scholar]
- Voets I. K. From Ice-Binding Proteins to Bio-Inspired Antifreeze Materials. Soft Matter 2017, 13, 4808–4823. 10.1039/c6sm02867e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs C. I.; Bailey T. L.; Graham B.; Stubbs C.; Fayter A.; Gibson M. I. Polymer Mimics of Biomacromolecular Antifreezes. Nat. Commun. 2017, 8, 1546. 10.1038/s41467-017-01421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.