Abstract
Background
Regular physical activity is widely recommended for patients with arterial hypertension as an essential component of lifestyle modification. Much less is known about the impact of physical exercise on the management of treatment of resistant hypertension (RH). The aim was to assess the effect of physical activity program intensified by mobile phone text reminders on blood pressure control in subjects with RH managed in the primary care.
Methods
In total, 53 patients with primary hypertension were qualified, including 27 who met the criteria for RH and 26 with well-controlled hypertension (WCH). Ambulatory 24-h blood pressure was monitored and body composition evaluated with bioimpedance and habitual physical activity profile was determined continuously over 72 h with accelerometer. All measurements were performed at baseline and after three and six months. The patients were asked to modify their lifestyle according to American Heart Association Guidelines that included regular aerobic physical activity tailored to individual needs.
Findings
Physical activity in RH increased significantly after six months compared with control subjects (P=0.001). Office systolic blood pressure (SBP) and diastolic blood pressure (DBP) in the RH group decreased significantly after three months but after six months only office DBP remained significantly lower. After three months 24-h SBP decreased by 3.1±11 mmHg (P=0.08) and DBP by 2.0±6 mmHg (P=0.17) in RH, whereas in WCH respective changes were +1.2±10 and −0.3±6 mmHg. After six months 24-h BP changes were similar.
Conclusion
Individualized structured physical activity program increases physical activity in the treatment of resistant hypertensives in primary care but the effect on 24-h blood pressure is only transient.
Key words: ambulatory blood pressure monitoring, physical activity, primary health care, resistant hypertension
Background
Regular physical activity has been recognized as a remedy for many diseases (Kokkinos and Myers, 2010; Booth et al., 2012). Physical activity improves the function of various organs, activates socially, enhances mental well-being, boosts general physical performance, and reduces body mass and mortality (Slentz et al., 2009). The relevant preventive effects of increased physical activity have been studied and confirmed in as many as 35 chronic diseases (Booth et al., 2012).
Individuals with hypertension, in particular those with ineffectively controlled blood pressure, that is with resistant hypertension (RH), have a higher risk of cardiovascular incidents compared with subjects with well-controlled arterial hypertension (WCH) (Calhoun et al., 2008; Mancia et al., 2013).
Treatment of RH remains an important clinical problem despite the advances in pharmacotherapy. RH has been related to 75% greater risk of stroke, 44% greater risk of ischemic heart disease and 30% greater risk of death for any cause as well as an increased risk of heart failure, end-stage renal disease and peripheral artery disease (Muntner et al., 2014). The prevalence of RH reaches 14–16% (Achelrod et al., 2015), and is rising mostly because of the aging of the population and increasing incidence of diabetes and obesity (Roberie and Elliott, 2012). RH is also common in primary care setting and accounts for 9.9–22.8% of all hypertensive patients (Gijὀn-Conde et al., 2014; Prejbisz et al., 2015).
According to a current definition, RH is diagnosed in patients who, after the administration of antihypertensive drugs from three different groups in therapeutic doses including a diuretic, did not achieve a target blood pressure (BP) <140/90 mmHg as well as in those who were administered four and more antihypertensives from three different groups, including a diuretic regardless of achieving a target BP (Calhoun et al., 2008). The population of patients with RH also includes the subjects who do not comply with the doctor’s recommendations. It is estimated that as many as 25% of patients may fail to observe systematically the drug regimen (Tomaszewski et al., 2014). The compliance may be even poorer with regard to recommended lifestyle modification, reduction of sodium and alcohol consumption, introduction of a high-fiber diet, avoiding obesity and increasing physical activity (Mancia et al., 2013; Kraus et al., 2015).
According to several large clinical studies, increased physical activity may have an effect on the reduction of mean BP by up to 4–9 mmHg (Cornelissen and Smart, 2013; Mancia et al., 2013). The benefits of moderate physical activity have also been found in patients with RH (Dimeo et al., 2012), but there were only a few intervention studies carried out in this field. Our initial hypothesis is that the patients with RH are resistant not only to pharmacotherapy, by definition, but also to lifestyle measures including increased level of physical activity.
The aim of this study was to assess the effects of a program of intensified physical activity introduced in primary health care combined with exercise training and short text messages sent to the patients’ mobile phones or motivational telephone conversations on BP in patients with treatment of RH.
Methods
The prospective lifestyle intervention study was carried out in a single primary care health center from October 2014 until the end of August 2015. The study protocol was approved by the local Ethics Committee and all the patients gave informed consent before being included. The research was conducted in accordance with the Helsinki Declaration. The participants included a population treated in primary health care in a community-based outpatient clinic in central Poland. The adult population of the Center catchment area is ~3700.
The patients qualified for the study were 18–70 years old and were diagnosed with arterial hypertension according to the 2013 guidelines of the European Society of Hypertension and the European Society of Cardiology (Mancia et al., 2013).
The exclusion criteria included any secondary cause of elevated BP, chronic kidney disease stage 3 and higher, that is estimated glomerular filtration rate (eGFR, 2009 Chronic Kidney Disease – Epidemiology Collaboration, CKD-EPI)<60 mL/min (Levin and Stevens, 2014), mental illness and/or impaired consciousness, significant mobility dysfunction, respiratory diseases, heavy liver failure, active malignant disease, unstable coronary disease or NYHA stage 2–4 heart failure, stroke or transient ischemic attack within the past 12 months.
All patients underwent an extensive diagnostics of RH with accordance to the statement from the American Heart Association (Calhoun et al., 2008) in the reference hypertension center (Department of Nephrology, Hypertension and Kidney Transplantation, Medical University of Lodz) for the secondary causes of arterial hypertension including renal, renovascular and endocrine causes, and pseudohypertension after the diagnosis of the treatment of RH had been established. For safety reasons the subjects were not qualified if they experienced any hypertension urgency or emergency requiring hospitalization for 12 months before the study.
A review of the medical records at the community-based health center allowed identifying patients who initially fulfilled the qualification criteria. After a short visit involving physical examination and analysis of the antihypertensive drug history, and previous diagnostics of arterial hypertension 80 subjects met inclusion criteria and signed an informed consent. After the visit laboratory tests were performed. Eventually 53 patients who fulfilled the clinical and laboratory criteria were enrolled into the study, including 27 who met the criteria for RH (Calhoun et al., 2008) and 26 subjects on antihypertensive medications with well-controlled BP (Mancia et al., 2013). Most patients who did not meet the criteria had eGFR lower than 60 mL/min. In addition, only the patients who had a long history of the visits in health center, were well known to family doctor and bought the prescribed medications at the pharmacy that is a part of our health center were enrolled. Moreover, the patients were also asked to bring the boxes of used and unused medications at each visit and the pills were counted.
Ambulatory blood pressure monitoring (ABPM) was performed using the Mobil-O-Graph device, version 12 (I.E.M. GmbH, Stolberg, Germany) (Jones et al., 2000). During the day the measurements were taken every 15 min and during the night every 30 min. The time of the day or the night was determined by the patient by pressing the button. We established the following definitions: the circadian dipper pattern as 10–20% reduction of BP measurements at night as compared with ABPM measurements during the day, the mild dipper pattern as 0–10% reduction, the extreme dipper pattern as a reduction of BP measurement by >20%, and the reverse dipper pattern as a rise of BP during the night. Office BP was measured using Accoson Greenlight 300 (A.C. Cossor & Son LTD, Essex, Great Britain).
The analysis of the physical activity profile and energy expenditure was carried out with BodyMedia SenseWear System accelerometer using BodyMedia SenseWear Armband Mini, triaxial accelerometer (BodyMedia Inc., Pittsburgh, PA, USA) attached to the patient’s arm for 3 consecutive days (Johannsen et al., 2010).
The body composition analysis using multi-frequency electrical bioimpedance (Nutriguard-M with dedicated Bianostic-AT electrodes; Data Input GmbH, Darmstadt, Germany) was carried out for all patients.
The interview, measurements (ABPM, body composition analysis, physical activity profile) and laboratory tests (complete blood count, creatinine, electrolytes, lipids, glucose, aspartate and alanine transaminases) were repeated after three and six months during the course of the study.
The patients from both groups received the recommendations concerning their diet and healthy lifestyle including physical activity in cardiovascular diseases. The recommendations were based on the American Heart Association’s Diet and Lifestyle Recommendations (www.heart.org, 2014) and prepared in writing in Polish language and presented to each patient at the visit and discussed in the form of a short conversation.
At each stage of the study the patients from RH group received also verbal instructions on how to intensify physical activity tailored to their needs and comorbidities. Additionally a meeting with a physical therapist was provided to the patients. During the meeting a demonstration was given how to perform the stretching and the exercises strengthening muscles. Easy-to-apply physical exercises were demonstrated and warm-up elements were discussed. During the 1-h-lasting meeting the patients were predominantly encouraged to engage in physical activity. The aim of this meeting was to decrease an anxiety related to physical exercises known as kinesiophobia (Lundberg et al., 2006). Then the information received was verified during a short conversation. The principles of lifestyle modification were also discussed during all study visits.
After three and six months of the study the patients from RH group received a self-assessment form to be completed at home, which included 10 questions. Questions concerned the difficulties in the implementation exercise, positive or negative attitudes towards physical activity, and the willingness to continue the exercise (The self-assessment form is available as Online Resource 1). On the basis of the information physical activity was modified and intensified.
In addition, the patients received text messages to their cell phones with reminders about the benefits of regular physical activity three times a week. The messages were tailored to the individual needs of each patient and their content was modified to avoid repeats (Cole-Lewis et al., 2010; de Jongh et al., 2012). Five patients from RH group did not use mobile phones on a daily basis. These patients were encouraged to undertake physical activity during a short landline phone conversation.
The results are presented as percentage values and arithmetic mean with a standard deviation. In order to compare the means of normally distributed variables between groups unpaired t-test was used. For non-normally distributed variables Mann–Whitney test was used. The results of repeated measurements were tested with analysis of variance (ANOVA). χ 2 test was used to analyze qualitative variables. For dichotomous variables evaluated repeatedly the Q Cochrane test was used. P<0.05 was taken as significant.
Results
Table 1 shows the baseline characteristics of the study groups. No significant differences between groups were observed with regard to age, sex, height, family history of the disease, prevalence of diabetes, ischemic heart disease, chronic kidney disease, alcohol consumption, tobacco smoking, circadian BP patterns or education. Significant differences between groups were found in case of body mass, body mass index (BMI), waist circumference, prevalence of headaches, sleep disturbances including snoring.
Table 1.
Baseline characteristics of the patients with resistant and well-controlled hypertension
| Resistant hypertension | Well-controlled hypertension | ||||||
|---|---|---|---|---|---|---|---|
| All (27) | Women [16 (59%)] | Men [11 (41%)] | All (26) | Women [17 (65%)] | Men [9 (35%)] | P value | |
| Age (years) | 55.5±9 | 56.5±9 | 54.2±10 | 54.8±9 | 55.7±9 | 53.1±9 | NS |
| Body weight (kg) | 89.4±13.6 | 85.3±14.7 | 95.4±9.5 | 76.3±11.6 | 73.2±11.8 | 82.1±9.3 | <0.001 |
| Height (m) | 1.66±0.1 | 1.63±0.06 | 1.7±0.08 | 1.65±0,1 | 1.6±0.05 | 1.72±0.05 | NS |
| BMI (kg/m2) | 32.5±5.1 | 32.25±5.2 | 32.9±5.3 | 28.2±4.3 | 28.4±4.9 | 27.7±3.0 | <0.005 |
| Waist circumference (cm) | 109.5±12.1 | 107.8±13 | 111.9±10 | 99.3±11.1 | 98.35±12.9 | 101±6.7 | <0.005 |
| Education | |||||||
| Basic | 15 (55.5%) | 8 (50%) | 7 (63.6%) | 11 (42.3%) | 8 (47%) | 2 (22.2%) | NS |
| Secondary | 9 (33.3%) | 6 (37.5%) | 3 (27.3%) | 11 (42.3%) | 5 (29.4%) | 6 (66.6%) | |
| Higher | 3 (11.1%) | 2 (12.5%) | 1 (9.1%) | 4 (15.38%) | 4 (23.53%) | 1 (11.1%) | |
| Circadian blood pressure pattern | |||||||
| Dippers | 14 (54%) | 6 (40%) | 8 (73%) | 15 (60%) | 9 (56%) | 6 (67%) | NS |
| Mild dippers | 4 (15%) | 3 (20%) | 1 (9%) | 7 (28%) | 4 (25%) | 3 (33%) | |
| Extreme dippers | 4 (15%) | 3 (20%) | 1 (9%) | 2 (8%) | 2 (13%) | 0 | |
| Reverse dippers | 4 (15%) | 3 (20%) | 1 (9%) | 1 (4%) | 1 (6%) | 0 | |
| Coronary heart disease | |||||||
| Yes | 3 (11%) | 3 (19%) | 0 | 0 | 0 | 0 | NS |
| No | 24 (89%) | 13 (81%) | 11 (100%) | 26 (100%) | 17 (100%) | 9 (100%) | |
| Diabetes | |||||||
| Yes | 11 (41%) | 6 (38%) | 5 (45%) | 8 (31%) | 7 (41%) | 1 (11%) | NS |
| No | 16 (59%) | 10 (63%) | 6 (55%) | 18 (69%) | 10 (59%) | 8 (89%) | |
| Chronic kidney disease | |||||||
| Yes | 5 (19%) | 4 (25%) | 1 (9%) | 1 (4%) | 1 (6%) | 0 | NS |
| No | 22 (81%) | 12 (75%) | 10 (91%) | 25 (96%) | 16 (94%) | 9 (100%) | |
| Chronic back pain | |||||||
| Yes | 17 (63%) | 11 (69%) | 6 (55%) | 10 (38%) | 8 (47%) | 2 (22%) | 0.08 |
| No | 10 (37%) | 5 (31%) | 5 (45%) | 16 (62%) | 9 (53%) | 7 (78%) | |
| Headache | |||||||
| Yes | 15 (56%) | 10 (63%) | 5 (45%) | 7 (27%) | 6 (35%) | 1 (11%) | <0.05 |
| No | 12 (44%) | 6 (38%) | 6 (55%) | 19 (73%) | 11 (65%) | 8 (89%) | |
| Sleep disorders, snoring | |||||||
| Yes | 22 (81%) | 14 (88%) | 8 (73%) | 13 (50%) | 8 (47%) | 5 (56%) | <0.05 |
| No | 5 (19%) | 2 (12%) | 3 (27%) | 13 (50%) | 9 (53%) | 4 (44%) | |
| Positive family history of cardiovascular disease | |||||||
| Yes (hypertension, diabetes, stroke) | 25 (93%) | 15 (94%) | 10 (91%) | 22 (85%) | 15 (88%) | 7 (78%) | NS |
| No | 2 (7%) | 1 (6%) | 1 (9%) | 4 (15%) | 2 (12%) | 2 (22%) | |
| Smoking | |||||||
| Currently, smoking | 3 (11%) | 1 (6%) | 2 (18%) | 5 (19%) | 3 (18%) | 2 (22%) | NS |
| Non-smoking | 23 (85%) | 14 (88%) | 9 (82%) | 18 (69%) | 13 (76%) | 5 (56%) | |
| Non-smoker from <5 years ago | 1 (4%) | 1 (6%) | 0 | 2 (12%) | 1 (2%) | 2 (22%) | |
| Alcohol drinking | |||||||
| Yes | 8 (30%) | 2 (13%) | 6 (55%) | 8 (31%) | 2 (12%) | 6 (67%) | NS |
| No | 19 (70%) | 14 (88%) | 5 (45%) | 18 (69%) | 15 (88%) | 3 (33%) | |
BMI=body mass index.
Data are presented as mean±SD or number (percentage).
Table 2 shows ambulatory and office BP during the study. A significant decrease of office BP was observed in RH group after three months, and the same was true for office diastolic blood pressure (DBP) after six months. There were no statistically significant differences after six months in ABPM values. Systolic blood pressure (SBP) and pulse pressure during the day differed significantly between the groups at baseline but not at the end of the study. During the last three months of the study nighttime ambulatory BP increased significantly in RH group. At the beginning of the study the differences of morning SBP and DBP between groups were numerically noticeable albeit statistically insignificant but that difference was no longer seen at the end of the study.
Table 2.
24-h, nighttime, daytime, morning systolic and diastolic blood pressure, pulse pressure in resistant hypertension and well-controlled hypertension groups at baseline and after three and six months of the study
| Parameter (mmHg) | Time of measurement | Resistant | Hypertension | Well-controlled hypertension | P value |
|---|---|---|---|---|---|
| Office SBP | At baseline | 150±24 | 132±8.5 | <0.001 | |
| After 3 months | 137.5±17 | P=0.004* | 129.7±10 | 0.1 | |
| After 6 months | 140±24 | P=0.08 | 130.7±11 | 0.2 | |
| Office DBP | At baseline | 90±14 | 80.5±7.5 | <0.005 | |
| After 3 months | 83±11 | P=0.001* | 77.7±7 | 0.1 | |
| After 6 months | 84.4±13 | P=0.04** | 80.7±8 | 0.2 | |
| SBP ABPM | At baseline | 127±17 | 119±12 | <0.05 | |
| After 3 months | 126±15 | P=0.20 | 120±11 | 0.1 | |
| After 6 months | 127±18 | P=0.60 | 119±9 | 0.1 | |
| DBP ABPM | At baseline | 75.4±11 | 72.6±7.1 | 0.3 | |
| After 3 months | 73.8±11 | P=0.10 | 71.9±6.5 | 0.5 | |
| After 6 months | 74.6±12 | P=0.40 | 71.9±5.2 | 0.4 | |
| Daytime SBP ABPM | At baseline | 131.3±16 | 122.4±12 | <0.05 | |
| After 3 months | 131.4±14 | P=0.30 | 124±12 | 0.1 | |
| After 6 months | 130±16 | P=0.30 | 123±10 | 0.1 | |
| Daytime DBP ABPM | At baseline | 77.8±11 | 75±7 | 0.3 | |
| After 3 months | 77.5±10 | P=0.30 | 74.7±7 | 0.3 | |
| After 6 months | 76.2±11 | P=0.20 | 74±5 | 0.4 | |
| Nighttime SBP ABPM | At baseline | 116.7±20 | 109.5±15 | 0.2 | |
| After 3 months | 111.6±17 | P=0.07 | 107±12 | 0.3 | |
| After 6 months | 119±25 | P=0.70 | 107±9 | 0.05 | |
| Nighttime DBP ABPM | At baseline | 67.4±13 | 64.8±9 | 0.8 | |
| After 3 months | 65.7±12 | P=0.40 | 63±6 | 0.4 | |
| After 6 months | 69±15 | P=0.90 | 64±6 | 0.2 | |
| Pulse pressure ABPM | At baseline | 53.2±11 | 46.4±9 | <0.05 | |
| After 3 months | 52.3±8 | P=0.20 | 48±9 | 0.1 | |
| After 6 months | 52.5±10 | P=0.40 | 47±9 | 0.1 | |
| Nighttime pulse pressure ABPM | At baseline | 49±11 | 44.2±9 | 0.4 | |
| After 3 months | 46±9 | P=0.03** | 44±9 | 0.4 | |
| After 6 months | 50±14 | P=0.80 | 43.5±8 | 0.1 | |
| Daytime pulse pressure ABPM | At baseline | 53.5±10 | 47.6±10 | <0.05 | |
| After 3 months | 54.7±9 | P=0.98 | 49.5±10 | 0.06 | |
| After 6 months | 53±9 | P=0.50 | 49±9 | 0.1 | |
| Morning SBP ABPM | At baseline | 136.6±25 | 125±19 | 0.06 | |
| After 3 months | 136±16 | P=0.40 | 125±20 | 0.05 | |
| After 6 months | 116.6±37 | P=0.07 | 120±17 | 0.3 | |
| Morning DBP ABPM | At baseline | 80.5±15 | 73.2±10 | 0.05 | |
| After 3 months | 80.3±14 | P=0.50 | 74±12 | 0.1 | |
| After 6 months | 75.2±19 | P=0.15 | 74±12 | 0.3 |
ABPM=automatic blood pressure monitoring; BP=blood pressure; DBP=diastolic blood pressure; Morning SBP and DBP=blood pressure mean during 1st hour after waking up; SBP=systolic blood pressure.
Data are presented as mean±SD.
*P<0.005 for the difference in the group of resistant hypertension versus baseline value.
**P<0.05 for the difference in the group of resistant hypertension versus baseline value.
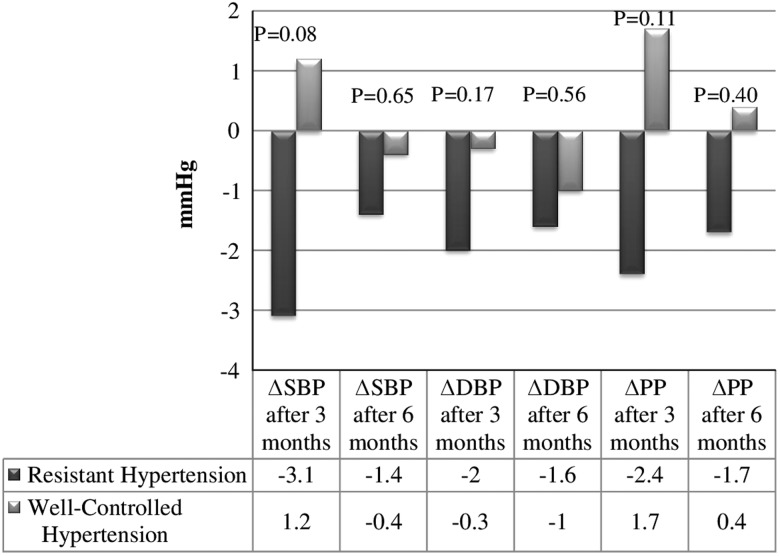
Figure 1 shows the changes of SBP, DBP and pulse pressure after three and six months of the study compared between patients with RH and WCH. After three months the changes between groups in the 24-h SBP were only borderline significant. No significant difference was demonstrated after six months. However, after excluding five patients who did not use cell phones and receive SMS reminders there was a statistically significant difference between groups in the changes of SBP and pulse pressure after three months (P=0.03 and P=0.04, respectively).
Figure 1.
Systolic blood pressure, pulse pressure in resistant hypertension and well-controlled hypertension groups in observation period. Δ=Change of parameter in observation period; DBP=diastolic blood pressure; PP=pulse pressure; SBP=systolic blood pressure. Data are presented as mean; P value of <0.05 is considered statistically significant
The statistically significant differences in RH were observed in office SBP and DBP (Friedman ANOVA, P=0.01 and P=0.02, respectively), nighttime SBP (P=0.02) and nighttime pulse pressure (P=0.002). No statistically significant differences were found in WCH group.
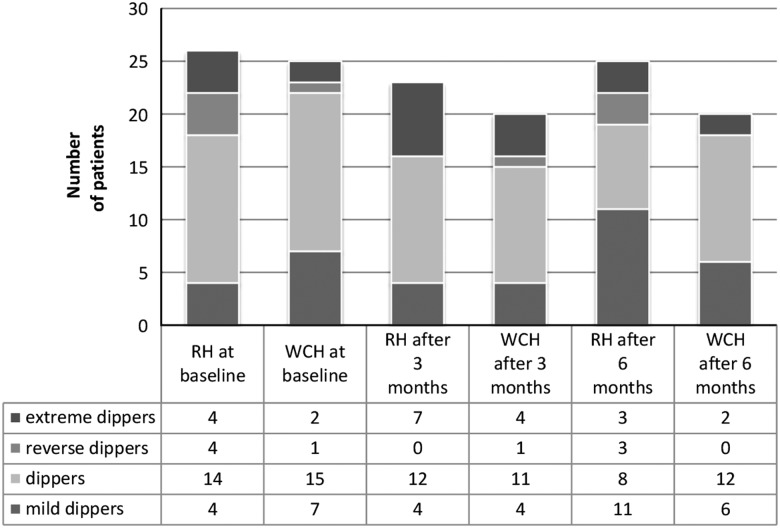
Figure 2 shows the circadian BP patterns in both groups. No statistically significant differences in the BP patterns were observed in different stages of the study. In RH group a non-significant reduction of the proportion of patients representing a ‘dipper’ profile was observed as well as an increase of the ‘mild dipper’ profile. The total number of the ‘dipper’ and ‘mild dipper’ patients increased but the number of patients in the ‘extreme dipper’ and the ‘reverse dipper’ category decreased at the end of the study. In WCH group the proportion of ‘dippers’, ‘mild dippers’ and ‘reverse dippers’ decreased.
Figure 2.
Circadian blood pressure patterns during the study. RH=resistant hypertension group; WHC=well-controlled hypertension group
Table 3 shows the physical activity profiles and the body composition analysis. After six months a statistically significant increase of the number of steps taken and metabolic equivalent and a decrease of both resting and sleeping time were observed in patients with RH. The body composition analysis demonstrated an increase of the total water content and the fat-free body mass in both groups and an increase of the body cell mass between months three and six in RH group (P=0.08). In the first three months of the study a statistically significant increase of extracellular mass in RH patients was observed.
Table 3.
Physical activity, energy expenditure and body composition in patients with resistant hypertension and well-controlled hypertension
| Parameter | Time of measurement | Resistant | Hypertension | Well-controlled hypertension | P value |
|---|---|---|---|---|---|
| Physical activity of moderate or higher intensity, min | At baseline | 290±254 | 373±220 | 0.06 | |
| After 3 months | 273±144 | P=0.97 | 424±293 | 0.001 | |
| After 6 months | 363±284 | P=0.30 | 421±279 | 0.4 | |
| Number of steps taken during 3 days | At baseline | 17 361±6815 | 21 374±9481 | 0.08 | |
| After 3 months | 20 807±8539 | P=0.05 | 243 314±11 135 | 0.23 | |
| After 6 months | 23067±7741 | P=0.002* | 25 779±11 201 | 0.34 | |
| MET average, during 3 days | At baseline | 1.325±0.3 | 1.48±0.2 | <0.05 | |
| After 3 months | 1.33±0.2 | P=0.80 | 1.52±0.3 | <0.05 | |
| After 6 months | 1.46±0.3 | P=0.001* | 1.55±0.3 | 0.33 | |
| Energy expenditure associated with physical activity during 3 days, (J) | At baseline | 6850±5812 | 7585±4708 | 0.27 | |
| After 3 months | 6500±3303 | P=0.90 | 8861±6505 | 0.41 | |
| After 6 months | 8327±5436 | P=0.20 | 8507±6098 | 0.98 | |
| Total energy expenditure during 3 days (J) | At baseline | 34 950±6193 | 33 054±6753 | 0.15 | |
| After 3 months | 35 310±5633 | P=0.90 | 34 426±9123 | 0.23 | |
| After 6 months | 37 769±7326 | P=0.06 | 34 172±8048 | 0.12 | |
| Rest time in 3 days, including a sedentary lifestyle (min) | at baseline | 1573±263 | 1520±175 | 0.24 | |
| After 3 months | 1511±263 | P=0.30 | 1573±312 | 0.77 | |
| After 6 months | 1458±292 | P=0.03** | 1483±246 | 0.77 | |
| Sleep during 3 days (min) | At baseline | 1278±228 | 1298±156 | 0.72 | |
| After 3 months | 1238±246 | P=0.80 | 1293±218 | 0.44 | |
| After 6 months | 1147±270 | P=0.02** | 1281±212 | 0.06 | |
| Total body water (l) | At baseline | 43±7.2 | 39.1±6.6 | <0.05 | |
| After 3 months | 43.6±7.4 | P=0.08 | 39.1±7.3 | <0.05 | |
| After 6 months | 44±7.3 | P=0.01** | 40.3±6.9 | 0.08 | |
| Lean body mass (kg) | At baseline | 58.8±9.8 | 53.4±9.1 | <0.05 | |
| After 3 months | 59.6±10.2 | P=0.11 | 53.4±10 | 0.02 | |
| After 6 months | 60.1±9.9 | P=0.01** | 55.1±9.4 | 0.08 | |
| Fat mass (kg) | At baseline | 29.9±9.1 | 22.6±8.1 | <0.005 | |
| After 3 months | 30.6±9.6 | P=0.06 | 23.8±8.1 | 0.01 | |
| After 6 months | 30.1±9.5 | P=0.77 | 23.3±9.1 | 0.01 | |
| Extracellular mass (kg) | At baseline | 27.8±4.7 | 25.3±4.0 | <0.05 | |
| After 3 months | 28.9±4.5 | P=0.005* | 25.4±4.4 | <0.005 | |
| After 6 months | 28.4±5.5 | P=0.36 | 26.1±4.4 | 0.07 | |
| Body cell mass (kg) | At baseline | 30.7±6.5 | 28.1±5.6 | 0.12 | |
| After 3 months | 30.6±6.4 | P=0.84 | 28±6.2 | 0.08 | |
| After 6 months | 31.7±6.1 | P=0.10 | 29±5.6 | 0.12 | |
| Basal metabolic rate (kcal) | At baseline | 1586±26 | 1502±177 | 0.12 | |
| After 3 months | 1584±23 | P=0.86 | 1500±194 | 0.09 | |
| After 6 months | 1616±195 | P=0.10 | 1531±178 | 0.12 |
MET=metabolic equivalent, 1 MET responsible resting oxygen consumption.
Data are presented as mean±SD; ‘Conversion factors to SI units are as follows: for basal metabolic rate, 4184; for total body water, 0.001’.
*P<0.005 for the difference in the group of resistant hypertension versus baseline value.
**P<0.05 for the difference in the group of resistant hypertension versus baseline value.
In RH patients body mass (89.4±13.6 versus 89.5±14 kg) and BMI (32.5±5.1 versus 32.6±5.3 kg/m2) did not change noticeably while waist circumference decreased (109.5±12 versus 108.6±12 cm; P=0.32). In WCH group, body mass (76.3±12 versus 78±12 kg; P=0.07) and BMI (28.2±4.3 versus 28.8±4.8 kg/m2; P=0.05) increased, whereas the waist circumference did not change (99.3±11 versus 99.4±11 cm).
Mean number of antihypertensive drugs used during the study in RH group slightly decreased from 4.2±0.75 to 3.9±1.2 but the difference was not significant (P=0.11). In WCH patients respective mean values were 1.7±0.5 and 1.8±0.6 (P=0.4).
Median number of drugs was unchanged in both groups in course of the study. At the end of the study the patients with RH completed the self-assessment form. In total, 85% of the patients positively evaluated the program and expressed their willingness to continue the physical activity. In total, 93% declared a subjective improvement of health and nearly all (96%) expressed their readiness to recommend the similar physical activity to their family members.
Discussion
The main finding of our study was that the successful implementation of the individualized structured program of increased physical activity in patients with RH led to moderate and transient BP reduction and to the apparently beneficial changes of body composition.
The study included the patients representing a typical population under the care of a community-based health center. A small size of the local community allowed to closely monitor the course of the study and its progress as well as to prevent non-compliance. The regular visits motivated the patients to intensify physical activity and might have reduced potential concerns related to physical training. Because of the nature of the conversations they were carried out individually but the topics that were discussed were basically the same in each case. The cell phone text messages were also used to encourage the patients to continue and increase the physical activity. This method is quite new but its positive impact of text reminders on the patients’ compliance concerning lifestyle changes has already been evaluated in several publications and the method has been gaining popularity (de Jongh et al., 2012; Vodopivec-Jamsec et al., 2012). Bobrow et al. found a small but positive effect of transmitting information via SMS to reduce BP in patients with arterial hypertension (Bobrow et al., 2016) but the recent study comparing a standard behavioral weight loss intervention versus a technology-enhanced weight loss intervention did not result in greater weight loss (Jakicic et al., 2016).
A study that referred to a similar population demonstrated that a 12-week aerobic exercise program was effective in decreasing BP. The BP reduction achieved during the day was 6±12 and 3±7 mmHg for SBP and DBP, respectively (Dimeo et al., 2012). In our population the respective changes of BP were smaller and non-significant reaching 1.5±16 mmHg and 1.6±11 mmHg. The smaller effect in our study may be explained by the lower 24-h mean SBP and DBP at baseline (127.2 and 75.4 mmHg, respectively) compared with 135.3 and 75.4 mmHg in a study of Dimeo et al.
We were not able to offer group activities and the exercises were not supervised or led by a coach for the whole duration of the study. Instead our simplified protocol included the methods that were widely available, inexpensive and that could be implemented without any additional preparation/training. Such program is more likely to be accepted by the patients who are generally reluctant to participate in group activities (Freene et al., 2014). The effectiveness of various methods aimed at increasing the physical activity was analyzed in Australia and that study confirmed the aptness relevance of our assumptions (Freene et al., 2014; 2015).
Dimeo et al. applied 30-min exercises such as walking on a treadmill with a gradual extension of the training duration, three times a week (Dimeo et al., 2012). Guimaraes et al. presented a slightly different approach implementing physical activity in the form of strength exercises based on using the persons own body mass (calisthenics) and walking in the water heated up to the temperature of 30–32°C. After 12 weeks of exercises three times a week, including 1-h meeting the lowering effects on BP were observed both during the day and night BP (Guimaraes et al., 2014). Ribeiro et al. found a significant effect of physical exercise on BP in individuals with high BP values at the start (Ribeiro et al., 2015). In our study a reference group included the individuals with well-controlled BP using ≤3 antihypertensives while in the aforementioned studies control patients had a RH with much higher BP at baseline. We considered a comparative group of well-controlled hypertensive patients with significantly better cardiovascular prognosis as a good background for demonstrating beneficial changes in RH group hence our study showed that the impact of the physical activity program is not inferior in patients with RH compared with the patients with well-controlled hypertension.
A nighttime BP during sleep has been established as an important prognostic factor in recent years, more important than 24-h mean values or morning BP (Salles et al., 2008; Hermida et al., 2013; 2014). Ling et al. postulated a beneficial influence of a regular aerobic activity on strengthening the effect of the SBP and pulse pressure drop at night (‘dipping’) despite the lack of the influence upon the 24-h SBP (Ling et al., 2014). Our study showed also the reduction of the percentage of the patients with the ‘dipper’ profile and an increase of the number of patients with the ‘mild dipper’ profile at the end of the study. It needs to be emphasized that the mean nighttime values of BP remained within the normal range during the whole study and at the end the proportion of the ‘reverse dipper’ and ‘extreme dipper’ patients known to have the highest risk of cardiovascular incidents (Kario and Shimada, 2004) was lower. The morning SBP tended to decrease [but the difference did not reach the level of statistical significance (P=0.07)] in RH group, which might be related to the change of the 24-h pressure profile into a more beneficial pattern as suggested by some authors (Xu et al., 2012).
The importance of diabetes and obesity in the pathogenesis of arterial hypertension and its resistance to treatment is well documented (Calhoun et al., 2008; Roberie and Elliott, 2012). Unfortunately RH group was too small to analyze the effect of obesity and diabetes on the effects of physical activity on the control of hypertension.
The study has other limitations. First, the impact of placebo and Hawthorne effect, that is the knowledge that the person participates in the study upon the results cannot be excluded. The patients were qualified to RH group on the basis of the current definition of RH based on office measurements (Calhoun et al., 2008), and therefore the group might have also included the patients experiencing the apparent RH, such as ‘white coat syndrome’ and medication non-adherence (Townsend and Epstein, 2016). Importantly the results were primarily analyzed with respect to ABPM that is as the best method for this population (Calhoun et al., 2008; Syrseloudis et al., 2011; Mancia et al., 2013).
The lower reduction of BP than in other studies might have been a consequence of a less intensive exercise program. In addition, the strongest effect on BP reduction was observed in the groups treated with isometric resistance exercises but that type of training was not used in our study (Cornelissen and Smart, 2013).
The strength of the study is the intervention that is simple, inexpensive and accessible in primary care. It is based on a tailor-made approach and provides an opportunity to select the most suitable type of activity. It is also well tolerated and well assessed by patients.
In conclusion the regular physical activity is a multidirectional form of intervention that has a beneficial impact on the patients with hypertension. Its benefits in patients with treatment of RH are however moderate and limited in time. It seems commendable to try to physically activate patients with RH in addition to a pharmacological therapy.
Financial Support
This work was supported by the Medical University of Lodz, Poland (M.N., grant number 503/1-151-02/503-0).
Conflicts of Interest
The authors declare no conflicts of interest to disclose.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guidelines on human experimentation (Bioethics Committee of the Medical University of Lodz) and with the Helsinki Declaration of 1975, as revised in 2008. Bioethics Committee of the Medical University of Lodz approved this project (Resolution No. RNN/169/14/14/EC of 10.28.2014).
Acknowledgments
The authors thank the patients without whom this study would not have been possibile. They also especially thank the staff of the Community-Based Health Center in Rząśnia and the staff of the Department of Nephrology, Hypertension and Kidney Transplantation in Lodz for their support.
References
- Achelrod D, Wenzel U Frey S (2015) Systematic review and meta-analysis of the prevalence of resistant hypertension in treated hypertensive populations. American Journal of Hypertension 28, 355–361. [DOI] [PubMed] [Google Scholar]
- American Heart Association. The American Heart Association’s diet and lifestyle recommendations. Retrieved 25 October 2014 from https://www.heart.org/.
- Bobrow K, Farmer AJ, Springer D, Shannyinde M, Yu LM, Brennan T, Rayner B, Namane M, Steyn K, Tarassenko L Levitt N. (2016) Mobile phone text messages to support treatment adherence in adults with high blood pressure (SMS-Text Adherence Support [StAR]): a single-blind, randomized trial. Circulation 133, 592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth FW, Roberts CK Laye MJ (2012) Lack of exercise is a major cause of chronic diseases. Comprehensive Physiology 2, 1143–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B Carey RM (2008) Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 51, 1403–1419. [DOI] [PubMed] [Google Scholar]
- Cole-Lewis H Kershaw T (2010) Text messaging as a tool for behavior change in disease prevention and management. Epidemiologic Reviews 32, 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen VA Smart NA (2013) Exercise training for blood pressure: a systematic review and meta-analysis. Journal of the American Heart Association 2, e004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jongh T, Gurol-Urganci I, Vodopivec-Jamsec V, Car J Atun R (2012) Mobile phone messaging for facilitating self-management of long-term illnesses. Cochrane Database of Systematic Reviews 12, CD007459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimeo F, Pagonas N, Seibert F, Arndt R, Zidek W Westhoff TH (2012) Aerobic exercise reduces blood pressure in resistant hypertension. Hypertension 60, 653–658. [DOI] [PubMed] [Google Scholar]
- Freene N, Waddington G, Chesworth W, Davey R Cochrane T (2014) Community group exercise versus physiotherapist-led home-based physical activity program: barriers, enablers, and preferences in middle-aged adults. Physiotherapy Theory and Practice 30, 85–93. [DOI] [PubMed] [Google Scholar]
- Freene N, Waddington G, Davey R Cochrane T (2015) Longitudinal comparison of a physiotherapist-led, home-based and group-based program for increasing physical activity in community-dwelling middle-aged adults. Australian Journal of Primary Health 21, 189–196. [DOI] [PubMed] [Google Scholar]
- Gijὀn-Conde T, Graciani A Banegas JR (2014) Resistant hypertension: demography and clinical characteristics in 6,292 patients in a primary health care setting. Revista Espaňola de Cardiologia (Engl Ed) 67 (4), 270–276. [DOI] [PubMed] [Google Scholar]
- Guimaraes GV, de Barros Cruz LG, Fernandes-Silva MM, Dorea EL Bocchi EA (2014) Heated water-based exercise training reduces 24-hour ambulatory blood pressure levels in resistant hypertensive patients: a randomized controlled trial (HEx trial). International Journal of Cardiology 172, 434–441. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Fernández JR Mojὀn A (2013) Sleep-time blood pressure: prognostic value and relevance as a therapeutic target for cardiovascular risk reduction. Chronobiology International 30, 68–86. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Mojὀn A, Smolensky MH, Portaluppi F Fernándes JR (2014) Sleep-time ambulatory blood pressure as a novel therapeutic target for cardiovascular risk reduction. Journal of Human Hypertension 28, 567–574. [DOI] [PubMed] [Google Scholar]
- Jakicic JM, Davis KK, Rogers RJ, King WC, Marcus MD, Helsel D, Rickman AD, Wahed AS Belle SH. (2016) Effect of wearable technology combined with a lifestyle intervention on long-term weight loss: the IDEA randomized clinical trial. JAMA 316, 1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen DL, Calabro MA, Stewart J, Franke W, Rood JC Welk GJ (2010) Accuracy of armband monitors for measuring daily energy expenditure in healthy adults. Medicine and Science in Sports and Exercise 42, 2134–2140. [DOI] [PubMed] [Google Scholar]
- Jones C, Taylor K, Chowienczyk P, Poston L Shennan AH (2000) A validation of the Mobil O Graph (version 12) ambulatory blood pressure monitor. Blood Pressure Monitoring 5, 233–238. [DOI] [PubMed] [Google Scholar]
- Kario K Shimada K (2004) Risers and extreme-dippers of nocturnal blood pressure in hypertension: antihypertensive strategy for nocturnal blood pressure. Clinical and Experimental Hypertension 26, 177–189. [DOI] [PubMed] [Google Scholar]
- Kokkinos P Myers J (2010) Exercise and physical activity: clinical outcomes and applications. Circulation 122, 1637–1648. [DOI] [PubMed] [Google Scholar]
- Kraus WE, Bittner V, Appel L, Blair SN, Church T, Després JP, Franklin BA, Miller TD, Pate RR, Taylor-Piliae RE, Vafiadis DK Whitse L. (2015) The national physical activity plan: a call to action from the American Heart Association: a science advisory from the American Heart Association. Circulation 131, 1932–1940. [DOI] [PubMed] [Google Scholar]
- Levin A Stevens P (2014) Summary of KDIGO 2012 CKD Guideline. Behind the scenes, need for guidance, and a framework for moving forward. Kidney International 85, 49–61. [DOI] [PubMed] [Google Scholar]
- Ling C, Diaz KM, Kretzschmar J, Feairheller DL, Sturgeon KM, Perkins A, Veerabhadrappa P, Williamson ST, Lee H, Grimm H, Babbitt DM Brown MD (2014) Chronic aerobic exercise improves blood pressure dipping status in African American nondippers. Blood Pressure Monitoring 19, 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg M, Larsson M, Östlund H Styf J (2006) Kinesiophobia among patients with musculoskeletal pain in primary healthcare. Journal of Rehabilitation Medicine 38, 37–43. [DOI] [PubMed] [Google Scholar]
- Mancia G, Fagard R, Narkiewicz K, Redὀn J, Zanchetti A, Bӧhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope RM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B Zannad F (2013) 2013ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Journal of Hypertension 31, 1281–1357. [DOI] [PubMed] [Google Scholar]
- Muntner P, Davis BR, Cushman WC, Bangalore S, Calhoun DA, Pressel SE, Black HR, Kostis JB, Probstfield JL, Whelton PK Rahman M (2014) Treatment – resistant hypertension and the incidence of cardiovascular disease and end-stage renal disease: results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension 64, 1012–1021. [DOI] [PubMed] [Google Scholar]
- Prejbisz A, Klocek M, Gąsowski J, Topór-Mądry R, Leśniak W, Kabat M, Czarnecka D, Kawecka-Jaszcz K, Narkiewicz K Januszewicz A (2015) Factors associated with resistant hypertension in a large cohort of hypertensive patients: the Pol-Fokus study. Polskie Archiwum Medycyny Wewnętrznej 125, 249–259. [DOI] [PubMed] [Google Scholar]
- Ribeiro F, Costa R Mesquita-Bastos J (2015) Exercise training in the management of patients with resistant hypertension. World Journal of Cardiology 7, 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberie DR Elliott WJ (2012) What is the prevalence of resistant hypertension in the United States? Current Opinion in Cardiology 27, 386–391. [DOI] [PubMed] [Google Scholar]
- Salles GF, Cardoso CR Muxfeldt ES (2008) Prognostic influence of office and ambulatory blood pressures in resistant hypertension. Archives of Internal Medicine 168, 2340–2346. [DOI] [PubMed] [Google Scholar]
- Slentz CA, Houmard JA Kraus WE (2009) Exercise, abdominal obesity, skeletal muscle, and metabolic risk: evidence for a dose response. Obesity 17, 27–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrseloudis D, Andrikou I, Andrikou E, Dimitriadis K Stefanadis C (2011) Ambulatory blood pressure monitoring in resistant hypertension. International Journal of Hypertension 2011, 285612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszewski M, White C, Patel P, Masca N, Damani R, Hepworth J, Samani NL, Gupta P, Madira W, Stanley A Williams B (2014) High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart 100, 855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend RR Epstein M (2016) Resistant hypertension: insights on evaluation and management in the Post-SPRINT (systolic blood pressure intervention trial) era. Hypertension 68, 1073–1080. [DOI] [PubMed] [Google Scholar]
- Vodopivec-Jamsec V, de Jongh T, Gurol-Urganci I, Atun R Car J (2012) Mobile phone messaging for preventive health care. Cochrane Database Systematic Reviews 12, CD007457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Zhang YQ Tan XR (2012) The dilemma of nocturnal blood pressure. Journal of Clinical Hypertension 14, 787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]