Abstract
Ace (Adhesin to collagen from Enterococcus faecalis) is a cell-wall anchored protein that is expressed conditionally and is important for virulence in a rat infective endocarditis (IE) model. Previously, we showed that rats immunized with the collagen binding domain of Ace (domain A), or administered anti-Ace domain A polyclonal antibody, were less susceptible to E. faecalis endocarditis than sham-immunized controls. In this work, we demonstrated that a sub nanomolar monoclonal antibody (mAb), anti-Ace mAb70, significantly diminished E. faecalis binding to ECM collagen IV in in vitro adherence assays and that, in the endocarditis model, anti-Ace mAb70 pre-treatment significantly reduced E. faecalis infection of aortic valves. The effectiveness of anti-Ace mAb against IE in the rat model suggests it might serve as a beneficial agent for passive protection against E. faecalis infections.
Keywords: Enterococcus faecalis, pathogenesis, ace collagen adhesion, infective endocarditis, protective vaccine, immunoprophylaxis
Anti-Ace mAb70 prevents E. faecalis endocarditis in rats.
INTRODUCTION
Enterococci are gram-positive cocci of intestinal origin and have been recognized as the 3rd most common cause of community onset infective endocarditis (IE) with Enterococcus faecalis accounting for majority (Tleyjeh et al.2005; Murdoch et al.2009; Slipczuk et al.2013). The therapeutic problems posed for patients with E. faecalis IE were well recognized as early as the 1950s. From the 1980’s onwards, E. faecalis strains have also been recognized as important causes of healthcare-associated infections (UTIs, bacteremia, intra-abdominal and wound infections and endocarditis) (Tleyjeh et al.2005; Murdoch et al.2009; Slipczuk et al.2013) and increasingly antibiotic resistant (Arias and Murray 2009; Nigo et al.2014). Thus, the study of potential virulence properties and immuno-protective agents may play an important role in discovering non-antibiotic means to treat, prevent or modulate E. faecalis infections and set the stage for preventative strategies for Enterococcus faecium, an even more difficult to treat organism.
Our previously published studies identified a family of genes encoding MSCRAMM-like proteins and one of these, called Ace (for Adhesin to collagen of E. faecalis), has been studied in detail (Nallapareddy et al.2000; Nallapareddy et al.2000; Nallapareddy and Murray 2006; Singh et al.2010). Similar to Cna from Staphylococcus aureus, Ace contain features characteristic of the LPXTG cell wall-anchored family of proteins (Symersky et al.1997; Rich et al.1999; Nallapareddy et al.2000). The A region of Ace contains the collagen binding domain and has significant sequence similarity to the corresponding domain of Cna.
Ace is ubiquitous (Duh et al.2001) in E. faecalis and conserved among diverse isolates, albeit with at least four variants due to variation in the number of repeats of the B domain (Nallapareddy et al.2000); we previously showed that Ace elicits an antibody response in patients with E. faecalis IE (Nallapareddy et al.2000) and that Ace is important for IE pathogenesis in a rat IE model (Singh et al.2010). Ace domain A has been shown to mediate the adherence of E. faecalis cells to collagen type I (CI), collagen type IV (CIV), laminin (Rich et al.1999; Nallapareddy et al.2000; Tomita and Ike 2004) and dentin (Kowalski et al.2006). Conditional in vitro surface expression of Ace (i.e. growth in brain heart infusion (BHI) at 46°C, with 40% serum (BHIS), or with bile) correlates with conditional adherence of E. faecalis strains to collagens and laminin (Nallapareddy et al.2000; Nallapareddy and Murray 2006; Pinkston et al.2011; Roh et al.2015). Previous studies have shown that affinity purified anti-Ace polyclonal antibodies from serum of patients with E. faecalis IE or from animals immunized with rAce domain A inhibited in vitro adherence of E. faecalis strains to collagen and laminin (Nallapareddy et al.2000; Nallapareddy et al.2000). Hall et al., showed that anti-Ace40 monoclonal antibody (targeting the ligand-binding domain A of Ace) inhibited binding of recombinant Ace to human CI and CIV and inhibited binding of Ace-coated fluorescent beads to epithelial cell (Hall et al.2007).
Utilizing an anti-Ace domain A mAb selected from a panel of mAbs developed against rAce domain A, designated anti-Ace mAb70 (Gao, Pinkston and Nallapareddy 2010), we previously demonstrated its utility in monitoring levels of Ace domain A protein expression to improve our understanding of factors regulating surface display (Gao, Pinkston and Nallapareddy 2010; Pinkston et al.2011; Gao et al.2013). In this study, we further characterize anti-Ace mAb70 for its affinity to rAce domain A, its ability to inhibit E. faecalis adhesion to collagen, and its effectiveness in preventing infection in a rat model of IE. Our findings demonstrate that anti-Ace mAb70 significantly reduces aortic valve infection by E. faecalis when given prophylactically in a rat model of IE.
MATERIALS AND METHODS
Bacterial strain and growth conditions
Enterococcus faecalis strain OG1RF (a strain we have extensively used for IE and whose genome has been sequenced and closed) (Murray et al.1993; Bourgogne et al.2006) was used in the rat endocarditis model. Enterococcus faecalis OG1RF was grown either on BHI agar (Difco Laboratories) for in vitro experiments or in BHI broth with 40% horse serum for in vivo experiments. Enterococcosel™ Agar (EA) (Becton Dickinson) supplemented with rifampicin (RIF) 100 μg mL−1 was used to plate tissue homogenates for bacterial recovery.
Anti-Ace mAb70 kinetic evaluation by Biacore T 100 system
To generate quantitative surface plasmon resonance (SPR) measurements of the equilibrium dissociation constant (KD) of anti-Ace mAb70, binding on and off rates to rAce domain A were measured using SPR and a Biacore T100 instrument (GE Healthcare). Two flow cells of a CM5 chip were coated with goat anti-mouse (Fc-specific) (Jackson ImmunoResearch) to approximately 8000 resonance units with an NHS/EDC Amine Coupling Kit (BR-1000-50; GE Healthcare). All measurements were made at 25°C using HBS EP (10 mM HEPES, 150 mM NaCl, 3 mM ethylenediaminetetraacetic acid, 0.05% v/v polysorbate 20, pH 7.4) as running buffer. For kinetic analysis, approximately 100 RU units of anti-Ace mAb 70 was captured on flow cell 2, followed by rAce domain A analyte run over both lanes 2 and 1 (in HBS EP). Analyte was tested over a 2-fold dilution series of 5 different molar concentrations from 100–6.25 nM. Flow cells were regenerated after each run with 100 mM phosphoric acid.
In vitro inhibition assay against CIV using anti-Ace mAb70
Microlon 600 ELISA plates (Greiner) were coated with Human Collagen IV (Sigma) at 10 μg mL−1 in PBS, 100 μL/well overnight at 4°C. Collagen-coated ELISA plates were blocked with 200 μL/well PBS-1% Bovine Serum Albumin (BSA) for 1 h at room temperature. Overnight bacterial cultures grown in 20 mL BHI at 37°C, 240 rpm, were diluted to OD600 = 0.05 into 125 mL centrifuge tubes containing 20 mL BHI broth and incubated an additional 60 min with shaking. Bacteria were harvested and resuspended in PBS–BSA containing either anti-Ace mAb70 or a murine gamma globulin (MGG) control (Jackson ImmunoResearch, West Grove, PA) at indicated concentrations (10 000–0.3 ng mL−1) to an optical density of 1.0. Bacterial suspensions were pipetted onto pre-washed (PBS) collagen-coated plates at 100 μL/well, covered and incubated an additional 1 h at room temperature under static conditions. Micro-well contents were aspirated and washed three-times with PBS. The remaining attached bacterial cells were fixed with 100 μL/well Bouin's solution for 30 min. Following an additional PBS wash step, a 1% solution of crystal violet was applied for another 30 min. The crystal violet was removed with sequential Milli-Q water flushes and solubilized with a 200 μL solution of 80:20 ethanol: acetone. Absorbance was determined with a Multiskan EX plate reader with 595 nm filter. Net adherence values were determined by absorbance subtraction of the PBS-only from the collagen IV coated wells and results were plotted.
Rat endocarditis
Endocarditis was produced in male Sprague–Dawley rats weighing ∼200 g using previous methodologies (Singh et al.2005; Singh et al.2010; Pinkston et al.2014). In brief, animals were anesthetized with isoflurane for placement of intravascular catheters. The right carotid artery exposed and a sterile polyethylene catheter (e.g. Intramedic PE 10; Clay Adams, Parsippany, NJ) was inserted through a small incision and advanced to 4 cm into the left ventricle. The catheter was ligated immediately and left in place for the duration of the experiment. The incision was closed with sutures. The animal protocol was pre-approved by UTHSC, Animal Welfare Committee, Houston (AWC), TX, USA and all the approved guidelines were followed during the course of this investigation.
Passive immunization and protection against E. faecalis infection
Rats were injected intravenously (i.v.) via the tail vein with 2 mg/kg of anti-Ace mAb70 or murine control IgG at 24 h post-catheterization and 1 h prior to bacterial inoculation (Singh et al.2010; Pinkston et al.2014). A total of 11 rats were given anti-Ace mAb70 and 10 rats were given control IgG (Pinkston et al.2014). Enterococcus faecalis OG1RF (8–9 × 107) grown in BHIS (Pinkston et al.2014) and premixed in saline was then injected i.v. via the tail vein, 25 h after catheter placement (Pinkston et al.2014) and 1 h after the injection of anti-Ace mAb70 or control IgG.
Animals were euthanized 24 h post infection. Hearts were aseptically removed from all euthanized animals. Aortic valves containing vegetations were removed from the hearts, weighed and homogenized in 1 mL of 0.9% saline. Sequential dilutions of homogenized tissues were carried out and the entire volume of each dilution including the undiluted sample was plated onto EA + RIF100 μg/mL plates to enumerate bacteria.
Statistics
Bacterial CFU/g from each rat vegetation were log-transformed and unpaired t-test was performed comparing anti-Ace mAb70 versus control IgG groups to obtain P values (Hagberg et al.1984). The geometric means of the bacterial CFU/g were also calculated in each group. Cultures yielding no growth were scored as sterile and were assigned a value of 1 CFU for statistical analysis of geometric means. Fisher's exact test was used for comparing the total number of infected/non-infected rats between anti-Ace mAb70 versus control IgG groups to obtain P values. Data/graphs were generated using Prism for Windows (version 4.00; GraphPad Software). Overall, differences were considered significant at a P level of <0.05.
RESULTS AND DISCUSSION
Affinity of anti-Ace mAb70 for rAce domain A and in vitro inhibition assay
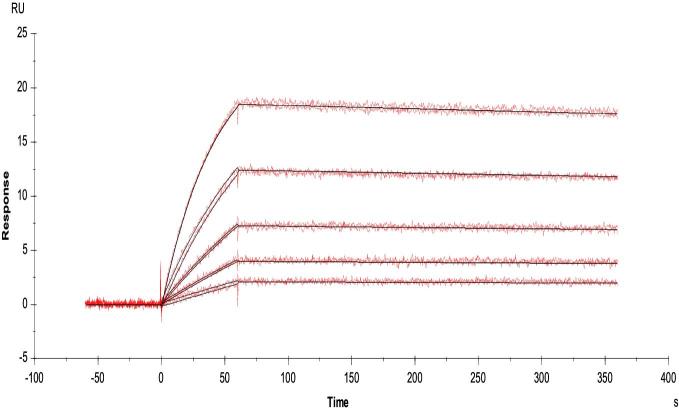
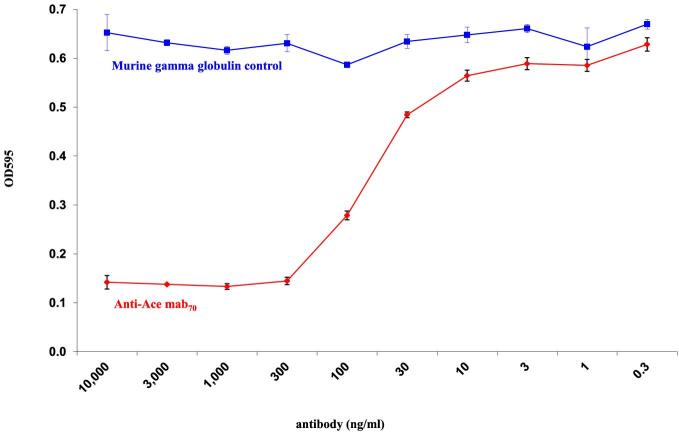
Utilizing the GE biacore evaluation software, we evaluated the kinetics of anti-Ace mAb70 interaction with rAce domain A, using the 1:1 binding model. Results demonstrated that anti-Ace mAb70 has a sub nanomolar affinity to rAce domain A, with an on rate (Kon) of 2.37 × 105 (1/Ms) and an off rate (Koff) of 1.66 × 10−4, yielding an overall affinity measurement (KD) of 0.7 nM (Fig. 1). We have previously demonstrated by flow cytometry analysis that anti-Ace mAb70 readily recognizes natively displayed Ace on the surface of E. faecalis cells at early time points, which is when the metalloprotease gelatinase (GelE) is not present (Pinkston et al.2011). Our results here demonstrated that anti-Ace mAb70 efficiently inhibited E. faecalis cells from adhering to collagen IV in vitro, compared to the MGG control (Fig. 2).
Figure 1.
Kinetic evaluation of anti-Ace mAb70 affinity for recombinant Ace domain A. To determine Kon and Koff kinetic measurements of the anti-Ace mAb70 binding domain with recombinant Ace domain A interaction, a dilution series (100–6.25nM) run in duplicate of Ace domain A (analyte) was evaluated for a 1:1 binding interaction with anti-Ace mAb70 immobilized to SPR surface (ligand). Results are representative of three independent experiments.
Figure 2.
Prevention of E. faecalis OG1RF adherence to immobilized collagen IV. Enterococcus faecalis adherence to immobilized collagen was evaluated at increasing levels of anti-Ace mAb70 concentration. Levels of cell adherence were measured by crystal violet absorbance at OD 595. Murine gamma globulin was used as control.
Passive immunization and protection against E. faecalis infection
In the rat endocarditis model, as seen in Fig. 3, we noted that 9 of 10 control IgG treated rats (90%) developed E. faecalis endocarditis compared with only 4 of 11 rats (36%) in the anti-Ace mAb70 treated group (Fisher's exact test P 0.0237). Control IgG treated rats showed 3.9 ± 1 log10 more CFU/g than the anti-Ace mAb 70 treated group (P 0.0035) in vegetations recovered from heart valves (Fig. 3).
Figure 3.
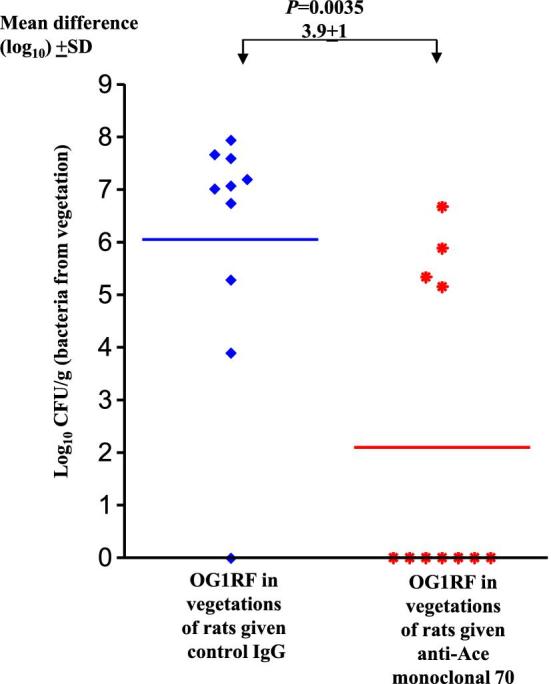
Passive immunization (anti-Ace mAb70 versus control IgG) in the rat endocarditis model. In the panel on the left, solid diamonds represent OG1RF log10 CFU/g recovered 24 h post-infection from vegetations from control IgG pre-treated rats. In the panel on the right, stars represent OG1RF log10 CFU/g recovered 24 h post infection from vegetations from anti-Ace mAb70 pre-treated rats. Horizontal bars represent the geometric means. Significantly fewer rats were infected by OG1RF in anti-Ace mAb70 administered rats (2 mg/kg) (P = 0.0237 by Fisher's exact test). Rats (anti-Ace mAb70 pre-treatment) showed a geometric mean ± SD decrease of 3.9 ± 1 log10 OG1RF CFU/g from vegetations versus control IgG pre-treatment (P = 0.0035 by unpaired t-test of log transformants).
Development of endocarditis can be initiated by injury to the valvular endothelium, disrupting the normal valve structure which exposes the underlying tissues, including ECM material resulting in deposition of host proteins, e.g. fibrin, as well as platelets at the site of injury leading to the formation of a sterile thrombotic vegetation. This endovascular lesion can be colonized by circulating bacteria in blood, leading to an infected vegetation. Both valvular and aortic tissues are collagen rich (Angrist and Oka 1963) and collagen is also found in sterile vegetations (Angrist and Oka 1963).
We previously demonstrated that polyclonal Ab to rAce A domain significantly prevents IE via both active immunization and using affinity purified Ig in passive immunization (Singh et al.2010). Because passive immunization studies would be much more practical for at-risk hospitalized patients and because mAb can be scaled up and are relatively safe, we evaluated monoclonal antibodies as a passive protective agent against E. faecalis experimental endocarditis and found it significantly prevented infection and reduced log10 CFU/g. However, in 4/11 rats protection was not seen. It has been reported that, in humans, response to vaccination is variable because different hosts respond variably in immunization studies (Kimman, Vandebriel and Hoebee 2007). We suspect similar response to immunogens/vaccines occurs in animals with some rats showing less or no protection.
We earlier described Ebp pili as another important factor in the pathogenesis of E. faecalis endocarditis as well as urinary tract infections and biofilm formation (Nallapareddy et al.2006; Singh, Nallapareddy and Murray 2007). More recently, we showed that monoclonal antibody to EbpC, the major (core) subunit of E. faecalis pili, labeled polymerized pilus structures, inhibited biofilm formation in plates, and significantly prevented the establishment of E. faecalis IE in a rat model. In addition, radiolabeled EbpC was detected at the site of E. faecalis infection, demonstrating molecular specificity during imaging of an established IE in rat model. The effectiveness of this anti-EbpC monoclonal provides further support of use of monoclonal antibodies as a preventative tool in IE.
In summary, we have demonstrated here that a monoclonal Ab, with subnanomolar affinity for the collagen adhesion domain A of Ace, (i) inhibited in vitro adherence of E. faecalis to CIV and (ii) conferred significant protection against endocarditis in passive immunization studies. Taken together, these results indicate that Ace is a promising target for prophylactic and therapeutic strategies against E. faecalis endocarditis and anti-Ace mAb70 provides novel data in support of the development of vaccines or immunotherapeutics that could be useful for the prevention of enterococcal infections.
Acknowledgements
We thank Karen Jacques-Palaz for technical assistance. This work was supported in part by NIH grant R01 AI047923-14 from NIAID to B.E.M.
Notes
K.V.S. has received grants from Paratek Pharmaceuticals and Merck.
B.E.M. has received grants from Paratek Pharmaceuticals and Merck, and served on the advisory board for Cempra and Paratek.
Conflict of interest. None declared.
REFERENCES
- Angrist AA, Oka M. Pathogenesis of bacterial endocarditis. JAMA 1963;183:249–52. [DOI] [PubMed] [Google Scholar]
- Arias CA, Murray BE. Antibiotic-resistant bugs in the 21st century–a clinical super-challenge. N Engl J Med 2009;360:439–43. [DOI] [PubMed] [Google Scholar]
- Bourgogne A, Hilsenbeck SG, Dunny GM et al. Comparison of OG1RF and an isogenic fsrB deletion mutant by transcriptional analysis: the Fsr system of Enterococcus faecalis is more than the activator of gelatinase and serine protease. J Bacteriol 2006;188:2875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duh RW, Singh KV, Malathum K et al. In vitro activity of 19 antimicrobial agents against enterococci from healthy subjects and hospitalized patients and use of an ace gene probe from Enterococcus faecalis for species identification. Microb Drug Resist 2001;7:39–46. [DOI] [PubMed] [Google Scholar]
- Gao P, Pinkston KL, Nallapareddy SR. Enterococcus faecalis rnjB is required for pilin gene expression and biofilm formation. J Bacteriol 2010;192:5489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Pinkston KL, Bourgogne A et al. Library screen identifies Enterococcus faecalis CcpA, the catabolite control protein A, as an effector of Ace, a collagen adhesion protein linked to virulence. J Bacteriol 2013;195:4761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L, Hull R, Hull S et al. Difference in susceptibility to gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect Immun 1984;46:839–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AE, Gorovits EL, Syribeys PJ et al. Monoclonal antibodies recognizing the Enterococcus faecalis collagen-binding MSCRAMM Ace: conditional expression and binding analysis. Microb Pathog 2007;43:55–66. [DOI] [PubMed] [Google Scholar]
- Kimman TG, Vandebriel RJ, Hoebee B. Genetic variation in the response to vaccination. Community Genet 2007;10:201–17. [DOI] [PubMed] [Google Scholar]
- Kowalski WJ, Kasper EL, Hatton JF et al. Enterococcus faecalis adhesin, Ace, mediates attachment to particulate dentin. J Endod 2006;32:634–7. [DOI] [PubMed] [Google Scholar]
- Murdoch DR, Corey GR, Hoen B et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009;169:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray BE, Singh KV, Ross RP et al. Generation of restriction map of Enterococcus faecalis OG1 and investigation of growth requirements and regions encoding biosynthetic function. J Bacteriol 1993;175:5216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallapareddy SR, Murray BE. Ligand-signaled upregulation of Enterococcus faecalis ace transcription, a mechanism for modulating host-E. faecalis interaction. Infect Immun 2006;74:4982–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallapareddy SR, Qin X, Weinstock GM et al. Enterococcus faecalis adhesin, ace, mediates attachment to extracellular matrix proteins collagen type IV and laminin as well as collagen type I. Infect Immun 2000;68:5218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallapareddy SR, Singh KV, Duh RW et al. Diversity of ace, a gene encoding a microbial surface component recognizing adhesive matrix molecules, from different strains of Enterococcus faecalis and evidence for production of ace during human infections. Infect Immun 2000;68:5210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallapareddy SR, Singh KV, Sillanpaa J et al. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J Clin Invest 2006;116:2799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigo M, Munita JM, Arias CA et al. What's new in the treatment of enterococcal endocarditis? Curr Infect Dis Rep 2014;16:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkston KL, Gao P, Diaz-Garcia D et al. The Fsr quorum-sensing system of Enterococcus faecalis modulates surface display of the collagen-binding MSCRAMM Ace through regulation of gelE. J Bacteriol 2011;193:4317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkston KL, Singh KV, Gao P et al. Targeting pili in enterococcal pathogenesis. Infect Immun 2014;82:1540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich RL, Kreikemeyer B, Owens RT et al. Ace is a collagen-binding MSCRAMM from Enterococcus faecalis. J Biol Chem 1999;274:26939–45. [DOI] [PubMed] [Google Scholar]
- Roh JH, Singh KV, La Rosa SL et al. The two-component system GrvRS (EtaRS) regulates ace expression in Enterococcus faecalis OG1RF. Infect Immun 2015;83:389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KV, Nallapareddy SR, Murray BE. Importance of the ebp (endocarditis- and biofilm-associated pilus) locus in the pathogenesis of Enterococcus faecalis ascending urinary tract infection. J Infect Dis 2007;195:1671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KV, Nallapareddy SR, Nannini EC. Fsr-independent production of protease(s) may explain the lack of attenuation of an Enterococcus faecalis fsr mutant versus a gelE-sprE mutant in induction of endocarditis. Infect Immun 2005;73:4888–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KV, Nallapareddy SR, Sillanpaa J et al. Importance of the collagen adhesin ace in pathogenesis and protection against Enterococcus faecalis experimental endocarditis. PLoS Pathog 2010;6:e1000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slipczuk L, Codolosa JN, Davila CD et al. Infective endocarditis epidemiology over five decades: a systematic review. PLoS One 2013;8:e82665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symersky J, Patti JM, Carson M et al. Structure of the collagen-binding domain from a Staphylococcus aureus adhesin. Nat Struct Mol Biol 1997;4:833–8. [DOI] [PubMed] [Google Scholar]
- Tleyjeh IM, Steckelberg JM, Murad HS et al. Temporal trends in infective endocarditis: a population-based study in Olmsted County, Minnesota. JAMA 2005;293:3022–8. [DOI] [PubMed] [Google Scholar]
- Tomita H, Ike Y. Tissue-specific adherent Enterococcus faecalis strains that show highly efficient adhesion to human bladder carcinoma T24 cells also adhere to extracellular matrix proteins. Infect Immun 2004;72:5877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]