Abstract
Endometriosis is an estrogen-dependent inflammatory disorder among reproductive-aged women associated with pelvic pain, anxiety, and depression. Pain is characterized by central sensitization; however, it is not clear if endometriosis leads to increased pain perception or if women with the disease are more sensitive to pain, increasing the detection of endometriosis. Endometriosis was induced in mice and changes in behavior including pain perception, brain electrophysiology, and gene expression were characterized. Behavioral tests revealed that mice with endometriosis were more depressed, anxious and sensitive to pain compared to sham controls. Microarray analyses confirmed by qPCR identified differential gene expression in several regions of brain in mice with endometriosis. In these mice, genes such as Gpr88, Glra3 in insula, Chrnb4, Npas4 in the hippocampus, and Lcn2 in the amygdala were upregulated while Lct, Serpina3n (insula), and Nptx2 (amygdala) were downregulated. These genes are involved in anxiety, locomotion, and pain. Patch clamp recordings in the amygdala were altered in endometriosis mice demonstrating an effect of endometriosis on brain electrophysiology. Endometriosis induced pain sensitization, anxiety, and depression by modulating brain gene expression and electrophysiology; the effect of endometriosis on the brain may underlie pain sensitization and mood disorders reported in women with the disease.
Keywords: endometriosis, brain, gene expression, electrophysiology, depression, anxiety, pain, central sensitization
Endometriosis disrupts brain gene expression and electrophysiology, inducing anxiety, depression, and pain sensitization in mice.
Introduction
Endometriosis is a common gynecological condition estimated to affect 10% to 15% of reproductive-aged women and up to 80% of women with chronic pelvic pain (CPP) [1]. CPP often debilitates women with endometriosis; it is associated with lost work time as well as significant physical and social debility [2]. Endometriosis likely produces pain by nociception. Endometriosis may compress nerves or stimulate them due to local inflammation near the lesions. Moreover, endometriosis lesions demonstrate increased nerve density compared to the surrounding peritoneum. Paradoxically, however, there is little correlation between the extent or location of endometriosis and the degree of pain. The differences may lie in the sensitivity to pain. Women with endometriosis are often hypersensitive to pain indicating central pain sensitization [3]; in this condition normal pain stimuli evoke exaggerated pain perception [4]. It is unknown if the endometriosis directly leads to central sensitization or if some women with the disease are already sensitized and experience pain from minimal disease. It is also unknown what mechanistic changes in the brain are associated with central pain sensitization in endometriosis. Here, we used an animal model of the disease to look at the effect of endometriosis on central nervous system (CNS) function, electrophysiology, and gene expression.
The insula, a brain region involved in the integration of interoceptive, affective, and cognitive signals, is a primary focus in pain neuroimaging studies because it is one of the most consistently activated regions during acute and chronic pain [5]. In contrast to women with relatively asymptomatic endometriosis, women with endometriosis-associated CPP exhibit nonpelvic hyperalgesia and decreased gray matter volume in key neural pain processing regions such as the insula [5]. Relative to age-matched pain-free controls, women with endometriosis-associated CPP displayed increased levels of combined glutamine-glutamate (Glx) within the anterior insula and greater anterior insula connectivity to the medical prefrontal cortex. Increased connectivity between these regions was positively correlated with anterior insula Glx concentrations, as well as clinical anxiety, depression, and pain intensity [6].
Chronic pain broadly impacts an individual's quality of life, resulting in depression, anxiety, and fatigue [7, 8]. Patients with anxiety and depression often display dysregulation of amygdala activity [9]. Chronic stress in humans also causes hyperactivity of the amygdala, which may be a path through which stress precipitates the emergence of anxiety and depressive disorders [10]. Moreover, the hippocampus, one of the crucial brain regions involved in learning and memory processing as well as in anxiety and depression, is an important candidate as substrate for the cognitive and affective consequences of neuropathic pain [11].
Although various psychological and behavioral symptoms are observed in patients with endometriosis, the underlying changes in the brain remain unknown. Identifying specific molecular mechanisms associated with pain, depression and anxiety in endometriosis patients is necessary to develop targeted treatment strategies for women who are experiencing these symptoms. The primary objective of this study was to determine if endometriosis could cause central pain sensitization, anxiety, and depression in a mouse model and to identify the molecular changes in the brain that are mechanistically responsible.
Materials and methods
Animal care and surgery
Female C57BL/6 mice at 9 weeks of age were obtained from Charles River Laboratories and kept under controlled conditions (12-h light and 12-h dark cycle, 22°C, food and water ad libitum). All mice were allowed 1 week of acclimation to this environment, prior to surgery. Experimental endometriosis was created as we have previously described in 40 mice [12]. Whole uterus was removed from C57BL/6 female mice at 9 weeks of age and washed in cold phosphate-buffered saline (PBS). The 2 uterine horns were divided and 1 of the horns was biopsied into 3 equally sized sections. The lumen of each section was opened longitudinally and kept in cold PBS until implantation. The uterine biopsies were sutured to the parietal peritoneum using 4–0 vicryl sutures. Sham surgeries were performed on an equal number of controls where the same procedure was followed except for the introduction of endometrium; sutures were affixed to the parietal peritoneum. This study was approved by Yale University's Institutional Animal Care and Use Committee, conforming to the US Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training.
Hot plate test
The hot plate test is a commonly used method for measuring nociception and response thresholds to thermal stimuli in rodents [13, 14]. The hot plate test was performed with a commercially available hot plate meter consisting of a clear, plexiglass cylinder placed on a hot plate. Both endometriosis and sham mice underwent hot plate testing every 2 weeks from the surgery day for a total of 12 weeks (N = 12 per group). Mice were allowed to walk on the hot plate (53.0°C ± 0.1°C) for up to 45 s (maximum allowed latency; to avoid tissue damage). Latency to jump, hind paw lick or flick was recorded, up to the maximum 45 s (if the animal did not emit such a response). Each animal was tested only once in each session.
Open field test
The open field test is performed to assess the degree of anxiety and locomotor activity in mice [15]. Both endometriosis (N = 12) and sham (N = 12) mice underwent open field testing every 2 weeks from the surgery day. The open field test arena is an open square box (50 cm × 50 cm × 40 cm) composed of a clear floor without bedding. The box is virtually demarcated into a central zone and peripheral zones. The experimental mouse was placed in one corner of the box and allowed to explore the arena for 18 min. Overall activity in the box (measured with video-track) was measured as well as the amount of time and distance traveled in the central zone. Mice with higher anxiety levels tend to spend more time in the periphery and less time in the central area. The overall distance moved by each mouse was considered an indicator of locomotor activity.
Tail suspension test
An automated tail suspension test device was used to measure the duration of behavioral immobility [16]. Increase in immobility time of animal reflected as depressive behavior. Both endometriosis and sham mice (N = 12 per group) underwent tail suspension testing every 4 weeks from the surgery day. The automated device consists of a box-like enclosure (box size: 32 cm × 33 cm × 33 cm) that was open on the front side, allowing videotaping. Acoustically and visually isolated mice were suspended by adhesive tape placed approximately 1 cm from the tip of the tail. A strain gauge connected to computer software detected any movements made by the mouse during a 6-min test session. The total duration of immobility was calculated as the time in which the force of the movements of the mice was below a preset threshold. An optimum threshold was determined by comparing manually scored videotapes with automated scores. The following settings were used in all experiments: threshold 0.2 N, resolution 10 ms.
Tissue collection
Twelve weeks following surgery, vaginal cytology was used to assess estrous cycle stage. Mice in the mid-diestrus stage were subsequently sacrificed. Endometriosis was confirmed at the time of sacrifice by identifying ectopic lesions in the abdominal cavity. Four different regions of each brain were collected by microdissection, including the insula, amygdala, hippocampus, and cerebral cortex. The equivalent Bregma coordinates from anterior to posterior (Paxinos and Franklin mouse atlas) for the insular cortex was from 1.94 to 1.18 mm, for the hippocampus and cortex from –1.34 to –1.94 mm and for the amygdala from –1.34 to –1.94 mm. The collected tissues were immediately divided and placed in either 4% formalin or RNAlater (Qiagen, Hilden, Germany). The tissues in RNAlater were stored for 24 h in +4°C and then transferred to –80°C until further molecular analysis was performed. The tissues in 4% formalin were then embedded in paraffin for histopathological examination.
RNA isolation
Total RNA was extracted from each specimen by means of the RNeasy Plus Micro Kit (Qiagen, Hilden, Germany) according to the manufacturer's specification. RNA quality was confirmed by A260/A280 ratio and agarose gel electrophoresis. The yield of RNA was determined with the use of a Nanodrop ND-2000 spectrophotometer (Nanodrop Technologies). Only RNA samples with appropriate size distribution, quantity, and an A260:A280 ratio of 1.8–2.1 were used for further analysis.
Measuring gene expression via affymetrix arrays
Total RNA from three mice in each group (endometriosis group and sham group) was pooled for microarray analysis. Pooled RNA samples from 4 different brain areas in both the endometriosis and sham control groups were processed at the Yale Center for Genome Analysis. cDNA was synthesized from 500 ng RNA using the Ambion WT kit (Life Technologies) and the single-stranded cDNA was prepared using the Affymetrix GeneChip WT Terminal Labeling Kit according to the manufacturer's instructions. The labeled mix was hybridized to GeneChip Mouse Exon 1.0 ST Array (Affymetrix, Santa Clara, CA) in the Gene Chip Hybridization Oven 640 overnight. Probe intensities were measured using the Affymetrix GeneChip Scanner, and the scanned images were analyzed with the use of Affymetrix Feature Extraction Software.
Quantitative real-time polymerase chain reaction
Total RNA (500 ng) from 20 mice per group was individually reverse transcribed in 20 μl of reaction mixture using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). The reaction mix was incubated for 5 min at 25°C, 30 min at 42°C, and 5 min at 85°C using the Eppendorf Mastercycler (Eppendorf North America). Quantitative real-time polymerase chain reaction (qRT-PCR) was prepared using the iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA). Each PCR reaction mixture consisted of 1 μl of cDNA template, 0.5 μl of forward primer (1 μM), 0.5 μl of reverse primer (1 μM), 3 μl of nuclease-free H2O, and 5 μl of iQ SYBR Green Supermix for a final reaction volume of 10 μl. The thermal cyclic conditions used were as follows: initial denaturation and enzyme activation for 3 min at 95°C followed by 40 cycles (denaturation for 15 s at 95°C, annealing/extension for 45 s at 55°C), followed by melt curve analysis. Relative gene expression was determined by analyzing data using the 2−ΔΔCT method to adjust for expression of a housekeeping gene β-actin. Specificity of the amplified products and the absence of primer-dimers were confirmed via melt curve analysis. All products obtained yielded the predicted melting temperature. All experiments were conducted in triplicate. Samples without cDNA template were used as negative controls. The primers used are listed in Supplementary Table S1.
Immunohistochemistry
Specimens of insula and amygdala from both endometriosis and sham mice were fixed in 4% formalin and embedded in paraffin. In amygdala, immunohistochemical staining was performed using rabbit polyclonal antibodies directed against Lcn2 and Nptx2 (ab63929 and ab69858; Abcam, Cambridge, MA, USA), while the expression of Gpr88 (ab64905, Abcam, Cambridge, MA, USA), Glra3 (Q91XP5, Biorbyt, CA, USA), Lct (Q6UWM7, Biorbyt, CA, USA), and Serpina3n (TA323305, Origene, Rockville, MD, USA) in insula were evaluated also using rabbit polyclonal antibodies (Supplementary Table S2). Tissue sections were deparaffinized followed by dehydration with xylene and ethanol. An antigen retrieval was carried out in 0.01 mol/L sodium citrate (pH 6.0) for 12 min, followed by washing in PBS with 0.1% Tween 20. Endogenous peroxidase was blocked with 3% hydrogen peroxide for 10 min. After a 60-min incubation with 10% normal goat blocking serum, sections were incubated with avidin and biotinylated peroxidase (Vectastain; Vector Laboratories, Burlingame, CA, USA) for 15 min respectively, followed by incubation overnight at 4°C with primary antibody. Goat immunoglobulin G (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as the negative control. Sections were then incubated with biotinylated goat anti-rabbit secondary antibody for 1 h, and diaminobenzidine (400 mg/mL) for 45 s. Hematoxylin was used for counterstaining, followed by dehydrating through alcohol, cleared in xylene, and mounted in Permount (Fisher Scientific Inc., MA, USA). The number of stained cells and the intensity of staining were evaluated in 5 high-power fields on each slide by 2 investigators blinded to the specimen source. Staining was quantified using the H-score method [17].
Electrophysiology
Mice from endometriosis and sham groups were anesthetized with isoflurane and then decapitated at 12 weeks after surgery (N = 7 per group). The brains were rapidly removed and immersed in an oxygenated cutting solution at 4°C containing (in mM): sucrose 220, KCl 2.5, CaCl2 1, MgCl2 6, NaH2PO4 1.25, NaHCO3 26, and glucose 10, and adjusted to pH 7.3 with NaOH. Coronal slices containing the amygdala or insular cortex (300 μm thick) were cut with a vibratome, trimmed to contain just the insula or amygdala, respectively. After preparation, slices were stored in a holding chamber with an oxygenated (with 5% CO2 and 95% O2) artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl 124, KCl 3, CaCl2 2, MgCl2 2, NaH2PO4 1.23, NaHCO3 26, glucose 10, pH 7.4 with NaOH. The slices were eventually transferred to a recording chamber constantly perfused with ACSF at 33°C at a rate of 2 ml/min after at least a 1 h recovery in the storage chamber.
Whole-cell patch clamp (at –60 mV) was performed to observe miniature excitatory postsynaptic currents (mEPSCs) and miniature inhibitory postsynaptic currents (mIPSCs) in layer II/III pyramidal cells of the insular cortex or medial amygdala neurons with a Multiclamp 700 A amplifier (Molecular devices, Sunnyvale, CA). The patch pipettes with a tip resistance of 4–6 MΩ were made of borosilicate glass (World Precision Instruments, Sarasota, FL) with a pipette puller (Sutter P-97) and back filled with a pipette solution containing (in mM): K-gluconate 135, MgCl2 2, HEPES 10, EGTA 1.1, Mg-ATP 2, Na2-phosphocreatine 10, and Na2-GTP 0.3, pH 7.3 with KOH. In the presence of tetrodotoxin (TTX, 0.5μM), mEPSCs were recorded under voltage clamp at –60 mV and mIPSCs were recorded at 0 mV from the same cells, respectively. Both input resistance and series resistance were monitored constantly during experiments. All data were sampled at 10 kHz and filtered at 6 kHz with an Apple Macintosh computer using Axograph X (AxoGraph Scientific, Sydney, Australia). mEPSC and mIPSC events were detected and analyzed with AxoGraph X and plotted with Igor Pro software (WaveMetrics, Lake Oswego, OR) as described previously [18].
Statistical analysis
For parametric continuous variables, the mean values between groups were compared using unpaired t-test. H-score was used to semiquantitatively compare immunohistochemistry specimens, and values were compared using the Mann–Whitney U-test. P values < 0.05 were considered statistically significant. All data are presented as means ± standard error of the mean. Changes in the cumulative probability curves for the amplitude of mEPSC and mIPSC in the electrophysiology studies were compared using the Kolmogorov–Smirnov test.
Results
Induction of endometriosis
Uterine segments from donor female mice were sutured in the pelvic region of recipient mice as described above. Induction of endometriosis was confirmed by the size and growth of endometriotic lesions in mice after 12 weeks of surgery and their absence in the sham control mice, as shown in Supplemental Figure S1.
Increased anxiety in endometriosis
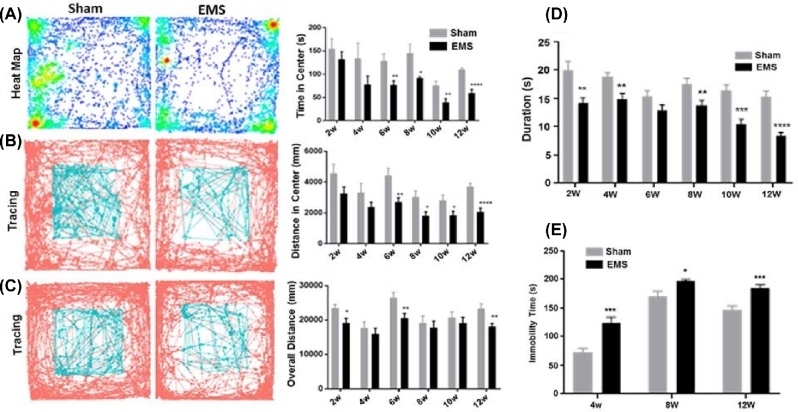
In the open field test, endometriosis mice spent less time (Figure 1A) and traveled less distance (Figure 1B) in the central area at all time points; these reductions were significant at 6, 8, 10, and 12 weeks after surgery, indicating that anxiety was induced in endometriosis mice starting 6 weeks from induction of the disease. We also found that the percentage of distance traveled in the center, after normalizing for total locomotion of each mouse, was higher from week 2 to week 10 in mice with endometriosis, as shown in Supplemental Figure S2. This gradual increase in percentage of distance traveled in the center further demonstrates that mice with endometriosis had anxiety.
Figure 1.
Behavioral studies. (A) Assessment of central time in open field test on week 2, 4, 6, 8, 10, and 12 after surgery. Compared to sham mice, endometriosis mice significantly spent less time in the central area at 6, 8, 10, and 12 weeks after surgery (*P < 0.05; **P < 0.01; ****P < 0.001). (B) Assessment of central distance in open field test on week 2, 4, 6, 8, 10, and 12 after surgery. Compared to sham mice, endometriosis mice traveled less time which is significant, in the central area at 6, 8, 10, and 12 weeks after surgery (*P < 0.05; **P < 0.01; ****P < 0.001). (C) Assessment of locomotor activity in an open field on week 2, 4, 6, 8, 10, and 12 after surgery. Compared to sham mice, endometriosis mice significantly traveled less overall distance at 2, 6, and 12 weeks after surgery (*P < 0.05; **P < 0.01). (D) Pain sensitivity assessed by hot plate test on week 2, 4, 6, 8, 10, and 12 after surgery. Endometriosis (EMS) mice had a significantly decreased duration at all time points except 6 weeks (**P < 0.01; ***P < 0.005; ****P < 0.001). (E) Assessment of depression behavior in tail suspension test on week 4, 8, and 12 after surgery. Endometriosis mice significantly decreased immobility time at all three time points (*P < 0.05; ***P < 0.005). All tests were performed on 12 mice from each group (N = 12).
Decreased locomotor activity in endometriosis
Endometriosis mice traveled less distance during the 18-min observation at all time points from the induction of disease; the reduction was significant at 2, 6, and 12 weeks after surgery (Figure 1C). Endometriosis mice were less active even after sufficient time to account for surgical recovery.
Hyperalgesia in endometriosis
Pain sensitization was tested with the hot plate test. Animals in the endometriosis group developed hyperalgesia compared to the sham control group. The duration of time prior to reaction in endometriosis mice was significantly shorter than the sham group at 2, 4, 8, 10, 12 weeks after surgery (Figure 1D), which suggested endometriosis-induced persistent pain hypersensitivity.
Increased depression in endometriosis
The tail suspension test was used to evaluate depression. In tail suspension testing, the immobility time of endometriosis mice was significantly longer than the sham group at all time points (Figure 1E). These results suggested persistent depression in endometriosis mice.
Altered CNS electrophysiology in endometriosis
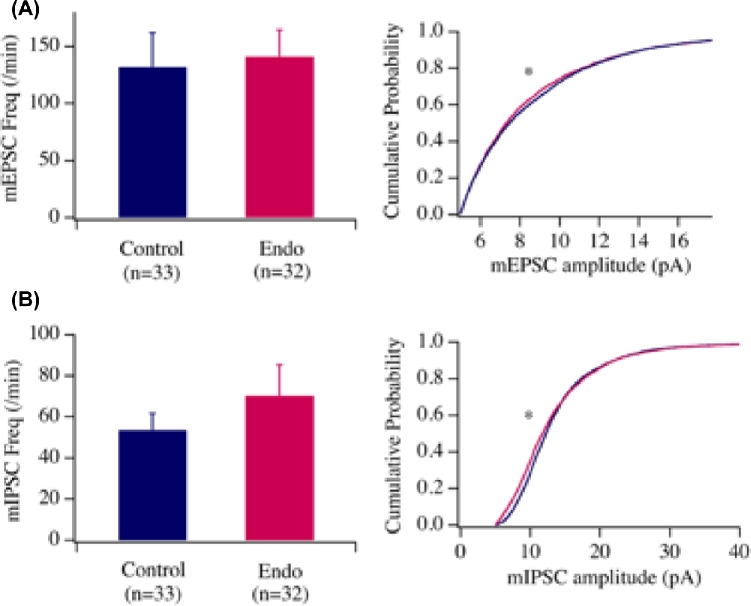
To understand the cellular mechanisms underlying the behavioral changes and verify the functionality of neurons affected by the altered expressions of genes relevant to animal behaviors in endometriosis mice, we examined whether changes in the neuronal circuitry occurred in one of the critical brain areas, the amygdala, responsible for pain, anxiety, and depression. We examined synaptic parameters in neurons in the central amygdala with whole-cell patch clamp approach as reported by Hou et al [19]. Specifically, we examined miniature excitatory and inhibitory postsynaptic currents with whole cell patch clamp in neurons in acute brain slices from amygdala in mice with endometriosis and sham mice. mEPSCs/mIPSCs were generated by random vesicle release of glutamate or GABA from presynaptic neurons in the absence of stimulation and the measurement of mEPSCs/mIPSC was used to analyze the efficacy of synaptic transmission. Changes in mEPSC frequency (Figure 2A; bar graph) are thought to result from modification of the presynaptic component of synaptic transmission, while amplitude (line graph) changes indicate alterations in the postsynaptic component. Our data showed that the frequency of mEPSCs (control: 131.8 ± 29.8 per min, n = 33 cells from 7 mice, endometriosis: 140.9 ± 23.6 per min, n = 32 from 7 mice; P > 0.05, t-test) and mIPSCs (Figure 2A) (control: 53.6 ± 8.1 per min, n = 33 cells from 7 mice, endometriosis: 70.3 ± 14.9 per min, n = 32 from 7 mice; P > 0.05, t-test) were not significantly different. However, the cumulative probability curves for the amplitude of mEPSC and mIPSC (Figure 2A and B respectively, line graphs) events recorded from both regions shifted significantly to the left in endometriosis mice as compared to the controls (P < 0.01, Kolmogorov–Smirnov test). Taken together, these results indicate that synaptic efficacy of glutamatergic and GABAergic transmission decreased at the postsynaptic sites in the neurons of the amygdala. Thus, we demonstrate impairment in glutamatergic and GABAergic transmission onto neurons in the amygdala from endometriosis mice.
Figure 2.
Whole cell recording of miniature excitatory and inhibitory postsynaptic currents (mEPSCs and mIPSCs) in the neurons in the amygdala in control and endometriosis mice. We tested the effects of endometriosis on glutamatergic and GABAergic transmissions onto amygdala neurons by examining miniature excitatory and inhibitory currents in these cells. The frequency of mEPSC and mIPSC was not significantly different between control and endometriosis groups. However, the cumulative probability of mEPSC and mIPSC amplitude was significantly decreased as shown by a Kolmogorov–Smirnov test in endometriosis group as compared with controls. These results suggest that the postsynaptic components of glutamatergic and GABAergic synapses on tested amygdala neurons were impaired. The frequency (bar graph) and the cumulative probabilities of amplitudes (line graph) of mEPSCs and mIPSCs were shown in (A) and (B), respectively. The cumulative probabilities of mEPSC and mIPSC events significantly shifted to the left in the Endo group as compared with the control group (mEPSC, P < 0.01, Kolmogorov–Smirnov test, 4523 events in control group and 4517 events in Endo group; mIPSC, P < 0.01, Kolmogorov–Smirnov test, 4004 events in control group and 4208 events in Endo group), suggesting the amplitudes of mEPSC and mIPSC events were smaller in the Endo groups than in controls. N = 32–33 brain cells from seven mice. “*” denotes statistical significance between control vs endometriosis mice.
Differentially expressed genes in the brains of endometriosis mice
A total of 2545 differentially expressed mRNAs were identified in the insula by microarray analysis after quantile normalization and data filtering (fold-change > 1.5), as well as 147 mRNAs in the amygdala, 178 mRNAs in the hippocampus, and 131 mRNAs in the cerebral cortex. A total of 1125 mRNAs were upregulated and 1410 were downregulated in the insula from endometriosis mice compared to sham mice; meanwhile, 70, 92, and 25 mRNAs were upregulated, and 77, 86, and 106 mRNAs were downregulated in amygdala, hippocampus, and cerebral cortex from endometriosis mice compared to sham mice, respectively. In the insula, which showed the greatest differential expression, 37 mRNAs were upregulated and 39 mRNAs were downregulated using a higher fold change of >3.0. The greatest increase in fold change was 9.01 (G-protein coupled receptor 88, Gpr88) in the endometriosis group compared to sham group, whereas the greatest decreased fold change was 12.43 (lactase, Lct). In the amygdala, the greatest fold change in up- and downregulated genes were 2.17 (zinc finger protein 458, zfp458) and 2.11 (N-acetylneuraminic acid phosphatase, Nanp), respectively. In the hippocampus, the greatest fold change in up- and downregulated genes was 3.30 (tryptophan hydroxylase 1, tph1) and 3.34 (defensin beta 11, defb11), respectively. In the cerebral cortex, the greatest fold change in up- and downregulated genes is 1.87 (zinc finger protein 873, zfp873) and 3.84 (cystein rich protein 61, cyr61), respectively.
Validation by qRT-PCR
In addition to the three samples used for microarray analysis, we also isolated total RNA from all of the paired mice brain areas for validation. The expression levels of the top 10 most upregulated and downregulated genes in the insula were verified by qRT-PCR as shown in Figure 3A and B, respectively. The relative fold changes in expression of all 20 genes, as detected by qRT-PCR, were consistent with the microarray data (Figure 3A and B).
Figure 3.
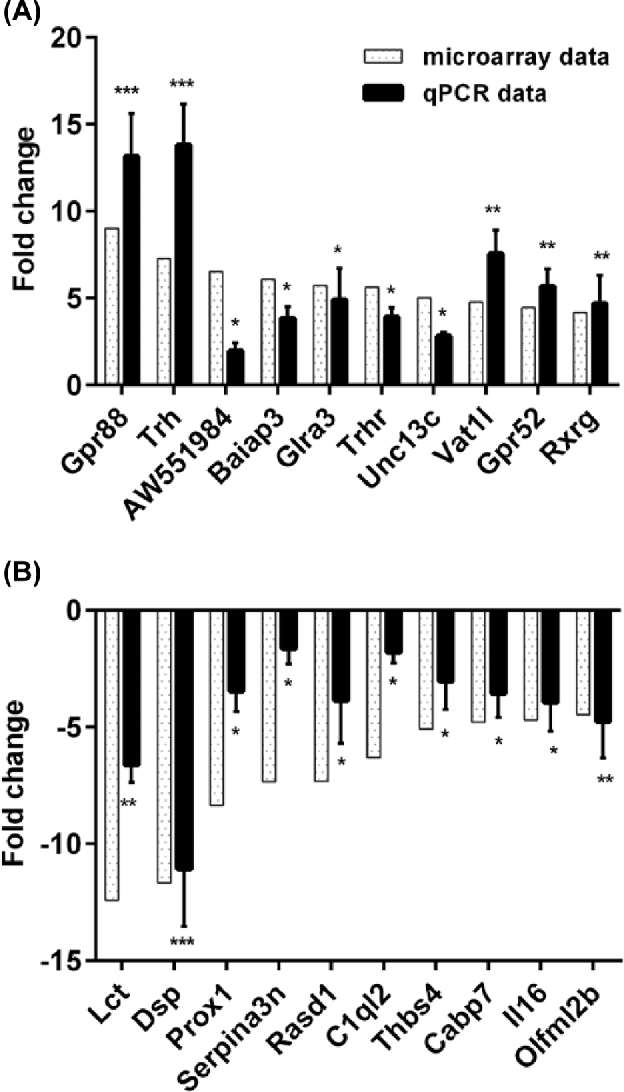
Gene expression changes in the insula. Comparison of qPCR and microarray data for top 10 upregulated genes (A) and downregulated genes (B) in insula. Relative fold changes in expression between endometriosis and sham mice, as detected by qPCR (light gray bars), were in agreement with microarray data (black bars) (*P < 0.05; **P < 0.01; ***P < 0.005). N = 20 per group.
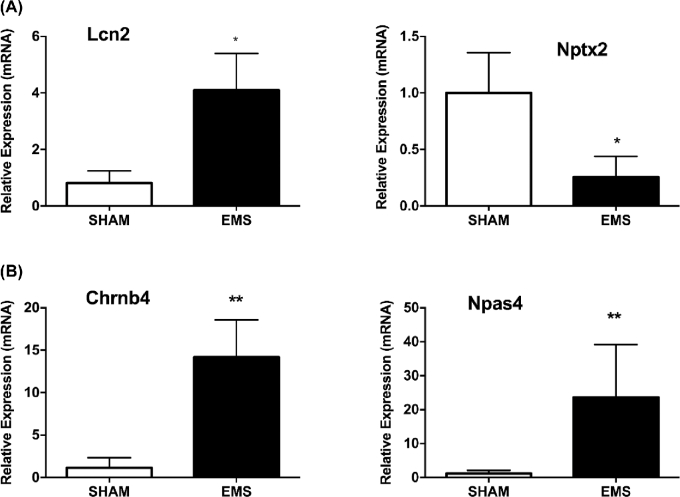
Other genes with a known function related to the behavior variations observed, which were identified as having significant fold changes in microarray analysis, were also validated by qRT-PCR. These include Lcn2 and Nptx2 in the amygdala (Figure 4A) and Chrnb4 and Npas4 in the hippocampus (Figure 4B). The qRT-PCR data were consistent with microarray data and established that Lcn2 is upregulated in amygdala of brain from mice with endometriosis compared to sham group, whereas Nptx2 is downregulated. As in hippocampus, both Chrnb4 and Npas4 were upregulated in mice with endometriosis compared to sham mice.
Figure 4.
Gene expression changes in the amygdala and hippocampus. Relative fold changes in expression between endometriosis and sham mice, as detected by qRT-PCR (A) Lcn2 and Nptx2 in amygdala (*P < 0.05). (B) Chrnb4 and Npas4 in hippocampus (**P < 0.01). N = 20 per group.
Validation of behavior-related protein expression
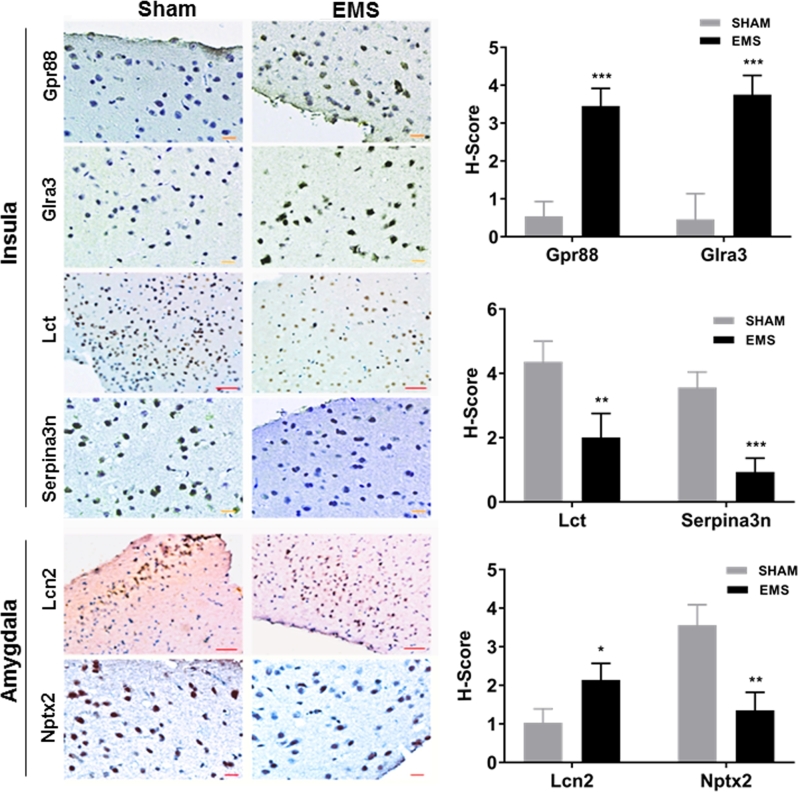
To confirm that the differential mRNA expression resulted in altered protein expression, six proteins were further validated by immunohistochemical staining. Among validated genes by qRT-PCR, six genes were chosen from the top 10 up- or downregulated genes based on their association with the behavioral and affective changes noted. An H-score was used to obtain a semiquantitative measure of expression. In the insula, the protein levels of Gpr88 and Glra3 (involved in anxiety and pain, respectively) increased significantly in the endometriosis group compared to the sham group (H-score fold changes 6.39 and 8.15, respectively). The protein levels of Lct and Serpina3n (also involved in pain) significantly decreased (H-score fold changes 2.17 and 3.85, respectively) in the endometriosis group compared to the sham group (Figure 5). Furthermore, in the amygdala, endometriosis led to a 2.08-fold increase in Lcn2 protein expression (P < 0.05); this protein has a known function in anxiety and depression. Similarly, we observed a 2.64-fold decrease in Nptx2 expression compared to sham surgery controls (P < 0.01); this protein plays a role in anxiety (Figure 5). All these results confirmed the microarray and qRT-PCR data, suggesting that these proteins may play a role in the behavioral and affective changes in endometriosis.
Figure 5.
Immunohistochemistry studies. Insula: the percentage of Gpr88 and Glra3 positive staining cells is higher and the staining is stronger in endometriosis mice compared with sham mice. The protein levels of Gpr88 and Glra3 in the insula of endometriosis mice increased 6.39- and 8.15-fold compared to sham mice (N = 20 per group). The percentage of Lct and Serpina3n positive staining cells is lower and the staining is lighter in endometriosis mice compare with sham mice. The protein levels of Lct and Serpina3n in insula of endometriosis mice decreased by 2.17- and 3.85-fold compared to sham mice (N = 10 per group), respectively (*P < 0.05; **P < 0.01; ***P < 0.005). Amygdala: the percentage of Lcn2 positive staining cells is higher and the staining is stronger, while the percentage of Nptx2 positive staining cells is lower and the staining is lighter in endometriosis mice compare with sham mice. The protein level of Lcn2 expression of endometriosis mice increased by 2.08- and Nptx2 decreased by 2.64-fold compared to sham mice (N = 20 per group). Scale: 50 μn for Lcn2 and Lct; 20 μn for Nptx2, Gpr88, Glra3, and Serpina3n.
Discussion
Women with endometriosis suffer from a wide spectrum of different types of pain, ranging from severe dysmenorrhea to chronic pelvic and other comorbid pain conditions [20]. Similarly, a large number of studies have demonstrated that endometriosis is associated with an elevated likelihood of developing depression and anxiety disorders [21]. In this study, we examined mouse models of endometriosis, which confirmed increased pain sensitivity, anxiety, and depression behaviors induced by endometriosis, similar to that reported in women with the disease [22, 23]. Moreover, we identified that endometriosis modulates gene expression in the insula, amygdala, and hippocampus. These areas of the brain play a key role in pain, anxiety, and depression. Our findings may help in the development of molecular targets and therapies to treat pain and control emotional disorders caused by endometriosis.
Pain and central sensitization
In the presence of ongoing tissue injury and/or inflammation as in endometriosis, the nociceptive system is sensitized, resulting in a decreased pain threshold and amplified sensory input, a phenomenon called central sensitization [24]. Central and peripheral sensitization have both been reported in endometriosis [25]. Central sensitization may be initiated by peripheral sensitization and maintained by continued input to the CNS from sensitized sensory afferent fibers. However, central actions can become independent of any peripheral inputs due to long-term “modification” of CNS functioning [26, 27]. The pain then remains long term, after the initiating pathophysiology resolves, explaining why removing ectopic lesions fail to relieve pain in some endometriosis patients.
The small, unmyelinated nerve fibers observed in the functional layer of the endometrium and ectopic endometriotic lesions of women have been identified as nociceptive in experimental and clinical studies [28]. It is believed that this nascent endometriosis-associated neural system has a widespread influence on the activity of neurons in the central neural system (CNS) and, hence, on pain perception in the patient. Nociceptors on these neurons transmit noxious stimuli and propagate these messages to the CNS. In the case of neuropathic pain, tactile afferents acquire synapses within the CNS, which enable the afferents to trigger central pain activity [29]. Persistent nociceptive input from endometriotic lesions is postulated to lead to central sensitization via increased responsiveness of spinal cord dorsal horn neurons processing input from the implants and affected adjacent viscera. Increased excitability of viscero-visceral convergent neurons to the spinal cord has been associated with persistent neuropathic pain and hyperalgesia in this setting. In this study, we found the duration in the hot plate test was significantly shorter in endometriosis mice compared with the sham group, which confirmed persistent pain hypersensitivity in endometriosis mice. This is in agreement with the results reported by Liu et al. [30] for rats which showed a significant reduction in latency in rats with induced endometriosis but not in sham rats, determined by hot plate test with respect to thermal stimulation. Previous reports showed that pain latencies in hot plate and tail flick tests were shorter in rats with endometriosis than in sham or atorvastatin-treated rats with endometriosis [31]. Both studies concluded that there was a significant reduction in latency in rats with induced endometriosis but not in sham surgery, similar to the reduction in duration time on hot plate by the mice with endometriosis observed in our study.
However, the molecular mechanisms that underlie central sensitization in endometriosis have not been characterized. Vicuna et al. demonstrated that mice lacking SerpinA3N developed more neuropathic mechanical allodynia than wild-type mice, and exogenous delivery of SerpinA3N attenuated mechanical allodynia in wild-type mice [32], which is consistent with our results that the expression of Serpina3n was downregulated in the insula of endometriosis mice. In addition, downregulation of Lct expression in the insula also may play an important role in endometriosis-induced pain sensitization, as previous studies have suggested that lactase deficiency occurs in abdominal pain syndromes [33, 34].
Furthermore, neurogenic inflammation also may initiate sensitization and myofascial pain in endometriosis. Harvey reported that GlyR alpha3 (Glra3) plays a critical role in pain hypersensitivity following spinal PGE2 injection, complete Freund's adjuvant, and zymosan-induced peripheral inflammation [35, 36], which is consistent with our results that upregulated Glra3 expression in the insula may play a role in pain sensitization in endometriosis.
Behavioral and affective changes in endometriosis
Mood disorders such as depression and anxiety are frequently observed in both patients and preclinical models of chronic pain. Epidemiological studies report an approximately 50% prevalence of major depressive disorder in patients with chronic pain [37]. Moreover, anxiety levels have been shown to predict pain severity and pain behavior in acute and chronic pain patients [38], and anxiety reduction techniques and anxiolytic drugs have been reported to be successful in ameliorating pain associated with medical procedures [39].
A large number of studies have reported an association between endometriosis and an elevated likelihood of developing mood disorders (depression and anxiety) [40, 41]. These conditions may impair women's social, educational, and employment opportunities as well as sexual relationships, reducing overall quality of life [42, 43]. We found that endometriosis mice spent less time and traveled less distance in the central area in the open field test; similarly, the immobility times of the mice with endometriosis were significantly longer than the sham group in tail suspension test. These data demonstrate that endometriosis induced anxiety and depression.
As almost all the behavioral tests used to assess anxiety- and depression-related behavior in rodents depend on the motor activity of the animals, it is always critical to control the effects of the neuropathic pain model on locomotor activity before performing any of these tests. This lack of locomotor deficit was most frequently tested by the distance traveled in an open field test. Our results showed mice with endometriosis have decreased locomotor activity compared to sham mice, corresponding to Seminowicz's study in a rat model of long-term neuropathic pain [44]. Additionally, sham and endometriosis mice varied at each time point, demonstrating that the results were not due to habituation.
Electrophysiological changes in endometriosis
At present, it is known that sciatic neuropathy evokes significant changes in the intrinsic electrophysiological properties of CeA neurons when they are viewed on a population basis, and decreases GABA-mediated inhibitory transmission within the CeA [45]. CeA effects on emotion-related behaviors are probably mediated by the medial part of the CeA (CeM) projections to the bed nucleus of the stria terminalis and the brainstem origins of ascending acetylcholine, dopamine, and norepinephrine fibers. Indeed, a pharmacological manipulation of GABA-A receptors in the CeA can modify the escape/avoidance behavior in neuropathic animals, suggesting that the CeA is implicated not only in sensory processing but also in the affective-motivational dimension of neuropathic pain [46]. Some of the CeA neurons cosynthesize GABA with the corticotropin-releasing factor, a neuropeptide which has a well-established role in anxiety and depression [47].
Besides the GABAergic transmissions, the glutamatergic system also plays critical roles in a neuropathic context. Glutamate mediates major excitatory transmission during long-term plasticity in both physiological and pathological conditions [48]. Specifically related to nociceptive or pain behaviors, metabotropic glutamate subtype receptors (mGluRs) have been involved in different types of synaptic modulation and plasticity from periphery to the spinal cord [49].
Central sensitization in dorsal horn neurons can be induced by an increase in excitatory synaptic transmission, mediated via the glutamate N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate receptors, or by a loss of inhibitory synaptic transmission (disinhibition), mediated via γ-aminobutyric acid (GABA) and glycine receptors. While the synaptic changes observed at the parabrachial nucleus (PB)-CeA synapses appear to be NMDA-receptor independent, the intra-CeA administration of NMDA or group I mGluR antagonists reduces the pain-induced place avoidance behavior [50]. This suggests that the amygdaloid NMDA receptors play a role in the maintenance of neuropathic pain by facilitating BLA inputs or by facilitating synaptic signaling between CeA interneurons rather than by facilitating ascending PB-CeA inputs.
Altered brain gene expression in endometriosis
Endometriosis led to changes in expression of several genes in the brain regions associated with pain, anxiety, and depression. Indeed, besides those identified genes we already discussed, many other genes shown to be differentially expressed in our study have been reported to be associated with behavior disorders. For example, Jha reported that lipocalin-2 (Lcn2) promotes stress-induced changes in spine morphology and function to regulate neuronal excitability and anxiety [51], which is in line with our results that the expression of Lcn2 was upregulated in amygdala of endometriosis mice. Similarly, our results showed downregulation of Nptx2 in amygdala of endometriosis mice compared to the sham group. A previous study suggested that Nptx2 played an essential role in controlling network dynamics, highlighting potential therapeutic targets for disorders with inhibition/excitation imbalances [52]. Moreover, Meirsman reported that Gpr88 expressed in A2AR neurons enhances ethological anxiety-like behaviors without affecting conflict anxiety and fear responses [53], whereas mice lacking Gpr88 show motor deficit, improved spatial learning, and low anxiety reversed by delta opioid antagonist [54], with the observed increase in Gpr88 expression in endometriosis mice being consistent with our results.
Conclusion
In summary, our study confirmed that pain sensitization, anxiety, and depression were induced by endometriosis. Endometriosis modulates gene expression in the insula, amygdala, and hippocampus which play a key role in behavioral changes of pain, anxiety, and depression. The effects of nociceptive signaling are integrated with endometriosis on brain function to create the experience of increased pain and its emotional and behavioral consequences. While medications can be used to treat endometriosis directly [55], the effects on the brain are largely ignored. Identified differential expressed genes may help in developing molecular targets to cure pain and control emotional disorders causing by endometriosis. Furthermore, our findings may provide useful suggestions for future research avenues in the study of the complex interaction between physical and psychological factors in endometriosis.
Supplementary data
Supplemental Figure S1. Endometriotic lesions in mice. Endometriosis was induced in mice experimentally as described in the methods. After 12 weeks from the date of induction, mice were sacrificed and examined for the growth of endometriotic lesions. The images show the endometriotic lesions in the induced mice. No lesions were identified in the sham controls (not shown).
Supplemental Figure S2. Percentage of distance traveled in the center at each time periods. The percentage of distance traveled in the center is significantly higher for sham group compared to mice with endometriosis. “*” denotes statistical significance (P < 0.00001) compared to sham group. N = 12 per group.
Supplemental Table S1. Primers used in qRT-PCR.
Supplemental Table S2. Antibodies used in Immunohistochemistry.
Notes
Conference Presentation: Presented in part at the 64th Annual Meeting of the Society for Reproductive Investigation (SRI), 15–18 March 2017, Orlando, Florida, USA.
Edited by Dr. Peter J. Hansen, PhD, University of Florida
Footnotes
Grant Support: This work was supported by the National Institutes of Health NIH RO1 HD052668 and Pfizer W5957335 awarded to HST.
References
- 1. Crosignani P, Olive D, Bergqvist A, Luciano A. Advances in the management of endometriosis: an update for clinicians. Hum Reprod Update 2006; 12:179–189. [DOI] [PubMed] [Google Scholar]
- 2. Simoens S, Hummelshoj L, D’Hooghe T. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update 2007; 13:395–404. [DOI] [PubMed] [Google Scholar]
- 3. Bajaj P, Bajaj P, Madsen H, Arendt-Nielsen L. Endometriosis is associated with central sensitization: a psychophysical controlled study. J Pain 2003; 4:372–380. [DOI] [PubMed] [Google Scholar]
- 4. Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature 1983; 306:686–688. [DOI] [PubMed] [Google Scholar]
- 5. As-Sanie S, Harris RE, Napadow V, Kim J, Neshewat G, Kairys A, Williams D, Clauw DJ, Schmidt-Wilcke T. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain 2012; 153:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. As-Sanie S, Kim J, Schmidt-Wilcke T, Sundgren PC, Clauw DJ, Napadow V, Harris RE. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain 2016; 17:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod 2007; 22:266–271. [DOI] [PubMed] [Google Scholar]
- 8. Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update 2005; 11:595–606. [DOI] [PubMed] [Google Scholar]
- 9. Chen SD, Wang YL, Liang SF, Shaw FZ. Rapid amygdala kindling causes motor seizure and comorbidity of anxiety- and depression-like behaviors in rats. Front Behav Neurosci 2016; 10:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Padival MA, Blume SR, Rosenkranz JA. Repeated restraint stress exerts different impact on structure of neurons in the lateral and basal nuclei of the amygdala. Neuroscience 2013; 246:230–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eisch AJ, Petrik D. Depression and hippocampal neurogenesis: a road to remission? Science 2012; 338:72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee B, Du H, Taylor HS. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol Reprod 2009; 80:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bannon AW, Malmberg AB. Models of nociception: hot-plate, tail-flick, and formalin tests in rodents. Curr Protoc Neurosci 2007; Chapter 8:Unit 8.9. [DOI] [PubMed] [Google Scholar]
- 14. Karl T, Pabst R, von Horsten S. Behavioral phenotyping of mice in pharmacological and toxicological research. Exp and Toxicol Pathol 2003; 55:69–83. [DOI] [PubMed] [Google Scholar]
- 15. Aldad TS, Gan G, Gao XB, Taylor HS. Fetal radiofrequency radiation exposure from 800-1900 Mhz-rated cellular telephones affects neurodevelopment and behavior in mice. Sci Rep 2012; 2:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steru L, Chermat R, Thierry B, Mico JA, Lenegre A, Steru M, Simon P, Porsolt RD. The automated tail suspension test: a computerized device which differentiates psychotropic drugs. Prog Neuropsychopharmacol Biol Psychiatry 1987; 11:IN1–IN671. [DOI] [PubMed] [Google Scholar]
- 17. Aldad TS, Rahmani N, Leranth C, Taylor HS. Bisphenol-A exposure alters endometrial progesterone receptor expression in the nonhuman primate. Fertil Steril 2011; 96:175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rao Y, Liu ZW, Borok E, Rabenstein RL, Shanabrough M, Lu M, Picciotto MR, Horvath TL, Gao XB. Prolonged wakefulness induces experience-dependent synaptic plasticity in mouse hypocretin/orexin neurons. J Clin Invest 2007; 117:4022–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hou WH, Kuo N, Fang GW, Huang HS, Wu KP, Zimmer A, Cheng JK, Lien CC. Wiring specificity and synaptic diversity in the mouse lateral central amygdala. J Neurosci 2016; 36:4549–4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Greene AD, Lang SA, Kendziorski JA, Sroga-Rios JM, Herzog TJ, Burns KA. Endometriosis: where are we and where are we going? Reprod 2016; 152:R63–R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pope CJ, Sharma V, Sharma S, Mazmanian D. A systematic review of the association between psychiatric disturbances and endometriosis. J Obstet Gynaecol Can 2015; 37:1006–1015. [DOI] [PubMed] [Google Scholar]
- 22. Facchin F, Barbara G, Saita E, Erzegovesi S, Martoni RM, Vercellini P. Personality in women with endometriosis: temperament and character dimensions and pelvic pain. Hum Reprod 2016; 31:1515–1521. [DOI] [PubMed] [Google Scholar]
- 23. Facchin F, Barbara G, Saita E, Mosconi P, Roberto A, Fedele L, Vercellini P. Impact of endometriosis on quality of life and mental health: pelvic pain makes the difference. J Psychosom Obstet Gynecol 2015; 36:135–141. [DOI] [PubMed] [Google Scholar]
- 24. Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009; 10:895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. As-Sanie S, Harris RE, Harte SE, Tu FF, Neshewat G, Clauw DJ. Increased pressure pain sensitivity in women with chronic pelvic pain. Obstet Gynecol 2013; 122:1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci 2003; 26:696–705. [DOI] [PubMed] [Google Scholar]
- 27. Ren K, Dubner R. Pain facilitation and activity-dependent plasticity in pain modulatory circuitry: role of BDNF-TrkB signaling and NMDA receptors. Mol Neurobiol 2007; 35:224–235. [DOI] [PubMed] [Google Scholar]
- 28. Tokushige N, Markham R, Russell P, Fraser IS. Different types of small nerve fibers in eutopic endometrium and myometrium in women with endometriosis. Fertil Steril 2007; 88:795–803. [DOI] [PubMed] [Google Scholar]
- 29. Asante A, Taylor RN. Endometriosis: the role of neuroangiogenesis. Annu Rev Physiol 2011; 73:163–182. [DOI] [PubMed] [Google Scholar]
- 30. Liu M, Liu X, Zhang Y, Guo SW. Valproic acid and progestin inhibit lesion growth and reduce hyperalgesia in experimentally induced endometriosis in rats. Reprod Sci 2012; 19:360–373. [DOI] [PubMed] [Google Scholar]
- 31. Simsek Y, Gul M, Yilmaz E, Ozerol IH, Ozerol E, Parlakpinar H. Atorvastatin exerts anti-nociceptive activity and decreases serum levels of high-sensitivity C-reactive protein and tumor necrosis factor-alpha in a rat endometriosis model. Arch Gynecol Obstet 2014; 290:999–1006. [DOI] [PubMed] [Google Scholar]
- 32. Vicuna L, Strochlic DE, Latremoliere A, Bali KK, Simonetti M, Husainie D, Prokosch S, Riva P, Griffin RS, Njoo C, Gehrig S, Mall MA et al. . The serine protease inhibitor SerpinA3N attenuates neuropathic pain by inhibiting T cell-derived leukocyte elastase. Nat Med 2015; 21:518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boey CC. Lactase deficiency among Malaysian children with recurrent abdominal pain. J Paediatr Child Health 2001; 37:157–160. [DOI] [PubMed] [Google Scholar]
- 34. Kumar S, Ranjan P, Mittal B, Singh R, Ghoshal UC. Lactase persistence/non-persistence genetic variants in irritable bowel syndrome in an endemic area for lactose malabsorption. J Gastroenterol Hepatol 2012; 27:1825–1830. [DOI] [PubMed] [Google Scholar]
- 35. Harvey RJ, Depner UB, Wassle H, Ahmadi S, Heindl C, Reinold H, Smart TG, Harvey K, Schutz B, Abo-Salem OM, Zimmer A, Poisbeau P et al. . GlyR alpha3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 2004; 304:884–887. [DOI] [PubMed] [Google Scholar]
- 36. Harvey VL, Caley A, Muller UC, Harvey RJ, Dickenson AH. A selective role for alpha3 subunit glycine receptors in inflammatory pain. Front Mol Neurosci 2009; 2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med 2003; 163:2433–2445. [DOI] [PubMed] [Google Scholar]
- 38. Kain ZN, Sevarino F, Alexander GM, Pincus S, Mayes LC. Preoperative anxiety and postoperative pain in women undergoing hysterectomy. A repeated-measures design. J Psychosom Res 2000; 49:417–422. [DOI] [PubMed] [Google Scholar]
- 39. Dellemijn PL, Fields HL. Do benzodiazepines have a role in chronic pain management? Pain 1994; 57:137–152. [DOI] [PubMed] [Google Scholar]
- 40. Chen LC, Chen MH. Reply to Laganà et al.'s comment on “Risk of developing major depression and anxiety disorders among women with endometriosis: a longitudinal follow-up study”. J Affect Disord 2017; 208:674. [DOI] [PubMed] [Google Scholar]
- 41. Lagana AS, Condemi I, Retto G, Muscatello MR, Bruno A, Zoccali RA, Triolo O, Cedro C. Analysis of psychopathological comorbidity behind the common symptoms and signs of endometriosis. Eur J Obstet Gynecol Reprod Biol 2015; 194:30–33. [DOI] [PubMed] [Google Scholar]
- 42. Culley L, Law C, Hudson N, Denny E, Mitchell H, Baumgarten M, Raine-Fenning N. The social and psychological impact of endometriosis on women's lives: a critical narrative review. Hum Reprod Update 2013; 19:625–639. [DOI] [PubMed] [Google Scholar]
- 43. De Graaff AA, D’Hooghe TM, Dunselman GA, Dirksen CD, Hummelshoj L, Simoens S. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod 2013; 28:2677–2685. [DOI] [PubMed] [Google Scholar]
- 44. Seminowicz DA, Laferriere AL, Millecamps M, Yu JS, Coderre TJ, Bushnell MC. MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain. NeuroImage 2009; 47:1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang Z, Tao W, Hou YY, Wang W, Lu YG, Pan ZZ. Persistent pain facilitates response to morphine reward by down regulation of central amygdala GABAergic function. Neuropsychopharmacol 2014; 39:2263–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pedersen LH, Scheel-Kruger J, Blackburn-Munro G. Amygdala GABA-A receptor involvement in mediating sensory-discriminative and affective-motivational pain responses in a rat model of peripheral nerve injury. Pain 2007; 127:17–26. [DOI] [PubMed] [Google Scholar]
- 47. Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol Psychiatry 2010; 15:574–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Usdin TB, Dimitrov EL. The effects of extended pain on behavior: recent progress. Neuroscientist 2016; 22:521–533. [DOI] [PubMed] [Google Scholar]
- 49. Koga K, Li S, Zhuo M. Metabotropic glutamate receptor dependent cortical plasticity in chronic pain. Curr Neuropharmacol 2016; 14:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ansah OB, Bourbia N, Goncalves L, Almeida A, Pertovaara A. Influence of amygdaloid glutamatergic receptors on sensory and emotional pain-related behavior in the neuropathic rat. Behav Brain Res 2010; 209:174–178. [DOI] [PubMed] [Google Scholar]
- 51. Jha MK, Lee S, Park DH, Kook H, Park KG, Lee IK, Suk K. Diverse functional roles of lipocalin-2 in the central nervous system. Neurosci Biobehav Rev 2015; 49:135–156. [DOI] [PubMed] [Google Scholar]
- 52. Pelkey KA, Barksdale E, Craig MT, Yuan X, Sukumaran M, Vargish GA, Mitchell RM, Wyeth MS, Petralia RS, Chittajallu R, Karlsson RM, Cameron HA et al. . Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuroen 2015; 85:1257–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Meirsman AC, Robe A, de Kerchove d’Exaerde A, Kieffer BL. GPR88 in A2AR neurons enhances anxiety-like behaviors. eNeuro 2016; 3:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Meirsman AC, Le Merrer J, Pellissier LP, Diaz J, Clesse D, Kieffer BL, Becker JA. Mice lacking GPR88 show motor deficit, improved spatial learning, and low anxiety reversed by delta opioid antagonist. Biol Psychiatry 2016; 79:917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Taylor HS, Giudice LC, Lessey BA, Abrao MS, Kotarski J, Archer DF, Diamond MP, Surrey E, Johnson NP, Watts NB, Gallagher JC, Simon JA et al. . Treatment of endometriosis-associated pain with Elagolix, an Oral GnRH antagonist. N Engl J Med 2017; 377:28–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.