Abstract
Glycogen synthase kinase 3 (GSK3) is a highly conserved protein kinase regulating key cellular functions. Its two isoforms, GSK3α and GSK3β, are encoded by distinct genes. In most tissues the two isoforms are functionally interchangeable, except in the developing embryo where GSK3β is essential. One functional allele of either of the two isoforms is sufficient to maintain normal tissue functions. Both GSK3 isoforms, present in sperm from several species including human, are suggested to play a role in epididymal initiation of sperm motility. Using genetic approaches, we have tested requirement for each of the two GSK3 isoforms in testis and sperm. Both GSK3 isoforms are expressed at high levels during the onset of spermatogenesis. Conditional knockout of GSK3α, but not GSK3β, in developing testicular germ cells in mice results in male infertility. Mice lacking one allele each of GSK3α and GSK3β are fertile. Despite overlapping expression and localization in differentiating spermatids, GSK3β does not substitute for GSK3α. Loss of GSK3α impairs sperm hexokinase activity resulting in low ATP levels. Net adenine nucleotide levels in caudal sperm lacking GSK3α resemble immature caput epididymal sperm. Changes in the association of the protein phosphatase PP1γ2 with its protein interactors occurring during epididymal sperm maturation is impaired in sperm lacking GSK3α. The isoform-specific requirement for GSK3α is likely due to its specific binding partners in the sperm principal piece. Testis and sperm are unique in their specific requirement of GSK3α for normal function and male fertility.
Keywords: GSK3α, sperm, isoform specific, hexokinase, male fertility
GSK3α in sperm is essential for male fertility.
Introduction
The enzyme GSK3, a serine/threonine protein kinase, was termed GSK-3 and was discovered after PKA and phosphorylase kinase (GSK-1 and GSK-2) along with two other GS kinases (GSK-4 and -5) based on relative elution from phosphocellulose chromatography of muscle extracts. Since then GSK3 has been found to be a key signaling component of a large number of cellular processes [1, 2]. An array of functions attributed to GSK3 includes insulin action, regulation of cell survival, apoptosis, embryonic development, Wnt/β-catenin and hedgehog signaling, and growth factor action. It is also a target for drug development due to involvement in several clinical disorders including cancer [3].
In mammals, GSK3 is ubiquitous and is expressed as two isoforms, GSK3α and GSK3β, encoded by different genes. The catalytic domains of the two isoforms are 98% identical, while their N- and C- termini are distinctive [4]. While there are reports ascribing distinct roles for each of the isoforms [5] under most circumstances, the two isoforms appear redundant and functionally interchangeable.
Knockout (KO) of Gsk3β in mice cause late embryonic lethality [6]. The inability of GSK3α to substitute for GSK3β in the developing embryo may be due to the nonoverlapping expression of the two isoforms. Conditional KO of the floxed Gsk3β alleles on a Gsk3α null background shows that complete loss of both isoforms impairs signaling and tissue function. However, one functional allele of Gsk3β or Gsk3α on a Gsk3α or Gsk3β null background, respectively, is sufficient to maintain normal tissue function [7] highlighting the functional redundancy of the two isoforms.
We discovered GSK3 in sperm as an enzyme responsible for activation of PP1γ2 [8, 9]. Both α and β isoforms of GSK3 are present in sperm. Immotile caput sperm contain fourfold higher GSK3 activity than motile caudal epididymal sperm. Both tyrosine phosphorylation (which stimulates catalytic activity) and serine phosphorylation of GSK3 (an inhibitory mechanism) increase significantly in sperm during their passage through the epididymis [10]. Incubation of motile or immotile sperm with compounds that activate PKA or inhibit protein phosphatase is accompanied by increases in GSK3 serine phosphorylation and motility stimulation [10] [4]. These data support the notion that GSK3 is involved in epididymal maturation and sperm motility. A recent report also documents the role for GSK3 and Wnt signaling in epididymal sperm maturation insofar as loss of Wnt signaling in sperm results in male infertility [5].
We recently showed that GSK3α null mice exhibit male infertility [11]. This finding raised a number of questions. It was not known whether infertility was due to loss of the enzyme in testis and sperm or a result of lack of the enzyme in the male reproductive tract or other tissues. Because both GSK3 isoforms are present in sperm, it was possible that infertility could also result from the loss of GSK3β. The goal of studies reported here was to determine whether there is an isoform-specific requirement for GSK3α in male fertility and, if so, if this requirement is testis- and sperm-specific. We used a Cre-LoxP strategy to affect tissue-specific KO of each of the two isoforms in differentiating postmeiotic testicular germ cells. While both isoforms of GSK3 are expressed at high levels in testis, GSK3β in particular is expressed at high levels compared to other tissues. Expression levels of both isoforms increased in postnatal developing testis at a time coincident with onset of spermatogenesis. The isoforms also shared spatial localization in developing and adult testis. However, KO of GSK3α in developing germ cells resulted in male infertility, whereas mice lacking GSK3β in testis and sperm were normal and fertile. This does not happen in females where loss of both or either of these two isoforms results in normal fertility [12]. Thus, GSK3β was unable to substitute for GSK3α whereas GSK3α substituted for the loss of GSK3β. Lowered net GSK3 catalytic activity was not responsible for infertility because sperm from mice lacking one allele each for GSK3α and GSK3β or sperm lacking GSK3α or GSK3β exhibited 50% lower GSK3 activity levels compared to wild-type (WT) sperm. The essential function of GSK3α in sperm may reflect its ability to bind to proteins in an isoform-specific manner. Sperm adenine nucleotide levels, energy metabolism, and protein phosphatase and kinase activities were affected suggesting impaired sperm maturation in the epididymis. Unlike other tissues, sperm and testis are unique in their essential requirement for GSK3α.
Materials and methods
Ethics statement
All procedures with WT, global, and conditional KO mice used in the present study were performed at the Kent State University animal facility. The protocol was approved by the NIH and National Research Council's publication “Guide for Care and Use of Laboratory Animals” and in accordance with the Kent State Animal Ethics Committee under the Institutional Animal Care and Use Committees.
Sperm isolation
The cauda epididymis and vas deferens from adult mice, aged 6–12 weeks, were isolated in PBS. The cauda epididymis was punctured with a 45-mm gauge and the sperm were allowed to swim out. Surgical scissors were also used to squeeze sperm out of the cauda and along the vas deferens. With occasional swirling, sperm were allowed to disperse into PBS for 5–10 min at 37°C. The sperm suspension was transferred to microcentrifuge tubes using a large-bore pipette tip. Approximately 10 μl of the suspension was diluted 20 times (1:20) in water for determination of sperm number using a Neubauer hemocytometer. To assess morphology, an aliquot of the sperm suspension was added to freshly prepared 4% paraformaldehyde (EM grade) in 1X PBS and incubated at 4°C for 30 min. Fixed sperm were mounted on poly-L-lysine-coated slides and sealed with coverslips. Sperm were observed under ×20 and ×60 objectives in an Olympus 81 differential interference contrast microscope.
SDS PAGE and western blot analysis
Testis and sperm extracts were boiled with Laemmli buffer for 5 min. The protein samples were then separated using 12% polyacrylamide gel and transferred to PVDF membranes (Millipore Corp). Following transfer, membranes were blocked with 5% nonfat dry milk diluted in TTBS (0.2 M Tris, pH 7.4, 1.5 M NaCl, 0.1% thimerosol, and 0.5% Tween 20). The blots were then incubated overnight with primary antibodies: GSK3α rabbit monoclonal antibody (Cell Signaling); GSK3β rabbit monoclonal antibody (Cell Signaling); anti phospho-tyrosine mouse monoclonal antibody 4G10 (Milipore) (Table 1). Blots were washed with TTBS twice for 15 min and twice for 5 min. The blots were finally developed with an enhanced chemiluminescence substrate.
Table 1.
Antibody information.
| Antibody name/Use | Company/Catalogue # | Dilution | Host |
|---|---|---|---|
| GSK3α | Cell Signaling (4337S) | 1:1000 (Western Blot) 1:200 (Immunofluorescence) | Rabbit monoclonal |
| GSK3β | Cell Signaling (9315S) | 1:1000 (Western Blot) | Rabbit monoclonal |
| Phospho-Tyrosine (4G10) | Milipore (05-321) | 1:2000 (Western Blot) | Mouse monoclonal |
| Hexokinase −1 | Cell Signaling (2024S) | 1:1000 (Western Blot) | Rabbit monoclonal |
| GSK3β | Novous Biologicals (NBP 47470) | 1:200 (Immunofluorescence) | Mouse monoclonal |
| PP1γ2 | Yenzym (In house) | 1:5000 (Western Blot) | Rabbit monoclonal |
| PPP1R2 (I2) | Yenzym (In house) | 1:1000 (Western Blot) | Rabbit monoclonal |
| PPP1R7 (sds22) | Yenzym (In house) | 1:1000 (Western Blot) | Rabbit monoclonal |
| PPP1R11 (I3) | Yenzym (In house) | 1:1000 (Western Blot) | Rabbit monoclonal |
| β-Actin | Gene script (A00702-200) | 1:2000 (Western Blot) | Mouse monoclonal |
RNA isolation, cDNA synthesis, and quantitative PCR
Total RNA was isolated from WT testis using Trizol reagent (Sigma), phenol-chloroform extraction (Amresco), and isopropanol precipitation. The pellet was washed with 75% ethanol and dissolved in DEPC-treated water. RNA concentration was measured in a Nanodrop ND-1000 Spectrophotometer (Nanodrop Technologies). To prepare cDNA from RNA (900 μg), QuantiTect Reverse Transcription Kit (Qiagen) was used. The PCR analysis was done using 1 μg of cDNA prepared from RNA of testis of WT. The following primer sets were used for Gsk3α: forward 5΄-ACCCTTGGACAAAGGTGTTC-3΄ (from exon 8/9 junction), and reverse 5΄-TCAGTCCTGGTGAACTGTCC-3΄ (from exon 10) expected to produce an amplicon 300 bp in size and for Gsk3β primer sets were used: forward 5΄-GTCCGACTGCGGTATTTCTTC-3΄ and reverse 5΄-CTCGATGGCAGATTCCAAAGG-3΄ expected to produce a 250-bp amplicon.
Quantitative PCR analysis of Gsk3α and Gsk3β mRNA expression was done using the Quanti Tect SYBR Green RT-PCR Kit. Quantitative RT-PCR (qRT-PCR) experiments were performed on the Rotor-Gene Q series. For SYBER green (Quanti-Tect SYBR Green RTPCR Kit) qRT-PCR, average threshold cycles were determined from triplicate reactions. PCR reactions were carried out in a 25 μl volume for 5 min at 95°C for initial denaturing, followed by 35 cycles at 95°C for 5 s and at 59°C for 20 s. Gapdh, a housekeeping gene was used as an internal control. Each primer set was first tested to determine optimal concentrations, and products were run on a 1% agarose gel to confirm the presence of the predicted amplification products. Data were analyzed by the dCt method considering the average Ct values of each sample. Error bars indicate standard deviation or standard deviation normalized to a reference sample, as indicated in the legends accompanying the figures.
Immunocytochemistry of spermatozoa
Caudal epididymal spermatozoa washed and suspended in PBS and were spun down at 700 g for 10 min at 4°C. The sperm pellet was resuspended fixed in 4% paraformaldehyde, EM grade (Electron Microscopy Sciences) at 4°C for 30 min, followed by permeabilization with 0.2% Triton-X (5 min). Fixed sperm were attached to poly-L-lysine-coated slides. The slides were washed three times with TTBS to remove excess paraformaldehyde and incubated for 3 h at room temperature in a blocking solution containing 5% normal goat serum in TTBS. Slides were then incubated overnight at 4°C with anti-GSK3α antibody (1:200 dilution; Cell Signaling; Rabbit monoclonal), GSK3β (1:200 dilution; Novus Biologicals; mouse monoclonal), washed three times 5 min each with TTBS, followed by incubation with the appropriate secondary antibody conjugated to Cy3 for 1 h at room temperature. The slides were then washed three times, 10 min each with TTBS, mounting medium was applied, and examined by bright field and fluorescence microscopy (Olympus 81).
GSK3 activity assay
Sperm suspensions were centrifuged at 700 g for 10 min at 4°C and pellets were resuspended again in 1X RIPA lysis buffer supplemented with 0.1% β-mercaptoethanol, 10 mM benzamidine, 1 mM phenyl-methyl-sulfonyl fluoride (PMSF), 0.1 mM N-tosyl-L-lysyl chloromethyl ketone (TPCK), 1 mM sodium orthovanadate, and 1 nM calyculin A. Sperm suspended in the lysis buffer was incubated in ice for 30 min followed by centrifugation at 16,000 g at 4°C for 20 min. The ensuing supernatant was collected and used for the GSK3 assay. GSK3 activity was measured by the amount of 32PO4 transferred from [32P] γ- adenosine triphosphate to phospho-glycogen synthase peptide-2 (GSK3 substrate, Millipore). The initial assay buffer contained 200 mM HEPES, 50 mM MgCl2, 8 μM DTT, 5 mM sodium ß-glycerophosphate, 0.4 mM ATP, and 4 μCi of gamma-P32 ATP. This assay buffer (5 μl) was added with 5 μl each of previously prepared cell extract and GS2 peptide (1 mg/ml). GSK3 activity was also measured in the presence of 1 mM LiCl. Lithium-sensitive kinase activity was considered to be due to GSK3 [13]. The reaction mixture was incubated at 30°C waterbath for 15 min and the reaction was stopped by cooling on ice for 10 min. Aliquot (12 μl) of the reaction mixture was applied to a phosphocellulose cation exchanger (P81; Whatman Inc, Clifton, NJ) paper cut into 1.5 cm × 1.5 cm squares and washed with 0.1% (vol/vol) phosphoric acid. After three washes (5 min each) in phosphoric acid, the squares were placed into scintillation vials with 2 ml of distilled water and counted in a scintillation counter. Each of the reaction set was done in triplicate. The GSK3 activity was measured as follows: Activity units/107 cells = (Lithium-sensitive cpm) × (Reaction vol/spot vol)/(sp. activity of P32 ATP) × reaction time). All assays were conducted in triplicate and the means of three or more separate experiments are shown.
Northern blot analysis
Mouse multiple tissue northern blot was obtained from Zyagen. The membrane was soaked in DEPC treated water for 5 min followed by 5 min in 1 X SSC. Following this treatment, the membrane was prehybridized in 10 ml of Ultrahyb hybridization buffer (Ambion) in a hybridization bottle at 42°C for 1 h. A full-length cDNA probe for Gsk3α or Gsk3β was random primer labeled using 32P-dCTP (MP Biomed) and a Rediprime nick translation kit (GE Healthcare, Piscataway, NJ). The radiolabeled probe was passed though Illustra NICK columns (GE Healthcare) to remove unincorporated dCTP. The eluted probe was diluted in 10 ml of hybridization buffer and added to the blot in a hybridization bottle. The blot was incubated overnight at 42°C, washed twice in 1% SSC with 0.1% SDS, twice in 0.5% SSC with 1% SDS, and twice in 0.1% SSC with 1% SDS. All the washes were at 42°C for 5 min. After washing, the moist membrane was wrapped in saran wrap and exposed to a phosphor-imager screen (Molecular Dynamics) and developed with Typhoon scanner (GE Healthcare).
Immunohistochemistry
Testis from conditional GSK3α KO, conditional GSK3β KO, and WT mice were collected and fixed in 4% paraformaldehyde in PBS at 4°C for 6 h. The fixed testes were transferred to 75% ethanol and dehydrated, permeabilized, and embedded in paraffin using a Shandon Tissue Processor (Thermo Electron Corp., Waltham, MA). Multiple 5-μm-thick sections of the whole testis were attached to poly-L-lysine-coated slides, deparaffinized, and rehydrated using a standard procedure. Antigen retrieval was performed using 1XAntigen Retrieval Citra Solution (BioGenex, San Ramon, CA). Sections immersed in Citra solution were microwaved three times for 2 min, with a cooling period of 1 min between each heating cycle. Slides were incubated for 1 h at room temperature in a blocking solution containing 10% normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA) in PBS. Slides were then incubated with primary antibodies for GSK3α and GSK3β (1:200) overnight at 4°C. For the GSK3αβ heterozygous testis sections both antibodies were used were used together. Slides were washed three times with 1X PBS, and incubated with the appropriate secondary antibody (1:250) conjugated with Cy3 or Alexa-fluor (Jackson ImmunoResearch Laboratories) for 2 h at room temperature. The slides were washed five times with PBS. Nuclei were labeled with Hoechst dye (Thermo Scientific Pierce, Oregon). The slides were mounted with Prolong Diamond Antifade Mountant (Thermo Scientific Pierce) mounting media, and examined using a Fluo View 500 Confocal Fluorescence Microscope (Olympus, Melville, NY). Control slides were processed in the same manner except that the primary antibody incubation was omitted.
ATP assay
Caudal sperm were isolated in PBS medium and then pelleted down at 700 g for 10 min. The sperm pellet was then resuspended in TYH medium (Pyruvate and Lactate free) and incubated separately with 10 mM glucose and 25 mM lactate. After 2-h incubation, triplicates of aliquots were diluted 1:10 in boiling Tris-EDTA buffer (0.1 M Tris- HCl and 4 mM EDTA; pH 7.75). The samples were prepared as described previously [11]. Diluted sample (100 μl) was then used for quantifying ATP using the Bioluminescence Assay Kit CLS II (Roche Applied Science) followed by measurement of luminescence in Luminometer (Turner Biosystems 20/20).
Hexokinase assay
Sperm lysates were prepared by collecting sperm directly from the cauda epididymis into PBS media. The samples were centrifuged and the pellet was resuspended in modified RIPA media (1X RIPA; Millipore, 1 M benzamidine, β-mercaptoethanol, phenyl methyl sulfonyl chloride, 1 mM activated sodium orthovanadate, and 1 mM calyculin). The resuspension was kept in ice for 30 min and was then centrifuged at 12,000 g for 15 min at 4°C. The supernatant was collected and used for the assay. 50 μl of sample was added with 950 μl of assay solution containing 20 mM Tris-HCl (pH 7.5), 20 mM MgCl2, 4 mM EDTA, one unit/ml G6PDH, 10 mM glucose, 0.6 mM βNADP+, and 0.1% Triton X-100. After 3 min of preincubation, hexokinase activity was initiated by addition of 4 mM ATP. The final NADPH production was measured at 340 nM by spectrophotometry after 12 min [14, 15]. For the control assay, glucose was omitted.
Quantitation of adenine nucleotides using the HPLC method
Mouse spermatozoa were isolated from cauda epididymis in a buffer containing 150 mM NaCl, 5.5 mM KCl, 0.4 mM MgSO4, 1 mM CaCl2, 10 mM NaHCO3, 10 mM HEPES-NaOH (pH 7.4). After washing, cells were resuspended in the same buffer at a concentration of 1 × 107/100 μl, supplemented with a final concentration of 0.5% ice-chilled perchloric acid and kept for 15 min on ice to remove proteins. The tube containing sperm cells was then centrifuged at 12,000 g at 4°C for 12 min and the supernatant was taken as the source of nucleotides. Before use, the supernatant was filtered (0.22 μm pore size). The filtered solution was neutralized with a final concentration of 100 mM KOH solution. A calibration curve was duly prepared using 0–50 μM of standard ATP/ADP/AMP solutions. HPLC (RT-1100, Agilent Technologies) analysis was performed after injection of 100 μl samples/standards into a Phenomenex Luna 5 μm C18 (2), 4.6 × 150 mm column, and the following buffers: Buffer A: 100 mM potassium phosphate buffer, pH 6.0 + 4 mM tetrabutyl ammonium sulfate, 5% methanol; Buffer B: 100 mM potassium phosphate buffer, pH 7.2 + 4 mM tetrabutyl ammonium sulfate, 30% methanol. Resolution of different nucleotide peaks was obtained at 260 nm wavelength under the following conditions: first 2 min 100% of buffer A, 2–15 min 0–100% gradient of buffer B, 15–20 min 100% buffer B, then 1 min using steep gradient of 0–100% buffer A, and finally washed with 100% buffer A. The content of ATP/ADP/AMP is represented as nmole/108 sperm [16, 17].
Co-immunoprecipitation
Crude lysates of mouse sperm were incubated for 2 h at 4°C with Protein G-Sepharose 4 Fast Flow beads (GE Healthcare) which were washed once with distilled water and twice with homogenization buffer (20 mM Tris-HCl pH 7.0, 1 mM EDTA, 1 mM EGTA, 10 mM Benzamidine, 1 mM PMSF, 0.1 mM of TPCK, and 0.1% 2-mercaptoethanol) for preclearing. It was then spun down at 10,000 × g for 1 min and the supernatant was incubated with 5 μg of the PP1γ2, PPP1R7, or PPP1R11 antibody or diluted rabbit pre-immune serum as a negative control, overnight with gentle rocking at 4°C. Following day, Protein G-Sepharose 4 Fast Flow beads (GE Healthcare) were washed once with distilled water and twice with homogenization buffer. Each extract/antibody solution was incubated with the beads by rocking for 2 h at 4°C. After incubation, the beads were washed five times with 1X TTBS. After washing, the beads were resuspended in 2X SDS reducing sample buffer (6% SDS, 25 mM Tris-HCl pH 6.5, 50 mM DTT, 10% glycerol and bromophenol blue), boiled for 10 min and centrifuged at 10,000 × g for 10 min. Supernatants were separated by SDS-PAGE, followed by western blot analysis.
In vitro fertilization
Two- to three-month-old females were injected intraperitoneally with 5 IU of pregnant mare's serum gonadotropin hormone. After exactly 52 h, the females were injected intraperitoneally with 5 IU of human chronic gonadotropin (hCG) hormone. In the next morning, 3- to6-month-old C57/BL6J WT, Gsk3α (+/–) and Gsk3α (–/–) male mice were sacrificed and caudal spermatozoa was isolated in HTF medium. The sperm then incubated for 1 h in 5% CO2 and 37°C. After 14 h from hCG injection, the females were sacrificed and the oviducts and ovaries were removed and placed on PBS media. Using the stereomicroscope, the fat tissues were removed from the oviducts and ovaries. The tissues were immersed in mineral oil that covers the fertilization dish. Using a dissecting needle, the ampulla was teared to release the cumulous-oocyte complexes (COCs) and the COCs were dragged from the mineral oil into 235 μl drop of HTF medium. After 1 h from the capacitation, 15 μl of sperm collection were transferred into the fertilization dish that has COCs and incubated in 5% CO2 and 37°C for 4 h. After 4 h, the eggs were moved from the fertilization drop using a mouth pipette into three wash drops and incubated again in 5% CO2 and 37°C overnight. In the next morning, the two-cell-stage embryos were counted and the percentage of fertility rate was calculated.
Results
Expression and localization of GSK3α and GSK3β in testis
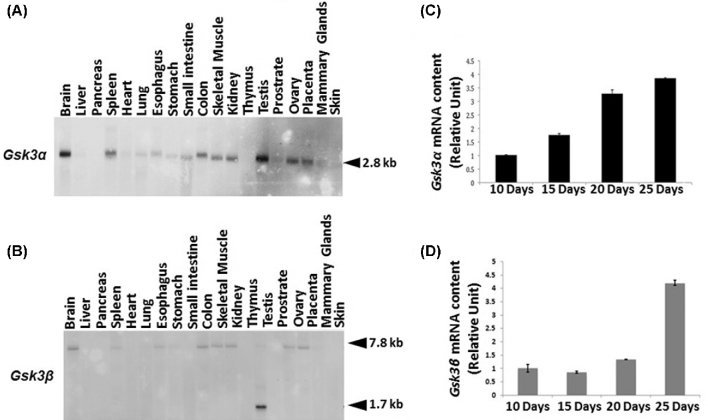
We have previously shown that both isoforms of GSK3 are present in sperm [11]. Presence of the enzymes in rat testis is documented [18]. However, expression and localization of the two isoforms in mouse testis is not known. We therefore determined mRNA expression and protein localization of the two isoforms in testis. Northern blot analysis of RNA from various mouse tissues was performed (Figure 1A and B). Messenger RNA for GSK3α (2.8 kb) is highest in testis and brain and present at lower levels in other tissues. Messenger RNA for GSK3β at 7.8 kb is present in several tissues confirming earlier data [19]. In addition, high levels of an mRNA of 1.7 kb is present exclusively in testis. The basis for the 1.7 kb mRNA seen in testis is not known. Quantitative PCR analysis of mRNA expression of the two GSK3 isoforms was performed in postnatal developing testis (Figure 1C and D). Levels of mRNA for both isoforms increased starting with 10-day old postnatal testis, reaching a maximum by day 20, coinciding with spermiogenesis and release of sperm into the lumen. This pattern of expression is seen for mRNAs for several testis and sperm proteins [20].
Figure 1.
(A, B) Northern blot analysis of multiple mouse tissue RNA shows messages for GSK3αand β are highest in testis. (C, D) Quantitative PCR showing increasing Gsk3α and Gsk3β mRNA levels in postnatal developing testis. The results are represented as fold change after normalizing the Gsk3α and Gsk3β mRNA levels with Gapdh mRNA. These data are representative of three independent experiments in triplicates, and error bars represent SE.
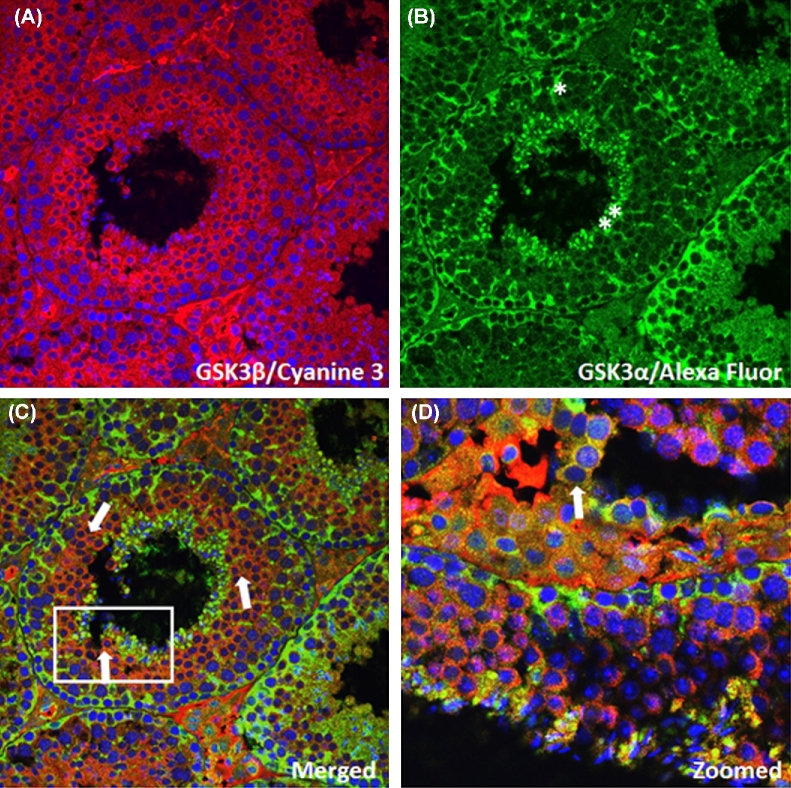
Immunofluorescence was performed with sections of adult mouse testis probed with GSK3α and GSK3β rabbit monoclonal antibodies (Figure 2A–D). This staining showed GSK3α expression predominantly in round and elongated spermatids and spermatozoa. GSK3α was also present in Sertoli cells, seen as spoke-like structures emanating from the periphery to the lumen (Figure 2A). Sertoli cells branch through germ cells at various stages of development [21, 22]. GSK3β staining was not seen in Sertoli cells, whereas it was present in secondary spermatocytes and spermatids (Figure 2B). Merged and magnified pictures show co-localization of GSK3α and GSK3β in secondary spermatocytes and spermatids (Figure 2C and D). There was an overlap in the spatial and temporal expression patterns of the two GSK3 isoforms in testis especially during the final stages of sperm formation.
Figure 2.
(A–D) Immunostaining of wild-type testis section with GSK3α and GSK3β monoclonal antibodies. (A) GSK3β expression using GSK3β monoclonal antibody; (B) GSK3α expression using GSK3α monoclonal antibody; (C) merged picture showing overlapping expression of GSK3α and GSK3β in testis; (D) zoomed picture showing overlapping expression of GSK3α and GSK3β during development of spermatozoa.
Loss of GSK3α but not GSK3β in sperm results in male infertility
The finding of male infertility in mice lacking GSK3α was surprising, given the large redundancy in function of GSK3 isoforms. We considered that GSK3 isoforms may also be functionally interchangeable in sperm but that there is requirement for a higher threshold level of GSK3 catalytic activity for normal sperm function. That is, loss of both alleles of Gsk3β or the loss of one allele of each of the two isoforms may also result in male infertility. Furthermore, it was possible that infertility could be due to global loss of GSK3α rather than due to its absence in testis or sperm. Conditional testis-specific KO of the GSK3 isoforms was required to examine these possibilities. A Cre-LoxP approach was used to inactivate each of GSK3 isoforms specifically in testicular germ cells [23, 24]. Mice with floxed Gsk3α or β alleles were crossed with mice expressing Cre directed by the Stra 8 promoter. Testis expression of Cre in Stra8-Cre mice is documented to occur in differentiating secondary spermatocytes onwards [21, 25]. We found that male mice lacking Gsk3α in testis [Gsk3α (–/ΔFl)] are infertile, similar to the global GSK3α null mice (Table 2). Except for male infertility there was no other observable phenotype in mice lacking GSK3α in testis. Mice lacking GSK3β in testis [Gsk3α (+/+) Gsk3β(–/ΔFl)] were fertile. Mice lacking one allele of Gsk3α [Gsk3α (+/–) Gsk3β (+/+)], and mice lacking one allele each of Gsk3α and β [Gsk3α (+/–) Gsk3β (+/–)] were also all fertile. Hence, male infertility results either from a global or testis-specific loss of GSK3α. Sperm from mice lacking GSK3α could not also fertilize in vitro. In the IVF experiment, we present the result for C57/BL6 WT, Gsk3α (+/–), and Gsk3α (–/–). For each experiment, we used three C57/BL6 WT females with one male. The fertilization rate using C57/BL6 WT male was 91.7% with a total number of fertilized eggs 78 out of 85. The fertilization rates for Gsk3α (+/–) and Gsk3α (–/–) were 81.4% and 0%, respectively. The total number of fertilized eggs using Gsk3α (+/–) male was 166 out of 204, while it was 0 out of 245 using Gsk3α (–/–) males suggesting that infertility of the GSK3α null male mice was not due to impaired sperm transport through the female reproductive tract (Supplemental Figure S1).
Table 2.
Fertility data of global Gsk3α KO, testis-specific Gsk3α KO, testis-specific Gsk3β KO.
| Mouse lines | Number of males tested for fertility (n) | Avg. age of mice (days) | Time of mating tests (weeks) | Number of males fertile | Number of litters | Avg. litter size | Fertility status |
|---|---|---|---|---|---|---|---|
| Gsk3α(–/–)β(+/+) | 18 | 60 | 4 | 0 | 0 | N/A | Infertile |
| Gsk3α(–/–)β(+/+) Cond. Gsk3α KO | 4 | 58 | 4 | 0 | 0 | N/A | Infertile |
| Gsk3α(+/+)β(–/–) Cond. Gsk3β KO | 5 | 56 | 4 | 5 | 5 | 6 | Fertile |
| Gsk3α(+/–)β(+/+) | 21 | 65 | 4 | 21 | 61 | 8 | Fertile |
| Gsk3α(+/–)β(+/−) | 3 | 62 | 4 | 3 | 3 | 5 | Fertile |
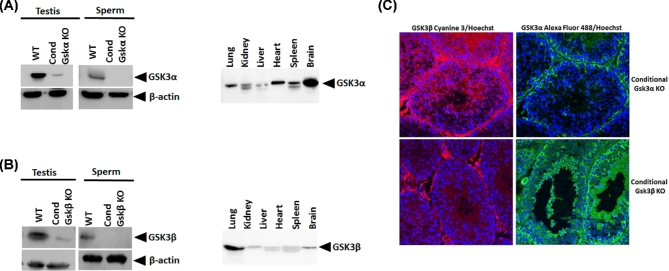
As anticipated, immunoblot analyses of testis and sperm extracts (Figure 3A and B) showed reduced levels of GSK3α or GSK3β in testis or their absence in sperm of conditional KO mice. Reduced, residual levels of GSK3 seen in immunoblots of testis extracts of conditional KO testis are likely from somatic cells and spermatogonia. Levels of GSK3α and GSK3β were normal in all tissues of conditional KO except in sperm where the respective GSK3 isoforms were absent. Lack of each of the respective GSK3 isoforms in developing germ cells in the conditional KO mice was also verified by immunohistochemistry of testis sections (Figure 3C).
Figure 3.
(A) Western blot results showing absence of GSK3α in Gsk3α conditional KO sperm compared to wild type. When compared with different other tissues of conditional Gsk3α KO, sperm shows complete absence of GSK3α. (B) Western blot comparing conditional Gsk3β KO and wild-type testis and sperm shows absence of GSK3β in KO sperm sample while very less amount of GSK3β expression in testis. When compared with other tissues of conditional Gsk3β KO sperm shows absence of Gsk3β in sperm. (C) Immunofluorescence result shows less expression of GSK3α in developing spermatids, while GSK3β is present in conditional Gsk3α KO testis section. For conditional GSK3β KO, there is no expression of GSK3β in testis section confirming the complete knockdown of GSK3β in germ cells.
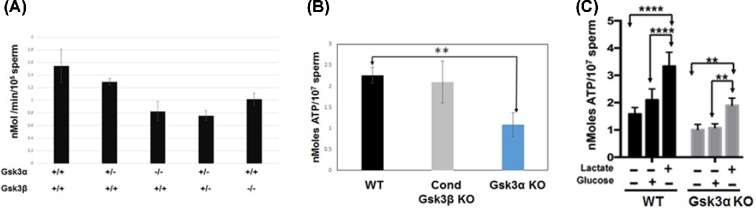
Next we considered the possibility that the requirement for GSK3α could be because of need for a net threshold GSK3 activity in sperm that GSK3β alone could not provide. We measured GSK3 activity in extracts of sperm from mice lacking GSK3α, GSK3β, or one allele each GSK3α and GSK3β. The activity was measured using GS peptide as substrate [10, 26]. GSK3 activity was reduced to 50% in sperm lacking two alleles of GSK3α or GSK3β or one allele each of the two isoforms compared to WT sperm (Figure 4A). This indicates that lowered total sperm GSK3 activity is likely not responsible for infertility of sperm lacking GSK3α.
Figure 4.
(A) GSK3 activity assay shows significantly reduced activity when one or both alleles of Gsk3α or Gsk3β are absent. Levels were measured using GS2 peptide as a substrate. Lithium-sensitive activity was considered due to GSK3. Unit activity is nmoles PO42− incorporated/min/105 sperm. Values are means ± SEM (n = 3). (B) ATP levels measurement with luciferase assay in conditional Gsk3β KO is almost comparable to WT mouse; however, in Gsk3αKO sperm the level is almost 50% less. (C) ATP assay using WT and Gsk3α KO sperm after treatment with substrates. There is significant decrease in ATP levels of Gsk3α KO sperm in both control and 5 mM glucose sample. However, after lactate treatment increase of ATP level in KO sperm is normal. Values expressed as percentage of control are means from five different experiments (n = 5). A nonparametric two-way ANOVA was used for comparison of all groups. *P < 0.05, **P, < 0.01, ***P < 0.001 defines significant differences between the groups.
ATP levels and hexokinase activity in sperm lacking GSK3 isoforms
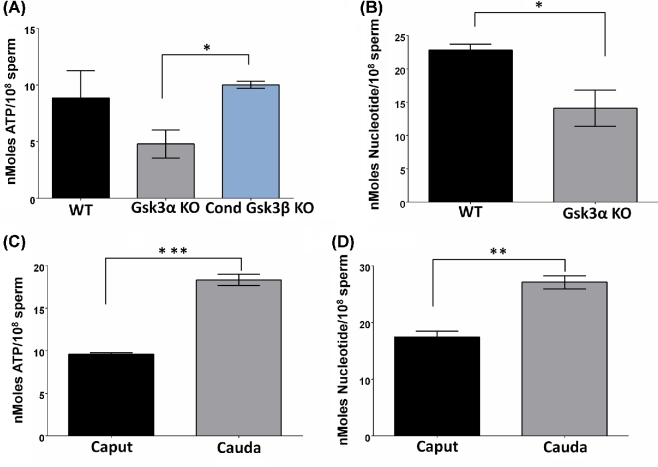
We previously showed that ATP levels in sperm from mice lacking GSK3α were about one half of that in sperm from WT mice [11]. We confirmed this observation here but also show that ATP levels in sperm lacking GSK3α rise in the presence of lactate but not with glucose as a metabolic substrate (Figure 4C) suggesting that ATP generation through glycolysis could be affected (Figure 4C). The levels of ATP in the presence of lactate or glucose were still lower in KO than in WT sperm (Figure 4C). ATP levels in sperm from mice lacking one allele each of GSK3α and β (Gsk3α+/–, Gsk3β+/–) were comparable to levels in WT sperm. ATP levels in sperm lacking GSK3β were also unaltered (Figure 4B). Reduction of ATP level occurred only upon loss of GSK3α in sperm. Reduced levels of ATP could result from its increased use and/or an impairment in its production. In either of these cases, the levels of ADP and AMP should be elevated when ATP levels are reduced. Levels of ATP determined by the luciferase assay (Figure 4B) were further verified by quantitation of adenine nucleotides by HPLC (Figure 5A). Contrary to expectation, both ADP and AMP levels were also lower in sperm from GSK3 null compared to WT mice. That is, net adenine nucleotide levels were lower in sperm lacking GSK3α (Figure 5B). Lowered ATP along with low ADP and AMP levels has previously been documented in caput epididymal sperm [27]. When compared to mature caudal sperm, immature caput sperm contained lower ATP (Figure 5C) (∼8 vs 18 nmoles) and also lower net adenine nucleotide levels (∼15 vs 30 nmoles) (Figure 5D).
Figure 5.
(A) Comparison of ATP levels in genetically GSK3α/β disrupted mice spermatozoa. Sperm cells (1 × 108/ml) were extracted using 0.05% chilled Perchloric acid and subsequently neutralized using KOH solution. All the data represent mean ± standard error (n = 3). (B) Effect of deletion of Gsk3α gene on relative Nucleotide (Ntd) Pool in mouse spermatozoa. All the data represent mean ± standard error (n = 3). (C) Comparison of ATP levels in bull caput and caudal spermatozoa. (D) Relative Nucleotide (Ntd) pool in bull caput and caudal spermatozoa. All the data represent mean ± standard error (n = 4). *P < 0.05, **P, < 0.01, ***P < 0.001 defines significant differences between the groups.
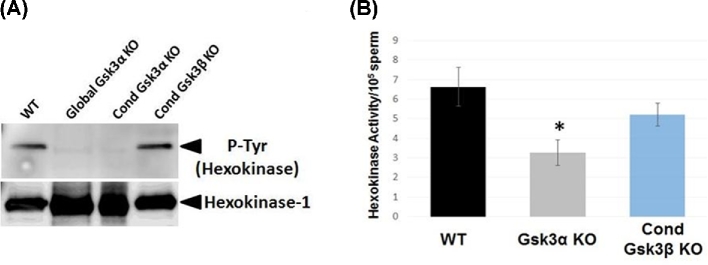
The band seen at 110 kDa with 4G10 antibodies is due to hexokinase which is constitutively tyrosine phosphorylated in mouse sperm [28]. Lack of tyrosine phosphorylation of the band thought to be hexokinase was one of the striking features of sperm lacking GSK3α. Sperm from testis-specific KO of GSK3α lack hexokinase phosphorylation similar to sperm from mice globally lacking GSK3α (Figure 6A). Hexokinase phosphorylation in sperm lacking GSK3β or sperm from mice lacking one allele each of GSK3α and β was unaltered and comparable to WT sperm. Since hexokinase was not phosphorylated, we examined if its activity was affected in sperm that lacked GSK3α. Hexokinase activity in extracts of sperm lacking GSK3α was 50% of that in WT sperm (Figure 6B). Hexokinase activity in sperm devoid of GSK3β was unaltered and comparable to WT sperm. These data, along with the data showing lower sperm ATP levels, suggest that glucose utilization could be impaired due to the absence of GSK3α in sperm.
Figure 6.
(A) Western blot probed with anti-phosphotyrosine mouse monoclonal antibody (4G10) shows absence of hexokinase phosphorylation in testis-specific Gsk3α KO sperm sample, whereas it is present in testis-specific Gsk3β KO sperm. Western blot with supernatant after 1X RIPA (modified) extraction from WT and global Gsk3a KO sperm shows absence of hexokinase phosphorylation in Gsk3α KO sperm. When probed with anti-Hexokinase rabbit monoclonal antibody for control, it shows presence of Hexokinase-1 in Gsk3α KO sperm. (B) Hexokinase activity measured in WT, Gsk3α KO, and conditional Gsk3β KO sperm shows almost 60% less activity in Gsk3α KO sperm, whereas in conditional Gsk3β KO hexokinase activity is almost same as WT. Values expressed as percentage of control are means from five different experiments (n = 5). A nonparametric two-way ANOVA was used for comparison of all groups. *P < 0.05 defines significant differences between the groups.
Changes in the binding of the protein phosphatase PP1γ2 in sperm lacking GSK3α
Reduced ATP and adenine nucleotide levels suggested that sperm maturation in the epididymis may have been affected in sperm lacking GSK3α. The association of PP1γ2 with its regulators (PPP1R2, R7, and R11) changes during epididymal sperm maturation [29]. In caput epididymal sperm, PP1γ2 is not bound to the regulators PPP1R2 and PPP1R7 but it is bound to PPP1R11. In mature caudal epididymal sperm, all three inhibitors are bound as heterodimeric or -trimeric complexes with PP1γ2. We examined the association status of PP1γ2 in caudal sperm from mice lacking GSK3α. PPP1R7 was not bound to PP1γ2, whereas PPP1R2 and PPP1R11 are bound to PP1γ2 in sperm from GSK3α KO mice (Figure 7). Lack of binding to PPP1R7 was verified by reciprocal IP with both PP1γ2 and PPP1R7 antibodies. All three regulators were bound to PP1γ2 in caudal epididymal sperm from WT mice (Figure 7). Thus, sperm from GSK3α KO mice resemble caput epididymal sperm with respect to lack of binding of PPP1R7 to PP1γ2.
Figure 7.
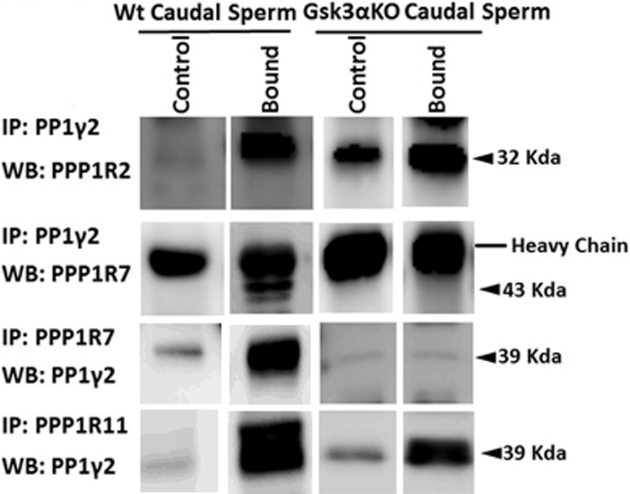
Co-immunoprecipitation of PP1γ2 and its regulators in WT and Gsk3α KO mice epididymal sperm. PP1γ2 is associated with PPP1R2, PPP1R7, and PPP1R11 in WT caudal sperm. In caput sperm, only PPP1R11 is associated with PP1γ2. A similar pattern was also observed in Gsk3α KO mouse epididymal sperm. Figure 7 shows that in caudal sperm from Gsk3α KO mice PPP1R7 is not bound to PP1γ2.
Intrasperm localization of GSK3α and GSK3β
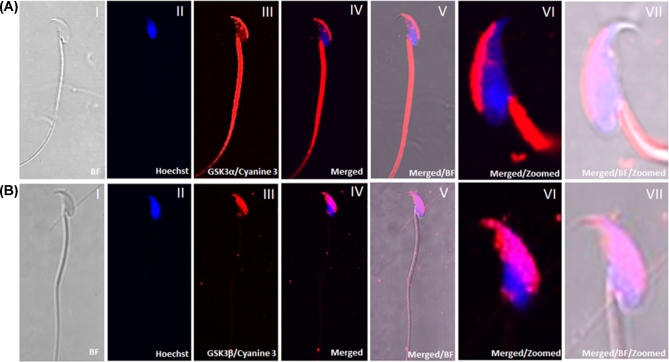
Immunostaining with GSK3α and GSK3β rabbit monoclonal antibody showed localization of the proteins in WT sperm (Figure 8). GSK3α is primarily localized to the acrosomal region in the head with additional staining in the tail, predominant in the mid-piece region. GSK3β was predominant in the equatorial and postacrosomal regions of the sperm head. GSK3β staining was absent or weak in the sperm mid-piece and the principal piece of the flagellum.
Figure 8.
Immunofluorescence using GSK3α and GSK3β monoclonal antibody shows expression of GSK3α (8A) and GSK3β (8B) in wild-type sperm. (A and B) (I) Bright field, (II) Hoechst/nucleus, (III) cyanine 3/GSK3α or GSK3β, (IV) merged, (VI) zoomed and merged with cyanine 3 to show the sperm head and Hoechst and (VII) zoomed and bright field merged with cyanine 3 and Hoechst. GSK3α is present in the tail and acrosomal region in the head. However, GSK3β is present in postacrosomal and equatorial region in head.
Discussion
In this study, we have conclusively shown that differentiating precursor sperm cells and mature sperm are unique in their requirement for GSK3α isoform for function. Lack of GSK3α, but not GSK3β, in differentiating spermatids and sperm results in male infertility. A possible reason for the lack of substitution of GSK3β for GSK3α could result from the isoforms having distinct spatio-temporal expression pattern. This possibility is unlikely in testis because both GSK3α and GSK3β share similar and overlapping expression (Figure 2) and both isoforms are present in developing postmeiotic cells. However, localization of GSK3α within sperm is distinct suggesting specific binding or targeting proteins for this isoform. It is notable that lack of GSK3β has no noticeable detrimental effect suggesting that GSK3α is able to compensate for the absence of GSK3β both in testis and sperm.
Reduced ATP levels in sperm lacking GSK3α is similar to sperm from targeted KOs of testis soluble adenylyl cyclase or sperm-specific PKA catalytic subunit where motility is compromised in mutant sperm [30]. It is not known whether low ATP levels in GSK3α null sperm is a consequence of lowered motility. Sperm hexokinase is constitutively tyrosine phosphorylated, a finding we corroborated in the GSK3β null or transheterozygous animals [28]. However, we found hexokinase hypophosphorylated on tyrosine in sperm lacking GSK3α. Sperm from GSK3α mice did not recover tyrosine phosphorylation after incubation for 1 h in buffer supporting capacitation. The absence of hexokinase phosphorylation is similar to that of sperm from t-complex mice, Septin-4 KO, and Tat-1 KO mice [31–33]. How mouse sperm hexokinase is phosphorylated and what is the role of this phosphorylation in glycolysis and ATP production are not known [34–36]. Hexokinase activity in sperm lacking GSK3α is reduced to 50% of that in WT sperm. It is possible that GSK3α is responsible for regulating hexokinase activity directly or indirectly by influencing its phosphorylation. Further studies are required to determine how GSK3α is related to hexokinase phosphorylation in mouse sperm.
A significant observation in this report is that sperm maturation in the epididymis appears compromised in GSK3α KO mice. First, ATP levels and levels of ADP and AMP are all lower in caudal sperm (Figure 5), a situation observed in immature caput epididymal sperm (Figure 5). Second, association of PP1γ2 with its regulators is altered in a manner that partially resembles immature caput epididymal sperm (Figure 7). Finally, high activity levels of PP1γ2 are similar to caput epididymal sperm [8, 37]. The change in association of PP1γ2 with PPP1R7 is likely a cause for elevated PP1γ2 catalytic activity. Thus, our data show that active GSK3α, followed by its inactivation, is required for normal sperm maturation in the epididymis. GSK3(α and β) is downstream of Wnt signaling. Recently, a role for GSK3 in epididymal sperm maturation was deduced in mice lacking Wnt signaling [5]. Taken together, these data support a key role for GSK3 along with the other key signaling enzymes PKA and PP1γ2 in development of motility and metabolism during sperm passage through the epididymis.
Isoform-specific binding of GSK3α and scaffold protein RACK1 is known. It has been shown that this interaction of RACK1 is with the N-terminal region of GSK3α [38, 39]. Despite losing its binding capacity with RACK1, deletion of N terminal region does not interfere with interaction of GSK3 with the Axin scaffold protein. We suggest that GSK3α has unique isoform-specific binding proteins in testis and spermatozoa. We are using yeast two hybrid, pull down using recombinant enzyme, and immunoprecipitation to identify these GSK3α binding proteins in sperm. The N-terminus of GSK3α is highly conserved in placental mammals. It is possible that specific binding is mediated through the conserved glycine-rich N-terminus of GSK3α [38]. These binding proteins may act as scaffolding proteins clustering GSK3α and its substrates.
In summary, our data demonstrate an isoform-specific requirement for GSK3α during final stages of spermatogenesis and in mature sperm. This requirement for GSK3α is unique compared to other tissues where the two isoforms of GSK3 are largely functionally interchangeable. Development of GSK3α-selective inhibitors may facilitate a male contraceptive. Identification of protein targets of GSK3α is also essential because mutations in these target proteins could be the basis for infertility due to impaired sperm function and maturation in the epididymis.
Supplementary data
Supplemental Figure S1. In vitro fertilization data shows sperm from Gsk3α (–/–) are unable to fertilize eggs whereas Gsk3α (+/+) and Gsk3α (+/–) show normal fertilization rate. All the data represent mean ± standard error (n = 3). ***P < 0.001 defines significant differences between the groups.
Notes
Edited by Dr. Jeremy P. Wang, MD, PhD, University of Pennsylvania
Footnotes
Grant support: This work is supported by National Institutes of Health grant R15 NIH HD068971 and R21 NIH HD086839 to Srinivasan Vijayaraghavan.
References
- 1. Kaidanovich-Beilin O, Woodgett JR. GSK-3: functional insights from cell biology and animal models. Front Mol Neurosci 2011; 4:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Medina M, Wandosell F. Deconstructing GSK-3: the fine regulation of its activity. Int J Alzheimer's Dis 2011; 2011:479249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res 2007; 32:577–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aparicio IM, Bragado MJ, Gil MC, Garcia-Herreros M, Gonzalez-Fernandez L, Tapia JA, Garcia-Marin LJ. Porcine sperm motility is regulated by serine phosphorylation of the glycogen synthase kinase-3alpha. Reproduction 2007; 134:435–444. [DOI] [PubMed] [Google Scholar]
- 5. Koch S, Acebron SP, Herbst J, Hatiboglu G, Niehrs C. Post-transcriptional Wnt signaling governs epididymal sperm maturation. Cell 2015; 163:1225–1236. [DOI] [PubMed] [Google Scholar]
- 6. Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature 2000; 406:86–90. [DOI] [PubMed] [Google Scholar]
- 7. McNeill H, Woodgett JR. When pathways collide: collaboration and connivance among signalling proteins in development. Nat Rev Mol Cell Biol 2010; 11:404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vijayaraghavan S, Stephens DT, Trautman K, Smith GD, Khatra B, da Cruz e Silva EF, Greengard P. Sperm motility development in the epididymis is associated with decreased glycogen synthase kinase-3 and protein phosphatase 1 activity. Biol Reprod 1996; 54:709–718. [DOI] [PubMed] [Google Scholar]
- 9. Smith GD, Wolf DP, Trautman KC, da Cruz e Silva EF, Greengard P, Vijayaraghavan S. Primate sperm contain protein phosphatase 1, a biochemical mediator of motility. Biol Reprod 1996; 54:719–727. [DOI] [PubMed] [Google Scholar]
- 10. Somanath PR, Jack SL, Vijayaraghavan S. Changes in sperm glycogen synthase kinase-3 serine phosphorylation and activity accompany motility initiation and stimulation. J Androl 2004; 25:605–617. [DOI] [PubMed] [Google Scholar]
- 11. Bhattacharjee R, Goswami S, Dudiki T, Popkie AP, Phiel CJ, Kline D, Vijayaraghavan S. Targeted disruption of glycogen synthase kinase 3a (Gsk3a) in mice affects sperm motility resulting in male infertility. Biol Reprod 2015; 92:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. da Rocha AM, Ding J, Slawny N, Wolf AM, Smith GD. Loss of glycogen synthase kinase 3 isoforms during murine oocyte growth induces offspring cardiac dysfunction. Biol Reprod 2015; 92:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ryves WJ, Fryer L, Dale T, Harwood AJ. An assay for glycogen synthase kinase 3 (GSK-3) for use in crude cell extracts. Anal Biochem 1998; 264:124–127. [DOI] [PubMed] [Google Scholar]
- 14. Nakamura N, Miranda-Vizuete A, Miki K, Mori C, Eddy EM. Cleavage of disulfide bonds in mouse spermatogenic cell-specific type 1 hexokinase isozyme is associated with increased hexokinase activity and initiation of sperm motility. Biol Reprod 2008; 79:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsao TS, Burcelin R, Charron MJ. Regulation of hexokinase II gene expression by glucose flux in skeletal muscle. J Biol Chem 1996; 271:14959–14963. [DOI] [PubMed] [Google Scholar]
- 16. Takei GL, Miyashiro D, Mukai C, Okuno M. Glycolysis plays an important role in energy transfer from the base to the distal end of the flagellum in mouse sperm. J Exp Biol 2014; 217:1876–1886. [DOI] [PubMed] [Google Scholar]
- 17. von Papen M, Gambaryan S, Schutz C, Geiger J. Determination of ATP and ADP secretion from human and mouse platelets by an HPLC assay. Transfus Med Hemother 2013; 40:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guo TB, Chan KC, Hakovirta H, Xiao Y, Toppari J, Mitchell AP, Salameh WA. Evidence for a role of glycogen synthase kinase-3beta in rodent spermatogenesis. J Androl 2003; 24:332–342. [DOI] [PubMed] [Google Scholar]
- 19. Yao HB, Shaw PC, Wong CC, Wan DC. Expression of glycogen synthase kinase-3 isoforms in mouse tissues and their transcription in the brain. J Chem Neuroanat 2002; 23:291–297. [DOI] [PubMed] [Google Scholar]
- 20. Lau KF, Miller CCJ, Anderton BH, Shaw PC. Expression analysis of glycogen synthase kinase-3 in human tissues. J Pept Res 1999; 54:85–91. [DOI] [PubMed] [Google Scholar]
- 21. Sinha N, Puri P, Nairn AC, Vijayaraghavan S. Selective ablation of Ppp1cc gene in testicular germ cells causes oligo-teratozoospermia and infertility in mice. Biol Reprod 2013; 89:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chakrabarti R, Kline D, Lu J, Orth J, Pilder S, Vijayaraghavan S. Analysis of Ppp1cc-null mice suggests a role for PP1gamma2 in sperm morphogenesis. Biol Reprod 2007; 76:992–1001. [DOI] [PubMed] [Google Scholar]
- 23. Bao JQ, Ma HY, Schuster A, Lin YM, Yan W. Incomplete cre-mediated excision leads to phenotypic differences between Stra8-iCre; Mov10l1(lox/lox) and Stra8-iCre; Mov10l1(lox/) mice. Genesis 2013; 51:481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu Q, Song R, Ortogero N, Zheng H, Evanoff R, Small CL, Griswold MD, Namekawa SH, Royo H, Turner JM, Yan W. The RNase III enzyme DROSHA is essential for microRNA production and spermatogenesis. J Biol Chem 2012; 287:25173–25190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sadate-Ngatchou PI, Payne CJ, Dearth AT, Braun RE. Cre recombinase activity specific to postnatal, premeiotic male germ cells in transgenic mice. genesis 2008; 46:738–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Markuns JF, Wojtaszewski JFP, Goodyear LJ. Insulin and exercise decrease glycogen synthase kinase-3 activity by different mechanisms in rat skeletal muscle. J Biol Chem 1999; 274:24896–24900. [DOI] [PubMed] [Google Scholar]
- 27. Hoskins DD, Munsterman D, Hall ML. The control of bovine sperm glycolysis during epididymal transit. Biol Reprod 1975; 12:566–572. [DOI] [PubMed] [Google Scholar]
- 28. Visconti PE, Ning X, Fornes MW, Alvarez JG, Stein P, Connors SA, Kopf GS. Cholesterol efflux-mediated signal transduction in mammalian sperm: cholesterol release signals an increase in protein tyrosine phosphorylation during mouse sperm capacitation. Dev Biol 1999; 214:429–443. [DOI] [PubMed] [Google Scholar]
- 29. Goswami Suranjana, Korrodi-Gregório Luís, Sinha Nilam, Bhutada Sumit, Bhattacharjee Rahul, Kline Douglas, Vijayaraghavan Srinivasan. Regulators of the protein phosphatase PP1g2, PPP1R2, PPP1R7, and PPP1R11, are involved in epididymal sperm maturation. Journal of Cellular Physiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, McKnight GS. Sperm-specific protein kinase A catalytic subunit Calpha2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci USA 2004; 101:13483–13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kissel H, Georgescu MM, Larisch S, Manova K, Hunnicutt GR, Steller H. The Sept4 septin locus is required for sperm terminal differentiation in mice. Dev Cell 2005; 8:353–364. [DOI] [PubMed] [Google Scholar]
- 32. Olds-Clarke P, Pilder SH, Visconti PE, Moss SB, Orth JM, Kopf GS. Sperm from mice carrying two t haplotypes do not possess a tyrosine phosphorylated form of hexokinase. Mol Reprod Dev 1996; 43:94–104. [DOI] [PubMed] [Google Scholar]
- 33. Toure A, Lhuillier P, Gossen JA, Kuil CW, Lhote D, Jegou B, Escalier D, Gacon G. The testis anion transporter 1 (Slc26a8) is required for sperm terminal differentiation and male fertility in the mouse. Hum Mol Genet 2007; 16:1783–1793. [DOI] [PubMed] [Google Scholar]
- 34. Nakamura N, Miranda-Vizuete A, Miki K, Mori C, Eddy EM. Cleavage of disulfide bonds in mouse spermatogenic cell-specific type 1 hexokinase isozyme is associated with increased hexokinase activity and initiation of sperm motility. Biol Reprod 2008; 79:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakamura N, Shibata H, O’Brien DA, Mori C, Eddy EM. Spermatogenic cell-specific type 1 hexokinase is the predominant hexokinase in sperm. Mol Reprod Dev 2008; 75:632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Travis AJ, Sui D, Riedel KD, Hofmann NR, Moss SB, Wilson JE, Kopf GS. A novel NH 2 -terminal, nonhydrophobic motif targets a male germ cell-specific hexokinase to the endoplasmic reticulum and plasma membrane. J Biol Chem 1999; 274:34467–34475. [DOI] [PubMed] [Google Scholar]
- 37. Smith GD, Wolf DP, Trautman KC, Vijayaraghavan S. Motility potential of macaque epididymal sperm: the role of protein phosphatase and glycogen synthase kinase-3 activities. J Androl 1999; 20:47–53. [PubMed] [Google Scholar]
- 38. Azoulay-Alfaguter I, Yaffe Y, Licht-Murava A, Urbanska M, Jaworski J, Pietrokovski S, Hirschberg K, Eldar-Finkelman H. Distinct molecular regulation of glycogen synthase kinase-3alpha isozyme controlled by its N-terminal region: functional role in calcium/calpain signaling. J Biol Chem 2011; 286:13470–13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zeidner LC, Buescher JL, Phiel CJ. A novel interaction between glycogen synthase kinase-3alpha (GSK-3alpha) and the scaffold protein receptor for activated C-kinase 1 (RACK1) regulates the circadian clock. Int J Biochem Mol Biol 2011; 2:318–327. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.