Abstract
We report here the pheromone of Megacyllene antennata (White) (Coleoptera: Cerambycidae), a species native to southwestern North America whose larvae feed in woody tissues of mesquite (Prosopis species; Fabaceae). Adult males sex-specifically produced a blend of eight common natural products, including the monoterpene alcohol (S)-α-terpineol; the monoterpenes (S)-limonene and terpinolene; the aromatic alcohols (R)-1-phenylethanol and 2-phenylethanol; and (E)-2-hexenol, (E)-2-hexenal, and 1-hexanol. Individual males produced the components in varying amounts, but (S)-α-terpineol and (E)-2-hexenal were always present and together constituted the majority of the blend. A synthetic reconstruction of the complete blend attracted both males and females of M. antennata during field bioassays, as did all subsets of the blend that included (S)-α-terpineol and (E)-2-hexenol. Adults were most strongly attracted to blends of the latter two compounds when in ratios approaching parity. Neither of the compounds were present in the bouquet of volatiles emitted by host plants of the larvae.
Keywords: longhorned beetle, mesquite borer, Sonoran desert, mate recognition, volatile attractant
Males of longhorned beetles in the genus Megacyllene have pores on the prothorax that, in other species in the subfamily Cerambycinae, are the source of hydroxyalkanones (ketols) and alkanediols that serve as aggregation-sex pheromones (sensu Cardé 2014) that attract both sexes (Ray et al. 2006, Millar and Hanks 2017). However, these chemicals appear to play varying roles in attraction for species in this genus. For example, the pheromone blend of the North American Megacyllene caryae (Gahan) includes both enantiomers of anti-2,3-hexanediol as minor components, but also (S)-2-methylbutan-1-ol and several compounds that are also common components of essential oils of plants, including (S)-(–)-limonene, 2-phenylethanol, (–)-α-terpineol, nerol, neral, and geranial (Lacey et al. 2008, Mitchell et al. 2012). Although adults of M. caryae are attracted to racemic anti-2,3-hexanediol (Millar et al. 2018), they also respond strongly to citral, an isomeric blend of neral and geranial, which was present in headspace extracts (e.g., Handley et al. 2015). In addition, adults of this species are attracted to racemic syn-2,3-hexanediol, despite its apparently not being produced by the beetles (Hanks and Millar 2013).
In contrast, the pheromone of the South American congener Megacyllene acuta (Germar) has only three components: (2S,3S)-2,3-hexanediol, (R)-3-hydroxyhexan-2-one, and (S)-2-methylbutan-1-ol (Silva et al. 2018). Additionally, its sympatric congener Megacyllene falsa (Chevrolat) is attracted by the blend of racemic 3-hydroxyhexan-2-one and 2-methylbutan-1-ol (Silva et al. 2018), indicating a pheromone of similar composition. Males of the North American Megacyllene robiniae (Forster) produce a blend of chemicals similar to that of M. acuta, including (2S,3R)-2,3-hexanediol, (R)-3-hydroxyhexan-2-one, and (R)-1-phenylethanol, but repeated field bioassays have shown that the adults are not attracted to a blend reconstructed from synthetic compounds (Hanks and Millar 2016).
We have further explored the chemical ecology of the genus Megacyllene by identifying the aggregation-sex pheromone of Megacyllene antennata (White) (Coleoptera: Cerambycidae), which is native to southwestern North America (Linsley 1964). This species is bivoltine in the area of our field studies, the Sonoran Desert, with adults emerging in early spring and summer (Craighead and Hofer 1921). Larvae of M. antennata bore within the sapwood and heartwood of mesquite trees (Prosopis spp.; Fabaceae), and commonly infest freshly cut mesquite wood (Linsley 1964). The species was historically an important pest in lumber operations, and the larvae can infest furniture and ornamental pieces constructed from mesquite wood (Craighead and Hofer 1921, Baker 1972). Here, we present evidence that male M. antennata produce neither the ketols nor diols associated with related cerambycine species, but instead an eight-component blend consisting of terpenoids, alcohols, and aldehydes.
Methods and Materials
Sources of Insects and Field Sites
Adult M. antennata were first captured for pheromone studies by blacklighting at a site in Sonora, Mexico, along Mexican Route 16 (‘SON’: 28.5752 lat., −109.6651 long.) on 8 July 2007. More specimens were captured during the targeted bioassays described below, which were conducted in Pima Co., AZ, in three residential neighborhoods in central Tucson (‘T1’: 32.24417, −110.92833; ‘T2’: 32.24583, −110.93389; ‘T3’: 32.25639, −110.94694), and two sites at the University of Arizona Santa Rita Experimental Range, ~13 km southeast of Green Valley (‘SRER1’: 31.79583, −110.87694, ‘SRER2’: 31.78694, −110.87111). Fabaceous host plants of M. antennata were present at all sites, including species of Prosopis in the Tucson neighborhoods, and thickets of velvet mesquite, Prosopis velutina Wooton, in the arroyos at SRER. Voucher specimens collected from bioassays were deposited with the University of Arizona Insect Collection, Tucson, AZ (UAIC #1114233–1114238).
Live males and females used for collection of headspace odors were housed separately in 0.03-m3 wire cages at room temperature (~22°C) and under natural light (11:13–14:10 [L:D] h), and provided 10% aqueous sucrose solution for moisture and nourishment (glass vial plugged with a cotton wick). Beetles were acclimated to the laboratory for 2–3 d prior to pheromone collections.
Sources of Chemicals
All compounds were purchased from commercial suppliers: (R)- and (S)-α-terpineol (both >98% pure), (E)-2-hexenol (96%), (E)-2-hexenal (98%), (R)- and (S)-limonene (both 96%), and 2-phenylethanol (98%) from Sigma-Aldrich (St. Louis, MO); (R)- and (S)-1-phenylethanol (both >97%) and 1-hexanol (99%) from Alfa Aesar (Ward Hill, MA); and terpinolene (85%) from TCI America (Portland, OR).
Analysis of Insect-Produced Compounds
Two male and one female M. antennata were collected from the SON site. Individuals were held in Mason canning jars (263 ml), with a small water-filled vial plugged with cotton to provide moisture. The jar lids were fitted with Swagelok fittings to allow attachment of air inlets and outlets. The outlet was fitted with a trap for volatiles consisting of a glass tube with a ~5-mm bed of activated charcoal (~50 mg; 100–200 mesh) held in place by glass wool plugs. Charcoal-filtered air was pulled through the jars (~300 ml/min), which were held under ambient laboratory temperature and light conditions. Collectors were changed daily and were eluted with 3 × 200-µl methylene chloride. The resulting extracts were analyzed at the University of California, Riverside by coupled gas chromatography-mass spectrometry using an HP 6890 gas chromatograph interfaced to an HP 5973 mass selective detector (Agilent, Santa Clara, CA). Analyses were carried out on a DB-5 column (30 m × 0.25 mm, 0.5 μm film thickness; J&W Scientific, Folsom, CA) programed from 40ºC for 1 min, 10ºC/min to 250ºC. Injections were made in splitless mode, with helium carrier gas, and the injector temperature was 250°C. The absolute configurations of chiral compounds were determined by analysis of extracts and authentic standards with an HP 5890 gas chromatograph equipped with a flame ionization detector, and fitted with a chiral stationary phase Cyclodex B column (30 m × 0.25 mm ID, J&W Scientific), programed from 50ºC for 1 min, 5ºC/min to 150ºC, injector temperature 100ºC. Analyses were repeated with a ramp of 2.5ºC/min if enantiomers did not completely separate. Absolute configurations were confirmed by coinjection of an aliquot of the insect extract with the appropriate standard.
An additional seven pheromone collections were conducted using beetles captured at field sites in Arizona, to estimate the ratios of the pheromone components. Similar headspace collection protocols were used, but chambers consisted of straight-walled glass jars (4 liters) that contained a few sheets of clean crumpled filter paper as a substrate to allow the beetles free movement and to provide a perch. We initially collected volatiles in three replicates from single individuals of both sexes, but later expanded our analysis to include representative aerations of grouped males (2, 3, and 10 individuals). Air was drawn through the jars by vacuum and purified on entry by activated charcoal granules in glass Pasteur pipettes. Volatiles were trapped in glass pipette cartridges containing the adsorbent polymer HayeSepQ (150 mg; Sigma-Aldrich) held in place with glass wool plugs. Loaded cartridges were rinsed with 1.5 ml dichloromethane to elute trapped volatiles, and the resulting extracts were concentrated to ~200 μl under a nitrogen stream, then analyzed with a Shimadzu GC-14A gas chromatograph with a flame ionization detector (Shimadzu Corp., Kyoto, Japan) fitted with a DB-5 column (30 m × 0.25 mm i.d., 0.25 μm film; Agilent). To confirm identifications, some samples were analyzed separately on a Focus GC coupled to a DSQII mass spectrometer (Thermo Fisher Scientific, Waltham, MA) fitted with an Rxi-5ms column (30 m × 0.25 mm i.d., 0.25 μm film; Restek, Bellefonte, PA). In each case, samples were injected splitless, the injector temperature was 250°C, and the oven was programed from 40°C for 1 min, 10°C/min to 220°C, and held for 3 min. Chemicals were identified by comparing their spectra and retention times with those of authentic standards and, when necessary, by coinjecting samples with standards. All collections of volatiles included system controls from jars without insects.
The volatile compounds collected from male M. antennata (see Results) also are produced by many species of plants (Matsui 2006, Degenhardt et al. 2009), which suggests that volatiles from host plants could potentially interfere with mate location. Thus, we sampled the volatiles released by damaged mesquite, looking specifically in the headspace for the pheromone components (S)-α-terpineol, (E)-2-hexenol, and (R)-1-phenylethanol (see Results). Volatiles were collected from leaves and cut branches of P. velutina using methods similar to those described above, but with each entire cut branch sealed in a plastic cooking bag (46 × 51 cm; True Liberty Bags, Windsor, CA). Air was pulled through bags (500 ml/min) after purification by passage through a pipette containing granular activated charcoal, which was inserted through the wall of the bag. Volatiles were collected on an adsorbent cartridge containing 150 mg HayeSepQ, which was inserted through the opposite wall of the bag. The pipettes were sealed to the bags with laboratory tape. Plant samples were collected from three P. velutina trees at the SRER2 site, and each sample was aerated separately.
Field Bioassays of Synthesized Compounds
Attraction of M. antennata to male-produced compounds was assessed with five independent bioassays that tested synthesized chemicals mixed to simulate the complete blend of beetle-produced volatiles, and subsets thereof. Lures were formulated based on the relative ratios of components in the first aerations of beetles from the SON site, unless stated otherwise (Table 1). We assessed attraction of beetles using black plastic cross-vane panel traps (AlphaScents, Portland, OR) that were coated with the lubricant fluoropolymer fluon (Northern Products, Woonsocket, RI; Graham et al. 2010). Traps were baited with polyethylene sachets (5.1 × 7.6 cm, Bagettes model 14770, Cousin Corp., Largo, FL) containing pheromone components dissolved in 1 ml of isopropanol, or a solvent control of 1 ml neat isopropanol. Traps were serviced daily, or every 2–3 d when inclement weather prevented access to field sites. During servicing, lures were switched between pairs of traps, or rotated along trap transects to control for positional effects. Lures were replaced when visibly depleted (7–10 d). Trap basins were also coated with fluon and filled with ~300 ml of water, which was also replaced when traps were serviced. Captured beetles soon became unresponsive in the water, but usually revived in the laboratory, and so could be used in subsequent experiments that measured rates of pheromone emission.
Table 1.
Relative proportions of volatile components found in an extract of headspace odors from males of M. antennata collected in Sonora, Mexico (as determined by peak area in chromatograms), and the quantities subsequently used to formulate synthetic lures
| Compound | Percent | Ratio | Lure (mg) |
|---|---|---|---|
| (S)-α-Terpineol | 41 | 100 | 50 |
| (E)-2-Hexenol | 27 | 66 | 33 |
| (S)-Limonene | 15 | 38 | 19 |
| (R)-1-Phenylethanol | 9 | 21.7 | 10.9 |
| (E)-2-Hexenal | 3 | 7 | 3.5 |
| Terpinolene | 3 | 7 | 3.5 |
| 1-Hexanol | 2 | 5.4 | 2.7 |
| 2-Phenylethanol | 0.5 | 1.5 | 0.8 |
Experiment 1 tested for attraction of beetles to the complete reconstruction of the blend of insect-produced volatiles. Two traps were placed ~10 m apart at each of the three Tucson sites, one baited with the complete blend and the other with a solvent control (Table 1). The experiment was conducted during the first of two annual emergences of M. antennata in 2014, 18 March to 4 April (mean high/low temperature 26/11°C, skies clear, no precipitation; The Weather Co., Atlanta, GA).
Experiments 2 and 3 were designed to be complementary, additive bioassays to assess the activity of the most consistent and abundant components in aeration extracts from males: (S)-α-terpineol, (E)-2-hexenol, and (R)-1-phenylethanol (see Results). In these experiments, (E)-2-hexenol was combined with two structurally similar six-carbon components also produced by males, (E)-2-hexenal and 1-hexanol (hereafter, this combination will be referred to as ‘C6-compounds’). Each experiment included five treatments containing 1) one dominant component, being either (S)-α-terpineol or C6-compounds, 2) that component plus (R)-1-phenylethanol, 3) both dominant components, 4) both dominant components plus (R)-1-phenylethanol, and 5) a solvent control. Experiment 2 focused on (S)-α-terpineol, whereas Experiment 3 focused on the C6-compounds. These experiments were conducted consecutively using five traps at the SRER1 site during the second emergence of adult beetles in 2014 (mean high/low temperature: 35/24°C, frequent thunderstorms, 15.2 cm precipitation; The Weather Co.), Experiment 2 during 17 July to 2 August and Experiment 3 during 2 to 26 August.
Experiment 4 was designed to confirm that (S)-α-terpineol and (E)-2-hexenol constituted a minimum attractive blend. At both the T1 and SRER1 sites, three traps were baited with one lure of (S)-α-terpineol, one lure of (E)-2-hexenol, or both lures together. Experiment 5 was conducted concurrently and assessed attraction of beetles to varying ratios of (S)-α-terpineol and (E)-2-hexenol. At each of the two SRER sites, five traps were baited with 10:1, 3:1, 1:1, 1:3, or 1:10 ratios of (S)-α-terpineol to (E)-2-hexenol, or a solvent control. Ratios were determined by mass, with 50 mg of one component mixed with 5, 17, or 50 mg of the other. Experiments 4 and 5 were conducted during the first emergence of adults in 2015 (mean high/low temperature across both experiments 26/11°C, skies usually clear, 2.3 cm total precipitation; The Weather Co.), Experiment 4 during 5 February to 12 March, and Experiment 5 during 10 February to 5 April.
Statistics
Treatment effects on numbers of beetles caught per replicate were tested using the nonparametric Friedman’s test blocked by site (R 3.4.2, muStat package; Wittkowski and Song 2012), because the numerous zero values violated ANOVA assumptions of normality (Sokal and Rohlf 1995). Each replicate consisted of a single collection period at a single study site, with each period spanning 1–3 d (see Field Bioassays of Synthesized Compounds). Replicates that contained no specimens in any treatment were not included in analyses. Pairs of treatments were compared using the native function in R for multiple pairwise Wilcoxon rank-sum tests, with familywise error controlled by the false discovery rate option (Benjamini and Hochberg 1995, R Core Team 2017). Within each experiment, we tested for deviations from the expected 1:1 sex ratio of captured beetles with the chi-square test.
Results
Analysis of Insect-Produced Compounds
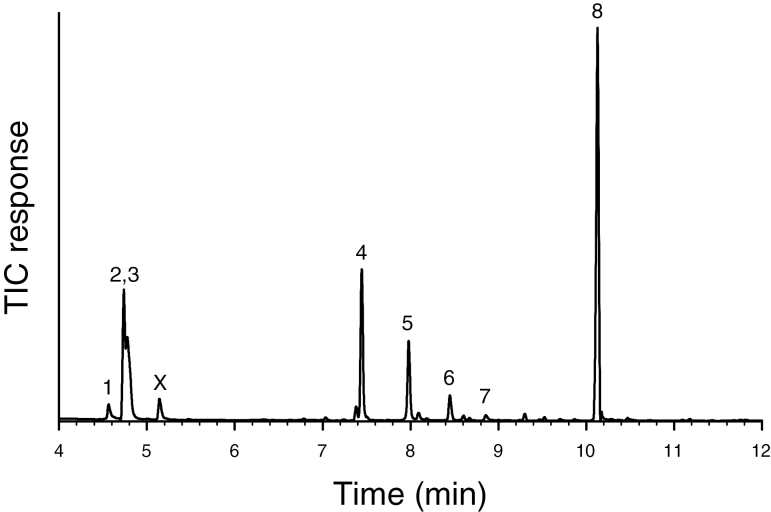
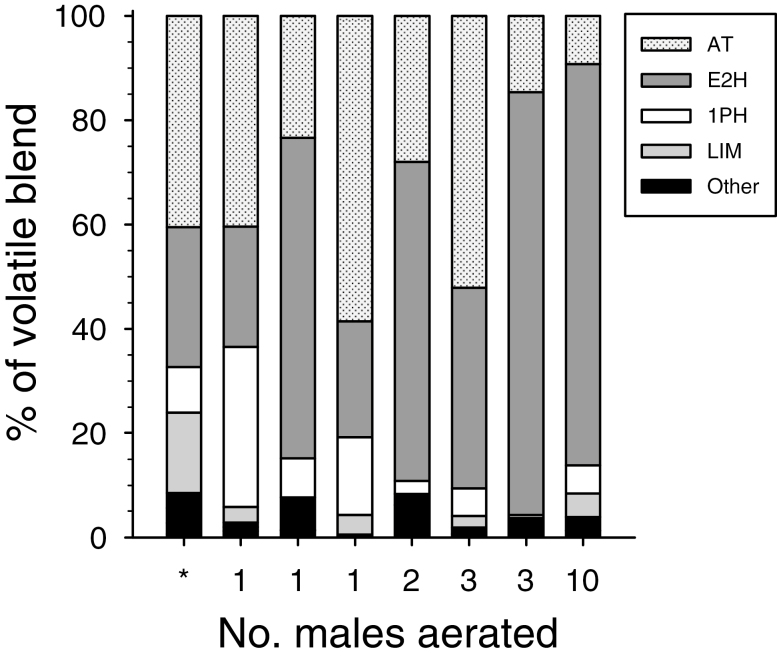
Volatiles produced by the males of M. antennata from Sonora, Mexico, included eight common natural products, including the monoterpene alcohol (S)-α-terpineol, the monoterpenes (S)-limonene and terpinolene, the aromatic alcohols (R)-1-phenylethanol and 2-phenylethanol, and the short-chain compounds (E)-2-hexenol, (E)-2-hexenal, and 1-hexanol (Table 1, Fig. 1). These compounds were tentatively identified by matching their mass spectra with database spectra, and then identifications were confirmed by matching their retention times and mass spectra to those of authentic standards. None of these chemicals were found in corresponding headspace collections from female beetles, nor from system controls, and we observed no evidence of the pheromone components associated with many species of cerambycines (i.e., 3-hydroxyalkan-2-ones, 2,3-alkanediols) at their predicted retention times (conservative estimate of detection limit, 0.5 ng). About two-thirds of the blend was composed of (S)-α-terpineol and (E)-2-hexenol in a relative proportion of 3:2 (Fig. 2).
Fig. 1.
Representative total ion chromatogram of headspace volatiles from adult males of M. antennata collected in Sonora, Mexico (DB-5 GC column). Compound identifications: 1) (E)-2-hexenal; 2) and 3) (E)-2-hexenol and hexanol; 4) limonene; 5) 1-phenylethanol; 6) terpinolene; 7) 2-phenylethanol; and 8) (S)-α-terpineol. Peak X is a contaminant.
Fig. 2.
Relative percent of volatile components in aeration extracts from three individual adult males of M. antennata, and from groups of 2, 3, and 10 males as determined by peak area in chromatograms. The bar marked with an asterisk represents the original analysis of aerations from two males collected in Sonora, Mexico, the ratios of which were used in formulating the blend used in trap lures (Table 1). The remaining bars represent analyses of extracts from beetles collected at sites in Arizona. AT: (S)-α-terpineol; E2H: (E)-2-hexenol; 1PH: (R)-1-phenylethanol; LIM: (S)-limonene; Other: (E)-2-hexenal, terpinolene, 1-hexanol, and 2-phenylethanol combined.
Extracts of volatiles produced by beetles collected at the Tucson sites also contained (S)-α-terpineol and (E)-2-hexenol, but in ratios that ranged from 2.5:1 to 1:10 (Fig. 2). 1-Phenylethanol was present in every aeration, but also in variable ratios (Fig. 2). The remaining components constituted <10% of the blends, and terpinolene was present only in the original extracts of the beetles from Sonora. Lastly, headspace odors of leaves and branches of P. velutina contained numerous common plant volatiles, but none of these matched the retention times of the components in the samples of volatiles produced by male M. antennata (Supplementary Fig. S1).
Field Bioassays of Synthesized Compounds
In total, 144 adult males and females of M. antennata were captured during the five field bioassays, with 37, 30, 26, 27, and 24 beetles caught in Experiments 1–5, respectively. The sex ratio deviated significantly from the expected 1:1 ratio only in Experiment 1 (81% female; χ2 = 7.2, P < 0.001). No other cerambycid species were trapped during the experiments, except for one beetle in Experiment 5 that was tentatively identified as a species of Anelaphus.
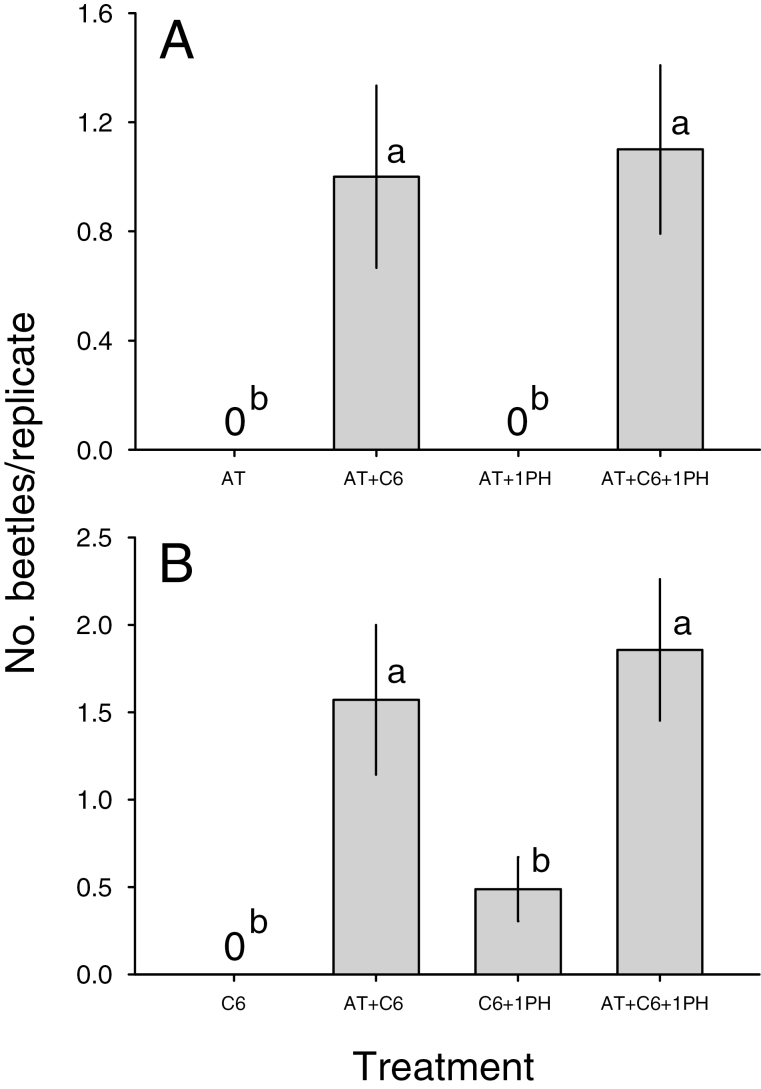
In Experiment 1, beetles were captured only by the trap baited with the complete pheromone blend, with a mean (±SE) of 1.1 ± 0.08 beetles/replicate compared with zero beetles in control traps (treatments significantly different; Friedman’s Q1,31 = 47.1, P < 0.001). In Experiments 2 and 3, beetles were only captured by traps baited with lures that included both (S)-α-terpineol and the C6-compounds (Fig. 3).
Fig. 3.
Mean (±SE) numbers of adults of M. antennata captured by traps baited with synthesized components of the male-produced pheromone during (A) Experiment 2 (treatments significantly different; Friedman’s Q3,9 = 16.8, P < 0.001) and (B) Experiment 3 (Q3,7 = 16, P = 0.001). Lures were formulated using the proportions of components shown in Table 1. Treatments within figures with different letters are significantly different (multiple Wilcoxon test, FDR-adjusted P < 0.05). AT = (S)-α-terpineol; C6 = blend of (E)-2-hexenol + (E)-2-hexenal + 1-hexanol; 1PH = (R)-1-phenylethanol.
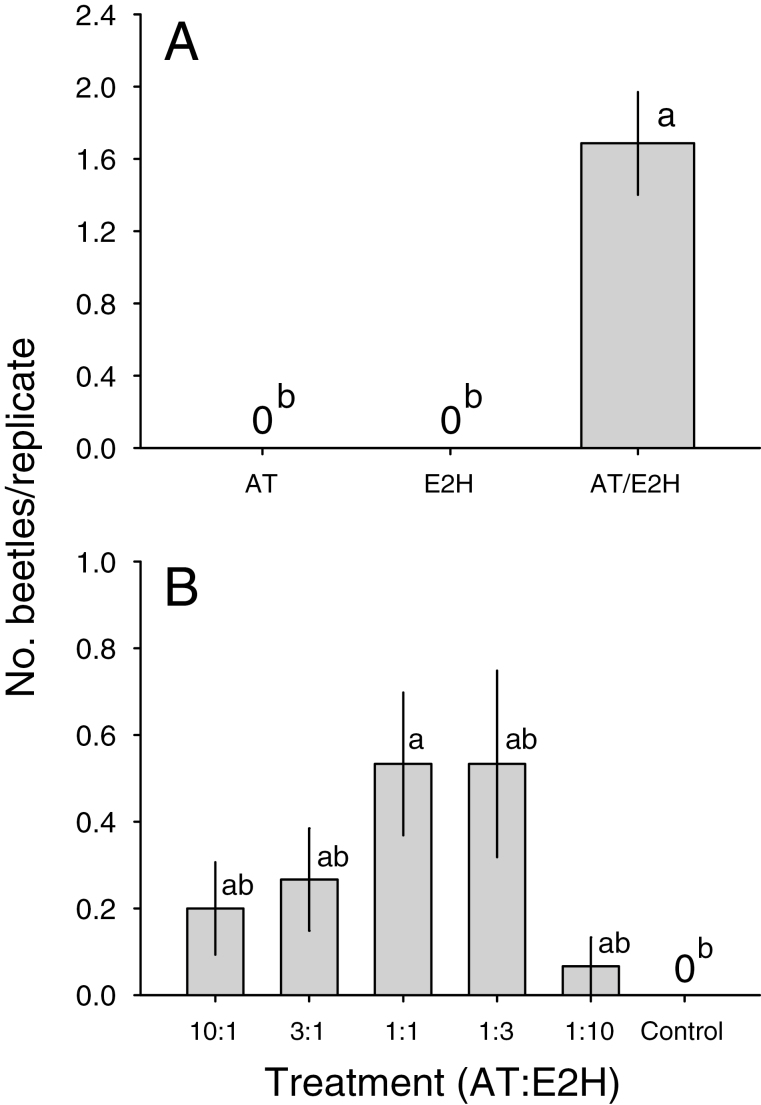
Experiment 4 confirmed that beetles were attracted by the combination of (E)-2-hexenol and (S)-α-terpineol, but not to the individual components (Fig. 4A). In Experiment 5, which tested ratios of (S)-α-terpineol to (E)-2-hexenol varying from 10:1 to 1:10, only the mean for the 1:1 treatment was significantly different from the control (Fig. 4B).
Fig. 4.
Mean (±SE) numbers of adults of M. antennata captured by traps baited with synthesized components of the male-produced pheromone, (S)-α-terpineol (‘AT’) and (E)-2-hexenol (‘E2H’), during (A) Experiment 4, baited with proportions following Table 1 (treatments significantly different; Friedman’s Q2,16 = 43, P < 0.001), and (B) Experiment 5 (marginally significant; Q4,15 = 11.0, P = 0.06), baited with varying proportions of AT to E2H. Treatments within figures with different letters are significantly different (multiple Wilcoxon test, FDR-adjusted P < 0.05).
Discussion
The experiments described above suggest that male M. antennata produce an aggregation-sex pheromone with a complex, but variable composition: only the compounds (S)-α-terpineol, (E)-2-hexenol, and (R)-1-phenylethanol were present in all extracts of headspace odors collected from males. The first two compounds together were dominant in all extracts, but again in highly variable ratios. Consistent with these findings, (S)-α-terpineol and (E)-2-hexenol comprised the minimum attractive blend during field bioassays, which was attractive at ratios approaching parity (i.e., 3:2 and 1:1). Nevertheless, beetles were captured by every trap baited with both (S)-α-terpineol and (E)-2-hexenol, and never in a solvent control. This suggests that attraction is not tightly coupled to the ratio of components, but it is important to note that the release rate of our lures under field conditions likely differs from the ratios loaded into the lure.
The behavioral function of the remaining components is uncertain. One possibility is that they serve to enhance the pheromone signal or inhibit the attraction of heterospecifics, as is the case for minor pheromone components produced by males of other cerambycid species (Mitchell et al. 2015). Thus, further research will be necessary to compare the full blend with its active subsets. The variable composition and ratios also suggest that the pheromone may be subject to regional variation; for example, terpinolene was only found in extracts from males of the population from Sonora, Mexico. However, both terpinolene and (S)-limonene could be degradation products of (S)-α-terpineol, which contains a tertiary alcohol that can readily eliminate water to produce either of these compounds.
The pheromone of M. antennata appears to be unusual in that it lacks the hydroxyketones or alkanediols that have been considered ‘signature’ compounds of the Cerambycinae (Millar and Hanks 2017). Although other species of Megacyllene emit some of the same alcohols and terpenoids observed here, they also include enantiomers of 3-hydroxyhexan-2-one or at least one isomer of 2,3-hexanediol in their blends (Lacey et al. 2008, Hanks and Millar 2016, Silva et al. 2018). In fact, these compounds have been identified from all of the more than 30 species of the tribe Clytini for which pheromones have been at least tentatively identified (Millar and Hanks 2017). In contrast, the pheromone produced by M. antennata appears to be composed of compounds which are typically associated with floral and green leaf volatiles. Moreover, the dominant compounds, (S)-α-terpineol and (E)-2-hexenol, would seem to be unlikely pheromone components because both are apparently ubiquitous in nature (El-Sayed 2018), which would seem to create opportunities for interference with the pheromone signal. However, our analyses of the volatiles of P. velutina showed that the host plant does not emit these two compounds from branches or leaves, a finding corroborated by other studies (Jardine et al. 2012). Thus, if adult males of M. antennata call near host material, the presence of (S)-α-terpineol and (E)-2-hexenol in combination with mesquite volatiles may comprise an unambiguous signal to conspecifics.
Terpenoids and green leaf volatiles are also present in the pheromones of numerous other insects, such as the pentatomid bug Podisus maculiventris (Say), males of which produce a pheromone composed of (R)-α-terpineol and (E)-2-hexenal that attracts both sexes (Aldrich 1988a,b). De novo terpenoid biosynthesis has been suggested to be rare in insects (cf. Scolytinae; Blomquist et al. 2010, Beran et al. 2016), and it is likely that most terpenoids isolated from insects are derived from isoprenoids in the diet of larvae or adults (e.g., Thompson and Mitlin 1979). Alternatively, terpenoid pheromone components could be partly or wholly produced by microbial symbionts (Yamada et al. 2015).
In summary, we have presented analytical and behavioral evidence that males of M. antennata produce an aggregation-sex pheromone that includes a synergistic blend of the monoterpene alcohol (S)-α-terpineol and the short-chain alcohol (E)-2-hexenol. Six other sex-specific compounds were detected in headspace extracts from males, but their functions have not yet been determined, and at least two of these compounds, limonene and terpinolene, may be artifacts from degradation of (S)-α-terpineol. Research to date confirms that the genus Megacyllene shows an unexpected shift in its pheromone chemistry in comparison to related species. Further work will be necessary to describe the evolutionary transition to terpene pheromones, their biosynthesis, and the extent of their production within the Cerambycidae.
Supplementary Material
Acknowledgments
We thank Jennifer Koop, David Koop, Paul Nabity, Barney Streit, Eugenio Nearns, and Ian Swift for assistance in field collections, Kevin Crawford for GCMS technical support, and Mitch McClaran, Mark Heitlinger, and the University of Arizona Santa Rita Experimental Range for access to field sites. Insect specimens from Mexico were collected under permit FAUT0097, Secretaría de Medio Ambiente y Recursos Naturales. This research was supported by National Institutes of Health postdoctoral training grant (5K12 GM000708-15 to R.F.M.), the Alphawood Foundation of Chicago (to L.M.H.), and the National Research Initiative of the U. S. Department of Agriculture Cooperative State Research, Education and Extension Service Grant (2006-35302-17457 to J.G.M. and L.M.H.).
References Cited
- Aldrich J. R. 1988a. Chemical ecology of the Heteroptera. Annu. Rev. Entomol. 33: 211–238. [Google Scholar]
- Aldrich J. R. 1988b. Chemistry and biological activity of pentatomoid sex pheromones, pp. 417–431. In H. G., Cutler (ed.), Biologically active natural products: potential use in agriculture. ACS Symposium Series No. 380, Washington, DC. [Google Scholar]
- Baker W. L. 1972. Eastern forest insects. USDA For. Serv. Misc. Pub. 1175: 178–179. [Google Scholar]
- Benjamini Y., and Hochberg Y.. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Royal Stat. Soc. B. 57: 289–300. [Google Scholar]
- Beran F., P., Rahfeld K., Luck R., Nagel H., Vogel N., Wielsch S., Irmisch S., Ramasamy J., Gershenzon D. G., Heckel, et al. 2016. Novel family of terpene synthases evolved from trans-isoprenyl diphosphate synthases in a flea beetle. Proc. Natl. Acad. Sci. U. S. A. 113: 2922–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomquist G. J., R. Figueroa-Teran M. Aw M. Song A. Gorzalski N. L. Abbott E. Chang, and Tittiger C.. 2010. Pheromone production in bark beetles. Insect Biochem. Mol. Biol. 40: 699–712. [DOI] [PubMed] [Google Scholar]
- Cardé R. T. 2014. Defining attraction and aggregation pheromones: teleological versus functional perspectives. J. Chem. Ecol. 40: 519–520. [DOI] [PubMed] [Google Scholar]
- Craighead F. C., and Hofer G.. 1921. Protection of mesquite cordwood and posts from borers. USDA Farmers Bull. 1197: 1–12. [Google Scholar]
- Degenhardt J., T. G. Köllner, and Gershenzon J.. 2009. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochem. 70: 1621–1637. [DOI] [PubMed] [Google Scholar]
- El-Sayed A. M. 2018. The Pherobase: database of pheromones and semiochemicals http://www.pherobase.com (accessed 29 March 2018).
- Graham E. E., R. F. Mitchell P. F. Reagel J. D. Barbour J. G. Millar, and Hanks L. M.. 2010. Treating panel traps with a fluoropolymer enhances their efficiency in capturing cerambycid beetles. J. Econ. Entomol. 103: 641–647. [DOI] [PubMed] [Google Scholar]
- Handley K., Hough-Goldstein J., Hanks L. M., Millar J. G., and D’Amico V.. 2015. Species richness and phenology of cerambycid beetles in urban forest fragments of northern Delaware. Ann. Entomol. Soc. Am. 108: 251–262. [Google Scholar]
- Hanks L. M., and Millar J. G.. 2013. Field bioassays of cerambycid pheromones reveal widespread parsimony of pheromone structures, enhancement by host plant volatiles, and antagonism by components from heterospecifics. Chemoecology. 23: 21–44. [Google Scholar]
- Hanks L. M. and Millar J. G.. 2016. Sex and aggregation-sex pheromones of cerambycid beetles: basic science and practical applications. J. Chem. Ecol. 42: 631–654. [DOI] [PubMed] [Google Scholar]
- Jardine K., G. A., Barron-Gafford J. P., Norman L., Abrell R. K., Monson K. T., Meyers M., Pavao-Zuckerman K., Dontsova E., Kleist C., Werner, et al. 2012. Green leaf volatiles and oxygenated metabolite emission bursts from mesquite branches following light-dark transitions. Photosynth. Res. 113: 321–333. [DOI] [PubMed] [Google Scholar]
- Lacey E. S., J. A. Moreira J. G. Millar, and Hanks L. M.. 2008. A male-produced aggregation pheromone blend consisting of alkanediols, terpenoids, and an aromatic alcohol from the cerambycid beetle Megacyllene caryae. J. Chem. Ecol. 34: 408–417. [DOI] [PubMed] [Google Scholar]
- Linsley E. G. 1964. The Cerambycidae of North America, Part V: taxonomy and classification of the subfamily Cerambycinae, Tribes Callichromini through Ancylocerini. Univ. Calif. Publ. Entomol. 22: 1–197. [Google Scholar]
- Matsui K. 2006. Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 9: 274–280. [DOI] [PubMed] [Google Scholar]
- Millar J. G., and Hanks L. M.. 2017. Chemical ecology of cerambycids, pp. 161–208. In Q., Wang (ed.), Cerambycidae of the world: biology and pest management. CRC Press/Taylor & Francis, Boca Raton, FL. [Google Scholar]
- Millar J. G., R. F., Mitchell J. A., Mongold-Diers Y., Zou C. E., Bográn M. K., Fierke M. D., Ginzel C. W., Johnson J. R., Meeker T. M., Poland, et al. 2018. Identifying possible pheromones of cerambycid beetles by field testing known pheromone components in four widely separated regions of the United States. J. Econ. Entomol. 111: 252–259. [DOI] [PubMed] [Google Scholar]
- Mitchell R. F., D. T. Hughes C. W. Luetje J. G. Millar F. Soriano-Agatón L. M. Hanks, and Robertson H. M.. 2012. Sequencing and characterizing odorant receptors of the cerambycid beetle Megacyllene caryae. Insect Biochem. Mol. Biol. 42: 499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R. F., P. F. Reagel J. C. Wong L. R. Meier W. D. Silva J. Mongold-Diers J. G. Millar, and Hanks L. M.. 2015. Cerambycid beetle species with similar pheromones are segregated by phenology and minor pheromone components. J. Chem. Ecol. 41: 431–440. [DOI] [PubMed] [Google Scholar]
- R Core Team.. 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. [Google Scholar]
- Ray A. M., E. S. Lacey, and Hanks L. M.. 2006. Predicted taxonomic patterns in pheromone production by longhorned beetles. Naturwissenschaften. 93: 543–550. [DOI] [PubMed] [Google Scholar]
- Silva W. D., J. G. Millar L. M. Hanks C. M. Costa M. O. G. Leite M. Tonelli, and Bento J. M. S.. 2018. Interspecific cross-attraction between the South American cerambycid beetles Cotyclytus curvatus and Megacyllene acuta is averted by minor pheromone components. J. Chem. Ecol. 44: 268–275. [DOI] [PubMed] [Google Scholar]
- Sokal R. R., and Rohlf F. J.. 1995. Biometry, 3rd ed. W. H. Freeman and Co, New York, NY. [Google Scholar]
- Thompson A. C., and Mitlin N.. 1979. Biosynthesis of the sex pheromone of the male boll weevil from monoterpene precursors. Insect Biochem. 9: 293–294. [Google Scholar]
- Wittkowski K. M., and Song T.. 2012. muStat: Prentice rank sum test and McNemar test. R package version 1.7.0, R Foundation for Statistical Computing, Vienna. [Google Scholar]
- Yamada Y., T. Kuzuyama M. Komatsu K. Shin-Ya S. Omura D. E. Cane, and Ikeda H.. 2015. Terpene synthases are widely distributed in bacteria. Proc. Natl. Acad. Sci. U. S. A. 112: 857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.