Abstract
Background
Opioids are commonly prescribed for relief in inflammatory bowel disease (IBD). Emerging evidence suggests that adolescents and young adults are a vulnerable population at particular risk of becoming chronic opioid users and experiencing adverse effects.
Objectives
This study evaluates trends in the prevalence and persistence of chronic opioid therapy in adolescents and young adults with IBD in the United States.
Method
A longitudinal retrospective cohort analysis was conducted with the Truven MarketScan Database from 2007 to 2015. Study subjects were 15–29 years old with ≥2 IBD diagnoses (Crohn’s: 555/K50; ulcerative colitis: 556/K51). Opioid therapy was identified with prescription claims within the Truven therapeutic class 60: opioid agonists. Persistence of opioid use was evaluated by survival analysis for patients who remained in the database for at least 3 years following index chronic opioid therapy use.
Results
In a cohort containing 93,668 patients, 18.2% received chronic opioid therapy. The annual prevalence of chronic opioid therapy increased from 9.3% in 2007 to 10.8% in 2015 (P < 0.01), peaking at 12.2% in 2011. Opioid prescriptions per patient per year were stable (approximately 5). Post hoc Poisson regression analyses demonstrated that the number of opioid pills dispensed per year increased with age and was higher among males. Among the 2503 patients receiving chronic opioid therapy and followed longitudinally, 30.5% were maintained on chronic opioid therapy for 2 years, and 5.3% for all 4 years.
Conclusion
Sustained chronic opioid use in adolescents and young adults with IBD is increasingly common, underscoring the need for screening and intervention for this vulnerable population.
Keywords: opioids, chronic opioid therapy, adolescents and young adults, Crohn’s disease, ulcerative colitis
INTRODUCTION
Opioid use among individuals with inflammatory bowel disease (IBD) has become a topic of increasing interest in light of the current opioid epidemic in the United States. Over the past 2 decades, the sale of prescription opioids quadrupled, and by 2015, 2 million people were dependent on prescription opioid medications.1 Between 1997 and 2015, more than 183,000 people died from overdoses related to prescription opioids in the United States, with rates increasing 4-fold.2, 3 Unintentional overdose on opioids has risen to become the leading cause of death for young adults aged 25–44 years.4 As death rates rise among young people taking opioids, it is important to understand the trends in opioid use among adolescents and young adults prescribed opioids for medical management.
In IBD management, opioids are commonly prescribed for temporary pain relief around the time of surgery and during acute disease exacerbations. Emerging evidence shows that a subset of individuals with IBD—approximately 6% of youth and 3%–13% of adults—receive chronic opioid therapy for uncontrolled disease activity and persistent pain.5, 6 Opioids are an important and effective method of addressing acute and procedural pain, but evidence of efficacy for chronic pain is limited.7 Chronic opioid therapy can have the unexpected result of narcotic bowel syndrome8 with a negative cycle of increased pain, opioid use, and dependence. Chronic opioid therapy among IBD patients has also been associated with psychiatric comorbidities and lower quality of life.5, 6, 9 Additionally, opiate use is a risk factor for serious infections, even after adjusting for severity of disease and use of immunosuppressive agents.10
A recent population-based study in Canada highlighted that IBD is an independent risk factor for chronic opioid therapy, particularly among children and young adults.11 Heavy opioid use in this sample also strongly predicted non-malignancy-related mortality. A cross-sectional study utilizing a US population–based registry showed that chronic opioid therapy was more common among pediatric IBD populations compared with the general pediatric population, particularly among those aged 15–18 years.5 This research highlights the increased risk and negative outcomes associated with chronic opioid therapy in IBD populations and the need to better understand opioid use among adolescents and young adults in the United States, where opioid use is higher than in Canada.
Adolescents and young adults with IBD are a particularly vulnerable population given their complex disease phenotype, self-management challenges around development of autonomy, and transition from pediatric to adult care.12–14 These factors may increase adolescents’ and young adults’ risk for uncontrolled disease activity and associated pain, and treatment with opioids. Prescription opioid use among adolescents and young adults is predictive of future opioid use and misuse,15, 16 regardless of the indication. Taken together, this research highlights the increased risk and negative outcomes associated with chronic opioid therapy among adolescents and young adults with IBD and the critical need to better understand opioid use among adolescents and young adults in the United States. This study is the first to evaluate trends in the prevalence and persistence of chronic opioid therapy longitudinally among adolescents and young adults with IBD in the United States in a large nationally representative insurance claims database.
METHODS
Study Design
We performed a longitudinal retrospective cohort analysis of Crohn’s disease (CD) and ulcerative colitis (UC) patients in the Truven MarketScan Commercial Claims and Encounters Database from 2007 to 2015. The Truven MarketScan Database consists of de-identified outpatient, inpatient, and pharmaceutical claims of approximately 40–50 million privately insured patients each year. These claims originate from more than 150 large employer-sponsored health insurance plans with patient coverage in all 50 states. The database includes patient characteristics (eg, age, sex, geographic region), financial variables (eg, inpatient, outpatient, and pharmaceutical costs), and pharmacy-level data (eg, National Drug Code, days’ supply, strength, administration method). All financial variables are scaled to February 2017 dollars using the Consumer Price Index (CPI).
Patient Identification
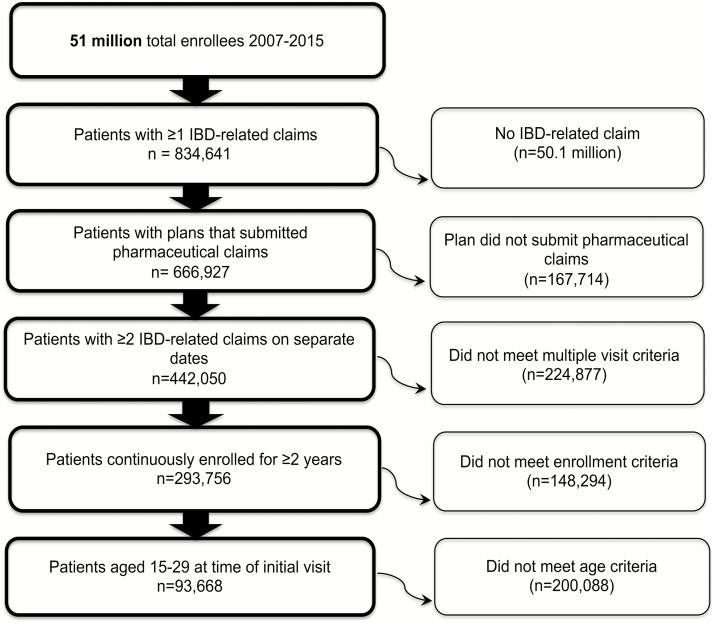
Patient inclusion criteria included (1) age 15–29 years17–19; (2) 2 or more distinct IBD diagnoses (defined under the International Classification of Diseases, Ninth Revision [ICD-9] as 555.xx for CD and 556.xx for UC, and the ICD-10 codes K50.xx and K51.xx, respectively; this stringent method of IBD classification has been examined and used previously in administrative data20–23); and (3) enrollment in a health plan that submitted at least 1 pharmaceutical claim. To classify a patient as CD or UC, the total number of distinct CD and UC diagnoses were summed, and if 80% or more of these diagnoses were 555.xx/K50.xx or 556.xx/K51.xx, the patient was classified as CD or UC, respectively. If neither was satisfied, the patient was classified as having interderminate colitis (IC) (see Figure 1 for a flow diagram of study population selection).
FIGURE 1.
Study population selection. This flow diagram outlines the study population selection in the Truven MarketScan Database. It demonstrates step by step how the chronic opioid therapy sample was created.
Chronic Opioid Therapy Classification
Chronic opioid therapy was determined using dispensed prescription drug claims. Opioid medications included in the Truven database included those categorized as therapeutic class 60: analgesics/antipyretics, opiate agonists (see Supplementary Table 1 for the full list of the medications included in this category). When examining the overall prevalence of chronic opioid therapy in our sample, a patient was classified as meeting criteria for chronic opioid therapy if s/he had ≥3 separate opioid drug claims on distinct dates within a 2-year rolling window (based on criteria established in a previous study utilizing the Truven database5). Only individuals with continuous enrollment in an insurance plan were included in this sample. Multiple opioid drugs dispensed on the same day were counted as a single opioid prescription. For this portion of the study, opioid drug claims within 30 days of a major abdominal operation were excluded.
When examining the prevalence of chronic opioid therapy per year from 2007 to 2015, criteria for chronic opioid therapy were modified to allow for assessment of longitudinal trends. In these analyses, a patient met criteria for chronic opioid therapy if s/he had at least 2 separate opioid drug claims on distinct dates within 1 year.
To account for fluctuations in total Truven enrollment, chronic opioid therapy was represented using a standardized index score (total number of opioid drug claims per year from members who met criteria for chronic opioid therapy in that specific year divided by the total number of members who met criteria for chronic opioid therapy in that year).
To evaluate persistence of opioid use, Kaplan-Meier survival analysis was conducted for those patients who met the modified chronic opioid therapy criteria (≥2 separate opioid prescriptions in 1 year) and remained in the database for at least 3 years following index chronic opioid therapy use. Patients who first met chronic opioid therapy criteria within 30 days after surgery were included in this analysis to study the long-term effects of their initial opiate exposure. Survival was defined as ≥2 separate opioid prescriptions per year. Failure was defined as <2 opioid prescriptions per year.
Variable Classification
Covariates of interest were classified using patient data during the study period. Demographic information included age, sex, geographic regions, and type of insurance plan. IBD medications included any pharmaceutical prescription or respective Healthcare Common Procedure Coding System/J-code for biologics (infliximab, adalimumab, certolizumab, golimumab, natalizumab, vedolizumab, ustekinumab) and any pharmaceutical prescription for corticosteroids (prednisone, prednisolone, methylprednisolone, hydrocortisone, budesonide), 5-aminosalicylic acid (5-ASA; mesalamine, sulfasalazine), or immunomodulator (6-mercaptopurine, methotrexate, azathioprine). Health care utilization included the number of IBD-related hospitalizations, IBD-related surgeries, emergency department (ED) visits, outpatient visits, and polypharmacy. Health care costs included high-cost patients (defined as annual health care costs >$50,000),24 total costs per year, and mean hospitalization cost. Comorbidities commonly associated with chronic opioid therapy were defined a priori based on clinical judgment and past research with population-based databases5 and the Truven database using ICD-9 and ICD-10 codes. These comorbidities included abdominal pain, joint pain, chronic back pain, migraines, chronic pain unspecified, osteoarthritis, rheumatoid arthritis, depression, anxiety, tobacco, substance abuse, and alcohol abuse (see Supplementary Table 2 for the full list of ICD-9/10 codes).
Statistical Analysis
Descriptive statistical analyses and Kaplan-Meier survival analyses were performed to assess the prevalence and persistence of chronic opioid therapy in this sample. Chi-square and 2-tailed Student t tests were used to compare the characteristics of individuals who received chronic opioid therapy with those who did not. Post hoc analyses included 2 Poisson regressions adjusting for age and sex to calculate the number of opioid pills dispensed per year, with age stratified as 15–19 years, 20–24 years, and 25–29 years. The first regression compared those who met the criteria for chronic opioid therapy with those who did not. The second regression compared those who had at least 1 opioid drug claim at any point between 2007 and 2015 with those who did not have an opioid drug claim. A multivariable logistic regression was used to estimate the outcome of chronic opioid therapy, adjusting for age bins (15–19 years, 20–24 years, 25–29 years), sex, geographical region, disease type, pain conditions, anxiety, depression, and substance abuse. The medication possession ratio (MPR) was also calculated for individuals meeting criteria for chronic opioid therapy and those who did not. MPR is the ratio of the days’ supply of opioids dispensed divided by the number of days individuals were observed in the database. Statistical significance was assessed at the level of α = 0.05. All analyses were performed using Stata/SE 14.2 (College Station, TX, USA).
RESULTS
Descriptive Statistics
Our sample contained 93,668 IBD patients aged 15–29 years. Of those, 17,084 (18.2%) received chronic opioid therapy at some point during the study period. Demographics of those who received chronic opioid therapy and those who did not are included in Table 1. Female sex and Crohn’s disease were significantly associated with chronic opioid therapy. Patients receiving chronic opioid therapy had greater use of biologic (36.8% vs 26.2%) and corticosteroid (75.2% vs 46.1%) medications.
TABLE 1:
Overall Sample Characteristics
| No Chronic Opioid Therapy (n = 76,584) | Chronic Opioid Therapy (n = 17,084) | P | |
|---|---|---|---|
| Sex, No. (%) | |||
| Female | 38,548 (50.3) | 10,134 (59.3) | <0.001 |
| Age | |||
| Mean (median), y | 23.0 (23) | 23.6 (24) | <0.001 |
| 15–19 y, No. (%) | 18,512 (24.2) | 3267 (19.1) | <0.001 |
| 20–24 y, No. (%) | 26,505 (34.6) | 5710 (33.4) | |
| 25–29 y, No. (%) | 31,567 (41.2) | 8107 (47.5) | |
| Geographic region, No. (%) | |||
| Northeast | 18,148 (23.7) | 2833 (16.6) | <0.001 |
| North Central | 18,910 (24.7) | 4087 (23.9) | |
| South | 27,151 (35.5) | 7076 (41.4) | |
| West | 10,805 (14.1) | 2800 (16.4) | |
| Other | 1570 (2.1) | 288 (1.7) | |
| Insurance,a No. (%) | |||
| EPO/PPO | 49,299 (69.3) | 10,934 (68.1) | <0.001 |
| HMO/Cap POSb | 9915 (13.9) | 2437 (15.2) | |
| HDHP/CDHP | 5915 (8.3) | 1123 (8.3) | |
| POS | 5040 (7.1) | 1331 (7.0) | |
| COMP | 954 (1.3) | 234 (1.5) | |
| IBD type, No. (%) | |||
| Crohn’s disease | 38,860 (50.7) | 10,039 (58.8) | <0.001 |
| Ulcerative colitis | 31,884 (41.6) | 5546 (32.5) | |
| Indeterminate colitis | 5840 (7.6) | 1499 (8.8) | |
| IBD medications, No. (%) | |||
| Biologics | 20,069 (26.2) | 6292 (36.8) | <0.001 |
| Corticosteroids | 35,301 (46.1) | 12,838 (75.2) | <0.001 |
| Immunomodulators | 21,955 (28.7) | 5789 (33.9) | <0.001 |
| 5-ASA | 44,020 (57.5) | 9910 (58) | 0.21 |
| Comorbidities, % | |||
| Pain | |||
| Abdominal pain | 56 | 85.5 | <0.001 |
| Joint pain | 21 | 46.4 | <0.001 |
| Chronic back pain | 6.3 | 22.9 | <0.001 |
| Migraines | 6.3 | 19 | <0.001 |
| Chronic pain, unspecified | 1.2 | 14.2 | <0.001 |
| Osteoarthritis | 1.5 | 6.2 | <0.001 |
| Rheumatoid arthritis | 1.3 | 3.8 | <0.001 |
| Psychiatric | |||
| Depression | 22.2 | 55.2 | <0.001 |
| Anxiety | 14.2 | 35.1 | <0.001 |
| Substance use | |||
| Tobacco | 5.7 | 22.4 | <0.001 |
| Substance abuse | 1.6 | 8.7 | <0.001 |
| Alcohol abuse | 1.9 | 5.6 | <0.001 |
aPatients were classified under the insurance plan of their first UC or CD diagnosis.
bHMO/Cap POS are capitated plans. All other plans are noncapitated.
Patients receiving chronic opioid therapy had significantly more comorbidities than those not meeting chronic opioid therapy criteria. Specifically, these patients had higher rates of comorbid pain, arthritis, anxiety, depression, and substance diagnoses compared with nonchronic opioid therapy patients (all P < 0.01).
Trends in Chronic Opioid Therapy
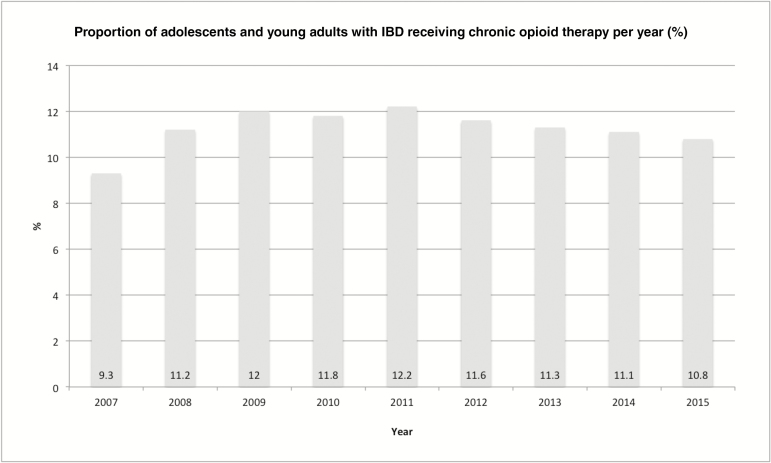
The prevalence of chronic opioid therapy increased from 9.3% in 2007 to a peak of 12.2% in 2011, then declined slightly to 10.8% in 2015 (P < 0.01) (Fig. 2). The number of opioid prescriptions per patient per year remained relatively stable between 2007 and 2015 (5.0–5.2; P > 0.05). The mean number of pills per prescription was 65.
FIGURE 2.
Prevalence of chronic opioid therapy. The prevalence of chronic opioid therapy among adolescents and young adults with IBD between 2007 and 2015 was calculated using the Truven MarketScan Database. The proportion of individuals meeting criteria for chronic opioid therapy increased from 9.3% in 2007 to a peak of 12.2% in 2011, then declined to 10.8% in 2015 (P < 0.01).
Persistence of Chronic Opioid Therapy
To evaluate chronic opioid use longitudinally, 2503 patients were identified who met chronic opioid therapy criteria in an index year and were enrolled in the data set for at least 3 subsequent years (years 2–4). Of these patients, 132 met criteria for chronic opioid therapy in all 4 years. Patients receiving chronic opioid therapy for 4 years were significantly more likely to take biologic and corticosteroid medications and to have higher rates of comorbid pain and psychiatric and substance diagnoses compared with patients who met criteria for chronic opioid therapy for 1–3 years (P < 0.01) (Table 2).
TABLE 2:
Longitudinal Chronic Opioid Therapy Sample Characteristics
| Chronic Opioid Therapy in Year 1; Enrolled ≥4 y (n = 2503) | Persistent Chronic Opioid Therapy ≥4 y (n = 132) | P | |
|---|---|---|---|
| Sex, No. (%) | |||
| Female | 1444 (57.7) | 87 (65.9) | 0.062 |
| Age | |||
| Mean (median), y | 22.9 (24) | 23.9 (26) | 0.009 |
| 15–19 y, No. (%) | 761 (30.4) | 30 (22.7) | |
| 20–24 y, No. (%) | 637 (45.5) | 26 (19.7) | 0.01 |
| 25–29 y, No. (%) | 1105 (44.1) | 76 (57.6) | |
| IBD type, No. (%) | 0.078 | ||
| Crohn’s disease | 1426 (57.0) | 83 (62.9) | |
| Ulcerative colitis | 851 (34.0) | 33 (25) | |
| Indeterminate colitis | 226 (9.0) | 16 (12.1) | |
| IBD medications, No. (%) | |||
| Biologics | 1096 (43.8) | 74 (56.1) | 0.006 |
| Corticosteroids | 2118 (84.6) | 128 (97.0) | <0.001 |
| Immunomodulators | 1107 (44.2) | 79 (59.8) | <0.001 |
| 5-ASA | 1748 (69.8) | 93 (70.4) | 0.88 |
| Comorbidities, % | |||
| Pain | |||
| Abdominal pain | 88.3 | 97.7 | 0.001 |
| Joint pain | 53.6 | 68.2 | 0.001 |
| Chronic back pain | 26.1 | 47.7 | <0.001 |
| Migraines | 20.8 | 46.2 | <0.001 |
| Chronic pain, unspecified | 9.6 | 40.9 | <0.001 |
| Osteoarthritis | 7.2 | 12.1 | 0.035 |
| Rheumatoid arthritis | 4.3 | 8.3 | 0.03 |
| Psychiatric | |||
| Depression | 31.4 | 56.1 | <0.001 |
| Anxiety | 35.5 | 68.9 | <0.001 |
| Substance use | |||
| Tobacco | 18.9 | 31.8 | <0.001 |
| Substance abuse | 7 | 20.5 | <0.001 |
| Alcohol abuse | 6.1 | 6.8 | 0.742 |
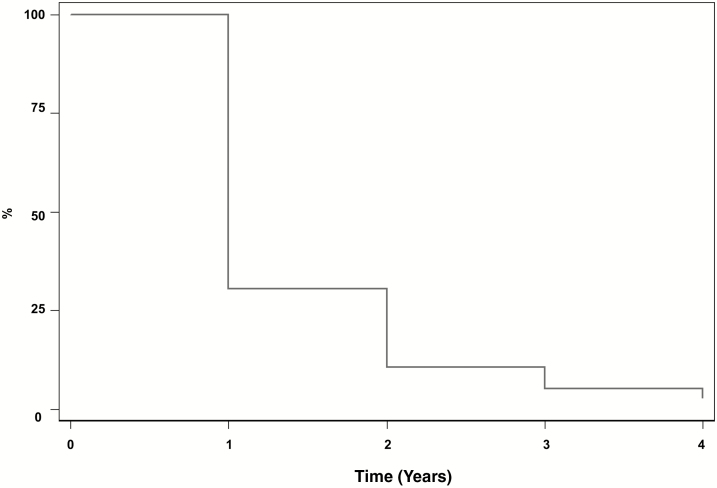
Kaplan-Meier survival analysis was used to evaluate the persistence of opioid therapy (Fig. 3). Results showed that 30.5% continued to receive chronic opioid therapy in year 2, 10.7% in year 3, and 5.3% in year 4. Of note, 54.65% of patients who met criteria for chronic opioid therapy in the index year never met criteria for chronic opioid therapy again during their enrollment in the database.
FIGURE 3.
Persistence of chronic opioid therapy. Kaplan-Meier analyses were conducted to determine persistence of chronic opioid therapy for adolescents and young adults with IBD who filled ≥2 opioid prescriptions in an index year and remained in the database for ≥3 subsequent years. Analyses showed that 30.5% continued to receive chronic opioid therapy in year 2, 10.7% in year 3, and 5.3% in year 4.
Health Care Utilization and Costs
There were significant differences between health care utilization and costs among patients who met criteria for chronic opioid therapy for 4 years, patients who met criteria for chronic opioid therapy for 1–3 years, and those who did not meet criteria for chronic opioid therapy (P < 0.001) (Table 3). Patients receiving chronic opioid therapy for 4 years went to the ED more (96.2% vs 88.1% in 1–3-year group vs 48.5% in the no chronic opioid therapy group) and were hospitalized more often (25% hospitalized ≥5 times per year vs 10.6% in the 1–3-year group vs 5.4% in the no chronic opioid therapy group). Patients who met chronic opioid therapy criteria for 4 years were also more frequently classified as high-cost patients, with annual health care costs exceeding $50,000 (28.8% vs 13.9% in the 1–3-year group vs 9.2% in the no chronic opioid therapy group). Those receiving chronic opioid therapy for 4 years had higher mean annual total costs of care (mean per-member, per-year cost of $44,766 vs $28,139 in 1–3-year group vs $20,473 in the no chronic opioid therapy group).
TABLE 3:
Health Care Utilization and Costs
| No Chronic Opioid Therapy; Enrollment for 2+ y (n = 76,584) | Chronic Opioid Therapy for 1–3 y; Enrollment ≥4 y (n = 2371) | Persistent Chronic Opioid Therapy ≥4 y (n = 132) | P | |
|---|---|---|---|---|
| Health care utilization | ||||
| IBD hospitalizations, % | ||||
| 0 | 74.4 | 36.9 | 15.2 | <0.001 |
| 1–2 | 15.5 | 42 | 43.7 | |
| 3–4 | 4.7 | 10.4 | 16.1 | |
| 5+ | 5.4 | 10.6 | 25 | |
| ≥1 IBD surgeries | 7.8 | 26.4 | 46.2 | <0.001 |
| Outpatient visits, mean | 12.3 | 16.3 | 26.4 | <0.001 |
| ≥1 ED visits | 48.5 | 88.1 | 96.2 | <0.001 |
| Polypharmacy,a mean | 3.9 | 4.2 | 5.9 | <0.001 |
| No. prescriptions, % | ||||
| 0–4 | 65 | 61.7 | 28 | |
| 5+ | 35 | 38.3 | 72 | <0.001 |
| Health care costs | ||||
| High-cost patients,b % | 9.2 | 13.9 | 28.8 | <0.001 |
| Total costs per year (SD), $ | 20,473 (40,105) | 28,139 (30,342) | 44,766 (42,823) | <0.001 |
| Hospitalization cost (SD), $ | 6351 (7041) | 7028 (7996) | 5284 (3597) | <0.001 |
aExcluding IBD medications.
bHigh-cost patients defined as annual health care costs >$50,000.
Post Hoc Analyses
Poisson regressions adjusting for age and sex were conducted post hoc to provide additional information about the number of opioid pills dispensed per year for individuals receiving chronic opioid therapy and those who received at least 1 opioid. Results demonstrated that among patients in our sample who met criteria for chronic opioid therapy, the number of opioid pills dispensed per year increased with age and was higher among males. Males 25–29 years old received approximately 309 pills per year. A similar trend was seen when looking at all 15–29-year-old patients in the database who received at least 1 opioid prescription. The number of pills per year increased with age; however, in this group, females had a slighter higher count than males. Of note, the opioid pill count was higher in every age and sex subgroup for individuals who met the criteria for chronic opioid therapy (Supplementary Fig. 1).
These results were further supported by the MPR analyses, which were calculated to provide additional information about the amount of opioid dispensed to individuals who met criteria for chronic opioid therapy. Results demonstrated that the median MPR was larger among adolescents and young adults receiving chronic opioid therapy compared with those who did not meet criteria for chronic opioid therapy. Individuals receiving chronic opioid therapy had an opioid MPR of 0.03, compared with 0.0007 among those who did not meet criteria for chronic opioid therapy. Of note, the top quartile of individuals in the chronic opioid therapy population had a higher opioid MPR of 0.1.
Lastly, multivariable logistic regression was conducted post hoc to provide information about predictors of chronic opioid therapy in this sample. Results showed that if a patient was diagnosed with depression, substance abuse, or a pain condition, the odds of receiving chronic opioid therapy was approximately 2–3 times higher (Table 4).
TABLE 4:
Multivariable Logistic Regression
| Independent Variable | Adjusted Odds Ratio for Chronic Opioid Therapy (95% CI) | Variable LRT P |
|---|---|---|
| Age, y | ||
| 15–19 | Referent | <0.001 |
| 20–24 | 1.31 (1.25–1.38) | |
| 25–29 | 1.57 (1.50–1.65) | |
| Sex | ||
| Female | 1.34 (1.30–1.40) | <0.001 |
| Region | ||
| Northeast | Referent | <0.001 |
| North Central | 1.40 (1.33–1.48) | |
| South | 1.73 (1.64–1.81) | |
| West | 1.76 (1.66–1.87) | |
| Unknown | 1.17 (1.03–1.34) | |
| IBD | ||
| CD | Referent | <0.001 |
| IC | 0.98 (0.92–1.04) | |
| UC | 0.65 (0.62–0.67) | |
| Paina | 2.44 (2.33–2.57) | <0.001 |
| Depression | 1.94 (1.79–2.10) | <0.001 |
| Anxiety | 1.48 (1.37–1.60) | <0.001 |
| Substance use | 2.65 (2.12–3.32) | <0.001 |
Abbreviations: CI, confidence interval; LRT, likelihood ratio test.
aThe pain variable includes common pain conditions associated with the prescription of opioids in the general population (joint pain, chronic back pain, migraines, unspecified chronic pain, osteoarthritis, and rheumatoid arthritis) (see Supplementary Table 2 for ICD-9/10 codes). Abdominal pain is excluded from this category due to our inability to differentiate IBD-related abdominal pain from other sources of abdominal pain in the sample.
DISCUSSION
To our knowledge, this is the first nationally representative longitudinal study in the United States to examine chronic opioid therapy among adolescents and young adults with IBD. This research is of particular importance given the current opioid epidemic and the growing awareness that adolescents and young adults are a vulnerable IBD population at risk of becoming chronic opioid users5, 11 with future opioid use and misuse in adulthood.15, 16 The current study results highlight that a significant proportion of adolescents and young adults in the United States received chronic opioid therapy between 2007 and 2015, with 18% receiving 3 or more opioid prescriptions within a 2-year period.
When examining the prevalence of chronic opioid therapy, rates have increased over time, peaking in 2011 (12% meeting criteria for chronic opioid therapy), mirroring national trends. Despite national efforts to decrease opioid prescriptions and use, rates of chronic opioid therapy persisted and remained high through 2015. Adolescents and young adults meeting criteria for chronic opioid therapy received a surprisingly large number of opioids, approximately 5 prescriptions per patient per year, with 65 pills per prescription. Post hoc analyses demonstrated that the number of opioid pills dispensed per year increased with age and was higher among males. Of note, males aged 25–29 years received approximately 309 pills per year.
It is also noteworthy to highlight that in a subsample of adolescents and young adults (enrolled in the database for at least 4 years), almost one-third of patients receiving chronic opioid therapy in 1 year continued to use opioids and meet criteria for chronic opioid therapy in the following year, and 5% in each of the subsequent 3 years. These results underscore that a small subset of adolescents and young adults with IBD are at particularly high risk for continued opioid use and related negative outcomes and merit increased clinical attention. Providers caring for IBD patients should receive additional education about this high-risk population of IBD patients including the unique developmental considerations of adolescents and young adults that could be contributing to continued opioid use (eg, development of autonomy and changing roles in disease management—shifting responsibilities from parents to adolescents and young adults). It will also be important to better understand the clinical profile and potential protective factors of the 54.65% of adolescents and young adults who never met criteria for chronic opioid therapy again after the index year. This information could support targeted intervention efforts for the small subset of patients who continue to struggle with persistent opioid use. Overall, increased awareness about this distinct group of high-risk adolescents and young adults with IBD will support greater collaboration between gastroenterologists and multidisciplinary teams to better monitor and wean opioids following surgery.
The study corroborates past research demonstrating that female sex, Crohn’s disease, and comorbid psychiatric and pain conditions are associated with chronic opioid therapy among children and adults with IBD.5, 6, 25 Our findings also support and extend past research demonstrating that chronic opioid therapy is related to increased health care utilization among children with IBD.5 In the current study, chronic opioid therapy was associated with higher health care utilization (eg, IBD surgeries, ED visits, hospitalizations) and health care costs among adolescents and young adults with IBD, especially among those with persistent chronic opioid therapy. Interestingly, 97% of individuals receiving chronic opioid therapy were on corticosteroids and only 56% were on biologic agents. Taken together, such findings suggest that this population may have severe disease with poor disease control, adding to past research that has demonstrated that adolescents and young adults with IBD are a vulnerable IBD population (eg, complex disease phenotype, self-management challenges).12–14 This underscores the importance of improved medical management and supporting adherence among adolescents and young adults. An important future direction would be for transition and young adult programs to target chronic opioid therapy prevention in their efforts to address the specific needs of adolescents and young adults with IBD.
A range of pain conditions (eg, abdominal, joint) were associated with chronic opioid therapy in this sample. This is noteworthy as the majority of adolescents and young adults with IBD experience pain at some point in their disease course, particularly abdominal pain during disease activity. Of note, research has shown that up to 50% of adults continue to experience abdominal pain during disease remission,26, 27 and similar trends have been documented in pediatric populations (up to 26%).28, 29 Such factors could potentially contribute to prolonged opioid use. Additionally, among young people with chronic pain and IBD, there is a greater risk for depression and anxiety,28 which this and other studies5 have shown are also associated with chronic opioid therapy. Chronic pain and affective disorders among young people are also associated with worse physical and psychological outcomes in adulthood.30 Of note, post hoc analyses have demonstrated that adolescents and young adults diagnosed with a pain condition, depression, or substance abuse were approximately 2–3 times more likely to receive chronic opioid therapy, underscoring that pain and psychiatric conditions may be a key predictor of chronic opioid use in this vulnerable population. Overall, there is a significant need to increase screening of adolescents and young adults in IBD clinics for chronic pain conditions and psychiatric disorders. Access to treatment for pain management and mental health challenges would support prevention efforts by targeting psychological comorbidities and reducing symptoms that may contribute to prolonged opioid use. Additionally, behavioral interventions as part of multidisciplinary pain management treatment could effectively support opioid weaning.
These efforts are particularly important among adolescents and young adults with IBD as opioid use during this period predicts future use and misuse, even among individuals who have little to no history of drug use or strong disapproval of illegal drug use at baseline.15 Furthermore, opioid use can interfere with employment (eg, through failed drug testing) during this crucial period.31 Given the current opioid epidemic and rising deaths among young adults, it is critical that future research explore the efficacy of interventions in reducing chronic opioid therapy in adolescents and young adults with IBD.
Limitations
The Truven database solely provides information about filled opioid prescriptions. No data are available on individuals’ consumption of opioids or the number of opioids prescribed and not filled. Similarly, opioid misuse (ie, use of opioids without a prescription) is not captured, likely leading to an underestimation of individuals taking chronic opioids. Second, this database is comprised of a commercially insured population. Although using an employer-based insurance database captures the largest group of individuals in the United States younger than age 65 years (2014: 47% of children, 59% of adults),32 young people who are on Medicaid or uninsured are not represented in this study population. This sampling bias may be particularly relevant for adolescents, as more children are enrolled in Medicaid than adults.33 Additionally, when looking at the persistence of chronic opioid therapy over time, only those who were continuously enrolled were included in the longitudinal analysis. This likely underestimates the population of individuals meeting criteria for chronic opioid therapy, as those who are unable to work due to chronic opioid therapy or IBD (eg, due to inability to pass an employer drug test, severe IBD) are excluded. Including young people only on commercial insurance likely leads to a study sample that is healthier, has a higher level of education and socioeconomic status, and/or lives in a family with more resources. Future research combining employer-based insurance and Medicaid databases would provide even more thorough coverage and further increase the accuracy of the rates of prevalence and persistence of chronic opioid therapy in the United States. Third, the definition of chronic opioid therapy used in this study has not been validated, although it has been used in published research.5 Furthermore, the algorithm for identification of IBD was validated in adults but has not been validated in adolescents. Although the algorithm appears to be fairly accurate, with a positive predictive value of 95%,23 there remains some potential for misclassification of IBD, specifically CD vs UC. Validation studies comparing the various definitions of chronic opioid therapy and IBD classification from administrative data in this age group would help determine the most accurate classification criteria going forward. Fourth, the database does not provide information about where the first narcotic prescription was written (eg, ED vs outpatient gastrointestinal clinic) or the indication for prescribed medications that could have resulted in misclassification. This was addressed by applying strict criteria for chronic opioid therapy based on past research.5 Investigators using databases who have access to these important prescription details should further explore whether such factors impact the development of chronic opioid therapy. Lastly, there were no data on specific clinical characteristics such as disease severity. Exploring clinical predictors of chronic opioid therapy over time in a more diverse IBD sample is an important area of future research.
CONCLUSIONS
This study demonstrates that in a large national sample of adolescents and young adults with IBD, a substantial proportion receive chronic opioid therapy. Rates of chronic opioid therapy have persisted over time in the United States despite national efforts to decrease opioid prescribing patterns. Inflammatory bowel disease providers should be made aware of the risk of opioid dependence among adolescents and young adults with IBD and the associated elevations in health care utilization and costs. The high prevalence of chronic opioid therapy among individuals with chronic pain, anxiety, and depression highlights the need for standardized psychosocial screening tools in IBD clinics for this vulnerable population, coupled with access to empirically supported behavioral interventions (eg, cognitive behavioral therapy). Overall, health care providers should be mindful of the potential long-term effects of starting adolescents and young adults with IBD on opioids and, when prescribed, utilize multidisciplinary pain management strategies to support timely weaning.
SUPPLEMENTARY DATA
Supplementary data are available at Inflammatory Bowel Diseases online.
ACKNOWLEDGMENTS
Data for this project were accessed using the Stanford Center for Population Health Sciences (PHS) Data Core. The PHS Data Core is supported by a National Institutes of Health National Center for Advancing Translational Science Clinical and Translational Science Award (UL1 TR001085) and by internal Stanford funding. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest: K.T.P. has received research support from Janssen, Takeda, and AbbVie Inc. (not related to this work). There are no other disclosures or conflicts of interest to report for the authors.
Supported by: This research was supported by NIDDK094868 (K.T.P.). The sponsor did not have any role in the design or analysis of this study.
REFERENCES
- 1. Hughes AW, Matthew, Lipari R, et al. Prescription drug use and misuse in the United States: results from the 2015 National Survey on Drug Use and Health. 2015. https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR2-2015/NSDUH-FFR2-2015.htm. Accessed January 8, 2017. [Google Scholar]
- 2. Centers for Disease Control and Prevention. Wide-Ranging Online Data for Epidemiologic Research (WONDER). 2016. http://wonder.cdc.gov. Accessed January 8, 2017. [Google Scholar]
- 3. Rudd R, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(5051):1445–1452. [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. CDC ten leading causes of death and injury. 2015. https://www.cdc.gov/injury/wisqars/LeadingCauses.html. Accessed July 8, 2017. [Google Scholar]
- 5. Buckley JP, Cook SF, Allen JK, et al. Prevalence of chronic narcotic use among children with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2015;13(2):310–315.e2. [DOI] [PubMed] [Google Scholar]
- 6. Cross RK, Wilson KT, Binion DG. Narcotic use in patients with Crohn’s disease. Am J Gastroenterol. 2005;100:2225–2229. [DOI] [PubMed] [Google Scholar]
- 7.American Pain Society. APS-AAPM clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. 2009. http://americanpainsociety.org/uploads/education/guidelines/chronic-opioid-therapy-cncp.pdf. Accessed July 8, 2017. [Google Scholar]
- 8. Grunkemeier DM, Cassara JE, Dalton CB, et al. The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol. 2007;5:1126–1139; quiz 1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanson KA, Loftus EV Jr, Harmsen WS, et al. Clinical features and outcome of patients with inflammatory bowel disease who use narcotics: a case-control study. Inflamm Bowel Dis. 2009;15:772–777. [DOI] [PubMed] [Google Scholar]
- 10. Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4:621–630. [DOI] [PubMed] [Google Scholar]
- 11. Targownik LE, Nugent Z, Singh H, et al. The prevalence and predictors of opioid use in inflammatory bowel disease: a population-based analysis. Am J Gastroenterol. 2014;109:1613–1620. [DOI] [PubMed] [Google Scholar]
- 12. American Academy of Pediatrics; American Academy of Family Physicians; American College of Physicians-American Society of Internal Medicine. A consensus statement on health care transitions for young adults with special health care needs. Pediatrics. 2002;110:1304–1306. [PubMed] [Google Scholar]
- 13. Brooks AJ, Smith PJ, Cohen R, et al. UK guideline on transition of adolescent and young persons with chronic digestive diseases from paediatric to adult care. Gut. 2017;66:988–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hommel KA, Greenley RN, Maddux MH, et al. Self-management in pediatric inflammatory bowel disease: a clinical report of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2013;57:250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miech R, Johnston L, O’Malley PM, et al. Prescription opioids in adolescence and future opioid misuse. Pediatrics. 2015;136:e1169–e1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McCabe SE, West BT, Veliz P, et al. Trends in medical and nonmedical use of prescription opioids among US adolescents: 1976–2015. Pediatrics. 2017;139(4):e20162387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wein S, Pery S, Zer A. Role of palliative care in adolescent and young adult oncology. J Clin Oncol. 2010;28(32):4819–4824. [DOI] [PubMed] [Google Scholar]
- 18. Ferrari A, Thomas D, Franklin AR, et al. Starting an adolescent and young adult program: some success stories and some obstacles to overcome. J Clin Oncol. 2010;28(32):4850–4857. [DOI] [PubMed] [Google Scholar]
- 19. Arnett JJ. Emerging adulthood. A theory of development from the late teens through the twenties. Am Psychol. 2000;55(5):469–480. [PubMed] [Google Scholar]
- 20. Yu H, MacIsaac D, Wong JJ, et al. Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment Pharmacol Ther. 2018;47(3):364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Govani SM, Noureldin M, Higgins PDR, et al. Defining an optimal adherence threshold for patients taking subcutaneous anti-TNFs for inflammatory bowel diseases. Am J Gastroenterol. 2018;113(2):276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kahn SA, Lin CW, Ozbay B, et al. Indirect costs and family burden of pediatric Crohn’s disease in the United States. Inflamm Bowel Dis. 2017;23(12):2089–2096. [DOI] [PubMed] [Google Scholar]
- 23. Liu L, Allison JE, Herrinton LJ. Validity of computerized diagnoses, procedures, and drugs for inflammatory bowel disease in a northern California managed care organization. Pharmacoepidemiol Drug Saf. 2009;18(11):1086–1093. [DOI] [PubMed] [Google Scholar]
- 24. Park KT, Colletti RB, Rubin DT, et al. Health insurance paid costs and drivers of costs for patients with Crohn’s disease in the United States. Am J Gastroenterol. 2016;111(1):15–23. [DOI] [PubMed] [Google Scholar]
- 25. Long MD, Barnes EL, Herfarth HH, et al. Narcotic use for inflammatory bowel disease and risk factors during hospitalization. Inflamm Bowel Dis. 2012;18(5):869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2012;107(10):1474–1482. [DOI] [PubMed] [Google Scholar]
- 27. Srinath AI, Walter C, Newara MC, et al. Pain management in patients with inflammatory bowel disease: insights for the clinician. Therap Adv Gastroenterol. 2012;5(5):339–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Watson KL KS, Boyle BM, Saps M. The prevalence and impact of functional abdominal pain disorders in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2017;65(2):212–217. [DOI] [PubMed] [Google Scholar]
- 29. Zimmerman LA, Srinath AI, Goyal A, et al. The overlap of functional abdominal pain in pediatric Crohn’s disease. Inflamm Bowel Dis. 2013;19:826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palermo TM, Eccleston C, Lewandowski AS, et al. Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: an updated meta-analytic review. Pain. 2010;148: 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kreuger A. Where have all the workers gone? An inquiry into the decline of the U.S. labor force participation rate. Brookings Papers on Economic Activity. Published 2017. https://www.brookings.edu/wp-content/uploads/2017/09/1_krueger.pdf. Accessed November 8, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Henry J. Kaiser Family Foundation. Health insurance coverage of nonelderly 0–64. 2014. http://www.kff.org/other/state-indicator/nonelderly-0–64/?currentTimeframe=1&selectedDistributions=employer--non-group--medicaid--other-public--uninsured--total&selectedRows=%7B%22wrapups%22:%7B%22united-states%22:%7B%7D%7D%7D&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed March 1, 2017. [Google Scholar]
- 33. Henry J. Kaiser Family Foundation. Medicaid Enrollees by Enrollment Group; 2014. http://www.kff.org/medicaid/state-indicator/distribution-of-medicaid-enrollees- by-enrollment-group/?dataView=0¤tTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed March 1, 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.