Abstract
Campylobacter jejuni, a human gastrointestinal pathogen, uses nitrate for growth under microaerophilic conditions using periplasmic nitrate reductase (Nap). The catalytic subunit, NapA, contains two prosthetic groups, an iron sulfur cluster and a molybdenum cofactor. Here we describe the cloning, expression, purification, and Michaelis-Menten kinetics (kcat of 5.91 ± 0.18 s−1 and a KM (nitrate) of 3.40 ± 0.44 μM) in solution using methyl viologen as an electron donor. The data suggest that the high affinity of NapA for nitrate could support growth of C. jejuni on nitrate in the gastrointestinal tract. Site-directed mutagenesis was used and the codon for the molybdenum coordinating cysteine residue has been exchanged for serine. The resulting variant NapA is 4-fold less active than the native enzyme confirming the importance of this residue. The properties of the C. jejuni enzyme reported here represent the first isolation and characterization of an epsilonproteobacterial NapA. Therefore, the fundamental knowledge of Nap has been expanded.
Keywords: NapA, nitrate reductase, pathogenicity, molybdenum, Campylobacter, heterologous expression
Molecular cloning, expression and kinetic analysis of the native Campylobacter jejuni enzyme and the cysteine 176 to serine variant.
HIGHLIGHTS
Campylobacter jejuni NapA has been cloned and expressed heterologously.
Campylobacter jejuni NapA has a kcat of 5.91 ± 0.18 s−1 and a KM (nitrate) of 3.40 ± 0.44 μM.
A NapA-C176S variant has been isolated and has a KM (nitrate) of 307 ± 16 μM and a kcat of 0.045 ± 0.001 s−1.
INTRODUCTION
Campylobacter jejuni has been classified as an emerging antibiotic-resistant pathogen worldwide (Johnson, Shank and Johnson 2017), where nearly 1% of Europe's population is infected, and the percentage is expected to be higher in developing countries (Epps et al.2013). Campylobacter jejuni is commonly present in the gastrointestinal tract (GIT) of chickens, and can be transmitted to humans via contaminated food and/or water (Liu et al.2012). In infants, C. jejuni infection causes severe diarrhea in 6–12% of cases and diarrhea is the second leading cause of mortality in children worldwide (Liu et al.2016). Campylobacter infection may also be detrimental to children as it is suggested to be an important contributor to growth deficits, especially in low-resource settings (Amour et al.2016). Due to the increased antibiotic resistance of C. jejuni and the impact on human health, specifically infants and children, physiological understanding of C. jejuni and development of new therapeutic strategies are crucial.
Aside from gastroenteritis infections, pathogens like C. jejuni have been attributed to methemoglobinemia, a disease primarily found in infants where methemoglobin is produced (Powlson et al.2008). Methemoglobinemia has also been linked to well water with high concentrations of nitrate (Knobeloch et al.2000). Re-evaluation of these studies indicates that the cases of incidence occurred with well water that was contaminated with feces (Powlson et al.2008). Due to fecal contamination, the water contained appreciable amounts of bacterial pathogens such as C. jejuni as well as nitrate, implying methemoglobinemia may be induced by bacterial nitrate reduction producing NO that converts hemoglobin to methemoglobin. The nitrate reduction by C. jejuni has not been fully investigated despite a potential correlation between excess nitrate in well water, the presence of C. jejuni and methemoglobinemia. Therefore, there is a need to understand nitrate reduction in C. jejuni at the molecular level.
Bacterial nitrate reduction is catalyzed by a class of pterin-containing molybdenum enzymes called nitrate reductase. There are three subclasses of prokaryotic nitrate reductases: periplasmic (Nap), respiratory (Nar) and assimilatory (Nas) (Stolz and Basu 2002). Of these, C. jejuni only harbors the genes for Nap (Pearson et al.2007). Nap is a dissimilatory enzyme complex, i.e. it is a catabolic complex that reduces nitrate. When Nap is coupled to the oxidation of formate or NADH, a proton gradient is generated enabling the production of ATP. Moreover, Nap can generate energy through nitrate respiration as part of the denitrification and dissimilatory nitrate reduction to ammonia pathways in bacteria (Stolz and Basu 2002; Sparacino-Watkins, Stolz and Basu 2014).
Unlike many pathogens, C. jejuni has no defining toxins and relies on other mechanisms for infection (Crofts et al.2018). The key metabolic pathways, like nitrate metabolism, that boost colonization may be one such mechanism. To this end, the oxidation of various electron donors such as FADH2, H2, formate, lactate or succinate can be coupled with nitrate (NO3−) reduction. Succinate is readily available in the host's GIT (Hofreuter 2014) where NO3− reduction has been recognized as an influential factor during C. jejuni host colonization (Weingarten, Grimes and Olson 2008; Liu et al.2012). Under inflammatory conditions the concentration of formate is increased and formate oxidation has been shown to be influenced by elevated nitrate levels (Hughes et al.2017).
During colonization in chickens, C. jejuni induces expression of the napAGHBLD operon (Woodall et al.2005) and during C. jejuni infection of mammalian cells, the expression of the catalytic subunit, NapA, is increased.(Liu et al.2012) A napA deletion decreased the adhesion of C. jejuni to human INT-407 cells and impacted motility and biofilm formation (Kassem et al.2012). Recently, Nap has been shown to be important in the pathogenesis of the intestinal pathogen Salmonella typhimurium that is also passed by poultry reservoirs to humans (Lopez et al.2015). It has been demonstrated that a NapA deletion reduces the ability to infect host cells signifying Nap influences pathogenesis (Lopez et al.2015). This result may indicate a common mechanism for intestinal pathogen survival via nitrate respiration using Nap.
Campylobacter jejuni NapA has not been biochemically characterized despite the significance of NO3− reduction by this pathogen. It has also been reported that there is a potential use of nitrate reductase genes for differentiating Campylobacter species (Miller et al.2007). Given the paucity of biochemical characterization of C. jejuni, fundamental information to understand nitrate reduction by C. jejuni is lacking. Here we report the cloning of napA, heterologous expression, and biochemical characterization of recombinant NapA (from here on referred to as the native enzyme). Using the expression system we have exchanged C176 (that putatively coordinates the molybdenum center) to serine. This variant has also been purified and biochemically characterized. In addition, we also compared the kinetic results with previously isolated NapA assayed by methyl viologen in solution.
MATERIALS AND METHODS
Plasmids and oligonucleotides are listed in Table S1 (supplementary material). Chromosomal DNA from the pathogenic C. jejuni strain RM1221 was used as the source of napA, napL and napD. Polymerase chain reaction (PCR) was used to amplify napLD maturation genes (YP_178877 and YP_178878) and the DNA fragment was inserted into the pTZS7R/T TA cloning vector (Thermo Fisher) yielding plasmid pBM8A. The napLD genes were excised using NcoI and EcoRI and then inserted into the pRSFDuet-1 expression vector (Novagen) to yield plasmid pBM9A.The napA gene (YP_178873) was amplified using PCR and the restriction enzyme-digested fragment was inserted into the pMCSG32 vector (DNASU) using NdeI and XmaI upstream from the TEV cleavable C-terminal hexa-histidine coding sequence, yielding the pMCSG32_napA plasmid. The napA gene was excised with NdeI and XhoI taking the tagged gene and inserting it into the same sites of pBM9A containing the maturation genes napLD to yield plasmid pBM10C. Thus, pBM10C allows coexpression of his tagged napA with untagged napLD in Escherichia coli from separate T7 promoters. All DNA constructs were confirmed by sequencing (ACGT Inc).
Escherichia coli K12 (New England Biolabs Shuffle T7 lysY #C3027) cells containing the pBM10C plasmid were maintained on LB medium supplemented with 30 μg/mL kanamycin. Inoculated cultures were grown overnight at 37°C, then transferred to 1 L of fresh autoinduction medium containing 12 g/L peptone, 24 g/L yeast extract, 1 g/L glucose, 2 g/L lactose, 0.50% (v/v) glycerol and 90 mM potassium phosphate buffer pH 7.00. The cultures were supplemented with kanamycin (30 μg/mL), Na2MoO4 (1 mM) and FeSO4 (0.5 mM), then incubated at room temperature for 48 h.
NapA (104 kDa) expression was induced by the lactose present in the medium. Expression was conducted at room temperature for 48 h while shaking. Cells were collected by centrifugation at 4400 × g at 4°C. The cell pellet was resuspended in ice cold buffer containing 50 mM HEPES, 300 mM sodium chloride and 10 mM imidazole at pH 7.00. Cells were lysed by ultrasonication using 30-s pulses in 45-s intervals over 10 min in an ice bath. The lysate was centrifuged at 7100 × g for 1 h at 4°C. The soluble fraction was loaded on a HisTrap HP 5 mL prepacked column (GE Life Sciences), and separation was conducted with an ÄKTA Prime Plus (GE Life Sciences) system. The column was washed with a step gradient of 20 and 50 mM imidazole. NapA was eluted with 250 mM imidazole. The fractions were pooled and concentrated during buffer exchange to 50 mM HEPES pH 7.00 using 30 kDa cutoff centrifugal filters (Millipore). The concentrated protein was loaded onto a HiPrep 16/60 Sephacryl S-200 size exclusion column (GE Healthcare). The resulting NapA fractions were pooled, concentrated and stored in buffer containing 50 mM HEPES pH 7.00 at –80°C. SDS-PAGE was used to screen fractions for NapA content and purity using standard protocols.
Mutagenesis of C. jejuni napA was conducted using the QuikChange II Site-Directed Mutagenesis Kit (Qiagen) with the pBM10C plasmid as template. The primers designed for a napA-C176S mutation are listed in Table S1 (Supporting Information). The PCR product was sequenced at ACGT Inc. The resulting plasmid, pBM10C-C176S, was expressed in E. coli, and NapA-C176S was purified in the same manner as the native NapA.
Nitrate reductase activity was measured spectrophotometrically by monitoring oxidation of reduced methyl viologen at 630 nm. Methyl viologen was reduced electrochemically in an inert atmosphere glovebox using a Metrohm PGASTAT204 potentiostat in a three electrode system with an Ag/AgCl reference electrode, a platinum wire auxiliary electrode and platinum mesh as the working electrode. The potential was held at –500 mV vs SHE (midpoint potential of methyl viologen is –449 mV vs SHE at pH 7.00; Watanabe and Honda 1982) until methyl viologen was reduced. Assays were conducted in an inert atmosphere glove box at 25°C using a Bio-Tek ELx808 Absorbance Microplate Reader. Assays were conducted with a total reaction volume of 300 μL. Nitrate addition initiated the reaction which was monitored for 5 min (NapA) or 15 min (NapA-C176S). The rate of methyl viologen oxidation was calculated using the Beer-Lambert law given the extinction coefficient of reduced methyl viologen (7800 M−1cm−1 at 630 nm). These rates were analyzed with a non-linear Michaelis–Menten model using OriginPro 2018 (OriginLab Inc.). Protein concentrations were determined using the Coomassie protein assay kit (Thermo Scientific) with bovine serum albumin standard (Pierce).
RESULTS
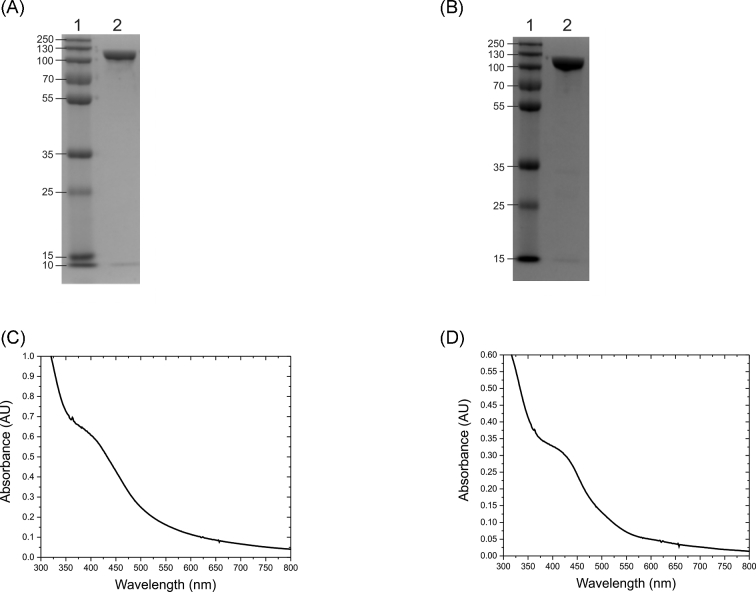
The napALD genes were coexpressed resulting in the production of recombinant NapA protein with the original C. jejuni N-terminal twin arginine translocase (TAT) leader sequence intact to preserve the NapA chaperone interactions. The napA gene contained a polyhistidine tag at the C-terminus that was kept intact for this investigation. NapA-hexaHis (hereafter referred to as NapA) was isolated by immobilized metal chromatography and purified to 95% homogeneity (Fig. 1A). The identity of the purified ∼100 kDa protein was confirmed to be NapA from C. jejuni by liquid chromatography-mass spectrometry (LC-MS).
Figure 1.
Characterization of native and variant C. jejuni NapA by gel electrophoresis and UV-vis spectroscopy. (A) SDS PAGE image of the NapA, (B) SDS PAGE image of the NapA-C176S variant where lane 1 is the molecular weight standards in kDa, (C) UV-vis spectrum of the as-prepared recombinant NapA in 50 mM HEPES pH 7.00, (D) UV-Vis spectrum of the as-prepared NapA-C176S variant in 50 mM HEPES pH 7.00.
The UV-Vis spectrum of C. jejuni NapA (Fig. 1C) shows a band at 400 nm similar to the band observed in the spectrum of Desulfovibrio desulfuricans NapA, indicating the presence of a [4Fe4S] cluster (Bursakov et al.1995). The metal (Mo and Fe) content in the enzyme was determined by inductively coupled plasma mass spectrometry (ICP-MS). The metal analyses indicate 92% Mo incorporation in active C. jejuni NapA. The Fe:Mo ratio was slightly higher than the theoretical value of 4:1 suggesting complete iron incorporation. (Table S2, Supporting Information)
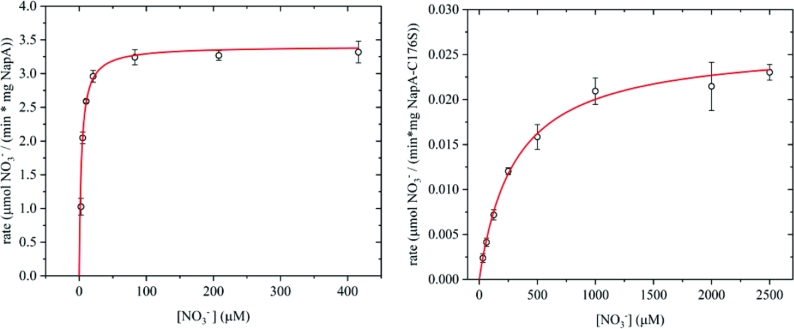
Campylobacter jejuni NapA displays Michaelis-Menten kinetics (Fig. 2), with a calculated maximum velocity (Vmax) of 3.40 ± 0.10 μmoles min−1 mg protein−1 and a KM for NO3− of 3.40 ± 0.44 μM. We determined a kcat of 5.91 ± 0.18 s−1 and calculated a kinetic putative second order rate (kcat/KM) constant of 1.74 × 106 M−1 s−1. A comparison of the KM and Vmax values with those reported for other characterized NapA (Table 1) reveals important differences in the kinetic properties. The major difference is the low KM of the heterologously produced C. jejuni NapA which indicates a high binding affinity for NO3−.
Figure 2.
Steady-state kinetic analysis using the Michaelis-Menten model. Left panel: steady-state kinetics of the reduction of nitrate by C. jejuni NapA. Right panel: steady-state kinetics of the reduction of nitrate by C. jejuni NapA-C176S variant. Note the Y-axes are different by ∼2 orders of magnitude and the solid lines represent Michaelis-Menten fit.
Table 1.
Kinetic parameters reported for NapA (A) or NapAB (AB) in various organisms using the methyl/benzyl viologen solution assay.
| Organism | pH | KM (μM) | kcat (s−1) | Vmax (μmoles nitrate min−1 mg−1) | kcat/KM (M−1 s−1) | Reference |
|---|---|---|---|---|---|---|
| Campylobacter jejuni (A) | 7.00 | 3.40 ± 0.44 | 5.91 ± 0.18 | 3.40 ± 0.10 | 1.74 × 106 | This work |
| Rhodobacter sphaeroides (A) | 7.00 | 120.00 | 70.20a | 39.00 | 5.85 × 105 | Sabaty et al. (2001) |
| Paracoccus pantotrophus (AB) | 7.00 | 112.00 | 58.00 | 5.18 × 105 | Gates, Richardson and Butt (2008) | |
| Paracoccus pantotrophus (AB) | 7.20 | 1300.00 | 240.00 | 1.85 × 105 | Butler et al. (1999) | |
| Magnetospirillum magnetotacticum (AB) | 7.00 | 3.20 | 2.50 | 7.81 × 105 | Taoka et al. (2003) | |
| Aliivibrio fischeri (AB) | 7.50 | 65.00 | 10.00a | 1.50 | 1.54 × 105 | Sadana and McElroy (1957) |
aValues calculated from kinetic parameters using the reported protein concentrations.
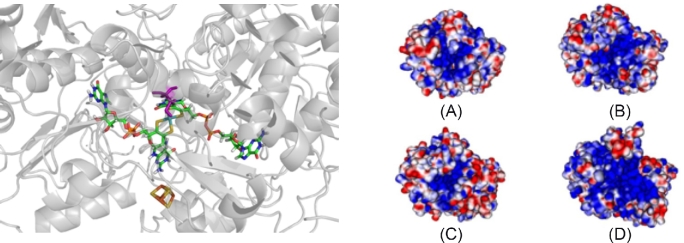
A three-dimensional model of the C. jejuni NapA structure was created using the structure of Rhodobacter sphaeroides NapA as the template (Sparacino-Watkins, Stolz and Basu 2014). The electrostatic potential was calculated for C. jejuni NapA, and, for comparison, the R. sphaeroides, D. desulfuricans and E. coli NapA structures (Fig. 3). In all structures, a dense region of positive charge is localized in the active site funnel, possibly used to facilitate the transport of NO3− into the cavity for catalytic transformation. The homology model (Sparacino-Watkins, Stolz and Basu 2014) suggests the substrate channel and catalytic pocket are more basic than other NapA proteins, which may influence the substrate binding as well as product release.
Figure 3.
Calculated structure and electrostatic potential map of NapA. Left panel: Pymol rendering of the metal centers of C. jejuni NapA homology model depicting the target for mutation, residue C176 (purple). Moco and the [4Fe4S] cluster are colored by element. The protein backbone is in gray. Right panel: the electrostatic potential plots for NapA showing the active site face of (a) D. desulfuricans, (b) E. coli, (c) R. sphaeroides and (d) C. jejuni. Desulfovibrio desulfuricans, E. coli and R. sphaeroides structure data were downloaded from PBD.org database. Comparison of the electrostatic maps reveals that NapA from C. jejuni is the most basic, as indicated by the blue color, while R. sphaeroides is the most acidic (red). Desulfovibrio desulfuricans and E. coli electrostatic potential maps are intermediate. The NapA structures were aligned and the electrostatic potentials were calculated in Pymol (–10 kT = red; 10 kT = blue; 0 kT = white).
Based on the homology model of C. jejuni NapA and the protein sequence alignment (Fig. S1, Supporting Information), we predicted that a strictly conserved cysteine at position 176 (C176), modeled in Fig. 3 (Sparacino-Watkins, Stolz and Basu 2014), is likely to coordinate the molybdenum center in C. jejuni. We hypothesize that a C176S mutation would reduce the catalytic activity of the enzyme. Similar mutations have been reported for related molybdenum enzymes including sulfite oxidase (coordinating Cys to Ser/Ala), biotin sulfoxide reductase (coordinating Ser to Cys/Ala/Thr), DMSO reductase (coordinating Ser to Cys/Ala/His), Nas (coordinating Cys to Ala) and Nap (coordinating Cys to Ser) (Garde, Kinghorn and Tomsett 1995; Garrett and Rajagopalan 1996; Trieber et al.1996; Hilton, Temple and Rajagopalan 1999; Pollock and Barber 2000; Hettmann et al.2004; Qiu et al.2010). These variants reduced or completely abolished activity compared to the native enzyme but there are no reports of determined rate constants (kcat or KM) (Table 2).
Table 2.
Reported mutations of the molybdenum coordinating residue in various mononuclear molybdenum enzymes.
| Enzyme | Mutation | Activity (% of WT) | Reference |
|---|---|---|---|
| C. jejuni NapA | C176S | 0.78% | This work |
| Chicken SO | C185S | Inactive | Qiu et al. (2010) |
| Chicken SO | C185A | Inactive | Qiu et al. (2010) |
| Human SO | C207S | <<1.00% | Garrett and Rajagopalan (1996) |
| R. sphaeroides BSOR | S121A | 0.30–3.00%a | Pollock and Barber (2000) |
| R. sphaeroides BSOR | S121T | 0.30–3.00%a | Pollock and Barber (2000) |
| R. sphaeroides BSOR | S121C | 0.30–3.00%a | Pollock and Barber (2000) |
| R. sphaeroides DMSOR | S147C | 37.00–41.00% | Hilton, Temple and Rajagopalan (1999) |
| E. coli DMSOR | S176A | <4.00%a | Trieber et al. (1996) |
| E. coli DMSOR | S176C | <4.00%a | Trieber et al. (1996) |
| E. coli DMSOR | S176H | <4.00%a | Trieber et al. (1996) |
| Aspergillus nidulans Nas | C150A | Inactive | Garde, Kinghorn and Tomsett (1995) |
| Cupriavidus necator Nap | C181S | Inactive | Hettmann et al. (2004) |
aActivity is indistinguishable from background, calculated from reported specific activity.
SO, sulfite oxidase; DMSOR, dimethyl sulfoxide reductase; BSOR, biotin sulfoxide reductase; Nas, assimilatory nitrate reductase; Nap, periplasmic nitrate reductase.
The NapA-C176S variant was observed to be pure to 97% homogeneity via SDS-PAGE (Fig. 1B). The identity of NapA and the presence of C176S substitution were confirmed by LC-MS (Fig. S2, Supporting Information) and metal content was determined by ICP-MS (Table S2, Supporting Information). Kinetic experiments with NapA-C176S indicated that this variant was active in reducing NO3− as a substrate but had significantly lower activity when compared to the native enzyme (Fig. 2). This mutation increased the KM (307 ± 16 μM) and reduced the kcat (0.045 ± 0.001 s−1) by two orders of magnitude.
DISCUSSION
NapA has been isolated from native organisms as a heterodimer with the diheme c-type cytochrome NapB, from E. coli (Jepson et al.2007), R. sphaeroides (Arnoux et al.2003), Achromobacter fischeri (Sadana and McElroy 1957), Cupriavidus necator (Coelho et al.2007) and Thiosphaera pantotropha (Berks et al.1994), or as a monomer from D. desulfuricans (Bursakov et al.1995). Whether the Nap is monomeric or heterodimeric depends on its ability to form salt bridges at the NapA:NapB interface (Simpson, McKinzie and Codd 2010). Two residues E76 and S801 (in R. sphareoides, Rs; Fig. S1, Supporting Information) have been identified as critical for the formation of the NapAB heterodimer (Simpson, McKinzie and Codd 2010). The sequence alignment of C. jejuni NapA with R. sphaeroides NapA shows the presence of a proline (P70) instead of a critical glutamate precluding the formation of the salt bridge, and thus C. jejuni NapA was expected to be a monomer in solution. Escherichia coli NapA also contains this proline substitution, and Jepson et al. (2007) reported that although NapA and NapB interact, the NapAB complex is not tight and the subunits purify independently. NapA representatives from each class of gram-negative Proteobacteria have been isolated except the Epsilonproteobacteria. The class of Epsilonproteobacteria includes notable human pathogens like Helicobacter and Campylobacter species. To date, this is the first active epsilonproteobacterial NapA to be isolated and have its enzymatic properties explored.
Successful heterologous production of a metalloprotein often requires the coexpression of genes encoding dedicated chaperones to ensure proper folding and metal center insertion. Expression of Pseudomonas strain G-179 NapA in E. coli, without the cognate maturation proteins, resulted in non-functional NapA found in inclusion bodies (Bedzyk, Wang and Ye 1999). Genetic experiments with Wolinella succinogenes show napL and napD to be critical for full activity in a similar Nap system (Kern, Mager and Simon 2007). These studies emphasize the importance of maturation proteins in obtaining functional enzyme. To maximize heterologous production of active NapA containing both a molybdenum cofactor and a [4Fe4S] cluster, the maturation proteins NapL and NapD were coexpressed in this study. In addition, the TAT leader sequence on NapA was not modified to preserve the possible interactions with these maturation proteins. The C. jejuni TAT sequence varies from the E. coli sequence (Fig. S1, Supporting Information); however, pure active enzyme was isolated despite this difference. The UV-Vis spectrum (Fig. 1C) is indicative of the iron-sulfur cluster, which is supported by the ICP-MS data. Furthermore, the high molybdenum incorporation confirms an effective expression system for active C. jejuni NapA has been achieved. To our knowledge, this represents the first example of a heterologously expressed functional periplasmic nitrate reductase.
The data presented here reveal that C. jejuni NapA is a very efficient NO3− reducing enzyme with a high kcat/KM value and low KM. The low KM indicates a high binding affinity for NO3− (Table 1), which is consistent with the electrostatic potential calculations (Fig. 3) (Sparacino-Watkins, Stolz and Basu 2014). The low KM could prove useful to the pathogen when competing for nitrate with the commensal nitrate utilizing organisms of the host microflora. Nitrate metabolism has been positively associated with colonization by Salmonella (Lopez et al.2015), E. coli (Winter et al.2013) and C. jejuni (Kassem et al.2012).
Both solution-based assay and protein film voltammetry (PFV) have been used in understanding the kinetic properties of Nap, although they exhibit some differences. For example, R. sphaeroides NapAB has a reported KM of 7.50 μM, which is comparable to the C. jejuni NapA KM of 3.40 μM (Frangioni et al.2004). Interestingly, Bertrand et al. (2007) argued the KM measured in solution assays potentially depends on all rates in the catalytic cycle and will depart from KM determined by PFV, if intermolecular electron transfer is the rate determining step in solution assays. The KM values determined in solution may be higher than those determined by PFV. For this reason, we only discuss solution-based parameters obtained by using reduced methyl viologen as an electron donor (Table 1).
Compared to other Nap proteins in Table 1, C. jejuni NapA has the second highest substrate affinity, second only to Magnetospirillum magnetotacticum. Interestingly, M. magnetotacticum prefers microaerobic environments like C. jejuni (Maratea and Blakemore 1981). Although M. magnetotacticum is not known to be pathogenic, its nap operon does appear to be phylogenetically closer to C. jejuni nap than its fellow α-proteobacteria (Sparacino-Watkins, Stolz and Basu 2014). Furthermore, C. jejuni NapA appears to be a more efficient NO3− reducer than NapA from M. magnetotacticum as well as all other reported Nap proteins (Table 1) by approximately one order of magnitude in the kcat/KM. Although the kcat/KM is higher, kcat itself is lower than the homologously expressed Naps in Table 1. A lower kcat may be attributable to substrate/product inhibition, attenuated electron transfer or conformational change. However, the exact reason for a lower kcat remains an open question.
Campylobacter jejuni NapA is expected to and does exhibit a higher affinity towards NO3−. We suggest there is a higher affinity for NO3− by C. jejuni NapA because it encounters low concentrations of NO3− under physiological conditions (Winter et al.2013; Lopez et al.2015). Although the inflammatory response increases nitrate concentration in the GIT of the host (Winter et al.2013; Lopez et al.2015; Hughes et al.2017), the concentration is below 1 mM (Winter et al.2013; Lopez et al.2015). Nap is expressed maximally at 1 mM and does not express above 6 mM while Nar expresses maximally at 10 mM and does not express below 1 mM suggesting that Nap will be the primary nitrate reductase expressed at a low nitrate concentration (Wang, Tseng and Gunsalus 1999). Therefore, a pathogen that uses Nap may have an advantage under these conditions and Nap may exhibit a high affinity for NO3− (Lopez et al.2015).
It has been suggested that Nap faces the periplasm and acts as a NO3− scavenger as Nap does not depend on NO3− transport (Simpson, Richardson and Codd 2010). It is interesting that C. jejuni NapA has a higher binding affinity for NO3− (low KM) and a higher efficiency for producing nitrite than the NapA proteins from the non-pathogenic microbes. Genetic experimentation suggests that NO3− metabolism is important in the physiology of C. jejuni (Woodall et al.2005; Kassem et al.2012; Liu et al.2012). The high efficiency of this enzyme compared to Nap proteins from non-pathogenic organisms suggests nitrate metabolism is important to this intestinal pathogen, but its role in pathogenicity is not completely clear. We suggest that NO3− is one of the metabolites or substrates that the pathogen relies on to ensure proper colonization into the gut of its host.
We have successfully altered the Mo coordinating residue in NapA, cysteine 176, to serine (Fig. S2, Supporting Information). The resulting variant is analogous to the coordination of Mo in DMSO reductase and trimethylamine N-oxide reductase (Li et al.2000). Similar alterations in other Mo enzymes show negligible or no activity (Table 2). In these cases, steady-state kinetic parameters, KM and kcat, were not reported. We have conducted Michaelis Menten kinetics on the C. jejuni NapA-C176S variant. This variant can reduce nitrate; however, the efficiency is lowered by four orders of magnitude. Interestingly, the KM changed by two orders of magnitude suggesting the serine residue impacts substrate affinity as well. We suggest that the mutation could induce a rearrangement of the active site altering substrate access or by destabilizing a key interaction that assists in substrate docking. The overall reduction in the catalytic rate is also modulated by this mutation, possibly in part due to a change in redox potential of the Mo center. Such a change in the reduction potential has been observed in model complexes (Uhrhammer and Schultz 2004).
In summary, C. jejuni NapA has been heterologously expressed in E. coli. To our knowledge, this is the first example of a functional NapA from an epsilonproteobacterium that has been overexpressed and purified. Kinetic analysis of C. jejuni NapA revealed a high substrate binding affinity and kinetic efficiency. The sequence alignment of NapA suggests C176 coordinates Mo. When this residue is exchanged for a serine, NO3− reductase activity is severely attenuated. The high substrate affinity (low μM range) of C. jejuni NapA suggests the Nap system has a role in scavenging for NO3− which has a relatively low concentration in the GIT with NO3− concentrations under 1 mM. Even during inflammation when nitric oxide synthase is overexpressed leading to a higher production of nitrate, the nitrate concentration does not exceed 1 mM (Winter et al.2013; Lopez et al.2015). These findings are in agreement with Lopez et al. and underscore the importance of Nap in the physiology of pathogens such as C. jejuni.
SUPPLEMENTARY DATA
Supplementary data are available at FEMSLE online.
Supplementary Material
Acknowledgements
We thank Drs A. Andrew Pacheco for discussions, and Lina Bird and Peter Chovanec for preliminary experiments. We thank Dr William Miller for genomic DNA of C. jejuni RM1221. We greatly appreciate all assistance from Dr Maissa Gaye for preliminary LC-MS experiments and Dr Gabriel Filippelli for preliminary ICP-OES experiments. Partial support of this work from PA CURE, DOE (DE-FG027ER64372) and National Institute of Health (GM061555 to PB) is gratefully acknowledged.
AUTHOR CONTRIBUTIONS
CSW conducted preliminary NapA experiments in various expression systems. KFS, GS, AM, JS, JRM aided in the molecular cloning aspects. JFS made intellectual contributions in biochemical aspects. DJB provided expertise and assistance with ICP-MS. JMM conducted native NapA kinetics and LC-MS confirmation of protein isolates. BM conducted bioinformatic analyses, molecular cloning, protein isolation, mutagenesis experiments, and NapA-C176S kinetics. BM and PB wrote the majority of the manuscript while all authors contributed to the editing.
Conflict of interest. None declared.
REFERENCES
- Amour C, Mduma E, Gratz J et al. Epidemiology and impact of campylobacter infection in children in 8 Low-Resource settings: Results from the MAL-ED Study. Clin Infect Dis 2016;63:1171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoux P, Sabaty M, Alric J et al. Structural and redox plasticity in the heterodimeric periplasmic nitrate reductase. Nat Struct Mol Biol 2003;10:928–34. [DOI] [PubMed] [Google Scholar]
- Bedzyk L, Wang T, Ye RW. The periplasmic nitrate reductase in Pseudomonas sp. strain G-179 catalyzes the first step of denitrification. J Bacteriol 1999;181:2802–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berks BC, Richardson DJ, Robinson C et al. Purification and characterization of the periplasmic nitrate reductase from Thiosphaera pantotropha. Eur J Biochem 1994;220:117–24. [DOI] [PubMed] [Google Scholar]
- Bertrand P, Frangioni B, Dementin S et al. Effects of slow substrate binding and release in redox enzymes: theory and application to periplasmic nitrate reductase. J Phys Chem B 2007;111:10300–11. [DOI] [PubMed] [Google Scholar]
- Bursakov S, Liu M-Y, Payne WJ et al. Isolation and preliminary characterization of a soluble nitrate reductase from the sulfate reducing organism Desulfovibrio desulfuricans ATCC 27774. Anaerobe 1995;1:55–60. [DOI] [PubMed] [Google Scholar]
- Butler CS, Charnock JM, Bennett B et al. Models for molybdenum coordination during the catalytic cycle of periplasmic nitrate reductase from Paracoccus denitrificans derived from EPR and EXAFS spectroscopy. Biochemistry 1999;38:9000–12. [DOI] [PubMed] [Google Scholar]
- Coelho C, González PJ, Trincão J et al. Heterodimeric nitrate reductase (NapAB) from Cupriavidus necator H16: purification, crystallization and preliminary X-ray analysis. Acta Crystallogr F 2007;63:516–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts AA, Poly FM, Ewing CP et al. Campylobacter jejuni transcriptional and genetic adaptation during human infection. Nat Microbiol 2018;3:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epps SVR, Harvey RB, Hume ME et al. Foodborne Campylobacter: infections, metabolism, pathogenesis and reservoirs. Int J Environ Res Pu 2013;10:6292–304, 6213 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangioni B, Arnoux P, Sabaty M et al. In Rhodobacter sphaeroides respiratory nitrate reductase, the kinetics of substrate binding favors intramolecular electron transfer. J Am Chem Soc 2004;126:1328–9. [DOI] [PubMed] [Google Scholar]
- Garde J, Kinghorn JR, Tomsett AB. Site-directed mutagenesis of nitrate reductase from Aspergillus nidulans. Identification of some essential and some nonessential amino acids among conserved residues. J Biol Chem 1995;270:6644–50. [DOI] [PubMed] [Google Scholar]
- Garrett RM, Rajagopalan KV. Site-directed mutagenesis of recombinant sulfite oxidase. J Biol Chem 1996;271:7387–91. [PubMed] [Google Scholar]
- Gates AJ, Richardson DJ, Butt JN. Voltammetric characterization of the aerobic energy-dissipating nitrate reductase of Paracoccus pantotrophus: exploring the activity of a redox-balancing enzyme as a function of electrochemical potential. Biochem J 2008;409:159–68. [DOI] [PubMed] [Google Scholar]
- Hettmann T, Siddiqui RA, Frey C et al. Mutagenesis study on amino acids around the molybdenum centre of the periplasmic nitrate reductase from Ralstonia eutropha. Biochem Bioph Res Co 2004;320:1211–9. [DOI] [PubMed] [Google Scholar]
- Hilton JC, Temple CA, Rajagopalan KV. Re-design of Rhodobacter sphaeroides dimethyl sulfoxide reductase. Enhancement of adenosine N1-oxide reductase activity. J Biol Chem 1999;274:8428–36. [DOI] [PubMed] [Google Scholar]
- Hofreuter D. Defining the metabolic requirements for the growth and colonization capacity of Campylobacter jejuni. Front Cell Infect Microbiol 2014;4:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ER, Winter MG, Duerkop BA et al. Microbial respiration and formate oxidation as metabolic signatures of inflammation-associated dysbiosis. Cell Host Microbe 2017;21:208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson BJN, Mohan S, Clarke TA et al. Spectropotentiometric and structural analysis of the periplasmic nitrate reductase from Escherichia coli. J Biol Chem 2007;282:6425–37. [DOI] [PubMed] [Google Scholar]
- Johnson TJ, Shank JM, Johnson JG. Current and potential treatments for reducing campylobacter colonization in animal hosts and disease in humans. Front Microbiol 2017;8:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem II, Khatri M, Esseili MA et al. Respiratory proteins contribute differentially to Campylobacter jejuni's survival and in vitro interaction with host's intestinal cells. BMC Microbiol 2012;12:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern M, Mager AM, Simon J. Role of individual nap gene cluster products in NapC-independent nitrate respiration of Wolinella succinogenes. Microbiology 2007;153:3739–47. [DOI] [PubMed] [Google Scholar]
- Knobeloch L, Salna B, Hogan A et al. Blue babies and nitrate-contaminated well water. Environ Health Perspect 2000;108:675–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H-K, Temple C, Rajagopalan KV et al. The 1.3 Å crystal structure of Rhodobacter sphaeroides dimethyl sulfoxide reductase reveals two distinct molybdenum coordination environments. J Am Chem Soc 2000;122:7673–80. [Google Scholar]
- Liu J, Platts-Mills JA, Juma J et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet North Am Ed 2016;388:1291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Gao B, Novik V et al. Quantitative proteomics of intracellular Campylobacter jejuni reveals metabolic reprogramming. PLoS Pathog 2012;8:e1002562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez CA, Rivera-Chavez F, Byndloss MX et al. The periplasmic nitrate reductase napABC supports luminal growth of Salmonella enterica serovar typhimurium during colitis. Infect Immun 2015;83:3470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maratea D, Blakemore RP. Aquaspirillium magnetotactium sp. nov, a Magnetic Spirillum. Int J Syst Evol Microbiol 1981;31:452–5. [Google Scholar]
- Miller WG, Parker CT, Heath S et al. Identification of genomic differences between Campylobacter jejuni subsp. jejuni and C. jejuni subsp. doylei at the nap locus leads to the development of a C. jejuni subspeciation multiplex PCR method. BMC Microbiol 2007;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson BM, Gaskin DJH, Segers RPAM et al. The complete genome sequence of Campylobacter jejuni strain 81116 (NCTC11828). J Bacteriol 2007;189:8402–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock VV, Barber MJ. Serine 121 is an essential amino acid for biotin sulfoxide reductase functionality. J Biol Chem 2000;275:35086–90. [DOI] [PubMed] [Google Scholar]
- Powlson DS, Addiscott TM, Benjamin N et al. When does nitrate become a risk for humans? J Environ Qual 2008;37:291–5. [DOI] [PubMed] [Google Scholar]
- Qiu JA, Wilson HL, Pushie MJ et al. The structures of the C185S and C185A mutants of sulfite oxidase reveal rearrangement of the active site. Biochemistry 2010;49:3989–4000. [DOI] [PubMed] [Google Scholar]
- Sabaty M, Avazeri C, Pignol D et al. Characterization of the reduction of selenate and tellurite by nitrate reductases. Appl Environ Microb 2001;67:5122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadana JC, McElroy WD. Nitrate reductase from Achromobacter fischeri. purification and properties: function of flavines and cytochrome. Arch Biochem Biophys 1957;67:16–34. [DOI] [PubMed] [Google Scholar]
- Simpson PJL, McKinzie AA, Codd R. Resolution of two native monomeric 90kDa nitrate reductase active proteins from Shewanella gelidimarina and the sequence of two napA genes. Biochem Bioph Res Co 2010;398:13–18. [DOI] [PubMed] [Google Scholar]
- Simpson PJL, Richardson DJ, Codd R. The periplasmic nitrate reductase in Shewanella: the resolution, distribution and functional implications of two NAP isoforms, NapEDABC and NapDAGHB. Microbiology 2010;156:302–12. [DOI] [PubMed] [Google Scholar]
- Sparacino-Watkins C, Stolz JF, Basu P. Nitrate and periplasmic nitrate reductases. Chem Soc Rev 2014;43:676–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz JF, Basu P. Evolution of nitrate reductase: molecular and structural variations on a common function. ChemBioChem 2002;3:198–206. [DOI] [PubMed] [Google Scholar]
- Taoka A, Yoshimatsu K, Kanemori M et al. Nitrate reductase from the magnetotactic bacterium Magnetospirillum magnetotacticum MS-1: Purification and sequence analyses. Can J Microbiol 2003;49:197–206. [DOI] [PubMed] [Google Scholar]
- Trieber CA, Rothery RA, Weiner JH. Consequences of removal of a molybdenum ligand (DmsA-Ser-176) of Escherichia coli dimethyl sulfoxide reductase. J Biol Chem 1996;271:27339–45. [DOI] [PubMed] [Google Scholar]
- Uhrhammer D, Schultz FA. Modulation of molybdenum-centered redox potentials and electron-transfer rates by sulfur versus oxygen ligation. Inorg Chem 2004;43:7389–95. [DOI] [PubMed] [Google Scholar]
- Wang H, Tseng C-P, Gunsalus RP. The napF and narG nitrate reductase operons in Escherichia coli are differentially expressed in response to submicromolar concentrations of nitrate but not nitrite. J Bacteriol 1999;181:5303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Honda K. Measurement of the extinction coefficient of the methyl viologen cation radical and the efficiency of its formation by semiconductor photocatalysis. J Phys Chem 1982;86:2617–9. [Google Scholar]
- Weingarten RA, Grimes JL, Olson JW. Role of Campylobacter jejuni respiratory oxidases and reductases in host colonization. Appl Environ Microbiol 2008;74:1367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SE, Winter MG, Xavier MN et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 2013;339:708–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodall CA, Jones MA, Barrow PA et al. Campylobacter jejuni gene expression in the chick cecum: Evidence for adaptation to a low-oxygen environment. Infect Immun 2005;73:5278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.