Abstract
The coordination of pituitary development is complicated and requires input from multiple cellular processes. Recent research has provided insight into key molecular determinants that govern cell fate specification in the pituitary. Moreover, increasing research aimed to identify, characterize, and functionally describe the presumptive pituitary stem cell population has allowed for a better understanding of the processes that govern endocrine cell differentiation in the developing pituitary. The culmination of this research has led to the ability of investigators to recapitulate some of embryonic pituitary development in vitro, the first steps to developing novel regenerative therapies for pituitary diseases. In this current review, we cover the major players in pituitary stem/progenitor cell function and maintenance, and the key molecular determinants of endocrine cell specification. In addition, we discuss the contribution of peripheral hormonal regulation of pituitary gland development, an understudied area of research.
Keywords: anterior pituitary, developmental biology, stem cells, hormones
Key transcription factors, extracellular molecular networks, and hormones work in concert to coordinate lineage specification and differentiation in the developing pituitary.
Introduction
The pituitary is an endocrine gland that dynamically regulates peripheral tissues to coordinate fundamental physiological functions such as growth, metabolism, sexual maturity and reproduction, pigmentation, the body's response to stress. The pituitary regulates these homeostatic processes by interpreting hypothalamic signals and, in response, releases hormones from specialized cells in the anterior pituitary. These cell types include somatotropes that produce growth hormone (GH), thyrotropes that produce thyroid stimulating hormone (TSH), lactotropes that produce prolactin (PRL), gonadotropes produce luteinizing hormone (LH) and follicle-stimulating hormone (FSH), melanotropes that produce melanocyte-stimulating hormone (αMSH), and corticotropes that produce adrenocorticotropic hormone (ACTH).
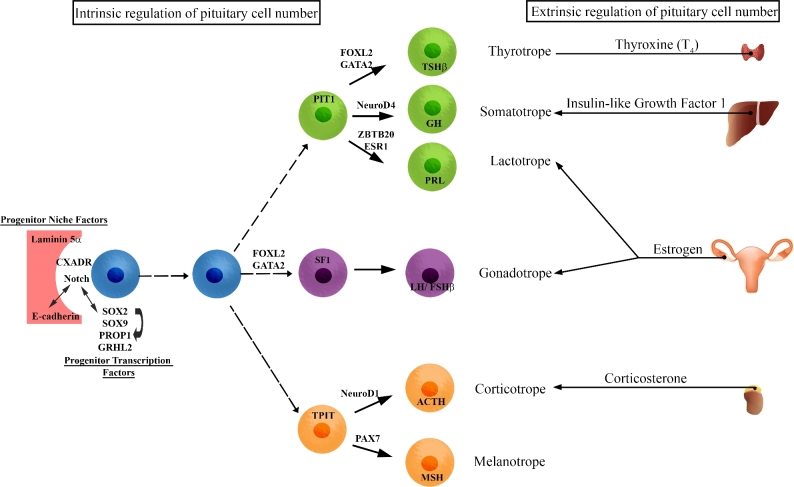
The use of genetic mouse models has provided novel insight to the molecular mechanisms that govern pituitary development and maintenance. These models have identified key transcription factors and signaling pathways that are necessary for normal pituitary development in both mouse and humans. More recently, the identification of a presumptive stem cell/progenitor population in the pituitary gland has provided insight into processes that underlie endocrine cell specification and organ homeostasis. These cells, identified by their expression of hallmark stemness markers, contribute to embryonic and postnatal development of the gland, as well as generation of endocrine cell types in response to physiological demands such as puberty, pregnancy, and injury. The current model of pituitary cell differentiation involves multipotent stem/progenitor cells giving rise to a progenitor population that functions as rapidly dividing transit-amplify cells. These cells then give rise to committed precursor cells that, under the influence of lineage-specific differentiation cues, will further mature into the hormone producing cells. The progression from stem/progenitor cell to a fully functional endocrine cell is dependent on a multifactorial process that integrates several factors including morphogenic factors, cell-to-cell signaling, key transcription factors, and hormonal influences (Figure 1).
Figure 1.
Coordination of pituitary progenitor differentiation into the endocrine cell types requires both intrinsic and extrinsic factors. During pituitary gland development, the hormone producing cell types are derived from a common progenitor cell. The balance of progenitor cell maintenance and differentiation is necessary for proper gland development. Maintenance of the progenitor cell compartment is dependent on transcription factors and niche components. Endocrine cell differentiation and cell number is coordinated by both transcription factors and peripheral hormones.
The importance of pituitary stem cell research is to potentially develop cell-based treatments for pituitary diseases that result in the absence or decreased levels of pituitary hormones such as combined pituitary hormone deficiency (CPHD), traumatic brain injury, or after surgical or radiation treatment of pituitary tumors. The goal is to treat patients with stem cells that can regenerate the endocrine cells types and restore normal pituitary functions. Recent studies have provided evidence that this type of advanced treatment is possible; however, more knowledge about pituitary development is necessary to improve these types of medical technologies. In this review, we aim to discuss the major determinants of cell lineage specification in the developing pituitary. We will review the well-known as well as the most novel factors that are important for the function and maintenance of the pituitary stem/progenitor cells, intrinsic factors that are necessary for lineage differentiation, and the extrinsic factors that contribute to pituitary expansion.
Identification, regulation, and function of the stem/progenitor cells population
Several groups have provided convincing evidence that stem/progenitor cells are present in the pituitary and are required for proper development and maintenance of the gland. Initial studies identifying this population of cells took advantage of inherent properties of stem cells including sphere-forming capabilities and side population isolation via verapamil sensitive dye efflux assays [1, 2]. These early studies identified a small number of cells that expressed a cohort of genes that are characteristic of stem cell function and maintenance in other organs [1, 2]. Subsequent research demonstrated that these populations of cells are capable of differentiating into all of the endocrine cell types during neonatal development of the pituitary and contribute to gland regeneration in the adult [3, 4] (Table 1).
Table 1.
Transcription factors and niche components identified in the pituitary progenitor compartment.
| Marker | Gene Name | Reference |
|---|---|---|
| Transcription factors | ||
| Sox2 | SRY-box containing gene 2 | [3, 4, 9] |
| Sox9 | SRY-box containing gene 9 | [1, 2, 4, 9, 96] |
| Prop1 | Prophet of Pit1, paired-like homeodomain transcription factor | [2, 12–16] |
| Grhl2 | Grainyhead like transcription factor 2 | [26] |
| Oct4 (Pou5f1) | Octamer-binding protein 4 | [1, 2, 97] |
| Prrx1 | Paired related homeobox protein 1 | [98, 99] |
| Prrx2 | Paired related homeobox protein 2 | [98, 99] |
| Ctnnb1 (β- Catenin) | Cadherin-associated protein beta | [100, 101] |
| Notch signaling components | ||
| Hes1 | Hairy and enhancer of split 1 | [22, 23, 28, 102] |
| Hey1 | Hairy/enhancer-of-split related with YRPW motif 1 | [28, 97] |
| Dll1 | Delta like canonical Notch ligand 1 | [15, 22, 23, 28] |
| Jag1 | Jagged 1 | [97, 102] |
| Notch1 | Notch 1 | [1, 97, 102] |
| Notch2 | Notch 2 | [15, 22, 23, 28, 97, 102] |
| Notch3 | Notch 3 | [23, 97, 102] |
| Extracellular receptors/cell surface antigens | ||
| Ly6a (Sca1) | Stem cell antigen 1 | [1, 97, 102] |
| SSEA-4 | Stage-specific embryonic antigen-4 | [2] |
| Cxadr | Coxsackievirus and adenovirus receptor | [2, 103] |
| Cxcr4 | C-X-C motif chemokine receptor 4 | [97, 103, 104] |
| Lgr5 | Leucine rich repeat containing G protein-coupled receptor 5 | [97] |
| CD44 | CD44 antigen | [97] |
| Gfra2 | GDNF family receptor alpha 2 | [2] |
| Cellular adhesion molecules | ||
| Cdh1 (E-cadherin) | Calcium-dependent adhesion protein | [2, 9, 26] |
| Lama5 (Laminin 5α) | Laminin subunit alpha 5 | [39] |
| Itgb1 (Integrin 1β) | Integrin subunit beta 1 | [104] |
| Other | ||
| S100b | S-100 protein subunit beta | [1, 2, 9, 16, 20, 103] |
| Nestin | Nestin | [1, 97] |
TFs that identify the stem cell population
SOX2
One of the earliest markers of the stem/progenitor population in the developing pituitary is sex determining region Y-box 2 (SOX2), a high mobility group box transcription factor, known to regulate stem cell plasticity during embryonic development [5, 6]. In humans and mice, loss of SOX2 in the pituitary results in varying degrees of hypopituitarism, demonstrating its essential role in normal pituitary development [6–8]. SOX2 is ubiquitously expressed in proliferating epithelial progenitors throughout Rathke's pouch (RP) as early as embryonic day 11.5 (e11.5) [9]. In postnatal development and in the adult, SOX2 expression decreases and becomes restricted to the marginal zone cells that surround the lumen. Interestingly, during early-postnatal maturation, clusters of SOX2-positive cells can also be found in the anterior lobe parenchyma [10]. This population of stem/progenitor is regionally distinct but remains in a homotypic network with the niche located in the marginal zone [11]. Studies have shown that all of the hormone-producing cell types are derived from a SOX2-expressing precursor cell, providing convincing evidence that these cells are in fact stem/progenitors in the pituitary [3, 4, 9]. SOX2-positive cells also express additional progenitor transcription factors including sex determining region Y-box 9 (SOX9), a marker of multipotent progenitors in other tissues [1, 2, 4, 9]. During early embryonic development of the gland, the progenitor population weakly expresses SOX9 but during postnatal development and in the adult the majority of SOX2 cells are also positive for SOX9 [9].
PROP1
The molecular markers that define the stem/progenitor cell population do not appear to be constant throughout maturation of the pituitary, suggesting a sequential development of the progenitor population and potentially a heterogeneous population of these cells. This theory is further supported by the temporal and regionally specific expression of paired like homeodomain factor 1 (PROP1), a pituitary specific transcription factor that has also been identified as a marker of the stem/progenitor cell population. PROP1 is weakly detected in the progenitor cell niche of RP at e11.5, a time in which SOX2 is highly expressed. However, by e13.5 the majority of luminal progenitors in RP are positive for both PROP1 and SOX2 [12–14]. Beginning in the late embryonic and early postnatal development, PROP1-positive cells are excluded from the intermediate lobe and become restricted to marginal zone and parenchyma of anterior lobe. In the adult, PROP1 is maintained in the marginal zone albeit at a significantly lower level [2, 13–16]. PROP1 mutations are the most common identified cause of hypopituitarism in humans [17, 18]. This phenotype is recapitulated Ames dwarf mice (Prop1df/df) that harbor a loss-of-function mutation in PROP1. Characterization of the progenitor cell population of these mice has clearly defined a role for PROP1 in maintenance and function of the stem/progenitor cell population in the pituitary. Prop1df/df mice display an inability of progenitor cells to migrate away from the marginal zone indicative of impaired epithelial-to-mesenchymal transition [14]. This is coincident with decreased expression of proliferation markers and decreased expression of NOTCH2, an important regulator of stem cell maintenance in the developing pituitary. Recently, lineage tracing studies have demonstrated that PROP1-expressing cells are able to differentiate into all of the anterior lobe cell types [19]. These data, in combination with previous studies that observed PROP1 in the adult pituitary stem cell niche, solidify PROP1 as a bona fide marker and regulator of the stem/progenitor population.
S100β
A subset of anterior lobe cells that express S100 protein, β polypeptide, neural (S100β) have been identified as folliculo-stellate cells. These cells are nongranular and display long cytoplasmic processes that aid in maintaining cellular networks. S100β-positive cells are not present during embryonic development and a subset of these cells are thought to function as a postnatal specific stem/progenitor cell population. It has been shown that a subpopulation of folliculo-stellate have colony-forming capacity and the ability to differentiate into endocrine cell types [20, 21]. In addition, multiple studies have demonstrated that S100β is detected in cells that express other stem cell makers including SOX2 and PROP1, although not exclusively. Interestingly, it appears that there is a progressive increase in SOX2/S100β-positive cells as the pituitary develops, with approximately 85% of SOX2 cells co-expressing S100β in the adult rat [2, 9, 16]. The fact that these cells are morphologically, temporally, and regionally distinct from other stem/progenitor cell populations in the pituitary may suggest that they constitute a specialized progenitor cell niche.
Stem cell niche factors
The maintenance of the stem/progenitor cell population is not only dependent on the expression of transcription factors that regulate cellular plasticity but also on the components of their specialized microenvironments. Factors that compose the stem cell niche are tissue specific and often include structural proteins that make up the extracellular matrix (ECM), cell surface receptors that function in cell-to-cell communication, soluble factors, supportive cells, and cell surface proteins. In the pituitary, relatively little is known about the niche components that contribute to stem/progenitor cell maintenance and function. However, a few factors that contribute to the structural integrity and cell–cell interactions in the pituitary stem/progenitor cell niche have been investigated.
Notch signaling
Notch signaling is a cell-to-cell contact-dependent pathway that regulates stem cell maintenance and cell fate choice in many organs including the developing pituitary. Activation of the pathway requires a cell expressing a Notch ligand to activate a Notch receptor on an adjacent cell. The interaction of the receptor and the ligand results in two successive proteolytic cleavages, the first by a disintegrin and metallopeptidase domain 10 (ADAM10) which releases the Notch extracellular domain of the receptor. Subsequently, the second cleavage by gamma secretase releases the Notch intracellular domain (NICD), which translocates to the nucleus and regulates transcription of target genes via binding of the co-factor protein recombination signal binding protein for immunoglobulin kappa J region (RBPJ). Core components of the Notch signaling pathway define the stem/progenitor cell compartment of the developing pituitary. The receptors Notch2 and Notch3, the ligands Jagged 1 (Jag1) and delta like canonical Notch ligand 1 (Dll1), as well as the canonical downstream target genes hes family basic helix-loop-helix factor 1 (Hes1) and hairy/enhancer-of-split related with YRPW motif 1 (Hey1) are detected in RP in proliferating progenitors during early pituitary development [22–24]. During postnatal development and in the adult, expression of these genes is downregulated but maintained in the presumptive stem cell compartment, following an expression pattern similar to that of other stem cell makers.
Genetic mouse models have provided useful insight into role of Notch signaling in the developing pituitary. Conditional loss of either the essential Notch cofactor Rbpj or the notch effector gene Hes1 during embryonic pituitary development results in a hypoplastic pituitary, decreased proliferation of pituitary progenitors, and premature cell cycle exit [23, 25]. A similar progenitor phenotype was observed in mice with a conditional loss of the Notch2 receptor (Notch2 cKO) specifically during postnatal pituitary development. These mice display decreased progenitor proliferation, and a decrease in expression of the well-known progenitor cell markers SOX2 and SOX9 as well as the novel pituitary progenitor marker grainyhead like 2 (GRHL2) [26]. Additionally, pituitary stem cells in culture rely on Notch signaling because loss of Rbpj or treatment with DAPT, a gamma secretase inhibitor, results in the inability of progenitors to expand and form pituispheres [15]. These findings suggest that Notch may directly regulate the expression transcription factors necessary for stem cell plasticity in the pituitary, as all of these have been shown to be directly regulated by Notch in other systems [27]. Furthermore, both mice with a loss of Notch2 or Rbpj have decreased expression of Prop1, which was also shown to be a direct Notch target in the pituitary [23, 28]. PROP1 is also thought to be a regulator of Notch2 expression suggesting a reciprocal relationship between these two factors [14, 22, 29, 30]. In agreement, overexpression of Notch in the pituitary inhibits differentiation of the hormone producing cell types and results in ectopic expression of SOX2 [31].
Active Notch signaling may also regulate the expression of cell adhesion molecules that are crucial for the maintenance of the stem/progenitor cell niche in the pituitary. Gene expression studies have shown that the pituitary progenitor population is enriched for both Notch signaling components and cadherin 1 (Cdh1; also known as Ecad), a cellular adhesion molecule, known to be an important regulator of stem-cell niche adhesion [2, 9, 32]. Decreased expression of E-cadherin was observed in Notch2 cKO pituitaries, specifically in the marginal zone of the intermediate lobe. Interestingly, an almost complete loss of SOX2-positive cells was observed in this region, indicating a severe disruption in the intermediate lobe progenitor niche [26]. Additional in vitro studies have established a link between Notch signaling and Ecad expression. Inhibition of cell-to-cell contact via downregulation of Ecad in pituitary monolayer cell culture results in decreased expression of the canonical Notch target, HES1 [33]. These findings may indicate a reciprocal relationship between pituitary niche adhesion molecules and Notch signaling activity.
Taken together, these studies demonstrate that Notch signaling is necessary for pituitary stem/progenitor maintenance and expansion. In particular, it appears that Notch signaling not only regulates the expression of transcription factors necessary for stem cell self-renewal but also adhesion molecules that modulate the stem cell microenvironment.
CXADR and ECM protein expression
Additional components that contribute to the stem cell niche have been relatively unexplored, but a series of studies have highlighted novel factors that may be important in the developing pituitary. Expression of Coxsackievirus and adenovirus receptor (CXADR), a transmembrane protein that associates with tight junction complexes, was recently identified in the progenitor cell compartment throughout pituitary development [34]. CXADR expression has been observed in neuroepithelial cells of the central nervous system and it thought to facilitate maturation of tight junctions during development [35]. However, the function of CXADR in the developing pituitary stem/progenitor cells remains unknown.
Extracellular matrix proteins are important for the proper development, morphology, and tumorigeneses in the pituitary [36]. Little is known about the specific ECMs that contribute to the stem/progenitor cell niche in the pituitary, but a few studies have demonstrated that ECMs may contribute to the function and maintenance of these cells. In vitro studies show S100β-positive cells interact with various ECM proteins including laminins, collagens, and integrins, and these interactions regulate proliferation and morphological characteristics of these cells [37, 38]. In addition, the ontogeny of laminin chain expression was examined during neonatal pituitary development. Interestingly, Laminin 5α, which is present in pluripotent embryonic stem cells, was detected in RP as well as in the marginal zone during postnatal pituitary development [39]. These data suggest that ECM components are important regulators of the stem/progenitor compartment in the developing pituitary.
Transcription factors that drive differentiation and lineage specification in the developing pituitary
As pituitary development progresses, stem/progenitor cells must exit the cell cycle and differentiate into one of the hormone producing cell types. The process of adopting a specific cell fate is facilitated by the expression of transcription factors that promote lineage specification. The use of genetically engineered mouse models and naturally occurring spontaneous mutations in both mice and humans have led to the discovery of several genes that are crucial for endocrine cell specification and function.
Corticotropes and melanotropes
The terminal differentiation of the two proopiomelanocortin (POMC) expressing cell types, melanotropes which cleave POMC into melanocyte-stimulating hormone (αMSH) and corticotropes which cleave POMC into adrenocorticotrophin hormone (ACTH), is driven by the expression of T-box 19 (TBX19; also known as TPIT). In humans, inactivating mutations in TPIT are associated with isolated adrenocorticotropic hormone deficiency (IAD), eluding the importance of TPIT in the proper differentiation of the POMC lineage in the pituitary. TPIT expression is detected in the caudoventral region of RP as early as e12.5 before the onset of POMC expression [40, 41]. Studies have demonstrated that TPIT regulates POMC transcription in corticotropes through synergistic interactions with other transcription factors including Paired-like homeodomain transcription factor 1 (PITX1), neurogenic differentation 1 (NeuroD1), and est variant 1 (ETV1) [42–44]. Tpit null mice display a decrease in the number of POMC-expressing corticotropes but do express other corticotrope-specific genes indicating a defect in the later stages of differentiation. In the absence of Tpit, the intermediate lobe is hypoplastic and melanotropes adopt an alternate cell fate indicated by the expression of glycoprotein hormones, α subunit (Cga; also known as αGSU) and nuclear receptor subfamily 5, group A, member 1 (NR5A1; also known as steroidogenic factor 1 (SF1)), markers of thyrotropes and gonadotropes [41]. Tpit overexpression in the αGSU-expressing cells initiates corticotrope differentiation in these cells and as a result expression of LHβ, FSHβ, and SF1 are decreased [40, 41]. These data demonstrate a role for TPIT not only in the promotion of corticotrope differentiation but also as an active suppressor of other lineage-specific makers.
Aside from the role of Notch signaling as regulator of stem/progenitor cell maintenance, it functions as a binary cell fate determinant in the developing pituitary. Specifically, it appears that Notch signaling regulates the balance between the TPIT and PIT1 lineages. Models of reduced Notch signaling in the pituitary show precocious differentiation of corticotrope cells [23, 28]. In addition, constitutive activation of Notch signaling in POMC-expressing cells prevents differentiation of melanotropes and corticotropes. This inability to differentiate is marked by increased expression of SOX2 in the intermediate lobe indicating these cells are confined to a progenitor like state. These studies demonstrate that Notch signaling is necessary to suppress aberrant corticotrope expansion and downregulation of this pathway is necessary for melanotrope and corticotrope differentiation.
Although TPIT is a common factor necessary for terminal differentiation in both corticotropes and melanotropes, additional lineage-specific factors such as NeuroD1 and paired box 7 (PAX7) have been shown to regulate development and maturation of these cell types. NeuroD1 is dynamically expressed in pituitary corticotropes and is not only a regulator of Pomc transcription but also is implicated in the differentiation of the corticotrope lineage. Loss of Neurod1 results in a minor delay in corticotrope differentiation that is recovered by e16.5, indicating that it may contribute to early differentiation of this lineage [42]. PAX7, a pioneering transcription factor, is an important regulator of melanotrope identity and melanotrope-specific POMC transcription. PAX7 is exclusively detected in the intermediate lobe cells that co-express TPIT beginning at e15.5 [45]. In Pax7 knockout mice pituitaries, melanotropes appear to adopt a corticotrope cell fate indicated by the downregulation of genes enriched in melanotropes, including proprotein convertase subtilisin/kexin type 2 (Pcsk2; also known as Pc2) and dopamine receptor D2 (Drd2), and a subsequent upregulation of genes specific to corticotropes including nuclear receptor subfamily 3, group C, member 1 (Nr3c1; also known as glucocorticoid receptor), Neurod1, and corticotropin releasing hormone receptor 1 (Crhr1). In addition, these studies demonstrate that PAX7 actually is not the main driver of melanotrope differentiation, rather is it necessary to allow accessibility of melanotrope-specific genes to other transcription factors that regulate differentiation such as TPIT [45, 46].
It is also important to note that cellular localization and cell network formation are important for the function and development of the POMC lineage. Loss of the cellular adhesion molecule Cadherin 2 (Cdh2; also known as Ncad) in POMC-expressing cells results in disrupted intermediate lobe boundaries, mis-localization of corticotropes, and an overall decrease in Pomc levels [47]. Additional studies have demonstrated the endocytic adaptor protein NUMB, known for its role in degradation of the Notch receptors and cell adhesion, is also necessary for intermediate lobe organization and progenitor cell localization [48]. Taken together, these studies identify additional regulatory proteins that contribute to the proper development of the POMC lineage.
PIT1 lineage: somatotropes, lactotropes, and thyrotropes
POU domain, class 1, transcription factor 1 (PIT1), is a major determinant of terminal differentiation of three hormone producing cell types: somatotropes, lactotropes, and thyrotropes. Mutations in PIT1 in both humans and mice results in CPHD, establishing an important role of PIT1 in pituitary development [49]. Genetic mouse models of PIT1 deficiency, including the Snell's dwarf and Jackson dwarf, exhibit pituitary hypoplasia coincident with a decrease in the number somatotropes, lactotropes, and thyrotropes [50]. These phenotypic changes are observed only during postnatal pituitary development suggesting that PIT1 is not required for the initial differentiation of these linages but rather it is necessary for postnatal expansion. PIT1 is first detected at e13.5 and increases in expression into adulthood as endocrine cells undergo major waves of expansion [51]. In terminally differentiated cells, PIT1 transcriptionally regulates the expression of Gh, Tshb, and Prl as well as the hypothalamic signaling receptor, growth hormone releasing hormone receptor (Ghrhr) [52, 53]. The Notch signaling pathway and PROP1 are currently thought to be the primary regulators of Pit1 expression. Ames dwarf mice and mouse models of reduced Notch signaling exhibit a similar decrease in the number of somatotropes, lactotropes, and thyrotropes in the developing pituitary [23, 28]. PROP1 is a direct transcriptional activator of Pit1 expression while Notch is thought to exert its effects on Pit1 indirectly through regulation of PROP1 expression [54]. Although it has been shown that PIT1 is necessary for terminal differentiation of somatotropes, lactotropes, and thyrotropes, the molecular mechanisms that govern specification of these different cells remain elusive.
Somatotropes
The bHLH factor neurogenic differentation factor 4 (NeuroD4), a downstream target of PIT1 necessary for somatotrope differentiation, is expressed by e13.5 in the mouse. Somatotropes are severely decreased in Neurod4 null mice embryonically. Although somatotrope differentiation mildly recovers during postnatal development, these mice fail to express GHRHR and display dwarfism [23]. The postnatal pituitary hypoplasia due to loss of somatotrope expansion in Neurod4 null mice is likely due in part to loss of hypothalamic input in this cell type. This is supported by a similar hypoplasia phenotype observed in little mice (Ghrhrlit/lit) which harbor a point mutation in GHRHR [55]. More recently, it was demonstrated that forkehead box O1 (FOXO1), is also important for embryonic somatotrope differentiation. Conditional loss of Foxo1 in the developing pituitary results in a decrease of Gh and Ghrhr mRNA levels, although adult animals appear unaffected. In addition, FOXO1 may be an upstream regulator of Neurod4 as its expression is reduced embryonically in these mice [56].
Thyrotropes
Two populations of thyrotrope cells are present during pituitary organogenesis: the first to appear is a PIT1-independent lineage located in the rostral tip specifically during embryonic development and a second PIT1-dependent lineage. Little is known about the regulation and function of rostral tip thyrotropes; however, factors have been identified that regulate the PIT1-dependent lineage. During early gland specification events, a set of common genes are expressed that are necessary for both thyrotrope and gonadotrope specification. These include two transcription factors, forkhead box L2 (FOXL2) and GATA binding protein 2 (GATA2), as well as Cga, the common α-glycoprotein subunit of TSH and the gonadotropins LH and FSH. FOXL2 regulates expression of αGSU and is the earliest maker of thyrotrope and gonadotrope specification [57]. Its expression is observed ventrally in RP as early as e10.5 before the onset of hormone expression in these lineages at e12 [58]. Loss-of-function studies have identified GATA2 as a regulator of differentiation of both thyrotropes and gonadotropes. Expression of a dominant negative form of GATA2 or a pituitary-specific knockout of Gata2 inhibits terminal differentiation of thyrotropes indicated by reduced express if Tshb [59–61]. In addition, Pit1 is increased in these pituitaries suggesting that PIT1 alone is not sufficient for the proper expression of TSHβ. In support of this observation, these studies also demonstrated that GATA2 and PIT1 act synergistically to transcriptionally regulate Tshb expression.
Lactotropes
Terminally differentiated lactotropes marked by the expression of PRL are detected by e15.5 in mice [62, 63]. Although lactotropes are detected before birth, lactotrope cell number remains low during embryonic pituitary development, with the majority of lactotrope expansion occurring during postnatal development. Interestingly, studies using targeted ablation of GH-producing cells demonstrated a significant decrease in lactotrope cells as well, indicating that mature lactotropes may be derived from a precursor cell that expressed GH referred to as somatolactotropes [64, 65]. However, these studies are somewhat controversial as lineage tracing studies suggest that the majority of lactotropes are derived from pituitary progenitor cells [66, 67]. Relatively little is known about the factors driving further lactotrope specification. More recently, zinc finger and BTB domain containing 20 (ZBTB20), a zinc finger containing transcription factor, was shown to be important for lactotrope specification. ZBTB20 null mice exhibit postnatal pituitary hypoplasia due to an absence of terminally differentiated lactotropes and a decreased number of somatotropes. These studies also demonstrate ZBTB20 as a transcriptional regulator of Prl expression [68]. In addition, it has been shown that lactotrope terminal differentiation and proliferation are regulated by hormonal signals such as estrogens. The decrease in PRL expression and lactotrope cell number observed in estrogen receptor alpha (Esr1; also known as ERα) disrupted mice (Esr1−/−) suggests that estrogenic signaling is necessary for maturation of lactotrope cells and may provide a mechanism to devise terminally differentiated lactotropes from somatolactotrope precursor cells [69, 70]. It has also been demonstrated that shortly after pregnancy there are an increased number of proliferating lactotropes and increased levels of circulating PRL, thought to be triggered by estrogen. However, it is unclear if the primary mechanism of increased PRL in response to estrogen in this capacity is due to increased lactotrope number or hypertrophy [71].
Gonadotropes
As mentioned previously, transcription factors GATA2 and FOXL2 are early markers of gonadotrope cells. FOXL2 interacts with Smad proteins to directly regulate transcription of Fshb in gonadotropes [72–74]. The necessity of FOXL2 in FSH expression is exemplified by FSHβ deficiency and subsequent reduced fertility in mice with a conditional deletion of Foxl2 in gonadotropes [57, 75]. GATA2 is not necessary for differentiation of this lineage but is important for function as reduced GATA2 expression is also associated with reduced FSH levels [60]. GATA2 is also a positive regulator of gonadotrope-specific genes including the orphan nuclear receptor NR5A1 (also known as SF1) [59]. NR5A1 expression is detected starting at e13.5, and is one of the first gonadotrope-specific makers before the onset of LHβ and FSHβ [76]. The loss of Nr5a1 globally or in the pituitary specifically results in decreased basal levels of gonadotropins; however, hormone expression can be induced in response to the hypothalamic stimulus gonadotropin releasing hormone (GnRH) [77]. It has also been demonstrated that GnRH-mediated transcriptional induction of Lhb requires synergism between SF1 and the transcription factors early growth response 1 (EGR1) and PITX1 both of which are known regulators of gonadotropins [78].
The Notch signaling pathway has been also implicated in gonadotrope differentiation. Overexpression of the Notch downstream target Hes1 and constitutively active Notch in Cga-expressing cells results in delayed gonadotrope differentiation [24, 79]. Notch2 cKO mice have increased expression of both Nr5a1 and Lhb but with no increase in gonadotrope number [28]. In contrast, no apparent gonadotrope phenotype was observed in mice with conditional loss of Rbpj, the essential Notch cofactor [23]. These studies indicate that active Notch signaling is sufficient to suppress gonadotrope differentiation; however, it does not appear to affect gonadotrope specification.
Hormonal influence on pituitary differentiation
The pituitary gland is a major hub of endocrine function and as such it is under feedback regulation from peripheral tissues. The release of hormones, such as sex steroids, thyroid hormones, glucocorticoids (GCs), and insulin-like growth factor 1 (IGF-1), from the corresponding peripheral tissues is the primary mechanism of feedback to the pituitary to ensure homeostatic balance. The endocrine cells of the pituitary are therefore functionally controlled by peripheral hormones, but these hormones may also regulate the differentiation and specification of cells in the pituitary. Here we discuss the most well-studied examples of peripheral hormones influence on pituitary development.
In response to activation of hypothalamic-pituitary-adrenal axis (HPA), pituitary corticotropes release ACTH which acts on the adrenal gland and subsequently results in the release of GCs that regulate peripheral tissues and negatively feedback to both the pituitary and hypothalamus. It is well established that GCs regulate the response to stress in the adult animal, but evidence suggests GC signaling also regulates corticotrope differentiation. In adult mice, it has been demonstrated that corticotrope cell number is increased in response to adrenalectomy [80]. The increase in cell number and the ability of dexamethasone to attenuate this response indicate that GCs are capable of regulating corticotrope differentiation. Furthermore, mice with a conditional deletion of the GC receptor (Nr3c1PomcCre) in the pituitary have increased Pomc mRNA levels and an apparent increase in corticotrope number at postnatal day 6 [81]. Taken together, these studies demonstrate a suppressive role of GCs in corticotrope differentiation in the adult and postnatal pituitary. In contrast, GCs have been shown to positively regulate somatotrope differentiation [82]. Studies in rat and chicken embryos demonstrate that in vivo exposure to GCs results in an increase in Gh levels and premature differentiation of somatotropes [83–85]. Coincidentally, terminal differentiation of somatotropes coincides with the onset of HPA axis activity during late embryonic development [86]. These studies indicate that GCs regulate differentiation of the both corticotropes and somatotropes during pituitary development.
Similar to the regulation of corticotrope number by GCs, peripheral hormones from target organs also regulate somatotrope differentiation. Secretion of GH from somatotropes stimulates the production of IGF-1 from the liver and feeds back negatively to hypothalamic-pituitary axis to suppress GH release. Studies in IGF-1 knockout mice demonstrate that loss of IGF-1 results in increased somatotrope number in adult mice, indicating IGF-1 is necessary to maintain the correct number of somatotropes [87].
As mentioned previously, the most well-known example of hormonal regulation on cellular differentiation in the pituitary is the effects of estrogen on lactotrope differentiation. However, other endocrine cells types in the pituitary are regulated by estrogenic signaling. In adults, it is well established that estrogens play a role in regulating gonadotropin secretion, but studies also indicate a role for estrogens during gonadotrope cell development. In the chick embryo, exposure to estradiol promotes an increase in gonadotrope differentiation and proliferation [88]. In support of this observation, adult rodents treated with tamoxifen, an estrogen receptor modulator, have an increased number of gonadotropes cells [89]. Furthermore, target organ ablation studies in which mice are gonadectomized show an increase in the gonadotrope population, most likely due to mobilization of the stem/progenitor cell population indicating that peripheral hormonal signals regulate cell number in the pituitary [80].
The influence of estrogens on pituitary development suggests that it may also be sensitive to exogenous estrogenic compounds. Specifically, exposure to the endocrine disrupting chemical (EDC) Bisphenol A (BPA) during critical windows of development affects gonadotrope differentiation. Female neonatal mice exposed to BPA or estradiol during gestation have increased gonadotrope cell numbers [90]. In addition, mice exposed to BPA and estradiol during postnatal development show decreased expression of sexually dichotomous genes in the pituitary, indicating postnatal sensitivity to hormonal regulation [91]. Several studies have also demonstrated that exposure to BPA during critical windows of development results in HPA axis dysfunction in adult mice [92–94]. The pituitary contribution to this dysfunction remains unclear but studies have reported changes in pituitary Crhr1 and Pomc mRNA levels [95]. Changes in gene expression and cell number after exposure to EDCs further demonstrate that the pituitary is sensitive to hormonal regulation during early developmental periods. While the mechanism/contribution of hormonal regulation has been relatively unexplored in the developing pituitary, this mode of regulation is important for gland development.
Conclusion
Current research has provided a substantial basis for understanding the molecular mechanisms that dictate pituitary cell fate decisions and organ homeostasis. However, in order to achieve the goal of using stem cell-derived treatment for pituitary disease, the contributing players in the stem/progenitor cell niche and peripheral regulation of pituitary development will need to be further dissected. Future research using single cell genomic approaches will be helpful in the identification of transitional cell types and novel molecular determinants that coordinate differentiation of the endocrine cell types. These studies will aid the field in identifying the developmental progression of each of the endocrine cell types thus allowing researchers recapitulate pituitary development.
Notes
Edited by Dr. T. Rajendra Kumar, PhD, University of Colorado Anschutz Medical Campus
Footnotes
Grant Support: The work in the Raetzman Lab is supported by National Institutes of Health Grants R01 DK076647 and T32 ES007326
References
- 1. Chen J, Hersmus N, Van Duppen V, Caesens P, Denef C, Vankelecom H. The adult pituitary contains a cell population displaying stem/progenitor cell and early embryonic characteristics. Endocrinology 2005; 146:3985–3998. [DOI] [PubMed] [Google Scholar]
- 2. Garcia-Lavandeira M, Quereda V, Flores I, Saez C, Diaz-Rodriguez E, Japon MA, Ryan AK, Blasco MA, Dieguez C, Malumbres M, Alvarez C V. A GRFa2/Prop1/Stem (GPS) cell niche in the pituitary. PLoS One 2009; 4:e4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andoniadou CL, Matsushima D, Mousavy Gharavy SN, Signore M, Mackintosh AI, Schaeffer M, Gaston-Massuet C, Mollard P, Jacques TS, Le Tissier P, Dattani MT, Pevny LH et al. Sox2(+) stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell 2013; 13:433–445. [DOI] [PubMed] [Google Scholar]
- 4. Rizzoti K, Akiyama H, Lovell-Badge R. Mobilized adult pituitary stem cells contribute to endocrine regeneration in response to physiological demand. Cell Stem Cell 2013; 13:419–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev 2003; 17:126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jayakody SA, Andoniadou CL, Gaston-Massuet C, Signore M, Cariboni A, Bouloux PM, Le Tissier P, Pevny LH, Dattani MT, Martinez-Barbera JP. SOX2 regulates the hypothalamic-pituitary axis at multiple levels. J Clin Invest 2012; 122:3635–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kelberman D, Rizzoti K, Avilion A, Bitner-Glindzicz M, Cianfarani S, Collins J, Chong WK, Kirk JMW, Achermann JC, Ross R, Carmignac D, Lovell-Badge R et al. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J Clin Invest 2006; 116:2442–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldsmith S, Lovell-Badge R, Rizzoti K. SOX2 is sequentially required for progenitor proliferation and lineage specification in the developing pituitary. Development 2016; 143:2376–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson ICAF. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci USA 2008; 105:2907–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gremeaux L, Fu Q, Chen J, Vankelecom H. Activated phenotype of the pituitary stem/progenitor cell compartment during the early-postnatal maturation phase of the gland. Stem Cells Dev 2012; 21:801–813. [DOI] [PubMed] [Google Scholar]
- 11. Mollard P, Hodson DJ, Lafont C, Rizzoti K, Drouin J. A tridimensional view of pituitary development and function. Trends Endocrinol Metab 2012; 23:261–269. [DOI] [PubMed] [Google Scholar]
- 12. Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O’Connell SM, Gukovsky I, Carrière C, Ryan AK, Miller AP, Zuo L, Gleiberman AS et al. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature 1996; 384:327–333. [DOI] [PubMed] [Google Scholar]
- 13. Yoshida S, Kato T, Susa T, Cai L, Nakayama M, Kato Y. PROP1 coexists with SOX2 and induces PIT1-commitment cells. Biochem Biophys Res Commun 2009; 385:11–15. [DOI] [PubMed] [Google Scholar]
- 14. Pérez Millán MI, Brinkmeier ML, Mortensen AH, Camper SA. PROP1 triggers epithelial-mesenchymal transition-like process in pituitary stem cells. Elife 2016; 5:e14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu X, Tollkuhn J, Taylor H, Rosenfeld MG. Notch-dependent pituitary SOX2 + stem cells exhibit a timed functional extinction in regulation of the postnatal gland. Stem Cell Rep 2015; 5:1196–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoshida S, Kato T, Yako H, Susa T, Cai L-Y, Osuna M, Inoue K, Kato Y. Significant quantitative and qualitative transition in pituitary stem/progenitor cells occurs during the postnatal development of the rat anterior pituitary. J Neuroendocrinol 2011; 23:933–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cogan JD, Wu W, Phillips JA 3rd, Arnhold IJ, Agapito A, Fofanova O V, Osorio MG, Bircan I, Moreno A, Mendonca BB. The PROP1 2-base pair deletion is a common cause of combined pituitary hormone deficiency. J Clin Endocrinol Metab 1998; 83:3346–3349. [DOI] [PubMed] [Google Scholar]
- 18. Wu W, Cogan JD, Pfäffle RW, Dasen JS, Frisch H, O’Connell SM, Flynn SE, Brown MR, Mullis PE, Parks JS, Phillips JA, Rosenfeld MG. Mutations in PROP1 cause familial combined pituitary hormone deficiency. Nat Genet 1998; 18:147–149. [DOI] [PubMed] [Google Scholar]
- 19. Davis SW, Keisler JL, Pérez-Millán MI, Schade V, Camper SA. All hormone-producing cell types of the pituitary intermediate and anterior lobes derive from Prop1-expressing progenitors. Endocrinology 2016; 157:1385–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lepore DA, Thomas GPL, Knight KR, Hussey AJ, Callahan T, Wagner J, Morrison WA, Thomas PQ. Survival and differentiation of pituitary colony-forming cells in vivo. Stem Cells 2007; 25:1730–1736. [DOI] [PubMed] [Google Scholar]
- 21. Higuchi M, Kanno N, Yoshida S, Ueharu H, Chen M, Yako H, Shibuya S, Sekita M, Tsuda M, Mitsuishi H, Nishimura N, Kato T et al. GFP-expressing S100β-positive cells of the rat anterior pituitary differentiate into hormone-producing cells. Cell Tissue Res 2014; 357:767–779. [DOI] [PubMed] [Google Scholar]
- 22. Raetzman L, Ross S, Cook S, Dunwoodie S, Camper S, Thomas P. Developmental regulation of Notch signaling genes in the embryonic pituitary: Prop1 deficiency affects Notch2 expression. Dev Biol 2004; 265:329–340. [DOI] [PubMed] [Google Scholar]
- 23. Zhu X, Zhang J, Tollkuhn J, Ohsawa R, Bresnick EH, Guillemot F, Kageyama R, Rosenfeld MG. Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. GenesDev 2006; 20:2739–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raetzman LT, Cai JX, Camper SA. Hes1 is required for pituitary growth and melanotrope specification. Dev Biol 2007; 304:455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Monahan P, Rybak S, Raetzman LT. The notch target gene Hes1 regulates cell cycle inhibitor expression in the developing pituitary. Endocrinology 2009; 150:4386–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Edwards W, Nantie LB, Raetzman LT. Identification of a novel progenitor cell marker, grainyhead-like 2 in the developing pituitary. Dev Dyn 2016; 245:1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Y, Hibbs MA, Gard AL, Shylo NA, Yun K. Genome-wide analysis of N1ICD/RBPJ targets in vivo reveals direct transcriptional regulation of Wnt, SHH, and hippo pathway effectors by Notch1. Stem Cells 2012; 30:741–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nantie LB, Himes AD, Getz DR, Raetzman LT. Notch signaling in postnatal pituitary expansion: proliferation, progenitors, and cell specification. Mol Endocrinol 2014; 28:731–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ward RD, Raetzman LT, Suh H, Stone BM, Nasonkin IO, Camper SA. Role of PROP1 in pituitary gland growth. Mol Endocrinol 2005; 19:698–710. [DOI] [PubMed] [Google Scholar]
- 30. Mortensen AH, MacDonald JW, Ghosh D, Camper SA. Candidate genes for panhypopituitarism identified by gene expression profiling. Physiol Genomics 2011; 43:1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goldberg LB, Aujla PK, Raetzman LT. Persistent expression of activated Notch inhibits corticotrope and melanotrope differentiation and results in dysfunction of the HPA axis. Dev Biol 2011; 358:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen S, Lewallen M, Xie T. Adhesion in the stem cell niche: biological roles and regulation. Development 2013; 140:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Batchuluun K, Azuma M, Yashiro T, Kikuchi M. Notch signaling-mediated cell-to-cell interaction is dependent on E-cadherin adhesion in adult rat anterior pituitary. Cell Tissue Res 2017; 368:125–133. [DOI] [PubMed] [Google Scholar]
- 34. Chen M, Kato T, Higuchi M, Yoshida S, Yako H, Kanno N, Kato Y. Coxsackievirus and adenovirus receptor-positive cells compose the putative stem/progenitor cell niches in the marginal cell layer and parenchyma of the rat anterior pituitary. Cell Tissue Res 2013; 354:823–836. [DOI] [PubMed] [Google Scholar]
- 35. Hotta Y, Honda T, Naito M, Kuwano R. Developmental distribution of coxsackie virus and adenovirus receptor localized in the nervous system. Dev Brain Res 2003; 143:1–13. [DOI] [PubMed] [Google Scholar]
- 36. Paez-Pereda M, Kuchenbauer F, Arzt E, Stalla GK. Regulation of pituitary hormones and cell proliferation by components of the extracellular matrix. Braz J Med Biol Res 2005; 38:1487–1494. [DOI] [PubMed] [Google Scholar]
- 37. Horiguchi K, Kouki T, Fujiwara K, Kikuchi M, Yashiro T. The extracellular matrix component laminin promotes gap junction formation in the rat anterior pituitary gland. J Endocrinol 2010; 208:225–232. [DOI] [PubMed] [Google Scholar]
- 38. Horiguchi K, Kikuchi M, Kusumoto K, Fujiwara K, Kouki T, Kawanishi K, Yashiro T. Living-cell imaging of transgenic rat anterior pituitary cells in primary culture reveals novel characteristics of folliculo-stellate cells. J Endocrinol 2010; 204:115–123. [DOI] [PubMed] [Google Scholar]
- 39. Ramadhani D, Tsukada T, Fujiwara K, Azuma M, Kikuchi M, Yashiro T. Changes in laminin chain expression in Pre- and postnatal rat pituitary gland. Acta Histochem Cytochem 2014; 47:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lamolet B, Pulichino A-M, Lamonerie T, Gauthier Y, Brue T, Enjalbert A, Drouin J. A pituitary cell-restricted T box factor, tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell 2001; 104:849–859. [DOI] [PubMed] [Google Scholar]
- 41. Pulichino A-M, Vallette-Kasic S, Tsai JP-Y, Couture C, Gauthier Y, Drouin J. Tpit determines alternate fates during pituitary cell differentiation. Genes Dev 2003; 17:738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Poulin G, Turgeon B, Drouin J. NeuroD1/beta2 contributes to cell-specific transcription of the proopiomelanocortin gene. Mol Cell Biol 1997; 17:6673–6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Poulin G, Lebel M, Chamberland M, Paradis FW, Drouin J. Specific protein-protein interaction between basic helix-loop-helix transcription factors and homeoproteins of the Pitx family. Mol Cell Biol 2000; 20:4826–4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Budry L, Couture C, Balsalobre A, Drouin J. The Ets factor Etv1 interacts with Tpit protein for pituitary pro-opiomelanocortin (POMC) gene transcription. J Biol Chem 2011; 286:25387–25396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Budry L, Balsalobre A, Gauthier Y, Khetchoumian K, L’honoré A, Vallette S, Brue T, Figarella-Branger D, Meij B, Drouin J. The selector gene Pax7 dictates alternate pituitary cell fates through its pioneer action on chromatin remodeling. Genes Dev 2012; 26:2299–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mayran A, Khetchoumian K, Hariri F, Pastinen T, Gauthier Y, Balsalobre A, Drouin J. Pioneer factor Pax7 deploys a stable enhancer repertoire for specification of cell fate. Nat Genet 2018; 50:259–269. [DOI] [PubMed] [Google Scholar]
- 47. Himes AD, Fiddler RM, Raetzman LT. N-Cadherin loss in POMC-expressing cells leads to pituitary disorganization. Mol Endocrinol 2011; 25:482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moran TB, Goldberg LB, Serviss SL, Raetzman LT. Numb deletion in POMC-expressing cells impairs pituitary intermediate lobe cell adhesion, progenitor cell localization, and neuro-intermediate lobe boundary formation. Mol Endocrinol 2011; 25:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li S, Crenshaw EB, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature 1990; 347:528–533. [DOI] [PubMed] [Google Scholar]
- 50. Camper SA, Saunders TL, Katz RW, Reeves RH. The Pit-1 transcription factor gene is a candidate for the murine Snell dwarf mutation. Genomics 1990; 8:586–590. [DOI] [PubMed] [Google Scholar]
- 51. Simmons DM, Voss JW, Ingraham HA, Holloway JM, Broide RS, Rosenfeld MG, Swanson LW. Pituitary cell phenotypes involve cell-specific Pit-1 mRNA translation and synergistic interactions with other classes of transcription factors. Genes Dev 1990; 4:695–711. [DOI] [PubMed] [Google Scholar]
- 52. Rhodes SJ, DiMattia GE, Rosenfeld MG. Transcriptional mechanisms in anterior pituitary cell differentiation. Curr Opin Genet Dev 1994; 4:709–717. [DOI] [PubMed] [Google Scholar]
- 53. McElvaine AT, Korytko AI, Kilen SM, Cuttler L, Mayo KE. Pituitary-specific expression and Pit-1 regulation of the rat growth hormone-releasing hormone receptor gene. Mol Endocrinol 2007; 21:1969–1983. [DOI] [PubMed] [Google Scholar]
- 54. Andersen B, Pearse R V, Jenne K, Sornson M, Lin SC, Bartke A, Rosenfeld MG. The Ames dwarf gene is required for Pit-1 gene activation. Dev Biol 1995; 172:495–503. [DOI] [PubMed] [Google Scholar]
- 55. Eicher EM, Beamer WG. Inherited ateliotic dwarfism in mice: characteristics of the mutation, little, on Chromosome 6. J Hered 1976; 67:87–91. [DOI] [PubMed] [Google Scholar]
- 56. Kapali J, Kabat BE, Schmidt KL, Stallings CE, Tippy M, Jung DO, Edwards BS, Nantie LB, Raeztman LT, Navratil AM, Ellsworth BS. Foxo1 is required for normal somatotrope differentiation. Endocrinology 2016; 157:4351–4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ellsworth BS, Egashira N, Haller JL, Butts DL, Cocquet J, Clay CM, Osamura RY, Camper SA. FOXL2 in the pituitary: molecular, genetic, and developmental analysis. Mol Endocrinol 2006; 20:2796–2805. [DOI] [PubMed] [Google Scholar]
- 58. Treier M, Gleiberman AS, O’Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev 1998; 12:1691–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dasen JS, O’Connell SM, Flynn SE, Treier M, Gleiberman AS, Szeto DP, Hooshmand F, Aggarwal AK, Rosenfeld MG. Reciprocal interactions of Pit1 and GATA2 mediate signaling gradient–induced determination of pituitary cell types. Cell 1999; 97:587–598. [DOI] [PubMed] [Google Scholar]
- 60. Charles MA, Saunders TL, Wood WM, Owens K, Parlow AF, Camper SA, Ridgway EC, Gordon DF. Pituitary-Specific Gata2 knockout: Effects on gonadotrope and thyrotrope function. Mol Endocrinol 2006; 20:1366–1377. [DOI] [PubMed] [Google Scholar]
- 61. Kashiwabara Y, Sasaki S, Matsushita A, Nagayama K, Ohba K, Iwaki H, Matsunaga H, Suzuki S, Misawa H, Ishizuka K, Oki Y, Nakamura H. Functions of PIT1 in GATA2-dependent transactivation of the thyrotropin promoter. J Mol Endocrinol 2008; 42:225–237. [DOI] [PubMed] [Google Scholar]
- 62. Dollé P, Castrillo JL, Theill LE, Deerinck T, Ellisman M, Karin M. Expression of GHF-1 protein in mouse pituitaries correlates both temporally and spatially with the onset of growth hormone gene activity. Cell 1990; 60:809–820. [DOI] [PubMed] [Google Scholar]
- 63. Matsubara M, Harigaya T, Nogami H. Effects of diethylstilbestrol on the cytogenesis of prolactin cells in the pars distalis of the pituitary gland of the mouse. Cell Tissue Res 2001; 306:301–307. [DOI] [PubMed] [Google Scholar]
- 64. Behringer RR, Mathews LS, Palmiter RD, Brinster RL. Dwarf mice produced by genetic ablation of growth hormone-expressing cells. Genes Dev 1988; 2:453–461. [DOI] [PubMed] [Google Scholar]
- 65. Borrelli E, Heyman RA, Arias C, Sawchenko PE, Evans RM. Transgenic mice with inducible dwarfism. Nature 1989; 339:538–541. [DOI] [PubMed] [Google Scholar]
- 66. Luque RM, Amargo G, Ishii S, Lobe C, Franks R, Kiyokawa H, Kineman RD. Reporter expression, induced by a growth hormone promoter-driven cre recombinase (rGHp-Cre) transgene, questions the developmental relationship between somatotropes and lactotropes in the adult mouse pituitary gland. Endocrinology 2007; 148:1946–1953. [DOI] [PubMed] [Google Scholar]
- 67. Fu Q, Gremeaux L, Luque RM, Liekens D, Chen J, Buch T, Waisman A, Kineman R, Vankelecom H. The adult pituitary shows stem/progenitor cell activation in response to injury and is capable of regeneration. Endocrinology 2012; 153:3224–3235. [DOI] [PubMed] [Google Scholar]
- 68. Cao D, Ma X, Cai J, Luan J, Liu A-J, Yang R, Cao Y, Zhu X, Zhang H, Chen Y-X, Shi Y, Shi G-X et al. ZBTB20 is required for anterior pituitary development and lactotrope specification. Nat Commun 2016; 7:11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Scully KM, Gleiberman AS, Lindzey J, Lubahn DB, Korach KS, Rosenfeld MG. Role of estrogen receptor-α in the anterior pituitary gland. Mol Endocrinol 1997; 11:674–681. [DOI] [PubMed] [Google Scholar]
- 70. Ogasawara K, Nogami H, Tsuda MC, J-Å Gustafsson, Korach KS, Ogawa S, Harigaya T, Hisano S. Hormonal regulation of prolactin cell development in the fetal pituitary gland of the mouse. Endocrinology 2009; 150:1061–1068. [DOI] [PubMed] [Google Scholar]
- 71. Yin P, Arita J. Differential regulation of prolactin release and lactotrope proliferation during pregnancy, lactation and the estrous cycle. Neuroendocrinology 2000; 72:72–79. [DOI] [PubMed] [Google Scholar]
- 72. Tran S, Lamba P, Wang Y, Bernard DJ. SMADs and FOXL2 synergistically regulate murine FSHβ transcription via a conserved proximal promoter element. Mol Endocrinol 2011; 25:1170–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang Y, Bernard DJ. Activin A induction of murine and ovine follicle-stimulating hormone β transcription is SMAD-dependent and TAK1 (MAP3K7)/p38 MAPK-independent in gonadotrope-like cells. Cell Signal 2012; 24:1632–1640. [DOI] [PubMed] [Google Scholar]
- 74. Li Y, Schang G, Boehm U, Deng C-X, Graff J, Bernard DJ. SMAD3 regulates follicle-stimulating hormone synthesis by pituitary gonadotrope cells in vivo. J Biol Chem 2017; 292:2301–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tran S, Zhou X, Lafleur C, Calderon MJ, Ellsworth BS, Kimmins S, Boehm U, Treier M, Boerboom D, Bernard DJ. Impaired fertility and FSH synthesis in gonadotrope-specific Foxl2 knockout mice. Mol Endocrinol 2013; 27:407–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ingraham HA, Lala DS, Ikeda Y, Luo X, Shen WH, Nachtigal MW, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev 1994; 8:2302–2312. [DOI] [PubMed] [Google Scholar]
- 77. Zhao L, Bakke M, Krimkevich Y, Cushman LJ, Parlow AF, Camper SA, Parker KL. Steroidogenic factor 1 (SF1) is essential for pituitary gonadotrope function. Development 2001; 128:147–154. [DOI] [PubMed] [Google Scholar]
- 78. Tremblay JJ, Drouin J. Egr-1 is a downstream effector of GnRH and synergizes by direct interaction with Ptx1 and SF-1 To enhance luteinizing hormone. Gene Transcription 1999; 19:2567–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Raetzman LT, Wheeler BS, Ross SA, Thomas PQ, Camper SA. Persistent expression of Notch2 delays gonadotrope differentiation. Mol Endocrinol 2006; 20:2898–2908. [DOI] [PubMed] [Google Scholar]
- 80. Nolan LA, Levy A. A population of non-luteinising hormone/non-adrenocorticotrophic hormone-positive cells in the male rat anterior pituitary responds mitotically to both gonadectomy and adrenalectomy. J Neuroendocrinol 2006; 18:655–661. [DOI] [PubMed] [Google Scholar]
- 81. Schmidt MV, Sterlemann V, Wagner K, Niederleitner B, Ganea K, Liebl C, Deussing JM, Berger S, Schütz G, Holsboer F, Müller MB. Postnatal glucocorticoid excess due to pituitary glucocorticoid receptor deficiency: differential short- and long-term consequences. Endocrinology 2009; 150:2709–2716. [DOI] [PubMed] [Google Scholar]
- 82. Vakili H, Cattini PA. The hidden but positive role for glucocorticoids in the regulation of growth hormone-producing cells. Mol Cell Endocrinol 2012; 363:1–9. [DOI] [PubMed] [Google Scholar]
- 83. Nogami H, Tachibana T. Dexamethasone induces advanced growth hormone expression in the fetal rat pituitary gland in vivo. Endocrinology 1993; 132:517–523. [DOI] [PubMed] [Google Scholar]
- 84. Porter TE. Regulation of pituitary somatotroph differentiation by hormones of peripheral endocrine glands. Domest Anim Endocrinol 2005; 29:52–62. [DOI] [PubMed] [Google Scholar]
- 85. Ellestad LE, Puckett SA, Porter TE. Mechanisms involved in glucocorticoid induction of pituitary GH expression during embryonic development. Endocrinology 2015; 156:1066–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wood CE, Walker C-D. Fetal and Neonatal HPA Axis. Comprehensive Physiology, vol. 6 Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2015:33–62. [DOI] [PubMed] [Google Scholar]
- 87. Hikake T, Hayashi S, Iguchi T, Sato T. The role of IGF1 on the differentiation of prolactin secreting cells in the mouse anterior pituitary. J Endocrinol 2009; 203:231–240. [DOI] [PubMed] [Google Scholar]
- 88. Wu YJ, Chen DW, Liu JL, Zhang JH, Luo HS, Cui S. Estradiol promotes pituitary cell proliferation and gonadotroph differentiation at different doses and with different mechanisms in chick embryo. Steroids 2009; 74:441–448. [DOI] [PubMed] [Google Scholar]
- 89. Aguilar R, Bellido C, Garrido-Gracia JC, Alonso R, Sánchez-Criado JE. Estradiol and its membrane-impermeable conjugate estradiol-BSA inhibit tamoxifen-stimulated prolactin secretion in incubated rat pituitaries. Reproduction 2006; 131:763–769. [DOI] [PubMed] [Google Scholar]
- 90. Brannick KE, Craig ZR, Himes AD, Peretz JR, Wang W, Flaws JA, Raetzman LT. Prenatal exposure to low doses of bisphenol a increases pituitary proliferation and gonadotroph number in female mice offspring at birth. Biol Reprod 2012; 87:82–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Eckstrum KS, Weis KE, Baur NG, Yoshihara Y, Raetzman LT. Icam5 expression exhibits sex differences in the neonatal pituitary and is regulated by estradiol and bisphenol A. Endocrinology 2016; 157:1408–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chen F, Zhou L, Bai Y, Zhou R, Chen L. Sex differences in the adult HPA axis and affective behaviors are altered by perinatal exposure to a low dose of bisphenol A. Brain Res 2014; 1571:12–24. [DOI] [PubMed] [Google Scholar]
- 93. Chen F, Zhou L, Bai Y, Zhou R, Chen L. Hypothalamic-pituitary-adrenal axis hyperactivity accounts for anxiety- and depression-like behaviors in rats perinatally exposed to bisphenol A. J Biomed Res 2015; 29:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhou R, Chen F, Feng X, Zhou L, Li Y, Chen L. Perinatal exposure to low-dose of bisphenol A causes anxiety-like alteration in adrenal axis regulation and behaviors of rat offspring: A potential role for metabotropic glutamate 2/3 receptors. J Psychiatr Res 2015; 64:121–129. [DOI] [PubMed] [Google Scholar]
- 95. Panagiotidou E, Zerva S, Mitsiou DJ, Alexis MN, Kitraki E. Perinatal exposure to low-dose bisphenol A affects the neuroendocrine stress response in rats. J Endocrinol 2014; 220:207–218. [DOI] [PubMed] [Google Scholar]
- 96. Castinetti F, Davis SW, Brue T, Camper SA. Pituitary stem cell update and potential implications for treating hypopituitarism. Endocr Rev 2011; 32:453–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chen J, Gremeaux L, Fu Q, Liekens D, Van Laere, S, Vankelecom, H. Pituitary progenitor cells tracked down by side population dissection. Stem Cells 2009; 27:1182–1195. [DOI] [PubMed] [Google Scholar]
- 98. Higuchi M, Kato T, Yoshida S, Ueharu H, Nishimura N, Kato Y. PRRX1- and PRRX2-positive mesenchymal stem/progenitor cells are involved in vasculogenesis during rat embryonic pituitary development. Cell Tissue Res 2015; 361:557–565. [DOI] [PubMed] [Google Scholar]
- 99. Higuchi M, Yoshida S, Ueharu H, Chen M, Kato T, Kato Y. PRRX1 and PRRX2 distinctively participate in pituitary organogenesis and a cell-supply system. Cell Tissue Res 2014; 357:323–335. [DOI] [PubMed] [Google Scholar]
- 100. Olson LE, Tollkuhn J, Scafoglio C, Krones A, Zhang J, Ohgi KA, Wu W, Taketo MM, Kemler R, Grosschedl R, Rose D, Li X et al. Homeodomain-Mediated β-Catenin-Dependent switching events dictate Cell-Lineage determination. Cell 2006; 125:593–605. [DOI] [PubMed] [Google Scholar]
- 101. Gaston-Massuet C, Andoniadou CL, Signore M, Jayakody SA, Charolidi N, Kyeyune R, Vernay B, Jacques TS, Taketo MM, Le Tissier P, Dattani MT, Martinez-Barbera JP. Increased wingless (Wnt) signaling in pituitary progenitor/stem cells gives rise to pituitary tumors in mice and humans. Proc Natl Acad Sci USA 2011; 108:11482–11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Chen J, Crabbe A, Van Duppen V, Vankelecom H. The notch signaling system is present in the postnatal pituitary: marked expression and regulatory activity in the newly discovered side population. Mol Endocrinol 2006; 20:3293–3307. [DOI] [PubMed] [Google Scholar]
- 103. Yoshida S, Kato T, Kato Y. EMT involved in migration of stem/progenitor cells for pituitary development and regeneration. J Clin Med 2016; 5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Horiguchi K, Ilmiawati C, Fujiwara K, Tsukada T, Kikuchi M, Yashiro T. Expression of chemokine CXCL12 and its receptor CXCR4 in folliculostellate (FS) cells of the rat anterior pituitary gland: the CXCL12/CXCR4 axis induces interconnection of FS cells. Endocrinology 2012; 153:1717–1724. [DOI] [PubMed] [Google Scholar]