Abstract
Objective
Cancer recurrence is a primary concern for patients with differentiated thyroid cancer; however, population-level data on recurrent or persistent disease do not currently exist. The objective of this study was to determine treated recurrent or persistent thyroid cancer by using a population-based registry, identify correlates of poor treatment-free survival, and define prognostic groups for treatment-free survival.
Methods
In this population-based study, we evaluated treatment-free survival in 9273 patients from the Surveillance, Epidemiology, and End Results Program–Medicare with a diagnosis of differentiated thyroid cancer between 1998 and 2012. Treated recurrence was defined by treatment of recurrent or persistent differentiated thyroid cancer with surgery, radioactive iodine, or radiation therapy at ≥1 year after diagnosis. Multivariable analysis was performed with Cox proportional hazards regression, survival trees, and random survival forests.
Results
In this cohort the median patient age at time of diagnosis was 69 years, and 75% of the patients were female. Using survival tree analyses, we identified five distinct prognostic groups (P < 0.001), with a prediction accuracy of 88.7%. The 5-year treatment-free survival rates of these prognostic groups were 96%, 91%, 85%, 72%, and 52%, respectively, and the 10-year treatment-free survival rates were 94%, 87%, 80%, 64%, and 39%. Based on survival forest analysis, the most important factors for predicting treatment-free survival were stage, tumor size, and receipt of radioactive iodine.
Conclusion
In this population-based cohort, five prognostic groups for treatment-free survival were identified. Understanding treatment-free survival has implications for the care and long-term surveillance of patients with differentiated thyroid cancer.
Understanding treatment-free survival has implications for the care of patients with differentiated thyroid cancer. In this study, five prognostic groups for treatment-free survival were identified.
For patients with differentiated thyroid cancer, death from thyroid cancer is rare, but recurrent or persistent thyroid cancer remains common (1–3). Confirmed or suspected thyroid cancer recurrence can be associated with psychological distress and heightened cancer-related worry (4, 5). Treatment of recurrent or persistent thyroid cancer increases the risk of complications including vocal fold paralysis, hypoparathyroidism, sialadenitis, and lacrimal duct damage (6–9).
Treatment-free survival, which is survival without the need for treatment of recurrent or persistent cancer, is an important outcome for patients. Yet population-level data on treatment-free survival do not currently exist. Recurrence is not routinely captured by large national cancer registries such as Surveillance, Epidemiology, and End Results (SEER) or the National Cancer Database (10). The applicability of recurrence data from previous single- and multi-institution studies may be limited by small sample size and selection bias (3, 11–13).
Understanding treatment-free survival at a population level is key to improving patient care, tailoring long-term surveillance, and, in some scenarios, reducing patient worry. We hypothesized that treatment-free survival in patients with differentiated thyroid cancer would vary greatly, allowing us to identify distinct prognostic groups. In this population-based study evaluating patients with differentiated thyroid cancer affiliated with SEER-Medicare, we obtained details on additional neck surgery, use of radioactive iodine, and use of radiation at ≥1 years after diagnosis to determine treated recurrence, identify correlates of poor treatment-free survival, and define prognostic groups for treatment-free survival.
Methods
Data source and study population
SEER-Medicare is a linkage of SEER data to Medicare claims. SEER is the primary source for cancer statistics in the United States, and it represents ~28% of the US population (14). Medicare represents ~15% of the US population, including most people ≥65 years old (15). The SEER program of cancer registries includes clinical and demographic details in addition to cause of death for patients with cancer. Medicare includes claims for covered health services. The linkage of these two data sets allows assessment of long-term cancer care in a population-based cohort (16).
Data from 43,813 patients with a diagnosis of differentiated thyroid cancer (papillary, follicular, Hürthle cell) between 1998 and 2012 were queried from SEER-Medicare. We evaluated patient enrollment in Medicare Part A and B, non–health maintenance organization (HMO), for the month before diagnosis, month of diagnosis, and 12 months after diagnosis. We did not include patients enrolled in Medicare who were also enrolled in HMOs because some of their care may not be captured by Medicare. To increase the likelihood that treatment of recurrent or persistent disease would be captured by the registry, only patients enrolled for ≥11 of the 14 months evaluated were included (N = 16,054). To avoid capturing treatment of another cancer, we excluded patients who also had a diagnosis of a nonthyroid malignancy (N = 11,336). Because we were evaluating treatment of thyroid cancer recurrence, only the patients who had an initial thyroid surgery recorded in both SEER and Medicare were selected for final analysis (N = 9273).
Institutional review board approval was not required Because this study involves research using publicly available data and cannot be tracked to human subjects.
Measures
Patient age at diagnosis was analyzed as a continuous variable. Patient race was categorized as white, black, and “other.” Because of the small sample size, Asian, American Indian/Alaska Native, Native Hawaiian, and Pacific Islander were grouped into the “other” category. The percentages of patients ≥25 years old with a high school diploma only and household income were categorized based on household zip code at the time of diagnosis, matched to the 2000 Census data and the American Community Survey 2008 to 2012. Tumor characteristics included stage, histology, and tumor size. Stage was based on SEER stage, with localized including tumors confined to the thyroid or extending into the capsule but not beyond. Regional included direct extension into blood vessels, nerves, muscles, thyroid cartilage, and so on, and tumors described as “fixed to adjacent tissues.” It also included regional lymph node involvement. Distant included distant lymph nodes and extension to bone, mediastinal tissues, and so on (3). Histology was restricted to International Classification of Diseases for Oncology classification codes for papillary, follicular, or Hürthle cell cancer (16). Tumor size was categorized based on categories previously defined by American Joint Committee on Cancer TNM staging: ≤1 cm, >1 cm and ≤2 cm, >2 cm and ≤4 cm, and >4 cm (17). Initial treatment characteristics included type of surgery, lymph node resection, receipt of radioactive iodine, and receipt of radiation. Surgery was categorized as lobectomy, which involves resection of one lobe of the thyroid, or total thyroidectomy, which includes resection of the entire thyroid or near-total thyroidectomy. Lymph node resection was classified as not resected or resected and negative for lymph node metastases or resected and positive for lymph node metastases. Receipt of radioactive iodine and receipt of radiation were dichotomized into yes or no.
Previous work found that delaying risk stratification until 8 to 12 months after initial treatment improves the predictive value of risk stratification (12). Because additional treatment in the first year after diagnosis may be secondary to incomplete initial treatment and may vary based on treating physician, in this study treated recurrence was defined as additional treatment ≥1 year after diagnosis. This additional treatment included surgery to resect lymph nodes in the neck, receipt of radioactive iodine, or receipt of radiation. Treatment-free survival was defined as the time interval from diagnosis to treatment of recurrent or persistent disease or to time of censoring. Disease-specific survival was defined as the time interval from diagnosis to death from thyroid cancer or time of censoring.
Statistical analysis
Survival tree analysis was used to construct distinct prognostic groups, such that within each group patients have similar treatment-free survival, but treatment-free survival differs between groups (17–19). In the tree paradigm, the covariate space is partitioned recursively in a binary fashion. The partitioning is intended to increase within-group homogeneity. For the treatment-free survival endpoint (censored), within-group homogeneity was measured via deviance based on a proportional hazards model (20). The two groups were recursively partitioned (each group being split on the same or other variables), creating a tree structure. At each step, to select the best split the tree-growing paradigm examined every possible cutoff point for each prognostic variable. This process was continued until the groups reached a minimum size (<10 patients in each group). Because the resulting tree was overgrown (thereby overfitting the data), a subtree was chosen via cost complexity pruning (20–22). The final tree contained “terminal” groups with similar treatment-free survival. Five- and 10-year treatment-free survival rates were calculated as summary measures for each terminal group. Although this method ensures that left and right terminal groups from the same parent are significantly different in terms of treatment-free survival, it is possible that terminal groups from distinct parents may have similar treatment-free survival. Therefore, further amalgamation of terminal groups with similar treatment-free survival was performed. For amalgamation, we first ordered the terminal groups based on hazard ratio of a terminal group relative to the leftmost terminal group of the final tree. The monotone ordering was then coded as a single ordered covariate, and the survival tree algorithm was used again to form the final prognostic groups (22).
Next, we performed random survival forest analyses of our data (17, 23, 24). A random survival forest is an ensemble of unpruned survival trees, induced from bootstrap samples of the data, with random feature selection used in the tree induction process. At each step of the splitting process, instead of evaluating all allowable splits on all variables, as is done when growing a single tree, a subset of the covariates is drawn at random. We grew 1000 trees in the forest with the treatment-free survival endpoint. Predictions for each patient were obtained by averaging the predictions across all trees in the forest. Furthermore, a byproduct of the forest is a ranking of variables in terms of their relative importance in the forest.
We also performed Cox proportional hazards regression analyses on this patient cohort with the same variables used in the tree and forest analyses.
Finally, to address potential confounding by indication with respect to radioactive iodine use, we performed a propensity scores–based analysis. The propensity scores were estimated as the conditional probability of receiving radioactive iodine based on a multivariable logistic regression model of radioactive iodine use (binary), with age, sex, race, education, income, SEER stage at diagnosis, histology, tumor size, initial surgery, lymph nodes resected, and receipt of radiation used as covariates. These scores were rank-ordered and grouped into quartiles. Subsequently, the propensity score quartiles were used in the Cox model, tree, and random forest analyses as an additional covariate.
Prediction accuracy for the Cox model, tree, and random forest were assessed with a generalized version of the C-statistic for censored outcomes (25). To safeguard against getting overly optimistic results by using the same data to build and test the models, we used out-of-bag predictions for obtaining the accuracy estimates (17, 25).
All analyses were performed in R (R Foundation for Statistical Computing) and SAS (SAS Institute Inc.) (26, 27). Specifically, for growing the survival tree and random survival forest, we used rpart and randomForestSRC packages in R, respectively (24, 28–30).
Results
Median follow-up was 6.4 years. A total of 1332 (14.4%) of the patients underwent treatment of recurrent or persistent disease at ≥1 year after diagnosis. Some patients received more than one treatment and more than one type of treatment. At ≥1 year after initial treatment, a total of 301 (3.3%) patients received additional neck surgery, 978 (10.6%) received treatment with radioactive iodine, and 435 (4.7%) received radiation treatment.
Table 1 shows the descriptive data and results from the Cox proportional hazards regression analysis relating patient, tumor, and initial treatment characteristics to treatment-free survival. The median patient age at time of diagnosis was 69 years (range 21 to 98), and 75% of the patients were female. Male sex [adjusted hazard ratio (AHR) 1.35; 95% CI, 1.20 to 1.52], regional (AHR 2.13; 95% CI, 1.82 to 2.49) and distant disease (AHR 4.43; 95% CI, 3.68 to 5.33), Hürthle cell cancer (AHR 1.36; 95% CI, 1.09 to 1.69), and larger tumor size (>1 cm and ≤2 cm AHR 1.36; 95% CI, 1.14 to 1.62; >2 cm and ≤4 cm AHR 1.69; 95% CI, 1.43 to 2.01; >4 cm AHR 2.11; 95% CI, 1.74 to 2.55) were significantly associated with worse treatment-free survival. Treatment characteristics including resection of positive lymph nodes (AHR 1.38; 95% CI, 1.14 to 1.67), receipt of radioactive iodine (AHR 1.47; 95% CI, 1.29 to 1.68), and receipt of radiation (AHR 1.68; 95% CI, 1.33 to 2.12) also were significantly associated with worse treatment-free survival. Results from the propensity score–based analyses were similar to those of the original analyses; therefore, we report only the latter. The generalized C-statistic for the Cox model was 72.4%.
Table 1.
Association of Patient, Tumor, and Initial Treatment Characteristics With Treatment-Free Survival
| Characteristic | N (%) | AHR (95% CI) |
|---|---|---|
| Patient characteristic | ||
| Age, y | ||
| Median (range) | 69 (21–98) | 1.00 (0.99–1.00) |
| Sex | ||
| Female | 6938 (74.8) | 1 (Reference) |
| Male | 2335 (25.2) | 1.35 (1.20–1.52) |
| Race | ||
| White | 7613 (82.1) | 1 (Reference) |
| Black | 680 (7.3) | 1.06 (0.85–1.34) |
| Other | 980 (10.6) | 1.07 (0.90–1.27) |
| Percentage with high school education only | ||
| <20% | 2195 (23.7) | 1 (Reference) |
| 20%–29.9% | 3168 (34.2) | 0.95 (0.82–1.10) |
| ≥30% | 3554 (38.3) | 0.92 (0.79–1.08) |
| Unknown | 356 (3.8) | |
| Household income | ||
| <$35,000 | 1627 (17.6) | 1.03 (0.86–1.23) |
| $35,000–$59,000 | 4188 (45.2) | 1.03 (0.90–1.18) |
| ≥$60,000 | 3098 (33.4) | 1 (Reference) |
| Unknown | 360 (3.9) | |
| Tumor characteristic | ||
| Stage | ||
| Localized | 6920 (74.6) | 1 (Reference) |
| Regional | 1902 (20.5) | 2.13 (1.82–2.49) |
| Distant | 451 (4.9) | 4.43 (3.68–5.33) |
| Histology | ||
| Follicular | 763 (8.2) | 1.19 (0.99–1.44) |
| Hürthle cell | 518 (5.6) | 1.36 (1.09–1.69) |
| Papillary | 7992 (86.2) | 1 (Reference) |
| Tumor size | ||
| ≤1 cm | 3580 (38.6) | 1 (Reference) |
| >1 cm and ≤2 cm | 2082 (22.5) | 1.36 (1.14–1.62) |
| >2 cm and ≤4 cm | 1972 (21.3) | 1.69 (1.43–2.01) |
| >4 cm | 1213 (13.1) | 2.11 (1.74–2.55) |
| Unknown | 426 (4.6) | 2.00 (1.56–2.57) |
| Initial treatment characteristic | ||
| Surgery | ||
| Lobectomy | 1922 (20.7) | 0.88 (0.73–1.05) |
| Total thyroidectomy | 7351 (79.3) | 1 (Reference) |
| Lymph node resection | ||
| Not resected | 6047 (65.2) | 1.01 (0.87–1.18) |
| Resected and negative | 2065 (22.3) | 1 (Reference) |
| Resected and positive | 1161 (12.5) | 1.38 (1.14–1.67) |
| Radioactive iodine | ||
| No | 5569 (60.1) | 1 (Reference) |
| Yes | 3704 (39.9) | 1.47 (1.29–1.68) |
| Radiation | ||
| No | 8918 (96.2) | 1 (Reference) |
| Yes | 355 (3.8) | 1.68 (1.33–2.12) |
Abbreviation: AHR, adjusted hazard ratio.
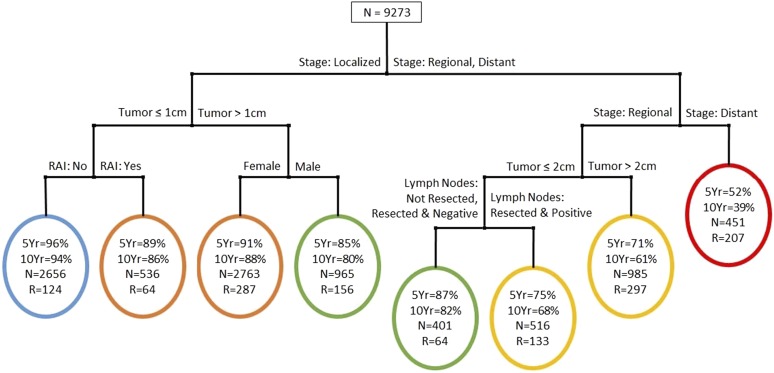
Figure 1 demonstrates the treatment-free survival tree with the 9273 patients from SEER-Medicare. At each level of the tree, we show the best splitter. Circles denote terminal groups in the tree. Within each terminal group, the first percentage denotes the 5-year treatment-free survival and the second percentage denotes the 10-year treatment-free survival. For each terminal group we also report the crude number of patients with treated recurrent or persistent disease and the total number of patients in the terminal group. The tree was initially split by stage (localized vs regional and distant). The localized group was subsequently split by tumor size (≤1 cm and >1 cm). The group with regional or distant stage was further split into regional vs distant. Based on the amalgamation method described earlier, we ultimately defined five prognostic groups for treatment-free survival (P < 0.001). At lowest risk for treated recurrence is group 1 (blue), which includes patients with localized stage, tumor size ≤1 cm, and no receipt of radioactive iodine. Group 2 (orange) includes patients with localized stage, tumor size ≤1 cm, and receipt of radioactive iodine along with patients with localized stage, tumor size >1 cm, and female sex. Group 3 (green) includes patients with localized stage, tumor size >1 cm, and male sex in addition to patients with regional stage, tumor size ≤2 cm, lymph nodes not resected or lymph nodes resected and negative. Group 4 (yellow) includes patients with regional stage, tumor size ≤2 cm, and lymph nodes resected and positive along with patients with regional stage and tumor size >2 cm. Group 5 (red) has the highest risk of treated recurrence and includes all patients with distant disease. The generalized C-statistic for the tree was 88.7%.
Figure 1.
Treatment-free survival tree. 5yr, 5-year treatment-free survival; 10yr, 10-year treatment-free survival; N, total number of patients in the terminal group; R, treated recurrent or persistent disease; RAI, radioactive iodine.
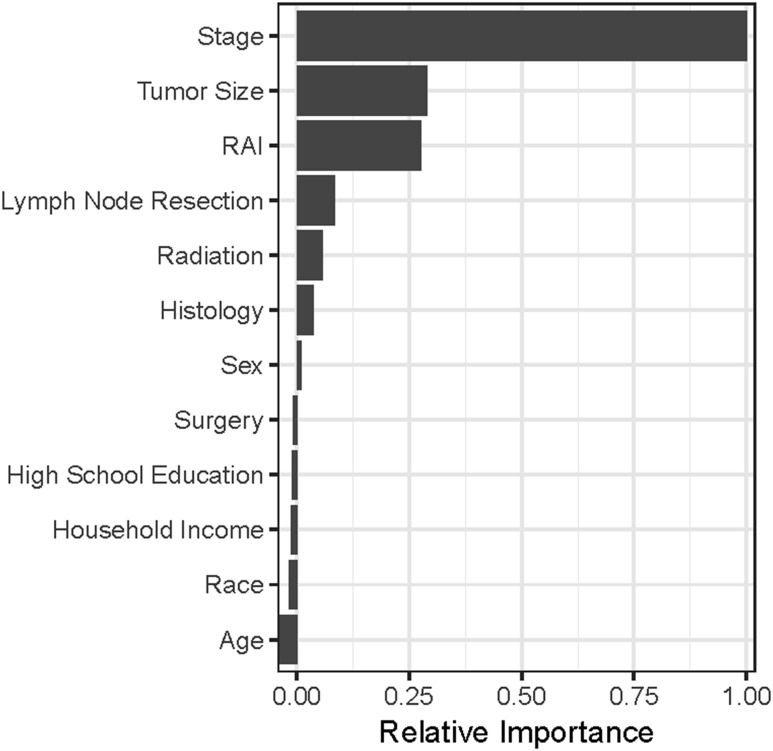
Figure 2 illustrates the relative importance of each of the patient, tumor, and treatment variables for predicting treatment-free survival based on the random forest analysis. Stage was most important in predicting treatment-free survival, followed by tumor size and receipt of radioactive iodine.
Figure 2.
Relative importance of each patient, tumor, or treatment characteristic to treatment-free survival based on the random survival forest analysis. RAI, radioactive iodine.
Table 2 lists the five prognostic groups and their respective 5-year and 10-year treatment-free survival along with their 5-year and 10-year disease-specific survival rates. The 5-year treatment-free survival rates of groups 1 to 5 were 96%, 91%, 85%, 72%, and 52%, respectively, and the 10-year treatment-free survival rates were 94%, 87%, 80%, 64%, and 39% (P < 0.001). The 5-year disease-specific survival rates were 100%, 99%, 99%, 95%, 76%, respectively, and the 10-year disease-specific survival rates were 99%, 98%, 96%, 89%, and 59%. Although treatment-free survival decreases incrementally with progression of the prognostic groups, the patients in prognostic groups 1 through 4 have a high disease-specific survival. In contrast, the patients with distant metastases (group 5, red), have both poor treatment-free survival and poor disease-specific survival.
Table 2.
Prognostic Groups for Treatment-Free Survival and Corresponding Disease-Specific Survival
| Treatment-Free Survival | Disease-Specific Survival | ||||
|---|---|---|---|---|---|
| Group | Description | 5-y (95% CI) | 10-y (95% CI) | 5-y (95% CI) | 10-y (95% CI) |
| 1 | Localized stage, tumor ≤1 cm, no radioactive iodine | 0.96 (0.95–0.97) | 0.94 (0.93–0.95) | 1.00 (0.99–1.00) | 0.99 (0.99–1.00) |
| 2 | Localized stage, tumor ≤1 cm, + radioactive iodine | 0.91 (0.90–0.092) | 0.87 (0.86–0.89) | 0.99 (0.98–0.99) | 0.98 (0.97–0.98) |
| or | |||||
| Localized stage, tumor >1 cm, female | |||||
| 3 | Localized stage, tumor >1 cm, male | 0.85 (0.83–0.87) | 0.80 (0.78–0.83) | 0.99 (0.98–1.00) | 0.96 (0.95–0.98) |
| or | |||||
| Regional stage, tumor ≤2 cm, lymph nodes not resected or resected and negative | |||||
| 4 | Regional stage, tumor ≤2 cm, lymph nodes resected and positive | 0.72 (0.70–0.75) | 0.64 (0.60–0.67) | 0.95 (0.94–0.96) | 0.89 (0.86–0.91) |
| or | |||||
| Regional stage, tumor >2 cm | |||||
| 5 | Distant stage | 0.52 (0.47–0.58) | 0.39 (0.33–0.46) | 0.76 (0.72–0.80) | 0.59 (0.53–0.66) |
Discussion
This study determined treated recurrent or persistent disease by using data from a population-based registry, identified correlates of poor treatment-free survival, and defined prognostic groups for treatment-free survival in patients with a diagnosis of differentiated thyroid cancer. Five distinct prognostic groups for treatment-free survival were identified, with stage, tumor size, and receipt of radioactive iodine emerging as important correlates of treatment-free survival.
In the past, studies on thyroid cancer recurrence were performed at the single- or multi-institution level (3, 11–13, 31–35). In addition to defining risk groups, previous smaller studies found a relationship between positive lymph nodes, male sex, and likelihood of recurrence (31, 32, 36–38). Although age is known to strongly correlate with risk of death from differentiated thyroid cancer, there are conflicting data on the relationship between age and risk of recurrence (19, 31, 33, 34). Strengths of previous single- and multi-institution studies include their ability to include tumor characteristics and biochemical markers not readily available in large national cancer registries and the relative homogeneity of their treatment protocols (3, 11–13). Limitations include small sample sizes, selection bias, and potential lack of applicability to the population at large.
Recurrent and persistent disease are not routinely captured by large national cancer registries such as SEER and the National Cancer Database (10). Our study uses Medicare claim data linked to a population-based cancer registry (SEER) to identify treatment of recurrent or persistent thyroid cancer. The results of our population-based study using SEER-Medicare data complement the findings from previous single- and multi-institution studies but also differ secondary to our use of a population-based sample and our focus on recurrence necessitating an intervention.
In addition to using of claim data to identify treatment of thyroid cancer recurrence in a population-based cohort, our study also uses a method to naturally identify risk groups. Although our initial assessment was with Cox proportional hazards regression, which allowed us to define correlates of poor treatment-free survival, limitations of Cox proportional hazards regression are the need to determine interaction a priori and the inability to group patients into well-characterized risk groups in a natural way. The survival tree analysis partitions the cohort into risk groups that are most homogeneous within and most heterogeneous between with regard to the defined outcome (i.e., treatment-free survival in our context). Terminal groups characterized by different branches can then be regrouped based on similar outcomes, via a post hoc amalgamation process. In this study we identified five distinct prognostic groups for treatment-free survival. Previous recurrence risk classification systems have fewer risk groups (3, 31, 35). Although some of the previously defined risk groups overlap with ours, unlike our study previous risk stratification systems do not separate patients with distant metastases into a separate “very high” risk group (3, 31, 35, 39). However, our large cohort size and risk stratification method (i.e., tree analysis) allowed us to identify patients at very high risk of treated recurrent or persistent disease (group 5). We also evaluated the role of initial treatment and likelihood of treated recurrence. Receipt of radioactive iodine and radiation correlated significantly with worse treatment-free survival based on Cox proportional hazards regression, and after stage and tumor size, receipt of radioactive iodine was the third most important correlate of treated recurrent or persistent disease in the survival forest analysis. We used propensity score analysis, which can reduce confounding by indication due to measured variables. However, the persistent relationship between receipt of radioactive iodine and worse treatment-free survival after the addition of propensity score analysis is unlikely to be secondary to a causal relationship but instead may occur because receipt of radioactive iodine correlates with additional unmeasured variables that may also be associated with greater likelihood of treated recurrence. For example, it is plausible that a higher postoperative thyroglobulin, a variable not captured by this data set, would be associated with both greater likelihood of receiving radioactive iodine and greater likelihood of recurrence.
Interestingly, although treatment-free survival decreased sequentially within each recurrence risk group, disease-specific survival remained excellent with the exception of group 5, which included patients with distant metastases. The fact that there is not a one-to-one correlation between recurrent or persistent disease and mortality is an expected finding for physicians who treat patients with differentiated thyroid cancer, but the explanation for this discordance remains unclear. The marked difference between treatment-free survival and disease-specific survival in the majority of the prognostic groups could be secondary to the efficacy of treatments for recurrence, our ability to detect low-volume indolent recurrence that may not affect survival, or pathogenic differences in cancers, with some cancers at greater risk for progression that leads to death.
As in other studies using claim data, limitations of our study include risk for coding errors and reporting bias. However, to reduce the risk of reporting bias, we restricted the cohort to patients who were enrolled in Medicare Part A and B, non-HMO (~80% or more), during the first year after their thyroid cancer diagnosis. An additional limitation is the fact that adequacy of initial treatment, response to initial treatment, biochemical evidence of recurrence, and structural recurrence not necessitating an intervention cannot be captured through the use of Medicare data. SEER-Medicare data can be used to identify treated recurrent or persistent disease, but recurrences that remain untreated will be missed (40). However, defining recurrence as additional treatment at ≥1 year after diagnosis reduces the likelihood of identifying correlates of incomplete initial therapy and focuses instead on the recurrent or persistent disease that is most clinically relevant. With use of this data set, we also lacked the ability to reliably capture use of tyrosine kinase inhibitors, thus potentially missing some patients with progressive recurrent or persistent disease. In addition, when using SEER data to capture initial treatment, such as initial treatment with radioactive iodine, there is a risk of underascertainment if adjuvant therapy occurred outside the hospital setting. Finally, although this cohort includes patients as young as 21 years, the median patient age at time of diagnosis was 69 years. SEER-Medicare represents a largely older patient cohort, and this bias may reduce the applicability of the findings to younger patients. Previous work in other cancer types has found that compared with younger patients, patients ≥70 years old may be less likely to have their recurrence treated (40). However, the potential limitations of using SEER-Medicare data are outweighed by the fact that this is a source of population-based recurrence data in the United States.
Recurrent or persistent disease is a primary concern for many patients with cancer but especially for patients with differentiated thyroid cancer because death from thyroid cancer is rare, but recurrent or persistent disease is common. The results of this study have implications for patients and their providers. Understanding population-based data on treatment-free survival will allow improved decision making at time of initial treatment. Patients with characteristics associated with both excellent treatment-free survival and disease-specific survival may be candidates for less intensive treatment, which has a lower risk of treatment-related complications. Similarly, patients with characteristics associated with both excellent treatment-free survival and disease-specific survival may be able to have their long-term surveillance transferred from specialists to primary care doctors sooner than those with characteristics associated with poor treatment-free survival. Because some patients with poor treatment-free survival still have excellent disease-specific survival, information on the prognostic groups for treatment-free survival and their corresponding disease-specific survival could be used to help reduce worry in many patients with recurrence. For patients with cancer, fear of recurrence can be associated with psychological distress, and treatment of recurrence can be associated with increased morbidity (4–8). Understanding the risk of recurrent or persistent disease, correlates of poor treatment-free survival, and prognostic groups for treatment-free survival is key to improving treatment decision making, tailoring the intensity of long-term surveillance, and in some cases reassuring worried patients.
Acknowledgments
The authors thank Ms. Brittany Gay, who assisted with creating the tables and editing the manuscript.
Financial Support: M.R.H. is supported by grants from the National Institutes of Health (R01 CA201198) and the Agency for Healthcare Research and Quality (R01 HS024512-01A1).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AHR
adjusted hazard ratio
- HMO
health maintenance organization
- SEER
Surveillance, Epidemiology, and End Results
References
- 1. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295(18):2164–2167. [DOI] [PubMed] [Google Scholar]
- 2. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA. 2017;317(13):1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, Brokhin M, Omry G, Fagin JA, Shaha A. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010;20(12):1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bresner L, Banach R, Rodin G, Thabane L, Ezzat S, Sawka AM. Cancer-related worry in Canadian thyroid cancer survivors. J Clin Endocrinol Metab. 2015;100(3):977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Misra S, Meiyappan S, Heus L, Freeman J, Rotstein L, Brierley JD, Tsang RW, Rodin G, Ezzat S, Goldstein DP, Sawka AM. Patients’ experiences following local-regional recurrence of thyroid cancer: a qualitative study. J Surg Oncol. 2013;108(1):47–51. [DOI] [PubMed] [Google Scholar]
- 6. Lamartina L, Borget I, Mirghani H, Al Ghuzlan A, Berdelou A, Bidault F, Deandreis D, Baudin E, Travagli JP, Schlumberger M, Hartl DM, Leboulleux S. Surgery for neck recurrence of differentiated thyroid cancer: outcomes and risk factors. J Clin Endocrinol Metab. 2017;102(3):1020–1031. [DOI] [PubMed] [Google Scholar]
- 7. Starmer H, Noureldine SI, Ozgursoy OB, Tufano RP. Voice outcomes following reoperative central neck dissection for recurrent/persistent thyroid cancer. Laryngoscope. 2015;125(11):2621–2625. [DOI] [PubMed] [Google Scholar]
- 8. Mendoza A, Shaffer B, Karakla D, Mason ME, Elkins D, Goffman TE. Quality of life with well-differentiated thyroid cancer: treatment toxicities and their reduction. Thyroid. 2004;14(2):133–140. [DOI] [PubMed] [Google Scholar]
- 9. da Fonseca FL, Yamanaka PK, Kato JM, Matayoshi S. Lacrimal system obstruction after radioiodine therapy in differentiated thyroid carcinomas: a prospective comparative study. Thyroid. 2016;26(12):1761–1767. [DOI] [PubMed] [Google Scholar]
- 10. In H, Bilimoria KY, Stewart AK, Wroblewski KE, Posner MC, Talamonti MS, Winchester DP. Cancer recurrence: an important but missing variable in national cancer registries. Ann Surg Oncol. 2014;21(5):1520–1529. [DOI] [PubMed] [Google Scholar]
- 11. Vaisman F, Momesso D, Bulzico DA, Pessoa CH, Dias F, Corbo R, Vaisman M, Tuttle RM. Spontaneous remission in thyroid cancer patients after biochemical incomplete response to initial therapy. Clin Endocrinol (Oxf). 2012;77(1):132–138. [DOI] [PubMed] [Google Scholar]
- 12. Castagna MG, Maino F, Cipri C, Belardini V, Theodoropoulou A, Cevenini G, Pacini F. Delayed risk stratification, to include the response to initial treatment (surgery and radioiodine ablation), has better outcome predictivity in differentiated thyroid cancer patients. Eur J Endocrinol. 2011;165(3):441–446. [DOI] [PubMed] [Google Scholar]
- 13. Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, Cooper DS, Haugen BR, Ladenson PW, Magner J, Robbins J, Ross DS, Skarulis M, Maxon HR, Sherman SI. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006;16(12):1229–1242. [DOI] [PubMed] [Google Scholar]
- 14. Surveillance, Epidemiology, and End Results Program (SEER) Available at: www.seer.cancer.gov. Accessed 29 January 2018.
- 15.Medicare.gov: the official U.S. government site for Medicare. Available at: www.medicare.gov. Accessed 1 February 2018.
- 16.National Cancer Institute, Division of Cancer Control and Population Sciences. SEER-Medicare database. 2017. Available at: healthcaredelivery.cancer.gov/seermedicare/overview/. Accessed 20 November 2017.
- 17. Banerjee M, Noone AM. Tree-based methods for survival data In: Biswas A, Datta S, Fine J, Segal M, eds. Statistical Advances in Biomedical Sciences: State of the Art and Future Directions. New York, NY: Wiley; 2008:265–285. [Google Scholar]
- 18. Banerjee M, George J, Song EY, Roy A, Hryniuk W. Tree-based model for breast cancer prognostication. J Clin Oncol. 2004;22(13):2567–2575. [DOI] [PubMed] [Google Scholar]
- 19. Banerjee M, Muenz DG, Chang JT, Papaleontiou M, Haymart MR. Tree-based model for thyroid cancer prognostication. J Clin Endocrinol Metab. 2014;99(10):3737–3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. LeBlanc M, Crowley J. Relative risk trees for censored survival data. Biometrics. 1992;48(2):411–425. [PubMed] [Google Scholar]
- 21. Breiman L, Frieman JH, Olshen RA, Stone CJ. Classification and Regression Trees. Belmont, CA: Wadsworth; 1984. [Google Scholar]
- 22. LeBlanc M. Tree-based methods for prognostication stratification In: Crowley J, ed. Handbook of Statistics in Clinical Oncology. New York, NY: Marcel Drekker, Inc.; 2001:457–472. [Google Scholar]
- 23. Breiman L. Random forests. Mach Learn. 2001;45(1):5–32. [Google Scholar]
- 24. Ishwaran H, Kogalur UB, Blackstone EH, Lauer MS. Random survival forests. Ann Appl Stat. 2008;2(3):841–860. [Google Scholar]
- 25. May M, Royston P, Egger M, Justice AC, Sterne JA; ART Cohort Collaboration . Development and validation of a prognostic model for survival time data: application to prognosis of HIV positive patients treated with antiretroviral therapy. Stat Med. 2004;23(15):2375–2398. [DOI] [PubMed] [Google Scholar]
- 26. SAS Institute Inc SAS version 9.4. Cary, NC: SAS Institute Inc.; 2002–2012. [Google Scholar]
- 27. R Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 28.Therneau T, Atkinson B, Ripley B. rpart: Recursive partitioning and regression trees. R package [computer program]. Version 4.1-11; 2017.
- 29. Ishwaran H, Kogalur UB. Random forests for survival, regression, and classification (RF-SRC). R package [computer program]. Version 2.5.1; 2017.
- 30. Ishwaran H, Kogalur UB. Random survival forests for R. R News. 2007;7(2):25–31. [Google Scholar]
- 31. Kim KM, Park JB, Bae KS, Kim CB, Kang DR, Kang SJ. Clinical prognostic index for recurrence of papillary thyroid carcinoma including intraoperative findings. Endocr J. 2013;60(3):291–297. [DOI] [PubMed] [Google Scholar]
- 32. Ito Y, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Kuma K, Miyauchi A. Risk factors for recurrence to the lymph node in papillary thyroid carcinoma patients without preoperatively detectable lateral node metastasis: validity of prophylactic modified radical neck dissection. World J Surg. 2007;31(11):2085–2091. [DOI] [PubMed] [Google Scholar]
- 33. Adam MA, Thomas S, Hyslop T, Scheri RP, Roman SA, Sosa JA. Exploring the relationship between patient age and cancer-specific survival in papillary thyroid cancer: rethinking current staging systems. J Clin Oncol. 2016;34(36):4415–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pitoia F, Jerkovich F, Smulever A, Brenta G, Bueno F, Cross G. Should age at diagnosis be included as an additional variable in the risk of recurrence classification system in patients with differentiated thyroid cancer. Eur Thyroid J. 2017;6(3):160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim Y, Roh JL, Gong G, Cho KJ, Choi SH, Nam SY, Kim SY. Risk factors for lateral neck recurrence of N0/N1a papillary thyroid cancer. Ann Surg Oncol. 2017;24(12):3609–3616. [DOI] [PubMed] [Google Scholar]
- 37. Kim SY, Kim BW, Pyo JY, Hong SW, Chang HS, Park CS. Macrometastasis in papillary thyroid cancer patients is associated with higher recurrence in lateral neck nodes. World J Surg. 2018;42(1):123–129. [DOI] [PubMed] [Google Scholar]
- 38. Kluijfhout WP, Drake FT, Pasternak JD, Beninato T, Vriens MR, Shen WT, Gosnell JE, Liu C, Suh I, Duh QY. Incidental positive lymph nodes in patients with papillary thyroid cancer is independently associated with recurrent disease. J Surg Oncol. 2017;116(3):275–280. [DOI] [PubMed] [Google Scholar]
- 39. Pitoia F, Bueno F, Urciuoli C, Abelleira E, Cross G, Tuttle RM. Outcomes of patients with differentiated thyroid cancer risk-stratified according to the American Thyroid Association and Latin American Thyroid Society risk of recurrence classification systems. Thyroid. 2013;23:1401–1407. [DOI] [PubMed] [Google Scholar]
- 40. Warren JL, Mariotto A, Melbert D, Schrag D, Doria-Rose P, Penson D, Yabroff KR. Sensitivity of Medicare claims to identify cancer recurrence in elderly colorectal and breast cancer patients. Med Care. 2016;54(8):e47–e54. [DOI] [PMC free article] [PubMed] [Google Scholar]