Abstract
The G protein–coupled receptors, free fatty acid (FFA) receptors 2 and 3 (FFA2 and FFA3), belonging to the free fatty acid receptor (FFAR) class, sense a distinct class of nutrients, short chain fatty acids (SCFAs). These receptors participate in both immune and metabolic regulation. The latter includes a role in regulating secretion of metabolic hormones. It was only recently that their role in pancreatic β cells was recognized; these receptors are known now to affect not only insulin secretion but also β-cell survival and proliferation. These observations make them excellent potential therapeutic targets in type 2 diabetes. Moreover, expression on both immune and β cells makes these receptors possible targets in type 1 diabetes. Furthermore, SCFAs are generated by gut microbial fermentative activity; therefore, signaling by FFA2 and FFA3 represents an exciting novel link between the gut microbiota and the β cells. This review enumerates the role of these receptors in β cells revealed so far and discusses possible roles in clinical translation.
Gut microbiota–derived short chain fatty acids modulate islet physiology via their cognate receptors, FFA2 and FFA3, presenting a link between the gut microbiota and the pancreatic β cell in diabetes.
“All disease begins in the gut.”
—Hippocrates
With recent advances in understanding gut microbiota and the far-reaching effects on host physiology, it can be generalized that all health begins in the gut. The influence of the gut microbiota occurs through interaction with the host through its structural components, metabolites, and multiple other suggested mechanisms (1). In 2003, short chain fatty acids (SCFAs), the major gut microbial metabolites, were recognized as ligands for the newly discovered G protein–coupled receptors (GPCRs), free fatty acid (FFA) receptors 2 and 3 (FFA2 and FFA3) (2–5), suggesting a mechanism whereby the gut microbiota can affect cellular processes. These discoveries have heralded great efforts to understand the physiological importance of these receptors. The combination of a very unique set of ligands (i.e., gut microbial byproducts), widespread tissue distribution, and amenability for therapeutic manipulation (that these are GPCRs) has made these receptors possible targets in several pathophysiological states. Although early research on these receptors mostly focused on their role as modulators of immune cell response (4–6), their importance as metabolic regulators was soon realized. Two patents by Arena Pharmaceuticals on these receptors also projected them as modulators of metabolic-related disorders (7, 8). Accompanying the identification of their expression in enteroendocrine cells, islets, and pancreatic β-cell lines (9–15), a search on the roles of these receptors in metabolic homeostasis was initiated that continues today. This review summarizes the research on FFA2 and FFA3 expressed in the islets of Langerhans, highlighting their interaction with the gut microbiota and the possibilities and hindrances to exploiting these receptors as therapeutic targets.
FFA2 and FFA3 Receptor Structure, Signaling, and Physiological Function
Pharmacology
FFA2 and FFA3 are closely related by sequence homology; however, some amino acid differences in the binding pocket confer ligand selectivity between these receptors (16). Nevertheless, the main endogenous SCFAs activate both FFA2 and FFA3, but with differing specificities and potencies based on the size of the aliphatic tail. For instance, human FFA2 is activated more potently by shorter SCFAs (acetate = propionate > butyrate > valerate), whereas FFA3 is activated by longer SCFAs (propionate = butyrate = valerate > acetate) (17) (Table 1). Stoddart et al. (16) demonstrated that some basic amino acid residues (arginine and histidine) in the transmembrane domains of FFA2 and FFA3 are crucial for the recognition, binding, and function of SCFAs at these two receptors. More recent reports have revealed that Arg180 and Arg255 are crucial for acetate/propionate-mediated FFA2 activation and that a possible interaction between these two arginine residues and His242 is pivotal in specifying the SCFA that binds to FFA2 as described for other free fatty acid receptors (FFARs) (16, 22).
Table 1.
Summary of Associated G Protein(s), Effector Mechanisms, and Endogenous and Synthetic Ligands for FFA2 and FFA3
| FFA2 | FFA3 | |
|---|---|---|
| G-protein signaling/effector | Gαi/o/cAMP | Gαi/o/cAMP |
| Gαq/11/Ca2+ | ||
| Endogenous ligands | SCFAs (C2-C6) | SCFAs (C3-C6) |
| Acetate (pEC50 4.5/3.6) | Butyrate (pEC50 4.0/3.7) | |
| Propionate (pEC50 4.2/3.7) | Propionate (pEC50 4.1/4.9) | |
| Synthetic agonists | CMTB (IC50 0.7/1.0) | MCP (pEC50 3.9/4.3) |
| PA (IC50 0.7/NA) | ||
| SCA14 (pEC50 3.2/NA) | ||
| SCA15 (pEC50 2.7/NA) |
pEC50 or IC50 values for human receptor/mouse receptor determined using different assays (18–21).
Abbreviations: CMTB, 4-chloro-α-(1methylethyl)-N-2-thiazolylbenzeneacetamide); MCP, 1-methylcyclopropanecarboxylic acid; NA, not available; PA, (S)-2-(4-chlorophenyl)-3,3-dimethyl-N-(5-phenylthiazol-2-yl)butanamide; pEC50, negative logarithm of effective ligand concentration giving half maximal response; SCA, small carboxylic acid.
It is noteworthy that this rank order of potency is not thought to be retained between human and mouse species orthologs (23). Acetate is 20-fold more potent at human FFA2 than at human FFA3 but almost equipotent at the two mouse orthologs (23). Similarly, propionate is more potent at mouse FFA3, but shows similar potency for the two human orthologs (23). Orthologs, where these SCFAs are more potent (mouse FFA3 and human FFA2), exhibit constitutive activity. The structural components of this constitutive activity have been defined. An extracellular ionic lock between the orthosteric binding site and acidic residues in extracellular loop 2 of human FFA3 and mouse FFA2 diminishes constitutive activity (16, 18, 19). Constitutive activity of the receptor orthologs may be part of the basis for the diverse effects observed for these receptors in mouse knockout (KO) models and/or disparate results in mouse vs human cell lines/tissues (24–29). Moreover, the paucity of clearly defined roles for these receptors underscores the need for developing specific, well-characterized, synthetic ligands active at each receptor to better define their biology (30, 31).
Signaling
FFA2 and FFA3 exhibit well-defined G protein–coupling specificities. FFA2 has been demonstrated to signal via two distinct Gα pathways: pertussis toxin (PTX)–sensitive Gαi/o and PTX-insensitive Gαq/11, whereas FFA3 signals selectively via Gαi/o pathway (3–5). It remains unreported what other signaling mechanisms common to GPCRs also occur upon SCFA binding to FFA2 and FFA3, such as signaling via the Gβγ complex and β arrestin, receptor desensitization, and receptor-receptor dimerization. Studies have indicated that SCFA binding to FFA2 recruits both β arrestin-1 and β arrestin-2, presumably playing a role in FFA2 internalization and possibly initiation of G protein–independent signaling (20). To our knowledge, there are no reported data on FFA3 and β arrestin signaling.
Physiological function
The expression of both FFA2 and FFA3 throughout the human body has led to their potential role in multiple physiological and pathological functions being proposed (14, 32) and will not be covered here. However, in light of the recently reported function of FFA2 and FFA3 in pancreatic β-cell physiology and the alluring role of the gut microbiota in metabolism, the central focus of this review is the role of FFA2 and FFA3 in β cells, and the possible relationship of the gut microbiota acting via SCFAs to affect β-cell function.
Role of FFA2 and FFA3 in Insulin Secretion
Glucose is the main stimulus for insulin secretion, but other nutrients, including amino acids and long-chain fatty acids, have established roles in modulating glucose-stimulated insulin secretion (GSIS) (33). Compared with these other nutrients, less attention has been devoted to understand the contribution of SCFAs to GSIS. Before the discovery of these receptors, studies were conflicting, and no consensus existed on how SCFAs (such as acetate) modulate GSIS (21). However, since the discovery of SCFA receptors, it has become clear that signaling through FFA3 inhibits GSIS in both human and mouse islets, and FFA2 signaling generally activates GSIS in mouse islets. The data in human islets for FFA2 are less clear.
Tang et al. (27) first reported that in β-cell lines and isolated islets from FFA2 and FFA3 KO mouse models, acetate signaling through FFA2 and FFA3 is a negative modulator of GSIS, acting through a PTX-sensitive Gαi/o pathway. Consistent with this, Priyadarshini and Layden (24) demonstrated that FFA3 activation modulates a PTX-sensitive inhibition of GSIS using mouse islets, SCFAs, and an FFA3 agonist, 1-methylcyclopropanecarboxylic acid (MCP). Additionally, islets from FFA3 KO mice exhibited increased insulin secretion as compared with islets from wild-type (WT) mice (24). Similar findings were reported by Veprik et al. (26). Because FFA3 signals via Gαi/o only, it is not surprising that activating FFA3 leads to the inhibition of GSIS.
Because FFA2 couples via two distinct Gα pathways (Gαq/11 or Gαi/o), it is not surprising that differences have emerged between early studies on how FFA2 influences GSIS. Although the first report indicated that FFA2 activation by acetate inhibits GSIS (27), two subsequent reports indicated that FFA2 activation enhances GSIS (25, 29). Priyadarshini et al. (25) demonstrated that acetate amplifies GSIS from WT islets and that this effect is absent in FFA2 KO islets. Complementary studies using isolated mouse islets were thus performed using synthetic FFA2 agonists to more clearly analyze the effects of FFA2 signaling on GSIS. Using WT and FFA2 KO islets, two small carboxylic acid (SCA) derivatives (SCA14, SCA15) and two phenylacetamide derivatives [4-chloro-α-(1methylethyl)-N-2-thiazolylbenzeneacetamide) (CMTB) and (S)-2-(4-chlorophenyl)-3,3-dimethyl-N-(5-phenylthiazol-2-yl)butanamide (PA)], Priyadarshini et al. (25) further found that FFA2 signaling could elicit both potentiation (by SCA14 and SCA15) and inhibition (by CMTB and PA) of GSIS. Moreover, CMTB- and PA-mediated inhibition of GSIS was retained in FFA2 KO islets, suggesting receptor-independent effects (25). These data showing that FFA2 couples with Gαq/11 and augments GSIS in islets and β-cell lines were largely confirmed by McNelis et al. (29); however, these authors also found that PA, but not acetate, leads to FFA2-mediated GSIS potentiation (29). The effects of FFA2 activity in islets from high-fat (HF) diet–fed FFA2 KO and WT mice revealed further complexity associated with FFA2 signaling. Although acetate and other agonists provided a similar pattern of GSIS in FFA2 KO and WT islets from mice on standard chow and an HF diet, PA unexpectedly increased GSIS from WT islets (25). These data indicated diet induced differential G protein coupling of FFA2, which was also suggested by McNelis et al. (29). They measured Gαq/11 and Gαi/o downstream second messengers (inositol 1,4,5-triphosphate; and cAMP, respectively) in response to PA-mediated FFA2 activation in islets from mice on 3 different dietary regimens: normal chow, short term HF feeding, and long term HF feeding (29). The authors found that inositol 1,4,5-triphosphate levels increased from normal chow to long-term HF feeding, whereas cAMP levels remained similar across the three dietary groups. Thus, an HF challenge possibly shifts the G protein coupling preference of FFA2 from Gαi/o to Gαq/11 (29). Although these studies indicate a role of FFA2 in potentiating insulin secretion, they also highlight the lack of our complete understanding of FFA2 signaling.
Role of FFA2 and FFA3 in Pancreatic β-Cell Mass Homeostasis
Insufficient β-cell mass is recognized as a primary defect in diabetes (both types 1 and 2). The process of maintaining the β-cell mass is dependent on numerous factors, including proliferation, apoptosis, hypertrophy, neogenesis, and transdifferentiation of β cells from other cell types (34). Not surprisingly, a large range of metabolic pathways has been suggested to contribute to these processes, and one pathway is through GPCRs. Similar to the mechanisms by which GPCRs contribute to GSIS, one can anticipate how GPCRs will influence β-cell mass based on their G protein coupling profiles. For instance, Gαq/11 GPCRs increase β-cell mass expansion in response to insulin resistance, Gαi/o GPCRs may reduce β-cell mass expansion (29, 35) and Gαs GPCRs such as GLP1 receptor and glucose-dependent insulinotropic peptide receptor have well-established stimulatory effects on β-cell mass expansion in rodent islets (36). Moreover, GPCRs primarily seem to influence β-cell mass through regulation of proliferation, apoptosis, and hypertrophy (36, 37).
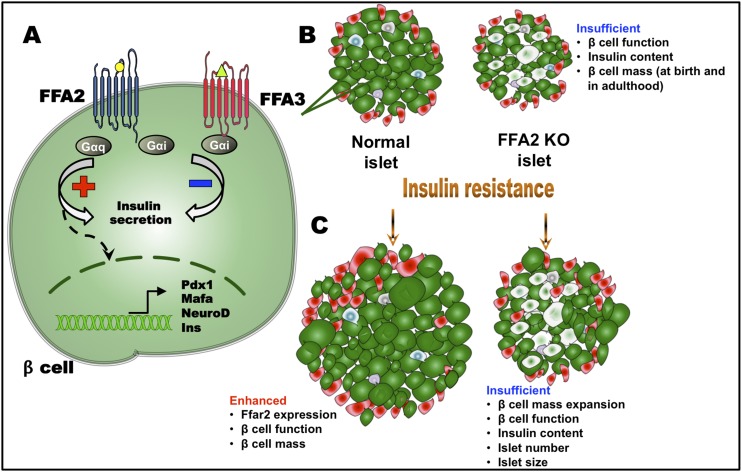
In exploring the role of FFA2 in β-cell mass, McNelis et al. (29) observed that FFA2 KO mice showed no difference in β-cell mass compared with WT mice when using immunohistochemical and morphometric analyses of pancreata. However, in mice fed an HF diet, FFA2 KO mice showed a decrease in both islet number and size, leading to reduced β-cell mass and total pancreatic insulin content (elaborated later in this article). Interestingly, Fuller et al. (38) reported similar findings during pregnancy studies, specifically a reduction in β-cell mass expansion in FFA2 KO mice. Villa et al. (39) and Fuller et al. (38) however, reported that both female and male FFA2 KO mice exhibited reduced β-cell mass compared with the WT littermate mice even under basal conditions. Exploring this latter observation, our research group examined young (postnatal days 1 and 21) and adult mice and showed impaired β-cell mass at birth and through adulthood in FFA2 KO mice. These findings suggested that impairments in β-cell mass develop during the prenatal period in FFA2 KO mice. Thus, FFA2 may contribute to the prenatal establishment of β-cell mass, and its deletion appears to result in enhanced postnatal β-cell death (39). Although our group and others have reported a role of FFA2 in β-cell mass, Tang et al. (27) reported no differences in islets morphology between WT and FFA2 KO mice.
Regarding FFA3, Veprik et al. (26) observed a role for FFA3 in the modulation of β-cell mass. Islet morphology studies showed that islets from FFA3 Tg mice (β cell–specific FFA3 overexpression transgenic mouse model) were larger, exhibited greater percentage of Ki-67+ cells, and consequently showed increased β-cell area compared with islets from WT mice. On the other hand, islets from FFA3 KO mice were significantly smaller than islets from WT mice, with a reduced number of both β and Ki-67+ cells leading to a reduced proliferation (26). However, these data are not necessarily consistent with a Gαi/o GPCR. Taken together, these studies reveal a potential role of both FFA2 and FFA3 in modulating β-cell mass and function; however, greater studies are needed, in particular, for FFA3.
Insights From Model Systems
The effects of distinct signaling cascades evoked by SCFA-FFA2/3 interaction (described previously and summarized in Table 1) on β-cell physiology have been evinced through studies in in vivo and ex vivo models as described previously (12, 21, 24–27, 29, 38, 39–42). Accordingly, these receptors have emerged as possible mediators in metabolic disorders, and we next review their observed role in metabolic states.
FFA2 and FFA3 in obesity and insulin resistance
On an HF diet, when the β cell is compensating to the increased demand for insulin secretion (43, 44), expression of mouse islet FFA2 is notably increased (25, 29). Islet FFA3 expression also trends higher under such a dietary challenge (M.P. and B.T.L, unpublished data, 2015). In fact, the earliest assertion of a role of these receptors in states of insulin resistance was reported in a legal patent, showing enhanced expression of these receptors in islets of db/db and ob/ob mice (7, 8). These expression changes suggest a role in β-cell responses to metabolic stress. As expected from a loss of a Gαq/11 receptor (36), FFA2 KO mice exhibit fasting hyperglycemia, reduced insulin levels, and glucose intolerance, despite exhibiting normal insulin sensitivity (29). These differences, however, are visible only under conditions of physiological stress (25, 29). In contrast, a phenotype of improved glucose tolerance with enhanced insulin secretion has been reported in FFA2 KO mice (27). These conflicting data suggest that the FFA2 KO phenotype may be conditional to several factors, such as differences in receptor-G protein coupling, its activity in insulin responsive tissues, characteristics of the gut microbiota, the strain of mice, and the duration of a dietary challenge (29, 32, 45).
In contrast, deletion of FFA3 has consistently shown improved insulin secretion and hence improved glucose tolerance (26, 27). More specifically, this FFA3 KO phenotype is observed either on HF only (27) or both on regular and HF diet (26). Correspondingly, β cell–specific FFA3 overexpression has been shown to deteriorate glucose responsiveness in mice (26). This phenotype represents a glucose-dependent β-cell secretory defect because no hypoglycemic responses were observed in these FFA3 models (26, 27).
During compensation to metabolic demand, enhanced β-cell function is accompanied by β-cell mass expansion (43). FFA2, as anticipated from a Gαq/11 coupled receptor (36), promotes β-cell mass expansion in mice on HF diet, as described previously (25, 29). Further studies from this group showed that FFA2 activation augments β-cell mass by a direct effect on β-cell proliferation and expression of β-cell differentiation genes (MafA, Pdx1, NeuroD) (Fig. 1). Of note, even on a regular diet, FFA2 signaling is necessary for establishment of normal β-cell mass, based on observations from our group. FFA2 KO mice present with a lower β-cell mass at birth that is maintained during adulthood (both in male and female mice) (38, 39). These data indicate that FFA2 expression in the β cell may regulate aspects of β-cell proliferation/survival (Fig. 1) (39). In contrast, FFA3, a Gαi/o receptor, should restrict β-cell mass expansion (36). However, lack of insulin in the β cells of β cell–specific FFA3 overexpression model is compensated by increased β-cell area and proliferation (26). Similarly, in FFA3 KO model, higher insulin secretion is matched by a complementary decline in β-cell area and proliferation (26). Thus, these β-cell mass changes appear to be secondary to changes on insulin secretion from FFA3.
Figure 1.
Modulation of islet responses by FFA2 and FFA3. (A) In the β cell, upon ligand activation, FFA2 couples primarily with Gαq/11 subunit and enhances glucose-stimulated insulin secretion. FFA2 can also couple with the Gαi/o subunit if activated by Gαi/o-biased ligands such as CMTB (see text). Activated FFA3 couples exclusively with Gαi/o and decreases insulin secretion. FFA2 signaling also upregulates expression of transcription of factors responsible for increased β-cell proliferation and mass. (B) Because of an absence of essential FFA2 signaling, FFA2 KO islet exhibits reduced β-cell function and insulin content and defective β-cell mass, which may be established at birth and continues to adulthood. (C) Upon challenge with insulin-resistant states such as HF diet consumption or pregnancy, FFA2 KO islets fail to compensate. FFA2 signaling thus contributes to normal and adaptive responses of the β cell to insulin resistance. Yellow circle represents acetate; green triangle represents propionate; plus and minus signs denote increase and decrease, respectively (21, 24–27, 29, 38, 39). Ins, insulin.
Because of the widespread expression of these receptors (14, 21), the most informative way of analyzing their β cell–specific effects will be to target β cell–specific expression. The β cell–specific FFA3 overexpression model of Veprik et al. (26) and β cell–specific FFA2 KO crossed with global FFA3 KO model of Tang et al. (27) are the only two specialized models studied to date. The latter model on HF diet has shown improved glucose tolerance and insulin secretion in response to a glucose challenge (27). More investigations using these types of models are needed to corroborate and expand upon these findings.
FFA2 and FFA3 in gestational diabetes
Expression of the SCFA receptors in rodent islets is modulated during pregnancy (15, 38; M.P. and B.T.L., unpublished data, 2014). FFA2, as anticipated from data in FFA2 KO male mice, enhances β-cell function and proliferation in female mice during pregnancy (38). FFA2 KO female mice had fasting hyperglycemia, impaired glucose tolerance, reduced insulin secretion, and insufficient expansion of the β-cell mass at gestational day 15 (a point of heightened insulin resistance during pregnancy) (38). These metabolic changes were not observed before pregnancy, indicating that greater mechanistic insight into FFA2 mediated β-cell regulation in pregnant mice is needed. It was reported that FFA2-mediated incretin secretion does not play a role in these gestational changes (38). Islet gene expression changes in FFA2 WT vs FFA2 KO female mice included several potential genes involved in β-cell mass modulation including Reg family members and the Tph1 gene as plausible contributors to these β-cell changes (M. Fuller, M.P., and B.T.L., unpublished data, 2014).
Islet FFA3 function in pregnant mice has not been elucidated. It is possible that FFA3 is involved in restricting β-cell mass expansion during pregnancy (35) because its expression in rodent islet increases later during pregnancy and in the postpartum period (M.P. and B.T.L., unpublished data, 2014). However, analysis of the interplay of receptor-specific signaling (FFA2 vs FFA3) during different phases of rodent pregnancy using β cell–specific KO models and in vitro functional assays is needed.
FFA2 and FFA3 in type 1 diabetes
FFA2 and FFA3 are expressed by various immune cell types and regulate intestinal immune homeostasis exerting a range of effects (32, 46–50), including possibly the intestinal immune barrier, which has been implicated in type 1 diabetes (T1D) (51, 52). Importantly, FFA2 expression in peripheral blood monocytes is upregulated in T1D patients (53) and that of FFA3 correlates with inflammation and metabolic markers (54). Despite these observations, limited studies have explored the role of these receptors in T1D.
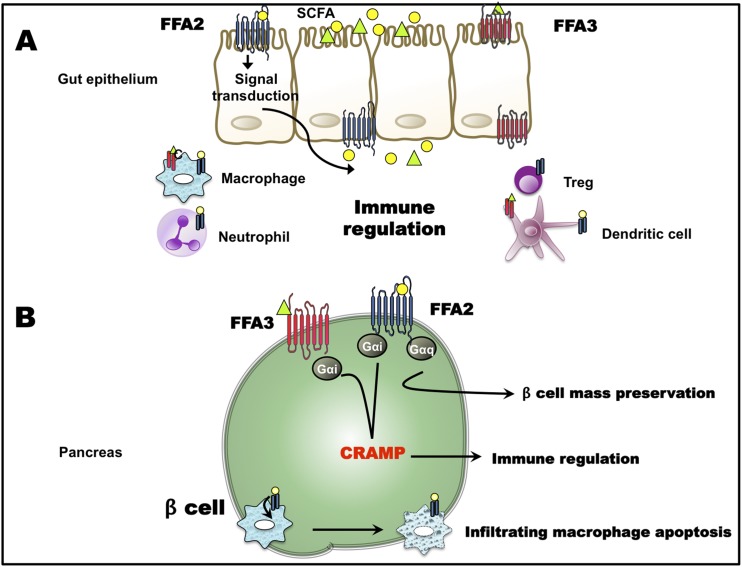
Mariño et al. (55) recently reported that the intestinal immune cell repertoire, primed by dietary SCFAs through the activation of FFA2, plays an important role in modulating islet inflammatory responses in the T1D model, NOD mice. Butyrate-enriched diet could partially protect FFA2 KO mice on a NOD background from T1D islet inflammation (55). This suggests that besides FFA2, the ketone body receptor GPR109a and FFA3 that can also be activated by butyrate, may also be conferring partial protection. In another T1D model that used multiple low-dose streptozotocin to induce diabetes, treatment with specific FFA2 agonists improved glucose tolerance and attenuated islet inflammation, primarily by inducing apoptosis of infiltrating macrophages, possibly through the activation of macrophage FFA2 (53). Another mechanism by which β cells can thwart autoimmune diabetes destruction may be through production of cathelicidin-related antimicrobial peptide (CRAMP), where this peptide has an immunoregulatory role (56). SCFAs regulate islet CRAMP production, and this regulation is susceptible to PTX treatment (56). Hence, a role of FFA3 (and/or FFA2) in CRAMP production is surmised (56). Taken together, FFA2 and FFA3 may prevent islet damage in T1D (Fig. 2). Whether this protection is through immune cell–specific or β cell–specific mechanisms or involves roles for FFA2 and FFA3 in both cell types needs to be investigated and requires using cell type–specific KO mouse models. Further, as islet destruction in T1D is extensive and rapid, dissecting the mechanism by in vitro methods will be crucial to understanding the role of these receptors in directly protecting β cells against T1D damage.
Figure 2.
Role of FFA2 and FFA3 in type 1 diabetes. (A) Activation of FFA2 and FFA3 on intestinal epithelial cells affects the production of inflammatory mediators/processes. Receptor signaling on various immune cell types results in immune modulation for example by differentiation and proliferation of regulatory T cells (Tregs) and consequent change in the inflammatory tone. (B) In the β cell, activation of FFA2 and FFA3 possibly stimulates production of CRAMP, which exerts anti-inflammatory effects. Also, FFA2 activation on infiltrating immune cells causes immune cell apoptosis. Along with these, FFA2 (and FFA3) signaling can preserve β-cell mass. Yellow circle represents acetate; green triangle represents propionate (32, 53, 55, 56).
FFA2 and FFA3: translation to human islets
Understanding of how these receptors modulate β-cell physiology in humans is crucial because their therapeutic value in diabetes is considered. However, this presents hurdles, as donor islets have variable expression of these receptors (S.R. Villa, M.P., and B.T.L., unpublished data, 2015). Further, donor dietary habits and health conditions, islet quality, and islet procurement time can each affect responses to glucose and ligand stimulation. Thus, while analyzing the role of these receptors on insulin secretion or β-cell survival using human islets, these variables must be considered.
In human islets, activation of FFA2 using agonists has provided results different than obtained with mouse islets. Moreover, human FFA2 may exhibit different coupling preferences as compared with mouse FFA2 (as noted previously). Data from our laboratory show that human FFA2 can simultaneously activate both Gαq/11 and Gαi/o pathways; hence, no net effect on GSIS in human islets is observed upon stimulation with specific synthetic agonists, such as PA, and the endogenous ligand acetate (25). An exception to this is FFA2 stimulation by the specific agonist CMTB that inhibits GSIS in human islets in a PTX-insensitive manner (25). Contradictory results from the study by McNelis et al. (29) showed that PA enhances GSIS from human islets in a PTX-insensitive manner, whereas Tang et al. (27) reported that acetate inhibited GSIS from human islets. These effects need to be further defined to establish whether they are FFA2-specific. As apparent, the data thus far are not clear as to the role of FFA2 in human islets.
Although additional data are needed to validate these insulin secretion findings, no studies have defined the role of these receptors in human β-cell proliferation and/or survival. This later point is especially important because β-cell proliferation in humans is less apparent than in mice and aspects of β-cell mass regulation may not always be as applicable to humans (57). Moreover, FFA3 function in human β cells is less explored than that of FFA2, but likely is more straightforward because of the less complex G-protein signaling. In sum, a focus of future research should be on understanding the basic mechanisms in human islets or human-specific model systems.
FFA2 and FFA3 Roles in Mediating the Gut–β-cell Axis
FFA2 and FFA3 respond to SCFA, gut microbial byproducts (1), and hence present one of the routes by which the gut microbiota can communicate with β cells. In the development of diabetes, insulin resistance, and obesity, alterations in gut microbial profiles are well documented in both humans and rodent models (58–66). It is challenging, however, to assess the relationship between altered gut microbiota and SCFA receptor function, especially in humans. Serum/fecal SCFA levels, receptor expression, and receptor activity in tissues (from biopsies) is the current surrogate available from human studies. For example, in inflammatory bowel syndrome patients as compared with normal subjects, altered immune cell SCFA receptor expression, fecal SCFA levels, and immune response to SCFA stimulation has been reported (67, 68). Alterations in serum and/or fecal SCFA levels have also been reported in individuals having metabolic disorders and in individuals with T1D (27, 69–71). However, correlation of these alterations in SCFA levels to the β-cell SCFA receptor function is not straightforward because FFA2 and FFA3 may also affect β-cell function through gut and neural pathways [reviewed in Tang and Offermanns (14)]. Thus, the causality of the relationship represents a major challenge in interpreting these observations (45).
To overcome this challenge, rodent models are being used to analyze the gut microbiota–SCFA (β-cell) receptor relationship in metabolic diseases. In the widely used whole body SCFA receptor KO models (for FFA2 and FFA3), gut microbiota profiles differ from those in the WT mice and, not surprisingly, are accompanied by differences in fecal SCFA levels between the models (29, 72–75). These differences are further amplified when the mice are challenged with a stressor, such as HF diet feeding or pregnancy. For example, McNelis et al. (29) observed an increased ratio of Firmicutes/Bacteroidetes in HF diet-fed mice with higher proportion of Gammaproteobacteria in the WT and Actinobacteria in the FFA2 KO mice. Bacterial function assessment revealed enrichment of fecal enzymes involved in butyrate and acetate production, consistent with the increase in serum butyrate in the WT mice and in serum acetate in the FFA2 KO mice (29). Functional implications of these differences are not clear but may represent a compensatory mechanism to accommodate for the absence of the acetate receptor, FFA2, in the KO mice. Fuller et al. (38) also reported an increased proportion of Actinobacteria in FFA2 KO mice, albeit before pregnancy (i.e., in absence of physiological stress). Changes in gut microbiota that accompanied pregnancy [although not as apparent as in humans (76)] occurred only in WT mice, not in FFA2 KO mice (38). These microbial changes were coupled with higher serum acetate and lower propionate levels during gestation, which is consistent with the role of FFA2 in enhancing insulin secretion. Moreover, the pregnancy model of Fuller et al. (38) emphasizes the role of FFA2 in linking changes in gut microbiota to enhanced β-cell function during pregnancy.
In NOD mice (a T1D model), a dependence upon gut microbiota derived SCFAs and β-cell SCFAs receptor signaling has also been highlighted (56). Specifically, butyrate generated by gut microbial fermentation can trigger the production of CRAMP (an antimicrobial peptide) from the islets, which governs the switching of inflammatory to regulatory immune cells in pancreas in this T1D mouse model and correlates negatively with disease incidence (56). Interestingly, male NOD mice possess a higher proportion of butyrate producers in the gut (56, 77, 78). Accordingly, systemic SCFA (butyrate) administration or fecal microbial transfer from male NOD mice diminished disease incidence in female NOD mice. Conversely, antibiotic treatment that targeted SCFA producers in male NOD mice increased disease progression (56). Further, SCFA-stimulated islet CRAMP production was susceptible to PTX treatment, suggesting the involvement of β-cell FFA3 (or FFA2) (56).
From these data, it is apparent that β-cell FFA2 and FFA3 present a link between the gut microbiota and β-cell function in both forms of diabetes. β cell–specific single and double receptor KO mice are required to further clarify these relationships. Humanization of these models (through fecal microbial transfer from diseased and normal human donors) will also define the presence of such links in humans.
Unresolved Questions and Future Perspectives
As described previously, FFA2 and FFA3 influence aspects of β-cell function. However, outcomes of several of the discussed reports are contradictory. Hence, there are several caveats associated with roles of FFA2 and FFA3 in β cells, including (1) overlapping ligand and expression profiles of these receptors (14, 21); (2) lack of commercially available receptor-specific and G protein–biased ligands (30); (3) absence of advanced mouse models (31); (4) challenges associated with precise determination of receptor expression (26, 49); (5) paucity of human studies correlating gut microbiota profiles/SCFA levels to insulin secretion; and (6) complex interplay of multiple factors, such as effects of these receptors in insulin-sensitive tissues, diet, gut microbiota, host hygiene, host genotype, and role of these receptors in determining host phenotype (45), each of which needs to be considered moving forward.
Most of the work on SCFA receptor β-cell physiology has been conducted on global KO models. Because compensatory effects of the other receptors and pathways can occur, it is crucial to develop/use β cell–specific single and double receptor KOs. Furthermore, FFA2 and FFA3 can affect the β cell via enteroendocrine, neural, and immune mechanisms (12, 14, 29, 40, 41, 53, 55, 56, 70, 75, 79–83). The role of these receptors in regulating enteroendocrine secretion is covered in another accompanying mini-review by Reimann and colleagues [see Lu et al., (84)]. It will be important to dissect each of these roles and their impact on the β cell. For example, comparison of receptor mutants generated by using β cell–specific vs myeloid-specific Cre may prove useful to elucidate immune cell– and β cell–specific roles of these receptors in both the pathophysiology of T1D and the inflammation associated with obesity and insulin resistance that may precede type 2 diabetes. To understand the functioning of human receptors (considering the differences between mouse and human orthologs), generation of humanized mice expressing the human orthologs globally or specifically in β cells are required. Also, ligands active at human vs mouse orthologs and at specific receptors (30, 31) need to be characterized for their effects on the β cell, both ex vivo using islets and in vivo in mouse models. Disparate observations may also arise because of differences in genetic background of mice that needs to be considered (32, 45, 65). And finally, diet (nutrient status) appears to be important in determining the activity of these receptors probably through interactions of the diet with the gut microbiota.
In conclusion, there is considerable evidence that these SCFA receptors influence β-cell physiology. Furthermore, they present potential therapeutic targets that can be targeted through drugs or modification of gut microbiota–diet axis. However, unraveling their true therapeutic potential requires further in depth research.
Acknowledgments
We appreciate Dr. Barton Wicksteed for the critical review of this article.
Financial Support: B.T.L. is supported by the National Institutes of Health Grant R01DK104927-01A1 and the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, VA merit Grant 1I01BX003382-01-A1.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- CMTB
4-chloro-α-(1methylethyl)-N-2-thiazolylbenzeneacetamide)
- CRAMP
cathelicidin-related antimicrobial peptide
- FFA
free fatty acid
- FFA2
free fatty acid receptor 2
- FFA3
free fatty acid receptor 3
- FFAR
free fatty acid receptor
- GPCR
G protein–coupled receptor
- GSIS
glucose-stimulated insulin secretion
- HF
high-fat
- KO
knockout
- MCP
1-methylcyclopropanecarboxylic acid
- PA
(S)-2-(4-chlorophenyl)-3,3-dimethyl-N-(5-phenylthiazol-2-yl)butanamide
- PTX
pertussis toxin
- SCA
small carboxylic acid
- SCFA
short chain fatty acid
- T1D
type 1 diabetes
- WT
wild-type
References
- 1. Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22(10):1079–1089. [DOI] [PubMed] [Google Scholar]
- 2. Sawzdargo M, George SR, Nguyen T, Xu S, Kolakowski LF, O’Dowd BF. A cluster of four novel human G protein-coupled receptor genes occurring in close proximity to CD22 gene on chromosome 19q13.1. Biochem Biophys Res Commun. 1997;239(2):543–547. [DOI] [PubMed] [Google Scholar]
- 3. Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278(13):11312–11319. [DOI] [PubMed] [Google Scholar]
- 4. Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, Parmentier M, Detheux M. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278(28):25481–25489. [DOI] [PubMed] [Google Scholar]
- 5. Nilsson NE, Kotarsky K, Owman C, Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun. 2003;303(4):1047–1052. [DOI] [PubMed] [Google Scholar]
- 6. Senga T, Iwamoto S, Yoshida T, Yokota T, Adachi K, Azuma E, Hamaguchi M, Iwamoto T. LSSIG is a novel murine leukocyte-specific GPCR that is induced by the activation of STAT3. Blood. 2003;101(3):1185–1187. [DOI] [PubMed] [Google Scholar]
- 7. Leonard JN, Chu ZL, Bruce MA, Boatman PD. Gpr41 and modulators thereof for the treatment of insulin-related disorders. Google Patents; 2014. Available at: https://patents.google.com/patent/US20080312277A1/en. Accessed May 7, 2018. [Google Scholar]
- 8. Leonard JN, Hakak Y. Gpr43 and modulators thereof for the treatment of metabolic-related disorders. Google Patents; 2006. Available at: https://patents.google.com/patent/US20070077602. Accessed May 7, 2018. [Google Scholar]
- 9. Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, Furness JB, Kuwahara A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324(3):353–360. [DOI] [PubMed] [Google Scholar]
- 10. Kebede MA, Alquier T, Latour MG, Poitout V. Lipid receptors and islet function: therapeutic implications? Diabetes Obes Metab. 2009;11(Suppl 4):10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Layden BT, Durai V, Lowe WL Jr. G-protein-coupled receptors, pancreatic islets, and diabetes. Nature Education. 2010;3(9):13. [Google Scholar]
- 12. Nøhr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, Sichlau RM, Grunddal KV, Poulsen SS, Han S, Jones RM, Offermanns S, Schwartz TW. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154(10):3552–3564. [DOI] [PubMed] [Google Scholar]
- 13. Tazoe H, Otomo Y, Kaji I, Tanaka R, Karaki SI, Kuwahara A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J Physiol Pharmacol. 2008;59(Suppl 2):251–262. [PubMed] [Google Scholar]
- 14. Tang C, Offermanns S. FFA2 and FFA3 in metabolic regulation. Handb Exp Pharmacol. 2017;236:205–220. [DOI] [PubMed] [Google Scholar]
- 15. Layden BT, Durai V, Newman MV, Marinelarena AM, Ahn CW, Feng G, Lin S, Zhang X, Kaufman DB, Jafari N, Sørensen GL, Lowe WL Jr. Regulation of pancreatic islet gene expression in mouse islets by pregnancy. J Endocrinol. 2010;207(3):265–279. [DOI] [PubMed] [Google Scholar]
- 16. Stoddart LA, Smith NJ, Jenkins L, Brown AJ, Milligan G. Conserved polar residues in transmembrane domains V, VI, and VII of free fatty acid receptor 2 and free fatty acid receptor 3 are required for the binding and function of short chain fatty acids. J Biol Chem. 2008;283(47):32913–32924. [DOI] [PubMed] [Google Scholar]
- 17. Milligan G, Stoddart LA, Smith NJ. Agonism and allosterism: the pharmacology of the free fatty acid receptors FFA2 and FFA3. Br J Pharmacol. 2009;158(1):146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hudson BD, Tikhonova IG, Pandey SK, Ulven T, Milligan G. Extracellular ionic locks determine variation in constitutive activity and ligand potency between species orthologs of the free fatty acid receptors FFA2 and FFA3. J Biol Chem. 2012;287(49):41195–41209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schmidt J, Smith NJ, Christiansen E, Tikhonova IG, Grundmann M, Hudson BD, Ward RJ, Drewke C, Milligan G, Kostenis E, Ulven T. Selective orthosteric free fatty acid receptor 2 (FFA2) agonists: identification of the structural and chemical requirements for selective activation of FFA2 versus FFA3. J Biol Chem. 2011;286(12):10628–10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee SU, In HJ, Kwon MS, Park BO, Jo M, Kim MO, Cho S, Lee S, Lee HJ, Kwak YS, Kim S. β-Arrestin 2 mediates G protein-coupled receptor 43 signals to nuclear factor-κB. Biol Pharm Bull. 2013;36(11):1754–1759. [DOI] [PubMed] [Google Scholar]
- 21. Priyadarshini M, Wicksteed B, Schiltz GE, Gilchrist A, Layden BT. SCFA receptors in pancreatic β cells: novel diabetes targets? Trends Endocrinol Metab. 2016;27(9):653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Srivastava A, Yano J, Hirozane Y, Kefala G, Gruswitz F, Snell G, Lane W, Ivetac A, Aertgeerts K, Nguyen J, Jennings A, Okada K. High-resolution structure of the human GPR40 receptor bound to allosteric agonist TAK-875. Nature. 2014;513(7516):124–127. [DOI] [PubMed] [Google Scholar]
- 23. Hudson BD, Christiansen E, Tikhonova IG, Grundmann M, Kostenis E, Adams DR, Ulven T, Milligan G. Chemically engineering ligand selectivity at the free fatty acid receptor 2 based on pharmacological variation between species orthologs. FASEB J. 2012;26(12):4951–4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Priyadarshini M, Layden BT. FFAR3 modulates insulin secretion and global gene expression in mouse islets. Islets. 2015;7(2):e1045182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Priyadarshini M, Villa SR, Fuller M, Wicksteed B, Mackay CR, Alquier T, Poitout V, Mancebo H, Mirmira RG, Gilchrist A, Layden BT. An acetate-specific GPCR, FFAR2, regulates insulin secretion. Mol Endocrinol. 2015;29(7):1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Veprik A, Laufer D, Weiss S, Rubins N, Walker MD. GPR41 modulates insulin secretion and gene expression in pancreatic β-cells and modifies metabolic homeostasis in fed and fasting states. FASEB J. 2016;30(11):3860–3869. [DOI] [PubMed] [Google Scholar]
- 27. Tang C, Ahmed K, Gille A, Lu S, Gröne HJ, Tunaru S, Offermanns S. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat Med. 2015;21(2):173–177. [DOI] [PubMed] [Google Scholar]
- 28. Ang Z, Er JZ, Tan NS, Lu J, Liou YC, Grosse J, Ding JL. Human and mouse monocytes display distinct signalling and cytokine profiles upon stimulation with FFAR2/FFAR3 short-chain fatty acid receptor agonists. Sci Rep. 2016;6(1):34145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McNelis JC, Lee YS, Mayoral R, van der Kant R, Johnson AM, Wollam J, Olefsky JM. GPR43 potentiates β-cell function in obesity. Diabetes. 2015;64(9):3203–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Milligan G, Bolognini D, Sergeev E. Ligands at the free fatty acid receptors 2/3 (GPR43/GPR41). Handb Exp Pharmacol. 2017;236:17–32. [DOI] [PubMed] [Google Scholar]
- 31. Milligan G, Shimpukade B, Ulven T, Hudson BD. Complex pharmacology of free fatty acid receptors. Chem Rev. 2017;117(1):67–110. [DOI] [PubMed] [Google Scholar]
- 32. Tan JK, McKenzie C, Mariño E, Macia L, Mackay CR. Metabolite-sensing G protein-coupled receptors-facilitators of diet-related immune regulation. Annu Rev Immunol. 2017;35(1):371–402. [DOI] [PubMed] [Google Scholar]
- 33. Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013;18(2):162–185. [DOI] [PubMed] [Google Scholar]
- 34. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. [DOI] [PubMed] [Google Scholar]
- 35. Berger M, Scheel DW, Macias H, Miyatsuka T, Kim H, Hoang P, Ku GM, Honig G, Liou A, Tang Y, Regard JB, Sharifnia P, Yu L, Wang J, Coughlin SR, Conklin BR, Deneris ES, Tecott LH, German MS. Gαi/o-coupled receptor signaling restricts pancreatic β-cell expansion. Proc Natl Acad Sci USA. 2015;112(9):2888–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ahrén B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes [published correction appears in Nat Rev Drug Discov. 2009;8:369–385] Nat Rev Drug Discov. 2009;8(5):369–385. [DOI] [PubMed] [Google Scholar]
- 37. Jain S, Ruiz de Azua I, Lu H, White MF, Guettier JM, Wess J. Chronic activation of a designer G(q)-coupled receptor improves β cell function. J Clin Invest. 2013;123(4):1750–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fuller M, Priyadarshini M, Gibbons SM, Angueira AR, Brodsky M, Hayes MG, Kovatcheva-Datchary P, Bäckhed F, Gilbert JA, Lowe WL Jr, Layden BT. The short-chain fatty acid receptor, FFA2, contributes to gestational glucose homeostasis. Am J Physiol Endocrinol Metab. 2015;309(10):E840–E851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Villa SR, Priyadarshini M, Fuller MH, Bhardwaj T, Brodsky MR, Angueira AR, Mosser RE, Carboneau BA, Tersey SA, Mancebo H, Gilchrist A, Mirmira RG, Gannon M, Layden BT. Loss of free fatty acid receptor 2 leads to impaired islet mass and beta cell survival. Sci Rep. 2016;6(1):28159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, Ghatei MA, Bloom SR, Frost G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes. 2015;39(3):424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sykaras AG, Demenis C, Case RM, McLaughlin JT, Smith CP. Duodenal enteroendocrine I-cells contain mRNA transcripts encoding key endocannabinoid and fatty acid receptors. PLoS One. 2012;7(8):e42373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53(Suppl 3):S16–S21. [DOI] [PubMed] [Google Scholar]
- 44. Mosser RE, Maulis MF, Moullé VS, Dunn JC, Carboneau BA, Arasi K, Pappan K, Poitout V, Gannon M. High-fat diet-induced β-cell proliferation occurs prior to insulin resistance in C57Bl/6J male mice. Am J Physiol Endocrinol Metab. 2015;308(7):E573–E582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu H, Tremaroli V, Bäckhed F. Linking microbiota to human diseases: a systems biology perspective. Trends Endocrinol Metab. 2015;26(12):758–770. [DOI] [PubMed] [Google Scholar]
- 46. Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bhutia YD, Ganapathy V. Short, but smart: SCFAs train T cells in the gut to fight autoimmunity in the brain. Immunity. 2015;43(4):629–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sina C, Gavrilova O, Förster M, Till A, Derer S, Hildebrand F, Raabe B, Chalaris A, Scheller J, Rehmann A, Franke A, Ott S, Häsler R, Nikolaus S, Fölsch UR, Rose-John S, Jiang HP, Li J, Schreiber S, Rosenstiel P. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J Immunol. 2009;183(11):7514–7522. [DOI] [PubMed] [Google Scholar]
- 49. Ang Z, Ding JL. GPR41 and GPR43 in obesity and inflammation - protective or causative? Front Immunol. 2016;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20(2):159–166. [DOI] [PubMed] [Google Scholar]
- 51. Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol. 2017;14(1):9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. D’Addio F, Fiorina P. Type 1 diabetes and dysfunctional intestinal homeostasis. Trends Endocrinol Metab. 2016;27(7):493–503. [DOI] [PubMed] [Google Scholar]
- 53. Shi G, Sun C, Gu W, Yang M, Zhang X, Zhai N, Lu Y, Zhang Z, Shou P, Zhang Z, Ning G. Free fatty acid receptor 2, a candidate target for type 1 diabetes, induces cell apoptosis through ERK signaling. J Mol Endocrinol. 2014;53(3):367–380. [DOI] [PubMed] [Google Scholar]
- 54. Pivovarova O, Hornemann S, Weimer S, Lu Y, Murahovschi V, Zhuk S, Seltmann AC, Malashicheva A, Kostareva A, Kruse M, Busjahn A, Rudovich N, Pfeiffer AF. Regulation of nutrition-associated receptors in blood monocytes of normal weight and obese humans. Peptides. 2015;65:12–19. [DOI] [PubMed] [Google Scholar]
- 55. Mariño E, Richards JL, McLeod KH, Stanley D, Yap YA, Knight J, McKenzie C, Kranich J, Oliveira AC, Rossello FJ, Krishnamurthy B, Nefzger CM, Macia L, Thorburn A, Baxter AG, Morahan G, Wong LH, Polo JM, Moore RJ, Lockett TJ, Clarke JM, Topping DL, Harrison LC, Mackay CR. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol. 2017;18(5):552–562. [DOI] [PubMed] [Google Scholar]
- 56. Sun J, Furio L, Mecheri R, van der Does AM, Lundeberg E, Saveanu L, Chen Y, van Endert P, Agerberth B, Diana J. Pancreatic β-cells limit autoimmune diabetes via an immunoregulatory antimicrobial peptide expressed under the influence of the gut microbiota. Immunity. 2015;43(2):304–317. [DOI] [PubMed] [Google Scholar]
- 57. Layden BT, Wicksteed B, Mauvais-Jarvis F. Incretin-based therapies: revisiting their mode of action. Endocrinology. 2017;158(6):1560–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rahat-Rozenbloom S, Fernandes J, Gloor GB, Wolever TM. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int J Obes. 2014;38(12):1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shepherd ML, Ponder MA, Burk AO, Milton SC, Swecker WS Jr. Fibre digestibility, abundance of faecal bacteria and plasma acetate concentrations in overweight adult mares. J Nutr Sci. 2014;3:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. [DOI] [PubMed] [Google Scholar]
- 61. Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TM, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes. 2014;4(6):e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li M, Gu D, Xu N, Lei F, Du L, Zhang Y, Xie W. Gut carbohydrate metabolism instead of fat metabolism regulated by gut microbes mediates high-fat diet-induced obesity. Benef Microbes. 2014;5(3):335–344. [DOI] [PubMed] [Google Scholar]
- 63. Murugesan S, Ulloa-Martínez M, Martínez-Rojano H, Galván-Rodríguez FM, Miranda-Brito C, Romano MC, Piña-Escobedo A, Pizano-Zárate ML, Hoyo-Vadillo C, García-Mena J. Study of the diversity and short-chain fatty acids production by the bacterial community in overweight and obese Mexican children. Eur J Clin Microbiol Infect Dis. 2015;34(7):1337–1346. [DOI] [PubMed] [Google Scholar]
- 64. Murphy EF, Cotter PD, Healy S, Marques TM, O’Sullivan O, Fouhy F, Clarke SF, O’Toole PW, Quigley EM, Stanton C, Ross PR, O’Doherty RM, Shanahan F. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut. 2010;59(12):1635–1642. [DOI] [PubMed] [Google Scholar]
- 65. Kreznar JH, Keller MP, Traeger LL, Rabaglia ME, Schueler KL, Stapleton DS, Zhao W, Vivas EI, Yandell BS, Broman AT, Hagenbuch B, Attie AD, Rey FE. Host genotype and gut microbiome modulate insulin secretion and diet-induced metabolic phenotypes. Cell Reports. 2017;18(7):1739–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. [DOI] [PubMed] [Google Scholar]
- 67. Agus A, Denizot J, Thévenot J, Martinez-Medina M, Massier S, Sauvanet P, Bernalier-Donadille A, Denis S, Hofman P, Bonnet R, Billard E, Barnich N. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci Rep. 2016;6(1):19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. [DOI] [PubMed] [Google Scholar]
- 69. Priyadarshini M, Thomas A, Reisetter AC, Scholtens DM, Wolever TM, Josefson JL, Layden BT. Maternal short-chain fatty acids are associated with metabolic parameters in mothers and newborns. Transl Res. 2014;164(2):153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature. 2016;534(7606):213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Knip M, Siljander H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat Rev Endocrinol. 2016;12(3):154–167. [DOI] [PubMed] [Google Scholar]
- 72. Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, Ian McKenzie C, Hijikata A, Wong C, Binge L, Thorburn AN, Chevalier N, Ang C, Marino E, Robert R, Offermanns S, Teixeira MM, Moore RJ, Flavell RA, Fagarasan S, Mackay CR. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6(1):6734. [DOI] [PubMed] [Google Scholar]
- 73. Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40(1):128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sivaprakasam S, Gurav A, Paschall AV, Coe GL, Chaudhary K, Cai Y, Kolhe R, Martin P, Browning D, Huang L, Shi H, Sifuentes H, Vijay-Kumar M, Thompson SA, Munn DH, Mellor A, McGaha TL, Shiao P, Cutler CW, Liu K, Ganapathy V, Li H, Singh N. An essential role of Ffar2 (Gpr43) in dietary fibre-mediated promotion of healthy composition of gut microbiota and suppression of intestinal carcinogenesis. Oncogenesis. 2016;5(6):e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA. 2008;105(43):16767–16772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Bäckhed HK, Gonzalez A, Werner JJ, Angenent LT, Knight R, Bäckhed F, Isolauri E, Salminen S, Ley RE. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150(3):470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, Antonopoulos D, Umesaki Y, Chervonsky AV. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39(2):400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339(6123):1084–1088. [DOI] [PubMed] [Google Scholar]
- 79. Brooks L, Viardot A, Tsakmaki A, Stolarczyk E, Howard JK, Cani PD, Everard A, Sleeth ML, Psichas A, Anastasovskaj J, Bell JD, Bell-Anderson K, Mackay CR, Ghatei MA, Bloom SR, Frost G, Bewick GA. Fermentable carbohydrate stimulates FFAR2-dependent colonic PYY cell expansion to increase satiety. Mol Metab. 2016;6(1):48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1-2):84–96. [DOI] [PubMed] [Google Scholar]
- 81. Inoue D, Kimura I, Wakabayashi M, Tsumoto H, Ozawa K, Hara T, Takei Y, Hirasawa A, Ishihama Y, Tsujimoto G. Short-chain fatty acid receptor GPR41-mediated activation of sympathetic neurons involves synapsin 2b phosphorylation. FEBS Lett. 2012;586(10):1547–1554. [DOI] [PubMed] [Google Scholar]
- 82. Forbes S, Stafford S, Coope G, Heffron H, Real K, Newman R, Davenport R, Barnes M, Grosse J, Cox H. Selective FFA2 agonism appears to act via intestinal PYY to reduce transit and food intake but does not improve glucose tolerance in mouse models. Diabetes. 2015;64(11):3763–3771. [DOI] [PubMed] [Google Scholar]
- 83. Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA. 2011;108(19):8030–8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lu VB, Gribble FM, Reimann F. Free-fatty acid receptors in enteroendocrine cells [published online ahead of print April 23, 2018]. Endocrinology. doi: 10.1210/en.2018-00261. [DOI] [PubMed]