Abstract
Context
Peripubertal obesity is associated with variable hyperandrogenemia, but precise mechanisms remain unclear.
Objective
To assess insulin resistance, hyperinsulinemia, and LH roles in peripubertal obesity–associated hyperandrogenemia.
Design
Cross-sectional analysis.
Setting
Academic clinical research unit.
Participants
Eleven obese (body mass index for age ≥95%) peripubertal girls.
Intervention
Blood samples were taken during a mixed-meal tolerance test (1900 to 2100), overnight (2100 to 0700), while fasting (0700 to 0900), and during an 80 mU/m2/min hyperinsulinemic-euglycemic clamp (0900 to 1100).
Main Outcome Measures
The dependent variable was morning free testosterone level; independent variables were insulin sensitivity index (ISI), estimated 24-hour insulin, and estimated 24-hour LH levels.
Results
All participants demonstrated insulin resistance and hyperinsulinemia. ISI, but not estimated 24-hour insulin level, correlated positively with morning free testosterone level when correcting for estimated 24-hour LH level and Tanner stage (rs = 0.68, P = 0.046). The correlation between estimated 24-hour LH and free testosterone levels approached significance after adjusting for estimated 24-hour insulin level and Tanner stage (rs = 0.63, P = 0.067). Estimated 24-hour insulin level did not correlate with free testosterone level after adjusting for estimated 24-hour LH level and Tanner stage (rs = 0.47, P = 0.20).
Conclusion
In insulin-resistant obese girls with hyperinsulinemia, free testosterone levels correlated positively with insulin sensitivity and, likely, circulating LH concentrations but not with circulating insulin levels. In the setting of relatively uniform hyperinsulinemia, variable steroidogenic-cell insulin sensitivity may correlate with metabolic insulin sensitivity and contribute to variable free testosterone concentrations.
Insulin sensitivity by hyperinsulinemic-euglycemic clamp, but not estimated 24-hour mean insulin concentration, was positively correlated with morning free testosterone concentration in obese adolescent girls.
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders in women, with a prevalence approximating 7% to 15% (1, 2). Hallmarks of PCOS include clinical and/or biochemical hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphology. PCOS is also associated with metabolic abnormalities, including obesity, insulin resistance, and glucose intolerance. In the setting of obesity, the manifestations and comorbidities of PCOS may be ameliorated by weight loss, suggesting that excess adiposity contributes to its pathophysiology (1). This relationship partly reflects insulin resistance with compensatory hyperinsulinemia, the latter contributing to hyperandrogenemia by augmenting ovarian and adrenal androgen production, and by inhibiting sex hormone binding globulin (SHBG) production (3, 4). Nonetheless, the relationships among obesity, insulin resistance, hyperinsulinemia, and hyperandrogenemia remain incompletely understood.
Peripubertal hyperandrogenemia can represent a precursor to PCOS (5, 6). Peripubertal girls with obesity demonstrate elevated levels of free testosterone (T) compared with their normal-weight counterparts (7–10), and weight loss is associated with reduced T levels in such girls (7). However, androgen concentrations are highly variable in girls with obesity (10), and the proximate causes of obesity-associated hyperandrogenemia remain unclear (11). We have previously reported that both morning LH and fasting insulin concentrations independently predict free T in such girls (10). However, fasting insulin level is not the gold standard method for measuring insulin sensitivity (12), and morning LH and fasting insulin concentrations are imprecise measures of average LH and insulin concentrations, respectively.
To address whether differences in insulin resistance and/or hyperinsulinemia account for variable free-T concentrations in obese peripubertal girls, in addition to a potentially independent role of LH in obesity-associated hyperandrogenemia, we designed a detailed study involving a hyperinsulinemic-euglycemic clamp (insulin clamp), frequent sampling for insulin and LH levels, and a mixed-meal tolerance test (MMTT). Our a priori hypothesis was that insulin resistance is a primary determinant of elevated free T, with greater degrees of insulin resistance being associated with higher free-T concentrations. In addition, we hypothesized that LH concentrations independently predict free-T concentrations.
Subjects and Methods
The University of Virginia (UVA) Institutional Review Board approved all study procedures. The study was registered with ClinicalTrials.gov (identifier NCT00928759). All study participants and custodial parents provided informed assent and consent, respectively.
Eleven adolescent girls with obesity [body mass index (BMI)-for-age percentile >95] were recruited from the local community and the UVA Pediatric Endocrinology and Fitness clinics. Participants’ ages ranged from 8.42 to 16.25 years, Tanner breast stages ranged from 1 to 5, and BMI z-scores ranged from 1.86 to 2.57 (Table 1). All participants had increased waist circumference for age (13), which correlates with insulin resistance in youth (14). Seven participants had hyperandrogenism by either hirsutism or elevated morning free-T level, as previously defined (15). Five participants were postmenarcheal but none by >4 years; of those who were at least 2 years postmenarcheal, three of four had oligomenorrhea (i.e., fewer than nine menses per year).
Table 1.
Participant Characteristics
| Summary Data a | Participant-Level Data (by Participant Number) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD or Median (IQR) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Tanner stage | 4 (2–5) | 1 | 2 | 2 | 3 | 4 | 4 | 5 | 5 | 5 | 5 | 5 |
| Age, y | 12.3 ± 2.4 | 11.00 | 8.42 | 10.92 | 12.83 | 8.92 | 11.25 | 13.58 | 13.75 | 14.08 | 14.58 | 16.25 |
| Bone age, y | 14.3 ± 2.3 | 13 | 11.5 | 13 | 14 | 11.5 | 12 | 17 | 15 | 16.5 | 16 | 18 |
| Menses | Pre | Pre | Pre | Pre | Pre | Pre | <2 yb | Oligo | Reg | Oligo | Oligo | |
| Hirsutism | No | No | No | No | No | No | Yes | Yes | No | Yes | Yes | |
| BMI, kg/m2 | 32.8 ± 5.9 | 30.7 | 23.3 | 25.6 | 30.4 | 28.9 | 33.3 | 39.8 | 38.6 | 30.8 | 41.3 | 37.8 |
| BMI-for-age percentile | 98.9 (97.7–99.5) | 97.7 | 97.7 | 96.8 | 98.2 | 99.5 | 99.3 | 99.5 | 99.4 | 97.7 | 99.5 | 98.9 |
| BMI z-score, SD | 2.29 (2.00–2.55) | 2.00 | 2.00 | 1.86 | 2.10 | 2.55 | 2.47 | 2.57 | 2.51 | 2.00 | 2.56 | 2.29 |
| Body fat, % | 37.8 ± 6.9 | 39.8 | 25.6 | 36.2 | 26.0 | 39.4 | 44.1 | 41.8 | 41.9 | 35.6 | 48.1 | 36.9 |
| Waist circumference, cm | 95.9 ± 10.7 | 91.5 | 78.0 | 80.8 | 87.6 | 91.0 | 103.2 | 107.3 | 106.9 | 107.1 | 101.5 | 100.1 |
| Total T by RIA, ng/dLc | 28.4 (15.4–47.1) | 28.4 | 10.0 | 10.0 | 107.3 | 33.7 | 15.4 | 93.3 | 22.3 | 31.4 | 20.6 | 47.1 |
| Total T by LC-MS/MS, ng/dLc | 33.5 ± 22.4 | 30 | 7 | 7 | 78 | 34 | 61 | 22 | 32 | 21 | 43 | |
| SHBG, nmol/Ld | 14.0 ± 5.0 | 25.0 | 14.4 | 14.7 | 11.5 | 10.7 | 14.4 | 13.3 | 19.2 | 12.2 | 13.5 | 5.2 |
| Free T by RIA, pg/mL | 5.9 (4.1–16.6) | 5.9 | 2.7 | 2.6 | 31.8 | 10.0 | 4.1 | 26.2 | 5.3 | 8.9 | 5.6 | 16.6 |
| Free T by LC-MS/MS, pg/mL | 9.5 ± 6.9 | 6.3 | 1.9 | 1.8 | 22.9 | 10.1 | 16.9 | 5.2 | 9.1 | 5.7 | 15.2 | |
| 17-hydroxyprogesterone, ng/dLe | 89.4 ± 43.0 | 55.6 | 76.9 | 65.5 | 116.6 | 79.0 | 51.4 | 179.3 | 38.5 | 74.2 | 99.3 | 147.1 |
| Androstenedione, ng/mLe | 2.25 ± 1.47 | 1.49 | 0.83 | 0.65 | 3.00 | 3.49 | 0.75 | 5.32 | 1.30 | 2.53 | 1.90 | 3.49 |
| IGF-1, ng/mLf | 382 ± 91 | 422 | 244 | 427 | 303 | 493 | 285 | 449 | 436 | |||
| DHEA-S, µg/dLf | 89 (49–165) | 37 | 42 | 84 | 97 | 49 | 61 | 268 | 165 | 150 | 89 | 341 |
| HbA1c, %f | 5.6 ± 0.4 | 5.5 | 5.1 | 5.6 | 5.6 | 5.7 | 5.4 | 5.9 | 6.3 | 5.0 | 5.7 | 5.7 |
Abbreviations: DHEA-S, dehydroepiandrosterone sulfate; IQR, interquartile range; LC-MS/MS, liquid chromatography-tandem mass spectrometry; oligo, <9 menses/y; pre, premenarcheal; reg, regular menses.
Shapiro-Wilk testing was used to determine which variables show evidence for skewness (i.e., appear not to follow a normal distribution). Apparently nonskewed data are presented as mean ± SD; apparently skewed data are shown as median (IQR).
<2 years since menarche.
Average of every-30-minute samples from 0700 to 0830 during study admission.
From 0700 sample during study admission.
From 0900 sample during study admission.
From 0900 single sample while fasting during screening visit.
No girl had congenital adrenal hyperplasia, hyperprolactinemia, hypothyroidism, or Cushing syndrome, and none met criteria for diabetes mellitus by HbA1c or fasting glucose level. No participant had taken any medication known to affect metabolism or the reproductive system within 3 months of the study.
Study procedures
As previously described (16), all participants underwent a detailed medical history and physical examination, including determination of Tanner (pubertal) stage for breast development. Screening blood tests were obtained between 0800 and 0900 to ensure good general health and to exclude unexpected hormonal abnormalities. Measures of adiposity included body composition [i.e., fat mass, fat free mass, and percentage of body fat by air displacement plethysmography (BOD POD; COSMED, Concord, CA)] and waist circumference, which were assessed while fasting in the Exercise Physiology Core Laboratory. Bone age was assessed by plain radiograph of the left hand and wrist.
All remaining study procedures were performed in the Clinical Research Unit (CRU). Premenarcheal girls (n = 6) underwent CRU admission at their convenience. Postmenarcheal participants were admitted on or after cycle day 7. Documentation of plasma progesterone level <1.5 ng/mL was required within 4 days (inclusive) before scheduled admission. For 3 days before CRU admission, participants were instructed to refrain from strenuous physical activity and to follow a weight-maintaining diet consisting of 55% carbohydrates (≥200 g/d), 30% fat, and 15% protein.
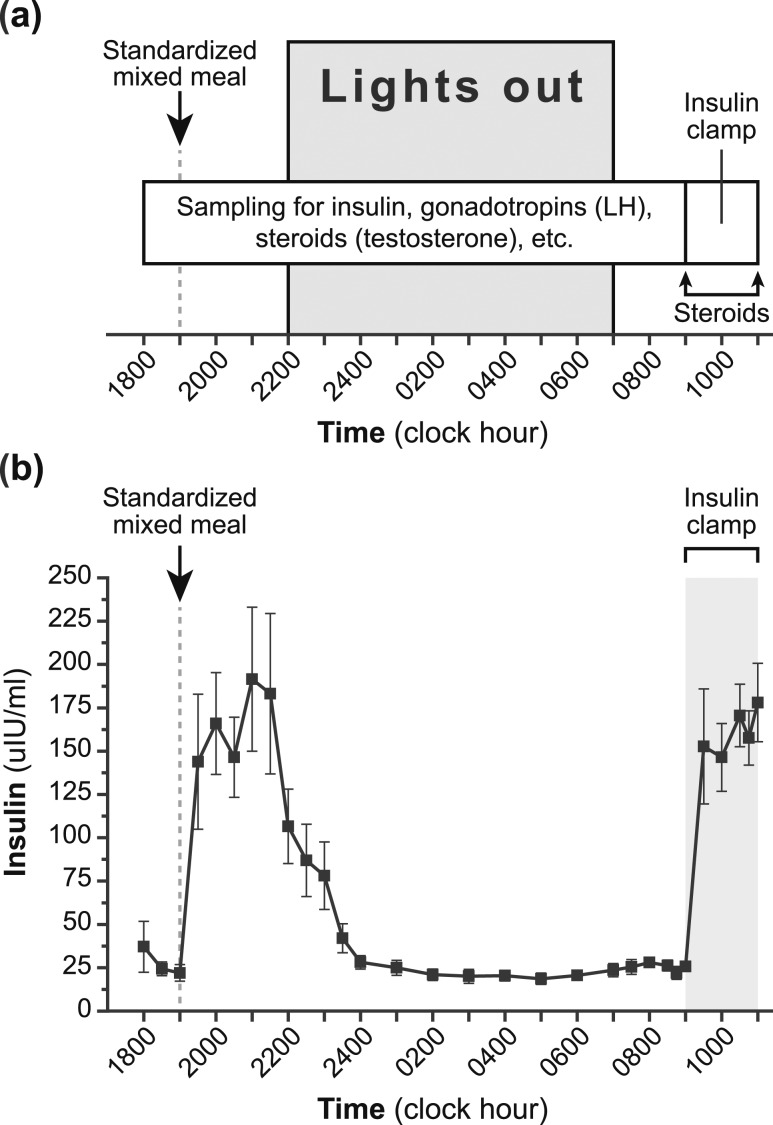
Participants were admitted to the CRU at 1600 after 4 hours of fasting [Fig. 1(a)]. Participants underwent a standardized MMTT [40% of estimated daily energy requirement (55% carbohydrates, 30% fat, and 15% protein)] at 1900. Blood was obtained via an indwelling forearm intravenous catheter to measure insulin and glucose concentrations every 30 minutes from 1800 to 2100. Participants fasted thereafter, and blood samples for insulin were obtained every 30 minutes from 2100 to 2400 and every hour from 2400 to 0700. Fasting insulin and glucose levels were obtained every 30 minutes from 0700 to 0830 and every 15 minutes from 0830 to 0900.
Figure 1.
Study protocol and insulin levels. (a) Schematic of the study protocol. (b) Insulin levels achieved during study (mean ± SEM). Specifically, insulin was measured before and after mixed-meal ingestion, during an observed overnight fast, and during a hyperinsulinemic-euglycemic clamp.
Frequent blood sampling for gonadotropins and sex steroids was performed overnight as follows: LH every 10 minutes (from 1800 to 0900); and FSH, estradiol, T, and progesterone every 20 minutes (from 1800 to 0740; combined into 2-hour pools). GH level—a driver of the physiological insulin resistance of puberty (17)—was measured every 20 minutes (combined into 2-hour pools) from 2200 to 0740. Lights were extinguished at 2200 and sleep encouraged until 0700; sleep was monitored using wrist actigraphy (Motion-Logger Basic-L; Ambulatory Monitoring, Inc., Ardsley, NY) (18). Serum SHBG level was measured at 0700. Samples for total-T measurements were collected every 30 minutes from 0700 to 0830; these samples were first pooled and then separated into two identical samples for measurement of total T by both radioimmunoassay (RIA) and liquid chromatography–tandem mass spectrometry (LC-MS/MS). Given cost and blood-volume constraints, we were unable to use LC-MS/MS for all T analyses, but LC-MS/MS was used to confirm the validity of the RIA assay.
For the insulin clamp, a catheter was placed in a forearm or antecubital vein for administration of insulin and glucose; another catheter was placed in a contralateral dorsal hand vein for frequent assessment of plasma glucose concentrations. “Arterialized” venous samples were obtained by warming the hand and forearm on the side containing the dorsal hand venous catheter. Baseline plasma glucose level after approximately 13.75 hours of fasting was calculated as the average of measurements from 0700 to 0900. Regular human insulin was infused intravenously from 0900 to 1100 at a dose of 80 mU/m2/min (19, 20), which was a dose expected to suppress hepatic glucose output. Every 5 minutes during insulin infusion, plasma glucose was immediately measured and used to adjust the glucose infusion rate (a 20% dextrose solution was administered) to maintain plasma glucose levels within 10% of baseline. Serum insulin concentrations were obtained every 15 minutes from 0830 to 0900, every 30 minutes from 0900 to 1030, and every 15 minutes from 1030 to 1100. The following steroid hormones were measured at the start and end of the insulin clamp (i.e., 0900 and 1100): progesterone, 17-hydroxyprogesterone, dehydroepiandrosterone, androstenedione, T, and estradiol.
Hormone assays
Most hormones were assayed in duplicate in the same assay by the UVA Ligand Assay and Analysis Core of the Center for Research in Reproduction. Samples with measured values below an assay’s functional sensitivity [i.e., the lowest standard to demonstrate accuracy within 20% and an intra-assay coefficient of variation (CV) <20%] were assigned the assay’s sensitivity. Total T was measured by RIA (Siemens, Healthcare Diagnostics, Los Angeles, CA) (sensitivity, 10 ng/dL; intra-assay CV, ≤6.1%; interassay CV, ≤8.1%). In 10 of 11 participants, total T was also measured by LC-MS/MS (Mayo Medical Laboratories, Rochester, MN) (sensitivity, 7 ng/dL; intra-assay CV, ≤6.0%; interassay CV range, 7.9% to 15.8% at 12.0 to 48.6 ng/dL) (21). Correlation between RIA and LC-MS/MS results was excellent [Spearman rank correlation (rs) ≥ 0.97, P < 0.0001]; and because LC-MS/MS results were not available for all participants, we used RIA results for primary analyses. SHBG, LH, and insulin levels were measured by chemiluminescence (Immulite 2000, Siemens Healthcare Diagnostics, Los Angeles, CA) (sensitivities, 0.2 nmol/L, 0.1 IU/L, and 2.6 µIU/mL, respectively; intra-assay CV, ≤4.8%; interassay CV, ≤7.9%). Other assay characteristics are described in detail in Supplemental Materials.
Data analysis
Morning total-T concentration was calculated as the average from 0700 to 0830. Free T was calculated from morning total-T and SHBG levels, using the following equation: FT = [T − (N)(FT)] / [(KT)(SHBG) − (KT)(T) + (N)(KT)(FT)]. In this equation, FT stands for free T (pmol/L); KT is the associated constant of SHBG for T (1.0 × 109); T is the total T concentration (ng/dL); SHBG is the SHBG concentration (nmol/L); and N is KA(CA) + 1, where KA is the association constant of albumin for T (3.6 × 104) and CA is the concentration of albumin (assumed to be 4.3 g/dL) (22). To convert free T in picomoles per liter to picograms per milliliter, divide by 3.467. Although this is not the gold standard method for estimating free-T level, it correlates well with free-T level measured by equilibrium dialysis (22). Glucose metabolized (23) was calculated as the average steady-state glucose infusion rate during the last 30 minutes of the clamp as a function of body weight (measured in milligrams per kilogram per minute). To normalize for variable insulin levels achieved with insulin infusion, the insulin sensitivity index (ISI) was calculated as glucose metabolized divided by the average steady-state insulin concentration (measured in micro international units per milliliter) during the last 30 minutes of the clamp (measured as milligrams per kilogram per minute per micro international units per milliliter multiplied by 100) (23).
Because blood withdrawal limits did not allow frequent assessments of insulin and LH levels over 24 hours, our a priori plan was to estimate 24-hour average insulin and LH levels. Based on 24-hour insulin profiles in normal participants with obesity (24), we assumed that periprandial and fasting values account for approximately two-thirds and one-third, respectively, of a 24-hour period. Accordingly, we estimated average 24-hour insulin as follows: (mean insulin level from 1800 to 2100 × 16/24) + (mean insulin level from 0700 to 0900 × 8/24). Because sleep vs wake LH secretion can differ markedly depending on pubertal stage, we assumed that 9 of 24 hours are reflected by mean LH level from 2200 to 0700 (sleep values) and 15 of 24 hours are reflected by mean LH level from 1800 to 2200 and 0700 to 0900 (wake values). Thus, average 24-hour LH level was estimated as follows: (sleep LH level × 9/24) + (wake LH × 15/24). Of note, estimated 24-hour insulin and LH concentrations were highly correlated with the average of all preclamp insulin and LH measurements, respectively (rs = 0.98 and 0.97, respectively; P < 0.0001 for both).
To interrogate unanticipated results from our primary analysis, we assessed (post hoc) the relationships among adiposity (BMI z-score and percentage of body fat), ISI, and insulin secretion. The latter included calculation of an insulinogenic index based on MMTT results as follows: change in insulin from time zero (fasting) to 30 minutes after mixed-meal ingestion divided by change in glucose from time zero to 30 minutes after mixed-meal ingestion. When calculated for an oral glucose tolerance test, the insulinogenic index correlates well with first-phase insulin responses to intravenous glucose (25), and a plot of insulinogenic index vs insulin sensitivity follows a curvilinear shape normally but flattens when individuals develop β-cell insufficiency (26, 27).
Statistical methods
As our a priori primary analysis, we used partial Spearman rank (nonparametric) correlation to examine the relationship between morning free-T level (dependent variable) and ISI (independent variable), controlling for estimated 24-hour LH level and Tanner stage (covariates). In essence, the partial correlation between free-T level and ISI represents the unique correlation between free-T level and ISI that is not shared by 24-hour LH level and Tanner stage. As secondary analyses, we similarly assessed the relationship between morning free-T and estimated 24-hour insulin levels while controlling for estimated 24-hour mean LH level and Tanner stage; and the relationship between morning free-T and estimated 24-hour LH levels while controlling for estimated 24-hour insulin level and Tanner stage. We also performed simple Spearman rank correlations to examine simple relationships between morning free-T level and ISI, estimated 24-hour insulin level, and estimated 24-hour LH level. We used Wilcoxon signed-rank tests to compare sex steroid concentrations before (at 0900) and at the end of the insulin clamp (at 1100).
Unless otherwise stated, data are presented as mean ± SD. When results of Shapiro-Wilk testing suggested non-normal data distribution, results are presented as median [interquartile range (IQR)]. For all tests, a two-sided P value ≤ 0.05 was used as the null hypothesis rejection rule. All statistical analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC).
Results
Free-T concentrations
Morning free-T concentrations were highly variable (median, 5.9 pg/mL; IQR, 4.1 to 16.6 pg/mL), with a 12.2-fold difference between maximum and minimum (range, 2.6 to 31.8 pg/mL; Table 2).
Table 2.
Study Results
| Summary Data a | Participant-Level Data (by Participant Number) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD or Median (IQR) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| MMTT | ||||||||||||
| Postprandial glucose, mg/dLb | 125 (123–136) | 125 | 136 | 142 | 143 | 98 | 87 | 130 | 125 | 123 | 124 | 126 |
| 2-hour postprandial glucose, mg/dL | 122.4 ± 20.4 | 113 | 139 | 132 | 156 | 94 | 84 | 127 | 118 | 136 | 117 | 130 |
| Periprandial insulin, µIU/mLc | 80 (60–149) | 80 | 149 | 60 | 127 | 60 | 56 | 89 | 195 | 80 | 59 | 165 |
| Postprandial insulin, µIU/mLb | 155.9 ± 80.0 | 129 | 207 | 94 | 186 | 95 | 61 | 138 | 312 | 132 | 89 | 272 |
| Insulinogenic index | 3.27 (2.91–4.15) | 3.12 | 4.85 | 1.35 | 3.00 | 4.15 | 3.69 | 2.91 | 8.85 | 2.78 | 3.27 | 3.81 |
| Overnight sampling, mean | ||||||||||||
| LH, mIU/mLd | 3.6 ± 2.7 | 5.5 | 0.3 | 0.2 | 3.2 | 5.1 | 1.5 | 4.7 | 1.6 | 8.7 | 6.2 | 2.7 |
| LH pulse frequency, pulses/15 he | 8.6 ± 4.4 | 8 | 1 | 3 | 10 | 9 | 6 | 9 | 8 | 17 | 13 | 11 |
| LH amplitude, mIU/mLe | 2.1 ± 1.3 | 4.4 | 0.1 | 0.5 | 2.0 | 3.8 | 2.1 | 2.5 | 1.4 | 3.0 | 2.2 | 1.6 |
| FSH, mIU/mLd | 2.8 ± 1.2 | 5.1 | 0.7 | 1.4 | 2.4 | 3.3 | 2.5 | 3.2 | 3.6 | 2.9 | 3.2 | 2.3 |
| GH, ng/mLf | 1.7 ± 0.5 | 2.3 | 2.1 | 1.0 | 1.7 | 1.8 | 1.4 | 2.5 | 1.9 | 1.2 | 1.1 | 2.1 |
| Progesterone, ng/mLd | 0.29 (0.24–0.55) | 0.28 | 0.29 | 0.26 | 0.55 | 0.31 | 0.23 | 0.63 | 0.47 | 0.24 | 0.20 | 0.57 |
| Total T, ng/dLd | 25.6 (14.8–41.4) | 25.6 | 10.0 | 10.0 | 84.6 | 26.8 | 14.8 | 102.3 | 20.9 | 38.3 | 22.7 | 41.4 |
| Free T, pg/mLd | 6.2 (3.9–14.6) | 5.3 | 2.7 | 2.6 | 24.9 | 7.9 | 3.9 | 28.8 | 4.9 | 10.9 | 6.2 | 14.6 |
| Estradiol, pg/mLd | 30.5 ± 9.5 | 31.5 | 17.9 | 16.7 | 45.3 | 25.5 | 30.4 | 32.5 | 29.7 | 26.0 | 32.8 | 47.4 |
| Sleep efficiency, %g | 79.4 ± 14.0 | 94.3 | 79.3 | 71.0 | 74.5 | 56.9 | 92.8 | 66.5 | 99.1 | 79.9 | ||
| Morning samples | ||||||||||||
| Morning total T by RIA, ng/dLh | 28.4 (15.4–47.1) | 28.4 | 10.0 | 10.0 | 107.3 | 33.7 | 15.4 | 93.3 | 22.3 | 31.4 | 20.6 | 47.1 |
| Morning total T by LC-MS/MS, ng/dLh | 33.5 ± 22.4 | 30 | 7 | 7 | 78 | 34 | 61 | 22 | 32 | 21 | 43 | |
| SHBG, nmol/Li | 14.0 ± 5.0 | 25.0 | 14.4 | 14.7 | 11.5 | 10.7 | 14.4 | 13.3 | 19.2 | 12.2 | 13.5 | 5.2 |
| Morning free T by RIA, pg/mLh | 5.9 (4.1–16.6)j | 5.9 | 2.7 | 2.6 | 31.8 | 10.0 | 4.1 | 26.2 | 5.3 | 8.9 | 5.6 | 16.6 |
| Fasting glucose, mg/dLk | 90.9 ± 6.0 | 94 | 95 | 95 | 89 | 91 | 83 | 96 | 103 | 86 | 85 | 85 |
| Fasting insulin, µIU/mLk | 24.9 ± 9.7 | 17 | 27 | 15 | 17 | 23 | 33 | 29 | 44 | 15 | 18 | 36 |
| 24-hour estimates, mean | ||||||||||||
| Estimated 24-hour insulin, µIU/mL | 76.1 ± 34.9j | 58.8 | 108.6 | 44.8 | 90.4 | 47.9 | 48.0 | 69.0 | 144.3 | 58.1 | 45.2 | 121.7 |
| Estimated 24-hour LH, mIU/mL | 3.1 (1.3–4.8)j | 4.7 | 0.3 | 0.2 | 3.1 | 4.7 | 1.3 | 4.8 | 1.6 | 8.5 | 6.8 | 3.0 |
| Insulin clamp | ||||||||||||
| M value, mg/kg/min | 2.82 (2.41–3.89) | 6.87 | 3.89 | 4.10 | 2.32 | 3.51 | 3.14 | 2.46 | 2.41 | 2.82 | 2.28 | 2.68 |
| Steady-state insulin, µIU/mL | 165.8 ± 53.1 | 158 | 145 | 189 | 60 | 138 | 211 | 103 | 226 | 168 | 189 | 237 |
| ISI, mg/kg/min per µIU/mL × 100 | 2.23 ± 1.09j | 4.34 | 2.69 | 2.17 | 3.84 | 2.55 | 1.49 | 2.38 | 1.07 | 1.68 | 1.21 | 1.13 |
Abbreviations: M, glucose metabolized.
Shapiro-Wilk testing was used to determine which variables show evidence for skewness (i.e., appear not to follow a normal distribution). Apparently nonskewed data are presented as mean ± SD; apparently skewed data are shown as median (IQR). End points of interest are listed according to the order of assessment during study admissions.
Average of every-30-minute samples from 1930 to 2100.
Average of every-30-minute samples from 1800 to 2100.
Average from 1800 to 0740.
From 1800 to 0900.
Average of 2-hour pools from 2200 to 0750.
Calculated as percentage of total sleep period (2200 to 0700) occupied by sleep (measured by wrist actigraphy).
Average of every-30-minute samples from 0700 to 0830.
Single sample from 0700.
Primary end point.
Average of every-30-minute samples from 0700 to 0900.
Insulin sensitivity and hyperinsulinemia as predictors of morning free-T concentration
The median periprandial insulin concentration (1800 to 2100) was 80 µIU/mL (IQR, 60 to 149 µIU/mL), and average fasting insulin level (0700 to 0900) was 24.9 ± 9.7 µIU/mL, yielding estimated average 24-hour insulin concentrations of 76.1 ± 34.9 µIU/mL (Table 2). Notably, insulin values returned to fasting levels by 5 to 6 hours after the start of mixed-meal ingestion [Fig. 1(b)]. During the insulin clamp, steady-state insulin concentrations were 165.8 ± 53.1 µIU/mL, or 6.6-fold higher than fasting values. Of interest, average insulin concentrations after MMTT (1930 to 2100) were 155.9 ± 80.0 µIU/mL, similar to insulin levels achieved during the insulin clamp (P = 0.70 by Wilcoxon signed-rank test). ISI was 2.23 ± 1.09 mg/kg/min per uIU/mL insulin × 100, with all ISI values suggesting insulin resistance (range, 1.07 to 4.34) (28).
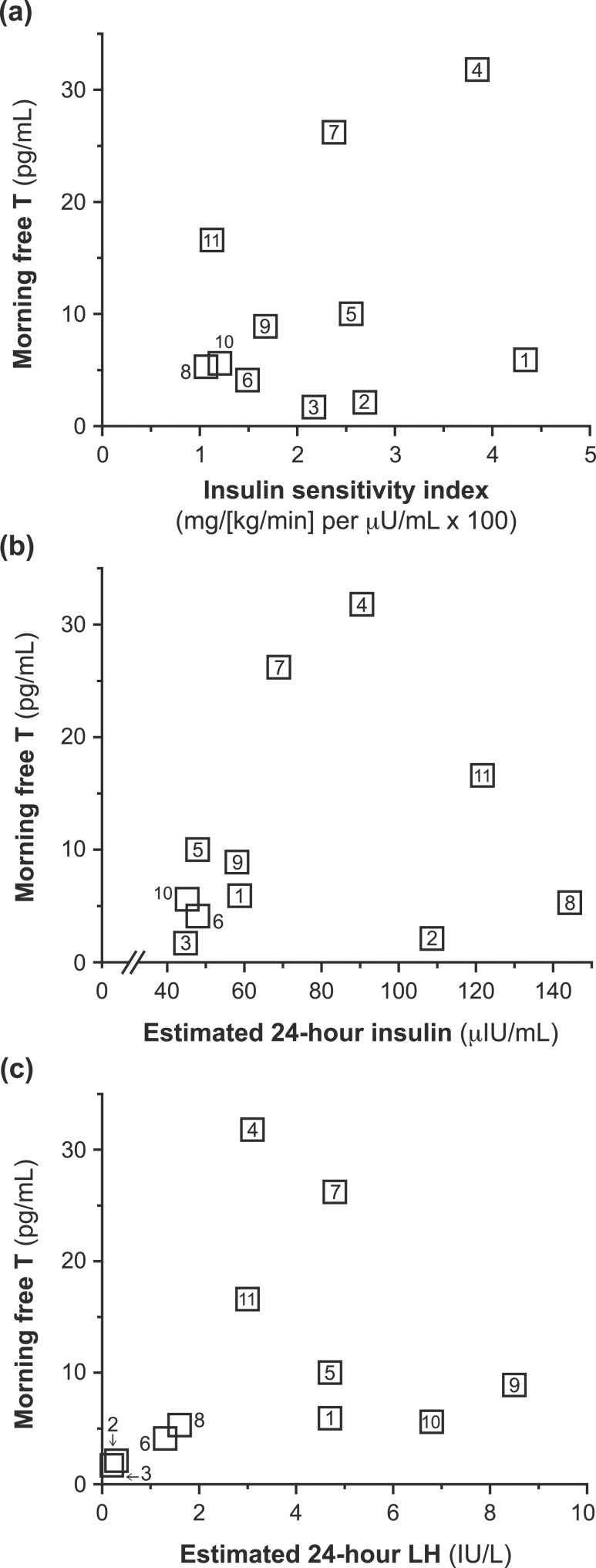
The simple correlation between ISI and free-T level was not statistically significant [rs = 0.20, P = 0.56; Fig. 2(a)]. However, correcting for differences in maturation (Tanner stage) and estimated 24-hour LH level (i.e., our preplanned primary analysis) unveiled a significant positive correlation between ISI and free-T level (rs = 0.68, P = 0.046). Similar results were obtained when using free-T levels calculated using LC-MS/MS (rs = 0.78, P = 0.023). Because SHBG level is known to decrease with hyperinsulinemia (29), we considered the possibility that this relationship with free T could primarily reflect changes in SHBG level. However, morning total-T level demonstrated a similar relationship with ISI when controlling for estimated 24-hour LH level and Tanner stage (RIA: rs = 0.65, P = 0.060; LC-MS/MS: rs = 0.74, P = 0.036). Similar results were obtained when using alternative strategies for estimating ISI (Supplemental Table 1).
Figure 2.
Potential predictors of free T (simple relationships). Simple relationship between (a) ISI and morning free-T level; (b) estimated 24-hour mean insulin and morning free-T levels; and (c) estimated 24-hour mean LH and morning free-T levels. Numbers associated with data points denote participant numbers that correspond to participant-level data.
Estimated 24-hour insulin level did not significantly correlate with free-T level [rs = 0.29, P = 0.39; Fig. 2(b)], even after adjusting for estimated 24-hour LH and Tanner stage (rs = 0.47, P = 0.20). Similar results were obtained when using alternative strategies for estimating periprandial insulin levels (Supplemental Table 2).
LH exposure as a predictor of morning free-T level
The median estimated 24-hour LH concentration was 3.1 µIU/mL (IQR, 1.3 to 4.8 µIU/mL; Table 2). Estimated 24-hour LH appeared to correlate with free-T level, although this did not reach statistical significance [rs = 0.60, P = 0.051; Fig. 2(c)]. Results were similar after adjusting for estimated 24-hour insulin and Tanner stage (rs = 0.63, P = 0.067).
Post hoc analyses: relationships among adiposity, ISI, and insulin secretion
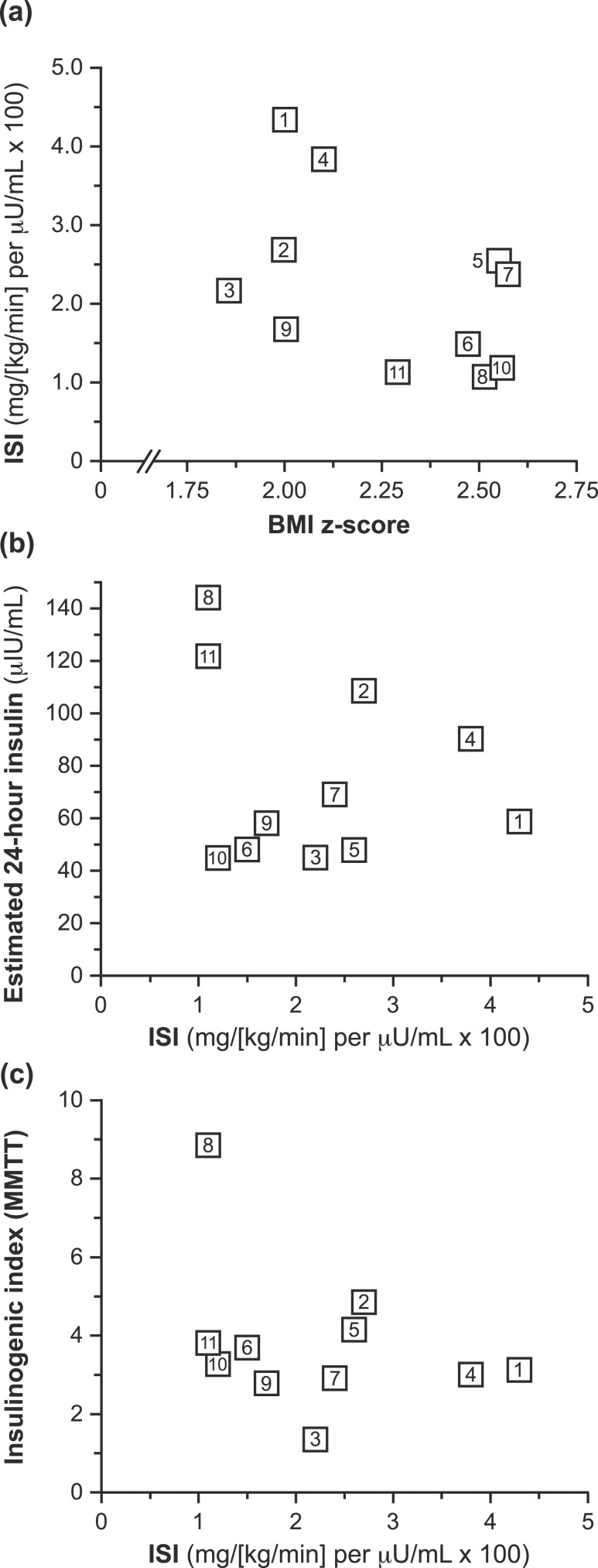
BMI z-score did not significantly correlate with ISI [rs = −0.35, P = 0.29; Fig. 3(a)], estimated 24-hour insulin (rs = −0.05, P = 0.89), fasting insulin (rs = 0.57, P = 0.068), or insulinogenic index (rs = 0.32, P = 0.34) in our cohort, even when correcting for Tanner stage (P > 0.10 for all). Similar results were observed when adiposity was expressed as percentage of body fat (P > 0.10 for all).
Figure 3.
Adiposity, insulin sensitivity, and insulin secretion. ISI was not significantly correlated with either (a) BMI z-scores or (b) estimated average 24-hour insulin concentrations. (c) A plot of ISI vs insulinogenic index during the MMTT did not clearly demonstrate the expected curvilinear relationship.
All girls exhibited insulin resistance, but ISI did not correlate with estimated 24-hour insulin level [rs = −0.11, P = 0.75; Fig. 3(b)], fasting insulin (rs = −0.52, P = 0.10), or insulinogenic index (rs = −0.26, P = 0.43), even when correcting for Tanner stage (P > 0.10 for all). A plot of MMTT insulinogenic index against clamp-derived ISI did not clearly demonstrate a curvilinear relationship [Fig. 3(c)] (26), suggesting the possibility of relative impairments of pancreatic β-cell function in some study participants. In support of this latter notion, we note that five girls had HbA1c values ≥5.7% and several girls (participants 6, 9, and 10) simultaneously exhibited low ISI, low estimated 24-hour insulin levels, and low insulinogenic index [Fig. 3(b) and 3(c)].
Results in girls with and without PCOS
Three girls (participants 8, 10, and 11) met criteria for PCOS [i.e., hyperandrogenism plus oligomenorrhea (≥2 years postmenarcheal)]. Although these girls had the lowest ISI values of the group, they had variable morning free-T levels (5.3, 5.6, and 16.6 pg/mL, respectively). Although two of these three girls had the highest estimated 24-hour insulin levels, their estimated 24-hour LH values were similar to those of other girls in the cohort. When we restricted analysis to the eight participants who did not meet criteria for PCOS, the relationship between ISI and morning free-T level (controlling for estimated 24-hour LH level and Tanner stage) remained significant (rs = 0.84, P = 0.034).
Acute steroid responses to hyperinsulinemia
No significant changes were observed across the 2-hour insulin clamp for progesterone, 17-hydroxprogesterone, dehydroepiandrosterone, or androstenedione (Supplemental Table 3; Supplemental Fig. 1). However, average total-T and estradiol levels decreased by 4.5 ng/mL and 5.3 pg/mL, respectively (P = 0.004 and 0.019, respectively).
Discussion
Clinical manifestations of PCOS frequently begin at puberty (5, 30), and adolescent hyperandrogenemia can be a precursor to full-blown PCOS (5, 6). However, little is known about the pathogenesis of adolescent hyperandrogenemia. Insulin resistance has long been associated with hyperandrogenemia (3, 4). However, prior adolescent studies have not addressed the relationships among free-T levels, insulin resistance (by insulin clamp), and estimates of 24-hour insulin and LH concentrations.
Our study confirmed that peripubertal girls with obesity can demonstrate highly variable free-T concentrations, in keeping with findings in prior reports (8, 10). Our current cohort exhibited marked free-T variability despite similar degrees of marked insulin resistance. Our a priori hypothesis was that insulin resistance (indicated by lower ISI value) would independently predict higher free-T level. However, in contrast to this hypothesis, greater insulin sensitivity (indicated by higher ISI value) was associated with higher free-T level after correcting for differences in pubertal stage and estimated 24-hour LH level. An analysis using total-T levels provided similar results, suggesting that T production is positively correlated with ISI.
The meaning of this unexpected relationship is unclear. Indeed, when we hypothesized that greater degrees of insulin resistance would be associated with higher free-T concentrations, we implicitly hypothesized that compensatory hyperinsulinemia would mediate this relationship. However, although ISI values in our cohort of girls (mean ± SEM, 2.23 ± 0.03) were similar to those in a previous report of obese girls with and without PCOS (mean ± SEM, 1.4 ± 0.7 and 2.7 ± 1.0, respectively) (31), we observed several findings suggesting that, as a group, our study cohort did not exhibit the expected relationship between insulin resistance and compensatory hyperinsulinemia. For example, ISI did not correlate with estimated 24-hour insulin or fasting insulin levels, even after correcting for pubertal stage, and we did not clearly observe the expected relationship between ISI and insulinogenic index (27). These and other findings [e.g., impaired fasting glucose (participant 8); elevated glucose 1 and/or 2 hours after MMTT (participants 1 and 4); and HbA1c values ≥5.7% (participants 5, 7, 8, 10, and 11)] suggest that some girls in our cohort may have had relative reductions in pancreatic β-cell function (32, 33). We suggest that a relationship between circulating insulin concentrations and free-T level was masked in our cohort, in part because all participants demonstrated hyperinsulinemia, but also because relative reductions in pancreatic β-cell function, at least in some participants, may have limited compensatory hyperinsulinemia in the setting of worsening insulin resistance. Regardless, we noted examples of participants with normal free-T values despite very high insulin levels [Fig. 2(b)]. Thus, whereas numerous studies confirm a role for hyperinsulinemia in obesity-associated hyperandrogenemia, our results corroborate prior studies suggesting that additional factors also play important roles in this regard.
We note that our results contrast with those of recent reports. In a study of obese adolescent girls with and without PCOS, free androgen index was negatively associated with glucose infusion rate during insulin clamp by simple (univariate) correlation (34). In a study of nonobese adolescent girls with and without PCOS, glucose infusion rate during insulin clamp did not significantly correlate with total-T concentrations (35). Reasons for the discordance between our results and these two larger studies are unclear, but we note that neither study included adjustments for differences in ambient LH concentrations, which we believe to be important determinant of androgen levels. Further clarification will require additional study.
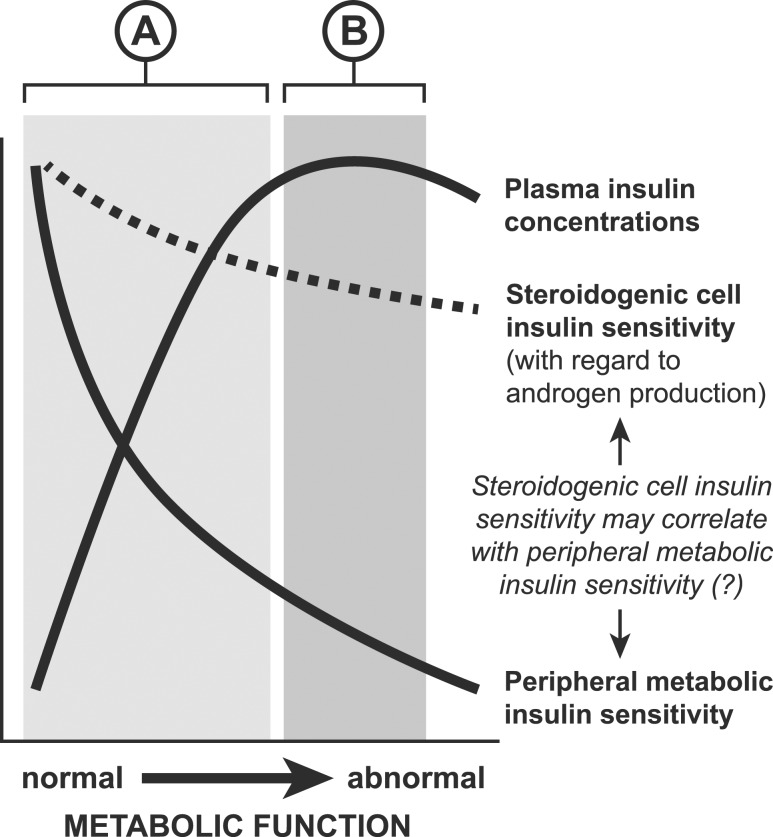
PCOS as an entity presents an interesting insulin paradox, whereby androgen-production pathways in ovarian and adrenal tissue remain sensitive to the stimulatory effects of hyperinsulinemia despite resistance to the metabolic effects of insulin (36). Although steroidogenic cell sensitivity to insulin likely varies among individuals, in vivo methods to quantify this are not available. We speculate that our unexpected findings may partly reflect variable steroidogenic-cell insulin sensitivity with respect to androgen production. In particular, if we stipulate that steroidogenic-cell insulin sensitivity with respect to androgen production correlates positively, albeit weakly, with metabolic insulin sensitivity—a stipulation that cannot currently be confirmed—a relationship between ISI and free-T level may be demonstrable when all participants are hyperinsulinemic and/or when hyperinsulinemia does not vary as a simple function of insulin sensitivity (e.g., when β-cell failure develops in the setting of insulin resistance; Fig. 4). Assessment of this speculative hypothesis requires additional study.
Figure 4.
A hypothetical model. This model stipulates steroidogenic cell insulin sensitivity is a determinant of androgen production, and that although steroidogenic-cell insulin sensitivity is substantially greater than peripheral (muscle) metabolic insulin sensitivity (in keeping with the insulin paradox), it correlates positively with peripheral metabolic insulin sensitivity. The figure denotes how plasma insulin levels generally increase as a function of worsening ISI [i.e., increasing peripheral (muscle) metabolic insulin resistance], but insulin concentrations may plateau (i.e., may not vary as a simple function of insulin sensitivity) due to emerging β-cell failure. The shaded area labeled “A” represents the putative range within which the strong relationship between insulin and T concentrations masks a positive correlation between insulin sensitivity and T. The shaded area labeled “B” represents the putative range within which hyperinsulinemia may be marked but may not vary as a simple function of insulin sensitivity, thus unmasking a positive correlation between insulin sensitivity and T.
In keeping with results reported in earlier studies (10, 16, 37), our findings suggest LH concentration is an important determinant of free T in girls with obesity. Of interest, estimated 24-hour LH values <2 IU/L were not accompanied by elevated free-T levels, even in two girls in our study who were at later pubertal stages. Thus, this suggests that a threshold amount of LH is required for the development of hyperandrogenemia. This finding aligns with in vitro data demonstrating that insulin alone does not stimulate androgen production as effectively as it does when administered along with LH (38). Interestingly, we observed marked free-T variability at LH values approximating 3 to 5 IU/L [Fig. 2(c)]; we speculate that participants in this range with higher free-T levels may have inherent or acquired exaggerated ovarian or adrenal responsiveness to LH or ACTH, respectively, and/or greater degrees of steroidogenic-cell insulin sensitivity.
Short-term insulin exposure via insulin clamp did not increase serum levels of androgens or their precursors in our participants, in keeping with findings of some previous studies (39–41). However, 2 hours of exogenous hyperinsulinemia may not be sufficient to increase ovarian and/or adrenal sex-steroid production, as implied by some, but not all, studies using 12 to 17 hours of insulin infusion (39, 42). We also note that the participants in our study exhibited marked hyperinsulinemia, and insulin concentrations during the insulin clamp were similar to those after a standardized mixed meal. Therefore, it is possible that the insulin clamp did not produce unusual hyperinsulinemia for these participants. It is important to note that our findings could also reflect an increase in hormone metabolism that either matched or exceeded any increase in secretion. The decrease in T and estradiol levels across the clamp was unexpected. We speculate that this observation reflects normal diurnal variations, because T and estradiol concentrations normally exhibit early-morning peaks and later-morning decreases (43, 44).
We recognize several limitations of the current study. First, our sample size was relatively small; the recruitment of adolescent girls to participate in detailed inpatient studies can be challenging. Because similar data do not currently exist, to our knowledge, we believe that these findings remain valuable nonetheless. Second, although all our participants had obesity, the sample cohort demonstrated heterogeneity in other ways. By design, participants exhibited variable maturational stage and, thus, variable LH concentrations. We note that some heterogeneity in the independent variables of interest is helpful as we try to discern the predictors of T concentrations in peripubertal girls with obesity. We also observed some variation in percentage of body fat, and peripheral insulin sensitivity partly relates to muscle mass. Regardless, the variability in percentage of body fat observed in our study seemed similar to other reported studies (34); body fat percentage did not correlate with ISI, estimated 24-hour insulin levels, or morning free-T levels in our cohort (P > 0.1 for all). Correlation analyses using fat-free mass or body surface area for ISI calculations yielded similar results to those of our primary analysis (Supplemental Table 1). Third, insulin and LH measurements over a limited time remain relatively imprecise to estimate integrated day-to-day insulin and LH concentrations. Although 24 hours of sampling may possibly provide a better estimate in this regard, doing so in pediatric participants is impractical. We also recognize that a standardized evening MMTT did not recapitulate each participant’s normal food intake in terms of timing, amount, and macronutrient composition; nor did it account for potentially different insulin responses at different times of day. Fourth, although we asked participants to refrain from strenuous activity for 3 days before the study, we did not assess baseline activity levels, which can influence insulin sensitivity and androgen concentrations (45, 46). However, participants in our cohort were generally sedentary, and we would expect that any influence of variable baseline activity (unmeasured) on free-T level would be mediated by effects on ISI and/or insulin levels (measured). Last, with regard to our post hoc analyses evaluating β-cell function, we adapted oral glucose tolerance test–derived assessments of insulin secretion (insulinogenic index) to MMMT. Although MMTT is not typically used to calculate estimates of insulin secretion, the MMTT is capable of generating elevated glucose responses in participants with prediabetes, and the MMTT can disclose progressive decreases in β-cell function measured using different methodologies (47).
We also note that our study cohort included three girls who met criteria for PCOS and who demonstrated the lowest ISI values. We recognize that the 80 mU/m2/min insulin infusion rate may not have fully suppressed hepatic glucose output in obese girls with PCOS, but any remaining hepatic glucose output was likely minimal (34). Despite evidence for marked insulin resistance in the three girls with PCOS, free-T values were variable and similar to others in the cohort, suggesting that additional factors contribute to free-T levels in adolescent girls with obesity. Also of interest, the independent relationship between ISI and morning free-T level was evident in the eight girls who did not meet criteria for PCOS, suggesting that our primary finding was not driven by inclusion of three girls with PCOS.
Numerous variables contribute to circulating androgen concentrations in adolescent girls, including ambient LH levels, insulin sensitivity, ambient insulin levels (e.g., degree of compensatory hyperinsulinemia), ovarian responses to stimulation by LH and/or insulin, adrenal responses to stimulation by ACTH and/or insulin, extraglandular steroidogenesis, and SHBG levels, to name only a few. Individuals exhibit variability in all these factors: In a given individual, some of these variables may tend to increase androgen levels to a greater or lesser degree, whereas other variables may tend to limit androgen levels to a greater or lesser degree. In some adolescents, the composite of all such effects renders hyperandrogenemia. Although we did not assess all plausible determinants of circulating free-T concentrations, our results imply that simultaneously addressing numerous relevant predictor variables is important for identifying the most important determinants of free-T levels in adolescents with obesity.
In summary, our results suggest that, among obese adolescent girls with significant insulin resistance and hyperinsulinemia, greater insulin sensitivity predicts higher circulating free-T concentrations. This finding may seem counterintuitive in light of previous studies; however, it would be consistent with a situation in which (1) steroidogenic-cell sensitivity to insulin augmentation of T production is an important determinant of circulating free-T levels; (2) metabolic insulin resistance in muscle roughly correlates with theca cell sensitivity to insulin (with respect to androgen production); and (3) compensatory insulin secretion reaches a plateau in the setting of severe insulin resistance, perhaps in part a reflection of developing β-cell insufficiency. Exploration of this hypothesis will require additional study.
Supplementary Material
Acknowledgments
We thank the patients and their families for their participation in this study; Lauren Lockhart, Michelle Abshire, and Anne Gabel for research coordination; the staff of the UVA Clinical Research Unit for protocol implementation; and Daniel Haisenleder and the staff of the UVA Center for Research in Reproduction Ligand Assay and Analysis Core for assay performance. This work used body composition assessment via waist circumference measurement and BOD POD instrument assessment in the Exercise Physiology Core, which is supported by the UVA School of Medicine.
Financial Support: This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (NIH) through cooperative agreement Grant P50 HD28934 as part of the National Centers for Translational Research in Infertility (C.M.B.S., J.T.P., J.C.M., C.R.M.); NIH Grant K23 HD070854 (C.M.B.S.); NIH Grant T32 HD007382 (K.L.K., J.S.C.); NIH Grant T32 DK007646 (A.D.A., E.G.H.); NIH Grant F32 HD066855 (J.S.C.); Endocrine Fellows Foundation Grant (A.D.A.); Endocrine Society Clinical Research Fellowship Award in Women’s Health (E.G.H.); and General Clinical Research Center Grant M01 RR00847.
Clinical Trial Information: ClinicalTrials.gov no. NCT00928759 (registered 26 June 2009).
Author Contributions: C.R.M. and J.C.M. designed the study. K.L.K., A.D.A., J.S.C., and E.G.H. assisted with participant recruitment and oversight of Clinical Research Unit admissions. K.L.K., A.D.A., J.S.C., E.G.H., and C.R.M. performed the hyperinsulinemic-euglycemic clamp procedures. C.M.B.S., K.L.K., A.D.A., J.S.C., E.G.H., and C.R.M. collected, organized, and analyzed the data. C.M.B.S. wrote the manuscript. All authors reviewed the manuscript. J.T.P. contributed to statistical considerations of study design and data interpretation. C.R.M. was the principal investigator of the study and was responsible for study design and oversight.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- CRU
Clinical Research Unit
- CV
coefficient of variation
- ISI
insulin sensitivity index
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- MMTT
mixed-meal tolerance test
- PCOS
polycystic ovary syndrome
- RIA
radioimmunoassay
- SHBG
sex hormone binding globulin
- T
testosterone
- UVA
University of Virginia
References
- 1. Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36(5):487–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCartney CR, Marshall JC. Clinical practice. Polycystic ovary syndrome. N Engl J Med. 2016;375(1):54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18(6):774–800. [DOI] [PubMed] [Google Scholar]
- 4. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33(6):981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Franks S. Adult polycystic ovary syndrome begins in childhood. Best Pract Res Clin Endocrinol Metab. 2002;16(2):263–272. [DOI] [PubMed] [Google Scholar]
- 6. Witchel SF. Puberty and polycystic ovary syndrome. Mol Cell Endocrinol. 2006;254-255:146–153. [DOI] [PubMed] [Google Scholar]
- 7. Reinehr T, de Sousa G, Roth CL, Andler W. Androgens before and after weight loss in obese children. J Clin Endocrinol Metab. 2005;90(10):5588–5595. [DOI] [PubMed] [Google Scholar]
- 8. McCartney CR, Prendergast KA, Chhabra S, Eagleson CA, Yoo R, Chang RJ, Foster CM, Marshall JC. The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. J Clin Endocrinol Metab. 2006;91(5):1714–1722. [DOI] [PubMed] [Google Scholar]
- 9. McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, Helm KD, Yoo R, Chang RJ, Foster CM, Caprio S, Marshall JC. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab. 2007;92(2):430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knudsen KL, Blank SK, Burt Solorzano C, Patrie JT, Chang RJ, Caprio S, Marshall JC, McCartney CR. Hyperandrogenemia in obese peripubertal girls: correlates and potential etiological determinants. Obesity (Silver Spring). 2010;18(11):2118–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson AD, Solorzano CM, McCartney CR. Childhood obesity and its impact on the development of adolescent PCOS. Semin Reprod Med. 2014;32(3):202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levy-Marchal C, Arslanian S, Cutfield W, Sinaiko A, Druet C, Marcovecchio ML, Chiarelli F ESPE-LWPES-ISPAD-APPES-APEG-SLEP-JSPEInsulin Resistance in Children Consensus Conference Group . Insulin resistance in children: consensus, perspective, and future directions. J Clin Endocrinol Metab. 2010;95(12):5189–5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taylor RW, Jones IE, Williams SM, Goulding A. Evaluation of waist circumference, waist-to-hip ratio, and the conicity index as screening tools for high trunk fat mass, as measured by dual-energy X-ray absorptiometry, in children aged 3-19 y. Am J Clin Nutr. 2000;72(2):490–495. [DOI] [PubMed] [Google Scholar]
- 14. Lee S, Bacha F, Gungor N, Arslanian SA. Waist circumference is an independent predictor of insulin resistance in black and white youths. J Pediatr. 2006;148(2):188–194. [DOI] [PubMed] [Google Scholar]
- 15. Collins JS, Beller JP, Burt Solorzano C, Patrie JT, Chang RJ, Marshall JC, McCartney CR. Blunted day-night changes in luteinizing hormone pulse frequency in girls with obesity: the potential role of hyperandrogenemia. J Clin Endocrinol Metab. 2014;99(8):2887–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, Marshall JC. Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: evidence for altered regulation in obese peripubertal girls. J Clin Endocrinol Metab. 2009;94(1):56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Styne DMGM. Physiology and disorders of puberty In: Shlomo MPK, Larsen PR, and Kronenberg HM, eds. Williams Textbook of Endocrinology. 13th ed.Philadelphia, PA: Saunders; 2016:1074–1218. [Google Scholar]
- 18. Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. [DOI] [PubMed] [Google Scholar]
- 19. Bacha F, Saad R, Gungor N, Janosky J, Arslanian SA. Obesity, regional fat distribution, and syndrome X in obese black versus white adolescents: race differential in diabetogenic and atherogenic risk factors. J Clin Endocrinol Metab. 2003;88(6):2534–2540. [DOI] [PubMed] [Google Scholar]
- 20. Yeckel CW, Weiss R, Dziura J, Taksali SE, Dufour S, Burgert TS, Tamborlane WV, Caprio S. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab. 2004;89(3):1096–1101. [DOI] [PubMed] [Google Scholar]
- 21. Bhasin S, Pencina M, Jasuja GK, Travison TG, Coviello A, Orwoll E, Wang PY, Nielson C, Wu F, Tajar A, Labrie F, Vesper H, Zhang A, Ulloor J, Singh R, D’Agostino R, Vasan RS. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96(8):2430–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. [DOI] [PubMed] [Google Scholar]
- 23. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223. [DOI] [PubMed] [Google Scholar]
- 24. Polonsky KS, Given BD, Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest. 1988;81(2):442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994;11(3):286–292. [DOI] [PubMed] [Google Scholar]
- 26. Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68(6):1456–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. DeFronzo RA, Tripathy D, Abdul-Ghani M, Musi N, Gastaldelli A. The disposition index does not reflect β-cell function in IGT subjects treated with pioglitazone. J Clin Endocrinol Metab. 2014;99(10):3774–3781. [DOI] [PubMed] [Google Scholar]
- 28. Gungor N, Saad R, Janosky J, Arslanian S. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr. 2004;144(1):47–55. [DOI] [PubMed] [Google Scholar]
- 29. Plymate SR, Jones RE, Matej LA, Friedl KE. Regulation of sex hormone binding globulin (SHBG) production in Hep G2 cells by insulin. Steroids. 1988;52(4):339–340. [DOI] [PubMed] [Google Scholar]
- 30. Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R. Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord. 2002;26(7):883–896. [DOI] [PubMed] [Google Scholar]
- 31. Lewy VD, Danadian K, Witchel SF, Arslanian S. Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. J Pediatr. 2001;138(1):38–44. [DOI] [PubMed] [Google Scholar]
- 32. Holman RR. Long-term efficacy of sulfonylureas: a United Kingdom Prospective Diabetes Study perspective. Metabolism. 2006;55(5, Suppl 1):S2–S5. [DOI] [PubMed] [Google Scholar]
- 33. Song DK, Hong YS, Sung YA, Lee H. Insulin resistance according to β-cell function in women with polycystic ovary syndrome and normal glucose tolerance. PLoS One. 2017;12(5):e0178120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cree-Green M, Bergman BC, Coe GV, Newnes L, Baumgartner AD, Bacon S, Sherzinger A, Pyle L, Nadeau KJ. Hepatic steatosis is common in adolescents with obesity and PCOS and relates to de novo lipogenesis but not insulin resistance. Obesity (Silver Spring). 2016;24(11):2399–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cree-Green M, Rahat H, Newcomer BR, Bergman BC, Brown MS, Coe GV, Newnes L, Garcia-Reyes Y, Bacon S, Thurston JE, Pyle L, Scherzinger A, Nadeau KJ. Insulin resistance, hyperinsulinemia, and mitochondria dysfunction in nonobese girls with polycystic ovarian syndrome. J Endocr Soc. 2017;1(7):931–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barber TM, McCarthy MI, Wass JA, Franks S. Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf). 2006;65(2):137–145. [DOI] [PubMed] [Google Scholar]
- 37. Blank SK, McCartney CR, Chhabra S, Helm KD, Eagleson CA, Chang RJ, Marshall JC. Modulation of gonadotropin-releasing hormone pulse generator sensitivity to progesterone inhibition in hyperandrogenic adolescent girls--implications for regulation of pubertal maturation. J Clin Endocrinol Metab. 2009;94(7):2360–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Franks S, Gilling-Smith C, Watson H, Willis D. Insulin action in the normal and polycystic ovary. Endocrinol Metab Clin North Am. 1999;28(2):361–378. [DOI] [PubMed] [Google Scholar]
- 39. Nestler JE, Clore JN, Strauss JF III, Blackard WG. The effects of hyperinsulinemia on serum testosterone, progesterone, dehydroepiandrosterone sulfate, and cortisol levels in normal women and in a woman with hyperandrogenism, insulin resistance, and acanthosis nigricans. J Clin Endocrinol Metab. 1987;64(1):180–184. [DOI] [PubMed] [Google Scholar]
- 40. Yamaguchi Y, Tanaka S, Yamakawa T, Kimura M, Ukawa K, Yamada Y, Ishihara M, Sekihara H. Reduced serum dehydroepiandrosterone levels in diabetic patients with hyperinsulinaemia. Clin Endocrinol (Oxf). 1998;49(3):377–383. [DOI] [PubMed] [Google Scholar]
- 41. Helm KD, McCartney CR, Okonkwo QL, Blank SK, Barrett EJ, Marshall JC. Hyperinsulinemia does not acutely enhance adrenal androgen production in women or men. Horm Metab Res. 2007;39(8):617–619. [DOI] [PubMed] [Google Scholar]
- 42. Tosi F, Negri C, Perrone F, Dorizzi R, Castello R, Bonora E, Moghetti P. Hyperinsulinemia amplifies GnRH agonist stimulated ovarian steroid secretion in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2012;97(5):1712–1719. [DOI] [PubMed] [Google Scholar]
- 43. Norjavaara E, Ankarberg C, Albertsson-Wikland K. Diurnal rhythm of 17 beta-estradiol secretion throughout pubertal development in healthy girls: evaluation by a sensitive radioimmunoassay. J Clin Endocrinol Metab. 1996;81(11):4095–4102. [DOI] [PubMed] [Google Scholar]
- 44. Mitamura R, Yano K, Suzuki N, Ito Y, Makita Y, Okuno A. Diurnal rhythms of luteinizing hormone, follicle-stimulating hormone, testosterone, and estradiol secretion before the onset of female puberty in short children. J Clin Endocrinol Metab. 2000;85(3):1074–1080. [DOI] [PubMed] [Google Scholar]
- 45. Bird SR, Hawley JA. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc Med. 2017;2(1):e000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mario FM, Graff SK, Spritzer PM. Habitual physical activity is associated with improved anthropometric and androgenic profile in PCOS: a cross-sectional study. J Endocrinol Invest. 2017;40(4):377–384. [DOI] [PubMed] [Google Scholar]
- 47. Shankar SS, Vella A, Raymond RH, Staten MA, Calle RA, Bergman RN, Cao C, Chen D, Cobelli C, Dalla Man C, Deeg M, Dong JQ, Lee DS, Polidori D, Robertson RP, Ruetten H, Stefanovski D, Vassileva MT, Weir GC, Fryburg DA; Foundation for the National Institutes of Health β-Cell Project Team . Standardized mixed-meal tolerance and arginine stimulation tests provide reproducible and complementary measures of beta-cell function: results from the Foundation for the National Institutes of Health Biomarkers Consortium Investigative Series. Diabetes Care. 2016;39(9):1602–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.