Abstract
IGF1—a small, single-chain, secreted peptide in mammals—is essential for normal somatic growth and is involved in a variety of other physiological and pathophysiological processes. IGF1 expression appears to be controlled by several different signaling mechanisms in mammals, with GH playing a key role by activating an inducible transcriptional pathway via the Jak2 protein kinase and the Stat5b transcription factor. Here, to understand aspects of Igf1 gene regulation over a substantially longer timeline than is discernible in mammals, Igf1 genes have been examined in 21 different nonmammalian vertebrates representing five different classes and ranging over ∼500 million years of evolutionary history. Parts of vertebrate Igf1 genes resemble components found in mammals. Conserved exons encoding the mature IGF1 protein are detected in all 21 species studied and are separated by a large intron, as seen in mammals; the single promoter contains putative regulatory elements that are similar to those functionally mapped in human IGF1 promoter 1. In contrast, GH-activated Stat5b-binding enhancers found in mammalian IGF1 loci are completely absent, there is no homolog of promoter 2 or exon 2 in any nonmammalian vertebrate, and different types of “extra” exons not present in mammals are found in birds, reptiles, and teleosts. These data collectively define properties of Igf1 genes and IGF1 proteins that were likely present in the earliest vertebrates and support the contention that common structural and regulatory features in Igf1 genes have a long evolutionary history.
These results define properties potentially present in Igf1 genes in the earliest vertebrates and suggest that common structural and regulatory features in Igf1 genes have a long evolutionary history.
IGF1 is a small, single-chain, secreted protein produced in most vertebrate species (1–6). In mammals, IGF1 plays key roles in prenatal development and in pre- and postnatal somatic growth and functions as a central mediator of the actions of GH (7–10). IGF1 also is involved in the control of intermediary metabolism independent of GH and in tissue regeneration and repair and has been implicated in cancer and other disease pathogenesis throughout life (11, 12). It is unknown whether these functions are shared among nonmammalian vertebrates.
IGF1/Igf1 genes and IGF1 proteins appear to be conserved among mammals (1–4, 13, 14). In mammals, these genes have been determined to consist of six exons and five introns and to share other features, including overall length (4, 13, 14). For example, the single-copy human IGF1 gene spans ∼85.1 kb on chromosome 12q23.2; several other primate IGF1 genes range from ∼83.1 to ∼85.4 kb (13), and mouse and rat Igf1 genes are ∼78.0 and ∼79.3 kb in length, respectively (14). Igf1 gene expression has been studied predominantly in the latter two species, in which multiple Igf1 mRNAs result from mechanisms that include gene transcription from two promoters, transcript initiation at several 5′ start sites in each promoter-specific leader exon, alternative RNA splicing, and differential polyadenylation (15–18). The IGF1/Igf1 mRNAs found in mammals encode six different versions of IGF1 protein precursors, with variation found in the N-terminal portions of their signal peptides and in the C-terminal parts of their extension peptides or E domains (4). Nevertheless, these differently characterized precursors contain a single mature IGF1 protein (13, 14, 19, 20).
GH is a critical regulator of IGF1/Igf1 gene expression in mammals, consistent with its physiological role in controlling somatic growth (7, 21–23). GH binds to the extracellular face of its specific transmembrane receptor and induces the enzymatic activity of the Jak2 tyrosine protein kinase (24, 25), which in turn leads to phosphorylation of intracellular tyrosine residues of the GH receptor and recruitment of several signal transduction cascades (24, 25). Among these pathways, the Stat5b transcription factor plays a key mediatory role in the stimulation of IGF1/Igf1 gene expression by GH (26). Subsequent to its activation by Jak2 on the GH receptor, dimerized Stat5b tracks to the nucleus and acutely binds to multiple putative enhancers in chromatin within the rat Igf1 locus and at other GH-responsive genes, leading to rapid activation of their transcription (27–30). Because inactivating mutations in the human STAT5B gene have been characterized in individuals with growth failure and IGF1 deficiency (31, 32) and genetic loss of Stat5b in mice causes growth deficiency (33, 34), it seems likely that similar molecular mechanisms exist in other mammalian species, a concept supported by conservation of putative Stat5b-binding elements in DNA within IGF1 loci in primates (13) and in other mammals (14).
The current analyses were undertaken to define the extent of Igf1 gene conservation or diversification among nonmammalian vertebrates as a means of gaining broader understanding of aspects of Igf1 gene regulation, including possible mechanisms of action of GH over a substantially longer evolutionary timeline than has been inferred in mammals. Information was pieced together from fragmentary data in several publicly available databases on Igf1 loci, genes, and predicted or identified IGF1 proteins from 21 nonmammalian vertebrates representing five classes and extending over ∼500 million years (Myr) of evolution. Results show close relationships within classes of terrestrial vertebrates with regard to IGF1 protein sequences and indicate that Igf1 genes in ray-finned fishes have diversified. Despite clear differences among various vertebrate classes, overall IGF1 protein structure and aspects of Igf1 gene organization are similar in all vertebrates analyzed. Taken together, these observations define essential features of Igf1 genes and IGF1 proteins that were likely present in the earliest vertebrates.
Materials and Methods
Database searches and analyses
Vertebrate genomic databases were accessed using the Ensembl Genome Browser (www.ensemble.org), the University of California, Santa Cruz (UCSC) Genome Browser (https://genome.ucsc.edu), and the Sequence Read Archive of the National Center for Biotechnology Information (NCBI; www.ncbi.nlm.nih.gov/sra). Initial searches were conducted with rat Igf1 gene DNA segments (Rattus norvegicus, genome assembly Rnor_6.0), using BlastN under normal sensitivity (maximum e-value of 10; mismatch scores: 1,−3; gap penalties: opening 5, extension, 2; filtered low complexity regions, and repeat sequences masked). Subsequent queries were performed with the same parameters using Igf1 DNA sequences from chicken (Gallus gallus, genome assembly Gallus_gallus-5.0), tetraodon (Tetraodon nigroviridis, genome assembly TETRAODON8), zebrafish (Danio rerio, genome assembly GRCz10), cave fish (Astyanax mexicanus, AstMex102), and spotted gar (Lepisosteus oculatus, genome assembly LepOcu1).
In addition, the Sequence Read Archive of the NCBI was searched with Igf1 gene fragments from different species in an attempt to fill in regions not detected within whole genome assemblies. Other follow-up queries were performed using conserved nonexon and nonpromoter segments of Igf1 loci identified in the evolutionary conserved region (ECR) browser (https://ecrbrowser.dcode.org/). As noted in Ensembl and in the UCSC Genome Browser, a variety of methods and approaches had been used for genome assembly and gene annotation among the 21 species queried (Table 1). Genomes from the following species were examined: Amazon molly (Poecilia formosa, PoeFor_5.1.2), anole lizard (Anolis carolinensis, AnoCar2.0), cave fish (Astyanax mexicanus, AstMex102), chicken (Gallus gallus, Gallus_gallus-5.0), Chinese softshell turtle (Pelodiscus sinensis, PelSin_1.0), cod (Gadus morhua, gadMor1), coelacanth (Latimeria chalumnae, LatCha1), duck (Anas platyrhynchos, BGI_duck_1.0), flycatcher (Ficedula albicollis, FicAlb_1.4), frog (Xenopus tropicalis, JGI 4.2), fugu (Takifugu rubripes, FUGU 4.0), lamprey (Petromyzon marinus, Pmarinus_7.0), medaka (Oryzias latipes, HdrR), platyfish (Xiphophorus maculatus, Xipmac4.4.2), spotted gar (Lepisosteus oculatus, LepOcu1), stickleback (Gasterosteus aculeatus, BROAD S1), tetraodon (Tetraodon nigroviridis, TETRAODON 8.0), tilapia (Oreochromis niloticus, Orenil1.0), turkey (Meleagris gallopavo, Turkey_2.0.1), zebra finch (Taeniopygia guttata, taeGut3.2.4), and zebrafish (Danio rerio, GRCz10). The highest scoring results in all cases mapped to the respective Igf1 gene. Protein sequences were obtained from the GENCODE/Ensemble databases, the NCBI Consensus CDS Protein Set (https://www.ncbi.nlm.nih.gov/CCDS/), and the Uniprot browser (http://www.uniprot.org/); when they were not available, DNA sequences were translated with the assistance of SerialCloner1.3 (e.g., platyfish). Data also were compared with human IGF1 protein sequences. Phylogenetic relationships among IGF1 proteins were determined using the MUSCLE 3.8.31 and PhyML 3.1/3.0 aLRT programs from Phylogeny.fr (http://www.phylogeny.fr/index.cgi) (35). Results are presented in text, tables, and figures as percentage identity over entire query regions, unless otherwise specified.
Table 1.
Vertebrate Genome Characteristics
| Species | Genome Length a | Genome Assembly b | Gene Annotation Methods b |
|---|---|---|---|
| Chicken | 1.13 | Contigs and scaffolds | RNA-seq data, cDNAs, long read RNAs |
| Turkey | 1.04 | Whole genome shotgun sequencing (30× coverage) | cDNAs, ESTs, chicken cDNAs, orthologous vertebrate protein sequences |
| Duck | 1.14 | Contigs and scaffolds | Orthologous vertebrate genes and protein sequences |
| Zebra finch | 1.20 | Whole-genome shotgun sequencing (6× coverage), plus 35 BAC clones | ESTs, avian protein sequences |
| Flycatcher | 1.12 | Contigs and scaffolds | RNA-seq data, orthologous vertebrate protein sequences |
| Turtle | 2.10 | Contigs and scaffolds | RNA-seq data, orthologous vertebrate protein sequences |
| Anole lizard | 1.78 | Contigs and scaffolds | RNA-seq data, cDNAs, ESTs, alignments of chicken protein sequences |
| Frog | 1.36 | Contigs and scaffolds, plus whole-genome shotgun sequencing (7.65× coverage) | cDNAs, orthologous vertebrate protein sequences |
| Tetraodon | 0.34 | Contigs and scaffolds, whole-genome shotgun sequencing (6× coverage) | Orthologous vertebrate protein sequencesc |
| Fugu | 0.39 | Contigs and scaffolds | Fugu proteins, orthologous vertebrate proteins, nonvertebrate protein sequences |
| Stickleback | 0.46 | Whole-genome shotgun sequencing (11× coverage) | cDNAs, ESTs, orthologous vertebrate protein sequences |
| Medaka | 0.70 | Whole-genome shotgun sequencing | cDNAs, ESTs, orthologous vertebrate protein sequences |
| Cod | 0.90 | Whole-genome shotgun and paired-end sequencing (25× coverage) | Whole-genome alignment to stickleback, orthologous vertebrate protein sequences |
| Cave fish | 1.00 | Contigs and scaffolds | RNA-seq data, orthologous vertebrate protein sequences |
| Tilapia | 0.97 | Whole-genome shotgun sequencing | RNA-seq data, orthologous vertebrate protein sequences |
| Amazon molly | 1.00 | Contigs and scaffolds | RNA-seq data, orthologous vertebrate protein sequences |
| Platyfish | 0.65 | Whole-genome shotgun sequencing (19.6× coverage) | RNA-seq data, platyfish protein sequences, orthologous vertebrate protein sequences |
| Zebrafish | 1.37 | Contigs and scaffolds; BAC and fosmid clones | RNA-seq data, cDNAs, zebrafish protein sequences |
| Spotted gar | 0.95 | Whole-genome shotgun sequencing | RNA-seq data, orthologous vertebrate protein sequences |
| Coelacanth | 2.90 | Whole-genome shotgun sequencing | RNA-seq data, cDNAs, coelacanth protein sequences |
| Lamprey | 1.92 | Whole-genome shotgun (5× coverage), BAC-end sequence reads | Lamprey cDNA and protein sequences, orthologous vertebrate protein sequences |
Abbreviations: BAC, bacterial artificial chromosome (contains foreign DNA); EST, expressed sequence tag; RNA-seq, RNA-based DNA sequencing.
Genome length is in gigabases.
Contigs and scaffolds refer to overlapping large DNA fragments; fosmid refers to specialized bacterial plasmid containing foreign DNA.
Information on assembly is incomplete.
Experimental strategy
Naming conventions adopted here include the abbreviation IGF1 for human or other primate genes and transcripts, Igf1 for all other genes and mRNAs, and IGF1 for all proteins. An initial assessment of nonmammalian vertebrate Igf1 genes and transcripts within the Ensembl and UCSC genome browsers revealed that most assignments seemed to be far simpler than rat Igf1 or human IGF1 (13, 14). For example, in mammalian IGF1/Igf1 genes, there are extensive 5′ and 3′ untranslated regions (UTRs) in the first and last exons, respectively (13, 14). Nevertheless, in the cod, coelacanth, frog, fugu, medaka, spotted gar, and stickleback, no 5′ UTRs had been identified for exon 1, and in the Amazon molly, Chinese softshell turtle (turtle), cod, frog, fugu, medaka, spotted gar, stickleback, and tilapia, no 3′ UTRs had been annotated for the last exon. Moreover, in the cod, medaka, stickleback, and tetraodon, Igf1 gene organization differed between the Ensembl and UCSC browsers, with the latter assigning fewer exons than the former, even though the same genome assemblies were being analyzed. In addition, even in the chicken Igf1, for which primary experimental data indicate the gene has five exons and two types of transcripts (36–38), the two genomic databases showed just four exons. Thus, primary goals were to characterize all genes as thoroughly as possible and then interpret these more comprehensive data sets.
An iterative strategy was developed that started with the exon assignments in the Ensembl or UCSC browser, which, depending on the species, were based on a combination of different approaches that had been used to characterize the specific genome (see Table 1). Next, BlastN searches were conducted against all 21 vertebrate genome assemblies for homologous genomic regions using parts of the rat Igf1 gene as queries. Results were mapped to each vertebrate Igf1 locus and were followed by secondary searches, first using chicken Igf1 exons and the chicken Igf1 gene promoter as queries and then relying on other genes that were evolutionarily more similar to specific target species to fill in potential gaps. Final follow-up searches were performed using Igf1 exons from potential outliers (i.e., species with “extra” exons) or using genomic segments outside exons or promoter regions found to be conserved between more distant species, as identified with the ECR browser. As a result of these series of steps, substantially more Igf1 gene complexity appeared to be present in all genomes than previously annotated.
Results
An overview of Igf1 genes in vertebrates
In most mammals, IGF1/Igf1 is a six-exon, five-intron gene in which two promoters and alternative splicing contribute to a multiplicity of different transcripts (13, 14). Igf1 organization in nonmammalian vertebrates appears to be more variable, as the gene is composed of four exons and three introns in seven of 21 genomes studied here, five exons and four introns in 12, six exons and five introns in one (cave fish), and eight exons and seven introns in one (spotted gar; Table 2). There is no homolog of mammalian IGF1/Igf1 exon 2 in any nonmammalian vertebrate analyzed and no corresponding promoter 2 was identified (Figs. 1‒3). The overall structure of the Igf1 gene and locus in the 21 vertebrates studied appeared to fall into two major classes on the basis of exon-intron organization. In the eight terrestrial vertebrates (anole lizard, chicken, duck, flycatcher, frog, turkey, turtle, and zebra finch), a large intron separates exons 2 and 3, the exons encoding the mature IGF1 protein (ranging from 2283 to 96,049 bp) (Fig. 2; Table 2); relatively large introns also divide exons 1 and 2 (from 1276 to 14,813 bp) and exons 3 and 4 (from 3402 to 17,783 bp) (Fig. 2; Table 2). In the nine teleosts with five exons and four introns (tetraodon, fugu, stickleback, medaka, cod, tilapia, Amazon molly, platyfish, and zebrafish), a large intron separates exons 2 and 3, which also encodes mature IGF1 (from 4905 to 13,287 bp), but smaller introns divide exons 1 and 2 (from 950 to 1754 bp), exons 3 and 4 (from 96 to 1273 bp), and exons 4 and 5 (from 615 to 2552 bp) (Fig. 3; Table 2). Of note, in all of these vertebrates except for the lamprey, the largest Igf1 intron divides exons 2 and 3 precisely after the first nucleotide of codon 26 of mature IGF1. This is the analogous codon and codon position of the intron separating exons 3 and 4 in mammals (3, 4). In lamprey, this intron interrupts codon 31 of mature IGF1 after the first nucleotide.
Table 2.
Organization of Vertebrate Igf1 Genesa
| Species | Exon 1 | Intron 1 | Exon 1a | Intron 1a | Exon 2 | Intron 2 | Exon 3 | Intron 3 | Exon 4 | Intron 4 | Exon 5 | Intron 5 | Exon 6 | Intron 6 | Exon 7 | Intron 7 | Exon 8 | Gene Length |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chicken | 309 | 2814 | 242 | 1245 | 157 | 34,138 | 182 | 9195 | 1713 | — | — | — | — | — | — | — | — | 49,995 |
| Turkey | 309 | 2804 | 247 | 1224 | 157 | 41,008 | 182 | 8351 | 1580 | — | — | — | — | — | — | — | — | 55,862 |
| Duck | 309 | 4307 | — | — | 157 | 35,379 | 182 | 10,387 | 1652 | — | — | — | — | — | — | — | — | 52,373 |
| Zebra finch | 327 | 4261 | — | — | 157 | 34,811 | 182 | 7272 | 988 | — | — | — | — | — | — | — | — | 47,998 |
| Flycatcher | 259 | 4251 | — | — | 157 | 35,453 | 182 | 6699 | 979 | — | — | — | — | — | — | — | — | 47,980 |
| Turtle | 311 | 3625 | — | — | 157 | 58,925 | 182 | 15,199 | 988 | — | — | — | — | — | — | — | — | 79,107 |
| Anole lizard | 323 | 9004 | — | — | 160 | 48,863 | 182 | 11,340 | 56 | 11,009 | 1464 | — | — | — | — | — | — | 82,195 |
| Frog | 267 | 2055 | — | — | 157 | 53,037 | 182 | 3402 | 521 | — | — | — | — | — | — | — | — | 59,621 |
| Tetraodon | 265 | 1168 | — | — | 160 | 4905 | 245 | 96 | 36 | 615 | 842 | — | — | — | — | — | — | 8332 |
| Fugu | 265 | 1176 | — | — | 160 | 6592 | 245 | 177 | 36 | 684 | 681 | — | — | — | — | — | — | 10,016 |
| Stickleback | 254 | 1336 | — | — | 160 | 6638 | 254 | 355 | 36 | 785 | 438 | — | — | — | — | — | — | 10,056 |
| Medaka | 259 | 950 | — | — | 160 | 13287 | 251 | 1273 | 36 | 900 | 151 | — | — | — | — | — | — | 17,267 |
| Cod | 295 | 1407 | — | — | 160 | 11,038 | 242b | 170 | 36 | 1319 | 62 | — | — | — | — | — | — | 14,729 |
| Cave fish | 404 | 1545 | — | — | 160 | 14,611 | 182 | 102 | 78 | 363 | 36 | 3653 | 2512 | — | — | — | — | 23,640 |
| Tilapia | 497 | 1339 | — | — | 160 | 10,576 | 248 | 173 | 33 | 2552 | 353 | — | — | — | — | — | — | 15,931 |
| Amazon molly | 415 | 1710 | — | — | 160 | 10,696 | 257 | 114 | 36 | 2077 | 60 | — | — | — | — | — | — | 15,524 |
| Platyfish | 284 | 1754 | — | — | 160 | 11,231 | 233 | 107 | 36 | 2314 | 311 | — | — | — | — | — | — | 16,522 |
| Zebrafish | 295 | 1079 | — | — | 160 | 11,576 | 182 | 521 | 36 | 2166 | 564 | — | — | — | — | — | — | 16,574 |
| Coelacanth | 303 | 14,813 | — | — | 163 | 96,049 | 188 | 17,783 | 844 | — | — | — | — | — | — | — | — | 130,143 |
| Lamprey | 202 | 1276 | — | — | 175 | 2283 | 149 | 7508 | 322 | — | — | — | — | — | — | — | — | 11,904 |
| Spotted gar | 280 | 1773 | — | — | 157 | 18,892 | 182 | 2052 | 31 | 469 | 25 | 133 | 18 | 403 | 49 | 3735 | 51 | 28,250 |
Length is in base pairs.
Three exons combined.
Figure 1.
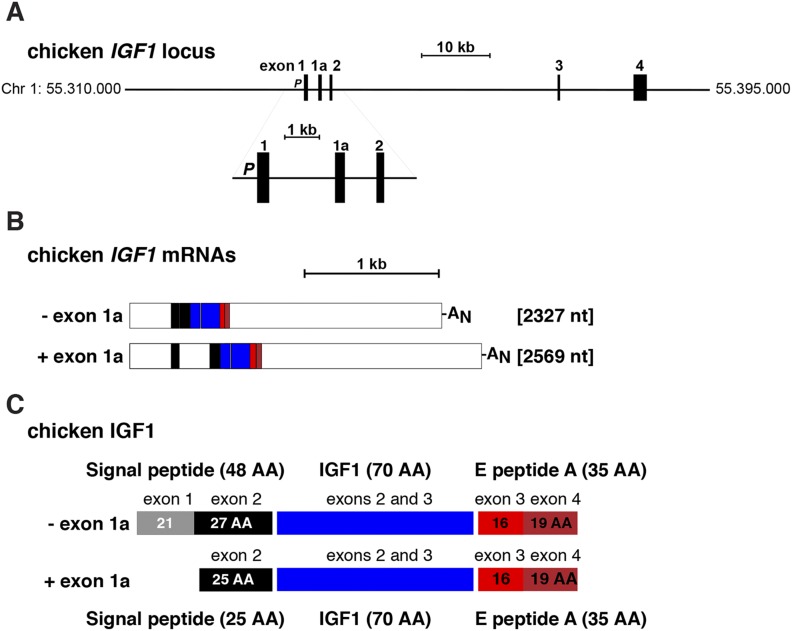
Organization of the chicken Igf1 gene and mRNAs. (A) Map of the chicken Igf1 locus, including chromosomal coordinates. Exons appear as boxes, and introns and flanking DNA appear as horizontal lines. The enlargement shows the Igf1 promoter (P) and exons 1, 1a, and 2. A scale bar is indicated. (B) Diagram of chicken Igf1 mRNAs. Coding regions are in color, and 5′ and 3′ untranslated regions are in white. AN represents the polyadenylic acid tail at the 3′ end of the transcript. A scale bar is shown. (C) Diagram of chicken IGF1 protein precursors, illustrating the derivation of each segment from different Igf1 exons. Mature, 70‒amino acid IGF1 is in blue, portions of the signal peptide are in gray and black, and components of E peptide are in shades of red. AA, amino acid; Chr, chromosome; nt, nucleotide.
Figure 2.
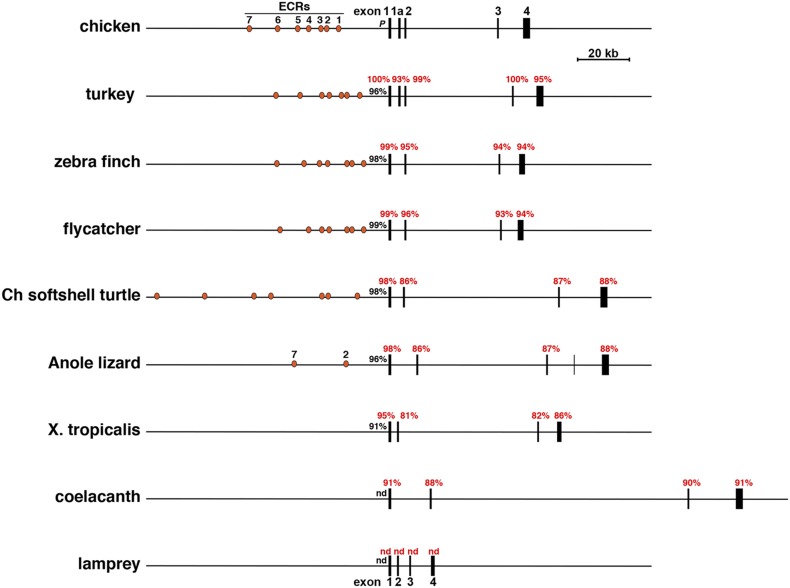
Comparison of selected vertebrate Igf1 genes. Schematics of chicken Igf1 and eight other Igf1 genes and loci are shown. Exons are illustrated as boxes, introns and flanking DNA as horizontal lines, and ECRs 1 to 7 as orange ovals. A scale bar is indicated. The percentage of nucleotide identity with different parts of chicken Igf1 is listed for each gene (red for exons, black for promoters). Ch, Chinese; nd, no identity detected; X, Xenopus.
Figure 3.
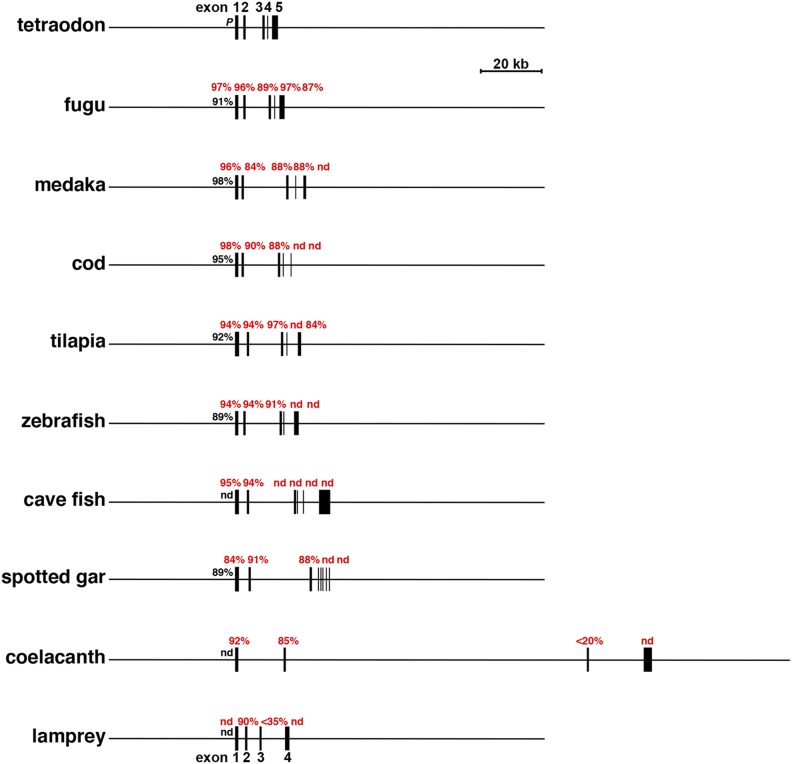
Comparison of additional vertebrate Igf1 genes. Maps of tetraodon Igf1 and nine other selected Igf1 genes and loci are shown. Exons are illustrated as boxes, and introns and flanking DNA are shown as horizontal lines. A scale bar is indicated. The percentage of nucleotide identity with different parts of chicken Igf1 is listed for each gene (red for exons, black for promoters). nd, no identity detected.
The overall lengths of the vertebrate Igf1 genes studied here varied over an ∼16-fold range, from 8332 bp in the tetraodon to 130,143 bp in the coelacanth (Figs. 2 and 3; Table 2), with the major determinants being the much greater lengths of introns in the latter (ranging from 14,813 to 96,049 bp) (Table 2), which also is seen in other species with longer IGF1 genes (e.g., the anole lizard, frog, and turtle) (Table 2). In addition, in contrast to the large variation in lengths of different parts of Igf1 genes among ray-finned fish, both exon and intron lengths were generally congruent in birds (Fig. 2; Table 2).
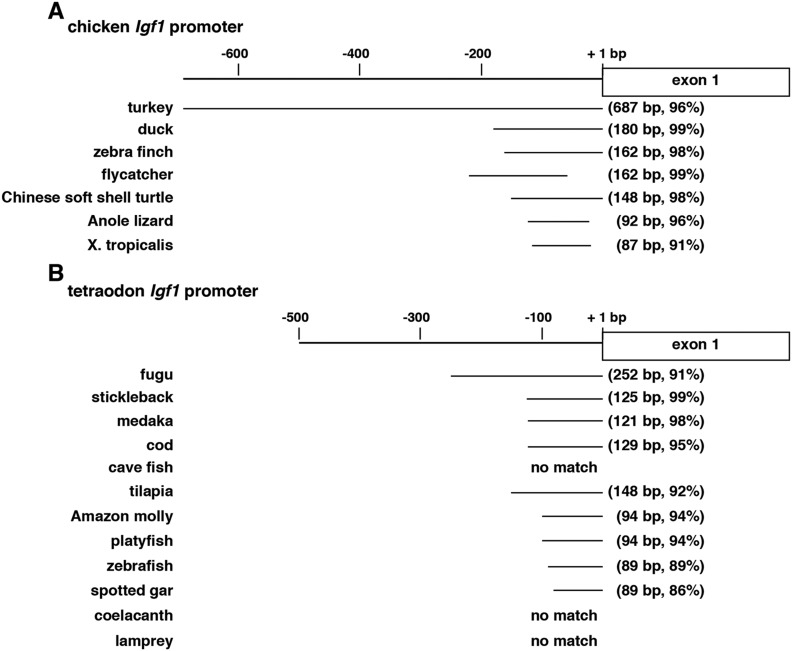
DNA conservation among Igf1 exons was variable among the species studied. In terrestrial vertebrates, overall nucleotide sequence identities with different chicken exons were fairly high, ranging from 93% to 100% in the turkey to a range of 81% to 95% for the frog (Fig. 2; Table 3), even though birds and anura diverged more than 200 Myr ago (39). DNA identity also was nearly as high between the chicken and the coelacanth (Fig. 2; Table 3). In contrast, there was minimal nucleotide conservation between chicken and lamprey Igf1 genes, the latter’s ancestor being phylogenetically older than the coelacanth’s (40, 41). Among ray-finned fish, DNA similarities were variable, being highest for Igf1 exons 1 and 2 (84% to 97% identity in all) (Fig. 3; Table 4) and exon 3 (86% to 97%, except for cave fish) (Fig. 3; Table 4) and lower for exons 4 and 5 (with no measurable identities in more than half of the species) (Fig. 3; Table 4). DNA sequence similarity also was high for the single Igf1 promoter among birds, ranging from 96% to 99% identity with the chicken, but was less conserved in other terrestrial species (91% to 98% over shorter DNA lengths) (Fig. 4A). In contrast, among fish there was no measurable similarity with the chicken Igf1 promoter, but it was substantial with tetraodon, in which 91% to 99% DNA sequence identity was detected in the proximal promoter region of all but one ray-finned fish species (i.e., cave fish) (Fig. 4B). Taken together, these results indicate that extensive diversification of potential promoter regulatory elements occurred during vertebrate speciation (but see analysis of gene promoters below).
Table 3.
Nucleotide Identity With Chicken Igf1 Exons (%)
| Species | Exon 1 (309 bp) a | Exon 1a (242 bp) | Exon 2 (157 bp) | Exon 3 (182 bp) | Exon 4 (1713 bp) a |
|---|---|---|---|---|---|
| Turkey | 100 | 93 | 99 | 100 | 95 (1516 bp) |
| Duck | 100 | 88 (186 bp)b | 97 | 99 | 94 (1310 bp) |
| Zebra finch | 99 | 85 (74 bp)b | 95 | 94 | 94 (955 bp) |
| Flycatcher | 99 | 82 (99 bp)b | 96 | 93 | 94 (960 bp) |
| Turtle | 98 | No match | 93 | 94 | 92 (666 bp) |
| Anole lizard | 98 | No match | 86 | 87 | 88 (336 bp)c |
| Frog | 95 | No match | 81 | 82 | 86 (121 bp) |
| Coelacanth | 91 | No match | 88 | 90 | 91 (187 bp) |
| Lamprey | No match | No match | No match | No match | No match |
Coding and noncoding DNA.
5′ and/or 3′ splice sites are not present.
Comparison is with exon 5.
Table 4.
Nucleotide Identity With Tetraodon Igf1 Exons (%)
| Species | Exon 1 (265 bp) a | Exon 2 (160 bp) | Exon 3 (245 bp) | Exon 4 (36 bp) | Exon 5 (842 bp) a |
|---|---|---|---|---|---|
| Fugu | 97 | 96 | 89 | 97 | 87 (578 bp) |
| Stickleback | 91 | 89 | 87 | 92 | 88 (214 bp) |
| Medaka | 96 | 84 | 88 | 88 | No match |
| Cod | 98 | 90 | 88 | No match | No match |
| Cave fish | 95 | 94 | No match | No match | No match |
| Tilapia | 94 | 94 | 97 | No match | 84 (353 bp) |
| Amazon molly | 88 | 95 | 86 | 92 | 92 (144 bp) |
| Platyfish | 88 | 95 | 86 | 92 | 91 (135 bp) |
| Zebrafish | 94 | 94 | 91 | No match | No match |
| Spotted gar | 84 | 91 | 88 | No match | No match |
| Coelacanth | 92 | 85 | <20 | No match | No match |
| Lamprey | No match | 90 | <35 | No match | No match |
Coding and noncoding DNA.
Figure 4.
Igf1 promoters in nonmammalian vertebrates. (A) Location of alignment of terrestrial vertebrate Igf1 promoters with the chicken Igf1 promoter. Length and percentage of identity are in parentheses for each species. (B) Location of alignment of aquatic vertebrate Igf1 promoters with the tetraodon Igf1 promoter. Length and percentage of identity are in parentheses for each species. No sequence alignments could be detected for the cave fish, coelacanth, or lamprey. X, Xenopus.
Igf1 genes in terrestrial vertebrates
Unlike genes of other avian species, chicken and turkey Igf1 genes have a noncoding exon 1a interposed between exons 1 and 2 (Fig. 1A and Fig. 2; Table 2). These two species are evolutionarily more related to each other than to the other birds studied (39, 42–45). Exon 1a, which appears to be alternatively expressed in one class of Igf1 mRNA in these species, interrupts the coding region within the signal peptide (Fig. 1B and 1C); however, because there is another in-frame methionine codon in exon 2, transcripts containing exon 1a would still encode the same mature IGF1 protein, and the signal peptide would include the identical C-terminal residues (Fig. 1C). Although DNA segments very similar to exon 1a are found in analogous locations in duck, zebra finch, and flycatcher Igf1 genes (Table 3), they lack either splice acceptor, splice donor, or both DNA elements and thus would not be included in IGF1 mRNAs in the latter birds. Collectively, these results point toward the presence of an exon 1a in a common ancestor of these avian species, but perhaps not in other vertebrates, because no comparable DNA sequence was detected in any other genomes assessed here (Table 3).
Igf1 genes in fish
At least one small coding exon is present in the Igf1 gene in all 11 teleosts studied here (e.g., exon 4: 31, 33, 36, or 78 nucleotides). A comparably sized Igf1 coding exon is found only in a single terrestrial vertebrate, the anole lizard (exon 4: 56 nucleotides) (Fig. 3; Table 2). No analogous exon was detected in the coelacanth or lamprey (Fig. 3; Table 2). These observations suggest that a different type of Igf1 gene may have emerged subsequent to the whole-genome duplication event that occurred in the common ancestor to all teleosts (46).
In the annotation of the cod genome in Ensembl, exon 3 is defined as consisting of three separate exons separated by 1 and 2 bp, respectively (see the note in Table 2), which is inconsistent with all known mechanisms of mRNA precursor splicing (47, 48). There is also no experimental evidence demonstrating that these are individual exons. Thus, these have been treated as a single exon in all analyses, especially because they collectively encode an open reading frame very similar to the Igf1 genes of other fish (Fig. 5B and Fig. 6B).
Figure 5.
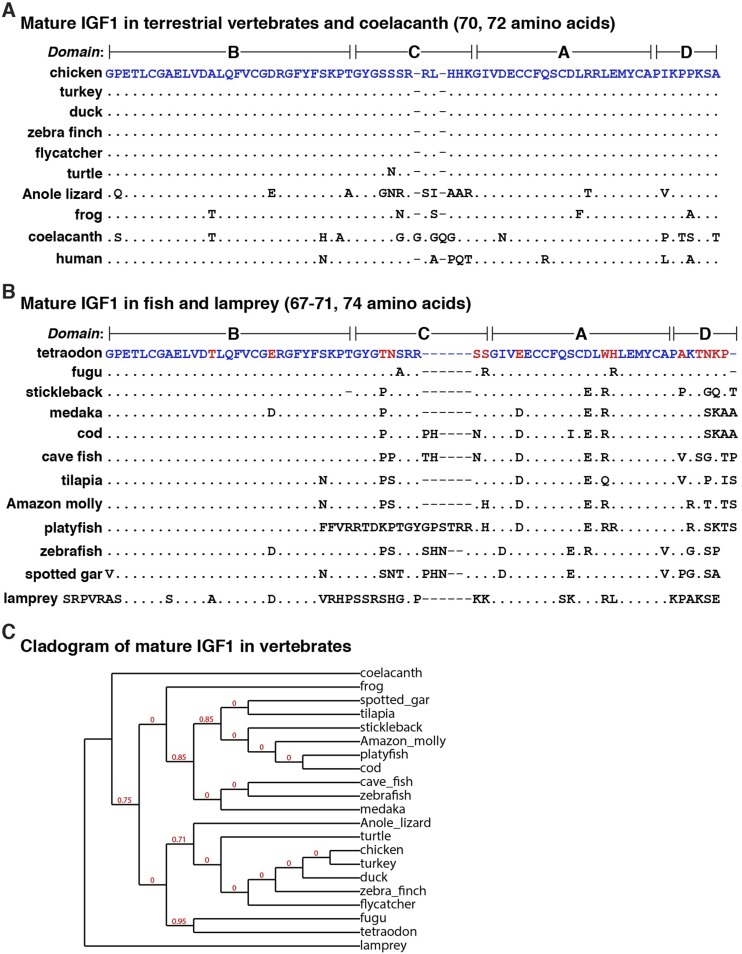
Alignments of vertebrate IGF1 proteins. (A) Amino acid sequences of IGF1 from eight terrestrial vertebrates, coelacanth, and human in single-letter code. Differences among species are indicated, with identities depicted by dots. Dashes indicate no residue and have been placed to maximize alignments. (B) Amino acid sequences of IGF1 from 11 ray-finned fish and lamprey in single-letter code. Differences among species are indicated, with identities depicted by dots. Blue text indicates amino acid identities between the chicken in (A) and the tetraodon in (B), and red text depicts differences. Dashes indicate no residue and have been placed to maximize alignments. (C) Cladogram of mature IGF1 in vertebrates. The numbers at the nodes indicate fractional differences, with 0 representing the highest levels of similarity. The length of each branch approximates the evolutionary distance.
Figure 6.
Alignments of vertebrate IGF1 signal peptides. (A) Amino acid sequences of IGF1 signal peptides from eight terrestrial vertebrates, coelacanth, and human in single-letter code. Differences are indicated, identities are depicted by dots, and a dash indicates no residue. The # next to the chicken and turkey indicates that a shorter IGF1 signal peptide of 25 amino acids is encoded by an alternative transcript in these two avian species (see Figs. 1 and 2). (B) Amino acid sequences of IGF1 signal peptides from 11 ray-finned fish and lamprey in single-letter code. Differences are indicated, with identities depicted by dots. A dash indicates no residue. Blue text depicts amino acid identities between the chicken in (A) and the tetraodon in (B), and red text indicates differences. The asterisk in front of the cave fish heading denotes the presence of an alternate signal peptide with the following 17 extra N-terminal amino acids: MTSKNKLLFVAWRRPAG. (C) Cladogram of the IGF1 signal peptide in vertebrates. The numbers at the nodes indicate fractional differences, with 0 representing the highest levels of similarity. The length of each branch approximates the evolutionary distance. Note that the IGF1 signal peptide in the coelacanth is more similar to that of other fish than is the mature IGF1 and that the lamprey is the outgroup in both comparisons (see Fig. 5C)
The cave fish is one of the teleosts with a different Igf1 gene structure, comprising six exons and five introns (Table 2). There appear to be two predicted classes of cave fish Igf1 mRNAs that either include or exclude exon 4. Exon 4 is 78 nucleotides in length and contains 26 codons, which are in frame with exons 3 and 5 and would contribute 26 amino acids to the C-terminal E peptide (see Fig. 7). The potential absence of exon 4 in some cave fish Igf1 transcripts would lead to an IGF1 protein precursor with a variant E peptide that is shorter than that detected in other teleosts (see the legend to Fig. 7). This specific type of alternative RNA splicing is not found in other vertebrates, and orthologues of 78-nucleotide cave fish exon 4 or 36-nucleotide exon 5 could not be identified in any other vertebrate genome studied here.
Figure 7.
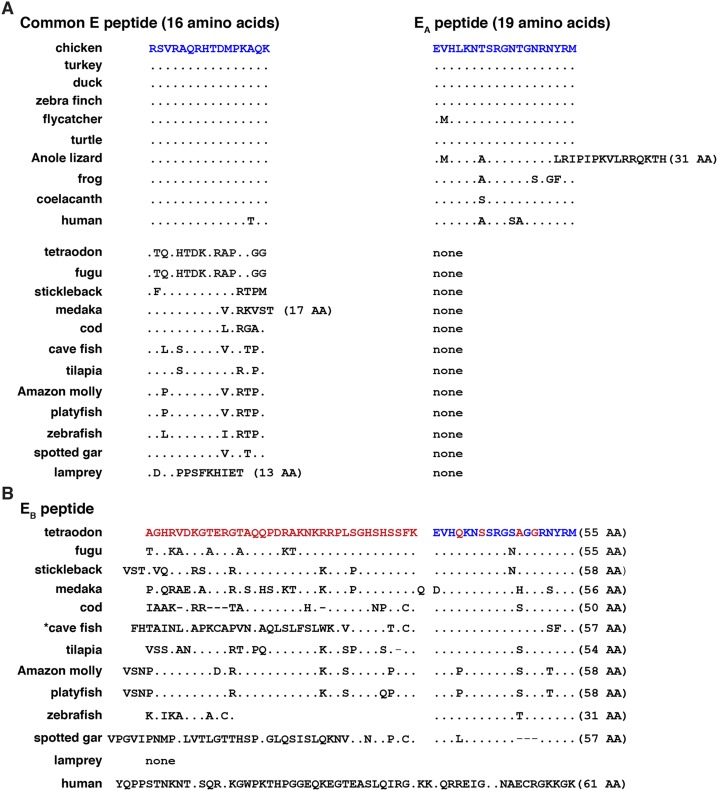
Alignments of vertebrate IGF1 E peptides. (A) Amino acid sequences of C-terminal common E (16 amino acids) and EA peptides (19 residues) in eight terrestrial vertebrates, coelacanth, human, 11 ray-finned fish, and lamprey in single-letter code. Differences are indicated, with identities depicted by dots. The number of amino acids is listed in parentheses if it differs from 16 residues for the common E region or from 19 residues for EA peptides. (B) Amino acid sequences of C-terminal EB peptides in 11 ray-finned fish, lamprey, and human in single-letter code. Differences are indicated, with identities depicted by dots. A dash indicates no residue. The number of amino acids is listed in parentheses. Blue text depicts amino acid identities between the chicken in (A) and the tetraodon in (B), and red text indicates differences. The asterisk in front of the cave fish heading depicts the presence of an alternate EB peptide with 26 fewer N-terminal amino acids. AA, amino acid.
The organization of the spotted gar Igf1 gene appears to be substantially different from that of the other Igf1 genes examined, as it is predicted to contain eight exons and seven introns, including very small exons 4 through 8 (31, 25, 18, 49, and 51 bp, respectively) (Table 2). DNA sequences similar to these exons could not be found in any of the other 20 vertebrate genomes analyzed here, with the exception of a 26-nucleotide stretch of exon 8 that was detected in cod Igf1 exon 5 (93% identity) and a 23-nucleotide fragment of exon 8 that mapped to coelacanth Igf1 exon 4 (96% identity). In contrast, homologous regions of 182-bp spotted gar Igf1 exon 3 were identified in all of the other species except for the anole lizard, frog, and lamprey.
Igf1 promoter structure and gene regulation in vertebrates
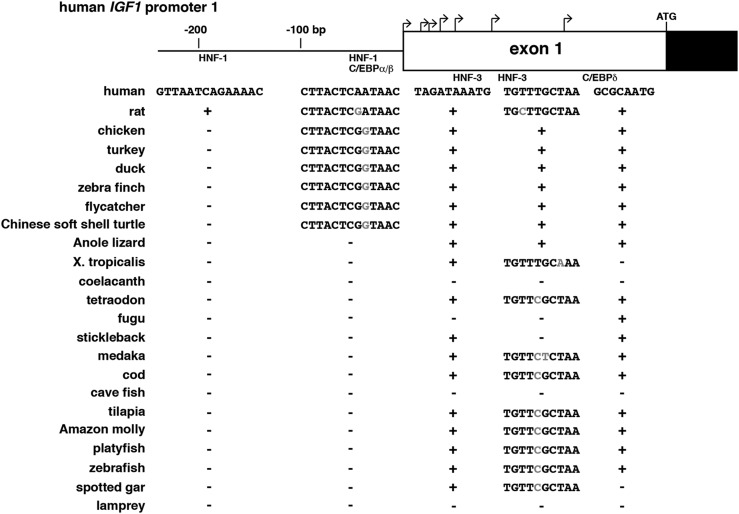
Functional experiments have identified portions of the two human IGF1 and rat Igf1 gene promoters that control their basal activity (20, 49–52); functional studies also have characterized segments of promoter 1 and the noncoding part of exon 1 that mediate actions of several hepatic-enriched transcription factors on IGF1/Igf1 gene expression in the liver, including HNF-1, HNF-3, C/EBPα, and C/EBPβ (53–55), and that serve as response elements for hormones that activate cAMP through the C/EBPδ transcription factor (56, 57). As noted previously, there is no equivalent to Igf1 promoter 2 in nonmammalian vertebrates. Although the homologs of vertebrate promoter 1 appear to be fairly dissimilar to those of their mammalian counterparts, there are a number of conserved features. Pictured in Fig. 8 are comparisons of the parts of proximal human and rat IGF1 promoter 1 and exon 1 that have been characterized as transcription factor‒binding sites in mammals with the orthologous promoter regions in all 21 nonmammalian vertebrate species studied. Several of these DNA elements are conserved in the majority. For example, potential binding sites for HNF-3 or C/EBPδ in distal exon 1 are found in 16 or 17 different vertebrates, respectively. In contrast, the most 5′ HNF-1 site in promoter 1 is not present, and the more 3′ site is detected only in the birds and turtle (Fig. 8). Of note, except for the chicken gene (36–38), no experiments have functionally evaluated Igf1 promoter function in any of the vertebrate species studied here.
Figure 8.
Comparison of Igf1 promoter elements. (A) Schematic of human IGF1 gene promoter 1 and exon 1. Bent arrows indicate transcription start sites in exon 1, and the location of the ATG codon is labeled. Coding DNA is in black and noncoding DNA in white. The locations of binding segments for transcription factors HNF-1, C/EBPα/β, HNF-3, and C/EBPδ are shown. The presence of sites identical to the human sequences in different species is indicated by + for each transcription factor site, and absence is depicted by −. Altered nucleotides within similar sites are shaded in gray.
In mammals, GH is the most important physiological activator of IGF1/Igf1 (58). Multiple GH-response elements have been identified functionally that bind the transcription factor Stat5b in a GH-activated way (28, 30), and several have been detected in analogous positions within the IGF1/Igf1 locus in other mammalian species (13, 14, 59). None of these elements appear to be present in the genomes of nonmammalian vertebrates, as analogues of the 209 to 351 bp regions containing these Stat5b sites in mammals (13, 14, 59) could not be identified through BlastN searches using the corresponding rat DNA sequences. This leaves open the question of whether or how GH stimulates Igf1 gene expression in nonmammalian vertebrate species. In fact, reports addressing these topics have reached variable conclusions (60–65).
Analysis of human IGF1 and chicken, frog, and zebrafish Igf1 genes for conserved DNA sequences in nonexonic and nonpromoter regions using the ECR browser revealed seven discrete segments that were 68% to 78% identical between human IGF1 and chicken Igf1 and that mapped from 18,844 to 52,921 nucleotides 5′ to chicken Igf1 exon 1 (Fig. 2). These DNA sequences were highly conserved among the birds, turtle, and anole lizard (85% to 100%) (Table 5) and were located in relatively similar positions in the Igf1 loci of these species (Fig. 2). However, they were not detected in frog, fish, or lamprey genomes, and none of these segments appeared to contain putative Stat5b binding sites. Moreover, to date, none of these regions have been tested functionally for enhancerlike transcriptional or other properties.
Table 5.
Nucleotide Identity With Chicken Igf1 5′ Conserved Sequences (%)
| Species | ECR 1 a (112 bp) | ECR 2 (139 bp) | ECR 3 (215 bp) | ECR 4 (199 bp) | ECR 5 (275 bp) | ECR 6 (354 bp) | ECR 7 (266 bp) |
|---|---|---|---|---|---|---|---|
| Turkey | 100 | 98 | 97 | 97 | 95 | 95 | 90 |
| Duck | 99 | 97 | 92 | 95 | 92 | 92 | 91 |
| Zebra finch | 99 | 98 | 92 | 90 | 91 | 90 | 90 |
| Flycatcher | 98 | 97 | 91 | 93 | 90 | 90 | 90 |
| Turtle | 90 (82 bp) | 91 (121 bp) | 88 (163 bp) | 87 | 90 (207 bp) | 84 (179 bp) | 87 (187 bp) |
| Anole lizard | No match | 90 (71 bp) | No match | No match | No match | No match | 85 (88 bp) |
| Frog | No match | No match | No match | No match | No match | No match | No match |
ECR with human IGF1 locus.
Conservation and variation in IGF1 protein sequences among vertebrates
In mammals, the 70-residue secreted mature IGF1 molecule, consisting of four domains termed B, C, A, and D, is found within several classes of protein precursors that differ at their N- and C-termini (4, 14). Among all terrestrial vertebrates studied here, the single IGF1 precursor is very similar to the chicken protein (Table 6). Mature IGF1 is not only 70 amino acids in length in all of these species, but it also is identical among birds and additionally is 89% identical to human IGF1 (Fig. 5A and 5C). A single difference with chicken IGF1 was found in the turtle, five changes were detected in frog IGF1, and 13 were noted in the anole lizard (Fig. 5A; Table 6).
Table 6.
Amino Acid Identities With Chicken IGF1
| Species | Signal Peptide (48 AA) | Mature IGF1 (70 AA) | Common E (16 AA) | EA Peptide (19 AA) |
|---|---|---|---|---|
| Turkey | 98a | 100 | 100 | 100 |
| Duck | 98 | 100 | 100 | 100 |
| Zebra finch | 96 | 100 | 100 | 100 |
| Flycatcher | 96 | 100 | 100 | 95 |
| Turtle | 92 | 99 | 100 | 100 |
| Anole lizard | 77b | 81 | 100 | 74c |
| Frog | 77 | 93 | 100 | 79 |
| Coelacanth | 56d | 80e | 100 | 95 |
Abbreviation: AA, amino acid.
Alternative is 28 AA.
49 AA.
EA domain is longer than the chicken’s (see Fig. 6).
50 AA.
72 AA.
Mature IGF1 varied in length among the aquatic vertebrates studied, primarily because of truncation or expansion of the C-region (e.g., platyfish), although in the lamprey, the N-terminal part of the protein also included five extra residues (Fig. 5B and 5C). Except for the B-domain, which was fairly well conserved, amino acid‒sequence similarity was reduced among the 11 ray-finned fish and coelacanth compared with birds, amphibians, or reptiles (Fig. 5B; Table 7).
Table 7.
Amino Acid Identities With Tetraodon IGF1
| Species | Signal Peptide (44 AA) | Mature IGF1 (67 AA) | Common E (16 AA) | EA Peptide (19 AA) | EB Peptide (55 AA) |
|---|---|---|---|---|---|
| Fugu | 98 | 95 | 100 | None | 85 |
| Stickleback | 89a | 90 | 13 | None | 80b |
| Medaka | 75 | 87c | 13d | None | 67b |
| Cod | 73 | 82c | 13 | None | 76b |
| Cave fishe | 59 | 81c | 13 | None | 51b |
| Tilapia | 95 | 85c | 13 | None | 75b |
| Amazon molly | 91 | 84c | 13 | None | 78b |
| Platyfish | 91 | 69c | 13 | None | 78b |
| Zebrafish | 55 | 81c | 13 | None | 77b |
| Spotted gar | 14a | 79c | 13 | none | 42b |
| Lamprey | 14a | 57c | 13d | None | None |
There are two different IGF1 signal peptides in mammals, resulting from alternative promoter usage, in which each promoter is linked to a specific leader exon (4, 14). Because no promoter 2 has been detected in nonmammalian vertebrates, there is a single class of signal peptides, ranging in length from 40 to 50 amino acids, that is derived from exons 1 and 2 in all species (Fig. 6A and 6B; Tables 6 and 7). A 48-residue signal peptide similar to that encoded by mammalian exons 1 and 3 has been well conserved among birds, with at most two amino acid substitutions among the five species evaluated (Fig. 6A and 6C; Table 6). Less similarity was observed among other terrestrial vertebrates. More divergent signal peptides also were found among the fish and lamprey, with variations observed in length (40, 44, 46, 48, or 50 residues, plus an extended alternative amino terminus seen in the cave fish) (Fig. 6B and 6C), and in amino acid sequence identity (minimal for the spotted gar and lamprey vs the tetraodon) (Fig. 6B and 6C; Table 7). It is not known how any of these alterations might affect signal peptide function because, except for the chicken, IGF1 protein biosynthesis and progenitor processing have not been examined experimentally (36).
Mammals express IGF1 protein precursors with different C-terminal extensions or E peptides (4, 14). These are composed of a common E region and either an EA, EB, or EC segment that are encoded by different single 3' exons or different combinations (4, 14). The pattern is radically different in nonmammalian vertebrates. Terrestrial species and the coelacanth encode very similar common E regions and EA peptides that appear to be homologous to those found in mammalian genomes and, as in mammals, are nearly all 16 and 19 amino acids in length, respectively (the exception is the anole lizard, with an EA segment of 31 residues) (Fig. 7A). These portions of the IGF1 protein precursor also are highly related in amino acid sequence, with the common E domain being identical in nine species and the EA peptide having only zero to four substitutions, except for the greater length in the anole lizard (Fig. 7A; Table 6). In contrast, the common E segment in ray-finned fish and the lamprey was more divergent, although in the spotted gar, tilapia, and cod, there were only two, three, and four amino acid differences with the chicken, respectively (Fig. 7A; Table 7). Unlike in terrestrial vertebrates and the coelacanth, in the genomes of ray-finned fish, an IGF1 EB-like segment was encoded by exons 3, 4, and 5 in eight species (the fugu, tetraodon, stickleback, medaka, cod, tilapia, Amazon molly, and platyfish), by exons 4 and 5 in the zebrafish, by exons 4 to 6 in the cave fish, and by exons 4 through 8 in the spotted gar. Although dissimilar in amino acid sequence to the human IGF1 EB domain and of different lengths, EB segments in fish also are enriched in basic residues (Fig. 7B; the cave fish also has a potential alternative EB peptide that lacks 26 N-terminal residues). Moreover, unlike in mammals, the C-terminal 19 amino acids of the EB region matched the sequence of the EA domain, and these residues were very similar to those in EA segments of terrestrial vertebrates (Fig. 7B).
Discussion
Igf1 genes in vertebrates
This study was undertaken to explore evolutionary aspects of IGF1 gene structure using public databases to gather information from the genomes of 21 nonmammalian vertebrate species representing five different classes and spanning ∼500 Myr of evolutionary diversification. In mammals, IGF1, a small secreted protein, plays a key role in mediating somatic growth in juveniles by functioning as a critical agent for the actions of GH (7–10). In these species, IGF1 also participates in metabolic regulation and tissue repair in adults (11, 12). The genomic data presented here suggest that unlike in mammals, in which a 6-exon, 5-intron IGF1/Igf1 gene encodes multiple mRNAs that are translated into several IGF1 protein precursors (4, 18), in most nonmammalian vertebrates there is a single class of transcripts and one protein progenitor. As in mammals, the genomes of all 21 nonmammalian vertebrates examined here also contain single-copy Igf1 genes; however, unlike in mammals, they appear to be organized into two broad structural categories (Figs. 2 and 3; Tables 2‒4). One class of genes, found primarily in terrestrial vertebrates, is composed of four exons and three introns (except for the chicken and turkey, both of which contain an alternatively expressed exon 1a), whereas the other major group, represented by ray-finned fish but also the anole lizard, contains five exons and four introns, with an additional small and poorly conserved coding exon located 3′ to exon 3 (Figs. 2 and 3; Tables 2‒4). In addition, the genomes of two of the ray-finned fish examined, the cave fish and spotted gar, encode Igf1 genes with extra exons compared with other species (Fig. 3; Table 2); with minimal exception, homologs of these additional exons are not found in other vertebrates.
Although Igf1 genes in these terrestrial vertebrates, fish, and lamprey have more extensive diversity than the genes in mammals, they share a number of common features with mammals. In all of the species studied, a large intron separates the two exons that encode the mature IGF1 protein (exons 3 and 4 in mammals and 2 and 3 in nonmammals) (Figs. 2 and 3; Table 2), with exon-intron and intron-exon junctions located in the same relative coding positions [interrupting the exons between the first and second nucleotides of codon 26 of mature IGF1 (codon 31 in the lamprey)]. Similarly conserved positioning of exon-intron and intron-exon boundaries with respect to the protein reading frame is found between exons 1 and 3 in mammals and exons 1 and 2 in nonmammals except for the coelacanth and lamprey and between exons 4 and 6 in mammals and exons 3 and 4 in the birds, anole lizard, turtle, and frog.
IGF1 proteins in vertebrates
Structural features of IGF1 protein precursors also appear to have been maintained between nonmammalian vertebrates and mammals. In most mammals (4, 14) and in most terrestrial vertebrates studied here, a similar signal peptide of 48 amino acids is present (Fig. 6A) that is substantially longer than the typical signal sequences of 20 to 25 residues found in most secreted proteins (66, 67). Longer-than-normal IGF1 signal peptides also were found in all of the other vertebrates evaluated (Fig. 6B), suggesting that this unusual conserved feature plays a functional role in IGF1 biosynthesis. In addition, in mammals, in all terrestrial vertebrates analyzed, and in the coelacanth, the IGF1 precursor contains a C-terminal extension consisting of a conserved N-terminal segment of 16 amino acids, termed the common E peptide, and an equally conserved 19-residue C-terminal component, the EA domain (Fig. 7A). Although the common E peptide is more divergent in ray-finned fish than in the other species, it still consists of 16 amino acids in most species (Fig. 7A). As a consequence of both a longer exon 3 and interposed exon 4, the fairly well-conserved EA region in teleosts has been offset to the carboxyl terminus by a 31‒ to 39‒amino acid segment rich in charged amino acids like the EB region in mammals (Fig. 7B). This EB-like portion of the IGF1 progenitor is more divergent in the cave fish and spotted gar, the two nonmammalian vertebrates with the most variant Igf1 genes (Fig. 7B).
Mature IGF1 in most mammals is a 70‒amino acid single-chain protein (4, 14) that is composed of four domains, termed B, C, A, and D, that are closely related to the analogous regions of IGF2 (4). The former three regions also are analogues of the B, C, and A chains of proinsulin (68). The B and A domains are highly similar among the 21 nonmammalian species studied here and are fairly well conserved with mammalian IGF1 (Fig. 5A and 5B). In contrast, the C segment is divergent in both amino acid sequence and length (Fig. 5A and 5B), perhaps consistent with its primary role as a linker separating and organizing the three-dimensional structure of the B and A domains (68).
Igf1 gene regulation during speciation
Although proximal Igf1 gene promoter regions differ among nonmammalian vertebrates (Fig. 4), fairly extensive DNA sequence identity in most species at discrete transcription factor‒binding sites for HNF-3 and C/EBPδ has been defined functionally in mammals (53–55), as well as in birds at a site for HNF-1 and C/EBPβ (Fig. 8). These data suggest that conserved and common transcriptional mechanisms may regulate some aspects of Igf1 gene expression in the majority of nonmammalian vertebrates and that these functional features are evolutionarily ancient, although this hypothesis needs to be tested experimentally.
One surprising outcome from analyzing these vertebrate Igf1 genes is the apparent absence of GH-regulated Stat5b-binding enhancer elements similar to those found in mammals (13, 14). Thus, although GH is thought to be involved in the molecular physiology of growth and potentially in Igf1 gene regulation in nonmammalian vertebrates (60–65), the mechanism of GH action is apparently different from that in mammals. However, because recent examination of multiple mammalian genomes has revealed that marsupials and monotremes also do not share the cohort of dispersed Stat5b DNA elements found in the IGF1/Igf1 loci of other mammals (14), it is possible that Stat5b-binding sequences are located elsewhere in Igf1 loci in nonmammalian vertebrates or that GH activates Igf1 via other transcription factors. Alternatively, because GH does not appear to robustly stimulate Igf1 gene expression in several of the vertebrate species examined here (64, 65), it is possible that GH regulation is a more recent mammalian adaptation (14). Because it is now possible to construct chimeric cell lines containing a specific Igf1 gene and locus and use them to study regulation by GH (59, 69), these hypotheses and others may be tested directly. For example, experiments could be performed to determine whether the evolutionary conserved elements between human IGF1 and avian Igf1 loci (see Fig. 2) play any roles in transcriptional control of Igf1 gene activity in the latter species.
Igf1 and whole-genome duplication events in teleost progenitors
Substantial evidence supports the idea that duplication of the entire genome occurred in a common ancestor of extant teleosts ∼320 to 350 Myr ago (46) and that this event was followed by rediploidization to various extents in the precursors of different modern teleost lineages (46). For example, ∼20% of duplicated genes have been retained in zebrafish as paralogues (70), but only 1% to 5% in the fugu or other pufferfish (71). From the analyses performed here, Igf1 does not appear to be one of these paralogous genes, and there is no evidence that even a portion of a duplicated Igf1 gene has been retained in any of the 11 species examined here. In contrast, there are two Igf2 genes in zebrafish but a single Igf2 in the fugu, tetraodon, and spotted gar. Nevertheless, there is evidence for divergence in Igf1 gene organization between fish and terrestrial vertebrates, as indicated by the presence of a small coding exon 4 in all of the teleosts studied here but in only a single nonaquatic nonmammalian vertebrate, the anole lizard (Table 2).
Maximizing utility of genome databases
Publically available genome databases contain abundant information about different genes from many different animal species, yet much of these data have not been fully annotated or evaluated in detail. Although information for most of the vertebrate Igf1 genes examined here was present in their genomes, it was either incompletely or incorrectly annotated in the Ensembl and UCSC browsers. It seems likely that many other genes in genome databases are not described accurately, raising the possibility that a focused study will provide many new insights into gene conservation or variation during speciation.
Although DNA sequence polymorphisms are defined primarily in humans (72), it seems likely that an extensive range could be found in nonmammalian vertebrates if enough genomes from different individuals were studied. In fact, in chickens, a number of polymorphisms have been identified within the Igf1 locus, and these sort with traits related to somatic growth, weight gain, muscle weight, and/or abdominal fat (73–78). Conceivably, as in humans, some of these alterations could modify gene expression if located within promoters, enhancers, or other regulatory elements (79). Single-nucleotide polymorphisms, DNA insertion-deletions, or copy-number variants may also play roles in population fitness or in other adaptations to changing environments; if present in multiple species, this may support a hypothesis regarding their contributions to the organismal fitness of evolutionary progenitors.
Key conclusions
Exon-intron organization is more similar to mammalian Igf1 genes in birds and other terrestrial vertebrates than in fish. Igf1 genes in nonmammalian vertebrates have a single promoter equivalent to mammalian promoter 1. Some regulatory DNA sequences functionally mapped to proximal mammalian promoter 1 are conserved in nonmammalian vertebrates, but dispersed Stat5b-binding elements that control GH-activated Igf1 gene transcription in mammals could not be identified in the vertebrate genomes studied here. The large signal peptide and mature IGF1 protein have been conserved in both mammals and nonmammalian vertebrates, but the C-terminal EB domain of the IGF1 precursor has diverged in teleosts and is absent in terrestrial vertebrates.
Final comments
The complex role of IGF1 in normal physiology and in disease pathogenesis is potentially reflected in its complicated structure and its variable patterns of expression in different cell types, tissues, and organs (15–18). Conservation of aspects of the Igf1 gene and of IGF1 among the 21 nonmammalian vertebrates analyzed here suggests that common, functionally important components may have been present in some of the evolutionarily earliest vertebrates (80, 81), an idea that, upon further investigation, may provide new insights into the comparative biology of IGF1 expression and action.
Acknowledgments
I thank my colleagues for comments on the manuscript.
Financial Support: Studies that led to this paper were initially supported by National Institutes of Health Research Grant R01 DK069703 (to P.R.).
Disclosure Summary: The author has nothing to disclose.
Glossary
Abbreviations:
- ECR
evolutionary conserved region
- Myr
million year
- NCBI
National Center for Biotechnology Information
- UCSC
University of California, Santa Cruz
- UTR
untranslated region
References
- 1. Daughaday WH, Kapadia M, Yanow CE, Fabrick K, Mariz IK. Insulin-like growth factors I and II of nonmammalian sera. Gen Comp Endocrinol. 1985;59(2):316–325. [DOI] [PubMed] [Google Scholar]
- 2. Daughaday WH, Rotwein P. Insulin-like growth factors I and II: peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev. 1989;10(1):68–91. [DOI] [PubMed] [Google Scholar]
- 3. Sussenbach JS, Steenbergh PH, Jansen E, Holthuizen P, Meinsma D, van Dijk MA, Gloudemans T. Structural and regulatory aspects of the human genes encoding IGF-I and -II. Adv Exp Med Biol. 1991;293:1–14. [DOI] [PubMed] [Google Scholar]
- 4. Rotwein P. Molecular biology of IGF-I and IGF-II In: Rosenfeld R, Roberts CJ, eds. The IGF System.Totowa, NJ: Humana Press; 1999:19–35. [Google Scholar]
- 5. Das R, Dobens LL. Conservation of gene and tissue networks regulating insulin signalling in flies and vertebrates. Biochem Soc Trans. 2015;43(5):1057–1062. [DOI] [PubMed] [Google Scholar]
- 6. Schwartz TS, Bronikowski AM. Evolution and function of the insulin and insulin-like signaling network in ectothermic reptiles: some answers and more questions. Integr Comp Biol. 2016;56(2):171–184. [DOI] [PubMed] [Google Scholar]
- 7. Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol. 2001;229(1):141–162. [DOI] [PubMed] [Google Scholar]
- 8. Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett N, Stewart TA. IGF-I is required for normal embryonic growth in mice. Genes Dev. 1993;7(12b, 12B):2609–2617. [DOI] [PubMed] [Google Scholar]
- 9. Woods KA, Camacho-Hübner C, Savage MO, Clark AJ. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med. 1996;335(18):1363–1367. [DOI] [PubMed] [Google Scholar]
- 10. LeRoith D. Clinical relevance of systemic and local IGF-I: lessons from animal models. Pediatr Endocrinol Rev. 2008;5(Suppl 2):739–743. [PubMed] [Google Scholar]
- 11. Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12(3):159–169. [DOI] [PubMed] [Google Scholar]
- 12. Gems D, Partridge L. Genetics of longevity in model organisms: debates and paradigm shifts. Annu Rev Physiol. 2013;75(1):621–644. [DOI] [PubMed] [Google Scholar]
- 13. Rotwein P. Variation in the insulin-like growth factor 1 gene in primates. Endocrinology. 2017;158(4):804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rotwein P. Diversification of the insulin-like growth factor 1 gene in mammals. PLoS One. 2017;12(12):e0189642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shimatsu A, Rotwein P. Mosaic evolution of the insulin-like growth factors: organization, sequence, and expression of the rat insulin-like growth factor I gene. J Biol Chem. 1987;262(16):7894–7900. [PubMed] [Google Scholar]
- 16. Lowe WLJ Jr, Roberts CTJ Jr, Lasky SR, LeRoith D. Differential expression of alternative 5′ untranslated regions in mRNAs encoding rat insulin-like growth factor I. Proc Natl Acad Sci USA. 1987;84(24):8946–8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoyt EC, Van Wyk JJ, Lund PK. Tissue and development specific regulation of a complex family of rat insulin-like growth factor I messenger ribonucleic acids. Mol Endocrinol. 1988;2(11):1077–1086. [DOI] [PubMed] [Google Scholar]
- 18. Hall LJ, Kajimoto Y, Bichell D, Kim SW, James PL, Counts D, Nixon LJ, Tobin G, Rotwein P. Functional analysis of the rat insulin-like growth factor I gene and identification of an IGF-I gene promoter. DNA Cell Biol. 1992;11(4):301–313. [DOI] [PubMed] [Google Scholar]
- 19. Rotwein P. Two insulin-like growth factor I messenger RNAs are expressed in human liver. Proc Natl Acad Sci USA. 1986;83(1):77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim SW, Lajara R, Rotwein P. Structure and function of a human insulin-like growth factor-I gene promoter. Mol Endocrinol. 1991;5(12):1964–1972. [DOI] [PubMed] [Google Scholar]
- 21. Mathews LS, Norstedt G, Palmiter RD. Regulation of insulin-like growth factor I gene expression by growth hormone. Proc Natl Acad Sci USA. 1986;83(24):9343–9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bichell DP, Kikuchi K, Rotwein P. Growth hormone rapidly activates insulin-like growth factor I gene transcription in vivo. Mol Endocrinol. 1992;6(11):1899–1908. [DOI] [PubMed] [Google Scholar]
- 23. Eleswarapu S, Gu Z, Jiang H. Growth hormone regulation of insulin-like growth factor-I gene expression may be mediated by multiple distal signal transducer and activator of transcription 5 binding sites. Endocrinology. 2008;149(5):2230–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Waters MJ, Brooks AJ. JAK2 activation by growth hormone and other cytokines. Biochem J. 2015;466(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Waters MJ. The growth hormone receptor. Growth Horm IGF Res. 2016;28:6–10. [DOI] [PubMed] [Google Scholar]
- 26. Woelfle J, Billiard J, Rotwein P. Acute control of insulin-like growth factor-I gene transcription by growth hormone through Stat5b. J Biol Chem. 2003;278(25):22696–22702. [DOI] [PubMed] [Google Scholar]
- 27. Woelfle J, Rotwein P. In vivo regulation of growth hormone-stimulated gene transcription by STAT5b. Am J Physiol Endocrinol Metab. 2004;286(3):E393–E401. [DOI] [PubMed] [Google Scholar]
- 28. Chia DJ, Varco-Merth B, Rotwein P. Dispersed chromosomal Stat5b-binding elements mediate growth hormone-activated insulin-like growth factor-I gene transcription. J Biol Chem. 2010;285(23):17636–17647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chia DJ, Rotwein P. Defining the epigenetic actions of growth hormone: acute chromatin changes accompany GH-activated gene transcription. Mol Endocrinol. 2010;24(10):2038–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Varco-Merth B, Mirza K, Alzhanov DT, Chia DJ, Rotwein P. Biochemical characterization of diverse Stat5b-binding enhancers that mediate growth hormone-activated insulin-like growth factor-I gene transcription. PLoS One. 2012;7(11):e50278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Feigerlova E, Hwa V, Derr MA, Rosenfeld RG. Current issues on molecular diagnosis of GH signaling defects. Endocr Dev. 2013;24:118–127. [DOI] [PubMed] [Google Scholar]
- 32. Scalco RC, Hwa V, Domené HM, Jasper HG, Belgorosky A, Marino R, Pereira AM, Tonelli CA, Wit JM, Rosenfeld RG, Jorge AA. STAT5B mutations in heterozygous state have negative impact on height: another clue in human stature heritability. Eur J Endocrinol. 2015;173(3):291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci USA. 1997;94(14):7239–7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93(5):841–850. [DOI] [PubMed] [Google Scholar]
- 35. Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36(Web Server issue):W465–W46 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kajimoto Y, Rotwein P. Structure and expression of a chicken insulin-like growth factor I precursor. Mol Endocrinol. 1989;3(12):1907–1913. [DOI] [PubMed] [Google Scholar]
- 37. Fawcett DH, Bulfield G. Molecular cloning, sequence analysis and expression of putative chicken insulin-like growth factor-I cDNAs. J Mol Endocrinol. 1990;4(3):201–211. [DOI] [PubMed] [Google Scholar]
- 38. Kajimoto Y, Rotwein P. Structure of the chicken insulin-like growth factor I gene reveals conserved promoter elements. J Biol Chem. 1991;266(15):9724–9731. [PubMed] [Google Scholar]
- 39. Nam K, Mugal C, Nabholz B, Schielzeth H, Wolf JB, Backström N, Künstner A, Balakrishnan CN, Heger A, Ponting CP, Clayton DF, Ellegren H. Molecular evolution of genes in avian genomes. Genome Biol. 2010;11(6):R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takezaki N, Figueroa F, Zaleska-Rutczynska Z, Takahata N, Klein J. The phylogenetic relationship of tetrapod, coelacanth, and lungfish revealed by the sequences of forty-four nuclear genes. Mol Biol Evol. 2004;21(8):1512–1524. [DOI] [PubMed] [Google Scholar]
- 41. Smith JJ, Kuraku S, Holt C, Sauka-Spengler T, Jiang N, Campbell MS, Yandell MD, Manousaki T, Meyer A, Bloom OE, Morgan JR, Buxbaum JD, Sachidanandam R, Sims C, Garruss AS, Cook M, Krumlauf R, Wiedemann LM, Sower SA, Decatur WA, Hall JA, Amemiya CT, Saha NR, Buckley KM, Rast JP, Das S, Hirano M, McCurley N, Guo P, Rohner N, Tabin CJ, Piccinelli P, Elgar G, Ruffier M, Aken BL, Searle SM, Muffato M, Pignatelli M, Herrero J, Jones M, Brown CT, Chung-Davidson YW, Nanlohy KG, Libants SV, Yeh CY, McCauley DW, Langeland JA, Pancer Z, Fritzsch B, de Jong PJ, Zhu B, Fulton LL, Theising B, Flicek P, Bronner ME, Warren WC, Clifton SW, Wilson RK, Li W. Sequencing of the sea lamprey (Petromyzon marinus) genome provides insights into vertebrate evolution. Nat Genet. 2013;45(4):415–421, 421e1–421e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Griffin DK, Robertson LB, Tempest HG, Vignal A, Fillon V, Crooijmans RP, Groenen MA, Deryusheva S, Gaginskaya E, Carré W, Waddington D, Talbot R, Völker M, Masabanda JS, Burt DW. Whole genome comparative studies between chicken and turkey and their implications for avian genome evolution. BMC Genomics. 2008;9(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Backström N, Karaiskou N, Leder EH, Gustafsson L, Primmer CR, Qvarnström A, Ellegren H. A gene-based genetic linkage map of the collared flycatcher (Ficedula albicollis) reveals extensive synteny and gene-order conservation during 100 million years of avian evolution. Genetics. 2008;179(3):1479–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McCormack JE, Harvey MG, Faircloth BC, Crawford NG, Glenn TC, Brumfield RT. A phylogeny of birds based on over 1,500 loci collected by target enrichment and high-throughput sequencing. PLoS One. 2013;8(1):e54848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Prum RO, Berv JS, Dornburg A, Field DJ, Townsend JP, Lemmon EM, Lemmon AR. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature. 2015;526(7574):569–573. [DOI] [PubMed] [Google Scholar]
- 46. Glasauer SM, Neuhauss SC. Whole-genome duplication in teleost fishes and its evolutionary consequences. Mol Genet Genomics. 2014;289(6):1045–1060. [DOI] [PubMed] [Google Scholar]
- 47. Gamazon ER, Stranger BE. Genomics of alternative splicing: evolution, development and pathophysiology. Hum Genet. 2014;133(6):679–687. [DOI] [PubMed] [Google Scholar]
- 48. van den Hoogenhof MM, Pinto YM, Creemers EE. RNA splicing: regulation and dysregulation in the heart. Circ Res. 2016;118(3):454–468. [DOI] [PubMed] [Google Scholar]
- 49. Jansen E, Steenbergh PH, van Schaik FM, Sussenbach JS. The human IGF-I gene contains two cell type-specifically regulated promoters. Biochem Biophys Res Commun. 1992;187(3):1219–1226. [DOI] [PubMed] [Google Scholar]
- 50. Steenbergh PH, Jansen E, van Schaik FM, Sussenbach JS. Functional analysis of the human IGF-I gene promoters. Mol Reprod Dev. 1993;35(4):365–367. [DOI] [PubMed] [Google Scholar]
- 51. An MR, Lowe WLJ Jr. The major promoter of the rat insulin-like growth factor-I gene binds a protein complex that is required for basal expression. Mol Cell Endocrinol. 1995;114(1-2):77–89. [DOI] [PubMed] [Google Scholar]
- 52. Mittanck DW, Kim SW, Rotwein P. Essential promoter elements are located within the 5′ untranslated region of human insulin-like growth factor-I exon I. Mol Cell Endocrinol. 1997;126(2):153–163. [DOI] [PubMed] [Google Scholar]
- 53. Nolten LA, van Schaik FM, Steenbergh PH, Sussenbach JS. Expression of the insulin-like growth factor I gene is stimulated by the liver-enriched transcription factors C/EBP alpha and LAP. Mol Endocrinol. 1994;8(12):1636–1645. [DOI] [PubMed] [Google Scholar]
- 54. Nolten LA, Steenbergh PH, Sussenbach JS. Hepatocyte nuclear factor 1 alpha activates promoter 1 of the human insulin-like growth factor I gene via two distinct binding sites. Mol Endocrinol. 1995;9(11):1488–1499. [DOI] [PubMed] [Google Scholar]
- 55. Nolten LA, Steenbergh PH, Sussenbach JS. The hepatocyte nuclear factor 3beta stimulates the transcription of the human insulin-like growth factor I gene in a direct and indirect manner. J Biol Chem. 1996;271(50):31846–31854. [DOI] [PubMed] [Google Scholar]
- 56. Umayahara Y, Billiard J, Ji C, Centrella M, McCarthy TL, Rotwein P. CCAAT/enhancer-binding protein delta is a critical regulator of insulin-like growth factor-I gene transcription in osteoblasts. J Biol Chem. 1999;274(15):10609–10617. [DOI] [PubMed] [Google Scholar]
- 57. Billiard J, Grewal SS, Lukaesko L, Stork PJ, Rotwein P. Hormonal control of insulin-like growth factor I gene transcription in human osteoblasts: dual actions of cAMP-dependent protein kinase on CCAAT/enhancer-binding protein delta. J Biol Chem. 2001;276(33):31238–31246. [DOI] [PubMed] [Google Scholar]
- 58. Rosenfeld RG, Hwa V. The growth hormone cascade and its role in mammalian growth. Horm Res. 2009;71(Suppl 2):36–40. [DOI] [PubMed] [Google Scholar]
- 59. Mukherjee A, Alzhanov D, Rotwein P. Defining human insulin-like growth factor I gene regulation. Am J Physiol Endocrinol Metab. 2016;311(2):E519–E529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang X, Carré W, Saxton AM, Cogburn LA. Manipulation of thyroid status and/or GH injection alters hepatic gene expression in the juvenile chicken. Cytogenet Genome Res. 2007;117(1-4):174–188. [DOI] [PubMed] [Google Scholar]
- 61. Pierce AL, Breves JP, Moriyama S, Hirano T, Grau EG. Differential regulation of Igf1 and Igf2 mRNA levels in tilapia hepatocytes: effects of insulin and cortisol on GH sensitivity. J Endocrinol. 2011;211(2):201–210. [DOI] [PubMed] [Google Scholar]
- 62. Wu G, Siegel PB, Gilbert ER, Yang N, Wong EA. Expression profiles of somatotropic axis genes in lines of chickens divergently selected for 56-day body weight. Anim Biotechnol. 2011;22(2):100–110. [DOI] [PubMed] [Google Scholar]
- 63. Reindl KM, Kittilson JD, Bergan HE, Sheridan MA. Growth hormone-stimulated insulin-like growth factor-1 expression in rainbow trout (Oncorhynchus mykiss) hepatocytes is mediated by ERK, PI3K-AKT, and JAK-STAT. Am J Physiol Regul Integr Comp Physiol. 2011;301(1):R236–R243. [DOI] [PubMed] [Google Scholar]
- 64. Beckman BR. Perspectives on concordant and discordant relations between insulin-like growth factor 1 (IGF1) and growth in fishes. Gen Comp Endocrinol. 2011;170(2):233–252. [DOI] [PubMed] [Google Scholar]
- 65. Fuentes EN, Valdés JA, Molina A, Björnsson BT. Regulation of skeletal muscle growth in fish by the growth hormone--insulin-like growth factor system. Gen Comp Endocrinol. 2013;192:136–148. [DOI] [PubMed] [Google Scholar]
- 66. von Heijne G. Signal sequences: the limits of variation. J Mol Biol. 1985;184(1):99–105. [DOI] [PubMed] [Google Scholar]
- 67. von Heijne G. The signal peptide. J Membr Biol. 1990;115(3):195–201. [DOI] [PubMed] [Google Scholar]
- 68. Blundell TL, Humbel RE. Hormone families: pancreatic hormones and homologous growth factors. Nature. 1980;287(5785):781–787. [DOI] [PubMed] [Google Scholar]
- 69. Alzhanov D, Mukherjee A, Rotwein P. Identifying growth hormone-regulated enhancers in the Igf1 locus. Physiol Genomics. 2015;47(11):559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Woods IG, Wilson C, Friedlander B, Chang P, Reyes DK, Nix R, Kelly PD, Chu F, Postlethwait JH, Talbot WS. The zebrafish gene map defines ancestral vertebrate chromosomes. Genome Res. 2005;15(9):1307–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kassahn KS, Dang VT, Wilkins SJ, Perkins AC, Ragan MA. Evolution of gene function and regulatory control after whole-genome duplication: comparative analyses in vertebrates. Genome Res. 2009;19(8):1404–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ott J, Wang J, Leal SM. Genetic linkage analysis in the age of whole-genome sequencing. Nat Rev Genet. 2015;16(5):275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhou H, Mitchell AD, McMurtry JP, Ashwell CM, Lamont SJ. Insulin-like growth factor-I gene polymorphism associations with growth, body composition, skeleton integrity, and metabolic traits in chickens. Poult Sci. 2005;84(2):212–219. [DOI] [PubMed] [Google Scholar]
- 74. Bennett AK, Hester PY, Spurlock DE. Polymorphisms in vitamin D receptor, osteopontin, insulin-like growth factor 1 and insulin, and their associations with bone, egg and growth traits in a layer--broiler cross in chickens. Anim Genet. 2006;37(3):283–286. [DOI] [PubMed] [Google Scholar]
- 75. Bian LH, Wang SZ, Wang QG, Zhang S, Wang YX, Li H. Variation at the insulin-like growth factor 1 gene and its association with body weight traits in the chicken. J Anim Breed Genet. 2008;125(4):265–270. [DOI] [PubMed] [Google Scholar]
- 76. Promwatee N, Laopaiboon B, Vongpralub T, Phasuk Y, Kunhareang S, Boonkum W, Duangjinda M. Insulin-like growth factor I gene polymorphism associated with growth and carcass traits in Thai synthetic chickens. Genet Mol Res. 2013;12(4):4332–4341. [DOI] [PubMed] [Google Scholar]
- 77. Boschiero C, Jorge EC, Ninov K, Nones K, do Rosário MF, Coutinho LL, Ledur MC, Burt DW, Moura AS. Association of IGF1 and KDM5A polymorphisms with performance, fatness and carcass traits in chickens. J Appl Genet. 2013;54(1):103–112. [DOI] [PubMed] [Google Scholar]
- 78. Bhattacharya TK, Chatterjee RN, Dushyanth K, Paswan C, Shukla R, Shanmugam M. Polymorphism and expression of insulin-like growth factor 1 (IGF1) gene and its association with growth traits in chicken. Br Poult Sci. 2015;56(4):398–407. [DOI] [PubMed] [Google Scholar]
- 79. Albert FW, Kruglyak L. The role of regulatory variation in complex traits and disease. Nat Rev Genet. 2015;16(4):197–212. [DOI] [PubMed] [Google Scholar]
- 80. Venditti C, Pagel M. Speciation as an active force in promoting genetic evolution. Trends Ecol Evol. 2010;25(1):14–20. [DOI] [PubMed] [Google Scholar]
- 81. Venditti C, Meade A, Pagel M. Multiple routes to mammalian diversity. Nature. 2011;479(7373):393–396. [DOI] [PubMed] [Google Scholar]