Abstract
Chronic exposure to stressors impairs the function of multiple organ systems and has been implicated in increased disease risk. In the rodent, the chronic variable stress (CVS) paradigm has successfully modeled several stress-related illnesses. Despite striking disparities between men and women in the prevalence and etiology of disorders associated with chronic stress, most preclinical research examining chronic stressor exposure has focused on male subjects. One potential mediator of the consequences of CVS is oxytocin (OT), a known regulator of stress neurocircuitry and behavior. To ascertain the sex-specific effects of CVS in the C57BL/6 mouse on OT and the structurally similar neuropeptide arginine vasopressin (AVP), the numbers of immunoreactive and mRNA-containing neurons in the paraventricular nucleus (PVN) and supraoptic nucleus (SON) were determined using immunohistochemistry and in situ hybridization, respectively. In addition, the mice underwent a battery of behavioral tests to determine whether CVS affects social behaviors known to be regulated by OT and AVP. Six weeks of CVS increased sociability in the female mouse and decreased PVN OT immunoreactivity (ir) and AVP mRNA. In the male mice, CVS decreased PVN OT mRNA but had no effect on social behavior, AVP, or OT-ir. CVS also increased the soma volume for PVN OT neurons. In contrast, OT and AVP neurons in the SON were unaffected by CVS treatment. These findings demonstrate clear sex differences in the effects of CVS on neuropeptides in the mouse, suggest a pathway through which CVS alters sociability and stress-coping responses in females and reveals a vulnerability to CVS in the C57BL/6 mouse strain.
Chronic variable stress induces a female-specific increase in sociability coupled with alterations in oxytocin and vasopressin expression within the paraventricular nucleus of the hypothalamus.
Exposure to chronic stress has a number of negative consequences for human health, including an increased risk of metabolic disorders (1), cardiovascular disease (2), and mood disorders (3). The widely used chronic variable stress (CVS) model has successfully replicated this vulnerability to chronic stress-related disease in the rodent, most classically through the induction of a depressive-like behavioral state (4). Importantly, despite sex differences in both the prevalence and presentation of chronic stress-related disorders (5, 6), most research using the CVS model has focused on male subjects. Thus, the implications of chronic stress exposure on female physiology remain largely unknown.
Much of the effect of the CVS procedure on health outcomes is associated with a dysregulation of hypothalamic-pituitary-adrenal (HPA) axis function. The unpredictability of the CVS paradigm prevents subjects from habituating to chronic stress exposure, often inducing a hyperactive stress response (7). Two potential facilitators of CVS-induced changes in HPA axis activity are the nonapeptides oxytocin (OT) and arginine vasopressin (AVP), which are synthesized and secreted from neurons in the paraventricular nucleus (PVN) and the supraoptic nucleus (SON) of the hypothalamus. Although these hormones are classically known for their control of smooth muscle contraction in parturition and lactation (8), mediation of social behavior (9, 10), fluid homeostasis, and the control of blood pressure (11), they are both important regulators of HPA axis activity (12, 13), with OT largely inhibiting HPA axis activity (13, 14), and AVP generally showing an enhancing effect (15).
Both OT and AVP are sensitive to the effects of acute and chronic stress. In the rat, acute stress activates PVN OT (16) and AVP (17) neurons and induces OT and AVP secretion, elevating levels in plasma (18, 19), in the PVN and SON (19–21), and within efferent targets such as the amygdala (21, 22). In the male rat, chronic stress exposure has also been associated with elevated PVN OT (23, 24) and AVP (25, 26) mRNA, as well as elevated OT immunoreactivity (ir), but not AVP-ir (27), in this region. OT might serve as a protective factor against some of the physiological and behavioral consequences of chronic stress exposure (28). In contrast, AVP might facilitate chronic stress-induced physiological (26), but not behavioral (29), changes. The effects of chronic stress on OT and AVP in females have remained largely uninvestigated. Recently, Steinman et al. (30) observed a female-specific decrease in PVN OT-ir but not mRNA in the rodent, Peromyscus californicus, after social defeat, a subchronic stress model. In contrast, CVS exposure during adolescence has also been shown to elevate PVN OT mRNA in adulthood in the female rat (31). Although CVS had no effect on PVN AVP mRNA in the female rat (25), it has been shown to alter AVP-ir in the female mouse PVN in a subregion-specific manner (32). It is currently unknown what effects CVS has on the OT system in the female mouse.
OT and AVP are important mediators of social behavior. Although OT is critical for the preference for social interaction (9), especially for female mammals (33), both OT and AVP are involved in social recognition (34). Thus, it is perhaps unsurprising that CVS, a procedure that reportedly alters PVN OT, has also been shown to induce changes in sociability in the rodent. To date, studies have provided conflicting accounts of the nature of CVS’s effects on social interaction, reporting both increased (35, 36) and decreased (37, 38) sociability. The present set of experiments sought to compare the effects of CVS on OT and AVP systems in the male and female mouse and to determine whether changes to these neuropeptides would be mirrored by changes in social behavior. The results showed sex-specific alterations to OT and AVP neurons within the PVN and female-specific changes to sociability. Together, these findings have demonstrated novel sex-specific consequences of CVS exposure in the mouse and suggest a mechanism by which CVS alters social behavior in a female-specific manner.
Materials and Methods
Subjects
Sixty-day-old male (n = 27) and female (n = 31) C57BL/6 (C57BL/6NCrl strain 027) mice were purchased from Charles Rivers Laboratories (Wilmington, MA). All subjects were pair housed with a same-sex cage mate with a 12-hour light:12-hour dark cycle (lights on at 6:00 am) and provided ad libitum access to food and water. The institutional animal care and use committee at Colorado State University approved all animal protocols, which were performed in accordance with the guidelines of Colorado State University, the National Institutes of Health, and the Association for Assessment and Accreditation of Laboratory Animal Care International.
Chronic variable stress
Mice were exposed to an average of two of eight different stressors daily for ∼6 weeks, as modified from previous studies (4, 39). The stressors consisted of damp bedding, novel empty cages without bedding, novel cages with bedding soiled by same-sex unfamiliar mice, 30 minutes of restraint, a 45° cage tilt, 8 hours of continuous loud (80 dB) pink noise, 36 hours of constant light, and exposure to cat odor (Table 1). The mice undergoing CVS were housed in a room separate from the controls. The slight variation in the length of CVS exposure resulted from limiting the testing of female mice to a day when both of the subjects in each cage were not in the proestrus phase of the estrous cycle to prevent activational effects of the acute increase in estradiol that occur on proestrus morning (40).
Table 1.
Description of Stressors Used in Chronic Variable Stress Model
| Stressor | Description |
|---|---|
| Social stress | Subjects placed in an empty, previously occupied cage for 3 h |
| Restraint stress | Subjects placed in a closed, ventilated tube for 30 min |
| Novel object exposure | Novel objects (8 marbles) placed in each cage overnight |
| Damp sawdust | Subjects remained in cages with damp bedding (125 mL of water added) for 3 h |
| Empty cage | Subjects placed in a cage without sawdust for 3 h |
| Light cycle disturbance | Subjects exposed to continuous overhead light for 36 h |
| Noise exposure | Subjects exposed to a pink noise (80 dB) for 8 h |
| Cage tilt | Cages tilted backward (45°) for 3 h |
| Predator odor | Subjects exposed to cat odor for 60 min |
Social behavioral testing
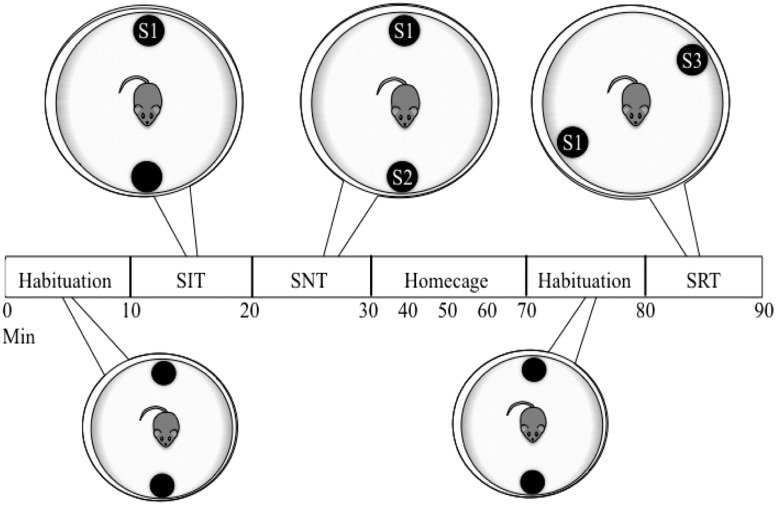
CVS-treated (males, n = 8; females, n = 10) and control (males, n = 8; females, n = 8) mice were evaluated using a series of social behavior tests. To facilitate social interaction (41), the subjects were separated from their cage mates and housed singly the day before testing. The next day, the mice were transported to an adjacent behavioral testing room 30 minutes before the behavioral assessment. Three behavioral tests (social interaction test, social novelty test, and social recognition test) were conducted between 12:30 and 3:30 pm in a circular (60-cm diameter) open-field arena containing two wire mesh cups (10-cm height × 9-cm diameter) holding individual 2- to 3-month-old ovariectomized stimulus mice (Fig. 1). The wire mesh permitted the mice to see, smell, and hear the stimulus animals but prevented direct contact. Their behavior was recorded by video (Bunker Hill Security, Harbor Freight Tools, Calabasas, CA) and scored by a researcher unaware of the condition. For the social interaction test, each subject was first placed in the center of the open field, which contained two empty cups on opposing sides of the field’s perimeter, and allowed to habituate to the arena for 10 minutes. At the end of this period, a stimulus mouse [first stimulus mouse (S1)] was placed under one cup, and the test mouse’s behavior was scored for 10 minutes. A social novelty test was conducted immediately afterward by introducing a novel stimulus mouse (S2) to the other empty cup. The behavior (time with cup containing S1, time with cup containing S2, time away from both cups, visits to each cup, and latency to approach S2) were then scored for another 10 minutes. Each subject was then returned to its home cage for 40 minutes. The arena and each cup were cleaned with a 70% ethanol solution, and the cups were placed back in each arena in new locations. For the social recognition test, each subject was reintroduced to the open field and empty cups for 10 minutes. Next, S1 and a new stimulus animal, third stimulus mouse (S3), were added to each of the empty cups in a random order. The test mouse’s behavior (time investigating cup containing S1, time with S3, time away from both cups, visits to each cup, and latency to approach S3) was recorded for another 10 minutes. The cups and open field arenas were cleaned with 70% ethanol before and after each test.
Figure 1.
Timeline for social behavior testing. Circles represent the round open field arena at each stage of behavioral testing, with wire mesh cups containing an ovariectomized stimulus mouse or no stimulus mouse (unmarked black circle). For each social behavior test, stimulus mice were added, and investigatory behavior was subsequently scored for 10 min. The mice were returned to their home cage for 40 min during the “home cage” period. SIT, social interaction test; SNT, social novelty test; SRT, social recognition test.
In situ hybridization
Immediately after social behavior testing, each subject was anesthetized with isoflurane and decapitated. The brains were flash frozen in 2-methylbutane (−40°C; Sigma-Aldrich Chemical Co., St. Louis, MO) and stored at −80°C until further processing. AVP and OT mRNA were then detected in PVN using in situ hybridization, as previously described (41). In brief, the probes for mouse AVP (5′GTAGACCCGGGGCTTGGCAGAATCCACGGACTCCCGTGTCCCA GCCAG 3′) and mouse OT (5′AAGCAGGCAGCAAGCGAGACT GGGGCAGGCCAT GGCGATGGTGCTCAG 3′) were end-labeled with [35S]deoxy-ATP using terminal deoxynucleotidyl transferase, and the radiolabeled probe was separated from free radioactivity using an Oligo Clean and Concentrator kit (Zymo Research, Irvine CA). Next, 16-µm-thick tissue sections through the hypothalamus were taken using a cryostat (Leica CM3050S; Leica Biosystems), thaw-mounted onto SuperFrost Plus slides (Fisher Scientific), and stored at −80°C until assayed. At the time of the assay, the tissue sections were thawed briefly and then fixed in 10% formalin, acetylated in triethanolamine containing 0.25% acetic anhydride, dehydrated in graded ethanol washes, delipidated in chloroform, air dried, and hybridized in radiolabeled (20 × 106 cpm/mL) hybridization solution (50% deionized formamide containing 100 mM dithiothreitol, 0.5% SDS, and 50% hybridization buffer [600 mM NaCl, 10 mM Tris HCl (pH 7.5), 1 mM EDTA, 10 mM NaH2PO4 (pH 8.0), 10% dextran sulfate, 0.04% 1× Denhardt solution, 0.05% yeast RNA, and 0.01% denatured salmon testis DNA]), and incubated overnight at 37°C. The next day, the slides were washed in 2× SSC and dehydrated in graded ethanol washes. The sections were tested for intensity of hybridization using a rapid exposure to X-Ray film (Carestream Kodak Biomax MR; Carestream, Rochester, NY) for 2 to 4 hours. The slides were then coated with NTB-3 nuclear tract emulsion (Carestream) and stored in darkness at 4°C for 10 hours for OT and 18 hours for AVP before being developed.
The tissue was lightly counterstained with cresyl violet to visualize cell nuclei and boundaries of the PVN but not allow interference of the stain with thresholding during grain counting, and the slides were coverslipped with Permount (Fisher Scientific). The sections were visualized using light microscopy with an Axio Examiner Z1 (Carl Zeiss Microscopy, Jena, Germany) with a 20× objective and subsequently photographed for analysis.
Photomicrographs showing hybridization for AVP or OT mRNA were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD). For each mouse, three to five images per hemisphere, encompassing the anterior, mid, and posterior regions of the PVN, were analyzed. The number of cells expressing mRNA was counted from the emulsion-coated tissues, and the average number of grains per cell was quantified using previously described approaches (42). Labeled cells were identified by the presence of a visible stained nucleus under a cluster of silver grains and at least a fivefold greater density of silver grains compared with the adjacent background area (Supplemental Fig. 1). In the case of multiple cell nuclei under a single cluster, the cluster was counted by the number of nuclei present. For grain counts, the average pixel size of the grains in a background field was determined from images taken in a cellular area adjacent to the PVN. The image was thresholded to match the number of silver grains visualized by the investigator. This same thresholding level was also used to calculate the number of grains covering a labeled cell. The average pixel size from an acellular area was divided into the total labeled area above the threshold in a labeled cell to convert the thresholded values to an approximate number of grains above a cell. All images were scored by an investigator who was unaware of both sex and treatment condition.
Immunohistochemistry
CVS-treated (males, n = 5; females, n = 6) and control (males, n = 6; females, n = 7) mice were examined for levels of OT-ir and AVP-ir in the PVN and SON. At ~80 minutes after exposure to a final stressor (20 minutes of restraint within a plexiglass restraint tube in their home cages), the mice were anesthetized with isoflurane, then transcardially perfused with 0.1 M PBS, followed by 4% paraformaldehyde. The control mice were similarly exposed to 20 minutes of restraint stress within their home cage, which we determined did not alter the baseline OT-ir (Supplemental Fig. 2). The brains were collected and postfixed for 24 hours in 4% paraformaldehyde solution at 4°C, and infiltrated with sucrose by storage in a 30% sucrose solution at 4°C until equilibrated. The tissue was sectioned into four series at 35-μm thickness on a cryostat at −16°C and examined for OT-ir or AVP-ir. The tissue was washed in 0.1 M PBS, blocked in 5% normal donkey serum (Jackson Laboratory, Bar Harbor, ME) for 1 hour at room temperature, and incubated overnight in rabbit anti-OT (1:5000; catalog no. T-4083.0400; RRID: AB_518523; Peninsula Laboratories, San Carlos, CA) or rabbit anti-AVP (1:250; catalog no. AHP372; RRID: AB_2062093; Bio-Rad/AbD Serotec, Hercules, CA), primary antibodies that have previously been validated (43, 44). The next day, the tissue was washed in a PBS-Triton X solution, incubated in donkey anti-goat Alexa Fluor 568 or donkey anti-rabbit Alexa Fluor 680 (Jackson Laboratory) for 2 hours at room temperature. After washing in PBS-Triton X, the tissue was mounted onto glass slides and coverslipped with ProLong Gold Antifade Mountant (Thermo Fisher, Waltham, MA). Fluorescence was visualized using an LSM 880 confocal scanning microscope (Carl Zeiss Microscopy, Jena, Germany) using a 10× objective (Plan-Apochromat 10×/0.45∞/0.17; Carl Zeiss Microscopy), and 25-µm-thick Z-stacks (1-µm-thick optical sections) were taken through the PVN. For immunohistochemical analysis, the Z-stack images were evaluated using Imaris, version 8.0 (Bitplane, Concord, MA). For each mouse, two to three images per hemisphere were captured for the PVN and the SON. For each image, the number of OT-ir or AVP-ir cells was determined using an Imaris automated counting application and manually checked and scored by an investigator who was unaware of both sex and treatment condition. The values from all images were averaged together for each subject. The images of OT-ir were also examined for cell volume using an Imaris surface application. The mean volume of the OT neurons was calculated per image and averaged together for each subject.
Vaginal cytology
Beginning ~2 weeks before testing, the female mice were examined by vaginal lavage daily between 10:00 and 11:00 am for estrous cyclicity, as previously reported (45). In brief, each female mouse was lavaged daily with saline (0.1% NaCl) solution. The samples were allowed to dry on glass slides and dipped in methylene blue (0.05%) for visualization using light microscopy. The male mice were similarly briefly restrained daily to minimize any sex differences in habituation to handling.
Statistical analysis
All statistical analyses were performed using SPSS Statistics, version 20 (IBM Corp., Armonk, NY). mRNA and ir were examined using two-way (CVS treatment × sex) ANOVA. Behavioral testing was analyzed using both two-way (CVS treatment × sex) ANOVA and three-way (CVS treatment × sex × test) repeated measures ANOVA. Significant behavioral changes across the tests after repeated measures ANOVA were analyzed using post hoc contrasts. Significance was set at P < 0.05.
Results
Social behavior
Social interaction test
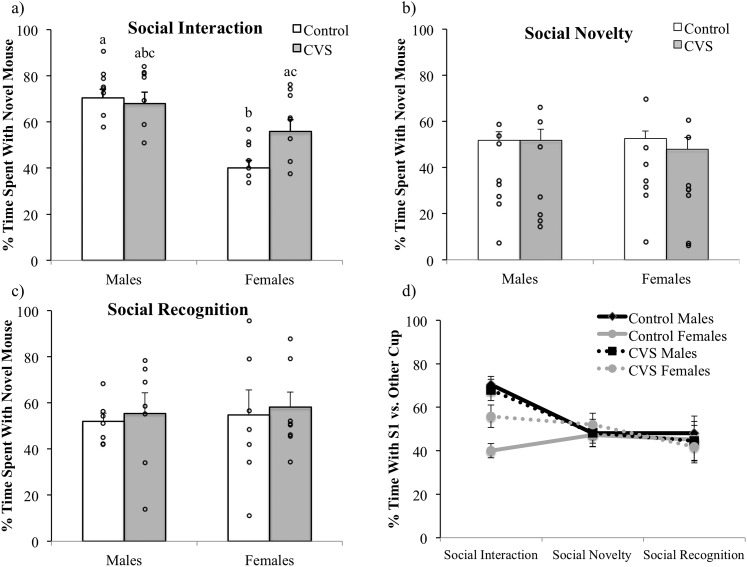
Sociability was assessed by examining the amount of time each mouse spent investigating a cup containing a novel mouse (S1) relative to an empty cup. Two-way ANOVA revealed a significant effect of sex, with male subjects spending a significantly greater percentage of time investigating the cup containing the stimulus animal S1 [expressed as (time with cup containing S1)/(total time with either cup) × 100; F(1,27) = 20.98; P < 0.001; Fig. 2a). In addition, a significant treatment by sex interaction was observed for this measure [F(1,27) = 5.53; P < 0.05], with a sex difference in the percentage of time spent with the cup containing S1 found for the control subjects but not between the CVS-treated male and female mice. For the total time in seconds spent with the cup containing S1, the analysis showed a main effect of sex [F(1,27) = 27.30; P < 0.001], with the males spending more time with S1. Furthermore, a trend toward an interaction between treatment and sex [F(1,27) = 4.04; P = 0.06] was observed. No significant differences were found between the groups in the number of visits to the cup containing S1 or in the latency to approach the cup containing S1. Examination of the total time in seconds spent away from both cups using two-way ANOVA showed that the female control mice [F(1,27) = 8.82; P < 0.01] and CVS-treated mice [F(1,27) = 4.19; P = 0.05] spent more time away from both cups.
Figure 2.
Social behaviors in the (a) social interaction test, (b) social novelty test, and (c) social recognition test in CVS-treated (males, n = 8; females, n = 10) and control (males, n = 8; females, n = 8) mice. (a) CVS exposure prevented the sex difference in social interaction, increasing the amount of time females spent investigating S1. No differences were observed in the (b) social novelty and (c) social recognition tests, (d) although only males appeared to show a preference for novelty, decreasing the amount of time spent with S1 relative to a novel mouse from the interaction test to the novelty and recognition tests. Each (a–c) bar and (d) point represents the mean and SEM of the percentage of time interacting with either cup preferentially spent with the novel stimulus mouse. Different lowercase letters denote significant comparisons; P < 0.05.
Social novelty test
To examine whether CVS treatment or sex would affect the subjects’ interest in a different novel mouse (S2) with the now-familiar mouse (S1) still present on the opposite side of the arena, the percentage of time spent with S2 [expressed as (time with cup containing S2)/(total time with cups containing S1 or S2) × 100] was analyzed using a two-way (CVS treatment × sex) ANOVA. No significant differences were observed in the percentage of time spent with novel mouse S2 (Fig. 2b), the total time in seconds spent with S2, the latency to visit the cup containing S2, or the total time spent away from both cups, although a tendency was found for the male mice to visit more with the cup containing S2 [F(1,26) = 3.50; P = 0.07].
Social recognition test
For the social recognition test, we assumed that the mice would spend a greater amount of time investigating a cup containing a novel stimulus mouse (S3) relative to a cup containing a familiar stimulus mouse (S1). Thus, the percentage of time spent with S3 [expressed as (time with cup containing S3)/(total time with cups containing S1 or S3) × 100] was analyzed using two-way (CVS treatment × sex) ANOVA. No significant differences were observed in the percentage of time spent with novel mouse S3 (Fig. 2c), the total time in seconds spent with S3, the latency to visit the cup containing S2, or the total time spent away from both cups. A significant effect of treatment [F(1,26) = 6.64; P < 0.05] was found for the number of visits to S3, with the CVS-treated mice visiting with S3 more often than the control mice.
Behaviors across tests
To assess the changes in subject behaviors across all three tests, a three-way (treatment × sex × test) ANOVA with repeated measures across test (time) was conducted, examining the percentage of time spent with the cup containing S1 of the total time spent with either cup (Fig. 2d). The results indicated a main effect of test [F(2,52) = 5.01; P < 0.05], test by sex interaction [F(2,52) = 3.78; P < 0.05], and a tendency toward an effect of sex (P = 0.06). Post hoc contrasts revealed that male mice spent significantly more time investigating S1 during the social interaction test than during the social novelty test (P < 0.0001) or the social recognition test (P < 0.01), with no differences between tests observable for the female mice.
In situ hybridization
Cell number and mRNA levels
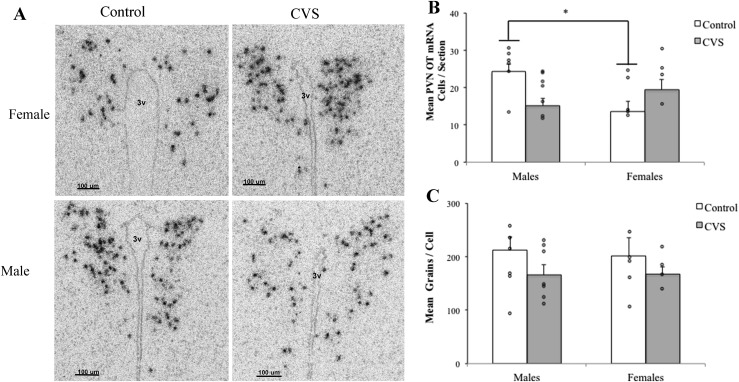
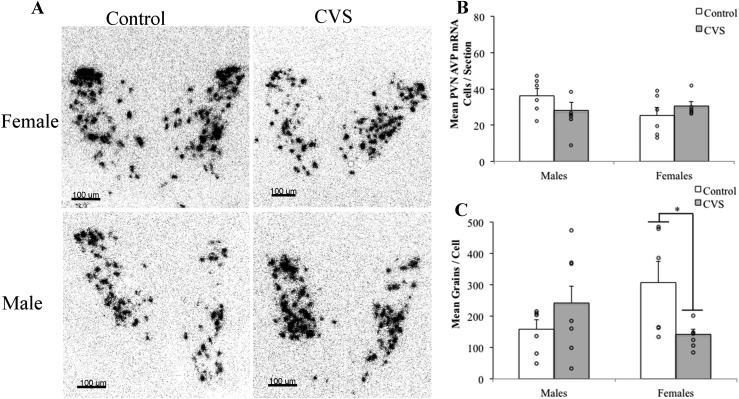
To examine changes in OT (Fig. 3) and AVP (Fig. 4) mRNA after CVS, two-way (CVS treatment × sex) ANOVA was performed for the mean PVN neuron number and the mean number of silver grains per cell. Analysis of OT mRNA revealed a significant treatment by sex interaction for the mean number of OT mRNA-containing cells [F(1,19) = 10.372; P < 0.01]. CVS decreased the number of OT mRNA-containing PVN neurons in males and removed the sex differences in the OT neuron number (Fig. 3B). In contrast, neither CVS exposure nor sex affected the mean number of OT mRNA silver grains per cell (Fig. 3C). Analysis of AVP mRNA revealed that, although CVS had no effect on the total number of PVN AVP mRNA-containing neurons, a significant interaction was found for treatment and sex on the average number of grains per cell [F(1,22) = 6.73; P < 0.05], with CVS decreasing the average number of AVP grains per cell in the female mice only (Fig. 4).
Figure 3.
The number of OT mRNA-containing neurons was reduced in the PVN of males after CVS. (A) Representative photomicrographs showing a greater number of OT mRNA neurons in nonstressed control males after in situ hybridization for OT mRNA in PVN (magnification ×20). (B) Quantitation of OT mRNA-expressing cell number per section or (C) average grains per cell. Each bar represents the mean and SEM of a unilateral PVN, averaged across three to six images encompassing the anterior, mid, and posterior subregions (n = 4 to 8 mice per group). Each point indicates the value for an individual mouse. (B) A significant interaction between treatment and sex indicated that CVS decreased the number of OT mRNA-containing PVN neurons in male mice, removing the sex differences in OT neuron number. (C) Neither CVS nor sex affected the mean number of grains per cell. *Significant sex difference (P < 0.05) in control groups. 3V, third ventricle.
Figure 4.
AVP mRNA was altered in the PVN of female mice after CVS. (A) Representative photomicrographs of PVN (magnification ×20) showing decreased silver grain numbers per cell in females after CVS. (B) CVS had no effect on the total number of PVN AVP neurons. (C) Two-way ANOVA revealed a significant interaction (P < 0.05) between treatment and sex. Post hoc analysis indicated that CVS decreased the cellular expression (grains per cell) in female subjects only (*P < 0.05). Each bar represents the mean and SEM of a unilateral PVN, averaged across three to six images per mouse, encompassing the anterior, mid, and posterior subregions (n = 4 to8 animals per group). Each point indicates the value for an individual subject. 3V, third ventricle.
Immunohistochemistry
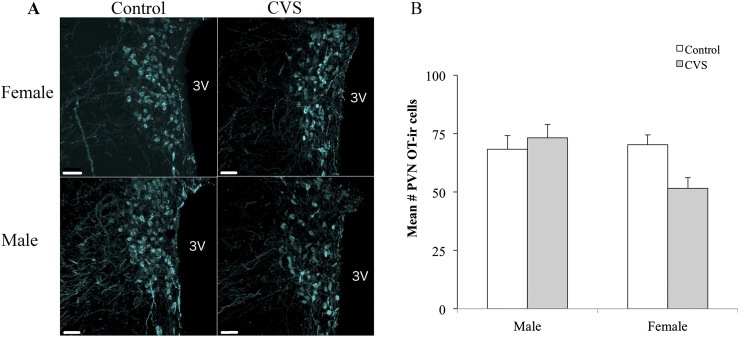
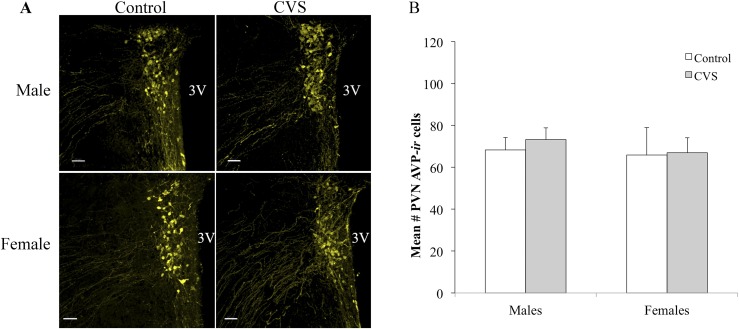
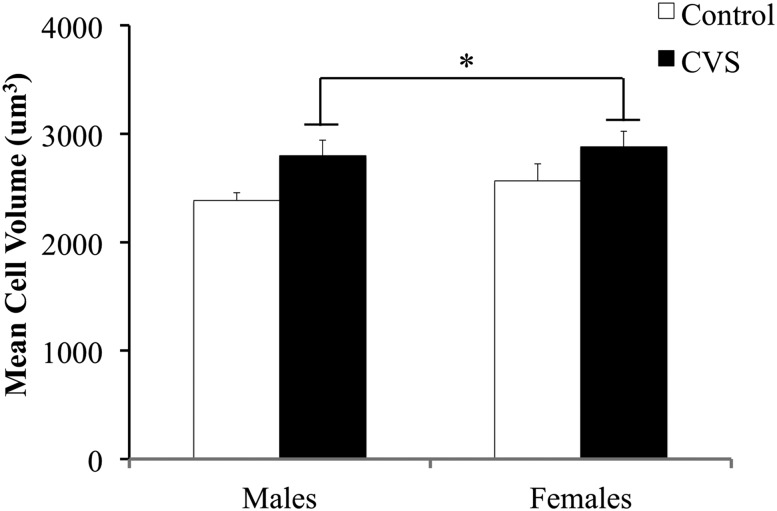
To assess the changes to AVP and OT protein expression after CVS, two-way (CVS treatment × sex) ANOVA was conducted analyzing the number of OT-immunoreactive and AVP-immunoreactive neurons within the PVN. A significant treatment by sex interaction was observed for PVN OT-ir [F(1,20) = 4.42; P < 0.05], with CVS-exposed female mice showing lower numbers of OT-immunoreactive neurons relative to the control females (Fig. 5). In contrast, no effect of sex or treatment was found on OT-immunoreactive neurons within the SON (control females: mean = 17.73 ± 1.95; CVS females: mean = 17.62 ± 1.71; control males: mean = 14.29 ± 1.60; CVS males: mean 17.89 ± 0.82). For AVP-ir, no effect of sex or treatment was found within the PVN (Fig. 6) or SON (control females: mean = 59.54 ± 7.53; CVS females: mean = 61.79 ± 5.80). Finally, the volume of OT neurons located in the PVN was examined for potential interacting effects of sex and CVS treatment using two-way (CVS treatment × sex) ANOVA. The neuronal volume increased after CVS [F(1,22) = 6.66; P < 0.05] but showed no significant differences between the male and female mice (Fig. 7).
Figure 5.
OT-ir was reduced in the PVN of female CVS-treated mice. (A) Representative photomicrographs showing reduced numbers of OT-immunoreactive neurons in the PVN. (B) Cell counts represent a unilateral PVN, averaged across three to six images per mouse encompassing the anterior, mid, and posterior subregions. Each bar represents the mean ± SEM (n = 5 to 7 mice per group). Each point indicates the value for an individual subject. 3V, third ventricle.
Figure 6.
CVS had no effect on the number of AVP-immunoreactive neurons in the PVN for male or female mice. (A) Photomicrographs showed no changes in the numbers of AVP-immunoreactive neurons in the PVN. (B) Cell counts represent a single hemisphere of the PVN, averaged across three to six images encompassing the anterior, mid, and posterior subregions. Each bar represents the mean ± SEM (n = 4 to 7 mice per group). Each point indicates the value for an individual subject. 3V, third ventricle.
Figure 7.
CVS increased OT neuronal volume in OT-immunoreactive neurons in the PVN. The mean volume obtained from all detectable neurons per image, averaged across three to six images of a single hemisphere of the PVN encompassing the anterior, mid, and posterior subregions. Each bar represents the mean ± SEM (n = 5 to 7 mice per group). 3V, third ventricle. *Effect of treatment; P < 0.05.
Discussion
Chronic exposure to stressors is associated with a number of consequences for human health (1–3) and is believed to increase susceptibility to diseases that often affect men and women differently (5, 6). In these experiments, we assessed the effects of CVS, a rodent model of chronic stress exposure (4), on both male and female C57BL/6 mice. This strain has traditionally been viewed as less susceptible to the effects of CVS (46, 47), although this conclusion has been based on the exclusive assessment of male mice. Consequently, in the present set of experiments, we investigated the effect of CVS on social behavior and the expression of the nonapeptides, OT and AVP, in male and female mice, which, in addition to their well-characterized effects on peripheral physiology, are also stress-sensitive hormones known to regulate social behavior (33, 34) and other central nervous system functions. Our results indicate that female C57BL/6 mice are more susceptible to the effects of CVS on both social behavior and AVP expression, resulting in a female-specific increase in sociability and a decrease in AVP mRNA levels within the PVN. Furthermore, we found a sex difference in OT mRNA-containing neurons in the PVN, with a greater number of OT-expressing cells identified in the control males relative to the females. CVS affected PVN OT mRNA and protein levels differently in the male and female mice, decreasing the number of OT mRNA-containing neurons exclusively in the male mice and decreasing the number of OT-immunoreactive cells in the female mice only. These findings reveal sex-specific effects of CVS exposure in the C57BL/6 mouse, refuting previous assumptions regarding the resilience of this strain to chronic stress based on data from males only and underscore the utility of the CVS model for uncovering sex-specific pathological changes to stress neurocircuitry and behavior.
Previous research has indicated that CVS exposure alters sociability, with reports of both increased (35) and decreased (37, 38) social interactions in the male rodent and a recent finding of increased sociability in the female mouse (36). Therefore, we investigated and compared the effects of CVS on social behavior in both male and female mice, an approach that has been largely overlooked in the reported data. Our observation that nonstressed control male mice appeared more sociable than control female mice is one that has been made previously for both mice (48) and rats (49), suggesting that male rodents typically show a greater preference for social interaction than do females. Exposure to CVS elevated the social interaction time in the female mice only, thereby removing the difference between the sexes in sociability. This female-specific increase in social interaction replicates recent work in the CD1 mouse (36) and is likely reflective of social buffering, a phenomenon in which socialization confers anxiolytic effects and reduces activation of the HPA axis (50). The strategy of seeking out social interaction as a coping mechanism has been proposed to occur more frequently and be more effective for women and female animal models (51, 52). The anxiolytic properties of social interaction are thought to be mediated by the release of OT in response to social stimulation (53). Thus, we next sought to determine whether CVS induced sex-specific alterations in the expression of OT and its structurally similar counterpart, AVP.
Parallel to our behavioral findings, the control male mice showed a larger number of OT mRNA-expressing neurons within the PVN relative to the control females. In addition, CVS caused a reduction in OT mRNA-expressing cells in the male mice but not the female mice. Although Dumais et al. (49) found no sex differences in the PVN OT mRNA levels in the rat, to the best of our knowledge, sex differences in mouse PVN OT mRNA levels have not been evaluated. Because Dumais et al. (49) determined the total PVN mRNA levels by measuring the optical density of hybridization over the entire PVN, we refined our analysis to examine the average number of silver grains per neuron, which is indicative of the relative levels of cellular expression. Although the number of silver grains per neuron was found to be similar between the groups, the control males had more PVN OT mRNA-expressing neurons than did the control females. This was not necessarily due to greater levels of OT synthesis per cell, because the grain counts revealed no sex differences. However, it might reflect increased synthesis in some OT neurons that were below the threshold of detection in the control mice. Thus, these data indicate that CVS might alter the number of PVN neurons that synthesize OT by increasing expression in only a population of OT neurons that are normally low-expressing neurons. As Althammer and Grinevich (54) have recently noted, the diversity in OT neuron type appears wider than previously believed, with variable genetic patterns, functions, and projections. It is therefore probable that the PVN OT neurons affected by CVS represent a specific subpopulation whose function and genetic profile have yet to be identified.
The CVS-induced decreases in OT mRNA in males and the sex difference in OT mRNA (females less than males) were not reflected by the absolute levels of peptide, because we found no sex differences in PVN OT-ir in our control mice. However, the effect of CVS on OT-ir was restricted to females. The presence of a greater number of neurons containing OT mRNA in male mice without corresponding changes in immunoreactivity suggests differences might be present in peptide storage vs mRNA. These differences in mRNA vs immunoreactive cell numbers can be explained by several potential mechanisms. One possibility is that the female mice synthesize less OT compared with the male mice, as reflected by decreased numbers of OT mRNA cells. In support of this hypothesis, male rats have previously been shown to have greater concentrations of plasma OT than female rats (55). However, these levels necessarily are dependent on secretion from OT neurons in the SON and the PVN. Alternatively, females might be more efficient at translating mRNA to peptide or turnover or trafficking of peptide might be different between the sexes. It appears that CVS upsets this balance, resulting in lower numbers of mRNA-expressing neurons in males and lower numbers of immunoreactive neurons in females. Whether this reduction in numbers of OT-immunoreactive neurons actually represents decreases in oxytocinergic neurotransmission remains to be determined; however, it is possible that reductions in OT-ir in females might represent increases in OT neurotransmission, as peptide is rapidly transported to axon terminals, thereby depleting immunoreactive OT from cell bodies. This latter hypothesis would be consistent with the increased social interaction seen in CVS-treated female mice.
In these studies, the female mice were intact and monitored for estrous cyclicity. Behavior testing was administered on a day of diestrus or estrus to minimize the effects of elevated levels of estradiol that occur on the morning of proestrus. The sex differences that we observed were therefore likely not the result of acute increases in estradiol but might reflect the cumulatively greater estradiol levels found in females compared with males. OT has been shown to be an estrogen-regulated gene (56, 57), with ~80% of PVN OT neurons expressing estrogen receptor (ER)β in the mouse (58). Moreover, ligands that bind ERβ have been shown to bind and upregulate the OT promoter in vitro (59). Sex differences in AVP levels can also be explained by estrogen exposure, because AVP is similarly regulated by estradiol (60). The levels of ERβ are highly correlated with PVN OT and AVP mRNA in the mouse, with a positive correlation additionally observed between ERα mRNA and levels of OT and AVP receptor in the medial amygdala, a region critical for social behavior (61). We have recently reported that female mice showed greater colocalization of ERβ with AVP neurons in the PVN (58). Thus, it is possible that CVS-induced sex differences in PVN AVP might stem from an ERβ-mediated response to CVS exposure. In contrast, it is also possible that sex differences can arise as a result of greater testosterone levels in males. However, the contribution of androgens to the effects we observed within the PVN is less certain, as, although AVP can be directly regulated by androgen receptors in a number of brain areas, this is likely not true for the PVN (62).
Despite the effect of CVS in reducing OT mRNA-containing PVN neurons in male subjects, the male mice showed no effect of stress exposure on OT-ir or on social interaction. This suggests that male mice might be less sensitive to the effects of changes in PVN OT levels on social behavior or that changes in OT-ir are a more sensitive measure for correlation with behavior. In either case, a threshold of OT expression likely exists that must be reached to identify an OT neuron as mRNA expressing or immunoreactive. The question arises regarding where this expression threshold is set in males compared with females and which approach (immunohistochemistry vs in situ hybridization) will most consistently identify the most OT neurons. Because our female subjects showed both increased sociability and decreased PVN OT-ir after CVS with no change in PVN OT mRNA expression, we hypothesized that these findings indicate a female-specific increase in OT neurosecretion from PVN neurons, ultimately inducing an increase in social interaction through OT projections to forebrain sites. PVN OT neurons project to a number of neural targets associated with the control of social behavior, including the medial amygdala and the central nucleus of the amygdala (63). This increased neurosecretion could generate a decrease in the number of neurons above the threshold of detection by immunohistochemistry through trafficking of neuropeptide from the cell soma to terminals. Of the OT neurons detectable by immunohistochemistry, CVS led to an increase in neuronal volume. Because chronic activation of OT neurons via dehydration or lactation induces cellular hypertrophy in this population (64), our morphological data support an increase in OT activity in response to CVS, albeit in both sexes. Future research is needed to determine the oxytocinergic pathways involved and corresponding OT receptor neuron plasticity after CVS in these regions.
Because AVP has been more commonly implicated in social recognition (34), a measure that was unaffected in our present study by CVS exposure, it was of interest to learn that CVS decreased PVN AVP mRNA in female mice only. This finding partially contradicts the results from a previous study that reported that CVS had no effect on PVN AVP mRNA using the female rat as a model (25) and suggests a species difference in the response of AVP to CVS. Research in the rat has indicated that female rats might be more sensitive to glucocorticoid regulation of AVP mRNA levels in the PVN (65). Thus, prolonged elevation in glucocorticoid levels due to chronic stress exposure (66) might have more of an impact on females’ PVN AVP expression. Our observed decrease in AVP mRNA in female mice after CVS, if translatable to a level of neurosecretion, might partially explain our subjects’ increase in sociability. Although less is known about the effects of AVP on social interaction, chronic social defeat, a paradigm that induces social avoidance, has upregulated PVN AVP mRNA in the male mouse (67). Furthermore, antagonism of the AVP receptor V1bR has been shown to increase sociability in male mice exposed to chronic social defeat (68), suggesting a role for this receptor in social aversion. Further investigation is needed to determine the functional implications of a female-specific decrease in PVN AVP mRNA after exposure to CVS and whether CVS induces a concomitant decrease in V1bR expression.
Several limitations exist within our present findings. The mice were exposed to three consecutive behavioral tests, assessing social interaction, the preference for a novel conspecific, and social recognition. Although the potential effect of stress from previous behavioral assessments is always a consideration with repeated testing (69), we opted to add analysis of social recognition to our adaptation of the social interaction/preference protocol to minimize previously observed sex- and estrous cycle-dependent effects on 24-hour social memory in the mouse (70). The utility of the social recognition test is reliant on rodents’ preference for novelty, which we did not observe in our subjects. The lack of preference for a novel social stimulus over a familiar one has previously been observed in male C57BL/6 mice and might be reflective of the mouse strain studied (71). Thus, any potential negative effects of previous behavioral testing on social memory were tempered by our inability to draw conclusions from our social recognition test. Nonetheless, our results on social recognition in the male mice are consistent with those previously observed in male C57BL/6 mice. Additionally, we chose to separate our subjects before behavioral testing as a method of encouraging social investigation. In contrast to previous investigation of sociability in female mice exposed to CVS (36), we limited the duration of isolation to 24 hours to minimize the period of isolation stress. However, further research is needed to parse out any potential effects of this manipulation. Finally, because we did not directly manipulate the OT or AVP levels in our subjects, the relationship between the alterations in these neuropeptides and social behavior is correlative in nature. This limitation does not diminish the implications of our present findings, which demonstrated that C57BL/6 mice exhibit a female-bias in vulnerability to the effects of CVS, with a significantly different response than that in male mice of the same strain.
In summary, female mice are uniquely affected by exposure to CVS. Specifically, female mice showed decreased PVN AVP mRNA and OT-ir, which might be indicative of elevated neurosecretion from this brain area. These changes to PVN neuropeptides likely contribute to the female-specific increase in social behavior after CVS. Because OT and AVP are both implicated in chronic stress-associated illnesses such as heart disease (72, 73) and metabolic syndrome (74, 75), the findings from the present study could have translational implications for the sex differences in the prevalence and pathophysiology observed for these disorders (76, 77). Our findings have demonstrated that the previously held assumption that C57BL/6 mice are resilient to CVS (46, 47) is likely reflective of a sex difference in the reactivity to CVS and the previously limited investigation of female subjects. Both rodents and humans have shown sex differences in the physiological response to stress and in the coping strategies used to mitigate it (51). The exclusive use of male subjects in stress research has, therefore, limited the conclusions that can be drawn about the response of a particular strain or species. Our present findings describe sex differences in the consequences of CVS exposure and further demonstrate the necessity of including female rodents in research using the CVS model of chronic stress.
Supplementary Material
Acknowledgments
Financial Support: This research was supported by the National Institutes of Health (Grant RO1-DK105826 to R.J. H.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AVP
arginine vasopressin
- CVS
chronic variable stress
- ER
estrogen receptor
- HPA
hypothalamic-pituitary-adrenal
- ir
immunoreactivity
- OT
oxytocin
- PVN
paraventricular nucleus
- S1
first stimulus mouse
- S2
second stimulus mouse
- S3
third stimulus mouse
- SON
supraoptic nucleus
References
- 1. Chandola T, Brunner E, Marmot M. Chronic stress at work and the metabolic syndrome: prospective study. BMJ. 2006;332(7540):521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol. 2008;51(13):1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Checkley S. The neuroendocrinology of depression and chronic stress. Br Med Bull. 1996;52(3):597–617. [DOI] [PubMed] [Google Scholar]
- 4. Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl). 1987;93(3):358–364. [DOI] [PubMed] [Google Scholar]
- 5. Regitz-Zagrosek V, Lehmkuhl E, Weickert MO. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin Res Cardiol. 2006;95(3):136–147. [DOI] [PubMed] [Google Scholar]
- 6. Altemus M. Sex differences in depression and anxiety disorders: potential biological determinants. Horm Behav. 2006;50(4):534–538. [DOI] [PubMed] [Google Scholar]
- 7. Herman JP. Neural control of chronic stress adaptation. Front Behav Neurosci. 2013;7:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ludwig M. Functional role of intrahypothalamic release of oxytocin and vasopressin: consequences and controversies. Am J Physiol. 1995;268(4 Pt 1):E537–E545. [DOI] [PubMed] [Google Scholar]
- 9. Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36(11):2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Albers HE. The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Horm Behav. 2012;61(3):283–292. [DOI] [PubMed] [Google Scholar]
- 11. McCann SM, Gutkowska J, Antunes-Rodrigues J. Neuroendocrine control of body fluid homeostasis. Braz J Med Biol Res. 2003;36(2):165–181. [DOI] [PubMed] [Google Scholar]
- 12. Gibbs DM, Vale W, Rivier J, Yen SS. Oxytocin potentiates the ACTH-releasing activity of CRF(41) but not vasopressin. Life Sci. 1984;34(23):2245–2249. [DOI] [PubMed] [Google Scholar]
- 13. Ochedalski T, Subburaju S, Wynn PC, Aguilera G. Interaction between oestrogen and oxytocin on hypothalamic-pituitary-adrenal axis activity. J Neuroendocrinol. 2007;19(3):189–197. [DOI] [PubMed] [Google Scholar]
- 14. Neumann ID, Krömer SA, Toschi N, Ebner K. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regul Pept. 2000;96(1-2):31–38. [DOI] [PubMed] [Google Scholar]
- 15. Evans MJ, Brett JT, McIntosh RP, McIntosh JE, McLay JL, Livesey JH, Donald RA. Characteristics of the ACTH response to repeated pulses of corticotrophin-releasing factor and arginine vasopressin in vitro. J Endocrinol. 1988;117(3):387–395. [DOI] [PubMed] [Google Scholar]
- 16. Miyata S, Itoh T, Lin S-H, Ishiyama M, Nakashima T, Kiyohara T. Temporal changes of c-fos expression in oxytocinergic magnocellular neuroendocrine cells of the rat hypothalamus with restraint stress. Brain Res Bull. 1995;37(4):391–395. [DOI] [PubMed] [Google Scholar]
- 17. Zavala JK, Fernandez AA, Gosselink KL. Female responses to acute and repeated restraint stress differ from those in males. Physiol Behav. 2011;104(2):215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams TD, Carter DA, Lightman SL. Sexual dimorphism in the posterior pituitary response to stress in the rat. Endocrinology. 1985;116(2):738–740. [DOI] [PubMed] [Google Scholar]
- 19. Torner L, Plotsky PM, Neumann ID, de Jong TR. Forced swimming-induced oxytocin release into blood and brain: effects of adrenalectomy and corticosterone treatment. Psychoneuroendocrinology. 2017;77:165–174. [DOI] [PubMed] [Google Scholar]
- 20. Bülbül M, Sinen O, Gemici B, İzgüt-Uysal VN. Opposite effects of central oxytocin and arginine vasopressin on changes in gastric motor function induced by chronic stress. Peptides. 2017;87:1–11. [DOI] [PubMed] [Google Scholar]
- 21. Neumann ID. Stimuli and consequences of dendritic release of oxytocin within the brain. Biochem Soc Trans. 2007;35(Pt 5):1252–1257. [DOI] [PubMed] [Google Scholar]
- 22. Ebner K, Wotjak CT, Landgraf R, Engelmann M. Forced swimming triggers vasopressin release within the amygdala to modulate stress-coping strategies in rats. Eur J Neurosci. 2002;15(2):384–388. [DOI] [PubMed] [Google Scholar]
- 23. Zheng J, Babygirija R, Bülbül M, Cerjak D, Ludwig K, Takahashi T. Hypothalamic oxytocin mediates adaptation mechanism against chronic stress in rats. Am J Physiol Gastrointest Liver Physiol. 2010;299(4):G946–G953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jankord R, Solomon MB, Albertz J, Flak JN, Zhang R, Herman JP. Stress vulnerability during adolescent development in rats. Endocrinology. 2011;152(2):629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lan N, Hellemans KG, Ellis L, Weinberg J. Exposure to chronic mild stress differentially alters corticotropin-releasing hormone and arginine vasopressin mRNA expression in the stress-responsive neurocircuitry of male and female rats prenatally exposed to alcohol. Alcohol Clin Exp Res. 2015;39(12):2414–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gray M, Innala L, Viau V. Central vasopressin V1A receptor blockade impedes hypothalamic-pituitary-adrenal habituation to repeated restraint stress exposure in adult male rats. Neuropsychopharmacology. 2012;37(12):2712–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Füchsl AM, Langgartner D, Reber SO. Mechanisms underlying the increased plasma ACTH levels in chronic psychosocially stressed male mice. PLoS One. 2013;8(12):e84161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peters S, Slattery DA, Uschold-Schmidt N, Reber SO, Neumann ID. Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology. 2014;42:225–236. [DOI] [PubMed] [Google Scholar]
- 29. Varga J, Domokos A, Barna I, Jankord R, Bagdy G, Zelena D. Lack of vasopressin does not prevent the behavioural and endocrine changes induced by chronic unpredictable stress. Brain Res Bull. 2011;84(1):45–52. [DOI] [PubMed] [Google Scholar]
- 30. Steinman MQ, Duque-Wilckens N, Greenberg GD, Hao R, Campi KL, Laredo SA, Laman-Maharg A, Manning CE, Doig IE, Lopez EM, Walch K, Bales KL, Trainor BC. Sex-specific effects of stress on oxytocin neurons correspond with responses to intranasal oxytocin. Biol Psychiatry. 2016;80(5):406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wulsin AC, Wick-Carlson D, Packard BA, Morano R, Herman JP. Adolescent chronic stress causes hypothalamo-pituitary-adrenocortical hypo-responsiveness and depression-like behavior in adult female rats. Psychoneuroendocrinology. 2016;65:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grassi D, Lagunas N, Calmarza-Font I, Diz-Chaves Y, Garcia-Segura LM, Panzica GC. Chronic unpredictable stress and long-term ovariectomy affect arginine-vasopressin expression in the paraventricular nucleus of adult female mice. Brain Res. 2014;1588:55–62. [DOI] [PubMed] [Google Scholar]
- 33. Campbell A. Attachment, aggression and affiliation: the role of oxytocin in female social behavior. Biol Psychol. 2008;77(1):1–10. [DOI] [PubMed] [Google Scholar]
- 34. Gabor CS, Phan A, Clipperton-Allen AE, Kavaliers M, Choleris E. Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behav Neurosci. 2012;126(1):97–109. [DOI] [PubMed] [Google Scholar]
- 35. Matrov D, Vonk A, Herm L, Rinken A, Harro J. Activating effects of chronic variable stress in rats with different exploratory activity: association with dopamine d(1) receptor function in nucleus accumbens. Neuropsychobiology. 2011;64(2):110–122. [DOI] [PubMed] [Google Scholar]
- 36. Dadomo H, Gioiosa L, Cigalotti J, Ceresini G, Parmigiani S, Palanza P. What is stressful for females? Differential effects of unpredictable environmental or social stress in CD1 female mice. Horm Behav. 2018;98:22–32. [DOI] [PubMed] [Google Scholar]
- 37. Kompagne H, Bárdos G, Szénási G, Gacsályi I, Hársing LG, Lévay G. Chronic mild stress generates clear depressive but ambiguous anxiety-like behaviour in rats. Behav Brain Res. 2008;193(2):311–314. [DOI] [PubMed] [Google Scholar]
- 38. Erburu M, Cajaleon L, Guruceaga E, Venzala E, Muñoz-Cobo I, Beltrán E, Puerta E, Tordera RM. Chronic mild stress and imipramine treatment elicit opposite changes in behavior and in gene expression in the mouse prefrontal cortex. Pharmacol Biochem Behav. 2015;135:227–236. [DOI] [PubMed] [Google Scholar]
- 39. Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175(1):43–50. [DOI] [PubMed] [Google Scholar]
- 40. Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17β throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94(6):1704–1708. [DOI] [PubMed] [Google Scholar]
- 41. Niesink RJ, van Ree JM. Short-term isolation increases social interactions of male rats: a parametric analysis. Physiol Behav. 1982;29(5):819–825. [DOI] [PubMed] [Google Scholar]
- 42. Li Y, McGivern RF, Nagahara AH, Handa RJ. Alterations in the estrogen sensitivity of hypothalamic proenkephalin mRNA expression with age and prenatal exposure to alcohol. Brain Res Mol Brain Res. 1997;47(1-2):215–222. [DOI] [PubMed] [Google Scholar]
- 43. Tanaka M, Cummins TR, Ishikawa K, Black JA, Ibata Y, Waxman SG. Molecular and functional remodeling of electrogenic membrane of hypothalamic neurons in response to changes in their input. Proc Natl Acad Sci USA. 1999;96(3):1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suzuki S, Handa RJ. Estrogen receptor-beta, but not estrogen receptor-alpha, is expressed in prolactin neurons of the female rat paraventricular and supraoptic nuclei: comparison with other neuropeptides. J Comp Neurol. 2005;484(1):28–42. [DOI] [PubMed] [Google Scholar]
- 45. Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol 2002;62(4a):609–614. [DOI] [PubMed] [Google Scholar]
- 46. Ducottet C, Belzung C. Correlations between behaviours in the elevated plus-maze and sensitivity to unpredictable subchronic mild stress: evidence from inbred strains of mice. Behav Brain Res. 2005;156(1):153–162. [DOI] [PubMed] [Google Scholar]
- 47. Tannenbaum B, Anisman H. Impact of chronic intermittent challenges in stressor-susceptible and resilient strains of mice. Biol Psychiatry. 2003;53(4):292–303. [DOI] [PubMed] [Google Scholar]
- 48. McPhie-Lalmansingh AA, Tejada LD, Weaver JL, Rissman EF. Sex chromosome complement affects social interactions in mice. Horm Behav. 2008;54(4):565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dumais KM, Bredewold R, Mayer TE, Veenema AH. Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex- specific ways. Horm Behav. 2013;64(4):693–701. [DOI] [PubMed] [Google Scholar]
- 50. Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychol Bull. 2014;140(1):256–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107(3):411–429. [DOI] [PubMed] [Google Scholar]
- 52. Tzeng WY, Wu HH, Wang CY, Chen JC, Yu L, Cherng CG. Sex differences in stress and group housing effects on the number of newly proliferated cells and neuroblasts in middle-aged dentate gyrus. Front Behav Neurosci. 2017;10:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54(12):1389–1398. [DOI] [PubMed] [Google Scholar]
- 54. Althammer F, Grinevich V. Diversity of oxytocin neurons: beyond magno- and parvocellular cell types? J Neuroendocrinol. 2017;e12549. [DOI] [PubMed]
- 55. Windle RJ, Forsling ML. Variations in oxytocin secretion during the 4-day oestrous cycle of the rat. J Endocrinol. 1993;136(2):305–311. [DOI] [PubMed] [Google Scholar]
- 56. Richard S, Zingg HH. The human oxytocin gene promoter is regulated by estrogens. J Biol Chem. 1990;265(11):6098–6103. [PubMed] [Google Scholar]
- 57. Burbach JP, Lopes da Silva S, Cox JJ, Adan RA, Cooney AJ, Tsai M-J, Tsai SY. Repression of estrogen-dependent stimulation of the oxytocin gene by chicken ovalbumin upstream promoter transcription factor I. J Biol Chem. 1994;269(21):15046–15053. [PubMed] [Google Scholar]
- 58. Oyola MG, Thompson MK, Handa AZ, Handa RJ. Distribution and chemical composition of estrogen receptor β neurons in the paraventricular nucleus of the female and male mouse hypothalamus. J Comp Neurol. 2017;525(17):3666–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hiroi R, Lacagnina AF, Hinds LR, Carbone DG, Uht RM, Handa RJ. The androgen metabolite, 5α-androstane-3β,17β-diol (3β-diol), activates the oxytocin promoter through an estrogen receptor-β pathway. Endocrinology. 2013;154(5):1802–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nomura M, McKenna E, Korach KS, Pfaff DW, Ogawa S. Estrogen receptor-β regulates transcript levels for oxytocin and arginine vasopressin in the hypothalamic paraventricular nucleus of male mice. Brain Res Mol Brain Res. 2002;109(1-2):84–94. [DOI] [PubMed] [Google Scholar]
- 61. Murakami G, Hunter RG, Fontaine C, Ribeiro A, Pfaff D. Relationships among estrogen receptor, oxytocin and vasopressin gene expression and social interaction in male mice. Eur J Neurosci. 2011;34(3):469–477. [DOI] [PubMed] [Google Scholar]
- 62. Viau V, Soriano L, Dallman MF. Androgens alter corticotropin releasing hormone and arginine vasopressin mRNA within forebrain sites known to regulate activity in the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2001;13(5):442–452. [DOI] [PubMed] [Google Scholar]
- 63. Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73(3):553–566. [DOI] [PubMed] [Google Scholar]
- 64. Lin S-H, Miyata S, Kawarabayashi T, Nakashima T, Kiyohara T. Hypertrophy of oxytocinergic magnocellular neurons in the hypothalamic supraoptic nucleus from gestation to lactation. Zool Sci. 1996;13(1):161–165. [DOI] [PubMed] [Google Scholar]
- 65. Ferrini MG, Grillo CA, Piroli G, de Kloet ER, De Nicola AF. Sex difference in glucocorticoid regulation of vasopressin mRNA in the paraventricular hypothalamic nucleus. Cell Mol Neurobiol. 1997;17(6):671–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Marin MT, Cruz FC, Planeta CS. Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiol Behav. 2007;90(1):29–35. [DOI] [PubMed] [Google Scholar]
- 67. Keeney A, Jessop DS, Harbuz MS, Marsden CA, Hogg S, Blackburn-Munro RE. Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary-adrenal axis function and hippocampal serotonin release in mice. J Neuroendocrinol. 2006;18(5):330–338. [DOI] [PubMed] [Google Scholar]
- 68. Litvin Y, Murakami G, Pfaff DW. Effects of chronic social defeat on behavioral and neural correlates of sociality: vasopressin, oxytocin and the vasopressinergic V1b receptor. Physiol Behav. 2011;103(3-4):393–403. [DOI] [PubMed] [Google Scholar]
- 69. Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S. The use of behavioral test batteries, II: effect of test interval. Physiol Behav. 2006;87(1):95–102. [DOI] [PubMed] [Google Scholar]
- 70. Sánchez-Andrade G, Kendrick KM. Roles of α- and β-estrogen receptors in mouse social recognition memory: effects of gender and the estrous cycle. Horm Behav. 2011;59(1):114–122. [DOI] [PubMed] [Google Scholar]
- 71. Pearson BL, Defensor EB, Blanchard DC, Blanchard RJ. C57BL/6J mice fail to exhibit preference for social novelty in the three-chamber apparatus. Behav Brain Res. 2010;213(2):189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chen X, Lu G, Tang K, Li Q, Gao X. The secretion patterns and roles of cardiac and circulating arginine vasopressin during the development of heart failure. Neuropeptides. 2015;51:63–73. [DOI] [PubMed] [Google Scholar]
- 73. Moghimian M, Faghihi M, Karimian SM, Imani A, Houshmand F, Azizi Y. Role of central oxytocin in stress-induced cardioprotection in ischemic-reperfused heart model. J Cardiol. 2013;61(1):79–86. [DOI] [PubMed] [Google Scholar]
- 74. Melander O. Vasopressin, from regulator to disease predictor for diabetes and cardiometabolic risk. Ann Nutr Metab. 2016;68(Suppl 2):24–28. [DOI] [PubMed] [Google Scholar]
- 75. Quintana DS, Dieset I, Elvsåshagen T, Westlye LT, Andreassen OA. Oxytocin system dysfunction as a common mechanism underlying metabolic syndrome and psychiatric symptoms in schizophrenia and bipolar disorders. Front Neuroendocrinol. 2017;45:1–10. [DOI] [PubMed] [Google Scholar]
- 76. Rochlani Y, Pothineni NV, Mehta JL. Metabolic syndrome: does it differ between women and men? Cardiovasc Drugs Ther. 2015;29(4):329–338. [DOI] [PubMed] [Google Scholar]
- 77. Sanghavi M, Gulati M. Sex differences in the pathophysiology, treatment, and outcomes in IHD. Curr Atheroscler Rep. 2015;17(6):511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.