Abstract
Background
The prevalence of cigarette smoking is significantly higher among those living at or below the federal poverty level. Cell phone-based interventions among such populations have the potential to reduce smoking rates and be cost-effective.
Methods
We performed a cost-effectiveness analysis of three smoking cessation interventions: Standard Care (SC) (brief advice to quit, nicotine replacement therapy and self-help written materials), Enhanced Care (EC) (SC plus cell phone-delivered messaging) and Intensive Care (IC) (EC plus cell phone-delivered counselling). Quit rates were obtained from Project ACTION (Adult smoking Cessation Treatment through Innovative Outreach to Neighborhoods). We evaluated shorter-term outcomes of cost per quit and long-term outcomes using cost per quality-adjusted life year (QALY).
Results
For men, EC cost an additional $541 per quit vs SC; however, IC cost an additional $5232 per quit vs EC. For women, EC was weakly dominated by IC—IC cost an additional $1092 per quit vs SC. Similarly, for men, EC had incremental cost-effectiveness ratio (ICER) of $426 per QALY gained vs SC; however, IC resulted in ICER of $4127 per QALY gained vs EC. For women, EC was weakly dominated; the ICER of IC vs SC was $1251 per QALY gained. The ICER was below maximum acceptable willingness-to-pay threshold of $50 000 per QALY under all alternative modelling assumptions.
Discussion
Cell phone interventions for low socioeconomic groups are a cost-effective use of healthcare resources. Intensive Care was the most cost-effective strategy both for men and women.
Trial registration number
; Results.
INTRODUCTION
Smoking cigarettes and tobacco use remain the largest preventable causes of morbidity and mortality in the USA, even though there has been a decrease in usage over the past decade.1 From 2005 to 2015, smoking prevalence in US adults dropped from 20.9% to 15.1%, but this decline has not been equally distributed across the population.1 The percentage of smokers in underprivileged communities is above average. For example, in 2015, 26.1% of people living below the federal poverty line were smokers compared with 13.9% of those living at or above the federal poverty line.1 These groups do not have readily available access to traditional smoking cessation strategies, such as nicotine replacement therapy, and access to general information on how to quit smoking, making it harder to quit.2 Providing cell phone-based cessation interventions may be a feasible and cost-effective strategy to reach these individuals.
A study by the Pew Research Center found that 95% of Americans own a cell phone and 92% of households that make less than $30 000 a year own one,3 suggesting that cell phone-delivered smoking cessation interventions might be an appropriate approach to reach underprivileged communities. Recent evidence from a Cochrane review indicates that cell phone-delivered smoking cessation treatments are efficacious.4 However, few studies included in this review targeted underserved, economically disadvantaged smokers.4 Another meta-analysis of studies that evaluated cell phones-based smoking cessation interventions found that the use cell phones to provide support via text messages in addition to traditional smoking cessation strategies resulted in quit rates of 36% higher than programmes that use only traditional smoking cessation strategies.5 Another meta-analysis found that behavioural support, in the form of supportive counselling delivered either over the phone or in person, can increase the chance of quitting up to 25%.6
To our knowledge, though studies have determined the effectiveness of cell phone-based smoking cessation interventions to improve quit rates among smokers, no study has estimated the cost-effectiveness of such interventions in the USA. The purpose of this study was to perform cost-effectiveness analysis (CEA) of smoking cessation interventions that included cell phone messaging and/or counselling.
METHODS
Overview of Project ACTION
The University of Texas MD Anderson Cancer Center approved the implementation of Project ACTION (Adult smoking Cessation Treatment through Innovative Outreach to Neighbors) to assess the efficacy of cell phones in helping people quit smoking in the Houston (Texas) area. While details about the study design of this project have been previously published,2 we will highlight the key concepts and study design here, along with the data relevant to our study. Project ACTION was designed to be easily accessible to those living at or below the poverty line. The project used a group-randomised design to test the efficacy of three different smoking-cessation interventions (clinicaltrials.gov ): (1) Standard Care (SC)—brief advice to quit smoking, nicotine replacement therapy and written self-help materials; (2) Enhanced Care (EC)—SC plus a cell phone-delivered text/graphic messaging component and (3) Intensive Care (IC)—EC plus a series of 11 cell phone-delivered proactive counselling sessions.
Intervention components
The SC approach consisted of general advice to quit smoking from a healthcare professional, self-help materials and nicotine replacement therapy. Nicotine replacement therapy included three boxes of 21 mg patches for 6 weeks use, a box of 14 mg patches for 2 weeks use and a box of 7 mg patches for 2 weeks use. The general advice to quit smoking, self-help materials and nicotine replacement therapy were administered by a healthcare professional at the start of enrolment.
The EC comprised the components in SC plus the use of cell phone-delivered text/graphic messages and access to a 24/7 quit smoking hotline. Messages started the week of participants’ scheduled quit date and continued for a 12-week period. During the first week after the quit date, participants received five messages a day. The number of messages gradually declined to one message per day by week 4 and stayed at this level until the end of the receipt of the intervention at week 12. The messages were designed to increase health knowledge, maintain/increase quit motivation, promote coping skills use and increase social support.
The IC included the components in EC and the addition of 11 scheduled over the phone counselling sessions over the 12-week treatment period. The first session took place 1 day prior to the quit date, the next four sessions were scheduled during the first week after quitting and the remaining six sessions were scheduled every other week until treatment ended. On average, each session lasted 15 min.
Participants
Participants for Project ACTION were 18 years of age or older, English or Spanish speaking, smoked at least 100 cigarettes during their lifetime, smoked at least 5 cigarettes per day on average and were willing to set a quit smoking date within a week from the date of enrollment.
Project ACTION recruited 626 participants from various neighbourhood sites (ie, community centres, churches and public housing complexes) throughout the large metropolitan area of Houston, Texas.2 Community sites were then randomly assigned to receive one of the three interventions. Though assignments were random, sites were stratified by type (ie, church, community centre and public housing complex) and racial/ethnic composition to have a balanced cohort in each intervention. Out of the 626 participants, 223 randomly received SC, 215 received ECi and 188 received IC.
Follow-up and data collection
All active participants were followed for 6 months after enrollment and were asked how their smoking habits had changed by cell phone assessments. All participants were given a $10 gift card for completing each of the monthly follow-ups. These gift cards were not included as a part of the CEA as they were deemed to be costs of performing the research.
Perspective for cost-effectiveness analysis
To perform CEA, we adopted a societal perspective, that is, we incorporated all costs and health effects incurred by healthcare systems as well as participants participating in the smoking cessation interventions, for example, work time loss.
Decision-analytic model
Two decision-analytic models were used to assess cost-effectiveness.7 Similar models and procedures were used in Cantor et al.8 The first model evaluated cost per successful quit. The second model estimated life expectancy and quality-adjusted life expectancy measured as quality-adjusted life years (QALYs) using quit rates from the study. The model estimating QALYs includes a lifetime time horizon to capture the long-term benefits gained by successful smoking cessation, as prescribed by the Panel for Cost-Effectiveness in Health and Medicine.9,10
Model parameters
Model parameters, including quit rates, were obtained from the data collected in the study and from other medical literature. Quit rates specific to intervention were stratified by gender (table 1).
Table 1.
Decision-analytic model parameters
| Parameters Quit rates (Intervention) |
Men % (95% CI) | Women % (95% CI) | Source |
|---|---|---|---|
| Standard Care | 33.04% (21.82% to 38.00%) | 36.94% (25.20% to 42.00%) | Clinical trial |
| Enhanced Care | 41.12% (29.62% to 47.71%) | 38.68% (27.71% to 45.88%) | |
| Intensive Care | 42.70% (36.25% to 60.26%) | 48.48% (38.70% to 59.34%) | |
| Proportion of smoking type | Men | Women | Rogers et al13 |
| Heavy smokers (>2 packs a day) | 2.92% | 1.89% | |
| Moderate smokers (1–2 packs a day) | 51.30% | 49.84% | |
| Light smokers (<1 pack a day) | 45.78% | 48.26% | |
| Life expectancy (for current and former smokers) | Rogers et al13 | ||
| Health-related utilities | Fiscella and Franks14 |
Quit rates were calculated by using the self-report quit status obtained at 6 months after study enrolment. This is common practice among other community-based studies on smoking cessation programmes.11,12 Participants who dropped out of the study before the 6-month follow-up were regarded as smokers who failed to quit (ie, intention-to-treat analysis). (Note: The two participants whose baseline surveys were missing were not included in the quit rates broken down by sex.)
The second decision-analytic model evaluated long-term health benefits from quitting smoking. Mortality rates for smokers and quitters were obtained from Rogers et al.13 Similar to Rogers et al, in our model, we stratified the gender-specific mortality rates by intensity—heavy (>2 packs a day), moderate (1–2 packs a day) and light (<1 packs a day)—of smoking for former/current smoker. Based on the intensity of smoking and current smoking status, our model followed individuals over their lifetime to estimate intervention-specific (ie, considering quit rates) long-term outcomes of life expectancy, quality-adjusted life expectancy and cost-effectiveness.
Utilities
The quality of life scores (ie, utilities) to evaluate QALYs for the various health outcomes were obtained from Fiscella and Franks.14 The utility scores were reported by sex and whether or not an individual is a current or former smoker (defined here as having quit smoking for 15 years or more), with former smokers having higher utilities. The model incorporates an increasing benefit in utility for every year that a former smoker has quit up to 15 years. The incremental utility scores did not vary by smoking intensity and the utility values are constant after the age of 70.
Cost of interventions
The decision-analytic models considered cost of interventions per participant. All costs are reported in 2014 US dollars, which was the year of the interventions. The analysis did not include the developmental costs for the text message system, following the guidelines of the US Health Panel on Cost-Effectiveness in Health and Medicine, as it was considered to be a one-time (sunk) cost.15Table 2 shows the breakdown of the cost calculations.
Table 2.
Intervention costs (US$ 2014) for Project ACTION (per participant)
| Components | Standard Care (n=223) | Enhanced Care (n=213) | Intensive Care (n=188) |
|---|---|---|---|
| Nicotine replacement therapy | $100.00 | $100.00 | $100.00* |
| Health brochures | $0.25 | $0.25 | $0.25† |
| Healthcare professional’s time | $3.05 | $3.05 | $3.05‡ |
| Participant’s time with a healthcare professional | $0.60 | $0.60 | $0.60§ |
| Hotline operator | – | $29.14 | $29.14¶ |
| Text message system technician | – | $14.57 | $14.57** |
| Phone counsellor | – | – | $62.47†† |
| Participant’s time on the phone | – | – | $19.94‡‡ |
| Total | $103.90 | $147.61 | $230.02 |
The nicotine replacement therapy was five boxes of nicotine patches.
The health brochures’ cost reflects printing and distribution cost.
The cost for the 5 min conversation with a healthcare professional time was determined by the hourly rate for a healthcare professional, $36.54, reported by the Bureau of Labor Statistics in 2014.
The cost for the participant to see a healthcare professional for a 5 min conversation was considered at Texas’s minimum wage in 2014, $7.25. Participant’s time with a healthcare professional. Conversations on average lasted 5 min.
The hotline operator was paid a salary of $11 744 including 28% fringe benefits over a 3-month period. The cost was divided by the number of participants in the Enhanced Care and Intensive Care groups.
The text message system technician was considered part-time and paid a salary of $5872 including 28% fringe benefits over a 3-month period. The cost was divided by the number of participants in the Enhanced Care and Intensive Care groups.
The phone counsellor was paid a salary of $11 744 including 28% fringe benefits over a 3-month period. The cost was divided by the number of participants in the Intensive Care group.
Participants in the Intensive Care group had 11 phone counselling sessions, which on average lasted 15 min long (range 10–20 min). Time was considered at Texas minimum wage, $7.25.
ACTION, Adult smoking Cessation Treatment through Innovative Outreach to Neighborhoods.
SC includes the cost of the health brochures, nicotine replacement therapy, the time of the healthcare professional and the participant’s time (based on minimum wage) during the 5 min conversation on quitting smoking. EC includes the costs of the components in the SC intervention, along with the cost for the hotline operator and text message system technician. IC includes the cost for EC with the additional cost for the phone counsellor and participant’s time during the sessions. The total cost per participant for SC, EC and IC was $103.90, $147.61 and $230.02, respectively.
A CEA from a societal perspective is a comprehensive approach that includes both direct healthcare costs as well as time costs, which places a value on the time participants were seeking healthcare. Our model includes the costs for the time participants spent in different components of the intervention(s). Specifically, the conversation with a healthcare professional and the counselling with a phone counsellor was considered to be an opportunity cost. In addition, it should be noted that although all components of the smoking cessation interventions were provided free of charge to all participants, these resources were included in the economic analysis.
Analysis
Incremental cost-effectiveness ratios (ICERs), the ratio of the difference in costs between the interventions to the difference in effectiveness between the same interventions, were used to evaluate cost-effectiveness. The cost-per-quit model estimated the ICER in terms of incremental cost per additional quit, and the model estimating longitudinal outcomes determined the ICER in terms of US dollars per QALY gained. Economic and clinical outcomes were discounted at a rate of 3%, as recommended.16
TreeAge Pro 2017 (TreeAge Pro 2017, R2.1. TreeAge Software, Williamstown, Massachusetts, USA; software available at http://www.treeage.com) was used to model the CEA. Our base case was a 45-year-old male or female smoker, based on the mean age of the cohort. Sensitivity analyses focused on the age of potential programme participants and the cost of intervention. We also varied quit rates of using 95% CIs and estimated cost-effectiveness in a two-way sensitivity analysis.
RESULTS
In the cost per quit analysis, irrespective of gender, the ICER for EC versus SC was $887 per additional quit and the ICER for IC versus EC was $1411 per additional quit. For men, the ICER for EC versus SC was $541 per additional quit and the ICER for IC versus EC was $5232 per additional quit. However, for women, the model shows that the EC strategy was extended dominated by the IC strategy, meaning that the ICER between EC over SC is greater than the ICER for IC over EC. The logical argument that comes from extended dominance is that if you would be willing to pay a larger amount for added effectiveness, you would definitely be willing to pay less for a larger amount of added effectiveness.17 Therefore, EC was not considered for further evaluation and the resulting ICER for IC versus SC for women was $1092 per additional quit.
As for long-term outcomes, the base case for 45-year-old men enrolled in the EC strategy yielded an additional 0.10 of QALYs over men in the SC strategy, while men in the IC strategy had a 0.02 increase in QALYs over men in the EC strategy (table 3). This resulted in 14.27 QALYs for men in SC and approximately 14.37 QALYs for men in both EC and IC. Overall life expectancy for men in SC is 32.70 years, 33.15 years for those in EC and 33.24 years for men in IC. The ICER for men enrolled in EC over those in SC is $426 per QALY gained and the ICER for men in IC over EC is $4127 per QALY gained.
Table 3.
Results for cohorts of Project ACTIONa
| Cost | Incr Cost | Eff | Incr Eff | ICER | |
|---|---|---|---|---|---|
| Base case results | |||||
| Men | |||||
| Standard Care | $103.90 | – | 14.27 | – | – |
| Enhanced Care | $147.61 | $43.71 | 14.37 | 0.10 | $426 |
| Intensive Care | $230.02 | $82.41 | 14.39 | 0.02 | $4127 |
| Women | |||||
| Standard Care | $103.90 | – | 15.17 | – | – |
| Enhanced Care | $147.61 | – | 15.19 | – | Ext. dominated |
| Intensive Care | $230.02 | $126.12 | 15.27 | 0.10 | $1251 |
Results are presented in the base case for cohorts, of a 45-year-old man or 45-year-old woman discounted at 3%.
ACTION, Adult Smoking Cessation Treatment through Innovative Outreach to Neighborhoods. ICER, incremental cost-effectiveness ratio, evaluated in discounted dollars per quality-adjusted life year. Incr Costs, incremental cost; Incr Eff, incremental effectiveness, evaluated in discounted quality-adjusted life years; Ext. dominated, extended dominated, an alternative strategy dominates in an extended sense over an alternative strategy; Eff, effectiveness, evaluated in discounted quality-adjusted life years.
The base case for 45-year-old women enrolled in the IC strategy gained an additional 0.10 QALY over women enrolled in the SC strategy (table 3). Women in SC are expected to have 15.27 QALYs, while women in IC are expected to have 15.27 QALYs. Overall life expectancy for women in SC is 36.06 years and 36.51 years for women in IC. The ICER for women enrolled in the IC over those in SC is $1251 per QALY gained.
Sensitivity analysis
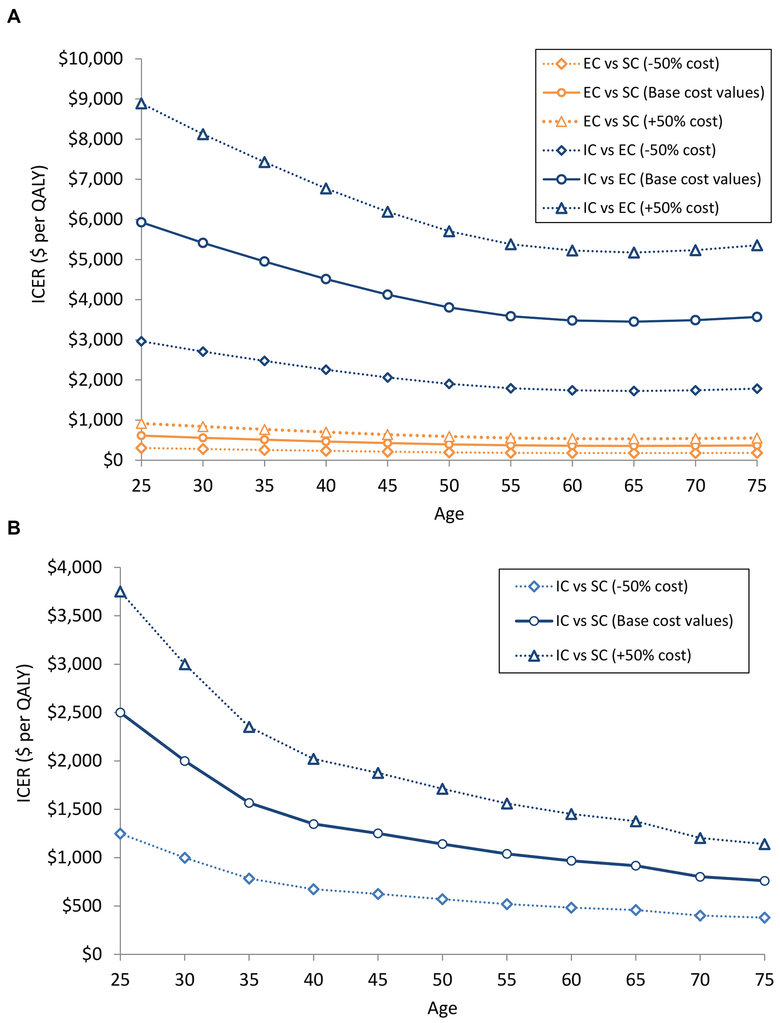
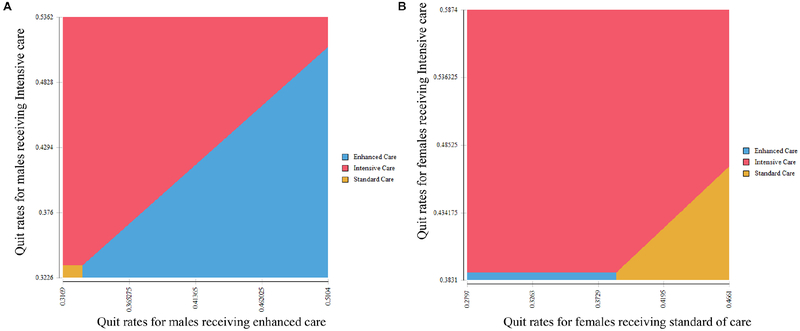
Sensitivity analysis on the QALY model shows the ICER of the interventions for men and women varying age and intervention cost (figure 1A and B). When we varied the cost values within the range of ±50%, we found that the policy conclusion of our study remained unaltered, that is, Intensive Care was a most cost-effective strategy among men and women across all age groups. Figure 1A and B reports the ICER for men enrolled in EC compared with SC and women enrolled in IC compared with SC. In both men and women, the ICER for interventions decreases the older the participant. In two-way sensitivity analysis (figure 2A and B), we found that the most cost-effective strategy changed with the change in the linear combination of the quit rates.
Figure 1.
Two-way sensitivity analysis. Figure shows the ICER, measured in dollars per quality-adjusted life year, for (A) men and (B) women, with variations on intervention costs and age of the hypothetical cohort. Results shown are ICERs based on increasing and decreasing intervention component costs by 50%. EC, Enhanced Care; IC, Intensive Care; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year; SC, Standard Care.
Figure 2.
Two-way sensitivity analysis for 45-year-old cohort of (A) men and (B) women. Figures show the cost-effective strategy based on a threshold of $50 000 per QALY gained as a linear combination of 6-month quit rates for the undominated strategies (determined in the base case analysis) vary. Base case values for quit rates and 95% CIs can be found in table 1. QALY, quality-adjusted life year.
DISCUSSION
In this study, using data from clinical trial and epidemiological studies, we determined the cost-effectiveness of cell phone-based smoking cessation interventions. IC was the most successful at getting participants to quit smoking, more cost-effective and resulted in the most quality-adjusted life-years among men and women, compared with EC and SC. The incremental cost-effective ratios (ICERs) were less than the willingness-to-pay threshold of $50 000 per QALY, indicating that even the most resource intensive cell phone-based intervention was cost-effective.18 We would recommend that a smoking-cessation programme that uses cell phones to deliver support be modelled after IC for a general population or one specifically geared towards women.
However, although both the EC and IC strategies were shown to be cost-effective, the QALYs gained for men receiving the IC strategy over men receiving EC were minimal—yielding essentially the same number of QALYs. Because of the additional cost for IC and the minimal added benefits from it, we would recommend a cell phone-based smoking-cessation programme targeting men to be modelled after the EC programme.
The sensitivity analysis showed that the ICER decreased with the increase in participant age indicating that the interventions were more cost-effective for older participants. However, because the ICER across the age group was well below the commonly recommended willingness-to-pay threshold of $50 000 per QALY, we recommend that cell phone-based smoking cessation programmes should be used irrespective of the age of the participants. Future studies could compare quit rates by age to determine the ages of individuals that are more likely to quit smoking with cessation programmes that use cell phones to provide support and determine ways to adapt interventions to make them even more cost-effective.
The quit rates associated with different smoking cessation programmes in Project ACTION are consistent with the studies observed in the previously published meta-analyses.5,6 Participants who received EC with access to a smoking hotline and supportive text messages were more likely to quit than those in SC. Furthermore, participants in IC who received additional support from a phone counsellor had a higher success at quitting than those in EC. Our study, however, used the interventions for smokers of low socioeconomic status. By recruiting participants from neighbourhood sites, we were successful at providing the smoking cessation interventions to underprivileged communities that benefit less from traditional cessation strategies.
Our results match our hypothesis that an IC strategy would yield a higher quit rate over EC and SC strategies and would be cost-effective. This agrees strongly with the findings in Cromwell et at,19 that more intensive smoking cessation programmes are more cost-effective, especially programmes that provide supportive counselling.
Limitations
Though most cost-effectiveness analyses on smoking cessation use 12-month quit rates in their models, it is not uncommon for 6-month quit rates to be used instead.20,21 Our model used self-reported quit rates instead of biochemically confirmed quit rates, which has a higher risk of participants misreporting their smoking status. However, the literature dictates that using self-reported quit rates here is acceptable because the sample of the study was a large community-based population of predominately low-income individuals, excluded minors and had a mean age of 45, and self-reporting was done in a private setting with interventions that were not face-to-face intensive; thus, biochemically confirmed quit rates would not be feasible or reliable.11 This approach is consistent with Society for Research on Nicotine and Tobacco (SRNT) guidelines for evaluating smoking cessation interventions in the community22.
As Project ACTION assessed quit rates and not decreases in smoking for those who did not discontinue use, it would be interesting to see how successful cell phone-delivered support was at reducing smoking in continuing smokers who failed to quit after the treatment. The individuals who reduced the number of cigarettes smoked could potentially incur health benefits. Although the literature on improved health outcomes related to smoking reduction has conflicting evidence, several studies have suggested decreased cardiovascular disease and chronic obstructive pulmonary disease rates for reducers.23,24
Project ACTION also did not take smoking intensity into account. With a larger sample size to ensure a sufficient number of heavy, moderate and light smokers, the effectiveness of different interventions at getting different types of smokers to quit could be analysed. These additional studies could shed light on the effect of the cell phone-based interventions on smoking cessation in distinct subgroups of smokers to inform a more targeted approach.
Summary
We found that cell phone messages and counselling are effective and cost-effective interventions for smoking cessation. The impact, however, is different for men and women—cost-effective interventions for men include text messaging; for women, the more cost-effective interventions also include counselling in addition to text messaging.
What this paper adds.
Cell phone-based smoking cessation interventions can enhance standard approaches, such as nicotine replacement therapy with written support material. However, the extent to which these more intensive interventions can promote smoking cessation in low socioeconomic communities is mostly unknown, as is their cost-effectiveness.
This is one of the first studies to determine the cost-effectiveness of providing cell phone-based smoking cessation interventions to low-income smokers.
Our results show that the cell phone-based interventions are both efficacious and cost-effective. The data indicate a greater role for both texting and counselling for women, while men did not benefit as much from the addition of over-the-phone counselling.
Acknowledgements
The authors thank Gary Deyter and Lacey Steagall from MD Anderson’s Department of Health Services Research for their editorial contributions that enhanced the manuscript.
Funding Financial support for this study was provided in part by grant CA141628 from the National Cancer Institute.
Footnotes
Competing interests None declared.
Patient consent Not required.
Ethics approval The University of Texas MD Anderson Cancer Center Institutional Review Board (IRB4).
Provenance and peer review Not commissioned; externally peer reviewed.
Baseline survey data are missing for two participants in Enhanced Care. These participants were figured in to calculate the costs per participants and overall quit rates, but excluded to calculate quit rates by sex.
REFERENCES
- 1.Jamal A, King BA, Neff LJ, et al. Current cigarette smoking among adults - United States, 2005-2015. MMWR Morb Mortal Wkly Rep 2016;65:1205–11. [DOI] [PubMed] [Google Scholar]
- 2.Vidrine DJ, Fletcher FE, Danysh HE, et al. A randomized controlled trial to assess the efficacy of an interactive mobile messaging intervention for underserved smokers: Project ACTION. BMC Public Health 2012; 12:696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mobile Fact Sheet. Washington DC: Pew Research Center, 2017. http://www.pewinternet.org/fact-sheet/mobile/2017 [Google Scholar]
- 4.Whittaker R, McRobbie H, Bullen C, et al. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev 2016;4:CD006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spohr SA, Nandy R, Gandhiraj D, et al. Efficacy of SMS text message interventions for smoking cessation: a meta-analysis. J Subst Abuse Treat 2015;56:1–10. [DOI] [PubMed] [Google Scholar]
- 6.Stead LF, Koilpillai P, Lancaster T. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database Syst Rev 2015;10:Cd009670. [DOI] [PubMed] [Google Scholar]
- 7.Cantor SB. Decision analysis: theory and application to medicine. Prim Care 1995;22:261–70. [PubMed] [Google Scholar]
- 8.Cantor SB, Deshmukh AA, Luca NS, et al. Cost-effectiveness analysis of smoking-cessation counseling training for physicians and pharmacists. Addict Behav 2015;45:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantor SB, Miller LAN. Time horizon Kattan MW, ed. Encyclopedia of Medical Decision Making. Thousand Oaks, CA: SAGE Publications, Inc, 2009:1138–9. [Google Scholar]
- 10.Lipscomb J, Weinstein MC, Torrance GW, et al. Time preference In: Gold MR, Siegel JE, Russell LB, Weinstein MC, eds. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press, 1996:214–35. [Google Scholar]
- 11.Velicer WF, Prochaska JO, Rossi JS, et al. Assessing outcome in smoking cessation studies. Psychol Bull 1992;111:23–41. [DOI] [PubMed] [Google Scholar]
- 12.Zhu SH, Anderson CM, Tedeschi GJ, et al. Evidence of real-world effectiveness of a telephone quitline for smokers. N Engl J Med 2002;347:1087–93. [DOI] [PubMed] [Google Scholar]
- 13.Rogers RG, Hummer RA, Krueger PM, et al. Mortality attributable to cigarette smoking in the United States. Popul Dev Rev 2005;31:259–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiscella K, Franks P. Cost-effectiveness of the transdermal nicotine patch as an adjunct to physicians’ smoking cessation counseling. JAMA 1996;275:1247–51. [PubMed] [Google Scholar]
- 15.Luce BR, Manning WG, Siegel JE, et al. Estimating costs in cost-effectiveness analysis In: Gold MR, Siegel JE, Russell LB, Weinstein MC, eds. Cost-Effectivness in Health and Medicine. New York: Oxford University Press, 1996:176. [Google Scholar]
- 16.Severens JL, Milne RJ. Discounting health outcomes in economic evaluation: the ongoing debate. Value Health 2004;7:397–401. [DOI] [PubMed] [Google Scholar]
- 17.Cantor SB. Cost-effectiveness analysis, extended dominance, and ethics: a quantitative assessment. Med Decis Making 1994;14:259–65. [DOI] [PubMed] [Google Scholar]
- 18.Weinstein MC. How much are Americans willing to pay for a quality-adjusted life year? Med Care 2008;46:343–5. [DOI] [PubMed] [Google Scholar]
- 19.Cromwell J, Bartosch WJ, Fiore MC, et al. Cost-effectiveness of the clinical practice recommendations in the AHCPR guideline for smoking cessation. Agency for Health Care Policy and Research. JAMA 1997;278:1759–66. [PubMed] [Google Scholar]
- 20.Richardson A, Graham AL, Cobb N, et al. Engagement promotes abstinence in a web-based cessation intervention: cohort study. J Med Internet Res 2013;15:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerriero C, Cairns J, Roberts I, et al. The cost-effectiveness of smoking cessation support delivered by mobile phone text messaging: Txt2stop. Eur J Health Econ 2013;14:789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benowitz NL, Jacob P, Ahijevych K, et al. Biochemical verification of tobacco use and cessation. Nicotine Tob Res 2002;4:149–59. [DOI] [PubMed] [Google Scholar]
- 23.Pisinger C, Godtfredsen NS. Is there a health benefit of reduced tobacco consumption? A systematic review. Nicotine Tob Res 2007;9:631–46. [DOI] [PubMed] [Google Scholar]
- 24.Begh R, Lindson-Hawley N, Aveyard P. Does reduced smoking if you can’t stop make any difference? BMC Med 2015;13:257. [DOI] [PMC free article] [PubMed] [Google Scholar]