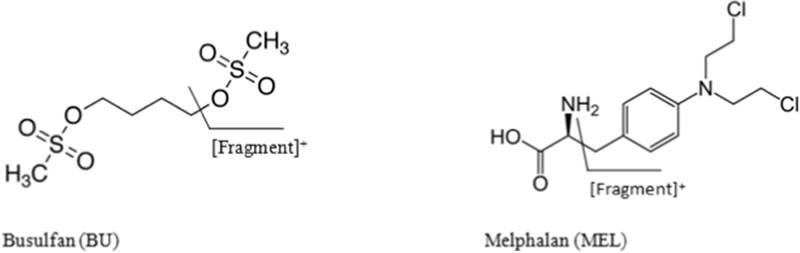Abstract
Busulfan and melphalan are cytotoxic DNA alkylating agents that are used in many hematopoietic stem cell transplantation (HCT) conditioning regimens. We report the development of an assay using turbulent flow liquid chromatography (TFLC) and tandem mass spectrometry to simultaneously measure the concentration of busulfan (Bu) and melphalan (Mel) in human plasma. The method involves precipitating proteins in the plasma specimen with an organic solvent containing deuterated internal standards of both compounds. Following centrifugation, an aliquot of the supernatant was injected into the TFLC mass spectrometry system operated in the positive ion mode. The analytical measurement range for both compounds was 10–5,000 ng/mL, and with validated dilutions the reportable range was extended to 25,000 ng/mL. Intra-day and inter-day (n=20 day) precision studies showed a coefficient of variation (CV) of less than 7% at several concentrations across the measurement range. To determine accuracy recovery studies were performed at several concentrations spanning the measurement range. Recoveries for both compounds were between 98–103%. Additionally, busulfan was compared with an existing assay and showed excellent correlation. Experiments were conducted to rule out matrix effects, carryover and interference from endogenous substances. The validated clinically reportable range (CRR) and assay precision will allow this assay to be used clinically to monitor and adjust Mel and Bu levels to ensure better therapeutic outcomes and also to support clinical trials aimed at better defining therapeutic ranges.
Keywords: busulfan, melphalan, turbulent flow liquid chromatography, tandem mass spectrometry, pharmacokinetic studies
1. Introduction
Therapeutic drug monitoring (TDM) using area under the curve calculations is a method to personalize therapy for patients with the overarching goal of increasing efficacy while minimizing toxicity. Busulfan (Bu) is an alkylating agent commonly used as a component of chemotherapy regimen prior to hematopoietic stem cell transplantation (HCT). TDM has been extensively used to prevent toxicities with these compounds, especially hepatic sinusoidal obstructive syndrome, while still allowing adequate myeloablation to maximize disease control [1,2,3].
Melphalan (Mel) is a DNA alkylating agent used to treat malignant diseases especially prior to HCT for multiple myeloma and amyloidosis. Myeloma patients being given high dose melphalan and HCT had a five-fold variability with a higher melphalan AUC correlating with longer overall survival compared to patients with a lower AUC, but also more mucositis [4].
A number of studies have shown the combination of busulfan and melphalan to be highly effective and a well-tolerated conditioning regimen for HCT, for acute leukemia [5,6,7], multiple myeloma [8] and advanced lymphoid malignancies [9]. With the potential for pharmacokinetic dosing of both agents and possibility of shortening length of stay, we sought to develop an assay for the simultaneous measurement of Mel and Bu concentrations from a single plasma sample. Previously published mass spectrometry methods for the measurement of Bu and Mel have measured each analyte individually [10, 11,12,13,14,15]. Some methods have been labor intensive, time consuming, and have required extensive sample preparation or derivatization [13, 14]. The method reported here is a simple, sensitive, and accurate assay to quantitate these compounds simultaneously without derivatization. The method employs TFLC coupled to heated electrospray ionization (HESI) tandem mass spectrometry. This method is very suitable for TDM of these two compounds simultaneously and to support PK studies of these agents.
2. Materials and methods
2.1. Chemicals and materials
Busulfan (Bu) was from Restek (Bellefonte, PA, USA) and melphalan (Mel) was from Sigma-Aldrich (Saint-Louis, MO, USA) to prepare calibrators. Additionally, Bu and Mel were obtained from Cerilliant (Round Rock, TX, USA) and Toronto Research Chemicals Inc. (Toronto, ON, Canada), respectively for QC material. Deuterated busulfan (Bu-d8) was from Cambridge Isotope Laboratories, Inc. (Andover, MA, USA) and deuterated melphalan (Mel-d8) was from Toronto Research Chemical. All compounds were greater than 99% pure. All solvents including methanol, 2-propanol, acetone, LCMS grade water, acetonitrile, ammonium hydroxide and formic acid were from Fisher Scientific (Fair Lawn, NJ, USA). Drug-free defibrinated human plasma was from UTAK Laboratories (Valencia, CA, USA). For the ion suppression, specificity and method comparison studies residual patient plasma specimens were used. This study was approved by the IRB committee of MSKCC.
2.2. Liquid chromatographic and mass spectrometric conditions
The turbulent flow chromatography system was a Thermo Scientific Aria TLX-2 turbulent flow system (Franklin, MA, USA) operated according to the manufacturer instructions as previously described [16]. This was connected to a Thermo Scientific TSQ Vantage mass spectrometer (San Jose, CA, USA) using an electrospray ionization probe and operated as previously described [16]. The parameters differed as follows; the source and ion transfer capillary temperature were maintained at 375 and 200°C while the spray voltage was set at 4,000 V. The auxiliary, ion sweep and sheath gas were set at at 20, 2 and 60 units, respectively.
2.3. calibrators and quality control materials
A solution of Mel (1 mg/mL) was prepared in methanol and stored at −20°C for both calibrators and QC. Additionally, a stock solution of Bu-d8 and Mel-d8 (1 mg/mL) was prepared and stored at −20°C. Internal standard solutions (1,250 ng/mL) were prepared in methanol from their stock solutions.
The calibrators were prepared by first preparing a solution containing 5,000 ng/mL of Bu and Mel in drug-free defibrinated plasma. This solution was diluted in human plasma to obtain calibrators at concentrations of 19.5, 78.1, 312.5, 1,250, 2,500, and 5,000 ng/mL. Quality control materials were made by pipetting the stock solution into defibrinated plasma to provide final concentrations of 250, 1,500, and 3,500 ng/mL. All solutions were stored at −80°C. Earlier work has shown that Bu and Mel are stable under freezing (−80°C), refrigerated (4°C), post-preparative and through multiple freeze-thaw cycles [17,18,19].
2.4. Specimen preparation
Specimens (100 μL) of calibrator, control, or patient specimen were transferred into a microcentrifuge tube containing internal standard solution (200 μL) in methanol as previously described [16]. The specimens were vortex-mixed and then centrifuged for 10 minutes at 13,000 x g and the supernatant transferred to an autosampler vial. The specimens were vortex mixed and then centrifuged for 10 min at 13,000 x g. The sample injection volume was optimized to 25 μL and was analyzed using the TFLC-MS/MS.
2.5. Method Validation
The validation protocol was adapted from guidance documents published by the U.S. Food and Drug Administration (FDA) [20], CLIA and CLSI [21] as previously described [16]. The validation included analysis of, precision, linearity, accuracy, carryover, selectivity, and ion suppression.
2.5.1. Specificity
The selectivity of the method was accessed for potential matrix interferences or co-eluting compounds present in the specimen. This was achieved by taking six drug-free patients specimens and evaluating them to ensure that there were no compounds that co-eluted at the retention times of Bu and Mel. The potential interference of commonly occurring substances, namely hemoglobin, lipids and bilirubin was determined. To perform this experiment a known concentration of Bu and Mel was added into patient specimens that were grossly hemolyzed, lipemic or icteric and the percent recovery was calculated. In addition, twenty drug-free plasma samples were spiked with both compounds and were analyzed for Bu and Mel. The ratios of the primary and secondary ions were monitored to check for unknown interferences.
2.5.2. Linearity
The assay linearity was determined using a 6-point calibration curve spanning the reportable range for for Bu and Mel. The specimens were extracted in triplicate as previously reported [16]. EP Evaluator software (South Burlington, VT, USA) determined clinical linearity using the criteria outlined in CLSI EP-6A [22].
2.5.3. Accuracy and precision
Inter-day imprecision was determined by analyzing three levels of quality control material in triplicate over a twenty day period. Intra-day imprecision was determined as previously described [16]. A dilution protocol was validated (two-fold and five-fold) in order to extend the reportable range of the assay. For the two-fold dilution, 100 μL of drug-free plasma was added to 100 μL of patient sample and vortexed. For the five-fold dilution, patient sample (100 μL) was added to drug-free plasma (400 μL) and vortexed briefly. A recovery experiment was used to evaluate the accuracy of the assay for Bu and Mel. Three concentrations spanning the AMR were prepared in blank plasma and analyzed in triplicate.
In addition, the Bu assay was compared with a previously validated Bu assay [23] and the results of the two were compared using regression analysis and a Bland-Altman difference plot [24] using Analyze-It software (Leeds, United Kingdom).
2.5.4. Matrix effects
Matrix effects were assessed by placing a tee fitting between the TFLC system and an infusion pump as previously described [16]. As previously reported a solution containing Mel and Bu was pumped into the HPLC eluant and the SRM for Mel and Bu was recorded. The drug-free plasma from six different patient specimens were extracted and analyzed by the TFLC-MS/MS system. Ion suppression would be detected as a depression in the baseline at the retention time where the drugs were to elute. According to established clinical guidelines, any variability in the baseline due to injection of specimen extracts should be less than 15%. [21].
2.5.5. Carryover
Carryover was assessed by analyzing the highest calibrator followed by a drug-free plasma sample on every run during the validation period. According to established clinical laboratory guidelines the carryover should be less than 25 percent of the value of the LLOQ. [20].
3. Data analysis and discussion
3.1. Liquid chromatography conditions
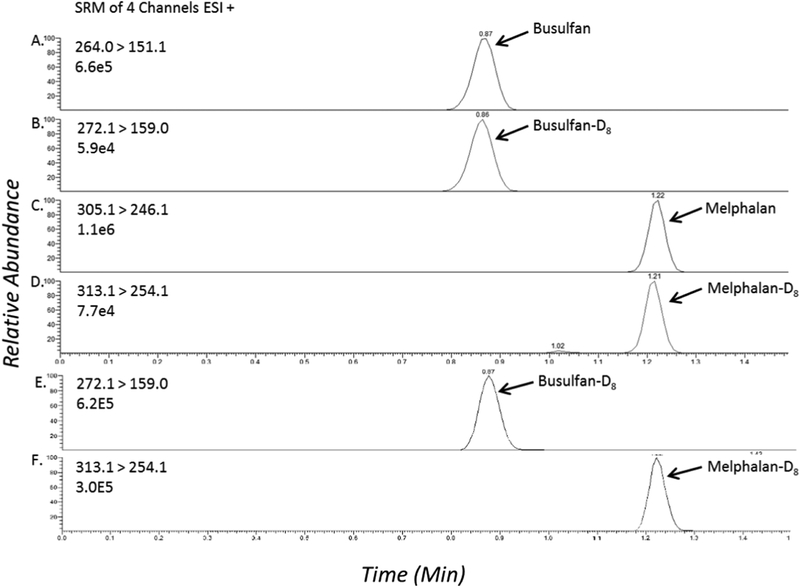
The TFLC settings were optimized to achieve maximum sensitivity for all compounds as previously described [16]. Modification to the previously reported parameters include a transfer step of 50% mobile phase B, a Hypersil Gold C18 HPLC column (3.0 × 50 mm, 5 μm), 25 μL injection volume and integration parameters of 15, 10, 1 and 30 seconds for integration window, peak noise factor, area noise factor and baseline window, respectively. Figure 2 shows ion chromatograms of spiked plasma samples containing Bu (5000 ng/mL) and Mel (5000 ng/mL). Figure 2 (panels E,F) shows blank plasma samples spiked with internal standards; Bu-D8 (625 ng/mL) and Mel-D8 (625 ng/mL). There were no impurities of the parent compounds present in the internal standard solutions (data not shown).
Figure 2:
TFLC-MS/MS ion chromatograms of (A) busulfan (B) busulfan-D8 (C) melphalan (D) melphalan-D8. Chromatograms (E, F) represent a blank plasma sample containing both internal standards.
The TFLC parameters are shown in Table 1.
Table 1:
TFLC loading and elution pump parameters for analysis of busulfan and melphalan
| TFLC Conditions | HPLC Conditions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Step | Seconds | Flow Rate (mL/min) | %A | %B | %C | Flow Rate (mL/Min) | Grad | %A | %B |
| 1 | 30 | 2.00 | 100 | - | - | 0.7 | S | 100 | |
| 2 | 45 | 0.15 | 100 | - | - | 0.7 | S | 100 | |
| 3 | 15 | 2.00 | - | - | 100 | 0.7 | R | 100 | - |
| 4 | 15 | 2.00 | - | - | 100 | 0.7 | S | 50 | 50 |
| 5 | 30 | 2.00 | - | - | 100 | 0.7 | S | 30 | 70 |
| 6 | 45 | 2.00 | 50 | 50 | - | 0.7 | S | 5 | 95 |
| 7 | 45 | 2.00 | 50 | 50 | - | 0.7 | S | 5 | 95 |
| 8 | 75 | 2.00 | 100 | 0.7 | S | 100 | |||
For the gradient (Grad), S is step and R is ramp.
3.2. Triple quadrupole mass spectrometry analysis
The method analysis was completed implementing the parameters set forth in section 2.2 and adapted from [16]. The SRM parameters were developed and optimized by utilizing positive electrospray ionization with direct infusion of all compounds. It was determined that the following transitions were optimal in SRM mode for the respective analytes; for busulfan the primary transition monitored was 264.0 > 151.1 and a secondary transition of 264.0 > 55.1 m/z was monitored. For Bu-d8 a primary transition of 272.1 > 159.0 and secondary transition of 272.1 > 62.2 m/z was used. For Mel, the primary transition was 305.1 > 246.0 and the secondary transition of 305.1 > 288.0 m/z was monitored. For Meld8 a primary transition was 313.1 > 254.1 and the secondary transition was 313.1 > 296.1 m/z. The optimum tube lens and CE voltages for Bu and Bu-d8 were 66 and 6 V, respectively. The optimum tube lens and CE voltages for Mel and Mel-d8 were 23 and 80 V, respectively. The declustering voltage and chrom filter peak width were found to be optimal for all compounds at 10 V and 5 seconds, respectively. Data analysis was performed using LCquan software version 2.6 (San Jose, CA, USA).
3.3. Assay validation
The assay method was validated as per Section 2.5.
3.3.1. Specificity
Figure 2 represents a conventional chromatogram (SRM) with all compounds displaying the retention time of each. The retentions times of Bu, Bu-d8, Mel, and Mel-d8 are 0.89, 0.90, 1.27, and 1.27 minutes, respectively. In the experiment to determine if matrix components interfere with the assay we found no compounds present with the same m/z and retention times as Bu and Mel. In the hemolysis, icterus, triglycerides (HIT) experiment to evaluate possible interferences, no interfering components were observed from hemoglobin, bilirubin, or triglycerides. Twenty patient samples from patients on these drugs were analyzed for Bu and Mel and the ratios of the primary and secondary ions deviated less than 20 percent of the expected ratio, consistent with CLSI guidelines [21].
3.3.2. Linearity
The assay was linear within the analytical measurement range of each analyte. The coefficient of correlations (r) was greater than 0.996 for the calibration curve of each compound using LCquan software. The LLOQs were found to be 10 ng/mL for both Bu and Mel. Melphalan had the lowest signal-to-noise ratio (S/N) at its LLOQ of the tested compounds (S/N > 200). This is greater than ten times the recommended S/N minimum recommended by clinical laboratory guidelines. [20].
3.3.3. Precision and accuracy
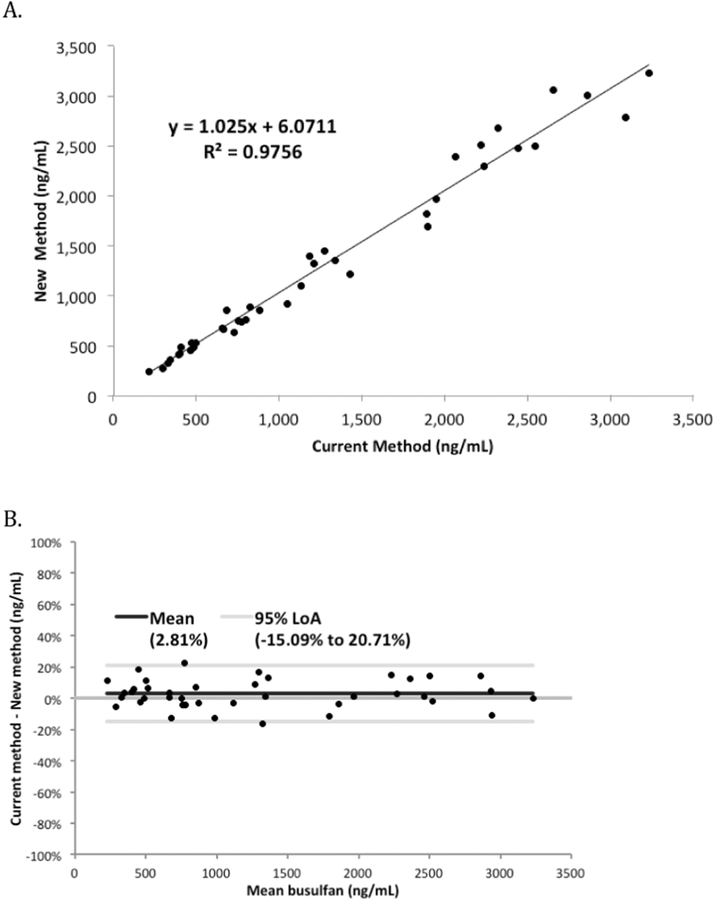
The coefficient of variation was <7% for three levels during the twenty-day precision experiment (Table 2). A recovery experiment was used to evaluate the accuracy of the assay for Bu and Mel. Three concentrations spanning the AMR were prepared in blank plasma and analyzed in triplicate, producing results ranging from 98.4–103.1% recovery for both compounds (Table 3). Therefore, this assay is sufficiently accurate and precise for the quantitation of Bu and Mel in human plasma. To further validate the accuracy of the busulfan assay a comparison of 40 residual samples obtained from routine clinical testing showed the current assay had excellent correlation with a previously validated assay; the slope, determined by linear regression was 1.025 and the correlation coefficient was (R2) was 0.976 (Figure 3A). A Bland-Altman plot of these data showed a slight positive bias (+2.8%) with the current assay when compared with the new assay across the linear range (Figure 3B). This slight bias will have no impact on therapeutic drug monitoring or pharmacokinetic analysis.
Table 2:
Inter-day and Intra-day Precision of the Assay
| Sample | Nominal Value (ng/mL) | Mean (ng/mL) Intra-day | Intra-day CV (%) | Mean (ng/mL) Inter-day | Inter-day CV (%) |
|---|---|---|---|---|---|
| Busulfan, LLOQ | 10.0 | 10.2 | 4.9 | 9.7 | 8.2 |
| Busulfan, 1 | 250.0 | 256.7 | 1.9 | 233.8 | 4.4 |
| Busulfan, 2 | 1,500 | 1,482.4 | 2.3 | 1,474.8 | 4.5 |
| Busulfan, 3 | 3,500 | 3,607.8 | 2.5 | 3,593.7 | 4.5 |
| Melphalan, LLOQ | 10.0 | 10.0 | 7.2 | 11.1 | 10.1 |
| Melphalan, 1 | 250.0 | 252.8 | 2.7 | 247.0 | 6.5 |
| Melphalan, 2 | 1,500.0 | 1,475.7 | 2.9 | 1,498.1 | 6.5 |
| Melphalan, 3 | 3,500.0 | 3,554.1 | 2.4 | 3,567.2 | 6.8 |
Intra-day and inter-day precision determined as described as stated in section 2.5.3.
Table 3:
Accuracy Determined by Compound Recovery
| Sample | Nominal Value (ng/mL) | Measured Value (ng/mL) | % Recovery (measured/nominal) |
|---|---|---|---|
| Busulfan, LLOQ | 10.0 | 10.2 ±0.5 | 102.1 ±4.9 |
| Busulfan, 1 | 250.0 | 256.7 ±4.9 | 102.7 ± 1.9 |
| Busulfan, 2 | 1,500.0 | 1,482.4 ±34.5 | 98.8 ±2.3 |
| Busulfan, 3 | 3,500.0 | 3,607.8 ±88.9 | 103.1 ±2.5 |
| Busulfan, 4* | 8,000.0 | 7,769.4 ±41.6 | 98.3 ±0.5 |
| Busulfan, 4** | 8,000.0 | 7,526.5 ± 139.5 | 95.2 ±1.9 |
| Melphalan, LLOQ | 10.0 | 10.0 ±0.7 | 100.0 ±7.2 |
| Melphalan, 1 | 250.0 | 252.8 ±6.9 | 101.1 ±2.7 |
| Melphalan, 2 | 1,500.0 | 1,475.7 ±42.5 | 98.4 ±2.9 |
| Melphalan, 3 | 3,500.0 | 3,554.1 ±85.9 | 101.5 ±2.4 |
| Melphalan, 4* | 8,000.0 | 7,747.2 ±251.4 | 99.1 ±3.2 |
| Melphalan, 4** | 8,000.0 | 7,726.5 ± 168.5 | 98.9 ±2.2 |
These samples were diluted 1:2 fold following the validated dilution procedure
These samples were diluted 1:5 fold following the validated dilution procedure
Figure 3.
(A) Correlation between the new busulfan TFLC-MS/MS assay and the prior busulfan TFLC MS/MS assay (current method). (B) Bland-Altman difference plots depicted as mean concentrations against the percental bias.
To expand the reportable range of the assay, several dilutions were validated; multiple concentration levels above than the analytical measurement range (AMR) were diluted (either two-fold or 5-fold) for both compounds so that the values fell into the AMR. The concentration of the elevated specimen was 8,000 ng/mL for Bu and Mel. All samples were analyzed in quintuplet and both the twofold and five-fold dilutions yielded results that were within 2% of the anticipated results.
3.3.4. Ion suppression & matrix interference
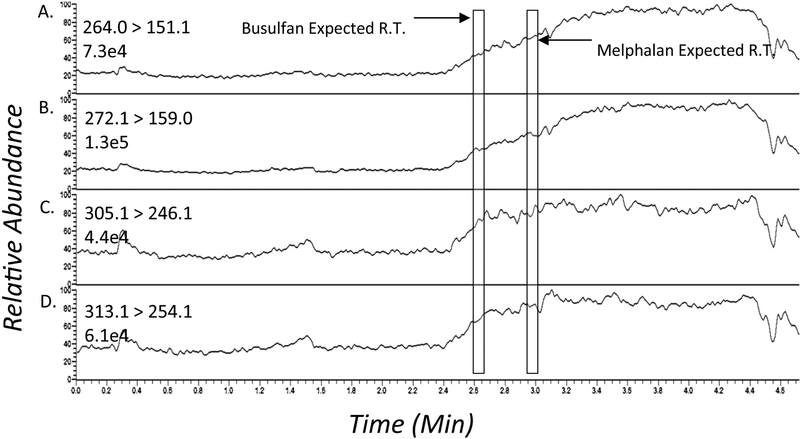
The post column infusion experiment did not show any significant ion suppression or enhancement. See figure 4 for representative chromatograms of the post column infusion experiments when a plasma extract was injected. There was no suppression of the baseline signal at the elution times of Mel or Bu. A total of six different patient extracts as well as a methanol blank were injected and yielded identical results (data not shown).
Figure 4:
Ion suppression profile after injection of a processed drug-free patient plasma sample; (A) busulfan (B) busulfan-D8 (C) melphalan (D) melphalan-D8. The analytes elute in the region indicated by the boxes.
3.3.5. Sample carryover.
The assessment of carryover took place by monitoring the response of analyte in a blank serum sample following the highest calibrator. This evaluation took place on each calibration curve completed during the assay development and validation period. No clinically significant carryover was observed (<20% of LLOQ) for either compound. [21].
4. Conclusion
Herein we describe the performance characteristics of a novel assay for the measurement of Bu and Mel in plasma, simultaneously. Implementing TFLC for sample cleanup shortens preparation times, allows for small sample volumes and a high throughput. This validated method has proven to be sensitive, robust and was validated based on CLIA guidelines including precision, accuracy, specificity, sensitivity and linearity, and recovery. The method was proven to be accurate, with recoveries ranging between 98–103% at concentrations spanning the AMR. Intra-day and inter-day imprecisions at several levels challenging the reportable range were less than 7% for both compounds. This assay is linear from 10–5,000 ng/mL for both Bu and Mel. There was neither ion suppression under the experimental conditions nor other interferences from the other compounds tested. The validated CRR and assay precision will allow this assay to be used for TDM and simultaneous pharmacokinetic studies for both drugs.
Figure 1:
Molecular structures and fragmentation patterns of busulfan and melphalan.
Highlights.
First reported mass spectrometry method for the measurement of busulfan and melphalan in human plasma in a single analysis.
The assay is fully clinically validated for use in patient care to monitor and adjust melphalan and busulfan levels and to support clinical trials.
The method was validated to be fast, reliable, convenient and sensitive for both compounds.
Acknowledgements
This work was supported through a NIH/NCI Cancer Center Support Grant P30 CA008748.
Abbreviations
- AMR
analytical measurement range
- Bu
busulfan
- Bu-d8
deuterated busulfan
- LLOQ
lower limit of quantitation
- HESI
heated electrospray ionization
- Mel
melphalan
- Mel-d8
deuterated melphalan
- SRM
selected reaction monitoring
- TFLC
turbulent flow liquid chromatography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- [1].Perkins JB, Kim J, Anasetti C, Fernandez HF, et al. , Maximally tolerated busulfan systemic exposure in combination with fludarabine as conditioning before allogeneic hematopoietic cell transplantation, Biology of Blood and Marrow Transplantation 187 (2012) 1099–1107. [DOI] [PubMed] [Google Scholar]
- [2].Pidala J, Kim J, Anasetti C, et al. , Pharmacokinetic targeting of intravenous busulfan reduces conditioning regimen related toxicity following allogeneic hematopoietic cell transplantation for acute myelogenous leukemia, Journal of hematology & oncology 31 (2010) 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang H, Graiser M, Hutcherson DA, et al. , Pharmacokinetic-directed high-dose busulfan combined with cyclophosphamide and etoposide results in predictable drug levels and durable long-term survival in lymphoma patients undergoing autologous stem cell transplantation, Biology of Blood and Marrow Transplantation 188 (2012) 1287–1294. [DOI] [PubMed] [Google Scholar]
- [4].Shaw PJ, Nath CE, Nivison-Smith I, et al. , Higher melphalan exposure is associated with improved overall survival for myeloma patients undergoing autologous transplant, Biology of Blood and Marrow Transplantation 182 (2012) S207. [Google Scholar]
- [5].Jakubowski AA, Small TN, Kernan NA, et al. , T cell–depleted unrelated donor stem cell transplantation provides favorable disease-free survival for adults with hematologic malignancies, Biology of Blood and Marrow Transplantation 179 (2011) 1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Goldberg JD, Linker A, Kuk D, et al. , T cell–depleted stem cell transplantation for adults with high-risk acute lymphoblastic leukemia: Long-term survival for patients in first complete remission with a decreased risk of graft-versus-host disease, Biology of Blood and Marrow Transplantation 192 (2013) 208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tamari R, Chung SS, Papadopoulos EB, et al. , CD34-selected hematopoietic stem cell transplants conditioned with myeloablative regimens and antithymocyte globulin for advanced myelodysplastic syndrome: limited graft-versus-host disease without increased relapse, Biology of Blood and Marrow Transplantation 2112 (2015) 2106–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Qazilbash Muzaffar H., et al. , A randomized phase III trial of busulfan+ melphalan vs melphalan alone for multiple myeloma, Blood 130 (2017) 399–399. [Google Scholar]
- [9].Kebriaei Partow, et al. , Intravenous busulfan plus melphalan is a highly effective, well-tolerated preparative regimen for autologous stem cell transplantation in patients with advanced lymphoid malignancies, Biology of Blood and Marrow Transplantation 173 (2011) 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Snyder M, Ritchie J, Quantification of busulfan in plasma using liquid chromatography electrospray tandem mass spectrometry (HPLC-ESI-MS/MS), Clinical Applications of Mass Spectrometry, Humana Press, New York, 2009, pp. 129–136. [DOI] [PubMed] [Google Scholar]
- [11].Chen L, Zhou Z, Shen M, Ma A, Quantitative analysis of busulfan in human plasma by LC-MSMS, Chromatographica 70 (2009) 1727–1732. [Google Scholar]
- [12].Mirkou A, Vignal B, Cohen S, Guillaumont M, Glehen O, Guitton J, Assays for the quantification of melphalan and its hydrolysis products in human plasma by liquid chromatography-tandem mass spectrometry, J. Chromatogr. B 877 (2009) 3089–3096. [DOI] [PubMed] [Google Scholar]
- [13].Nath C, Zeng L, Eslick A, Trotman J, Earl J, An isocratic UV HPLC assay for analysis of total and free melphalan concentrations in human plasma, Acta Chromatogr. 20 (2008) 383–398. [Google Scholar]
- [14].Pinguet F, Joulia J, Martel P, Grosse P, Astre C, Bressolle F, High-performance liquid chromatographic assay for melphalan in human plasma application to pharmacokinetic studies, J. Chromatogr. B 686 (1996) 43–49. [DOI] [PubMed] [Google Scholar]
- [15].Davies I, Allanson J, Causon R, Rapid determination of the anti-cancer drug melphalan (AlkeranTM) in human serum and plasma by automated solid phase extraction and liquid chromatography tandem mass spectrometry, Chromatographica 52 (2000) S92–S97. [DOI] [PubMed] [Google Scholar]
- [16].Schofield RC, V Ramanathan L, Murata K, Grace M, Fleisher M, Pessin MS & Carlow DC, Development and validation of a turbulent flow chromatography and tandem mass spectrometry method for the quantitation of methotrexate and its metabolites 7-hydroxy methotrexate and DAMPA in serum. Chromatogr. B 1002 (2015) 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bosanquet A, Stability of melphalan during preparation and storage, J. Pharm. Sci 74 (1985) 348–351. [DOI] [PubMed] [Google Scholar]
- [18].Karstens A, Kramer I, Experimental study to determine the physic-chemical stability of diluted busulfan solutions, Eur. J. Hosp. Pharm. Sci 13 (2007) 41–47. [Google Scholar]
- [19].Balasubramanian P, Srivastava A, Chandy M, Stability of busulfan in frozen plasma and whole blood samples, Clin. Chem 47 (2001) 766–768. [PubMed] [Google Scholar]
- [20].FDA guidance for industry bioanalytical method validation, US department of health and human service, Food and Drug Administration, Center for drug evaluation and research (CDER), 2001. [Google Scholar]
- [21].CLSI. Liquid Chromatography-Mass Spectrometry Methods; Approved Guideline. CLSI document C62-A. Wayne, PA: Clinical and Laboratory Standards Institute; 2013. [Google Scholar]
- [22].CLSI. Evaluation of the Linearity of Quantitative Measurement Procedures: A Statistical Approach; Approved Guideline. CLSI document EP6-A. Wayne, PA: Clinical and Laboratory Standards Institute; 2013. [Google Scholar]
- [23].Schofield R, Murata K, Bechtel LK, Measuring Busulfan in Blood Plasma Using On-Line Extraction Coupled to LC-MS/MS, Poster session presented at the Mass Spectrometry Applications in the Clinical Laboratory Conference. San Diego, CA: 2011. [Google Scholar]
- [24].Bland JM & Altman D (1986). Statistical methods for assessing agreement between two methods of clinical measurement. The Lancet. 327 (1986) 307–31. [PubMed] [Google Scholar]