Abstract
Background:
The field of cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) has suffered from a lack of clinical trials to validate its expanding use.
Objective:
To evaluate published and ongoing clinical trials seeking to better define role of CRS/HIPEC in the treatment of peritoneal surface malignancies.
Methods:
Systematic review by PubMed search was performed using terms “Clinical trial,” “intraperitoneal chemotherapy,” and “HIPEC.” ClinicalTrials.gov and EudraCT registries were searched for active clinical trials. Eligibility included CRS/HIPEC trials investigating adult patient populations from published clinical reports and/or trials currently accruing or at completion.
Results:
Thirteen published trials and 57 active clinical trials were included for review.
Conclusions:
Published and ongoing U.S. and international clinical trials for CRS and HIPEC are defining important parameters that include improving patient selection, strategic sequences of treatment, cytoreductive strategies, chemotherapeutics, optimal hyperthermic temperature and timing, and toxicity profiles. Main barriers or limitations to trial development remain patient enrollment, trial design, and oncologic community collaboration. Overall progress is positive with increasing number of clinical trials throughout the world. Collaboration between surgeons and the wider oncologic community will be crucial to validate this important treatment strategy.
Keywords: clinical trials, cytoreductive surgery, HIPEC, peritoneal carcinomatosis, peritoneal metastasis
1 |. INTRODUCTION
The use of cytoreductive surgery (CRS) dates back to the 1930s, when J.V. Meigs began performing aggressive debulking and demonstrated improved survival in patients with ovarian cancer with peritoneal metastasis (PM).1 The development of this aggressive surgical approach toward PM, previously considered a terminal stage for most cancers, has faced an arduous process toward gaining acceptance in the at-large oncologic community. Investigations through the 1960s and 70s with further application to pseudomyxoma peritonei (PMP) and ovarian cancer supported the role of CRS in selective management of peritoneal disease.1 The development of cytoreduction continued throughout the 20th century, and was optimized when Sugarbaker proposed six specific peritonectomy procedures for complete cytoreduction.2 Separately, uses of adjuvant therapy began to expand during the 1970s, with trials investigating the pharmacokinetics and effectiveness of intraperitoneal (IP) chemotherapy.3 Hyperthermia was introduced to this paradigm when Spratt et al demonstrated the benefit of heated IP perfusion in canines and Palta et al developed an IP therapy filtration system.4,5
Historically, the first patient was treated with hyperthermic intraperitoneal chemotherapy (HIPEC) in 1979 for recurrent PMP, having previously undergone CRS.6 Phase I and II clinical trials in the 1980s subsequently demonstrated the effectiveness of IP chemotherapy with improved survival in patients largely suffering from appendiceal or ovarian disease.7–9 Koga et al, Toi et al, and Fujimoto et al expanded investigations toward its use in metastatic gastric cancer, while Sugarbaker et al prospectively compared the use of IP 5-fluorouracil (5-FU) to systemic therapy for colorectal and appendiceal cancer.10–15 The benefits of heated IP chemotherapy were also demonstrated in clinical trials by Zimm et al and Howell et al.7,8 Currently, CRS and HIPEC have become the standard of care for pseudomyxoma peritonei (PMP) and peritoneal mesothelioma.16 Conducting clinical trials to validate potential treatment efficacy for PM toward other malignancies has proven difficult.17,18
Despite the successes of these previous investigations, there have been few prospective, controlled clinical trials for HIPEC. Challenges include trial design, bias within the oncologic community, and poor patient enrollment. In the United States, Stojadinovic et al, in collaboration with the U.S. Military Cancer Institute, American College of Surgeons Oncology Group, and National Cancer Institute, attempted to enroll 328 patients for a randomized controlled trial (RCT) investigating the role of CRS and HIPEC for the management of colorectal PM, however, were only able to recruit a single patient. Lack of patient accrual is a common factor in the failure of most clinical trials for CRS and HIPEC, despite a growing need for high-level clinical evidence to validate this treatment approach for peritoneal malignancies. Limited clinical trials, as well as significant retrospective data already published, have provided a foundation from which more prospective clinical trials have developed.
2 |. METHODS
Initially, a PubMed search was conducted using terms “Clinical trial,” “intraperitoneal chemotherapy,” and “HIPEC.” Literature presenting the data from clinical trials between 2000 and 2016 were included in the review of published clinical studies.
The ClinicalTrials.gov and EudraCT clinical trials registries were searched for currently active, clinical trials, either within the U.S. or internationally. Search terms “HIPEC,” “intraperitoneal chemotherapy,” “peritoneal malignancy” were used. These databases were searched between January and March 2017 for updated trial information. Included studies were limited to those actively recruiting patients or those that have completed the trial period, but continue to record follow-up data. Excluded trials included completed but unpublished trials, trials for the creation of HIPEC data registries, those focused on adjuvant or systemic therapy only, and treatment of metastasis outside of the peritoneal cavity. Only trials investigating adult populations were included. Inclusion was not limited by trial start date or length of follow-up, however, the earliest American trial began patient recruitment in 2010, with an end date of 2020, and earliest European trial began in 2010. Trials were reviewed for data related to age of recruited patients, investigated malignancy, compared interventions, primary and secondary endpoints, and duration of follow-up (Figure 1). Published studies were assessed for bias and reported descriptively at both study design and outcome levels.19
FIGURE 1.
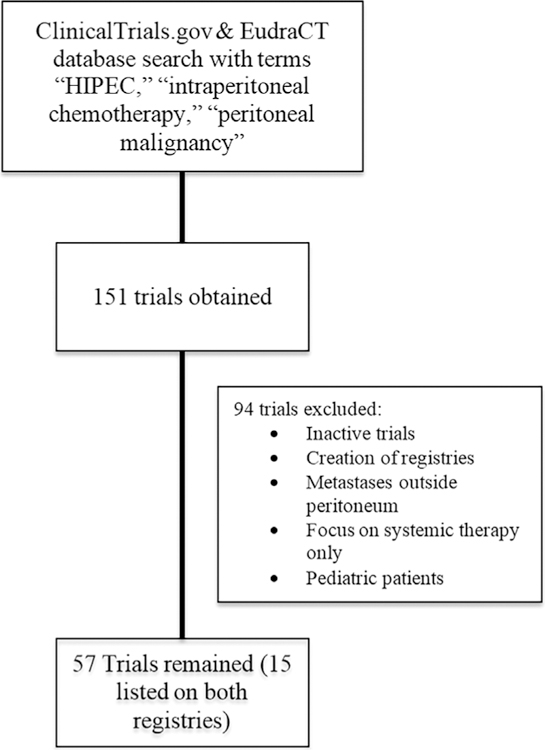
Flow chart demonstrating results of clinical trial database search and exclusion criteria
3 |. RESULTS OF LITERATURE AND CLINICAL TRIAL REVIEW
PubMed search yielded 13 clinical trials published after 2000. Initially 113 and 38 studies were obtained from the ClinicalTrials.gov and EudraCT registries, respectively. After exclusion criteria were applied, 57 clinical trials remained for inclusion in this review.
4 |. DISCUSSION
4.1 |. Published clinical studies
Validation of CRS and HIPEC as part of the multimodal disease management system of PM has been hampered by a lack of prospective, clinical trials. To date, a handful of modern published clinical trials exist which address colorectal, appendiceal, ovarian, and gastric cancer with PM, and peritoneal mesothelioma (Table 1).
TABLE 1.
All studies on the ClinicalTrials.gov and EudraCT registries investigating the role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on peritoneal malignancies
| Authors | Type of study | Malignancy | # of patients | Control arm | Experimental arm | Major finding |
|---|---|---|---|---|---|---|
| Verwaal et al23 | Single institution, randomized, phase III | Colorectal | 105 | Systemic chemotherapy ± palliative surgery | CRS + HIPEC | Improved survival in CRS/HIPEC arm |
| Yan et al30 | Single institution, non-randomized, phase II | PMP | 50 | N/A | CRS + PIC | Improved survival in patients with less extensive previous surgery and DPAM |
| Yang et al33 | Single institution, randomized, phase III | Gastric | 68 | CRS only | CRS + HIPEC | Improved survival in experimental arm |
| Gaducci et al37 | Multi-institution, randomized, phase III | Ovarian | 113 | Debulking + IV cisplatin, epirubicin, CPP | Debulking + IP chemotherapy | Slight improvement in PFS and OS in experimental arm |
| Markman et al39 | Multi-institution, randomized, phase III | Ovarian | 523 | Debulking + IV cisplatin, paclitaxel | Debulking + IV carboplatin, paclitaxel + IP cisplatin | Significant improvement in PFS, borderline improvement in OS in experimental arm |
| Yen et al41 | Single institution, randomized, phase III | Ovarian | 118 | CRS + IV CPP, epirubicin or adriamycin + IV cisplatin | CRS + IV CPP, epirubicin or adriamycin + IP cisplatin | Equivalent survival, lower hematologic toxicity in experimental arm |
| Armstrong et al42 | Multi-institution, randomized, phase III | Ovarian Primary Peritoneal |
415 | CRS + IV paclitaxel, cisplatin | CRS + POD1 IP cisplatin, POD8 paclitaxel | Improved survival in experimental arm |
| Chi et al43 | Multi-institution, retrospective, cohort analysis | Ovarian | 378 | Primary CRS | CRS, including upper abdomen | Increased PFS and OS in experimental arm |
| Spiliotis et al44 | Single institution, randomized, phase III | Ovarian | 120 | CRS + IV therapy | CRS + IV therapy, HIPEC | Improved survival in experimental arm |
| Glehen et al46 | Single institution, non-randomized, phase II | Colorectal Ovarian Gastric Peritoneal mesothelioma PMP Miscellaneous |
56 | N/A | CRS + HIPEC | Improved survival in patients with complete macroscopic resection |
| Loggie et al48 | Single institution, non-randomized, phase II | Peritoneal mesothelioma | 12 | N/A | CRS + HIPEC | Improved OS as compared to historical controls |
| Deraco et al50 | Single institution, non-randomized, phase II | Peritoneal mesothelioma | 19 | N/A | CRS + HIPEC | Improved resolution of malignant ascites and OS as compared to historical controls |
| Hesdorffer et al51 | Single institution, non-randomized, phase I/II | Peritoneal mesothelioma | 27 | N/A | CRS + PIC, IP gamma-IFN followed by repeat CRS + HPEC, radiation | Demonstrated feasibility and efficacy of multi-modal therapy |
CRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy; PMP, pseudomyxoma peritonei; DPAM, diffuse peritoneal adenomucinosis; PIC, peri-operative intraperitoneal chemotherapy; IV, intravenous; IP, intraperitoneal; POD, post-operative day; PFS, progression-free survival; OS, overall survival; CPP, cyclophosphamide; N/A, not applicable.
One such trial that made a significant impact in current cancer treatment guidelines is that of Verwaal et al.20,21 As a result, the current National Cancer Comprehensive Network (NCCN) Guidelines for colon cancer, state that “if R0 resection can be achieved, surgical resection of isolated peritoneal disease may be considered at experienced centers.21” In total, 105 patients were randomized to a control arm of systemic chemotherapy with 5-FU and leucovorin or irinotecan for up to 26 weeks and palliative surgery when necessary, or an experimental arm of CRS and HIPEC with mitomycin-C (MMC), followed by adjuvant chemotherapy within 3 months of surgery. This study demonstrated a significant improvement in median survival, 12.6 months in the control group, compared to 22.4 months in the CRS/HIPEC group (P = 0.032). Their data showed a 2-year survival rate in the experimental arm that doubled that of the control arm. Additionally, patients with PM involving greater than six regions of the abdomen had worse survival compared to those with less than five regions based on Peritoneal Cancer Index (PCI), as previously reported by Sugarbaker et al and Elias et al.22,23 Verwaal et al later published follow-up data from the original RCT in 2008.24 Median follow-up time was 8 years (72–115 months). Median progression-free survival was 7.7 months for patients with standard therapy and 12.6 months after CRS and HIPEC with adjuvant chemotherapy (P = 0.02). Disease-specific survival was 12.6 months for control group and 22.2 months for CRS/HIPEC group (P = 0.28). Ultimately, this group determined that the major impact on survival was completeness of cytoreduction; patients with complete cytoreduction had a median survival of 48 months and 5-year survival of 45%, with no treatment-related deaths occurring. While this trial was groundbreaking, it was not without its faults. This study did not exclude patients with more extensive disease (>6 or 7 regions), a group previously shown to have poor rates of complete cytoreduction and survival in prior studies by Sugarbaker et al and Elias et al.22,23 The investigators also included patients with appendiceal neoplasms, possibly confounding the reported survival statistics.
The Verwaal group analyzed a subset of these patients whom underwent standard treatment, with systemic therapy only.25 At this time, many were pushing for clinical trials investigating the use of CRS/HIPEC for PMs from colorectal cancer; however, full benefit of this standard therapy was not yet fully understood to allow for sufficient comparison. This subgroup analysis demonstrated the effectiveness of standard therapy, including conventional surgery and systemic chemotherapy. The median survival of 12.6 months (37–58 months) was longer than that of previous studies, likely due to patient selection.26 Importantly, the subset of these patients that underwent a more radical resection had a survival of 17.3 months, demonstrating a potential survival benefit for extensive resection beyond that of standard treatment, a factor not fully investigated in the initial publication.
PMP has been traditionally managed with CRS and HIPEC; early benefits were demonstrated through retrospective data and comparisons to historical controls.27–30 In 2006, Yan et al published the results of an open, non-randomized phase II study of 50 patients with PMP, including patients with diffuse peritoneal adenomucinosis (DPAM), peritoneal mucinous carcinomatosis with intermediate features (PMCA-I), and peritoneal mucinous carcinomatosis (PMCA).31,32 All underwent CRS and HIPEC with MMC and early post-operative chemotherapy (EPIC) with 5-FU. Overall, they demonstrated overall 1-, 3-, and 5-year survival rates of 89%, 69%, and 69%, respectively. When analyzed by Ronnett classification, 5-year survival rates were 100%, 69%, and 0% for DPAM, PMCA-I, and PMCA, respectively. This study while fruitful, demonstrated the difficulties in conducting randomization within a clinical trial, as well as the impact of tumor biology on prognosis despite maximal therapy.31 The small number of patients, and the number who could be followed, may have affected the survival statistics. Additionally, this multi-institutional study lacked consistent operative technique due to varied surgeon experience with this complex procedure.
Up to 30% of patients with primary gastric cancer may present with synchronous PM, spurring the development of clinical trials utilizing CRS and HIPEC as an aggressive treatment approach.33 In a phase III trial, Yang et al enrolled 68 patients with PM of gastric origin for randomization to treatment with CRS alone or CRS with HIPEC.34 Median survival in the CRS group was 6.5 months as compared to 11 months in the CRS and HIPEC cohort (P = 0.046). Furthermore, PCI and completeness of cytoreduction (CCR) scores, were both found to have a significant impact on survival.35 In the 23 patients with a high PCI (≥20), the median overall survival of the CRS and HIPEC group was 13.5 months as compared to 3-month survival in the CRS-only group (P = 0.012). Although a small study cohort, the addition of HIPEC to complete cytoreduction showed improved survival.34
In 2006, the National Cancer Institute (NCI) issued an announcement stating IP chemotherapy should be regarded as a standard adjuvant therapy for stage III epithelial ovarian cancer.36 This shift in treatment paradigm was the result of the aggregate data from eight clinical trials published from 1994 to 2006, in which combined IV/IP or IP-only therapy was compared to systemic therapy only, after surgical debulking.37–43 The most recent of these studies (2006), a phase III RCT by the Gynecologic Oncology Group, randomly assigned 415 patients with stage III ovarian carcinoma or primary peritoneal carcinoma, after maximal debulking and with no residual mass greater than 1.0 cm, to treatment with adjuvant systemic paclitaxel/cisplatin or systemic paclitaxel with IP paclitaxel/cisplatin. The authors demonstrated a median progression-free survival of 23.8 months in this group as compared to 18.3 months in the control arm (P = 0.05), and median overall survival of 65.6 and 49.7 months, respectively (P = 0.03).43 This study did not attempt to assess duration of treatment on clinical outcome, with many patients halting IP treatment early due to toxicity or adverse events. The authors propose that most benefit may come from the early cycles of therapy only; an aspect that would require further randomized testing.
Chi et al later demonstrated the improved median overall survival of 54 months in patients who underwent optimal cytoreduction by including upper abdominal cytoreduction, as compared to 43 months in patients who underwent more traditional resection, involving just the lower abdominopelvic region. Yet despite maximal cytoreduction, most patients recurred within 2 years, many with platinum-resistant disease, highlighting the need for effective adjuvant therapy.44 Spiliotis et al conducted a prospective randomized phase III study investigating the efficacy of HIPEC for epithelial ovarian cancer (EOC).45 EOC often presents at an advanced stage with spread throughout the abdominopelvic region due to indolent spread.46 From 2006–2013, this group randomized 120 women with stage IIIC/IV recurrent EOC, who previously underwent CRS/debulking and adjuvant chemotherapy. Patients were randomized to CRS and HIPEC with subsequent systemic chemotherapy or CRS with systemic chemotherapy only. For HIPEC, paclitaxel and cisplatin were used in platinum-sensitive disease, while doxorubicin and paclitaxel or MMC were used for platinum-resistant disease. In the HIPEC treatment group, mean survival were 26.9 and 26.4 months in the stage IIIC and IV groups, respectively. In those treated with CRS and systemic chemotherapy only, mean overall survival were 14.2 and 11.9 months in patients with stage IIIC and IV, respectively. The addition of HIPEC produced a significant survival benefit for both stage IIIC and IV disease with both platinum-sensitivity and platinum-resistance. Even after the positive results of this study, there remained questions regarding the ideal intraperitoneal chemotherapy regimen to optimize survival in these patients.
Glehen et al conducted a phase II, prospective, non-randomized clinical trial between 1998 and 2001 investigating the role of aggressive cytoreduction, including peritonectomy, and “intraperitoneal chemohypothermia (IPCH)” in the management of PM of various primary malignancies.47 The 56 enrolled patients (36 synchronous and 20 metachronous) included 26 colonic adenocarcinoma, 7 PMP, 7 ovarian carcinoma, 6 gastric adenocarcinoma, 5 peritoneal mesothelioma, 3 small bowel adenocarcinoma, and 2 of unknown primary. The 27 patients who underwent a complete macroscopic resection (R0 and R1) had a 2-year survival rate of 79.0%, as compared to 44.7% in the 29 patients who received an R2 resection (mean survival 558.2 days vs 360.1 days, respectively, P = 0.006). This earlier study helped spur the development of further trials since its completion, but its results are subject to interpretation partly due to the small cohort of patients and heterogeneity of peritoneal malignancies.
Peritoneal mesothelioma is exceedingly rare, with approximately 2500 cases of malignant mesothelioma per year.48 Concordantly, clinical trials investigating the role of CRS and HIPEC with this disease have included a small number of patients, but have demonstrated significant results. Following a series of small, phase I trials published by the National Cancer Institute, Loggie et al published results of a phase II prospective, non-randomized clinical trial of 12 malignant peritoneal mesothelioma (MPM) patients, 8 with symptomatic ascites, who were treated with CRS and HIPEC (MMC) between 1997 and 2001.49,50 All patients had resolution of ascites. This group demonstrated an encouraging 34.2 months median overall survival and 33% 5-year survival, as compared to 5–12 months median survival for historically untreated cases.49 Deraco et al similarly conducted a phase II trial of 19 patients with MPM treated with CRS and HIPEC (cisplatin + MMC or cisplatin + doxorubicin) between 1995 and 2002.51 Three-year progression-free and overall survival were 66% and 69%, respectively.51 Lastly, Hesdorffer et al published a phase I/II feasibility study of 27 patients treated with CRS and IP chemotherapy (cisplatin and doxorubicin) 2–3 weeks post-operatively, as well as IP gamma IFN-1b and whole abdominal radiation.52 Median disease-free and overall survivals were 30 and 70 months, respectively.
These studies, although investigating treatment of PM from various malignancies, demonstrated benefit of complete cytoreduction. Patients achieved improved survival with satisfactory quality of life from CRS, in some instances even without HIPEC. The addition of HIPEC, though increasing toxicity in some trials, also added a survival benefit for those undergoing full cytoreduction. It is becoming clearer from these studies that while these treatment strategies have improved the management of peritoneal metastases, there remain questions as to the ideal chemotherapies, timing, duration, and use of these therapies combined with systemic therapy and surgery. These aspects are under investigation in the expanding number of U.S. and international clinical trials.
4.2 |. Active clinical trials
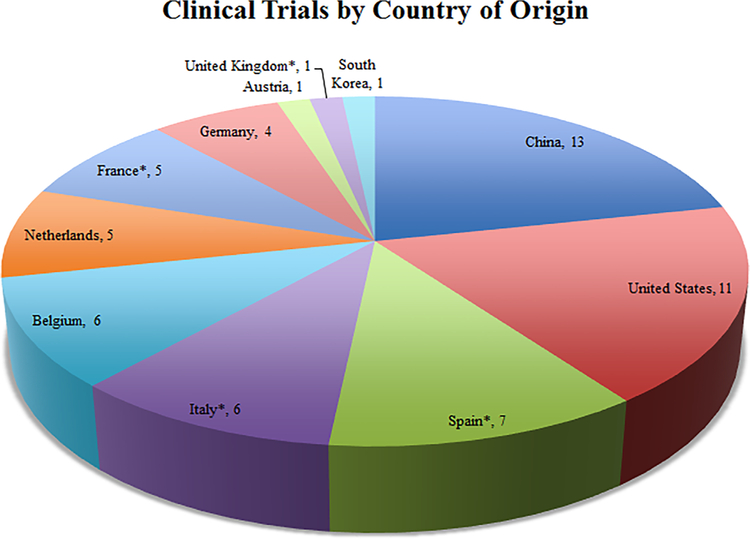
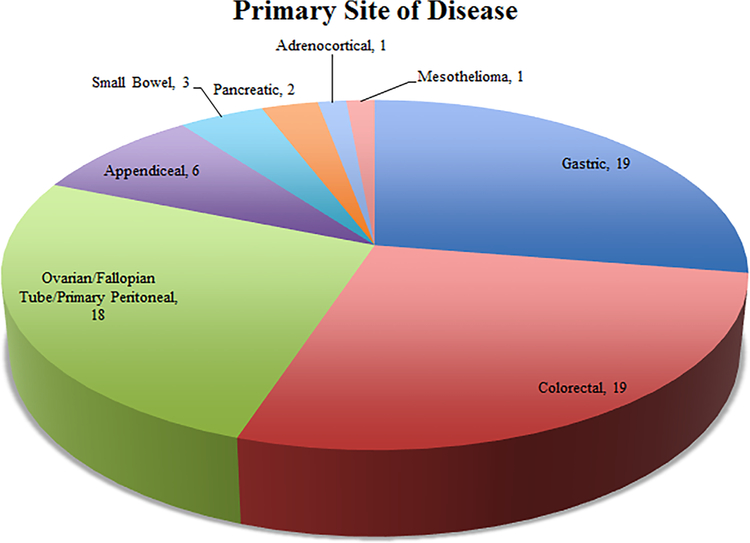
Despite the successes of previous clinical trials, further studies are required to define the role of CRS and HIPEC in various peritoneal malignancies. Currently, there are a number of clinical trials either actively recruiting or continuing to accrue follow-up data (Table 2). These studies are being conducted internationally, with over 10 countries represented, almost half occurring in the U.S. (Figure 2). The number of trials occurring in the E.U. and past difficulties with patient recruitment has led to significant collaboration across institutions to meet accrual requirements (Figure 3). These clinical trials seek to answer the remaining questions in defining the role of CRS and HIPEC in the treatment of peritoneal metastases. While we highlight the technical aspects and potential impact of a number of studies, all 57 included clinical trials can be found in Table 2.
TABLE 2.
Previously published clinical studies investigating cytoreductive surgery and hyperthermic intraperitoneal chemotherapy
| ClinicalTrials.gov ID/EudraCT # | Phase | Country | Primary institution/group | Malignancy |
|---|---|---|---|---|
| II | US | Bay Area Gynecology Oncology | Ovarian, fallopian tube, uterine, mesothelioma, GI, cervical, primary peritoneal | |
| II | US | Mercy Medical Center | Ovarian, fallopian tube, primary peritoneal | |
| II | US | City of Hope Medical Center | Ovarian, uterine, fallopian tube, primary peritoneal | |
|
2006–003466-34 |
III | Netherlands | NKI-AVL | Ovarian, fallopian, tube, primary peritoneal |
|
2011–001715-31 |
III | Spain | Fundacion para la Formacion e Invetigacion Sanitaria de la Región de Murcia | Ovarian, fallopian, tube, primary peritoneal |
| 2012-003244-76 | I/II | Austria | Medizinische Universität Wien | Ovarian, fallopian, tube, primary peritoneal |
| 2009-017437-22 | II | Italy | Azienda Ospedaliera Di Perugia | Ovarian, colorectal, small bowel, gastric, appendiceal, peritoneal mesothelioma, sarcomatosis (retroperitoneal) |
|
2012-002872-15 |
III | Italy | Catholic University of the Sacred Heart | Recurrent ovarian |
| I | US | Loma Linda University Cancer Center | Recurrent ovarian | |
| II | US | Memorial Sloan Kettering Cancer Center | Recurrent ovarian | |
|
2015-000418-23 |
II | Belgium | University Hospital, Ghent | Ovarian |
| 2012-004103-12 | II | France | Centre Jean Perrin | Ovarian |
|
2012-002616-22 |
III | Italy | A.O. Ospedale Papa Giovanni XXIII | Ovarian fallopian tube |
| 2007-005674-31 | II | Italy | Azienda Ospedaliera Senese | Ovarian |
|
2011-006319-69 |
III | Spain | Hospital General de la Ciudad Real | Ovarian |
| 2014-002850-38 | II | Spain | Fundación para la Invetigación Biomédica de Córdoba | Ovarian |
|
2010-023035-42 |
III | Spain/Italy | UNICANCER | Recurrent ovarian |
|
2009-012859-21 |
II/III | Spain/UK | NCIC Clinical Trial Group | Ovarian |
| III | China | Affiliated Tumor Hospital of Guangzhou Medical University | Gastric | |
| II | US | M.D. Anderson Cancer Center | Gastric | |
| I | US | Loma Linda University Cancer Center | Gastric | |
|
2006-006088-22 |
III | Germany | Charite University | Gastric |
| II/III | Germany | University Hospital Tuebingen | Gastric | |
| 2013-000138-37 | II | Netherlands | NKI-AvL | Gastric |
| III | China | Affiliated Tumor Hospital of Guangzhou Medical University | Gastric | |
| III | China | Affiliated Tumor Hospital of Guangzhou Medical University | Gastric | |
| III | China | Sixth Affiliated Hospital, Sun Yat-sen University | Gastric | |
| II | China | Zhejiang Cancer Hospital | Gastric | |
| III | China | Nanfang Hospital of Southern Medical University | Gastric | |
| III | China | Tang-Du Hospital | Gastric | |
| II | China | Wuhan University | Gastric | |
| II | China | Peking University | Gastric | |
| III | China | The Affiliated Nanjing Drum Tower Hospital of Nanjing University Medical School | Gastric | |
| III | France | Hospices Civils de Lyon | Gastric | |
| Ib/II | South Korea | Yonsei University | Gastric | |
| 2014-000882-34 | II | Belgium | University Hospital Ghent | Appendiceal, colonic, small bowel, gastric |
| II | US | University of California, San Diego | Appendiceal | |
|
2009-014040-11 |
II | Germany | University of Regensburg | Colorectal appendiceal |
| II | US | Memorial Sloan Kettering Cancer Center | Colorectal appendiceal | |
|
2012-000701-77 |
II | Belgium | University Hospital, Ghent | Colorectal appendiceal |
| II | China | Fudan University | Colorectal | |
| III | China | Zhejiang University | Colorectal | |
| II | China | Wuhan University | Colorectal | |
|
2016-001865-99 |
II/III | Netherlands | Catharina Ziekenhuis Eindhoven | Colorectal |
|
2014-002794-11 |
III | Netherlands | Academisch Medisch Centrum—Universiteit van Amsterdam | Colorectal |
| 2010-020787-37 | III | Netherlands | Universitair Medisch Centrum Groningen | Colorectal |
| II | Belgium | University Hospital, Ghent | Colorectal | |
| 2012-000701-77 | II | Belgium | University Hospital, Ghent | Colorectal |
| 2014-000882-34 | II | Belgium | University Hospital, Ghent | Colorectal |
| III | France | Gustav Roussy, Cancer Campus, Grand Paris | Colorectal | |
| I/II | France | Hospices Civils de Lyon | Colorectal | |
| 2006-006175-20 | III | France/Spain | UNICANCER | Colorectal |
| III | Italy | University of Roma La Sapienza | Colorectal | |
|
2015-001801-15 |
III | Spain | Maimónides Biomedical Research Institute of Córdoba | Colorectal |
|
2015-002288-41 |
I/II | Germany | University Hospital Tuebingen | Pancreatic |
| II | US | Carolinas Medical Center | Pancreatic | |
| II | US | National Cancer Institute | Adrenocortical |
FIGURE 2.
Number of controlled, clinical trials actively enrolling patients or continuing to accrue follow-up data, based on the ClinicalTrials.gov and EudraCT registries. *Denotes inclusion in studies being conducted in more than one country
FIGURE 3.
Primary site of disease being investigated in the studies in the ClinicalTrials.gov and EudraCT registries
4.2.1 |. Colorectal/appendiceal
The ICARuS (Intraperitoneal Chemotherapy After cytoreductive Surgery) trial (), at Memorial Sloan Kettering Cancer Center (MSKCC), seeks to determine the benefit of HIPEC with MMC versus early, post-operative, normothermic IP chemotherapy (EPIC) with floxuridine after optimal CRS in patients with appendiceal and colorectal cancers. Enrollment began in March 2013, with an estimated completion date of March 2018. The primary outcome measure of the study is disease-free survival, within 3 years, though secondary measures will monitor surgical and chemotherapy-related toxicities (grades 3–5) up to 60 days post-operative. Data from this study may guide surgeons and oncologists in developing the most effective treatment timeline incorporation IP therapy.
The PRODIGE 7 study, a randomized, multicenter, phase III trial at the Insitut du Cancer de Monpellier Val d’Aurelle (Monpellier, France), was designed to evaluate the added benefit of HIPEC to complete CRS. Accrual of the 270 patients with colorectal cancer and limited peritoneal dissemination was completed in 2013, with ongoing collection of survival data. Patients who underwent complete cytoreduction were randomized intraoperatively to receive HIPEC or saline lavage only. In this study, oxaliplatin (460 mg/m2) in 2 L/m2 of dextrose 5% over 30 min at a minimal temperature of 42°C was used. One hour before the HIPEC, 20 mg/m2 of leucovorin and 400 mg/m2 of 5-fluorouracil were given intravenously. While this study is promising, it does come with potential issues. The participation of multiple institutions with varying degrees of experience and lack of an inter-institutional protocol for usage and timing of systemic therapies may cloud the definitive role of HIPEC in the management of these patients. In addition, it is possible patients randomized to the no-HIPEC arm could later receive another surgery with HIPEC after they recur. Similarly, the CAIRO6 Study (Netherlands) will focus on the role of perioperative systemic therapy on survival in patients undergoing CRS and HIPEC for CRC (). This phase II/III study will randomize patients to undergo neoadjuvant systemic therapy with FOLFOX (5-fluorouracil, leucovorin, oxaliplatin) or CAPOX (capecitabine, oxaliplatin) with bevacizumab followed by CRS and HIPEC, then adjuvant systemic therapy with FOLFOX or CAPOX.53,54 The control arm will undergo CRS and HIPEC only. The primary measures will be Clavien-Dindo grade III-V postoperative complications and overall survival, with up to 3 years follow-up.55 An important contribution of these studies will be showing the value of full cytoreductive surgery while also receiving systemic chemotherapy. Therefore, these may become landmark studies highlighting the importance of multidisciplinary management for patients with PM from CRC.
In Amsterdam, at the Academisch Medisch Centrum— Universiteit van Amsterdam, the COLOPEC study aims to determine the effectiveness of adjuvant HIPEC in preventing PM (). Up to 176 patients with T4 or intra-abdominally perforated colon cancer that have undergone curative resection, will be randomized to receive adjuvant HIPEC with oxaliplatin or not. Patients will be followed for recurrence-free survival up to 18 months post-operatively, as well as endpoints related to safety/toxicity, disease-free survival, overall survival, and presence of concomitant liver or lung metastases. The ProphyloCHIP study (France) began patient enrollment in 2010, with a goal of 130 patients (). This multicenter, randomized, phase III study aims to compare the effectiveness of exploratory laparotomy and HIPEC as a prophylactic, follow-up procedure, as compared to standard surveillance in patients at high-risk of developing PM from CRC after initial surgery and adjuvant chemotherapy. After primary resection of disease, patients will undergo 6 months of standard, adjuvant chemotherapy (currently FOLFOX-4 regimen), if work-up is negative, patients will be randomized to standard surveillance or laparotomy with HIPEC. The primary outcome measure will be 3-year disease-free survival, with secondary measures of 3-year, 5-year, and peritoneal disease-free survival. These two studies will assist physicians in determining the role of HIPEC as a prophylactic measure, both initially and in the adjuvant setting.
Wake Forest University Health Sciences, in collaboration with the National Cancer Institute (NCI), is conducting a phase II, non-blinded, randomized trial investigating the hematologic toxicity profiles and effectiveness of CRS and HIPEC with MMC or HIPEC with oxaliplatin for the management of PM from appendiceal carcinoma or primary peritoneal cavity malignancies (). Because CRS and HIPEC are considered the mainstay of treatment for these types of malignancies, this group is seeking to identify the safer IP chemotherapeutic.17,18 The primary outcome measure will be the difference in rate of grade 3 or 4 hematologic toxicities between the two groups, with secondary outcome measures of disease-free survival and quality of life assessments (3 years). As of December 2015 this study has recruited 116 patients. This study may aid in determination of the most effective intraperitoneal agent in the treatment of appendiceal carcinoma, as well as a potential for reduction in treatment toxicity.
4.2.2 |. Ovarian
A multi-center, phase II RCT at MSKCC is currently enrolling patients with ovarian, fallopian tube, and primary peritoneal cancers (). This study evolved from an initial small, phase I study demonstrating the safety of CRS and HIPEC in patients with recurrent, platinum-sensitive, epithelial ovarian cancers undergoing secondary cytoreduction.56 The study will randomize patients to secondary CRS with or without carboplatin-based HIPEC, followed by systemic chemotherapy. The HIPEC arm will receive 5 cycles, and the CRS-only arm 6 cycles of platinum-based systemic therapy. Patient enrollment began in January of 2013, with a goal of 98 patients. Similarly, UNICANCER, comprised of HIPEC centers in Belgium, France, and Spain, are recruiting patients for a phase III study (). Much like the MSKCC trial, this group is investigating the effect of CRS with or without HIPEC on overall survival (primary outcome) and relapse-free survival (secondary outcome) of patients with resectable, recurrent, platinum-sensitive ovarian cancer isolated to the peritoneum. With an initial start date of April 2011, this group aims to enroll 444 patients and complete data collection, with up to 4 years of follow-up, by December 2020. Studies such as these remain crucial, as, despite the previously discussed NCI announcement regarding the benefits of IP chemotherapy for ovarian cancer, this modality has been met with skepticism by the community-at large. These issues have led to significant issues with patient accrual in both studies.
Mercy Medical Center (Baltimore, MD) is currently investigating the combination of CRS and HIPEC (carboplatin) with adjuvant chemotherapy (carboplatin and paclitaxel) as compared to CRS with adjuvant, combination systemic (paclitaxel) and IP (cisplatin and paclitaxel) chemotherapy in patients with stage III/IV ovarian, fallopian tube, or primary peritoneal cancer (). The 48 patients recruited for this phase II study will be newly diagnosed, without prior intervention. The goal of this trial will be to determine the safety of HIPEC in the perioperative period, with a primary outcome measure of 30-day postoperative complication rates as compared to those undergoing systemic, adjuvant therapy. Secondary outcome measures will track progression-free survival (at 24 months), overall survival (up to 5 years), and quality of life. This study is unique in that it acknowledges the effectiveness of IP chemotherapy in the management of PM of ovarian or fallopian tube origin, but seeks to identify the safest timing of IP delivery.
4.2.3 |. Gastric
Gastric cancer remains the third leading cause of cancer-related death in China despite improvements in screening and aggressive management.57 This has led to a number of studies developed in China to employ HIPEC in managing this disease. Peng et al from the Sixth Affiliated Hospital of Sun Yat-Sen University in China will soon begin a randomized phase III trial to investigate the addition of HIPEC to standard treatment for patients with primary gastric cancer (). All patients will undergo neoadjuvant chemotherapy, followed by gastric resection with D2 lymphadenectomy, and adjuvant chemotherapy. The experimental arm will receive HIPEC, while controls will receive only peritoneal lavage with distilled water. This group seeks to determine the safety and efficacy of HIPEC prophylactic measure to prevent recurrence due to potential peritoneal seeding at initial resection.58 Their estimated enrollment of 640 patients will be followed for progression-free survival for 1–3 years and overall survival up to 5 years. Cui et al of the Affiliated Tumor Hospital of Guangzhou Medical University in China are currently recruiting patients for a similar study. This randomized phase III trial, which began in July 2014 and estimates enrolling 582 patients, will similarly study the effect of HIPEC after gastrectomy with D2 lymphadenectomy for locally advanced gastric cancer (). Primary outcome will be overall survival for up to 5 years, with secondary measures of recurrence-free, locoregional-free, and hepatic metastases-free survival. A similar phase III study from the Affiliated Nanjing Drum Tower Hospital of Nanjing University Medical School is unique in that it will be double-blinded (), investigating the role of HIPEC in stage T3-T4 gastric cancer treatment. This group hopes to show improved overall survival (at 24 months), while also monitoring complication rates, time to progression, and time to distant metastases. While HIPEC has previously been shown as an effective tool to prolong survival in patients with PM from gastric cancer, and is currently recommended in treatment guidelines from the Health Committee of China, its role as a prophylactic measure has yet to be determined.58
While the United States has a lower incidence of gastric cancer, it accounts for 1.3% of new cancer diagnoses annually.59 Senthil et al at Loma Linda University Cancer Center aim to enroll 15 patients for a phase I trial to determine the safety and tolerability of HIPEC with MMC and cisplatin within the 90-day postoperative period (). Enrollment of patients with T3/T4 and/or have clinically positive nodes is ongoing with a study completion date of December 2018. M.D. Anderson Cancer Center is currently recruiting up to 18 patients for a phase II study investigating the efficacy of HIPEC with MMC and cisplatin, in addition to gastrectomy and cytoreduction for patients with PM from gastric cancer ().
The GASTRICHIP study, conducted by Glehen et al at Hospices Civils de Lyon (France), is a prospective, open, randomized, multicentric, phase III trial (). This trial will compare the control arm of gastrectomy, D1–2 lymphadenectomy and systemic therapy (5-FU/folinic acid) to the experimental arm randomized to additionally receive HIPEC (oxaliplatin). Beginning in 2013, the primary outcome measure will be 5-year overall survival, and secondary measures of recurrence-free survival, locoregional-free survival, and treatment-related morbidity and mortality. Patients will have resectable T3 or T4 gastric adenocarcinoma, with out without positive peritoneal cytology discovered during diagnostic laparoscopy. This study follows on previously published data by Glehen et al reporting the findings of a retrospective, multicentric study including 159 patients with gastric PM.60 Charite University in Germany has began a phase III trial, the GASTRIPEC study, in March 2014. All patients will receive neoadjuvant therapy, followed by CRS, and adjuvant therapy, but patients in the experimental arm will also receive HIPEC. Primary outcome will be overall survival (up to 2.5 years), with secondary measures that include time to progression, quality of life, time to distant metastases, toxicity, and requirement of second surgery. These studies will similarly demonstrate the benefit of HIPEC as a prophylactic measure in patients that may not present with macroscopic PM.
4.2.4 |. Pancreatic
Beckert et al of University Hospital Tübingen (Tübingen, Baden-Württemberg, Germany) seek to expand the accepted use of CRS and HIPEC to metastatic pancreatic adenocarcinoma (PANHIPEC) (EUDRA-CT 2015–002288-41).61 Only 15–20% of patients with pancreatic adenocarcinoma are eligible for curative resection and 66–92% will eventually develop recurrent disease, typically locoregionally.62 The primary endpoint of this study is 30-day mortality, with secondary endpoints of safety and toxicity based on Common Terminology Criteria for Adverse Events (CTCAE) 4.0. This study seeks to validate this aggressive treatment strategy for pancreatic cancer, as often, due to anatomic location and advanced disease at diagnosis, an R0 resection may not be possible. The investigators seek to weigh the increased risk of morbidity and mortality with the survival benefit demonstrated in previous studies.63,64
Carolinas Medical Center plans to similarly investigate the surgical outcomes and clinicopathological results of treating patients with T1-T3, resectable pancreatic ductal adenocarcinoma with pancreaticoduodenectomy and HIPEC with gemcitabine, in conjunction with perioperative systemic therapy (). This phase II, proof-of-concept study is enrolling a small cohort of ten patients and will primarily examine peritoneal disease-free survival, as well as overall survival. These patients will be compared to historical controls that have been treated with 6 months of adjuvant gemcitabine as by their institutional protocol.
4.2.5 |. Modifying intraperitoneal drug delivery
Further studies are being conducted internationally that seek to improve the delivery of chemotherapeutics during HIPEC, as well as eliminate remaining disease after maximal cytoreduction. These studies investigate the dosage, temperature, pressure, and timing of intraperitoneal chemotherapy in an effort to define the most effective treatment strategies.
The Fondazione IRCCS Istituto Nazionale dei Tumori (Milan, Italy) is investigating the ability to improve the uptake of chemotherapy by neoplastic tissue after CRS. This will be achieved by using high intra-abdominal pressure (IAP) (18–22 mmHg). Currently enrolling up to 38 patients, this phase II study will randomize each patient to undergo CRS and HIPEC, with low IAP (8–12 mmHg) in the control arm and high pressure in the experimental arm (). Postoperatively, tumor tissue concentration of cisplatin, collected within 15 min of procedure, will be compared to that of normal tissue. Secondary outcome measures will track pharmacokinetic advantage and patient physiologic parameters, toxicity, and postoperative complications.
A phase III at Hasselt University, in collaboration with Ziekenhuis Oost-Limburg (Belgium), is enrolling up to 60 patients in order to compare the effectiveness of a concentration-based versus body surface area-based protocol for dosing intraperitoneal chemotherapy. Many institutions utilize a body surface area-based (BSA) approach, however, this group postulates that sex, pathophysiologic changes, and presence of ascites can affect the initial homogenous drug concentration delivered to the patient. Others using a concentration-based approach, face unpredictability in the levels of plasmatic chemotherapy and the toxicity profile of chosen dosage. These two methods will be compared by randomizing patients to receiving either a BSA-based or concentration-based regimen of HIPEC after CRS, for duration of 30 min. Primary outcome measures will be an assessment of pharmacologic advantage by an area-under-the-curve ratio of IP fluid oxaliplatin concentration over time, as well as drug excretion in urine, intraoperative drug concentration within tumor nodules, and 3 months overall morbidity and mortality. This study will aid surgeons in determining the ideal conditions for IP chemotherapy delivery.
Studies into applicability of minimally invasive approaches to CRS have also begun. This approach has been demonstrated as feasible and safe in both prospective and retrospective studies at high volume centers.65–68 Currently, diagnostic laparoscopy is most commonly used for determination of tumor burden prior to open CRS. Laparoscopic CRS may improve short-term morbidity in patients undergoing this procedure, while maintaining the surgical precision of an open laparotomy. University of California, San Diego Moores Cancer Center is compiling data from a phase I clinical trial to confirm the potential of this approach for reduction in 30-day post-operative morbidity and mortality, while maintaining adequate ability for full cytoreduction (). Ideally, this method would allow for faster recovery and return to systemic therapy, which could facilitate a complete response to CRS and HIPEC.
4.3 |. Designing the ideal trial
There are obviously significant ethical dilemmas and issues of equipoise in designing the optimal clinical trial. Physicians and patients must weight the safety and efficacy of treatment options when determining the proper strategy for management of PM. CRS and HIPEC have been shown to prolong overall and disease-free survival in a number of retrospective studies and previous clinical trials, yet the ideal study remains unperformed. Oncologists and surgeons desire a study that will clearly define the proper sequence and duration of the currently available strategies. Additionally, patients must be stratified prior to treatment in order to compare therapies and identify subgroups that will benefit from a specific modality. Typically this may be done based upon performance status and burden of disease. Accurate determination of tumor burden for treatment stratification has now become possible with improvements in imaging and a clear understanding of the importance of the radiologist in the management team.69,70 Furthermore, with improved implementation of genomic profiling, tumor biology could direct stratification, with treatments tailored to the specific tumor susceptibilities for individual patients.71 A number of questions need to be answered via clinical trials in order to accomplish this daunting task: what is the role of HIPEC for each diagnosis? Which drugs should be used? At what temperature should they be delivered? How long should drug(s) remain in the abdomen? Which technique is more effective, open or closed? Is there a role for repeat CRS and HIPEC in all diagnoses?
Using the example of PM from CRC, one such possibility for a clinical trial would be based on patient stratification using the peritoneal surface disease severity score (PSDSS) to determine resectability of those with an initial diagnosis of PM.72–74 Upon diagnosis patients would receive 2–3 months of best systemic therapy. Patients would then be restaged and if still surgical candidates, randomized to one of three arms: continued systemic therapy, CRS only followed by systemic therapy, or CRS and HIPEC followed by systemic therapy. With intention-to-treat, patients cross over to CRS and HIPEC at first signs of progression (Figure 4). This model would allow for direct unprejudiced comparison between traditional chemotherapy and CRS/HIPEC, while also clarifying the role of HIPEC in improving survival and delaying disease progression. As some degree of bias is nearly always present in clinical studies, more rigorously designed balanced trials at levels of study design and outcome, in part, should yield valid, higher-level evidence.75
FIGURE 4.
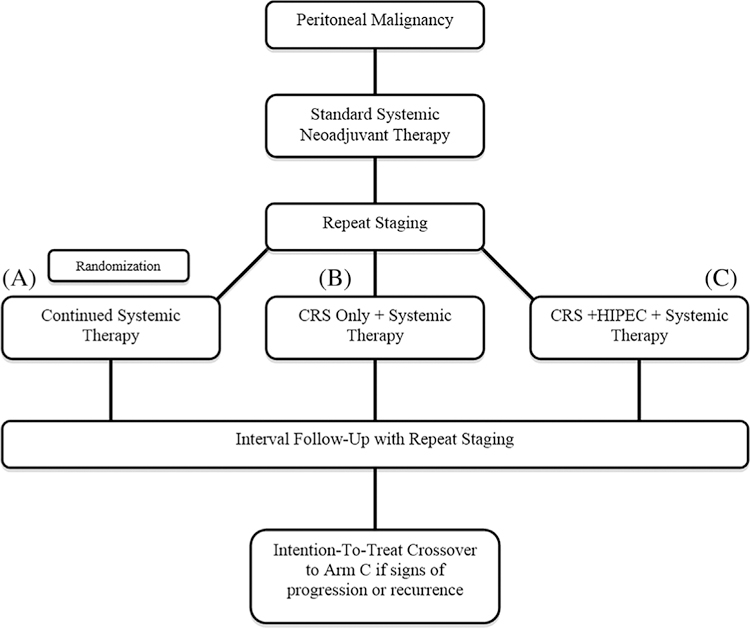
Algorithm for design of an ideal trial to clarify the benefit of both cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of peritoneal malignancies. Patients undergo systemic therapy followed by randomization to (A) continued systemic therapy, (B) CRS and systemic therapy, or (C) CRS, HIPEC, and systemic therapy
This systematic review of clinical studies in CRS and HIPEC demonstrates the strength of existing evidence. Limitations of this review may be the inclusion of only published trials with outcomes reporting statistically significant, favorable findings, and therefore more likely to be published, which may introduce bias. The eligibility criteria proposed methodologically herein may limit some of this inherent bias. Existing evidence to-date should act as a stimulus for conducting appropriate and necessary trials. The ongoing clinical trials discussed here will vary in their impact on the field. Many compare variations of CRS and HIPEC in both control and experimental arms, as it would be potentially unethical to withhold a life-prolonging therapy to one randomized group. This likely introduces a selection bias into a number of these studies, as they will limit the included patients to a subset of patients with PM known to benefit from this treatment strategy.
5 |. CONCLUSION
CRS and HIPEC are a solution looking for a problem. This disease management strategy has demonstrated survival benefit in a number of peritoneal surface malignancies. Modern prospective, randomized trials are currently underway across the world. Validation through clinical trials will further define important parameters that include improving patient selection, strategic sequences of treatment, cytoreductive strategies, chemotherapeutics, optimal hyperthermic temperature and timing, and toxicity profiles for CRS and HIPEC. Main barriers to trial development remain patient enrollment, trial design (feasibility vs “ideal” study parameters), oncologic community collaboration, and financial considerations. Regardless, overall progress is positive with increasing number of clinical trials throughout the world. Collaboration between surgeons and the wider oncologic community will be crucial to validate this important treatment strategy.
REFERENCES
- 1.Neuwirth MG, Alexander HR, Karakousis GC. Then and now: cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC), a historical perspective. J Gastrointest Oncol 2016;7:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221: 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dedrick RL, Myers CE, Bungay PM, DeVita VT. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep 1978;62:1–11. [PubMed] [Google Scholar]
- 4.Spratt JS, Adcock RA, Sherrill W, Travathen S. Hyperthermic peritoneal perfusion system in canines. Cancer Res 1980;40:253–255. [PubMed] [Google Scholar]
- 5.Palta JR. Design and Testing of a Therapeutic Infusion Filtration System Columbia, MO: University of Missouri; 1977. [Google Scholar]
- 6.Spratt JS, Adcock RA, Muskovin M, Sherrill W, McKeown J. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res 1980;40:256–260. [PubMed] [Google Scholar]
- 7.Zimm S, Cleary SM, Lucas WE, et al. Phase I/pharmacokinetic study of intraperitoneal cisplatin and etoposide. Cancer Res 1987;47: 1712–1716. [PubMed] [Google Scholar]
- 8.Howell SB, Zimm S, Markman M, et al. Long-term survival of advanced refractory ovarian carcinoma patients with small-volume disease treated with intraperitoneal chemotherapy. J Clin Oncol 1987;5: 1607–1612. [DOI] [PubMed] [Google Scholar]
- 9.Colombo N, Speyer JL, Green M, et al. Phase II study of carboplatin in recurrent ovarian cancer: severe hematologic toxicity in previously treated patients. Cancer Chemother Pharmacol 1989; 23:323–328. [DOI] [PubMed] [Google Scholar]
- 10.Sugarbaker PH, Gianola FJ, Speyer JC, Wesley R, Barofsky I, Meyers CE. Prospective, randomized trial of intravenous versus intraperitoneal 5-fluorouracil in patients with advanced primary colon or rectal cancer. Surgery 1985;98:414–422. [PubMed] [Google Scholar]
- 11.Sugarbaker PH. Treatment of peritoneal carcinomatosis from colon or appendiceal cancer with induction intraperitoneal chemotherapy. Cancer Treat Res 1996;82:317–325. [DOI] [PubMed] [Google Scholar]
- 12.Koga S, Hamazoe R, Maeta M, Shimizu N, Kanayama H, Osaki Y. Treatment of implanted peritoneal cancer in rats by continuous hyperthermic peritoneal perfusion in combination with an anticancer drug. Cancer Res 1984;44:1840–1842. [PubMed] [Google Scholar]
- 13.Koga S. Prophylactic and therapeutic continuous hyperthermic peritoneal perfusion for peritoneal metastases of gastric cancer. Gan No Rinsho 1985;31:1103–1105. [PubMed] [Google Scholar]
- 14.Fujimoto S, Shrestha RD, Kokubun M, et al. Intraperitoneal hyperthermic perfusion combined with surgery effective for gastric cancer patients with peritoneal seeding. Ann Surg 1988;208:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toi M, Shiramizu T, Yonemura T, et al. Intraperitoneal cisplatin in peritoneal carcinomatosis patients. Gan No Rinsho 1985;31:522–526. [PubMed] [Google Scholar]
- 16.Morano WF, Aggarwal A, Love P, Richard SD, Esquivel J, Bowne WB. Intraperitoneal immunotherapy: historical perspectives and modern therapy. Cancer Gene Ther 2016;23:373–381. [DOI] [PubMed] [Google Scholar]
- 17.Sugarbaker PH, Graves T, DeBruijn EA, et al. Early postoperative intraperitoneal chemotherapy as an adjuvant therapy to surgery for peritoneal carcinomatosis from gastrointestinal cancer: pharmacological studies. Cancer Res 1990;50:5790–5794. [PubMed] [Google Scholar]
- 18.Feldman AL, Libutti SK, Pingpank JF, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol 2003;21:4560–4567. [DOI] [PubMed] [Google Scholar]
- 19.Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. Br J Surg 2009; 96:342–349. [DOI] [PubMed] [Google Scholar]
- 20.Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003; 21:3737–3743. [DOI] [PubMed] [Google Scholar]
- 21.Network NCC. NCCN Guidelines Version 2. 2016 Colon Cancer Available at: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
- 22.Sugarbaker PH, Schellinx ME, Chang D, Koslowe P, von Meyerfeldt M. Peritoneal carcinomatosis from adenocarcinoma of the colon. World J Surg 1996;20:585–591. [DOI] [PubMed] [Google Scholar]
- 23.Elias DM, Ouellette JF. Intraperitoneal chemohyperthermia: rationale, technique, indications, and results. Surg Oncol Clin N Am 2001;10:915–933. [PubMed] [Google Scholar]
- 24.Verwaal VJ, Bruin S, Boot H, van Slooten GW, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 2008;15:2426–2432. [DOI] [PubMed] [Google Scholar]
- 25.Bloemendaal ALA, Verwaal VJ, van Ruth S, Boot H, Zoetmulder FAN. Conventional surgery and systemic chemotherapy for peritoneal carcinomatosis of colorectal origin: a prospective study. Eur J Surg Oncol 2005;31:1145–1151. [DOI] [PubMed] [Google Scholar]
- 26.Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg 2002;89:1545–1550. [DOI] [PubMed] [Google Scholar]
- 27.Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol 1999;6:727–731. [DOI] [PubMed] [Google Scholar]
- 28.Elias D, Laurent S, Antoun S, et al. Pseudomyxoma peritonei treated with complete resection and immediate intraperitoneal chemotherapy. Gastroenterol Clin Biol 2003;27:407–412. [PubMed] [Google Scholar]
- 29.van Ruth S, Acherman YI, van de Vijver MJ, Hart AA, Verwaal VJ, Zoetmulder FA. Pseudomyxoma peritonei: a review of 62 cases. Eur J Surg Oncol 2003;29:682–688. [DOI] [PubMed] [Google Scholar]
- 30.Güner Z, Schmidt U, Dahlke MH, Schlitt HJ, Klempnauer J, Piso P. Cytoreductive surgery and intraperitoneal chemotherapy for pseudomyxoma peritonei. Int J Colorectal Dis 2005;20:155–160. [DOI] [PubMed] [Google Scholar]
- 31.Yan TD, Links M, Xu ZY, Kam PC, Glenn D, Morris DL. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei from appendiceal mucinous neoplasms. Br J Surg 2006;93:1270–1276. [DOI] [PubMed] [Google Scholar]
- 32.Ronnett BM, Zahn CM, Kurman RJ, Kass ME, Sugarbaker PH, Shmookler BM. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to ‘pseudomyxoma peritonei’. Am J Surg Pathol 1995;19:1390–1408. [DOI] [PubMed] [Google Scholar]
- 33.Yonemura Y, Endou Y, Shinbo M, et al. Safety and efficacy of bidirectional chemotherapy for treatment of patients with peritoneal dissemination from gastric cancer: selection for cytoreductive surgery. J Surg Oncol 2009;100:311–316. [DOI] [PubMed] [Google Scholar]
- 34.Yang XJ, Huang CQ, Mei LJ, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 2011;18:1575–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugarbaker PH. Management of peritoneal surface malignancy: the surgeon’s role. Langenbeck’s Arch Surg 1999;384:576–587. [DOI] [PubMed] [Google Scholar]
- 36.NCI clinical announcement: intraperitoneal chemotherapy for ovarian cancer [press release] National Cancer Institute 2006.
- 37.Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 1996;335:1950–1955. [DOI] [PubMed] [Google Scholar]
- 38.Gadducci A, Carnino F, Chiara S, et al. Intraperitoneal versus intravenous cisplatin in combination with intravenous cyclophosphamide and epidoxorubicin in optimally cytoreduced advanced epithelial ovarian cancer: a randomized trial of the Gruppo Oncologico Nord-Ovest. Gynecol Oncol 2000;76:157–162. [DOI] [PubMed] [Google Scholar]
- 39.Kirmani S, Braly PS, McClay EF, et al. A comparison of intravenous versus intraperitoneal chemotherapy for the initial treatment of ovarian cancer. Gynecol Oncol 1994;54:338–344. [DOI] [PubMed] [Google Scholar]
- 40.Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol 2001;19:1001–1007. [DOI] [PubMed] [Google Scholar]
- 41.Polyzos A, Tsavaris N, Kosmas C, et al. A comparative study of intraperitoneal carboplatin versus intravenous carboplatin with intravenous cyclophosphamide in both arms as initial chemotherapy for stage III ovarian cancer. Oncology 1999;56:291–296. [DOI] [PubMed] [Google Scholar]
- 42.Yen MS, Juang CM, Lai CR, Chao GC, Ng HT, Yuan CC. Intraperitoneal cisplatin-based chemotherapy vs. intravenous cisplatin-based chemotherapy for stage III optimally cytoreduced epithelial ovarian cancer. Int J Gynaecol Obstet 2001;72:55–60. [DOI] [PubMed] [Google Scholar]
- 43.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006;534:34–43. [DOI] [PubMed] [Google Scholar]
- 44.Chi DS, Eisenhauer EL, Zivanovic O, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol 2009;114:26–31. [DOI] [PubMed] [Google Scholar]
- 45.Spiliotis J, Halkia E, Lianos E, et al. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol 2015;22:1570–1575. [DOI] [PubMed] [Google Scholar]
- 46.Kurman RJ, Shih IEM. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer—shifting the paradigm. Hum Pathol 2011;42:918–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glehen O, Mithieux F, Osinsky D, et al. Surgery combined with peritonectomy procedures and intraperitoneal chemohyperthermia in abdominal cancers with peritoneal carcinomatosis: a phase II study. J Clin Oncol 2003;21:799–806. [DOI] [PubMed] [Google Scholar]
- 48.Taub RN, Keohan ML, Chabot JC, Fountain KS, Plitsas M. Peritoneal mesothelioma. Curr Treat Options Oncol 2000;1:303–312. [DOI] [PubMed] [Google Scholar]
- 49.Loggie BW, Fleming RA, McQuellon RP, Russell GB, Levine EA. Prospective trial for the treatment of malignant peritoneal mesothelioma. Am Surg 2001;67:999–1003. [PubMed] [Google Scholar]
- 50.Park BJ, Alexander HR, Libutti SK, et al. Treatment of primary peritoneal mesothelioma by continuous hyperthermic peritoneal perfusion (CHPP). Ann Surg Oncol 1999;6:582–590. [DOI] [PubMed] [Google Scholar]
- 51.Deraco M, Casali P, Inglese MG, et al. Peritoneal mesothelioma treated by induction chemotherapy, cytoreductive surgery, and intraperitoneal hyperthermic perfusion. J Surg Oncol 2003;83:147–153. [DOI] [PubMed] [Google Scholar]
- 52.Hesdorffer ME, Chabot JA, Keohan ML, et al. Combined resection, intraperitoneal chemotherapy, and whole abdominal radiation for the treatment of malignant peritoneal mesothelioma. Am J Clin Oncol 2008;31:49–54. [DOI] [PubMed] [Google Scholar]
- 53.Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004;22:23–30. [DOI] [PubMed] [Google Scholar]
- 54.Souglakos J, Kalykaki A, Vamvakas L, et al. Phase II trial of capecitabine and oxaliplatin (CAPOX) plus cetuximab in patients with metastatic colorectal cancer who progressed after oxaliplatin-based chemotherapy. Ann Oncol 2007;18:305–310. [DOI] [PubMed] [Google Scholar]
- 55.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zivanovic O, Abramian A, Kullmann M, et al. HIPEC ROC I: a phase I study of cisplatin administered as hyperthermic intraoperative intraperitoneal chemoperfusion followed by postoperative intravenous platinum-based chemotherapy in patients with platinum-sensitive recurrent epithelial ovarian cancer. Int J Cancer 2014;136: 699–708. [DOI] [PubMed] [Google Scholar]
- 57.Bu Z, Ji J. A current view of gastric cancer in China. Transl Gastroint Cancer 2013;2:1–4. [Google Scholar]
- 58.Beeharry MK, Liu WT, Yao XX, Yan M, Zhu ZG. A critical analysis of the cytoreductive surgery with hyperthermic intraperitoneal chemotherapy combo in the clinical management of advanced gastric cancer: an effective multimodality approach with scope for improvement. Transl Gastroenterol Hepatol 2016;20:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lowe KA, Danese MD, Gleeson ML, Langeberg WJ, Ke J, Kelsh MA. Racial and ethnic variability in the prevalence and incidence of comorbidities associated with gastric cancer in the United States. J Gastrointest Canc 2016;47:168–181. [DOI] [PubMed] [Google Scholar]
- 60.Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 2004;22:3284–3292. [DOI] [PubMed] [Google Scholar]
- 61.Beckert S, Löffler M, Meisner C, et al. Open-label pilot phase I/II study of hyperthermic intraperitoneal chemotherapy in addition to macroscopic complete resection (R0/R1) of pancreatic adenocarcinoma. J Peritoneum 2016;1:75. [Google Scholar]
- 62.Castellanos JA, Merchant NB. Intensity of follow-up after pancreatic cancer resection. Ann Surg Oncol 2014;21:747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tentes AA, Stamou K, Pallas N, Karamveri C, Kyziridis D, Hristakis C. The effect of hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) as an adjuvant in patients with resectable pancreatic cancer. Int J Hyperthermia 2016;32:895–899. [DOI] [PubMed] [Google Scholar]
- 64.Tentes AA, Kyziridis D, Kakolyris S, et al. Preliminary results of hyperthermic intraperitoneal intraoperative chemotherapy as an adjuvant in resectable pancreatic cancer. Gastroenterol Res Pract 2012;2012:506571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fagotti A, Petrillo M, Costantini B, et al. Minimally invasive secondary cytoreduction plus HIPEC for recurrent ovarian cancer: a case series. Gynecol Oncol 2014;132:303–306. [DOI] [PubMed] [Google Scholar]
- 66.Esquivel J, Averbach A, Chua TC. Laparoscopic cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with limited peritoneal surface malignancies: feasibility, morbidity and outcome in an early experience. Ann Surg 2011;253:764–768. [DOI] [PubMed] [Google Scholar]
- 67.Esquivel J, Averbach A. Laparoscopic cytoreductive surgery and HIPEC in patients with limited pseudomyxoma peritonei of appendiceal origin. Gastroenterol Res Pract 2012;2012:981245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lotti M, Capponi MG, Piazzalunga D, et al. Laparoscopic HIPEC: a bridge between open and closed-techniques. J Minim Access Surg 2016;12:86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aherne EA, Fenlon HM, Shields CJ, Mulsow JJ, Cronin CG. What the radiologist should know about treatment of peritoneal malignancy. AJR 2017;208:531–543. [DOI] [PubMed] [Google Scholar]
- 70.Laghi A, Bellini D, Rengo M, et al. Diagnostic performance of computed tomography and magnetic resonance imaging for detecting peritoneal metastases: systematic review and meta-analysis. Radiol Med 2017;122:1–15. [DOI] [PubMed] [Google Scholar]
- 71.Gleeson EM, Feldman R, Mapow BL, et al. Appendix-derived pseudomyxoma peritonei (PMP): molecular profiling toward treatment of a rare malignancy. Am J Clin Oncol 2017. 10.1097/COC.0000000000000376 [DOI] [PubMed]
- 72.Pelz JO, Chua TC, Esquivel J, et al. Evaluation of best supportive care and systemic chemotherapy as treatment stratified according to the retrospective peritoneal surface disease severity score (PSDSS) for peritoneal carcinomatosis of colorectal origin. BMC Cancer 2010;10:689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoon W, Alame A, Berri R. Peritoneal Surface Disease Severity Score as a predictor of resectability in the treatment of peritoneal surface malignancies. Am J Surg 2014;207:403–407. [DOI] [PubMed] [Google Scholar]
- 74.Esquivel J, Lowy AM, Markman M, et al. The American Society of Peritoneal Surface Malignancies (ASPSM) multiinstitution evaluation of the Peritoneal Surface Disease Severity Score (PSDSS) in 1,013 patients with colorectal cancer with peritoneal carcinomatosis. Ann Surg Oncol 2014;21:4195–4201. [DOI] [PubMed] [Google Scholar]
- 75.Paradis C. Bias in surgical research. Ann Surg 2008;248:180–188. [DOI] [PubMed] [Google Scholar]