Abstract
Pathogenic germline variation in the microRNA processing gene DICER1 gives rise to an autosomal dominant, tumor-predisposition disorder. Conditional deletion of Dicer1 in murine dental epithelium shows that it controls tooth patterning, size, number and shape. The human dental phenotype of people with germline pathogenic variation in DICER1 is unknown. 57 DICER1-carriers and 55 family controls were evaluated at the NIH Clinical Center dental clinic as part of a comprehensive medical evaluation. Digital panoramic radiographs, bite-wing radiographs and oral photographs were collected. A single observer, blind to DICER1 status, reviewed the dental records and determined the presence or absence of eleven dental characteristics as described in the clinic notes, radiographs, or oral photographs. Subjective phenotypes were reviewed on radiographs by two examiners (blind to DICER1 status) for the presence or absence of the dental characteristics to reduce inconsistencies. By simple association, bulbous crown, periodontitis and taurodontism were all significant (P<0.05). Logistic regression with chi-square maximum likelihood estimates showed that bulbous crown and periodontitis remained significant. Recognition of these phenotypes may aid identification of individuals and families at risk for DICER1-associated neoplasms. These findings may also guide dental care for individuals with germline DICER1 pathogenic variation.
Keywords: DICER1, taurodontism, syndromes, bulbous crowns, periodontal disease, tooth morphology
Introduction
Loss-of-function variants in DICER1 are observed in the general population at a frequency of ~1/10,600 (Kim, Field, Schultz, Hill, & Stewart, 2017) and give rise to an autosomal dominant, tumor-predisposition disorder (Doros et al., 2014). DICER1 is involved in microRNA (miRNA) processing. The hallmark tumor of the disorder is the pleuropulmonary blastoma (PPB), a sarcoma recognized in 1988 (Manivel et al., 1988) and the most common lung cancer of childhood. Linkage analysis of familial PPB with DICER1 was reported in 2009 (Hill et al., 2009). Other manifestations of the disorder include kidney tumors, sex-cord stromal tumors, thyroid cancer, multinodular goiter and macrocephaly (Doros et al., 2014; Khan, Bauer, Doros, et al., 2017; Khan, Bauer, Schultz, et al., 2017). Ocular abnormalities and structural anomalies of the kidney and collecting system have recently been recognized in DICER1 families (Huryn et al., 2018; Khan et al., 2018). Early recognition of families and children at risk is imperative so that appropriate cancer surveillance can be implemented for carriers of pathogenic DICER1 variation (Schultz et al., 2018).
The full clinical phenotype and spectrum of severity arising from germline pathogenic variation in DICER1 is still being determined. MiRNAs are short (~22 nucleotides), single-stranded RNA sequences that regulate gene expression. DICER1 is an RNase III-family endonuclease that cleaves precursor miRNA (pre-miRNA) into active miRNA. The tumors associated with DICER1 arise from a cell harboring a loss-of-function DICER1 variant (usually germline) and a (usually somatic) variant in one of five “hotspot” codons (Brenneman et al., 2015). Conditional deletion of Dicer1 in murine dental epithelium shows that it controls tooth patterning, size, number and shape (Cao et al., 2010; Michon, Tummers, Kyyronen, Frilander, & Thesleff, 2010; Oommen et al., 2012); in murine mesenchyme, Dicer1 is a key regulator of multiple craniofacial structures (Barritt et al., 2012; Yu et al., 2017).
The human dental phenotype of DICER1 heterozygotes has not been previously described. Characterization of the oral features of the disorder, if any, may aid in the recognition of the disorder by dental professionals. Doing so would improve dental care and increase the likelihood that at-risk individuals and families would undergo DICER1 testing. The aim of this study is thus to characterize and, if appropriate, quantify dental anomalies in children and adults with germline DICER1 pathogenic variation, compared with family controls, with a goal of developing a more complete phenotypic description.
Materials and Methods
Study eligibility, recruitment and phenotyping.
his prospective, family-based, observational study, “DICER1-Related Pleuropulmonary Blastoma Cancer Predisposition Syndrome: A Natural History Study” (National Cancer Institute IRB approved protocol 11-C-0034) is a collaboration with the International Pleuropulmonary Blastoma/DICER1 Registry. All participants (or parents/legal guardians) provided written consent. Participants were evaluated at the National Institutes of Health (NIH) Clinical Center and Dental Clinic (Bethesda, MD) from November 2011 to May 2016. Patients were eligible for the study if they had a history of known DICER1-associated tumors or carried a germline DICER1 pathogenic variant (hereafter, “DICER1-carriers”). Family members without a germline DICER1 variant served as controls. To avoid analysis of deciduous teeth, participants were ≥13 years old. Participants were excluded from this analysis if edentulous or with negative DICER1 sequencing on blood (i.e., suspected to be DICER1 mosaic or tumor-confined).
All participants underwent DICER1 sequencing and completed extensive medical-history questionnaires (including history of chemotherapy, radiation, and facial trauma) prior to evaluation at the NIH Clinical Center. Individuals invited to the NIH Clinical Center underwent a three-day comprehensive medical and dental evaluation. Dental history, including orthodontic therapy and dental trauma, was obtained. Digital panoramic radiographs, bite-wing radiographs and oral photos were collected through Patterson Eaglesoft Version 17.00 in JPEG format (Patterson Dental, Laurel, MD). Panoramic radiographs were acquired by the same dental assistant with a Planmeca Promax 2D imaging system (Planmeca USA, Inc., Roselle, IL). The radiographs were taken with exposure parameters recommended by the manufacturer based on the size of the person: 66kV/9mA for adult females or small males, 68kV/13mA adult males, and 70kV/14mA for large adults.
Clinical assessment of dental anomalies.
We developed a list of eleven dental characteristics to be examined (Table 1). A single observer, blind to the DICER1 status of the participants, reviewed the dental records and determined the presence or absence of the eleven characteristics as described in the clinic notes, radiographs, or oral photographs. Subjective phenotypes (e.g., bulbous crown and short roots) were reviewed on radiographs (three times) by two examiners blinded to DICER1 status for the presence or absence of the dental characteristics to reduce inconsistencies. Box 1 and Supplementary Materials list definitions and details on select dental phenotypes.
Table 1.
Tests of simple association (two-sided Fisher’s Exact) and logistic regression (chi-square maximum likelihood estimates), adjusting for sex, age, chemotherapy, radiation, history of orthodontic treatment and history of trauma co-variates for eleven dental characteristics. P-values in bold are significant at <0.05. 95% confidence intervals which exclude 1.0 are statistically significant (P<0.05). See Supplementary Materials for additional details about dental phenotypes.
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Periodontitis | 0.04 | 0.003 | 33.8 | 3.4–336.8 |
| Bulbous Crown | 0.005 | 0.01 | 4.6 | 1.4–15.6 |
| Taurodontism | 0.03 | 0.1 | 6.9 | 0.6–73.4 |
| Short Roots | 0.3 | 0.2 | 2.0 | 0.7–5.9 |
| Shovel-Shaped | 0.5 | 0.2 | 1.8 | 0.7–4.8 |
| Supernumerary Teeth | 1.0 | 0.2 | 4.4 | 0.4–50.4 |
| Obliterated Pulp Chamber | 0.3 | 0.4 | 1.7 | 0.6–5.2 |
| Pulp Stones | 0.4 | 0.4 | 1.7 | 0.5–5.9 |
| Chips or Wear | 1.0 | 0.7 | 1.4 | 0.3–6.9 |
| Enamel Defect | 0.2 | 0.7 | 1.3 | 0.4–4.2 |
| Congenitally Missing Teeth | 1.0 | 1.0 | 1.0 | 0.07–15.0 |
Box 1.
Anatomical crown: That portion of tooth normally covered by, and including, enamel
Bulbous crown: Clinical and radiographic assessment that demonstrates rounded, bulb-like crown and relative concomitant cervical constriction at the junction between the tooth crown and roots, in contrast to a normal tooth in which there is a gradual and sloping transition from the root to the crown.
Enamel: Hard calcified tissue covering dentin of the crown of tooth
Periodontal Disease: Inflammatory process of the gingival tissues and/or periodontal membrane of the teeth, resulting in an abnormally deep gingival sulcus, possibly producing periodontal pockets and loss of supporting alveolar bone. Periodontal Disease includes gingivitis and periodontitis
Pulp: Connective tissue that contains blood vessels and nerve tissue which occupies the pulp cavity of a tooth
Pulp chamber: The space in a tooth containing the dental pulp. The portion of the cavity within the crown of the tooth is the pulp chamber; the portion within the root is the pulp canal or root canal
Taurodontism: enlargement of the body and pulp chamber of a multirooted tooth with apical displacement of the pulpal floor and bifurcation of the roots
Taurodontism was determined objectively using the Filho method (Melo Filho et al., 2015): the taurodontic index (TI) was calculated by measuring the height of the pulp chamber and dividing by the height from the root of the pulp chamber to the root apex and then multiplying that ratio by 100. A TI >20 was deemed a taurodontic tooth. The measurements were done on Patterson Eaglesoft radiograph imaging software (Patterson Dental, Laurel, MD). Dental status and presence of caries were estimated by counting the number of decayed, missing, and filled teeth (DMFT) of the participants on a panoramic radiograph. DMFT scores were based on 28 teeth; wisdom teeth were excluded to account for individuals who had unerupted or missing wisdom teeth.
Statistical analysis.
Simple tests for association using Fisher’s Exact test and logistic regression of the eleven dental characteristics on DICER1-carriers and family controls was analyzed using SAS software version 9.4 (SAS, Inc, Cary, NC). Logistic regression covariates included sex, age, chemotherapy, radiation, history of orthodontic treatment and history of trauma. Statistical significance was set at P <0.05 with 95% confidence intervals constructed. No Bonferroni corrections for simultaneous multiple inferences were performed.
Results
DICER1-carriers and controls well-matched for age.
A total of 182 participants were evaluated in dental clinic; 112 were eligible for this analysis. Seventy were excluded due to lack of dental records, age < 13 years, DICER1 mosaic/sporadic status, or edentulism. The majority of DICER1-carriers and controls were of European ancestry. Of the 112 participants included in the analysis (Table 2), there were 57 DICER1-carriers and 55 family controls. There were no statistically significant differences in age (P=0.7), but there was a significant difference in sex between DICER1-carriers and controls (Table 2; P=0.02).
Table 2.
Demographics of the NCI DICER1 syndrome cohort included in this analysis. There was not a statistically significant difference in age (P-value for age< 18 years, P=0.7), but there was a significant difference in sex between DICER1-carriers and controls (P=0.02).
| Total Sample | ||
|---|---|---|
| Controls (n=55) |
DICER1 (n=57) |
|
| Female gender | 22 (40.0%) | 36 (63.2%) |
| Age < 18 years | 3 (5.5%) | 5 (8.8%) |
| Mean Age±SD Range | 43±14.8 14–74 |
42±17.3 13–74 |
Bulbous crown and periodontitis nominally significant after logistic regression.
In the 112 DICER1-carriers and controls, the frequency of missing data for the eleven dental characteristics ranged from 0 – 6% of subjects. Table 1 shows the results of tests of simple association and logistic regression for the eleven characteristics. By simple association, bulbous crown (Figure 1), periodontitis and taurodontism (Figure 2) were all statistically significant (p<0.05). Logistic regression with chi-square maximum likelihood estimates showed that bulbous crown (p=0.01; odds ratio = 4.6; 95% confidence interval = 1.4–15.6) and periodontitis (p=0.003; odds ratio = 33.8; 95% confidence interval = 3.4 – 336.8) remained statistically significant.
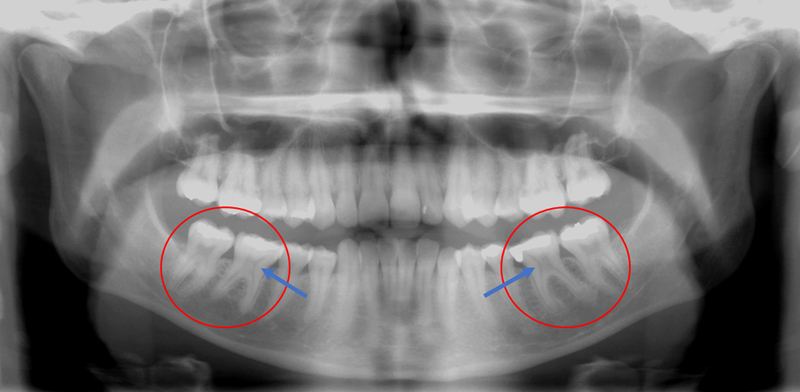
Figure 1:
65-year-old female DICER1-carrier with bulbous crowns, especially prominent in the right maxillary molars and premolars.
Figure 2:
22-year-old female DICER1-carrier with taurodontism prominent in first molars, characterized by enlarged pulp and relatively short roots.
Discussion
Mouse modeling has shown the importance of Dicer1 in dental development (Cao et al., 2010; Michon et al., 2010; Oommen et al., 2012). In humans with pathogenic germline variation in DICER1, we observed a significant excess of bulbousness, particularly in the crown. In the DcrK14-/ mouse, differences in crown morphology (e.g., simpler, smaller more symmetric cusps) may be the analogous phenotype (Michon et al., 2010). Bulbous crowns and tooth enlargement in which the relation between cusps and major grooves is eliminated is known as globodontia (Gorlin, 1998). It is a rare entity and is key feature in otodental syndrome, an autosomal dominant disorder that may arise from deletion of FGF3 (Gregory-Evans et al., 2007). This is more extreme than observed in the DICER1-carriers.
By simple association, we also observed a significant excess of taurodontism; this was not observed after adjustment using logistic regression. Although taurodontism has been reported to be associated with other genes (e.g., WNT10A, GREM2, EDA) (Kantaputra et al., 2018), we did not see any clinical evidence of these phenotypes in our DICER1 cohort. Taurodontism has not been observed in Dicer1 mouse models. Other features observed in Dicer1 mouse models such as supernumery teeth, enamel defects and abnormal morphology of molars were also observed in DICER1-carriers, although the excess was not significant. In addition, the human clinical findings were less severe compared with the mouse model.
We observed a significant excess of periodontal disease in the DICER1-carriers using logistic regression, despite controlling for sex, age, chemotherapy, radiation, history of orthodontic treatment and history of trauma. Since our controls were family members, it seems less likely that this finding was driven by differences in oral care habits, fluoride exposure or diet. Periodontal disease is more common in men than in women (Eke et al., 2012). However, since we have a statistical excess of female DICER1-carriers than controls, this difference strengthens our observation. Some possible explanations (mouth pain or tumors, cognitive differences, altered oral anatomy) are not routinely observed in DICER1 carriers and are not likely. Tooth shape, such as the bulbous crowns we observed in people with a pathogenic germline variant in DICER1, can influence periodontal phenotype (Stellini, Comuzzi, Mazzocco, Parente, & Gobbato, 2013). How bulbous crowns might increase the risk for periodontal disease needs to be elucidated. Alternatively, periodontal disease is a state of chronic inflammation (Carrizales-Sepulveda, Ordaz-Farias, Vera-Pineda, & Flores-Ramirez, 2018). Evidence from Dicer1 mouse models suggests that failures of immune surveillance mechanisms can lead to chronic inflammatory states (De Cauwer, Mariotte, Sibilia, Bahram, & Georgel, 2018). This non-canonical role (outside miRNA processing) for DICER1 has not been well-studied, especially in humans. However, it is important to note that disorders of chronic inflammation (e.g., auto-immune diseases) are not known at this time to be associated with the human DICER1 phenotype. Lastly, it is possible that variation in DICER1 affects barrier function, increasing risk for periodontal disease.
Clinically, our observations may be useful in the recognition of DICER1-carriers. Bulbous crowns (with thin roots) can be diagnostically useful in Schimke immunoosseous dysplasia, a rare disorder of short stature, nephropathy and T-cell deficiency (Gendronneau, Kerouredan, Taque, Sixou, & Bonnaure-Mallet, 2014; Morimoto et al., 2012). In the general population, the frequency of periodontal disease is approximately 8.5% (age 20–64 years; US National Health and Nutrition Examination Survey, 1999–2004), taurodontism is 2.5% (Jaspers & Witkop, 1980) and that of bulbousness is unknown. Very few disorders will feature all three dental findings; if present, a practitioner might consider the DICER1 syndrome.
Limitations of the study include a modest sample size and some subjective phenotypes. We acknowledge that there were significantly more female DICER1-carriers than controls but as noted this difference strengthens our observation. We did not correct for multiple testing in this hypothesis-generating study.
In conclusion, we observed a significant excess of crown bulbousness and periodontal disease in DICER1-carriers. Recognition of these phenotypes may aid identification of individuals and families at risk for DICER1-associated neoplasms. These findings may also guide the dental care of people with pathogenic germline variation in DICER1 and provide a framework to investigate the role of microRNAs in the pathogenesis of dental abnormalities.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government. Data from this study is available to share as per NIH policy.
Supplementary Material
Acknowledgments:
This work was supported by the Intramural Research Programs of the National Institute of Dental and Craniofacial Research and the Division of Cancer Epidemiology and Genetics of the National Cancer Institute, Bethesda, MD. The authors also wish to thank the Pine Tree Apple Tennis Classic and St. Baldrick’s Foundation for their ongoing support of children’s cancer research. The authors wish to thank the many patients, families and treating physicians who participate in the NCI DICER1-related Pleuropulmonary Blastoma Cancer Predisposition Syndrome study, the International OTST Registry and/or the International PPB Registry.
Footnotes
Conflict of Interest:
Dr. Stewart is a clinical consultant for Genome Medical, Inc.
References
- Barritt LC, Miller JM, Scheetz LR, Gardner K, Pierce ML, Soukup GA, & Rocha-Sanchez SM (2012). Conditional deletion of the human ortholog gene Dicer1 in Pax2-Cre expression domain impairs orofacial development. Indian Journal of Human Genetics, 18(3), 310–319. doi: 10.4103/0971-6866.107984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman MA, Field A, Yang J, Williams G, Doros L, Rossi CT, … Hill DA (2015). Temporal order of RNase IIIb and loss-of-function mutations during development determines phenotype in DICER1 syndrome: a unique variant of the two-hit tumor suppression model. F1000 Research, 4, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Wang J, Li X, Florez S, Huang Z, Venugopalan SR, … Amendt BA (2010). MicroRNAs play a critical role in tooth development. Journal of Dental Research, 89(8), 779–784. doi: 10.1177/0022034510369304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrizales-Sepulveda EF, Ordaz-Farias A, Vera-Pineda R, & Flores-Ramirez R (2018). Periodontal Disease, Systemic Inflammation and the Risk of Cardiovascular Disease. Heart, Lung and Circulation, 27(11), 1327–1334. doi: 10.1016/j.hlc.2018.05.102 [DOI] [PubMed] [Google Scholar]
- De Cauwer A, Mariotte A, Sibilia J, Bahram S, & Georgel P (2018). DICER1: A Key Player in Rheumatoid Arthritis, at the Crossroads of Cellular Stress, Innate Immunity, and Chronic Inflammation in Aging. Frontiers in Immunology, 9, 1647. doi: 10.3389/fimmu.2018.01647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doros L, Schultz KA, Stewart DR, Bauer AJ, Williams G, Rossi C, … Hill DA (2014). DICER1-related disorders In Pagon RA (Ed.), GeneReviews. Seattle (WA: ): University of Washington. [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ, & Cdc Periodontal Disease Surveillance workgroup: James Beck, G. D. R. P. (2012). Prevalence of periodontitis in adults in the United States: 2009 and 2010. Journal of Dental Research, 91(10), 914–920. doi: 10.1177/0022034512457373 [DOI] [PubMed] [Google Scholar]
- Gendronneau M, Kerouredan O, Taque S, Sixou JL, & Bonnaure-Mallet M (2014). Dental abnormalities and preventive oral care in Schimke immuno-osseous dysplasia. European Archives of Paediatric Dentistry, 15(3), 217–221. doi: 10.1007/s40368-013-0099-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlin RJ (1998). Otodental syndrome, oculo-facio-cardio-dental (OFCD) syndrome, and lobodontia: dental disorders of interest to the pediatric radiologist. Pediatric Radiology, 28(10), 802–804. doi: 10.1007/s002470050469 [DOI] [PubMed] [Google Scholar]
- Gregory-Evans CY, Moosajee M, Hodges MD, Mackay DS, Game L, Vargesson N, … Gregory-Evans K (2007). SNP genome scanning localizes oto-dental syndrome to chromosome 11q13 and microdeletions at this locus implicate FGF3 in dental and inner-ear disease and FADD in ocular coloboma. Human Molecular Genetics, 16(20), 2482–2493. doi: 10.1093/hmg/ddm204 [DOI] [PubMed] [Google Scholar]
- Hill DA, Ivanovich J, Priest JR, Gurnett CA, Dehner LP, Desruisseau D, … Goodfellow PJ (2009). DICER1 mutations in familial pleuropulmonary blastoma. Science, 325(5943), 965. doi: 10.1126/science.1174334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huryn LA, Turriff A, Harney LA, Carr AG, Chevez-Barrios P, Gombos DS, … Stewart DR (2018). DICER1 Syndrome: Characterization of the Ocular Phenotype in a Family-Based Cohort Study. Ophthalmology. doi: 10.1016/j.ophtha.2018.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspers MT, & Witkop CJ Jr. (1980). Taurodontism, an isolated trait associated with syndromes and X-chromosomal aneuploidy. American Journal of Human Genetics, 32(3), 396–413. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6992564 [PMC free article] [PubMed] [Google Scholar]
- Kantaputra PN, Hutsadaloi A, Kaewgahya M, Intachai W, German R, Koparal M, … Ketudat Cairns JR (2018). WNT10B mutations associated with isolated dental anomalies. Clinical Genetics, 93(5), 992–999. doi: 10.1111/cge.13218 [DOI] [PubMed] [Google Scholar]
- Khan NE, Bauer AJ, Doros L, Schultz KA, Decastro RM, Harney LA, … Stewart DR (2017). Macrocephaly associated with the DICER1 syndrome. Genetics in Medicine, 19(2), 244–248. doi: 10.1038/gim.2016.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NE, Bauer AJ, Schultz KAP, Doros L, Decastro RM, Ling A, … Stewart DR (2017). Quantification of Thyroid Cancer and Multinodular Goiter Risk in the DICER1 Syndrome: A Family-Based Cohort Study. The Journal of Clinical Endocrinology and Metabolism, 102(5), 1614–1622. doi: 10.1210/jc.2016-2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NE, Ling A, Raske ME, Harney LA, Carr AG, Field A, … Stewart DR (2018). Structural renal abnormalities in the DICER1 syndrome: a family-based cohort study. Pediatric Nephrology, 33(12), 2281–2288. doi: 10.1007/s00467-018-4040-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Field A, Schultz KAP, Hill DA, & Stewart DR (2017). The prevalence of DICER1 pathogenic variation in population databases. International Journal of Cancer, 141(10), 2030–2036. doi: 10.1002/ijc.30907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manivel JC, Priest JR, Watterson J, Steiner M, Woods WG, Wick MR, & Dehner LP (1988). Pleuropulmonary blastoma. The so-called pulmonary blastoma of childhood. Cancer, 62(8), 1516–1526. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3048630 [DOI] [PubMed] [Google Scholar]
- Melo Filho MR, Nogueira dos Santos LA, Barbosa Martelli DR, Silveira MF, Esteves da Silva M, de Barros LM, … Martelli-Junior H (2015). Taurodontism in patients with nonsyndromic cleft lip and palate in a Brazilian population: a case control evaluation with panoramic radiographs. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, 120(6), 744–750. doi: 10.1016/j.oooo.2015.08.005 [DOI] [PubMed] [Google Scholar]
- Michon F, Tummers M, Kyyronen M, Frilander MJ, & Thesleff I (2010). Tooth morphogenesis and ameloblast differentiation are regulated by micro-RNAs. Developmental Biology, 340(2), 355–368. doi: 10.1016/j.ydbio.2010.01.019 [DOI] [PubMed] [Google Scholar]
- Morimoto M, Kerouredan O, Gendronneau M, Shuen C, Baradaran-Heravi A, Asakura Y, … Boerkoel CF (2012). Dental abnormalities in Schimke immuno-osseous dysplasia. Journal of Dental Research, 91(7 Suppl), 29S–37S. doi: 10.1177/0022034512450299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oommen S, Otsuka-Tanaka Y, Imam N, Kawasaki M, Kawasaki K, Jalani-Ghazani F, … Ohazama A (2012). Distinct roles of microRNAs in epithelium and mesenchyme during tooth development. Developmental Dynamics, 241(9), 1465–1472. doi: 10.1002/dvdy.23828 [DOI] [PubMed] [Google Scholar]
- Schultz KAP, Williams GM, Kamihara J, Stewart DR, Harris AK, Bauer AJ, … Hill DA (2018). DICER1 and Associated Conditions: Identification of At-risk Individuals and Recommended Surveillance Strategies. Clinical Cancer Research, 24(10), 2251–2261. doi: 10.1158/1078-0432.CCR-17-3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellini E, Comuzzi L, Mazzocco F, Parente N, & Gobbato L (2013). Relationships between different tooth shapes and patient’s periodontal phenotype. Journal of Periodontal Research, 48(5), 657–662. doi: 10.1111/jre.12057 [DOI] [PubMed] [Google Scholar]
- Yu Y, Zuo X, He M, Gao J, Fu Y, Qin C, … Bian Z (2017). Genome-wide analyses of non-syndromic cleft lip with palate identify 14 novel loci and genetic heterogeneity. Nature Communications, 8, 14364. doi: 10.1038/ncomms14364 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.