Abstract
Purpose:
Dynamic susceptibility contrast (DSC) imaging requires high temporal sampling which poses limits on achievable spatial coverage and resolution. Additionally, more encoding intensive multi-echo acquisitions for quantitative imaging are desired to mitigate contrast leakage effects, which further limits spatial encoding. We present an accelerated sequence that provides whole brain coverage at an improved spatio-temporal resolution, to allow for dynamic quantitative R2 and R2* mapping during contrast enhanced imaging.
Methods:
A multi-echo spin and gradient echo (SAGE) sequence was implemented with simultaneous multi-slice (SMS) acquisition. Complementary k-space sampling between repetitions and joint-virtual coil (JVC) reconstruction were used along with a dynamic phase-matching technique to achieve high quality reconstruction at 9-fold acceleration, which enabled 2×2×5 mm whole-brain imaging at repetition time of 1.5–1.7s. The multi-echo images from this sequence were fit to achieve quantitative R2 and R2* maps for each repetition, and subsequently used to find perfusion measures including cerebral blood flow (CBF) and cerebral blood volume (CBV).
Results:
Images reconstructed using JVC show improved image quality and g-factor compared to conventional reconstruction methods, resulting in improved quantitative maps with a 9-fold acceleration factor and whole brain coverage during the dynamic perfusion acquisition.
Conclusion:
The method presented shows the advantage of using a JVC-GRAPPA reconstruction to allow for high acceleration factors while maintaining reliable image quality for quantitative perfusion mapping, with the potential to improve tumor diagnostics and monitoring.
Keywords: Parallel imaging, simultaneous multi-slice, joint reconstruction, virtual coil, perfusion, dynamic susceptibility contrast
Introduction
Perfusion weighted magnetic resonance imaging scans are commonly used to monitor brain tumor progression and treatment. Perfusion imaging can give valuable information about the tumor perfusion and permeability1–4. Dynamic susceptibility contrast (DSC) imaging is a perfusion scan that uses an intravenous injection of gadolinium (Gd) chelated contrast agent and relies on T2* contrast to examine the changes in image intensity over time. The transient dynamics after the bolus contrast injection provides perfusion measures such as cerebral blood volume (CBV), cerebral blood flow (CBF), and mean transit time (MTT).
Studies have demonstrated that changes in DSC imaging parameters can be observed after tumor treatment, showing promise in the potential for early prediction of local recurrence or malignant transformation that correlates well with overall survival and progression free survival5–7. However, this data is not always clear and overall correlations are modest8–10. There are several limiting factors in DSC imaging that may contribute to incomplete tumor understanding, including poor image quality at low spatial resolution due to limited encoding speed as well as errors in quantitation of parameters. In particular, the blood brain barrier (BBB) is not always intact in tumor subjects, leading to contrast leakage effects that diminish the accuracy of CBV, when not properly considered. A compromised BBB often requires the use of a Gd “preload” prior to the DSC acquisition to reduce the T1-shortening effect7,12,13 from contrast leakage during the transient flow, which adds to the overall dose. Recent concerns about Gd retention14, especially in patients such as those with brain metastases who require frequent monitoring, motivates the development of single dose DSC sequences, without the need for a preload. A promising development in this direction is in the use of a multi-echo sequence to measure quantitative T2 or T2* maps, rather than obtaining only weighted images, and use these time series maps directly to eliminate the need for T1-leakage correction or a pre-bolus injection15–17.
In addition to improving DSC by measuring quantitative T2 or T2* maps, DSC can be performed with either gradient echo images or spin echo images, and more recent sequences can achieve both images in one acquisition17,18. Gradient echo images give T2* contrast with excellent contrast-to-noise ratio and sensitivity to macro-vessel sizes, while spin echo imaging utilizes T2 contrast that have lower contrast-to-noise ratio but are more sensitive to the microvasculature. Studies have shown that measurements of both spin and gradient echo images may help to improve diagnoses8,19, and together these images can be used to form vessel size imaging (VSI) maps20. VSI techniques show promise in further understanding tumor vasculature, and have provided new biological insights into anti-angiogenic therapy of glioma21.
A multi-echo sequence that has gained interest for DSC is the spin and gradient (SAGE) echo method22,23 which acquires multiple echo planar imaging (EPI) echoes with a mixture of spin and gradient echo contrasts. These images can be used to simultaneously compute both T2 and T2* quantitative maps (or R2 and R2* maps) by fitting the data to the signal equation. While the SAGE method greatly improves signal quantitation and provides exciting new vasculature information that facilitates vessel size and vessel architectural imaging, its added encoding burden further hinders the ability for DSC to achieve sufficient resolution and volume coverage at a fast enough temporal sampling rate to adequately capture the passage of contrast agent through a tumor and accurately measure perfusion characteristics24. Therefore, SAGE scans are usually acquired with a limited number of slices (~15) and relatively low resolution (3×3×5 mm), which is near the upper end of the range of suggested resolution for DSC25. Low spatial resolution images can impact the accuracy of the arterial input function (AIF) calculation due to partial volume effects26, as well as cause difficulties in visualizing tumor activity and vascular changes in smaller tumors. Multi-echo methods such as SAGE that eliminate the T1 leakage effect also have shown promise for improving automated AIF calculations27,28.
Additionally, the EPI encoding that is needed for rapid acquisition of SAGE data results in artifacts such as relaxation-induced blurring and B0 distortions, as well as long echo times. Accelerated encoding and parallel imaging can alleviate this to some extent, but even with modern multi-channel receivers, perfusion EPI sequences are limited to 2- or 3-fold acceleration, so that the SNR penalty and artifact problems do not degrade image quality. Virtual coil (VC) concept methods have been used to provide improved in-plane spatial encoding using image phase prior information29–31. However, VC requires the phase to be consistent between the reference data and imaging data, which does not hold true in typical dynamic EPI acquisitions, especially for gradient echo acquisitions where shot-to-shot B0 variations can induce significant phase differences. Joint reconstruction of multiple images with different contrasts has been used to take advantage of smooth temporal dynamics in approaches such as k-t GRAPPA32, k-t SENSE33–35, PEAK GRAPPA36, and joint VC (JVC) GRAPPA37 with success in reducing SNR loss for typical Cartesian acquisitions. Recently, joint EPI techniques such as PEAK-EPI38 with shifted k-space sampling have shown promise for accelerating perfusion imaging acquisitions.
A blipped, “controlled aliasing in parallel imaging results in higher acceleration” (CAIPIRINHA) technique, introduced as “blipped-CAIPI” SMS39 acquisition has previously been shown as a valuable method for improving slice coverage while limiting the g-factor and SNR penalty. Here, we implement the blipped-CAIPI SMS in a modified SAGE sequence to allow for whole-brain coverage and maintain a high temporal resolution. In combination with slice acceleration, the acquisition is accelerated in-plane to shorten echo times as well as EPI related blurring and distortion. To maintaining high acceleration factors without a high g-factor penalty and image quality degradation, a joint-virtual coil (JVC) GRAPPA reconstruction is introduced that takes advantage of temporal smoothness to maintain reliable image quality even at high acceleration factors. Complementary k-space sampling between time points is used to further improve the GRAPPA kernel and therefore image reconstruction. A unique phase matching step is implemented in the reconstruction to allow JVC reconstruction even with dynamic, multi-echo imaging where the background phase changes over time. The proposed technique was demonstrated in healthy subjects and a comparison between the proposed JVC-GRAPPA and conventional GRAPPA reconstruction was performed. In addition, the proposed technique was validated against conventional multi echo sequences as well as a conventional, non-SMS SAGE acquisition.
Methods
Sequence Acquisition
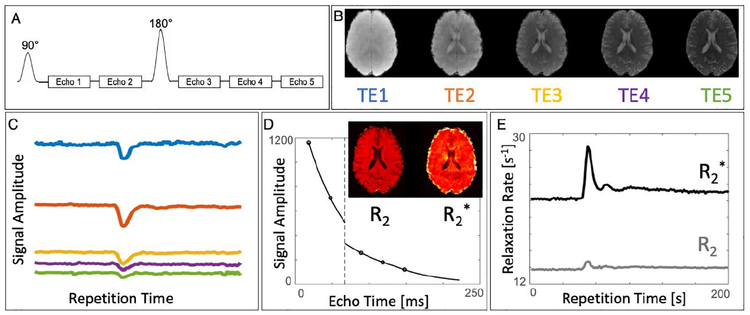
A DSC protocol with a 5-echo SAGE sequence was implemented on Siemens 3T Skyra and Prisma systems (Siemens AG, Munich, Germany). The protocol was acquired on four healthy subjects with written informed consent using a 32-channel head coil during a gadolinium injection. The sequence, illustrated in Figure 1A, was acquired with the blipped-CAIPI SMS technique to increase slice coverage and capture the entire brain in a single repetition. As described previously22, the SAGE sequence involves a 90° excitation pulse followed by two gradient echo single-shot EPI readouts, followed by a 180° refocusing pulse, two asymmetric spin echo readouts, and one spin echo readout. A single slice demonstrating the different contrasts that are acquired using this sequence are shown in Figure 1B. The entire volume is acquired dynamically during the injection of a Gd contrast agent, as shown in Figure 1C which displays a signal in the slice over the entire acquisition for all five echoes. As described in more detail below, the echoes from each time point are subsequently fit to the signal equation to find R2 and R2* maps (Figure 1D), so that the dynamic R2 and R2* maps (Figure 1E) can be used directly for pharmacokinetic analysis and parameter quantification.
Figure 1:
A) The 5-echo SAGE sequence: excitation, two gradient-echo EPI readouts, refocusing, two asymmetric spin-echo EPI readouts and one spin echo EPI readout. B) Five separate image contrasts at different echo times can be reconstructed from each repetition. C) Acquired dynamically, the sequence can be used to capture all five echoes during the injection of contrast agent. D) Each time point can be fit to the signal equation to find R2 and R2* maps, resulting in E) dynamic quantitative R2 and R2* maps during contrast injection.
Sequence parameters were primarily held constant between Prisma/Skyra acquisitions, though the higher gradient performance of the Prisma (gradient strength of 80/45 mT/m, respectively) allowed for slightly shorter echo spacing, TR, and TE, as noted below. The sequence parameters used here include: in-plane resolution = 2×2 mm, FOV = 230×230 mm, slice thickness = 5 mm, 27 slices, effective echo spacing = 0.61/0.72 ms, TR = 1.5/1.7s, TE = [15 40 77 102 128]/[18 47 89 118 148] ms (Prisma/Skyra), with a total acceleration factor (AF) = 9 (MB = 3, Rip =3). Total scan time was approximately 4 min (1 min FLEET40 training data, 3 min of data acquisition).
K-space sampling was shifted in the phase encode direction to improve coverage. Optimal k-space shifting for VC is found when a shift of Rip/4 Δky is used, to create uniform sampling between real and virtual coils. In this case with Rip=3, shifts were 0.75Δky and −0.75Δky alternating over repetitions, allowing for increased k-space coverage for joint-GRAPPA reconstruction across TRs, while at the same time achieving uniform k-space sampling spacing for VC at each TR. The shifted sampling was implemented by adding or subtracting the corresponding gradient moment to the prewinding blip before the EPI readout. In addition, echo time shifting (ETS) was used to ensure the effective echo time remained the same over repetitions41. Figure 2 illustrates the k-space sampling scheme, where the solid lines show the acquired k-space lines, and the dotted lines show the “virtual” k-space sampling lines that are found by using complex conjugate symmetry and phase prior information to create the virtual coils, as described below. By acquiring different lines across repetitions, the kernel created from the joint reconstruction across repetitions is improved.
Figure 2:
Joint virtual coil sampling and reconstruction illustrated in k-space. Solid lines represent acquired EPI data, dotted lines represent the corresponding virtual coil (VC) lines derived from the source data. The k-space sampling is shifted from the center ky line to achieve uniform sampling between real and virtual coils across k-space. The sampling is also shifted across repetition time to improve the GRAPPA kernel for JVC reconstruction. For the target point shown in red, many source and virtual coil points can be used from all coils, repetitions, and surrounding points (shown here is a 3×4 kernel).
For further validation, the proposed SAGE sequence was also acquired in one healthy subject and compared to a conventional, non-SMS SAGE acquisition – all other parameters were held constant, for a total acceleration factor of 3-fold (Rip = 3 and MB = 1) and 9 slices. A conventional multi-GRE sequence and a conventional multi-SE sequence were also acquired for comparison of R2 and R2* maps. Sequence parameters for multi-GRE included a 9-slice acquisition with TE = [15 25 35 45 55 65], TR = 1s, FOV = 230×230mm, resolution = 1.8×1.8×5mm, for a total scan time of 2m10s (no acceleration). The multiple spin-echo acquisition was a 2D single slice image with 20 echoes, first TE=12ms, echo spacing = 12ms, TR = 2s, FOV = 230×230mm, resolution = 1.8×1.8×5mm, for a total scan time of 4m18s.
Image Reconstruction
All reconstructions shown were performed in MATLAB (MathWorks, Natick, MA) to assess the comparative performance of conventional GRAPPA and JVC-GRAPPA methods. Coil sensitivity maps were found from the reference data using ESPIRiT42 for complex coil combination of images. Figure 3 shows the reconstruction pipeline used to find the images for all echoes and repetitions. All data and reference data were ghost corrected first using the slice-collapsed data. Split-slice GRAPPA was used to separate collapsed slices, then the resulting data was slice shifted and a second ghost correction step was performed using slice specific data, before performing in-plane GRAPPA. The two-step ghost correction is employed here to improve the ghost correction performance of the SMS acquisition as per Setsompop et. al.43.
Figure 3:
Overview of the reconstruction process: After ghost correction, conventional slice-GRAPPA and in-plane GRAPPA are performed. Then, an additional phase matching step is used for each image. For this step, the smoothed difference between the image phase and reference image phase is found, and then added coil-by-coil back to the reference data to create matched reference data for each image. Then, virtual coils are created from the phase-matched reference and acquired data, followed by a joint slice-GRAPPA and joint in-plane GRAPPA, where the additional images used are stacked along the channel axis.
For the JVC reconstruction, a phase-matching step was needed to ensure that the background phase remained the same between the data and reference scan, to correctly enforce the phase prior needed for the virtual coil concept. Because the reference data is acquired at the beginning of the scan and does not have matching background phase information to each repetition due to shifted sampling, physiological motion, or B0 drift, it is difficult to apply standard virtual coil methods29 to dynamic EPI acquisitions. Therefore, an accurate way to estimate background phase dynamically was developed to allow for reliable reconstructions without a large g-factor penalty. The background phase estimate is performed by finding the phase difference between the reference data and the initial conventional GRAPPA reconstructed data. To achieve a good estimate, the phase difference is calculated on the high-SNR complex coil-combined data rather than on a coil-by-coil basis, since the phase difference should be common across all coils. Moreover, a smooth phase prior information of this phase difference is enforced through apodization in k-space using a 2D Tukey window to create a smooth high-SNR difference map. This difference was added back to the reference data on a coil by coil basis, to create a phase-matched reference data for the joint-virtual coil reconstruction. After matching the phase, standard virtual coil methods using conjugate symmetry were applied to each coil, effectively doubling the number of channels available for GRAPPA-reconstruction. The joint-reconstruction creates GRAPPA kernel weights using multiple neighboring time points for each image reconstruction. The phase-matched reference data and actual data to be used in the joint-reconstruction were stacked along the channel dimension to find GRAPPA kernel weights over multiple contrasts. In the present study, the GRAPPA kernel used was across just two repetitions to limit temporal smoothing and reconstruction time. Performance of the reconstruction was assessed using g-factor maps. Reconstruction code along with an example data set is available upon request.
Post-processing
The 5-echo images were motion corrected across the time series and masked using FSL44. The masked multi-echo images were then used to calculate R2 and R2* maps by performing a four-parameter least-squares fit to the signal equation22. As described in Schmiedeskamp et. al.22, a four-parameter fit is used to separately find S0I before the 180° pulse and S0II after the 180° pulse, due to slice profiles mismatches of the 90° and 180° pulses, but a three-parameter fit can be used by estimating the ratio S0I / S0II. Here, we use a 3D polynomial fit to estimate a smoothed S0I / S0II after an initial, fast four-parameter fit for one volume, and then a three-parameter least-squares fit is used to improve the robustness of the maps. The quality of the maps was assessed by the normalized root-mean-squared error (RMSE) of the fit and the standard deviation across time during baseline (before contrast injection). Subsequently, the R2 and R2* maps were processed with NordicICE (NordicNeuroLab, Norway)45,46 to generate relative CBF and CBV maps, using automated AIF determination45,47 and T2* leakage correction.
For validation of the R2 and R2* maps to conventional mapping methods, the multi-GRE images were fit using an exponential fit to the signal equation. The multi-SE images were fit with stimulated echo compensation using the StimFit48 software available online.
Results
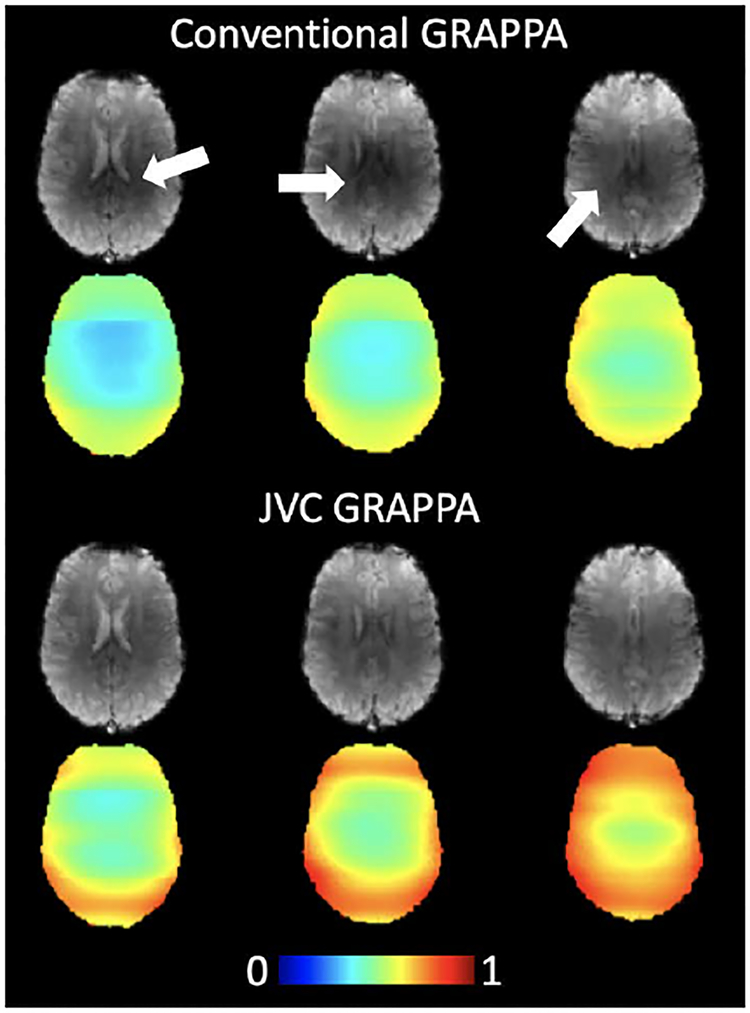
Figure 4 shows images from three slices in one subject using both conventional-GRAPPA and JVC-GRAPPA reconstruction. The darker areas in the middle of the brain in the conventional GRAPPA images are largely mitigated in the images reconstructed with JVC-GRAPPA. Maps showing 1/g-factor penalty are also shown for both cases, where g-factor in these slices is an average of 1.9 for conventional reconstruction and 1.5 for JVC-GRAPPA reconstruction, a 20% improvement. The max g-factor in these slices (Gmax) also decreased by approximately 18%, showing an improvement from 3.1 to 2.6 when using JVC-GRAPPA. Over all subjects in the whole brain, the g-factor penalty decreased on average by 20% (from 2.2 to 1.7) with the Gmax decreasing by 15% (from 3.8 to 3.2).
Figure 4:
Three representative slices from the second echo time (TE = 47 ms) with corresponding 1/g-factor maps, scaled from 0 to 1. The darker center images (marked by white arrows) seen in the conventional GRAPPA reconstruction is mitigated using JVC-GRAPPA reconstruction, which also markedly reduces the g-factor penalty.
Figure 5 shows R2 and R2* maps for both conventional and JVC reconstructions from a baseline time point of one subject in three representative slices near the center of the brain, an area that is often hardest to reconstruct due to low-SNR and low coil variation for the multi-channel receiver array used in this acquisition. The maps from JVC reconstructed images show substantial improvement compared to the conventional reconstructed images, allowing an acceleration factor of 9-fold with relaxation rates comparable to published values49–51. The normalized RMSE of the fit is displayed below the maps, in these slices the average RMSE dropped from an average of 8% to 6% when going from conventional reconstruction to JVC reconstruction, showing the improvement using the JVC reconstruction. Over all subjects in the center slice, center 20×20 voxels, the average normalized RMSE dropped from 13% to 5% when using JVC-GRAPPA reconstruction compared to conventional-GRAPPA reconstruction.
Figure 5:
Fitted R2 (top) and R2* (middle) maps from three representative slices from the center of the brain, using both conventional GRAPPA and JVC-GRAPPA reconstruction. JVC-GRAPPA reconstruction greatly improves the quality of the fitted maps, with blue arrows pointing to areas that show especially poor fit in the conventional GRAPPA reconstruction. These improvements are reflected in the normalized RMSE of the fit (bottom).
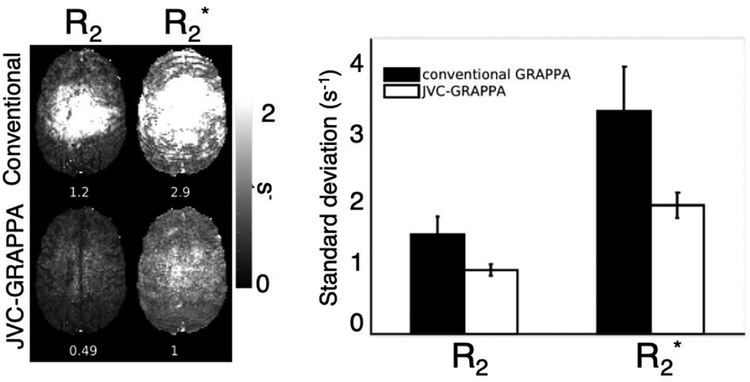
Figure 6 demonstrates the variation of the R2 and R2* maps in one slice across 20 time points during baseline scanning before injection, where the changes over time are expected to be low. The maps derived from JVC-GRAPPA reconstructed images show improved standard deviation, with the R2 standard deviation decreasing from 1.2 s−1 to 0.49 s−1 and the R2* standard deviation decreasing from 2.9 s−1 to 1 s−1 in this slice. Over the whole brain over all subjects, R2 and R2* standard deviation decreased from 1.4 s−1 to 0.9 s−1 and from 3.1 s−1 to 1.8 s−1, respectively, as shown in the bar graph in Figure 6.
Figure 6:
Variation during baseline between reconstruction methods. (Left): The standard deviation during baseline scanning is significantly reduced in both the R2 and R2* maps when using JVC-GRAPPA instead of conventional GRAPPA reconstruction. (Right): Across the whole brain over all subjects, a decrease in standard deviation in the maps over time is seen for both R2 and R2* when using JVC-GRAPPA reconstruction.
Figure 7 shows R2 and R2* maps derived from conventional multi-echo methods, conventional SAGE, and the proposed JVC-GRAPPA SMS SAGE. The R2* and R2 maps from conventional acquisitions show similar values to the SAGE acquisitions with higher SNR, though it is important to take into consideration the scan time and number of slices achieved. Similar R2 and R2* values were also seen between conventional SAGE and JVC-GRAPPA SMS SAGE, the JVC-GRAPPA SMS acquisition has only a small SNR/g-factor penalty at 3× the acceleration as conventional SAGE images.
Figure 7:
Conventional R2 and R2* maps from a single slice multi-echo SE and 9-slice multi-echo GRE acquisition, calculated from 4.3min and 2.1min acquisitions respectively (left). R2 and R2* maps calculated from a conventional SAGE (middle) in a 9-slice, 1.5s acquisition, and maps calculated from whole-brain 1.5s JVC-GRAPPA SMS SAGE (right) show similar values.
Figure 8 shows relative CBF and CBV maps obtained from the time-series of the R2 and R2* maps from the one slice shown in Figure 5, using both conventional and JVC reconstruction. As in the R2 and R2* maps, significant errors can be seen in the CBV and CBF maps derived from the conventional GRAPPA reconstruction images, which are generally resolved when the JVC reconstruction method is used.
Figure 8:
Normalized CBF and CBV maps calculated from the R2* map and R2 map time courses. The maps derived from conventional-GRAPPA reconstructed images show erroneously high CBV values, especially in the center of the brain where the g-factor penalty is highest. In contrast, maps derived from JVC-GRAPPA reconstructed images yield perfusion maps that exhibit the typical contrast expected across brain regions.
Discussion
The presented method allows for quantitative R2 and R2* maps with whole brain coverage at a temporal resolution of less than 2 s, which can be used to calculate quantitative perfusion parameters directly. These quantitative perfusion maps are not confounded by T1 leakage, thus only post processing T2* leakage correction is needed, eliminating the need for an additional preload injection of gadolinium contrast agent. In addition, the sequence was shown to give reliable R2 and R2* maps in 1.5s, even at an acceleration factor of 9-fold, and compares well with previous methods and conventional mapping methods.
Achieving higher spatial resolution dynamic images is an important improvement in perfusion imaging, as this allows for smaller tumors or metastases to be adequately captured without losing temporal resolution needed to adequately capture the passage of the contrast agent. In addition, whole brain coverage advances the ability to visualize tumors outside of the narrower slice coverage that is typically used for DSC imaging, covering only the area surrounding a known tumor location, which could be difficult when imaging particularly large gliomas or capturing metastases in multiple locations in the brain. Whole brain coverage is also applicable in other areas of research with DSC, such as Alzheimer’s disease, where it is desirable to capture hypoperfusion across the entire brain52. Supporting Information Table S1 demonstrates the tradeoffs in resolution, slice coverage, SNR, and quantitative mapping from using a more conventional single echo DSC acquisition compared to the SAGE acquisition, with and without slice acceleration. The benefit of the accelerated acquisition here is not limited to the chosen protocol but can be flexibly used to improve other DSC protocols, such as an example 2.8 mm isotropic acquisition case acquired with a repetition time of 1.9 s and TE = [13 31 48 66 84] ms, demonstrated in Figure 9. This acquisition demonstrates a tradeoff between higher in-plane resolution and temporal resolution with shorter echo times and thinner slices. Isotropic resolution whole-brain datasets can greatly benefit DSC-MRI, by decreasing slice thickness to reduce partial volume effects, or lowering the in-plane resolution to shorten the echo time and improve SNR and AIF determination53.
Figure 9:
The protocol is flexible and can be adapted to a variety of sequence parameters, as demonstrated in this whole brain SAGE acquisition with isotropic resolution (2.8 mm) in a TR of 1.9 s. The results here show three pane views of the R2 maps (top) and the R2* maps (bottom). Arrows show regions where conventional GRAPPA results in particularly poor image quality, which is improved using JVC-GRAPPA reconstruction.
While the presented work focuses on DSC acquisitions, dynamic contrast enhanced (DCE) MRI is also used to study permeability of brain tumors. While DSC examines perfusion dynamics, DCE examines the leakage of contrast across the BBB over a period of time after contrast injection to derive measures such as the transfer coefficient (Ktrans) to assess tumor permeability. DSC and DCE protocols are typically acquired in two separate scans as a result of somewhat competing imaging requirements, with DSC necessitating an acquisition that can provide the transverse relaxation time (T2/T2*) contrast weighting and DCE requiring T1 contrast weighting53. However, the data from this sequence can be used to decouple the T2* and T1 signal components by extrapolating to TE=023,53,54, providing a T1-weighted signal time-series that can be used to find permeability parameters from DCE analysis. This eliminates two separate injections of Gd contrast agent to achieve both perfusion and permeability information. These potential benefits will be explored in our planned work on a tumor population.
The phase matching step for implementation of JVC-GRAPPA was imperative for improvement of the images in this dynamic EPI acquisition, as demonstrated in Figure 10. The figure shows without matching the background phase, the phase prior assumption for creating virtual coils is no longer valid due to changes in phase information over time, and applying virtual coil to this data can result in artifacts and errors in the reconstructed images, leading to image quality that in some cases that is worse than the images reconstructed from standard-GRAPPA alone. Similarly, joint-GRAPPA reconstruction with matching background phase information for all repetitions allows for neighboring repetitions to improve the GRAPPA kernel while maintaining accurate underlying data consistency for each time point.
Figure 10:
The importance of phase matching is demonstrated in this representative second echo image. Without phase matching, JVC-GRAPPA (middle) causes severe signal dropout in the center of the image (white arrows) due to incorrect phase information, where with phase matching, JVC-GRAPPA (right) improves the signal dropout compared to conventional GRAPPA reconstruction (left).
While JVC-GRAPPA offers improved image quality, it does come with some limitations. First, the reconstruction time is increased for JVC-GRAPPA because an initial conventional GRAPPA step is needed to find the matching phase information. In addition, using virtual coil and joint reconstructions increases the number of channels used in the kernel, resulting in more kernels that can slow down reconstruction time. In our current implementation, the JVC-GRAPPA reconstruction takes approximately 4 times longer than conventional GRAPPA reconstruction. In future work, a better optimized implementation may aid in accelerating the reconstruction time. Other studies have investigated the benefit of simplified SAGE (sSAGE) that uses only three echoes (two gradient echo and one spin echo), which saves reconstruction time and also still mitigates the T1 leakage effect54,55. However, this sSAGE sequence does not significantly reduce scan time, and therefore all five echoes were used in this preliminary study to achieve high quality quantitative R2 and R2* maps. Future studies may benefit from acquiring fewer echoes to improve reconstruction and post-processing time.
Joint-reconstruction can lead to smoothing across repetitions, especially during the peak of contrast agent injection. Here, we limit the joint-reconstruction to two repetitions to limit both temporal smoothing and reconstruction time, but this reconstruction could be further optimized by using different weightings or regularization between contrasts. We also investigated joint-reconstruction over multiple echo times, but found that there was non-negligible signal contamination between images when reconstructed jointly, as neighboring echo times can have very different contrast unlike the relatively smooth changes between repetitions. Using joint-TE can result in incorrect signal intensity in the weighted images, leading to poor quantification of R2 and R2* as well as the underlying perfusion parameters. Further work exploring the benefits of joint-reconstruction while maintaining data consistency may help to further accelerate the acquisition and improve resolution.
Temporal information and complementary sampling across repetitions was explored here, but could be further investigated by using other advanced reconstruction methods. For example, k-space based reconstruction methods such as LORAKS56 and joint-LORAKS may allow for improved exploitation of joint or prior information across echoes and repetition. Furthermore, a SENSE-based reconstruction method may allow for more control over data consistency and weighting between different joint contrasts. In addition, using a low-rank model to represent the perfusion time course and using a small number of basis functions could help to further improve reconstruction57–60, and possibly allow for direct reconstruction of perfusion maps from the data. Future studies further exploiting the temporal component may help to further accelerate this perfusion acquisition and allow for higher spatio-temporal resolution.
Conclusion
The method presented here shows the advantage of using a JVC-GRAPPA reconstruction in this quantitative DSC perfusion sequence to allow for high acceleration factors while maintaining reliable image quality. The results show that the protocol has the potential to improve tumor diagnostics and monitoring by enabling whole-brain and temporally efficient SAGE DSC-MRI data acquisition.
Supplementary Material
Supporting Information Table S1: Comparison between standard single gradient echo DSC and a 5-echo SAGE acquisition, with and without slice acceleration.
Acknowledgements
This work was supported in part by NIH research grants: F32EB026304, R01MH116173, R01EB020613, R01EB019437, U01EB025162, P41EB015896, and the shared instrumentation grants: S10RR023401, S10RR019307, S10RR019254, S10RR023043.
References
- 1.Maeda M, Itoh S, Kimura H, et al. Tumor vascularity in the brain: evaluation with dynamic susceptibility-contrast MR imaging. Radiology. 1993;189(1):233–238. doi: 10.1148/radiology.189.1.8372199. [DOI] [PubMed] [Google Scholar]
- 2.Cha S, Johnson G, Wadghiri YZ, et al. Dynamic, contrast-enhanced perfusion MRI in mouse gliomas: Correlation with histopathology. Magn Reson Med. 2003;49(5):848–855. doi: 10.1002/mrm.10446. [DOI] [PubMed] [Google Scholar]
- 3.Kalpathy-Cramer J, Gerstner ER, Emblem KE, Andronesi OC, Rosen B. Advanced Magnetic Resonance Imaging of the Physical Processes in Human Glioblastoma. Cancer Res. 2014;74(17):4622–4637. doi: 10.1158/0008-5472.CAN-14-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skinner JT, Moots PL, Ayers GD, Quarles CC. On the Use of DSC-MRI for Measuring Vascular Permeability. Am J Neuroradiol. 2016;37(1):80–87. doi: 10.3174/ajnr.A4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts HC, Roberts TPL, Brasch RC, Dillon WP. Quantitative Measurement of Microvascular Permeability in Human Brain Tumors Achieved Using Dynamic Contrast-enhanced MR Imaging: Correlation with Histologic Grade. Am J Neuroradiol. 2000;21(5):891 LP–899. http://www.ajnr.org/content/21/5/891.abstract. [PMC free article] [PubMed] [Google Scholar]
- 6.Weber MA, Zoubaa S, Schlieter M, et al. Diagnostic performance of spectroscopic and perfusion MRI for distinction of brain tumors. Neurology. 2006;66(12):1899–1906. 10.1212/01.wnl.0000219767.49705.9c\npapers2://publication/doi/10.1212/01.wnl.0000219767.49705.9c. [DOI] [PubMed] [Google Scholar]
- 7.Bjornerud A, Sorensen AG, Mouridsen K, Emblem KE. T 1 - and T* 2 -Dominant Extravasation Correction in DSC-MRI: Part I—Theoretical Considerations and Implications for Assessment of Tumor Hemodynamic Properties. J Cereb Blood Flow Metab. 2011;31(10):2041–2053. doi: 10.1038/jcbfm.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donahue KM, Krouwer HGJ, Rand SD, et al. Utility of simultaneously acquired gradient-echo and spin-echo cerebral blood volume and morphology maps in brain tumor patients. Magn Reson Med. 2000;43(6):845–853. doi:. [DOI] [PubMed] [Google Scholar]
- 9.Li SP, Padhani AR. Tumor response assessments with diffusion and perfusion MRI. J Magn Reson Imaging. 2012;35(4):745–763. doi: 10.1002/jmri.22838. [DOI] [PubMed] [Google Scholar]
- 10.Brastianos PK, Carter SL, Santagata S, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5(11):1164–1177. doi: 10.1158/2159-8290.CD-15-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song YS, Choi SH, Park CK, et al. True progression versus pseudoprogression in the treatment of glioblastomas: A comparison study of normalized cerebral blood volume and apparent diffusion coefficient by histogram analysis. Korean J Radiol. 2013;14(4):662–672. doi: 10.3348/kjr.2013.14.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boxerman JL, Schmainda KM, Weisskoff RM. Relative Cerebral Blood Volume Maps Corrected for Contrast Agent Extravasation Significantly Correlate with Glioma Tumor Grade, Whereas Uncorrected Maps Do Not. Am J Neuroradiol. 2006;27:859–867. [PMC free article] [PubMed] [Google Scholar]
- 13.Leu K, Boxerman JL, Ellingson BM. Effects of MRI Protocol Parameters, Preload Injection Dose, Fractionation Strategies, and Leakage Correction Algorithms on the Fidelity of Dynamic-Susceptibility Contrast MRI Estimates of Relative Cerebral Blood Volume in Gliomas. Am J Neuroradiol. 2017;38(3):478–484. doi: 10.3174/ajnr.A5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270(3):834–841. doi: 10.1148/radiol.13131669. [DOI] [PubMed] [Google Scholar]
- 15.Vonken EPA, van Osch MJ, Bakker CJ, Viergever MA. Measurement of cerebral perfusion with dual-echo multi-slice quantitative dynamic susceptibility contrast MRI. J Magn Reson Imaging. 1999;10(2):109–117. doi: [DOI] [PubMed] [Google Scholar]
- 16.Newbould RD, Skare ST, Jochimsen TH, et al. Perfusion mapping with multiecho multishot parallel imaging EPI. Magn Reson Med. 2007;58(1):70–81. doi: 10.1002/mrm.21255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eichner C, Jafari-Khouzani K, Cauley S, et al. Slice accelerated gradient-echo spin-echo dynamic susceptibility contrast imaging with blipped CAIPI for increased slice coverage. Magn Reson Med. 2014;72(3):770–778. doi: 10.1002/mrm.24960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jafari-Khouzani K, Emblem KE, Kalpathy-Cramer J, et al. Repeatability of Cerebral Perfusion Using Dynamic Susceptibility Contrast MRI in Glioblastoma Patients. Transl Oncol. 2015;8(3):137–146. doi: 10.1016/j.tranon.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Speck O, Chang L, DeSilva NM, Ernst T. Perfusion MRI of the human brain with dynamic susceptibility contrast: Gradient-echo versus spin-echo techniques. J Magn Reson Imaging. 2000;12(3):381–387. doi:. [DOI] [PubMed] [Google Scholar]
- 20.Kiselev VG, Strecker R, Ziyeh S, Speck O, Hennig J. Vessel size imaging in humans. Magn Reson Med. 2005;53(3):553–563. doi: 10.1002/mrm.20383. [DOI] [PubMed] [Google Scholar]
- 21.Emblem KE, Mouridsen K, Bjornerud A, et al. Vessel architectural imaging identifies cancer patient responders to anti-angiogenic therapy. Nat Med. 2013;19(9):1178–1183. doi: 10.1038/nm.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmiedeskamp H, Straka M, Newbould RD, et al. Combined spin- and gradient-echo perfusion-weighted imaging. Magn Reson Med. 2012;68(1):30–40. doi: 10.1002/mrm.23195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmiedeskamp H, Andre JB, Straka M, et al. Simultaneous Perfusion and Permeability Measurements Using Combined Spin- and Gradient-Echo MRI. J Cereb Blood Flow Metab. 2013;33(5):732–743. doi: 10.1038/jcbfm.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knutsson L, Ståhlberg F, Wirestam R. Aspects on the accuracy of cerebral perfusion parameters obtained by dynamic susceptibility contrast MRI: a simulation study. Magn Reson Imaging. 2004;22(6):789–798. doi: 10.1016/j.mri.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Welker K, Boxerman J, Kalnin A, Kaufmann T, Shiroishi M, Wintermark M. ASFNR recommendations for clinical performance of MR dynamic susceptibility contrast perfusion imaging of the brain. Am J Neuroradiol. 2015;36(6):E41–E51. doi: 10.3174/ajnr.A4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen JJ, Smith MR, Frayne R. The impact of partial-volume effects in dynamic susceptibility contrast magnetic resonance perfusion imaging. J Magn Reson Imaging. 2005;22(3):390–399. doi: 10.1002/jmri.20393. [DOI] [PubMed] [Google Scholar]
- 27.Willats L, Calamante F. The 39 steps: evading error and deciphering the secrets for accurate dynamic susceptibility contrast MRI. NMR Biomed. 2013;26(8):913–931. doi: 10.1002/nbm.2833. [DOI] [PubMed] [Google Scholar]
- 28.Newton AT, Pruthi S, Stokes AM, Skinner JT, Quarles CC. Improving perfusion measurement in DSC-MR imaging with multiecho information for arterial input function determination. Am J Neuroradiol. 2016;37(7):1237–1243. doi: 10.3174/ajnr.A4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blaimer M, Gutberlet M, Kellman P, Breuer FA, Köstler H, Griswold MA. Virtual coil concept for improved parallel MRI employing conjugate symmetric signals. Magn Reson Med. 2009;61(1):93–102. doi: 10.1002/mrm.21652. [DOI] [PubMed] [Google Scholar]
- 30.Blaimer M, Jakob PM, Breuer FA. Regularization method for phase-constrained parallel MRI. Magn Reson Med. 2014;72(1):166–171. doi: 10.1002/mrm.24896. [DOI] [PubMed] [Google Scholar]
- 31.Blaimer M, Heim M, Neumann D, Jakob PM, Kannengiesser S, Breuer FA. Comparison of phase-constrained parallel MRI approaches: Analogies and differences. Magn Reson Med. 2016;75(3):1086–1099. doi: 10.1002/mrm.25685. [DOI] [PubMed] [Google Scholar]
- 32.Huang F, Akao J, Vijayakumar S, Duensing GR, Limkeman M. k-t GRAPPA: Ak-space implementation for dynamic MRI with high reduction factor. Magn Reson Med. 2005;54(5):1172–1184. doi: 10.1002/mrm.20641. [DOI] [PubMed] [Google Scholar]
- 33.Tsao J, Boesiger P, Pruessmann KP. k-t BLAST andk-t SENSE: Dynamic MRI with high frame rate exploiting spatiotemporal correlations. Magn Reson Med. 2003;50(5):1031–1042. doi: 10.1002/mrm.10611. [DOI] [PubMed] [Google Scholar]
- 34.Jung H, Sung K, Nayak KS, Kim EY, Ye JC. k-t FOCUSS : A General Compressed Sensing Framework for High Resolution Dynamic MRI. Magn Reson Med. 2009;61:103–116. doi: 10.1002/mrm.21757. [DOI] [PubMed] [Google Scholar]
- 35.Ponce IP, Blaimer M, Breuer FA, Griswold MA, Jakob PM, Kellman P. Auto-calibration approach for k-t SENSE. Magn Reson Med. 2014;71(3):1123–1129. doi: 10.1002/mrm.24738. [DOI] [PubMed] [Google Scholar]
- 36.Jung B, Ullmann P, Honal M, Bauer S, Hennig J, Markl M. Parallel MRI with extended and averaged GRAPPA kernels (PEAK-GRAPPA): Optimized spatiotemporal dynamic imaging. J Magn Reson Imaging. 2008;28(5):1226–1232. doi: 10.1002/jmri.21561. [DOI] [PubMed] [Google Scholar]
- 37.Bilgic B, Kim TH, Liao C, et al. Improving parallel imaging by jointly reconstructing multi-contrast data. Magn Reson Med. 2018;80(2):619–632. doi: 10.1002/mrm.27076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramb R, Mader I, Jung B, Hennig J, Zaitsev M. High resolution CBV assessment with PEAK-EPI: k-t-undersampling and reconstruction in echo planar imaging. Magn Reson Med. 2017;77(6):2153–2166. doi: 10.1002/mrm.26298. [DOI] [PubMed] [Google Scholar]
- 39.Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012;67(5):1210–1224. doi: 10.1002/mrm.23097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polimeni JR, Bhat H, Witzel T, et al. Reducing sensitivity losses due to respiration and motion in accelerated echo planar imaging by reordering the autocalibration data acquisition. Magn Reson Med. 2016;75(2):665–679. doi: 10.1002/mrm.25628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mugler JP, Brookeman JR. Off-resonance image artifacts in interleaved-EPI and GRASE pulse sequences. Magn Reson Med. 1996;36(2):306–313. doi: 10.1002/mrm.1910360218. [DOI] [PubMed] [Google Scholar]
- 42.Uecker M, Lai P, Murphy MJ, et al. ESPIRiT - An eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn Reson Med. 2014;71(3):990–1001. doi: 10.1002/mrm.24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Setsompop K, Cohen-Adad J, Gagoski BA, et al. Improving diffusion MRI using simultaneous multi-slice echo planar imaging. Neuroimage. 2012;63(1):569–580. doi: 10.1016/j.neuroimage.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(SUPPL. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 45.Bjørnerud A, Emblem KE. A Fully Automated Method for Quantitative Cerebral Hemodynamic Analysis Using DSC–MRI. J Cereb Blood Flow Metab. 2010;30(5):1066–1078. doi: 10.1038/jcbfm.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bjornerud A, Sorensen AG, Mouridsen K, Emblem KE. T 1 - and T* 2 -Dominant Extravasation Correction in DSC-MRI: Part I—Theoretical Considerations and Implications for Assessment of Tumor Hemodynamic Properties. J Cereb Blood Flow Metab. 2011;31(10):2041–2053. doi: 10.1038/jcbfm.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mouridsen K, Christensen S, Gyldensted L, Østergaard L. Automatic selection of arterial input function using cluster analysis. Magn Reson Med. 2006;55(3):524–531. doi: 10.1002/mrm.20759. [DOI] [PubMed] [Google Scholar]
- 48.Lebel RM, Wilman AH. Transverse relaxometry with stimulated echo compensation. Magn Reson Med. 2010;64(4):1005–1014. doi: 10.1002/mrm.22487. [DOI] [PubMed] [Google Scholar]
- 49.Stanisz GJ, Odrobina EE, Pun J, et al. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Reson Med. 2005;54(3):507–512. doi: 10.1002/mrm.20605. [DOI] [PubMed] [Google Scholar]
- 50.Li T-Q, Yao B, van Gelderen P, et al. Characterization of T 2 * heterogeneity in human brain white matter. Magn Reson Med. 2009;62(6):1652–1657. doi: 10.1002/mrm.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmiedeskamp H, Straka M, Bammer R. Compensation of Slice Profile Mismatch in Combined Spin- and Gradient-Echo Echo-Planar Imaging Pulse Sequences. Magn Reson Med. 2012;67:378–388. doi: 10.1002/mrm.23012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eskildsen SF, Gyldensted L, Nagenthiraja K, et al. Increased cortical capillary transit time heterogeneity in Alzheimer’s disease: a DSC-MRI perfusion study. Neurobiol Aging. 2017;50:107–118. doi: 10.1016/j.neurobiolaging.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Quarles CC, Bell LC, Stokes AM. Imaging vascular and hemodynamic features of the brain using dynamic susceptibility contrast and dynamic contrast enhanced MRI. Neuroimage. 2018;(October 2017):1–24. doi: 10.1016/j.neuroimage.2018.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stokes AM, Skinner JT, Yankeelov T, Quarles CC. Assessment of a simplified spin and gradient echo (sSAGE) approach for human brain tumor perfusion imaging. Magn Reson Imaging. 2016;34(9):1248–1255. doi: 10.1016/j.mri.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stokes AM, Quarles CC. A simplified spin and gradient echo approach for brain tumor perfusion imaging. Magn Reson Med. 2016;75(1):356–362. doi: 10.1002/mrm.25591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haldar JP. Low-Rank Modeling of Local k-Space Neighborhoods (LORAKS) for Constrained MRI. IEEE Trans Med Imaging. 2014;33(3):668–681. doi: 10.1109/TMI.2013.2293974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lingala SG, Hu Y, DiBella E, Jacob M. Accelerated Dynamic MRI Exploiting Sparsity and Low-Rank Structure: k-t SLR. IEEE Trans Med Imaging. 2011;30(5):1042–1054. doi: 10.1109/TMI.2010.2100850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Velikina JV, Jung Y, Field AA, Samsonov AA. High Resolution Dynamic Susceptibility Contrast Perfusion Imaging Using Multi-Echo Spirals and Temporal Compressed Sensing-Based Reconstruction. Proc Intl Soc Mag Reson Med. 2013;21:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ulas C, Preibisch C, Sperl J, Pyka T, Kalpathy-Cramer J, Menze B. Accelerated Reconstruction of Perfusion-Weighted MRI Enforcing Jointly Local and Nonlocal Spatio-temporal Constraints. 2017;XX(X):1–12. doi: 10.1016/j.bbagen.2007.12.006. [DOI] [Google Scholar]
- 60.Guo Y, Lingala SG, Zhu Y, Lebel RM, Nayak KS. Direct estimation of tracer-kinetic parameter maps from highly undersampled brain dynamic contrast enhanced MRI. Magn Reson Med. 2017;78(4):1566–1578. doi: 10.1002/mrm.26540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table S1: Comparison between standard single gradient echo DSC and a 5-echo SAGE acquisition, with and without slice acceleration.