Abstract
Background
Computed tomography pulmonary angiography (CTPA) is the gold standard for the diagnosis of pulmonary embolism (PE). However, contrast is contraindicated in some patients. The purpose of this study was to determine the diagnostic accuracy of unenhanced multidetector CT (MDCT) for diagnosis of central PE using CTPA as the gold standard.
Methods
The records of patients with suspected PE seen between 2010 and 2013 were retrospectively reviewed. Inclusion criteria were an acute, central PE confirmed by CTPA and non-enhanced MDCT before contrast injection. Patients with a PE ruled out by CTPA served as a control group. MDCT findings studied were high-attenuation emboli in pulmonary artery (PA), main PA dilatation > 33.2 mm, and peripheral wedge-shaped consolidation. Receiver operating characteristic (ROC) analysis was used to determine the sensitivity and specificity of unenhanced MDCT to detect PE. Wells score of all patients were calculated using data extracted from medical records prior to imaging analysis.
Results
Thirty-two patients with a PE confirmed by CTPA and 32 with a PE ruled out by CTPA were included. Among the three main MDCT findings, high-attenuation emboli in the PA showed best diagnostic performance (Sensitivity 72.9%; Specificity 100%), followed by main PA dilatation > 33.2 mm (sensitivity 46.9%; specificity 90.6%), and peripheral wedge-shaped consolidation (sensitivity 43.8%; specificity 78.1%). Given any one or more positive findings on unenhanced MDCT, the sensitivity was 96.9% and specificity was 71.9% for a diagnosis of PE in patients. The area under the curve (AUC) of a composite measure of unenhanced MDCT findings (0.909) was significantly higher than that of the Wells score (0.688), indicating unenhanced MDCT was reliable for detecting PE than Wells score.
Conclusions
Unenhanced MDCT is an alternative for the diagnosis of acute central PE when CTPA is not available.
Keywords: CTPA, High attenuation, Pulmonary artery dilatation, Wedge-shaped consolidation
Background
Acute pulmonary embolism (PE) has an annual incidence of approximately 3–6 cases per 10,000 persons in the general population [1, 2], and is the third leading cause of death responsible for an average of 650,000 deaths annually in the United States [3–5].
Currently, the diagnostic strategy of PE mainly evaluates the hemodynamic status first, followed by clinical risk assessment system (Wells score and Geneva score). After confirmation of PE or ruling out non-PE patients using hemodynamic and clinical risk assessment test, the suspected PE patients may perform radiological assessment using multi-detector contrast-enhanced computed tomography angiography (CTPA), which is the gold standard for the imaging diagnosis of PE [6–10]. However, excessive use of CTPA may result in excessive radiation exposure. Furthermore, even though Wells score and revised Geneva score can rule-out non-PE patients [11–16], it cannot be used for definitive PE diagnosis [17]. The Wells score-revised Geneva score stratification method can be further combined with the D-dimer test [18], which is a useful, non-invasive approach for the diagnosis of PE. The predictive value of the D-dimer test depends greatly on the clinical pretest probability estimated by the Wells score [2, 6, 7, 19–22].
The use of contrast agents is contraindicated in certain patients, such as those with renal insufficiency [23]. Generally, physicians faced with this clinical situation will have the Wells score available for ruling out the PE, but evaluation tools or tests for detecting PE are lacking. Rapid diagnosis of PE has been shown to reduce the mortality rate [6], and waiting for laboratory tests of renal function before performing CTPA may delay diagnosis. Unenhanced multidetector CT (MDCT) might be used as alternative methods to get images as soon as possible. An acute PE can occasionally be detected as high-attenuation emboli in the pulmonary artery (PA) on unenhanced CT [24, 25]. Furthermore, acute central PE is associated with more severe hemodynamic changes and higher mortality than distal PE and chronic PE, and timely intervention is vital in achieving good treatment outcomes [26]. The ability of the radiologists to establish an accurate diagnosis of PE base on MDCT information may be helpful in a situation where CTPA cannot be performed or is not available. Only a few reports have addressed the utility of non-contrast CT images in PE detection focusing on high attenuation emboli found in PA [24, 27–29]. None of the previous studies have attempted to evaluate the diagnostic performance of multiple unenhanced MDCT findings or determine the most sensitive criteria for determining a diagnosis based on multiple unenhanced MDCT findings.
The purpose of this study was to determine the sensitivity and specificity of unenhanced MDCT for the diagnosis of PE using CTPA as the gold standard. We also sought to determine what unenhanced MDCT findings are most useful for diagnosis of PE, and compare the accuracy of MDCT and Wells score for diagnosing PE, again using CTPA as the gold standard. Our hypothesis was that unenhanced MDCT may present as an alternative approach for diagnosis of PE when CTPA is not available.
Methods
Patients
The study protocol was approved by the institutional review board of Chi-Mei Medical Center, and informed consent was waived based on the retrospective nature of this study.
The medical records of all patients who were admitted to the emergency department of our medical center with suspected PE between 2010 and 2013 were retrospectively reviewed. Acute central PE was the focus of this study because it is associated with more severe hemodynamic changes and higher mortality, and requires prompt intervention to have a good outcome as compared to distal PE and chronic PE [26]. Acute central PE was defined as a clot in the main, left, or right PA. Patients with a chronic PE were excluded. Chronic PE was defined as complete obstruction with an eccentric of calcified thrombus; post-stenotic dilatation of pulmonary artery, peripheral PA affected segments may be narrowed; PA calcification; right ventricular enlargement or hypertrophy is seen and lung mosaic perfusion pattern is present [30]. Patients who did not receive a CTPA or MDCT, and those without sufficient data in the medical records to calculate a Wells score were also excluded.
We have a CTPA protocol at our hospital. When a CTPA is considered necessary, first a non-enhanced CT is performed, followed by CTPA, and finally by a venous phase contrast enhanced CT. The case group consisted of patients with an acute, central PE confirmed by CTPA, who had also undergone non-enhanced MDCT of the chest. A control group with no evidence of PE confirmed by CTPA was randomly selected from the same time period. Records were first identified by ICD-9 code (415.1; pulmonary embolism and infarction includes acute and chronic, central and peripheral pulmonary embolism), and the images of those records identified were reviewed by radiologists on a picture archiving and communications system (PACS) workstation for identification of patients with an acute, central PE confirmed by CTPA.
Imaging analysis
All imaging studies were performed on a Toshiba Aquilion 64 Slice CT, and the scanning protocol at the time included both unenhanced and enhanced scans. Both images were collected for evaluation. Parameters varied among the unenhanced and enhanced examinations, with a slice thickness ranging from 3 to 5 mm. All MDCT images were reviewed by two experienced radiologists (with 3 and 15 years of experience in reading CTPA, respectively) who were blinded to the patients’ medical history and examination and laboratory findings. The radiologists reviewed the records independently. When their independent observations did not agree, they attempted to achieve a consensus. If no consensus was achieved the patient was excluded. Only non-contrast images were reviewed by the radiologists to avoid possible misleading due to contrast-enhanced results. Three important radiologic features on unenhanced MDCT images were chosen to compare with the Wells score: High-attenuation emboli in pulmonary artery (PA), main PA dilatation > 33.2 mm, and peripheral wedge-shaped consolidation. Again, only when all features were agreed upon by the two radiologists was a patient included in the study.
Wells score
Wells score was calculated based on seven variables are previously described [20]. The variables and their score were: 1) clinical symptoms of deep venous thrombosis (DVT) (score = 3.0); 2) no alternative diagnosis (score = 3.0); 3), heart rate > 100 (score = 1.5); 4) immobilization or surgery in the previous 4 weeks (score = 1.5); 5) previous DVT/PE (score = 1.5); 6) hemoptysis (score = 1.0); 7) malignancy (score = 1.0). The scores of the seven variables were summed to determine the Wells score. PE was considered unlikely if the Wells score was < 4.5, and considered likely if the score was ≥4.5. The combination of a Wells score < 4.5 and a negative SimpliRED D-dimer result was considered to exclude a PE [20]. Data were extracted from the medical records. If data of any of the seven variables was not available, the patient was excluded.
Statistical analysis
The gold standard for the diagnosis of PE was CTPA. Categorical data were expressed as numbers and percentages. Fisher’s exact test was performed to examine the associations of Wells score items and unenhanced MDCT image findings with PE. Logistic regression analyses were performed to examine the associations of a diagnosis of PE based on CTPA with Wells score and the number of findings on unenhanced MDCT, as well as with each item of unenhanced MDCT. In order to select significant individual features that might help detect PE and to examine whether unenhanced MDCT findings were independently associated with PE diagnosis, multivariate logistic regression was performed by including both Wells score and number of MDCT findings, age, and sex of patients. The number of positive findings on unenhanced MDCT was considered as a continuous variable, and was included as one independent variable in the logistic regression model with PE diagnosis as the dependent variable. Odds ratios (ORs) were obtained from logistic regression. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic performance of unenhanced MDCT in detecting PE. The area under the ROC curve (AUC) for unenhanced MDCT was compared with that of the Wells score by using the method proposed by DeLong et al. [31]. Sensitivity, specificity, positive likelihood ratio (PLR), positive predictive value (PPV), negative likelihood ratio (NLR), and negative predictive value (NPV), as well as their 95% confidence intervals (95% CIs), for diagnosis of PE were calculated for each unenhanced MDCT finding. ROC curve analyses were performed by using MedCalc for Windows, version 12.5 (MedCalc Software, Ostend, Belgium). Descriptive statistics and regression analysis were performed by IBM SPSS statistical software version 22 for Windows (IBM Corp., New York, USA). A value of p < 0.05 was considered statistically significant.
Results
A total of 181 patients were diagnosed with a PE during the study period. After applying the exclusion criteria, 32 patients with an acute central PE confirmed by CTPA, and 32 patients with a PE ruled-out by CTPA were included in the analysis. There was no significant difference between ages of individuals with and without a PE (mean age: 67.1 ± 16.6 versus 65.3 ± 14.6 years, respectively, p = 0.66). Approximately 41% of the patients with a PE were male, while 46.9% of those without a PE were male (p = 0.80).
Wells criteria and unenhanced MDCT findings in patients with and without a PE are shown in Table 1. Based on Wells score, all 64 patients had an alternative diagnosis that was less likely than a PE. Approximately 75% of patients with a PE had a Wells score > 4.5, while 56.3% of patients without a PE had a Wells score > 4.5. The primary findings on unenhanced MDCT of the chest in patients with a PE were high attenuation within the pulmonary artery (35.9%), main PA dilatation > 3.2 mm (28.1%), and peripheral wedge-shaped consolidation (43.8%) (Table 1; Figs. 1, 2 and 3).
Table 1.
Wells criteria and unenhanced multidetector computed tomography (MDCT) findings in patients with and without pulmonary embolism (PE)
| With PE diagnosed by CTPA | Without PE confirmed by CTPA | p-value | |
|---|---|---|---|
| Wells criteria | |||
| DVT | 11 (34.4%) | 0 (0%) | < 0.001 |
| Alternative diagnosis less likely than PE | 32 (100%) | 32 (100%) | NA |
| Heart rate > 100 beats/minute | 19 (59.4%) | 14 (43.8%) | 0.317 |
| Recent surgery or immobilization | 3 (9.4%) | 5 (15.6%) | 0.708 |
| Previous PE/DVT | 3 (9.4%) | 4 (12.5%) | 1.000 |
| Hemoptysis | 2 (6.3%) | 0 (0%) | 0.492 |
| Malignancy history | 6 (18.8) | 4 (12.5%) | 0.732 |
| Wells score | 0.188 | ||
| ≥ 4.5 | 24 (75.5%) | 18 (56.3%) | |
| < 4.5 | 8 (25.0%) | 14 (43.8%) | |
| Unenhanced MDCT | |||
| High attenuation in pulmonary artery (PA) | 23 (71.9%) | 0 (0%) | < 0.001 |
| Main PA dilatation > 33.2 mm | 15 (46.9%) | 3 (9.4%) | 0.002 |
| Peripheral wedge-shape consolidation | 14 (43.8%) | 7 (21.9%) | 0.109 |
| Number of findings | < 0.001 | ||
| 0 | 1 (3.1%) | 23 (71.9%) | |
| 1 | 13 (40.6%) | 8 (25.0%) | |
| 2 | 15 (46.9%) | 1 (3.1%) | |
| 3 | 3 (9.4%) | 0 (0%) | |
DVT deep vein thrombosis, MDCT multidetector computed tomography, PA pulmonary artery, PE pulmonary embolism
NA: Not applicable since all patients had alternative diagnosis less likely than PE
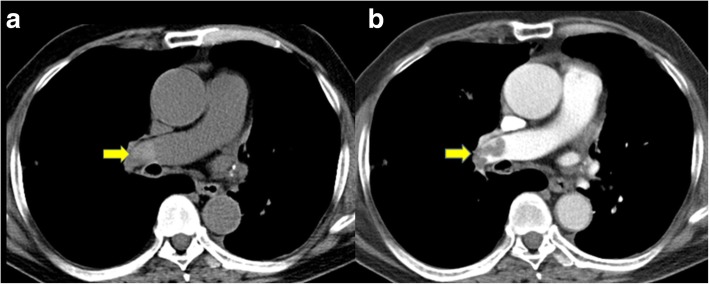
Fig. 1.
This patient was seen in the emergency department with dyspnea and diagnosed with an acute pulmonary embolism by CTPA. a Non-contrast computed tomography showed high attenuation emboli in the right pulmonary artery (arrow). b Post-contrast image showed filling defects in the right pulmonary artery
Fig. 2.
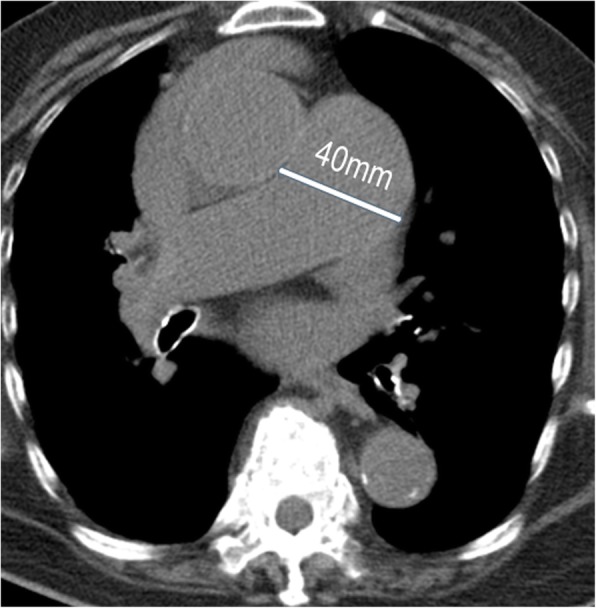
This patient was seen in the emergency department for dyspnea. Computed tomography showed a dilated pulmonary artery (diameter > 33.2 mm)
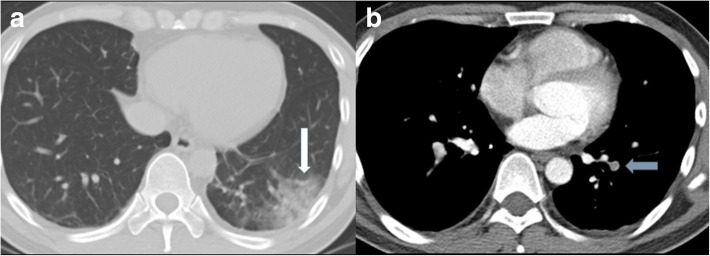
Fig. 3.
This patient was seen in the emergency department due to hemoptysis, and was diagnosed with an acute pulmonary embolism by CTPA. a A wedged-shaped opacification was seen in the left lower lobe (arrow). b Post-contrast image showed a centrally located embolism surrounded by contrast material (polo mint sign, arrow)
To compare the association of individual findings on unenhanced MDCT with PE diagnosis, we performed multivariate regression analysis. After adjusting for age, gender, and number of unenhanced MDCT findings, multivariable analysis indicated that diagnosis of PE was only associated with a greater number of findings on unenhanced MDCT (adjusted odds ratio [aOR] = 26.34; 95% CI: 4.91, 141.29; p < 0.001), and main PA dilatation > 33.2 mm (aOR = 10.59; 95% CI: 2.39, 47.02; p = 0.002) (Table 2). No associations were found for any of the Wells criteria.
Table 2.
Association between PE diagnosis by enhanced MDCT with Wells score and unenhanced MDCT findings
| OR (95% CI) | p-value | aOR (95% CI) | p-value | |
|---|---|---|---|---|
| Wells score (≥ 4.5 vs. < 4.5) a | 1.68 (1.17, 2.41) | 0.005 | 2.10 (0.99, 4.42) | 0.052 |
| Number of findings on unenhanced MDCTb | 21.11 (4.91, 90.77) | < 0.001 | 26.34 (4.91, 141.29) | < 0.001 |
| High attenuation in pulmonary artery (PA) b | NA | |||
| Main PA dilatation > 33.2 mm b | 8.53 (2.15, 33.79) | 0.002 | 10.59 (2.39, 47.02) | 0.002 |
| Peripheral wedge-shape consolidation b | 2.78 (0.93, 8.27) | 0.066 | 2.79 (0.84, 9.20) | 0.093 |
aOR adjusted odds ratio, MDCT multidetector computed tomography, PE pulmonary embolism
NA: Not applicable since there were no non-PE patients for this finding
aThe multivariate model included age, gender, and number of findings on unenhanced MDCT
bThe multivariate model included age, gender, and Wells score
The performance of unenhanced MDCT for the diagnosis of PE is shown in Table 3. High-attenuation emboli in pulmonary artery had the highest sensitivity (71.9%; 95% CI: 53.3, 86.3%; AUC = 0.859) and specificity (100%; 95% CI: 89.1, 100%) for the diagnosis of PE, followed by a main PA dilatation > 33.2 mm (sensitivity = 46.9%; specificity = 90.6%; AUC = 0.687), and peripheral wedge-shaped consolidation (sensitivity = 43.8%; specificity = 78.1%; AUC = 0.609). The optimal cut-off point for the number of findings on unenhanced MDCT was ≥1. The sensitivity was 96.9% (95% CI: 83.8, 99.9%) and specificity was 71.9% (95% CI: 53.3, 86.3%) for a diagnosis of PE in patients when there was at least one positive finding on unenhanced MDCT (Table 3).
Table 3.
Diagnostic performance based on unenhanced MDCT findings
| Unenhanced MDCT | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | PLR | NLR | AUC |
|---|---|---|---|---|---|---|---|
| High attenuation emboli in PA | 71.9 (53.3, 86.3) | 100 (89.1, 100) | 100 (85.2, 100) | 78.0 (62.4, 89.4) | NE | 0.28 | 0.859 |
| Main PA dilatation > 33.2 mm | 46.9 (29.1, 65.3) | 90.6 (75.0, 98.0) | 83.3 (58.6, 96.4) | 63.0 (47.5, 76.8) | 5.0 (3.4, 7.4) | 0.6 (0.2, 1.8) | 0.687 |
| Peripheral wedge-shape consolidation | 43.8 (26.4, 62.3) | 78.1 (60.0, 90.7) | 66.7 (43.0, 85.4) | 58.1 (42.1, 73.0) | 2.0 (1.3, 3.1) | 0.7 (0.3, 1.5) | 0.609 |
| Number of positive findingsa | 96.9 (83.8, 99.9) | 71.9 (53.3, 86.3) | 77.5 (61.5, 89.2) | 95.8 (78.9, 99.9) | 3.4 (2.7, 4.3) | 0.04 (0.01, 0.3) | 0.909 |
AUC area under ROC curve, MDCT, multidector computed tomography, NE not estimated, PA pulmonary artery, PLR positive likelihood ratio, NLR negative likelihood ratio, PPV positive predictive value, NPV negative predictive value
aThe optimal cut-of-point was ≥1
The AUC of a composite measure of unenhanced MDCT (i.e., number of findings on unenhanced MDCT) (AUC = 0.909; 95% CI: 0.811, 0.967) was significantly higher than that of the Wells score (AUC = 0.688; 95% CI: 0.560, 0.798) (p = 0.002), indicating better diagnostic performance of unenhanced MDCT than Wells score for detecting a PE (Fig. 4).
Fig. 4.
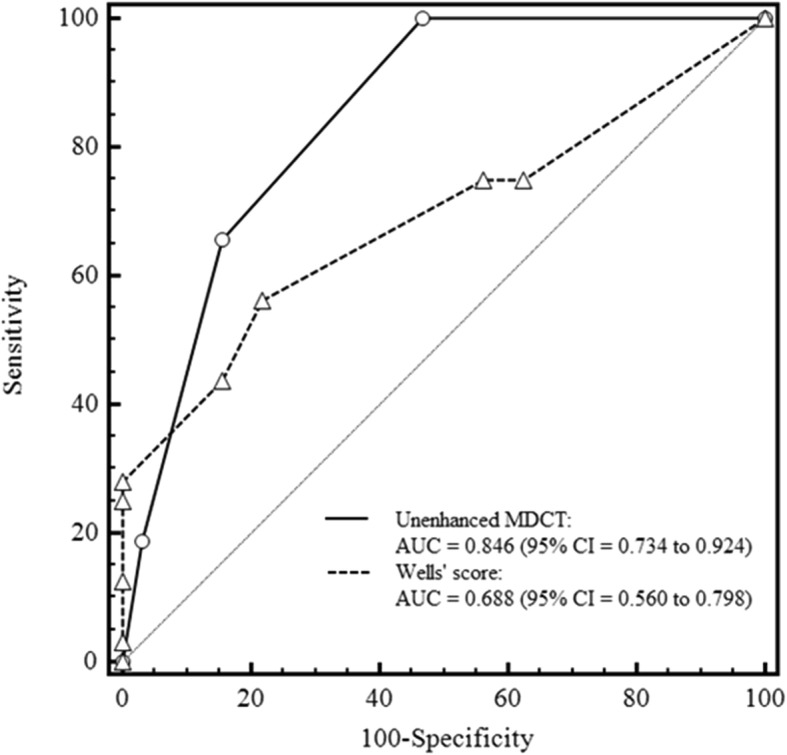
Receiver operating characteristic (ROC) curve for unenhanced MDCT and Wells score used for the diagnosis of pulmonary embolism
Discussion
The purpose of this study was to determine the value of unenhanced MDCT as screening tool for central acute PE using CTPA as the gold standard. The sensitivity was 96.9% and specificity was 71.9% for a diagnosis of PE in patients with at least one positive finding on unenhanced MDCT. High attenuation within the PA had a PPV of 100% and NPV of 78.0% for diagnosis of a central acute PE. Furthermore, the diagnostic performance of unenhanced MDCT was significantly better than that of Wells score. These results suggest that unenhanced MDCT may be useful for a rapid diagnosis of PE when CTPA is not available or contraindicate.
Acute PE is a life-threatening condition and prompt diagnosis is critical for good outcomes. While CTPA is the gold standard for diagnosis, it is not always available and contraindicated in certain patients. Wells score is typically used as alternative, and while useful for ruling out PE it is not sensitive for diagnosis of a PE [17]. For this reason, we examined the value of an alternative, unenhanced MDCT, as a screening to for the diagnosis of PE in the emergency room setting. The finding of high attenuation emboli in the PA on unenhanced MDCT has received most attention and evaluated by several studies in the context of PE diagnosis. Moreover, wedge-shaped subpleural consolidation and dilated central pulmonary arteries observed in unenhanced MDCT had been indicated as indirect signs for acute PE [32]. To the best of our knowledge, the current study is the first in performing and proposing a multi-component evaluation strategy based on several imaging findings on unenhanced MDCT. High attenuation emboli in PA indeed showed best diagnostic performance among the three analyzed findings in the present study, and the sensitivity was further improved by inclusion of other unenhanced MDCT findings. Further investigations performed in a more general setting or in a prospective manner are required for confirming the favorable diagnostic performance shown by multi-component unenhanced MDCT findings before advice on implementation of the strategy can be made.
Although we showed that high-attenuation emboli in PA had a sensitivity of 71.9% and specificity of 100% for diagnosis of a PE, other studies reported slightly lower sensitivity or incidence of the unenhanced MDCT finding. Tatco et al. [24] reported that this sign had an overall sensitivity of only 36% for detecting central PE, which is significantly lower that the sensitivity found in our study. Cobelli et al. [28] reported that emboli in central PA could be detected on unenhanced CT in 41.2% of their hospitalized patients with clinical suspicion of PE, and Kanne et al. [27] found that 46.1% of their unenhanced scans with central clots and 6% of all unenhanced CT scans carried out in their institution were positive for PE. High-attenuation emboli in PA had a higher sensitivity for PE in the current study, probably because we focused on acute emboli suspected in the emergency room, and the diagnosis was only made when there was consensus of two radiologists. The high attenuation of thrombi on CT is due to the higher level of hemoglobin in clots as compared to that of circulating blood [24, 33].
PA enlargement and wedge-shaped consolidation are well-known indicators suggestive of PE [34]. The use of PA enlargement and wedge-shaped consolidation in combination with high attenuation emboli in the PA is responsible for the overall high sensitivity of unenhanced MDCT for diagnosis of PE, and when at least one positive finding was noted on unenhanced CT the sensitivity approached 100%.
When emboli are located in segmental, subsegmental, and more peripheral arteries, the sensitivity of unenhanced CT is limited [35, 36]. Motion artifact, partial volume averaging, and low signal-to-noise ratio almost always affect imaging of the peripheral arteries and contribute to false negative results [36, 37]. In addition, any anatomical structure adjacent to the PA can cause areas of hyper-attenuation during respiratory or cardiac motion, which can mimic the hyperdense lumen sign. When volume averaging with atherosclerotic disease involving the pulmonary arteries is performed, false positive results may be obtained [24].
Limitations
There are several limitations to this study, including its retrospective nature and the small sample size. All patients in this study were selected from the emergency department which may be one source of bias. The age of the clot and the patient’s hematocrit level at the time of imaging, and other factors which may interfere with visualization were not assessed. In addition, the study examined only acute central PE, and thus the technique may not be of value for imaging of other types of PE. High attenuation emboli in the PA were the greatest source of discrepancy between radiologists because of the non-quantitative and subjective nature of this finding, and we did not determine inter-rater accuracy of diagnosis. We acknowledge that the included patient numbers were not large, future studies with larger sample size is necessary for further validation. However, the results from this study does demonstrate that unenhanced MDCT is an alternative approach for the diagnosis of PE.
Conclusions
Unenhanced MDCT is an alternative approach for the diagnosis of acute central PE when CTPA is inaccessible or contraindicated. In our study, non-enhanced MDCT has shown better performance than Well’s score for confirming acute thrombi in the main right or left pulmonary arteries, but cannot rule out pulmonary thromboembolism.
Acknowledgements
Not applicable
Abbreviations
- aOR
Adjusted OR
- AUC
Area under the ROC curve
- CTPA
Computerized tomography pulmonary angiography
- DVT
Deep vein thrombosis
- MDCT
Unenhanced multidetector computerized tomography
- NPV
Negative predictive value
- OR
Odds ratio
- PE
Pulmonary embolism
- PPV
Positive predictive value
- ROC
Receiver operator characteristic
Authors’ contributions
CH C: study concepts, study design, clinical studies: data acquisition: CH Chien, FC Shih, CY Chen, CH Chen, WL Wu, CW Mak. All authors have read and approved the final version of the manuscript.
Funding
Not applicable
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Ethics approval and consent to participate
The study protocol was approved by the Chi-Mei Medical Center institutional review board, and informed consent was waived based on the retrospective nature of this study.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chiao-Hsuan Chien, Phone: 886-6-2812811, Email: kalanono@gmail.com.
Fu-Chieh Shih, Email: b8601140@yahoo.com.tw.
Chin-Yu Chen, Email: chency@seed.net.tw.
Chia-Hui Chen, Email: chiachchen@gmail.com.
Wan-Ling Wu, Email: chofeculture@yahoo.com.tw.
Chee-Wai Mak, Email: makcw33@hotmail.com.
References
- 1.Spencer FA, Emery C, Lessard D, Anderson F, Emani S, Aragam J, et al. The Worcester venous thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med. 2006;21:722–727. doi: 10.1111/j.1525-1497.2006.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rohacek M, Buatsi J, Szucs-Farkas Z, Kleim B, Zimmermann H, Exadaktylos A, et al. Ordering CT pulmonary angiography to exclude pulmonary embolism: defense versus evidence in the emergency room. Intensive Care Med. 2012;38:1345–1351. doi: 10.1007/s00134-012-2595-z. [DOI] [PubMed] [Google Scholar]
- 3.Shahriar Z, Stephan R, Shweta M, Arun S, Mathew T, Brijal P, et al. Could the number of CT angiograms be reduced in emergency department patients suspected of pulmonary embolism? World J Emerg Med. 2012;3:172–176. doi: 10.5847/wjem.j.issn.1920-8642.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanter DS, Mikkola KM, Patel SR, Parker JA, Goldhaber SZ. Thrombolytic therapy for pulmonary embolism. Frequency of intracranial hemorrhage and associated risk factors. Chest. 1997;111:1241–1245. doi: 10.1378/chest.111.5.1241. [DOI] [PubMed] [Google Scholar]
- 5.Ma Y, Yan S, Zhou L, Yuan DT. Competitive assessments of pulmonary embolism: noninvasiveness versus the golden standard. Vascular. 2016;24:217–224. doi: 10.1177/1708538115589893. [DOI] [PubMed] [Google Scholar]
- 6.Gruettner J, Viergutz T, Bolte M, Henzler T, Schoenberg SO, Sudarski S, et al. Importance of risk factors for the evaluation of patients with a suspected pulmonary embolism. Exp Ther Med. 2015;9:2281–2284. doi: 10.3892/etm.2015.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med. 2010;363:266–274. doi: 10.1056/NEJMra0907731. [DOI] [PubMed] [Google Scholar]
- 8.Sun S, Semionov A, Xie X, Kosiuk J, Mesurolle B. Detection of central pulmonary embolism on non-contrast computed tomography: a case control study. Int J Cardiovasc Imaging. 2014;30:639–646. doi: 10.1007/s10554-013-0356-x. [DOI] [PubMed] [Google Scholar]
- 9.Stein PD, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354:2317–2327. doi: 10.1056/NEJMoa052367. [DOI] [PubMed] [Google Scholar]
- 10.Hou DJ, Tso DK, Davison C, Inacio J, Louis LJ, Nicolaou S, et al. Clinical utility of ultra high pitch dual source thoracic CT imaging of acute pulmonary embolism in the emergency department: are we one step closer towards a non-gated triple rule out? Eur J Radiol. 2013;82:1793–1798. doi: 10.1016/j.ejrad.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Raja AS, Ip IK, Dunne RM, Schuur JD, Mills AM, Khorasani R. Effects of performance feedback reports on adherence to evidence-based guidelines in use of CT for evaluation of pulmonary embolism in the emergency department: a randomized trial. AJR Am J Roentgenol. 2015;205:936–940. doi: 10.2214/AJR.15.14677. [DOI] [PubMed] [Google Scholar]
- 12.Wells PS, Anderson DR, Rodger M, Stiell I, Dreyer JF, Barnes D, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001;135:98–107. doi: 10.7326/0003-4819-135-2-200107170-00010. [DOI] [PubMed] [Google Scholar]
- 13.Ceriani E, Combescure C, Le Gal G, Nendaz M, Perneger T, Bounameaux H, et al. Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:957–970. doi: 10.1111/j.1538-7836.2010.03801.x. [DOI] [PubMed] [Google Scholar]
- 14.Douma RA, Mos IC, Erkens PM, Nizet TA, Durian MF, Hovens MM, et al. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism: a prospective cohort study. Ann Intern Med. 2011;154:709–718. doi: 10.7326/0003-4819-154-11-201106070-00002. [DOI] [PubMed] [Google Scholar]
- 15.Lucassen W, Geersing GJ, Erkens PM, Reitsma JB, Moons KG, Büller H, et al. Clinical decision rules for excluding pulmonary embolism: a meta-analysis. Ann Intern Med. 2011;155:448–460. doi: 10.7326/0003-4819-155-7-201110040-00007. [DOI] [PubMed] [Google Scholar]
- 16.Gibson NS, Sohne M, Kruip MJ, Tick LW, Gerdes VE, Bossuyt PM, et al. Further validation and simplification of the Wells clinical decision rule in pulmonary embolism. Thromb Haemost. 2008;99:229–234. doi: 10.1160/TH07-05-0321. [DOI] [PubMed] [Google Scholar]
- 17.Kline Jeffrey A. Diagnosis and Exclusion of Pulmonary Embolism. Thrombosis Research. 2018;163:207–220. doi: 10.1016/j.thromres.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 18.van Belle A, Büller HR, Huisman MV, Huisman PM, Kaasjager K, Kamphuisen PW, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA. 2006;295:172–179. doi: 10.1001/jama.295.2.172. [DOI] [PubMed] [Google Scholar]
- 19.Parikh N, Morris E, Babb J, Wickstrom M, McMenamy J, Sharma R, et al. MDCT diagnosis of acute pulmonary embolism in the emergent setting. Emerg Radiol. 2015;22:379–384. doi: 10.1007/s10140-014-1290-5. [DOI] [PubMed] [Google Scholar]
- 20.Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83:416–420. doi: 10.1055/s-0037-1613830. [DOI] [PubMed] [Google Scholar]
- 21.Klok FA, Mos IC, Nijkeuter M, Righini M, Perrier A, Le Gal G, et al. Simplification of the revised Geneva score for assessing clinical probability of pulmonary embolism. Arch Intern Med. 2008;168:2131–2136. doi: 10.1001/archinte.168.19.2131. [DOI] [PubMed] [Google Scholar]
- 22.Le Gal G, Righini M, Roy PM, Sanchez O, Aujesky D, Bounameaux H, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165–171. doi: 10.7326/0003-4819-144-3-200602070-00004. [DOI] [PubMed] [Google Scholar]
- 23.Luk L, Steinman J, Newhouse JH. Intravenous contrast-induced nephropathy-the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24:169–175. doi: 10.1053/j.ackd.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Tatco VR, Piedad HH. The validity of hyperdense lumen sign in non-contrast chest CT scans in the detection of pulmonary thromboembolism. Int J Cardiovasc Imaging. 2011;27:433–440. doi: 10.1007/s10554-010-9673-5. [DOI] [PubMed] [Google Scholar]
- 25.Wittram C, Maher MM, Yoo AJ, Kalra MK, Shepard JA, McLoud TC. CT angiography of pulmonary embolism: diagnostic criteria and causes of misdiagnosis. Radiographics. 2004;24:1219–1238. doi: 10.1148/rg.245045008. [DOI] [PubMed] [Google Scholar]
- 26.Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galiè N, Pruszczyk P, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC) Eur Heart J. 2008;29:2276–2315. doi: 10.1093/eurheartj/ehn475. [DOI] [PubMed] [Google Scholar]
- 27.Kanne JP, Gotway MB, Thoongsuwan N, Stern EJ. Six cases of acute central pulmonary embolism revealed on unenhanced multidetector CT of the chest. AJR Am J Roentgenol. 2003;180:1661–1664. doi: 10.2214/ajr.180.6.1801661. [DOI] [PubMed] [Google Scholar]
- 28.Cobelli R, Zompatori M, De Luca G, Chiari G, Bresciani P, Marcato C. Clinical usefulness of computed tomography study without contrast injection in the evaluation of acute pulmonary embolism. J Comput Assist Tomogr. 2005;29:6–12. doi: 10.1097/01.rct.0000148274.45419.95. [DOI] [PubMed] [Google Scholar]
- 29.Kanne P, Thoongsuwan N, Stern EJ. Detection of central pulmonary embolism on computed tomography densitometry images before computed tomography pulmonary angiography. J Comput Assist Tomogr. 2003;27:907–910. doi: 10.1097/00004728-200311000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Castañer E, Gallardo X, Ballesteros E, Andreu M, Pallardó Y, Mata JM, et al. CT diagnosis of chronic pulmonary thromboembolism. Radiographics. 2009;29:31–50. doi: 10.1148/rg.291085061. [DOI] [PubMed] [Google Scholar]
- 31.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 32.Coche EE, Muller NL, Kim KI, Wiggs BR, Mayo JR. Acute pulmonary embolism: ancillary findings at spiral CT. Radiology. 1998;207:753–758. doi: 10.1148/radiology.207.3.9609900. [DOI] [PubMed] [Google Scholar]
- 33.Wolverson MK, Crepps LF, Sundaram M, Heiberg E, Vas WG, Shields JB. Hyperdensity of recent hemorrhage at body computed tomography: incidence and morphologic variation. Radiology. 1983;148:779–784. doi: 10.1148/radiology.148.3.6878700. [DOI] [PubMed] [Google Scholar]
- 34.Devaraj A, Sayer C, Sheard S, Grubnic S, Nair A, Vlahos I. Diagnosing acute pulmonary embolism with computed tomography: imaging update. J Thorac Imaging. 2015;30:176–192. doi: 10.1097/RTI.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 35.Mohamed ND, Othman MHM, Hassan LS, Yousef HAZ. The accuracy of non-contrast chest computed tomographic scan in the detection of pulmonary thromboembolism. J Curr Med Res Prac. 2019;4:61–66. doi: 10.4103/JCMRP.JCMRP_13_19. [DOI] [Google Scholar]
- 36.Kligerman SJ, Mitchell JW, Sechrist JW, Meeks AK, Galvin JR, White CS. Radiologist performance in the detection of pulmonary embolism: features that favor correct interpretation and risk factors for errors. J Thorac Imaging. 2018;33:350–357. doi: 10.1097/RTI.0000000000000361. [DOI] [PubMed] [Google Scholar]
- 37.Hutchinson BD, Navin P, Marom EM, Truong MT, Bruzzi JF. Overdiagnosis of pulmonary embolism by pulmonary CT angiography. AJR Am J Roentgenol. 2015;205:271–277. doi: 10.2214/AJR.14.13938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.