Key Points
Ponatinib therapy heightens arterial thrombosis and platelet reactivity.
Concurrent pioglitazone treatment reverses heightened thrombosis risk and platelet reactivity induced by ponatinib.
Introduction
Chronic myeloid leukemia (CML) is treated by oral tyrosine kinase inhibitors (TKIs). However, ABL1 kinase T315I+ CML is resistant to most tyrosine TKIs. Ponatinib (Iclusig; Takeda Oncology), an ABL kinase inhibitor, is not blocked by the T315I mutation and is effective as salvage therapy. In the PACE trial, which characterized ponatinib, the incidence of arterial occlusive events was 29% (myocardial infarction, 14%; stroke, 11%; and limb ischemia, 11%; some patients had >1 vascular event) after 4 years of treatment.1,2
Ponatinib has the widest inhibitory spectrum of CML tyrosine kinases.3 It inhibits ABL1 (T315I), fibroblast growth factor receptors 1 to 4, vascular endothelial growth factor receptors 1 to 3, FLT3, KIT, platelet-derived growth factor receptor, SRC, MEKK3, and Tie2, which are important vascular and myeloid cell receptors involved in cell growth, proliferation, angiogenesis, and repair.3-5 ABL kinases (ABL1 and ABL2) themselves influence vascular development, function, and survival and regulate angiopoietin1/Tie2-mediated activities.6,7 Loss of ABL1 kinase leads to vascular dysfunction, apoptosis, tissue damage, infarction, and microvascular thrombosis, and its deletion is embryonically lethal.8 We examined the in vivo influence of ponatinib on murine arterial thrombosis and platelet reactivity. Our investigations reveal that ponatinib increases in vivo arterial thrombosis risk and platelet hyperreactivity, but concurrent pioglitazone administration reverses both.
Methods
Normal C57CL/6J mice 18 to 20 weeks old were purchased from Jackson Laboratories. All experimentation was performed with Case Western Reserve University Institutional Animal Care and Use Committee–approved laboratory animal protocols. Ponatinib and imatinib were provided by Ariad Pharmaceuticals, which assayed in vivo drug level by liquid chromatography with tandem mass spectrometry. Pioglitazone hydrochloride and ponatinib were purchased from Tocris Bioscience. Polyclonal antibodies to nitrotyrosine (Millipore #06-284 at 1 µg/mL) and caspase-3 (Cell Signaling #9661 at 1:50 dilution) were obtained for immunoperoxidase staining of aortic sections. Immunoperoxidase staining reactions were developed with reagents from Cell Signaling (#8059) and Thermo Fisher (#34065). Collagen-rich peptide (CRP) was a gift of Deborah Newman of the Blood Center of Wisconsin. Human α-thrombin (3000 U/mL) and adenosine 5′-diphosphate were purchased from Haematologic Technologies and Sigma, respectively.
All TKIs to mice were administered by gavage twice daily for 14 days. Murine carotid artery thrombosis studies were performed using Rose Bengal as previously reported.8,9 Plasma prothrombin time, activated partial thromboplastin time, thrombin generation, murine blood collection, platelet washing, and flow cytometry were performed as previously reported.8,9 Fluorescein isothiocyanate- and phycoerythrin-labeled rat anti-mouse CD62P (#M130-1) and CD41/61 (#M023-2) antibodies, respectively, were purchased from Emfret Analytics. Alexa Fluor 488 fibrinogen (#F13191) was obtained from Molecular Probes. Antibody to Stat5 conjugated with horse radish peroxidase was purchased from Santa Cruz Biotechnology (#SC-74442 HRP). Immunoblot band density was determined with ImageJ. Statistical analysis was performed by 1-way analysis of variance (ANOVA) and Student t test.
Results and discussion
Influence of ponatinib on carotid artery thrombosis
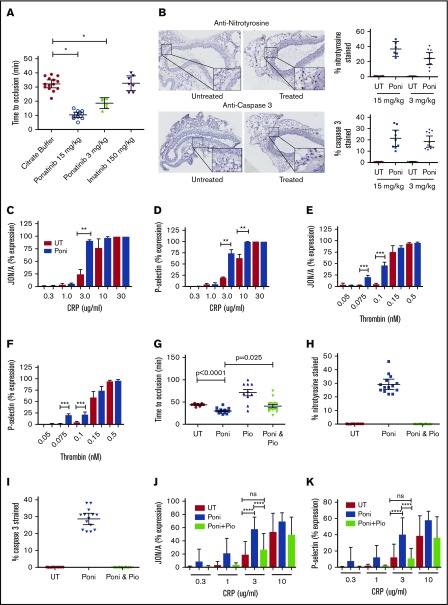
To examine global thrombosis risk after TKI therapy, an in vivo murine arterial thrombosis model was established. Mice treated with ponatinib at 15 or 3 mg/kg twice daily by gavage for 14 days had significantly shorter times to carotid artery occlusion compared with buffer-treated mice (mean ± 95% confidence interval [CI], 10.4 ± 2.9 or 18.7 ± 3.7 vs 32.3 ± 4.8 minutes, respectively; P < .0001; Figure 1A). Because mice treated with 15 mg/kg of ponatinib twice per day exhibited weight loss and lethargy, subsequent studies were performed mostly with ponatinib at 3 mg/kg twice daily. No difference in time to carotid artery occlusion was observed with imatinib at 180 mg/kg twice daily compared with controls (buffer-treated mice; mean ± 95% CI, 32.7 ± 5.6 vs 32.3 ± 4.8 minutes in controls; P = .85). Drug levels on day 4 of treatment at 2 and 24 hours showed that the concentrations of ponatinib (3 mg/kg twice daily) and imatinib (180 mg/kg twice daily) in murine blood were comparable to those of human samples (Table 1).1,10 The shortened time to thrombosis in ponatinib-treated mice could not be explained by changes in blood coagulation parameters, because they had normal complete blood and platelets counts, normal prothrombin and activated partial thrombosplastin times, and normal contact activation- and tissue factor–mediated thrombin generation times (supplemental Figures 1 and 2).
Figure 1.
Influence of ponatinib on thrombosis propensity, vessel wall, and platelets. (A) Aged mice were untreated (UT) or treated with the indicated TKI at the concentration shown for 14 days by oral gavage twice daily. Data shown are the mean ± 95% CI for the time to carotid artery vessel occlusion for each of the treatment conditions. Each symbol in the graph represents the investigation of a single mouse. (B) The vessel wall and adventitia of ponatinib (Poni)-treated mice (3 or 15 mg/kg twice daily for 14 days) were examined for the presence of increased reactive oxygen species (anti-nitrotyrosine) and apoptosis (anti–caspase 3) by immunohistochemistry. The histology on the left represents mouse aorta cross sections examined on a Leica SCN 400 Slide Scanner equipped with a Hamamatsu line sensor color camera and a 40×/0.65 numerical aperture objective and reviewed by digital microscope (Leica Biosystems) at 10× magnification. The insets in the right lower part of each histologic panel are a fourfold increase in the size of the section of each tissue shown. The brown intracellular material seen is the presence of antibodies to nitrotyrosine or caspase 3, respectively, in the tissue of mice treated with Poni. Graphs represent the mean ± 95% CI of the percentage of stained cells to the total number of cells present per high-powered field, as determined by manual counting after treatment with 2 different concentrations of Poni shown. Each symbol on the graph represents analysis from 1 complete slide. (C-D) Washed platelets from UT (red bars) and Poni-treated (blue bars) (3 mg/kg per day) mice were examined for the threshold concentration for CRP-induced integrin activation (JON/A) and P-selectin expression (mean ± standard error of the mean [SEM] of 3 separate experiments with washed platelets from 2 mice per experiment. (E-F) Washed platelets from UT (red bars) and Poni-treated (blue bars) (3 mg/kg per day) mice were examined for the threshold concentration for human α-thrombin–induced integrin activation (JON/A) and P-selectin expression (mean ± SEM of 3 JON/A and 5 P-selectin experiments with washed platelets from 2 mice per experiment). (G) Aged mice were UT or treated with the indicated agent for 14 days by oral gavage. Poni treatment was at 3 mg/kg (twice daily) and pioglitazone (Pio) (10 mg/kg) was administered daily. Data shown are the mean ± 95% CI for the time to carotid artery vessel occlusion for each of the treatment conditions shown. Each symbol in the graph represents the investigation of a single mouse. P values were determined by Student t test. (H-I) Graphs represent the mean ± 95% CI of the percentage of stained cells for nitrotyrosine (H) or caspase 3 (I), respectively, to the total number of cells present after treatment with ponatinib (3 mg/kg twice daily by gavage) in the absence or presence (Poni and Pio) of simultaneous daily treatment with Pio (10 mg/kg). Preparation of slides and analysis were performed as described in panel B. Each symbol on the graph represents analysis from 1 complete slide. (J-K) Washed platelets from untreated (red bars), 3 mg/kg twice daily Poni-treated (blue bars), and combined Poni- and 10 mg/kg daily Pio-treated (light green bars) mice were examined for the threshold concentration for CRP-induced integrin activation (JON/A) and P-selectin expression (mean ± SEM of 5-6 separate experiments with washed platelets from 2 mice per experiment. *P < .0001, **P < .01, ***P < .025, ****P < .04. ns, not significant.
Table 1.
Concentrations of TKI levels in murine plasma at steady-state condition after 4 days of treatment and 2 and 24 hours after oral administration
| Agent | 2 h, ng/mL* | 24 h, ng/mL* |
|---|---|---|
| Ponatinib, 3 mg/kg twice daily | 176 ± 47† | 11.42 ± 3.4‡ |
| Imatinib, 180 mg/kg twice daily | 17 367 ± 4840 | 876 ± 60 |
Values represent mean ± standard deviation (SD) of 3 values for each agent collected on days 4-5 at 2 and 24 hours after dosing on day 4. Groups of treated mice were given the agent twice daily for 4 d before sample collection.
Value is 33 ± 8.8 nM (mean ± SD).
Value is 2.1 ± 0.6 nM (mean ± SD).
Influence of ponatinib on vessel wall homeostasis
Because recent studies have indicated ponatinib influences vessels,4,5,11 aortas were examined for evidence of increased reactive oxygen species (ROS) and apoptosis in treated mice by nitrotyrosine and caspase 3 expression, respectively. There was increased nitrotyrosine and caspase 3 expression in the adventitia of mice treated with either 15 or 3 mg/kg of ponatinib (Figure 1B). Vessel apoptosis of the adventitia is associated with increased nicotinamide adenine dinucleotide phosphate oxidase–derived ROS.12,13
Influence of ponatinib on platelet activity
Mean treated mice tail bleeding time (± SEM) was 55 ± 12 minutes, shorter than the 102 ± 9.3 minutes for untreated mice (P < .007; n ≥ 20 in each group; supplemental Figure 3). Platelet glycoprotein VI activation after CRP treatment revealed that in vivo ponatinib-treated platelets were hyperreactive. CRP-induced expression of JON/A (the epitope of the activated heterodimeric complex of α2ββ3 integrins on murine platelets; Figure 1C) and P-selectin (CD62; Figure 1D) were significantly higher, at 3 μg/mL of CRP in ponatinib-treated (3 mg/kg orally daily) vs untreated mice as examined ex vivo by flow cytometry. The concentration of CRP needed to induce platelet JON/A or P-selectin expression after treatment was significantly lower (2-way ANOVA P < .0001). Likewise, the thresholds for α-thrombin–induced membrane expression of the JON/A epitope at 0.075 and 0.1 nM (P < .0125) and P-selectin at 0.075 and 0.1 nM (P < .0125 and .025, respectively) were significantly lower in ponatinib-treated (3 mg/kg per day) vs untreated animals (2-way ANOVA P < .0001; Figure 1F). In previous in vitro investigations, CRP-induced, but not thrombin-induced, platelet activation was inhibited by treating washed platelets with 100 nM of ponatinib.14 In our studies, platelets were exposed to lower in vivo ponatinib concentrations (2-33 nM; Table 1). These platelets were hyperreactive when treated with similar concentrations of CRP and α-thrombin. Ponatinib-treated mice had normal adenosine 5′-diphosphate–induced platelet activation (supplemental Figure 4). In contrast, imatinib treatment inhibited murine platelets, because the CRP threshold dose was higher (supplemental Figure 5).
Influence of pioglitazone on ponatinib
The thiazolidinedione pioglitazone, a peroxisome proliferator–activated receptor γ agonist, increases molecular remission in CML and is associated with a reduction in cardiovascular events in high-risk populations of CML.15-17 Peroxisome proliferator–activated receptor γ upregulates vasculoprotective transcription factors Sirt1 and KLF4, which downregulates vessel wall tissue factor expression.18 We therefore examined if pioglitazone is beneficial and reverses the ponatinib-induced prothrombotic state. Ponatinib-treated mice (3 mg/kg by mouth twice daily) had a mean occlusion time (± SEM; 30 ± 2 minutes) that was significantly shorter than that of untreated mice (44 ± 1.1 minutes; P < .0001; Figure 1G). Pioglitazone (10 mg/kg per day) delayed the mean time to thrombosis (± SEM) in normal mice (71.3 ± 7.2 minutes; Figure 1G). When ponatinib (3 mg/kg by mouth twice daily) was administered concurrently with oral pioglitazone, the mean time to thrombosis (± SEM) was longer (41 ± 3.7 minutes; P < .025) compared with ponatinib treatment alone (Figure 1G).
Next, we investigated if concurrent pioglitazone and ponatinib treatment in mice ameliorated vessel wall ROS and apoptosis and corrected platelet hyperreactivity. Pioglitazone (10 mg/kg per day) treatment with ponatinib eliminated vessel ROS (Figure 1H) and apoptosis (Figure 1I). Pioglitazone treatment alone had no effect on CRP-induced platelet reactivity (supplemental Figure 6). However, in vivo pioglitazone treatment was sufficient to reduce Stat5 antigen in mouse platelets (supplemental Figure 7). Pioglitazone cotreatment of ponatinib-treated platelets also normalized the ability of 3 μg/mL of CRP to induce earlier and higher expression of the platelet epitopes JON/A and P-selectin (Figure 1J-K; ANOVA P < .0001). Thus, pioglitazone treatment blocked both deleterious vessel wall changes and reduced platelet reactivity in ponatinib-treated mice.
These combined investigations indicate that ponatinib at human therapeutic concentrations in mice exhibited deleterious effects on the vessel wall and selectively modulated agonist-induced platelet reactivity. Pioglitazone has the ability to neutralize these deleterious effects. This finding suggests that pioglitazone, along with its possible ability to increase molecular remission in CML, may be a useful adjunct in some patients with cardiovascular risk factors receiving ponatinib therapy.15,16
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported in part by a research grant from Ariad Pharmaceuticals and a Velosano award from the Cleveland Clinic Foundation and the Case Comprehensive Cancer Center, as well as National Institutes of Health grants CA223301 (from the National Cancer Institute), AI130131 (from the National Institute of Allergy and Infectious Diseases), HL052779, HL126645, HL144113, and U01HL143402 (from the National Heart, Lung, and Blood Institute) and grant BC150596P1 from the US Department of the Army (A.H.S.).
Authorship
Contribution: This work was mostly performed by A.M. under the planning and guidance of A.H.S.; S.C.M., G.L.F., and E.X.S. performed the arterial thrombosis assays and treated the mice; A.H.S. designed all experiments and performed the analysis; and all authors reviewed the manuscript and edited the manuscripts created by A.H.S.
Conflict-of-interest disclosure: A.H.S. was supported in part by Ariad Pharmaceuticals for the initial performance of the project; this support was by contract with Case Western Reserve University. The remaining authors declare no competing financial interests.
Correspondence: Alvin H. Schmaier, Case Western Reserve University, University Hospitals Cleveland Medical Center, 2103 Cornell Rd, WRB2-130, Cleveland, OH 44106-7284; e-mail: schmaier@case.edu.
References
- 1.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. ; PACE Investigators . A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369(19):1783-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. . Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132(4):393-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moslehi JJ, Deininger M. Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol. 2015;33(35):4210-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X, Cai Y, Goines J, et al. . Ponatinib combined with rapamycin causes regression of murine venous malformation. Arterioscler Thromb Vasc Biol. 2019;39(3):496-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi JP, Wang R, Yang X, et al. . Ponatinib (AP24534) inhibits MEKK3-KLF signaling and prevents formation and progression of cerebral cavernous malformations. Sci Adv. 2018;4(11):eaau0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chislock EM, Ring C, Pendergast AM. Abl kinases are required for vascular function, Tie2 expression, and angiopoietin-1-mediated survival. Proc Natl Acad Sci USA. 2013;110(30):12432-12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hossain MB, Shifat R, Johnson DG, et al. . TIE2-mediated tyrosine phosphorylation of H4 regulates DNA damage response by recruiting ABL1. Sci Adv. 2016;2(4):e1501290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nieman MT, Warnock M, Hasan AA, et al. . The preparation and characterization of novel peptide antagonists to thrombin and factor VIIa and activation of protease-activated receptor 1. J Pharmacol Exp Ther. 2004;311(2):492-501. [DOI] [PubMed] [Google Scholar]
- 9.Fang C, Stavrou E, Schmaier AA, et al. . Angiotensin 1-7 and Mas decrease thrombosis in Bdkrb2-/- mice by increasing NO and prostacyclin to reduce platelet spreading and glycoprotein VI activation. Blood. 2013;121(15):3023-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narasimhan NI, Dorer DJ, Niland K, Haluska F, Sonnichsen D. Effects of food on the pharmacokinetics of ponatinib in healthy subjects. J Clin Pharm Ther. 2013;38(6):440-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paez-Mayorga J, Chen AL, Kotla S, et al. . Ponatinib activates an inflammatory response in endothelial cells via ERK5 SUMOylation. Front Cardiovasc Med. 2018;5:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pagano PJ, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement by angiotensin II. Proc Natl Acad Sci USA. 1997;94(26):14483-14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meijles DN, Pagano PJ. Nox and inflammation in the vascular adventitia. Hypertension. 2016;67(1):14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loren CP, Aslan JE, Rigg RA, et al. . The BCR-ABL inhibitor ponatinib inhibits platelet immunoreceptor tyrosine-based activation motif (ITAM) signaling, platelet activation and aggregate formation under shear. Thromb Res. 2015;135(1):155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prost S, Relouzat F, Spentchian M, et al. . Erosion of the chronic myeloid leukaemia stem cell pool by PPARγ agonists. Nature. 2015;525(7569):380-383. [DOI] [PubMed] [Google Scholar]
- 16.Rousselot P, Prost S, Guilhot J, et al. . Pioglitazone together with imantinib in chronic myeloid leukemia: a proof of concept study. Cancer. 2017;123:1791-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kernan WN, Viscoli CM, Furie KL, et al. ; IRIS Trial Investigators . Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374(14):1321-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Zhang Y, Xiao F, et al. . The peroxisome proliferator-activated receptor γ agonist pioglitazone prevents NF-κB activation in cisplatin nephrotoxicity through the reduction of p65 acetylation via the AMPK-SIRT1/p300 pathway. Biochem Pharmacol. 2016;101:100-111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.