Key Points
DNMT3AR882, TET2, ASXL1, and SRSF2 mutations identified at the time of diagnosis are associated with delayed count recovery.
Persistence of preleukemic mutations in remission at high variant allele frequency is associated with delayed count recovery.
Introduction
Induction chemotherapy debulks the leukemic burden in acute myeloid leukemia (AML) patients. Count recovery driven by hematopoietic progenitor cells (HPCs) usually occurs during the fourth or fifth week after the start of chemotherapy in patients who achieve complete remission (CR). However, a subset of patients experiences substantial delays in recovery that can increase the risk of infection and bleeding in the short term.1,2 Such delays are also associated with inferior relapse-free survival and overall survival in the long term.3 Prior studies have identified several factors associated with prolonged cytopenias, but our understanding of the underlying mechanisms remains incomplete.4-7
Recent studies demonstrated that ∼40% to 50% of AML patients who are in CR after induction chemotherapy continue to carry a subset of leukemia-associated mutations.8-10 The most common persistent mutations were in the genes DNMT3A, TET2, ASXL1, and SRSF2 (DTAS).9 These mutations have been implicated as candidate drivers of clonal hematopoiesis (CH) and preleukemic conditions, including myelodysplastic syndrome.11 These mutations are initially acquired in hematopoietic stem cells and are subsequently propagated to HPCs and terminally differentiated blood cells. In this study, we hypothesized that persistence of these mutations in HPCs might compromise their capacity for hematopoietic reconstitution following chemotherapy. To test this hypothesis, we determined the impact of preleukemic mutations identified at the time of diagnosis and their persistence during remission on time to hematologic recovery in patients who achieved morphologic remission in the bone marrow (BM) after 1 cycle of induction chemotherapy.
Methods
Study cohort
We retrospectively identified 323 consecutive adult patients with newly diagnosed AML who received induction chemotherapy at Princess Margaret Cancer Centre between September 2014 and October 2018 and achieved at least a morphologic leukemia-free state. Patients who required a second induction or received granulocyte colony-stimulating factor were excluded. We reviewed medical records to obtain information on patient demographics, complete blood counts, disease characteristics, and chemotherapy regimen.
Sequencing analysis
Targeted next-generation sequencing (NGS) using an amplicon-based targeted 54-gene panel (TruSight Myeloid Sequencing Panel; Illumina, San Diego, CA) was performed on DNA isolated from peripheral blood (PB) or BM samples collected at the time of diagnosis. Detection of persistent mutations in DNA isolated from remission PB samples was performed using a custom 37-gene error-corrected NGS platform based on the Duplex Sequencing method.12
Definitions and statistical analysis
Time to neutrophil and platelet recovery were defined as the number of days from the start of chemotherapy to the day when absolute neutrophil count and platelet count were ≥0.5 × 109/L and ≥50 × 109/L, respectively. These cutoffs are consistent with the definition of CR with partial hematologic recovery.13 Count recovery curves were constructed using the Kaplan-Meier method, and the difference in curves was tested for statistical significance using the log-rank test. Hazard ratios (HRs) were calculated according to Altman et al.14 Multivariable Cox regression analysis was used to adjust for potential confounding variables. P < .05 was considered significant. Data were processed and analyzed using MedCalc (version 18.11.6) software.
See supplemental Methods for further details.
Results and discussion
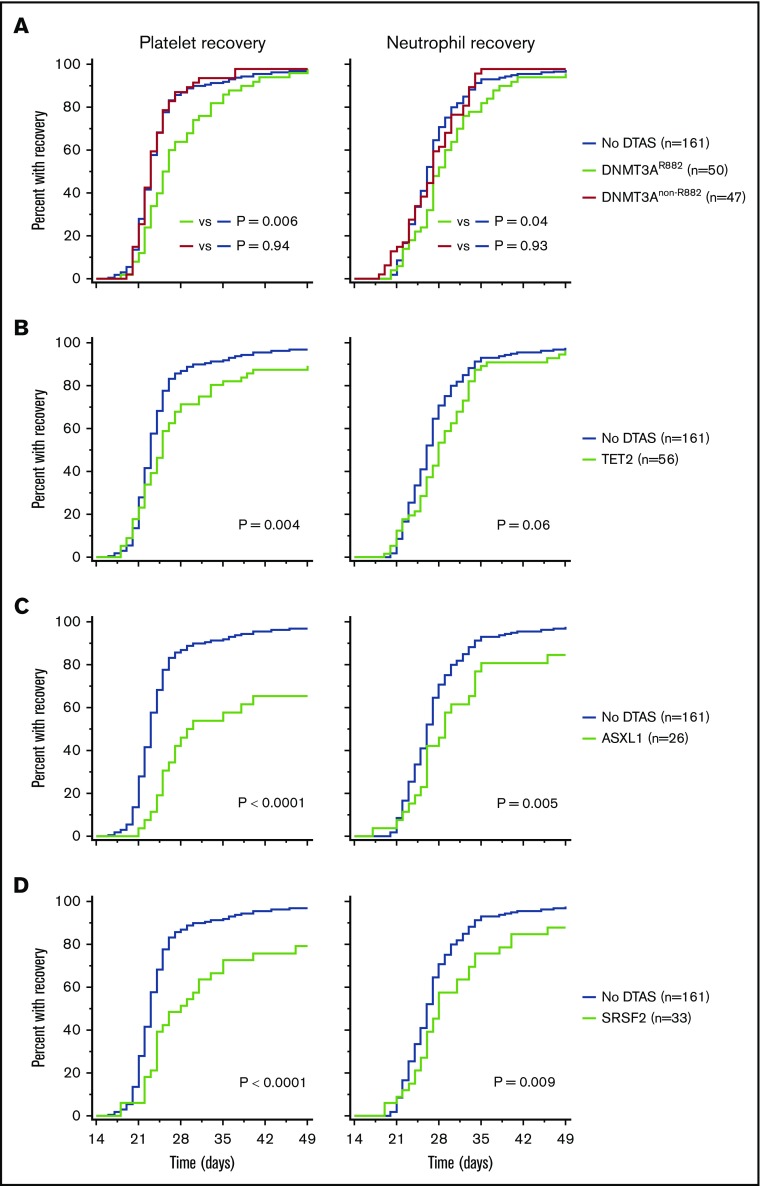
Clinical characteristics of the 323 patients in the study cohort are listed in supplemental Table 1. To test our hypothesis, we first queried whether time to recovery was delayed in patients with ≥1 of the DTAS mutations at diagnosis compared with those without DTAS mutations. We focused on DTAS mutations because of their well-characterized involvement in preleukemia and frequent persistence in remission.8-10 In our cohort, the proportion of patients with DNMT3A, TET2, ASXL1, or SRSF2 mutation at diagnosis was 30% (n = 97), 17% (n = 56), 8% (n = 26), and 10% (n = 33), respectively. In the subset of patients with DNMT3A mutations, 52% (n = 50/97) harbored missense mutations in the hotspot R882 codon. Time to platelet recovery was significantly delayed in DNMT3AR822-mutated patients relative to those without DTAS mutations (median, 25 days [10th-90th percentile, 21-37] vs median, 23 days [10th-90th percentile, 20-30]; HR, 0.64; P = .006; Figure 1A). Time to neutrophil recovery was also significantly delayed (median, 28 days [10th-90th percentile, 22-38] vs median, 26 days [10th-90th percentile, 22-34]; HR, 0.72; P = .04; Figure 1A). In contrast, DNMT3Anon-R882 mutations were not associated with delayed platelet or neutrophil recovery (Figure 1A). The presence of TET2 mutations was also associated with a longer time to platelet recovery (median, 25 days [10th-90th percentile, 20-38] vs median, 23 days [10th-90th percentile, 20-30]; HR, 0.63; P = .004) and a trend toward delayed neutrophil recovery (median, 28 days [10th-90th percentile, 21-35] vs median, 26 days [10th-90th percentile, 22-34]; HR, 0.74; P = .06) (Figure 1B). Similarly, the presence of ASXL1 or SRSF2 mutations was strongly correlated with a longer time to platelet recovery (ASXL1: median, 27 days [10th-90th percentile, 23-52] vs median, 23 days [10th-90th percentile, 20-30]; HR, 0.37; P < .0001; SRSF2: median, 25 days [10th-90th percentile, 22-39] vs median, 23 days [10th-90th percentile, 20-30]; HR, 0.44; P < .0001) and neutrophil recovery (ASXL1: median, 28 days [10th-90th percentile, 22-37] vs median, 26 days [10th-90th percentile, 22-34]; HR, 0.57; P = .005; SRSF2: median, 28 days [10th-90th percentile, 22-40] vs median, 26 days [10th-90th percentile, 22-34]; HR, 0.61; P = .009) (Figure 1C-D). When combined into 1 group, the subset of patients with mutations in DNMT3A R882, TET2, ASXL1, or SRSF2 (DR882TAS) at diagnosis experienced a significantly longer time to platelet recovery (median, 25 days [10th-90th percentile, 20-39] vs median, 23 days [10th-90th percentile, 20-30]; HR, 0.56; P < .0001) and neutrophil recovery (median, 28 days [10th-90th percentile, 21-37] vs median, 26 days [10th-90th percentile, 22-34]; HR, 0.69; P = .002) compared with those without DR882TAS mutations (supplemental Figure 1). In contrast, the presence of NPM1, NRAS, KRAS, or FLT3 mutations, which are not preleukemic, was not associated with delayed recovery (supplemental Figure 2).8-10 To determine whether the presence of DR882TAS mutations was an independent risk factor, we performed multivariable Cox regression analysis and confirmed its statistical significance after adjusting for age, type of AML, presence of myelodysplasia-related changes, cytogenetic risk category, and chemotherapy regimen (supplemental Table 2).
Figure 1.
Association between DTAS mutations and hematologic recovery following induction chemotherapy in AML patients. Cumulative proportion of patients with platelet and neutrophil recovery in those with DNMT3A (A), TET2 (B), ASXL1 (C), and SRSF2 (D) mutations compared with those without any DTAS mutations. The P values were calculated using the log-rank test.
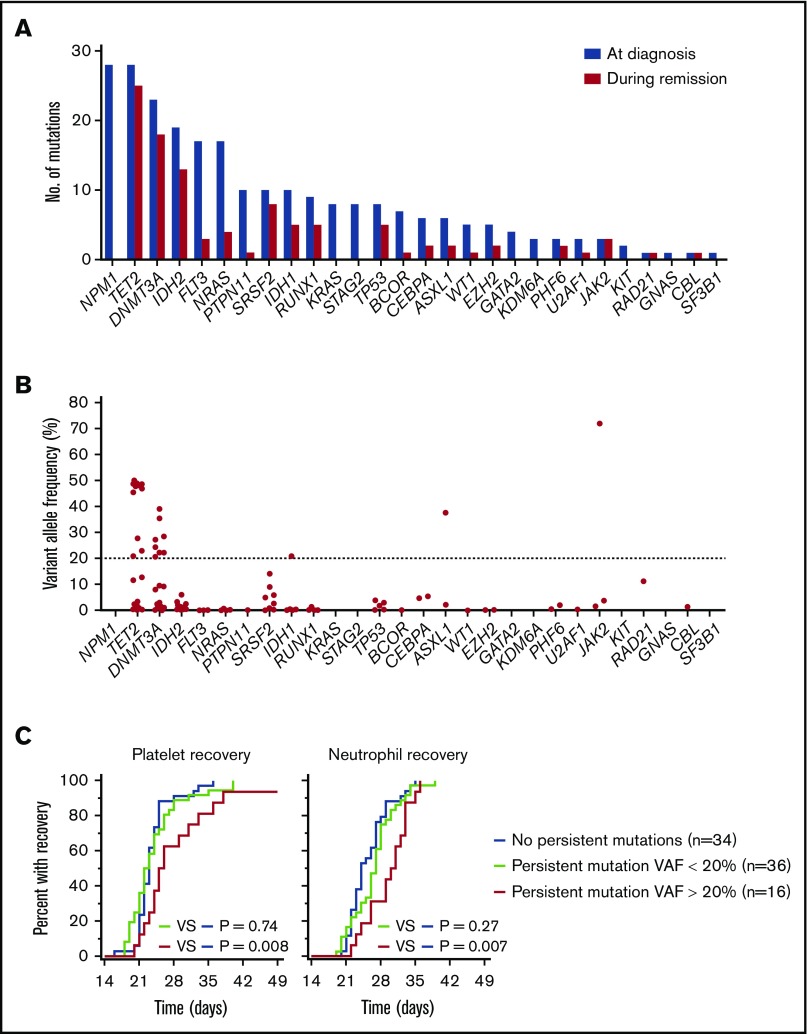
Our findings above support a model in which persistence of preleukemic mutations in remission compromises hematopoietic reconstitution following chemotherapy. To investigate further, we used error-corrected NGS to detect mutation persistence in a subset of 86 patients with available remission samples. A total of 277 putative oncogenic mutations was identified at diagnosis in 80 of the 86 patients (93% with ≥1 mutation). A total of 103 (37%) of these mutations persisted in remission with ≥1 supporting duplex consensus read. The most common persistent mutations were in TET2 (n = 25) and DNMT3A (n = 18) (Figure 2A). The persistent mutations were found in 52 patients (60% of patients), of whom 27 harbored ≥1 mutation. We divided this subset of 52 patients into 2 groups; the variant allele frequency (VAF) of the persistent mutation was <20% in the “low VAF” group and >20% in the “high VAF” group. For patients with >1 persistent mutation, the highest VAF was considered. The majority of the persistent mutations with a VAF > 20% were in the genes TET2 (n = 13) and DNMT3A (n = 8); the remaining mutations were in IDH1 (n = 1), ASXL1 (n = 1), and JAK2 (n = 1) (Figure 2B). In contrast, the persistent mutations with a VAF < 20% were distributed across 20 genes (Figure 2B). Intriguingly, DNMT3AR882 mutations were more likely to persist at a higher VAF than were DNMT3Anon-R882 mutations (supplemental Figure 3). This distinction between the 2 types of DNMT3A mutations has been reported10 and provides a plausible explanation for the lack of association between the presence of DNMT3Anon-R882 mutations at diagnosis and delayed count recovery (Figure 1A).
Figure 2.
Association between persistence of mutations in remission on hematologic recovery following induction chemotherapy in AML patients. (A) Number of mutations in each of the indicated genes found at diagnosis and during remission. (B) VAF of each mutation in each gene during remission. Each data point represents an individual mutation detected in remission. The horizontal dotted line indicates the cutoff used to distinguish between “high” and “low” VAF. See text for details. (C) Cumulative proportion of patients with platelet recovery (≥50 × 109/L) and neutrophil recovery (≥0.5 × 109/L) in patients with persistence of mutations at “high” or “low” VAF compared with those without any detectable mutations in remission. The P values were calculated using the log-rank test.
Patients in the high VAF group experienced a significantly longer time to platelet recovery (median, 26 days [10th-90th percentile, 21-38] vs median, 23 days [10th-90th percentile, 21-28]; HR, 0.42; P = .008) and neutrophil recovery (median, 31 days [10th-90th percentile, 23-35] vs median, 24 days [10th-90th percentile, 21-32]; HR, 0.42; P = .007) than did patients with no detectable mutations in remission (Figure 2C). In contrast, patients in the low VAF group experienced no significant delays in recovery (Figure 2C). These findings suggest that the impact of persistent mutations on count recovery is correlated with size of the preleukemic clone(s) and is not merely a manifestation of residual leukemia. In support of this hypothesis, we found that detection of minimal/measurable residual disease in BM by multiparameter flow cytometry was not significantly correlated with delayed recovery (supplemental Figure 4). On multivariable Cox regression analysis adjusting for potential confounders, persistence of mutations above the 20% VAF cutoff remained independently associated with delayed recovery (supplemental Table 3).
Our results collectively support a model in which the persistence of preleukemic mutations in HPCs compromises their capacity for hematopoietic reconstitution, leading to a delay in count recovery. This model is supported by mouse studies of Dnmt3a, Tet2, and Asxl1 mutations, in which the mutant hematopoietic stem and progenitor cells exhibit a reduced capacity for terminal differentiation.15-18 Although most individuals with CH do not have abnormalities in their numbers of PB cells under steady-state conditions, our results indicate that the impact of preleukemic mutations may only become apparent under stress conditions (ie, postchemotherapy). This hypothesis is supported by a recent study that reported a greater requirement for red blood cell transfusion during chemotherapy for nonhematologic cancers in patients with CH compared with those without CH.19 Our study provides new insights into the impact of preleukemic mutations on HPC function during stress hematopoiesis in patients with AML.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the leukemia bank at the Princess Margaret Cancer Centre for collection and storage of samples.
This work was supported by the Princess Margaret Cancer Foundation.
Authorship
Contribution: T.M., G.S.D.-R., and S.M.C. compiled the data; T.M. and S.M.C. performed statistical analyses; M.K., T.S., and S.K.-R. performed sequencing and bioinformatics analysis of diagnostic samples; J.Z. and S.V.B. performed sequencing and bioinformatics analysis of remission samples; A.A. collected and stored patient samples; V.G., C.J.M., M.D.M., A.D.S., H.S., K.W.L.Y., D.M., and A.C.S. were involved in the clinical care of the patients; and A.T. performed flow cytometric analysis of the samples.
Conflict-of-interest disclosure: T.M. has received honoraria from Novartis. V.G. receives research funding and honoraria from and is a consultant for Novartis and has received research funding from Incyte. A.D.S. receives research funding from Medivir AB, is a paid consultant for Jazz Pharmaceuticals and Otsuka Pharmaceuticals, and is a paid consultant and is on the advisory committee for Novartis. C.M. has received honoraria from Novartis. K.W.L.Y. receives research funding from Agenysys, Astex, GlaxoSmithKline, Onconova, and Genetch/Roche and serves on advisory committees for Celgene, Novartis, and Otsuka. D.M. received honoraria from and is a consultant for Novartis. S.V.B. is a coinventor on a patent describing methods for circulating tumor DNA analysis, which has been licensed to Roche Molecular Diagnostics. A.C.S. is a consultant for Amgen, Celgene, Shire, Teva, Novartis, Otsuka, Jazz Pharmaceuticals, and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Steven M. Chan, Department of Medical Oncology and Hematology, Princess Margaret Cancer Centre, 101 College St, Room 8-354, Toronto, ON M5G 1L7, Canada; e-mail: steven.chan@uhnresearch.ca.
References
- 1.Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64(2):328-340. [DOI] [PubMed] [Google Scholar]
- 2.Gaydos LA, Freireich EJ, Mantel N. The quantitative relation between platelet count and hemorrhage in patients with acute leukemia. N Engl J Med. 1962;266(18):905-909. [DOI] [PubMed] [Google Scholar]
- 3.Estey EH, Shen Y, Thall PF. Effect of time to complete remission on subsequent survival and disease-free survival time in AML, RAEB-t, and RAEB. Blood. 2000;95(1):72-77. [PubMed] [Google Scholar]
- 4.Rosinski SL, Ravandi F, Faderl S, et al. . Count recovery in AML patients achieving a complete response. Blood. 2009;114(22):2062. [Google Scholar]
- 5.Buckley SA, Othus M, Vainstein V, Abkowitz JL, Estey EH, Walter RB. Prediction of adverse events during intensive induction chemotherapy for acute myeloid leukemia or high-grade myelodysplastic syndromes. Am J Hematol. 2014;89(4):423-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu R, Wei H, Wang Y, et al. . The number of CD34+CD38+CD117+HLA-DR+CD13+CD33+ cells indicates post-chemotherapy hematopoietic recovery in patients with acute myeloid leukemia. PLoS One. 2017;12(7):e0180624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerbing RB, Alonzo TA, Sung L, et al. . Shorter remission telomere length predicts delayed neutrophil recovery after acute myeloid leukemia therapy: a report from the Children’s Oncology Group. J Clin Oncol. 2016;34(31):3766-3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jongen-Lavrencic M, Grob T, Hanekamp D, et al. . Molecular minimal residual disease in acute myeloid leukemia. N Engl J Med. 2018;378(13):1189-1199. [DOI] [PubMed] [Google Scholar]
- 9.Rothenberg-Thurley M, Amler S, Goerlich D, et al. . Persistence of pre-leukemic clones during first remission and risk of relapse in acute myeloid leukemia. Leukemia. 2018;32(7):1598-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morita K, Kantarjian HM, Wang F, et al. . Clearance of Somatic mutations at remission and the risk of relapse in acute myeloid leukemia. J Clin Oncol. 2018;36(18):1788-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silver AJ, Jaiswal S. Clonal hematopoiesis: pre-cancer PLUS. Adv Cancer Res. 2019;141:85-128. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy SR, Schmitt MW, Fox EJ, et al. . Detecting ultralow-frequency mutations by duplex sequencing [published correction appears in Nat Protoc. 2014;9(12):2903]. Nat Protoc. 2014;9(11):2586-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloomfield CD, Estey E, Pleyer L, et al. . Time to repeal and replace response criteria for acute myeloid leukemia? Blood Rev. 2018;32(5):416-425. [DOI] [PubMed] [Google Scholar]
- 14.Machin D, Gardner MJ. Time to event studies In: Altman D, Machin D, Bryant T, eds., et al.. Statistics with Confidence: Confidence Intervals and Statistical Guidelines, 2nd ed London, United Kingdom: BMJ Books; 2000:93-104. [Google Scholar]
- 15.Ko M, Bandukwala HS, An J, et al. . Ten-Eleven-Translocation 2 (TET2) negatively regulates homeostasis and differentiation of hematopoietic stem cells in mice. Proc Natl Acad Sci USA. 2011;108(35):14566-14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Challen GA, Sun D, Jeong M, et al. . Dnmt3a is essential for hematopoietic stem cell differentiation. Nat Genet. 2011;44(1):23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagase R, Inoue D, Pastore A, et al. . Expression of mutant Asxl1 perturbs hematopoiesis and promotes susceptibility to leukemic transformation. J Exp Med. 2018;215(6):1729-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeong M, Park HJ, Celik H, et al. . Loss of Dnmt3a immortalizes hematopoietic stem cells in vivo. Cell Reports. 2018;23(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arends CM, Galan-Sousa J, Hoyer K, et al. . Hematopoietic lineage distribution and evolutionary dynamics of clonal hematopoiesis. Leukemia. 2018;32(9):1908-1919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.