Abstract
Background:
Previous studies assessing the risk of stroke in the general population performed screening with Doppler ultrasonography only for high-risk patients and neglected low- and moderate-risk patients. The aims of this study were to explore the current prevalence of intracranial arterial stenosis (ICAS) and analyze its association with different levels of stroke risk and risk factors based on the risk assessment scale for stroke used in China.
Methods:
A total of 3654 participants who underwent transcranial Doppler ultrasound (TCD) were eligible for inclusion. Information regarding demographic characteristics and risk factors such as alcohol consumption and hypertension was collected through interviews and questionnaires and used to analyze the association of ICAS with different levels of stroke risk and risk factors.
Results:
The mean age of 501 subjects diagnosed with at least one ICAS was higher than that of participants without ICAS (57.13 ± 9.56 years and 55.52 ± 9.35 years, respectively). After adjusting for confounding factors, gender, education, residence, hypertension and personal history of stroke were associated with ICAS. The odds ratios for ICAS in patients with hypertension and a personal history of stroke were 1.655 [95% confidence interval (CI): 1.341–2.043] and 1.854 (95% CI: 1.371–2.508), respectively. In addition, participants in the low- and moderate-risk stroke groups accounted for an unexpectedly high proportion of individuals with ICAS (up to 38.3%). Results from multivariate analyses indicated that the adjusted odds ratios for ICAS in patients with moderate and high stroke risks versus those with a low stroke risk were 1.603 (95% CI: 1.171–2.195) and 1.612 (95% CI: 1.272–2.042), respectively.
Conclusion:
The prevalence of ICAS is high in northeast China and increases with the level of stroke risk. However, the proportion of patients with ICAS among those with low and moderate stroke risks should also be noted.
Keywords: intracranial arterial stenosis, risk factor, stroke screening, transcranial Doppler ultrasound
Introduction
Intracranial arterial stenosis (ICAS) is commonly associated with ischemic strokes worldwide.1,2 Previous studies have demonstrated that ICAS is more prevalent in individuals with Asian, African and Hispanic ancestries than in Caucasian individuals.3–6 In Asian populations, ICAS accounts for more than 30% of ischemic stroke events every year,7–9 while only 8–10% of all ischemic strokes are due to ICAS in Caucasian populations. This demonstrates that the distribution of vascular stenosis varies greatly among different nationalities.6,7,9,10
In China, stroke has become the primary contributor to death and disability, as evidenced in data from studies assessing the burden of stroke,11,12 which also indicated that 33–46.6% of ischemic stroke events were attributable to ICAS.13–15 One study also revealed that patients with ICAS had more severe strokes and an increased risk of stroke recurrence.15 Therefore, the risk factors for ICAS and the relevant risk populations in China should be evaluated. Early detection, prevention, and treatment of ICAS may effectively reduce the incidence of stroke in this group of individuals.
However, the risk factor profiles for stroke and ICAS have not been fully elucidated.13,15–18 Wong and colleagues were the first to report a door-to-door study on the risk factors of ICAS using transcranial Doppler ultrasound (TCD) in China.16 They found that the prevalence of ICAS increased with the number of significant risk factors in 2007, including hypertension, heart disease, family history of stroke, and glycosuria. The same results were reported in a study conducted by Zhang and colleagues.17 Previous studies have also demonstrated a strong correlation between patients with ICAS and the Framingham stroke risk profile (FSRP) score;19 however, the risk assessment scale for stroke that was propounded by the Stroke Screening and Prevention Program of the National Health and Family Planning Commission of China exhibits better availability and reliability as an assessment tool for the risk of stroke in the Chinese population than does the modified FSRP.20,21 According to the criteria of the risk assessment scale for stroke used in China in 2013,20 participants who met the requirements of the high-risk stroke group were requested to undergo further TCD or carotid ultrasound examination, whereas those with low and moderate risks were likely to be neglected. Therefore, our study aimed to determine the current prevalence of ICAS and analyze its association with different levels of stroke risk and risk factors based on the risk assessment scale for stroke using TCD.
Methods
Study design and population
A survey was conducted as a population-based cross-sectional study in Jilin Province, northeast China, as part of the Stroke Screening and Prevention Program of the National Health and Family Planning Commission of China.20 The main targeted population for this study was residents aged ⩾40 years who had registered households in Dehui City of Jilin Province and were residing in these households for ⩾6 months. The survey was conducted from January 2016 to March 2016, primarily because a large number of immigrants living in this area went home to celebrate the Spring Festival during this period. This facilitated the appropriate representation of individuals aged ⩾40 years in the area and reduced selection bias. The survey data were derived from a previous study that used the multistage stratified random cluster sampling method for random sample selection and investigated the prevalence of stroke in northeast China.22 Selected five villages and five communities were also randomly sampled from 30 villages (rural) and 10 towns (urban) by using PPS. The planned sample size was 3356, and we aimed to randomly select residents aged ⩾40 years from each household in the selected five villages and five communities, which were sampled from both rural and urban areas. A total of 4100 participants aged ⩾40 years were willing to participate, and the lost-to-follow-up rate was <10%; a total of 4052 individuals participated after the exclusion of 48 individuals with missing data. From the 4052 participants, 398 were excluded on the basis of the following criteria based on TCD: (1) poor or closed temporal window penetration and (2) unwillingness to cooperate or refusal to undergo TCD examination. After the exclusions, a total of 3654 individuals were eligible for inclusion. The study protocol was approved by the Human Ethics and Research Ethics committees of the First Hospital of Jilin University (approval No: 19K059-001), and written informed consent was provided by all participants. Figure 1 presents a flow chart of the study.
Figure 1.
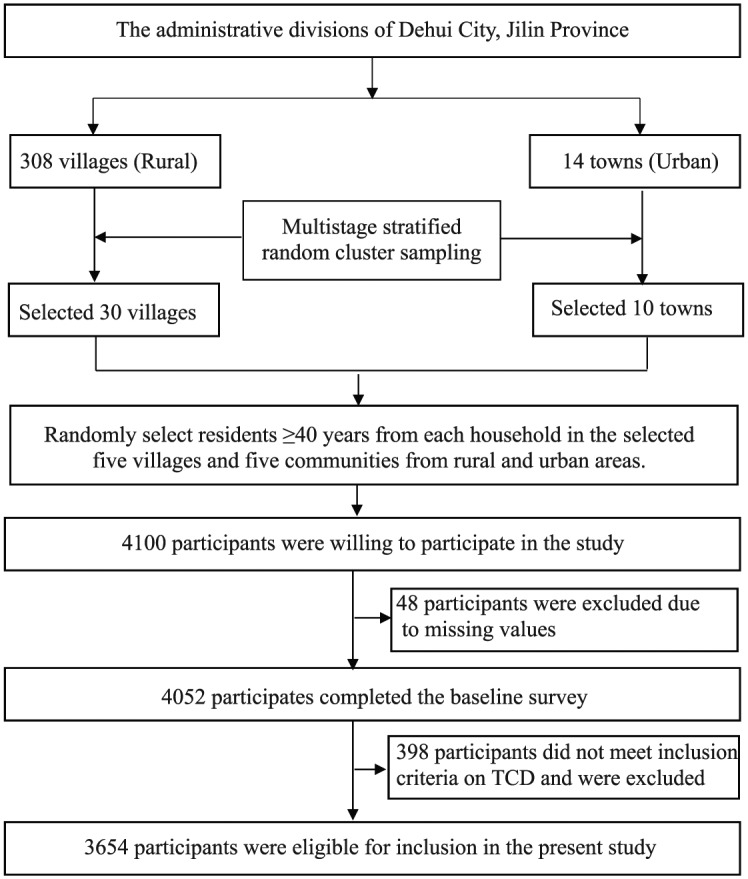
Study flowchart.
Transcranial Doppler ultrasound
TCD was used as a noninvasive screening tool for the assessment of ICAS and performed by two experienced neurologists. The diagnostic criterion for ICAS was based on the peak systolic flow velocity (PSV) as outlined in publications by Wong and colleagues.14,16 Briefly, the peak systolic flow velocity criteria were as follows: ⩾140 cm/s for the middle cerebral artery (MCA), ⩾120 cm/s for the anterior cerebral artery, ⩾100 cm/s for the posterior cerebral artery and vertebrobasilar artery, and ⩾120 cm/s for the siphon internal carotid artery. MCA stenosis was classified as follows:14 mild stenosis, systolic peak velocity 140–209 cm/s; moderate stenosis, 210–280 cm/s; and severe stenosis, >280 cm/s. In addition, we also considered the participant’s age, spectrum shape, and turbulence or musical sound.
Risk factors
All participants completed a face-to-face questionnaire designed by the Stroke Screening and Prevention Program of the National Health and Family Planning Commission of China. Information regarding demographic characteristics; eight major stroke risk factors (hypertension, atrial fibrillation, diabetes mellitus, hyperlipidemia, smoking, lack of exercise, obesity and family history of stroke); alcohol consumption; and personal history of coronary heart disease (CHD) was collected. Hypertension was defined as a previous history of hypertension or the use of antihypertensive drugs within the last 2 weeks, a systolic blood pressure (SBP) of ⩾140 mmHg and/or a diastolic blood pressure (DBP) of ⩾90 mmHg. Diabetes mellitus was defined as a personal history of diabetes or the use of oral hypoglycemic medication or insulin and a fasting blood glucose (FBG) level of ⩾7.0 mmol/L (fasting for ⩾8 h). Atrial fibrillation was diagnosed using standard 12-lead electrocardiography (ECG). According to the standard of the risk assessment scale for stroke proposed by the Stroke Screening and Prevention Program of the National Health and Family Planning Commission of China,20 these three risk factors were considered chronic diseases. Hyperlipidemia was defined as a history of hyperlipidemia or the fulfillment of one of the following criteria: a total cholesterol level (TC) of ⩾6.2 mmol/L, a low-density lipoprotein cholesterol (LDL-C) level of ⩾4.1 mmol/L, a high-density lipoprotein cholesterol (HDL-C) level of <1.04 mmol/L, and triglyceride (TG) level of ⩾2.3 mmol/L.23 Smokers were defined as individuals who had smoked continuously or cumulatively for ⩾6 months and smoked ⩾1 cigarette per day. Lack of exercise referred to little or light physical activity (<3 times a week, <30 min per exercise, and duration of ⩾1 year). Individuals were considered overweight or obese if they had a BMI of ⩾26 kg/m2.20 A family history of stroke referred to a history of stroke in first-degree relatives. Alcohol consumption was defined by the consumption of alcohol ⩾3 times per week or the consumption of ⩾40 g.
The low-risk stroke population comprised participants with fewer than three of the eight risk factors and no chronic diseases. The moderate-risk stroke population included participants with fewer than three of the eight risk factors and more than one of the three chronic diseases. The high-risk stroke population comprised individuals with more than three of the eight risk factors or a history of transient ischemic attack (TIA) or stroke.20
Statistical methods
All statistical tests were performed using the IBM SPSS statistical software package, version 22.0 (SPSS Inc., New York, USA). Continuous parametric variables are described as mean ± standard deviation (SD) and compared using t tests (continuous variables with a normal distribution). Categorical variables, including gender, risk factors, and the classification of stenotic arteries, are expressed as percentages and were compared using Chi-square tests. We estimated the prevalence rate and 95% confidence interval (CI) for ICAS in the different subgroups. Logistic models were used to (1) test the association between the different risk populations and ICAS after adjusting for gender, age and education level and (2) to analyze the interaction between subgroups (gender and age) and risk populations. Furthermore, we examined the risk factors for ICAS and determined their odds ratios (ORs) and 95% CIs. All statistical tests were two-sided, and a p value of <0.05 was considered statistically significant.
Results
A total of 3654 participants (mean age: 55.74 ± 9.40) aged ⩾40 years were included in our analysis; 501 (13.7%) exhibited more than one arterial stenosis and 3153 did not exhibit ICAS. Among the participants with ICAS, 48.9% were male. Table 1 lists the basic characteristics of individuals according to the presence or absence of ICAS. Participants with ICAS (mean age: 57.13 ± 9.56 years) were older than those without (mean: 55.52 ± 9.35 years). Moreover, they exhibited a lower educational level and more frequently inhabited rural areas. Meanwhile, the prevalence of major associated risk factors such as hypertension, smoking, alcohol consumption, and personal history of stroke was higher in individuals with ICAS than in those without.
Table 1.
Basic characteristics of participants according to the presence or absence of intracranial arterial stenosis (ICAS).
| Characteristics | Total (n = 3654) | Without ICAS (n = 3153) | With ICAS (n = 501) | p value |
|---|---|---|---|---|
| Age (years) | 55.74 ± 9.40 | 55.52 ± 9.35 | 57.13 ± 9.56 | <0.001 |
| Male sex (n, %) | 1465 (40.1) | 1220 (83.3) | 245 (48.9) | <0.001 |
| Education (n, %) | ||||
| Elementary or below | 1177 (32.3) | 949 (30.1) | 230 (45.9) | |
| Middle school | 1574 (43.1) | 1383 (43.9) | 191 (38.1) | <0.001 |
| High school or above | 901 (24.7) | 821 (26.0) | 80 (16.0) | |
| Urban | 2054 (56.2) | 1851 (58.7) | 203 (40.5) | <0.001 |
| Hypertension (n, %) | 2055 (56.2) | 1709 (54.2) | 346 (69.1) | <0.001 |
| Atrial fibrillation (n, %) | 44 (1.2) | 34 (1.1) | 10 (2.0) | 0.08 |
| Diabetes (n, %) | 311 (8.5) | 264 (8.4) | 47 (9.4) | 0.452 |
| Dyslipidemia (n, %) | 1763 (48.2) | 1511 (47.9) | 252 (50.3) | 0.323 |
| Smoking (n, %) | 1791 (49.0) | 1492 (47.3) | 299 (59.7) | <0.001 |
| Drinking (n, %) | 989 (27.1) | 835 (26.5) | 154 (30.7) | 0.046 |
| Lack of exercise (n, %) | 861 (23.6) | 751 (23.8) | 110 (22.0) | 0.362 |
| Overweight or obesity (n, %) | 1165 (31.9) | 993 (31.5) | 172 (34.3) | 0.206 |
| Personal history of CHD (n, %) | 238 (6.5) | 201 (6.4) | 37 (7.4) | 0.395 |
| Personal history of stroke (n, %) | 278 (7.6) | 207 (6.6) | 71 (14.2) | <0.001 |
CHD, coronary heart disease.
The prevalence of ICAS based on demographic characteristics is outlined in Table 2. Among the 501 patients with ICAS, 331 (66.1%), 90 (18.0%), 28 (5.6%) and 52 (10.4%) exhibited mild stenosis, moderate stenosis, severe stenosis and complete occlusion, respectively. The severity of vascular stenosis was higher in men and participants who inhabited rural areas than in women and participants who inhabited urban areas. Moreover, the severity of vascular stenosis significantly increased with age and decreased with educational level. Overall, there were obvious differences in the basic demographic characteristics and classification of vascular stenosis.
Table 2.
Prevalence of different severities of intracranial arterial stenosis (ICAS) according to demographic characteristics.
| Variable | Mild stenosis (n = 331) | Moderate stenosis (n = 90) | Severe stenosis (n = 28) | Occlusion (n = 52) | p value |
|---|---|---|---|---|---|
| Age (years) (n, %) | |||||
| 40–49 | 98 (8.5) | 20 (1.7) | 6 (6.5) | 9 (0.8) | 0.027 |
| 50–59 | 109 (8.9) | 27 (2.20) | 11 (0.9) | 17 (1.4) | |
| 60–69 | 94 (9.7) | 31 (3.2) | 6 (0.6) | 18 (1.9) | |
| ⩾70 | 30 (9.4) | 12 (3.8) | 6 (1.9) | 7 (2.2) | |
| Gender (n, %) | |||||
| Female | 176 (8.0) | 42 (1.9) | 18 (0.8) | 20 (0.9) | <0.001 |
| Male | 42 (1.9) | 48 (3.3) | 11 (0.8) | 31 (2.1) | |
| Education (n, %) | |||||
| Elementary or below | 155 (13.1) | 40 (3.4) | 14 (1.2) | 21 (1.8) | <0.001 |
| Middle school | 120 (7.6) | 36 (2.3) | 12 (0.8) | 23 (1.5) | |
| High school or above | 56 (2.6) | 14 (1.6) | 3 (0.3) | 7 (0.8) | |
| Residence (n, %) | |||||
| Rural | 198 (12.4) | 55 (3.4) | 18 (1.1) | 27 (1.7) | <0.001 |
| Urban | 133 (6.5) | 35 (1.7) | 11 (0.5) | 24 (1.2) |
Table 3 summarizes the prevalence of ICAS in the low-risk (22.3%; 95% CI: 18.7–26.0), moderate-risk (16.0%; 95% CI: 12.7–19.2) and high-risk (61.7%; 95% CI: 57.4–65.9) stroke populations. There are two classification systems for vascular stenosis. According to the number of stenoses, the 501 participants with ICAS were categorized into single ICAS (n = 265) and multiple ICAS (n = 236) groups. In the single ICAS group, patients were further divided into low-risk (65/265; 24.5%), moderate-risk (40/265; 15.1%) and high-risk (160/265; 60.4%) groups. According to the severity of arterial stenosis, the patients were classified into mild stenosis (n = 331), moderate stenosis (n = 90), severe stenosis (n = 28) and occlusion (n = 52) groups. The prevalence of mild, moderate and severe stenosis varied across the low-, moderate- and high-risk stroke populations. However, there were no differences in the prevalence of single and multiple ICAS among the different risk populations.
Table 3.
Prevalence of intracranial arterial stenosis (ICAS) and its different classifications in individuals with different levels of stroke risk.
| Classification | Different stroke risk population [%
(95% CI)] |
|||
|---|---|---|---|---|
| Low-risk stroke population (%) | Moderate-risk stroke population (%) | High-risk stroke population (%) | p value | |
| Total | 22.3 (18.7–26.0) | 16.0 (12.7–19.2) | 61.7 (57.4–65.9) | |
| Single ICAS | 24.5 (19.3–29.7) | 15.1 (10.8–19.4) | 60.4 (54.5–66.3) | 0.447 |
| Multiple ICAS | 19.9 (14.8–25.0) | 16.9 (12.1–21.8) | 63.1 (56.9–69.3) | |
| Mild stenosis | 27.2 (22.4–32.0) | 16.9 (12.9–21.0) | 55.9 (50.5–61.3) | 0.001 |
| Moderate stenosis | 13.3 (6.2–20.5) | 16.7 (8.8–24.5) | 70.0 (60.3–79.7) | |
| Severe stenosis | 21.4 (5.2–37.6) | 14.3 (0.5–28.1) | 64.3 (45.4–83.2) | |
| Occlusion | 7.7 (0.2–15.2) | 9.6 (1.3–17.9) | 82.7 (72.1–93.3) | |
CI, confidence interval.
Table 4 presents the estimated ORs and 95% CIs for ICAS in the three risk populations. In the unadjusted model, ORs for ICAS increased with an increase in the risk level (i.e. from low risk to high risk). These findings were maintained after adjustment for confounding factors such as gender, age and educational level. For men and participants aged ⩾60 years, ORs for ICAS increased with an increase in the risk level (Table 4). In the multivariable logistic regression model, risk factors independently associated with ICAS included gender, educational level, place of residence, hypertension and personal history of stroke. There was no significant association between ICAS and age, smoking and alcohol consumption (Table 5).
Table 4.
Odds ratios and 95% confidence intervals for intracranial arterial stenosis (ICAS) in patients with different levels of stroke risk.
| Different stroke risk population | Total (n = 3654) | Female (n = 2189) | Male (n = 1465) | <60 years (n = 2369) | ⩾60 years (n = 1285) |
|---|---|---|---|---|---|
| Crude model | |||||
| Low risk | Reference | Reference | Reference | Reference | Reference |
| Moderate risk | 1.690 (1.243–2.297) | 1.648 (1.110–2.467) | 1.644 (1.019–2.651) | 1.441 (0.975–2.132) | 2.221 (1.279–3.855) |
| High risk | 1.887 (1.500–2.374) | 1.740 (1.287–2.353) | 1.867 (1.299–2.683) | 1.650 (1.255–2.169) | 2.410 (1.515–3.832) |
| Adjusted model | |||||
| Low risk | Reference* | Reference† | Reference† | Reference‡ | Reference‡ |
| Moderate risk | 1.603 (1.171–2.195) | 1.673 (1.116–2.508) | 1.770 (1.088–2.879) | 1.476 (0.993–2.193) | 2.216 (1.269–3.867) |
| High risk | 1.612 (1.272–2.042) | 1.565 (1.150–2.129) | 1.933 (1.336–2.795) | 1.443 (1.091–1.907) | 2.285 (1.432–3.646) |
Adjusted for gender, age (years) and educational level.
Adjusted for age (years), residence and educational level.
Adjusted for gender, education, residence and educational level.
Table 5.
Associations between related risk factors and intracranial arterial stenosis (ICAS) according to multivariable logistic regression analysis.
| Category | Subcategory | Fully adjusted model* OR (95% CI) | p value |
|---|---|---|---|
| Gender | Female | Reference | |
| Male | 1.446 (1.141–1.834) | 0.002 | |
| Age | 40–49 | Reference | |
| 50–59 | 0.944 (0.734–1.215) | 0.656 | |
| 60–69 | 0.927 (0.707–10215) | 0.582 | |
| ⩾70 | 1.039 (0.721–10498) | 0.836 | |
| Education | Elementary or below | Reference | |
| Middle school | 0.751 (0.584–0.966) | 0.026 | |
| High school or above | 0.644 (0.451–0.919) | 0.015 | |
| Residence | Urban | Reference | |
| Rural | 1.528 (1.716–1.985) | 0.002 | |
| Drinking | No | Reference | |
| Yes | 0.912 (0.704–1.182) | 0.488 | |
| Smoking | No | Reference | |
| Yes | 1.154 (0.923–1.441) | 0.209 | |
| Hypertension | No | Reference | |
| Yes | 1.655 (1.341–2.043) | <0.001 | |
| Personal history of stroke | No | Reference | |
| Yes | 1.854 (1.371–2.508) | <0.001 |
CI, confidence interval.
Parameters with a p value of <0.1 in univariate analysis were eligible for inclusion in the multiple logistic regression model.
Adjusted for gender, age, educational level, place of residence, alcohol consumption, smoking, hypertension and personal history of stroke.
Discussion
This study demonstrated that the prevalence of ICAS in patients aged ⩾40 years and residing in northeast China was higher than that reported in previous studies investigating ICAS diagnosed using TCD.16,17,24,25 The prevalence in the present study was 13.7%, while that in several Asian studies based on TCD varies from 5.9% to 24.5%.7,18,26,27 Few studies have shown the prevalence of ICAS in asymptomatic non-Asian patients. Suri and colleagues evaluated an elderly American population using TCD and found that the prevalence of ICAS was 15.6%, which was higher than that in some previous studies of Asian populations. The authors attributed the discrepancy in the findings to differences in the ages of their population and the Asian populations.28 This may explain the varied distribution of ICAS in different geographical regions and ethnicities.3,6,7,9,10 The participants in the low- and moderate-risk stroke groups accounted for an unexpectedly high proportion of individuals with ICAS (up to 38.3%). To our knowledge, this was the most recent survey to analyze the relationship between risk factors based on the risk assessment scale for stroke and ICAS prevalence in northeast China.
Previous studies have demonstrated that the prevalence of ICAS progressively increases with increasing numbers of risk factors.16–18 Results from a study conducted by Wong and colleagues in Liangbei County, located in central China, revealed a positive gradient relationship between four risk factors (hypertension, glycosuria, heart disease and family history of stroke) and the prevalence of ICAS.16 In addition, Zhang and colleagues17 summarized a dramatic increase in the prevalence of ICAS from 5.8% in participants without any related risk factors to 50% in participants exposed to five risk factors (⩾50 years, hypertension, diabetes, left ventricular hypertrophy and high-sensitivity C-reactive protein > 2.2 mg/dl) in China. Moreover, data from the Kailuan study found that the ideal cardiovascular health metrics played a protective role in cardiovascular diseases, and that ischemic stroke exhibited a significant inverse relationship with the number of cardiovascular health metrics and the prevalence of asymptomatic polyvascular disease.29 It is well known that ICAS plays an important role in the occurrence of ischemic stroke in Asian populations, with 30–50% of strokes being associated with ICAS.3 Past studies have demonstrated that the risk assessment scale for stroke propounded by the Stroke Screening and Prevention Program in China has better availability and reliability than does the modified FSRP scale,20,21 particularly for Chinese populations. Specifically, results from the risk assessment scale for stroke showed that Cronbach’s α, split-half reliability and the test–retest correlation were 0.701, 0.826, and 0.94, respectively; these values indicate that this scale may be useful for primary stroke risk assessment. Our results also indicated an association between the risk assessment scale for stroke and ICAS.
The adjusted ORs for participants in the moderate- and high-risk stroke populations were 1.603 (1.171–2.195) and 1.612 (1.272–2.042), respectively, consistent with the values derived in previous studies.16–18 In addition, the accumulating effects of related risk factors led to an increased risk of ICAS development, and men and individuals aged ⩾60 years exhibited an increase in ORs for ICAS with an increase in the stroke risk. This suggests that men and aging individuals are more susceptible to risk factors associated with ICAS. In addition, there was no association between an age of <60 years and ICAS development. This may be related to the interactions between confounding factors other than the traditional risk factors, and early screening techniques may help in the detection of an association in this age group. We also analyzed the influence of demographic characteristics on the severity of ICAS and found differences according to age, gender, residence and educational level.
Participants in the high-risk stroke population comprised a higher proportion of the classifications of ICAS than did the low- and moderate-risk stroke populations. The low-, moderate- and high-risk stoke populations contributed to 61.7%, 16.0% and 22.3% of all ICAS cases, respectively. However, according to the Stroke Screening and Prevention Program of the National Health and Family Planning Commission in China, those participants who met the requirements of the high-risk stroke group were requested to undergo further TCD or carotid ultrasound examination,20 while those in the low- and moderate-risk stroke groups were likely to be excluded from analysis. In fact, participants in the low- and moderate-risk stroke groups accounted for a large proportion of individuals with ICAS (up to 38.3%). Therefore, awareness of routine screening and prevention in these populations is of vital importance. Ovesen and colleagues30 reported that ICAS independently increased the risk of recurrent ischemic events in patients with TIAs or ischemic stroke. Wang and colleagues15 investigated 2864 patients and demonstrated that the risk of recurrent stroke remains higher among individuals with severe stenosis and risk factors associated with ICAS. Our results and findings from previous studies suggest that early detection is important for the development of effective primary prevention measures or treatments for asymptomatic ICAS, and helps in lowering the incidence of ICAS. Additional studies with larger samples are required to explore the relationship between ICAS and different risk populations using the risk assessment scale for stroke.
From the perspective of prevention, it is critical to clarify the associated risk factors for asymptomatic ICAS.25 In the present study, we observed that gender, educational level, residence and modifiable risk factors (e.g. hypertension and personal history of stroke) were independent risk factors for ICAS. Our results and findings from previous research revealed that men are at a higher risk of ICAS development than are women.9,27,29,31 Interestingly, men reportedly smoke and consume excessive alcohol more frequently than do women; however, other studies showed no correlation between gender and ICAS, with some even reporting a higher prevalence of ICAS in women.16,17,25,32 A recent study suggested that this may be the effect of sex hormones.33 As mentioned above, the relationship between gender and ICAS has not been fully elucidated. Our results also demonstrated that participants with lower educational levels and those living in rural areas exhibited a higher prevalence of ICAS, probably because highly educated people or individuals residing in urban areas are more aware of prevention and defense against risk factors. Therefore, publicity and education concerning the prevention of ICAS should be increased and improved on an urgent basis, especially in rural areas, where residents are not aware about early physical examination. Another explanation for the differences in ICAS prevalence between rural and urban areas is that Jilin Province is located in the northernmost section of China, near the sub-frigid zone, and has a distinct temperate monsoon climate with an extremely dry and cold winter. Therefore, rural residents do not have the same opportunities as urban residents in terms of visiting the gym for exercise. Different behaviors of residents in rural and urban regions (i.e. smoking and alcohol consumption) may be another reason for the difference.34,35
In the present study, hypertension was recognized as a strong independent risk factor for ICAS, consistent with the findings in previous studies.16–19,25, 27,29,32,33 However, hyperlipidemia or diabetes mellitus were not considered risk factors for ICAS, as reported in a study conducted by Uehara and colleagues.36 In addition, Wang and colleagues found that interarm differences in DBP may be an early sign of ICAS in the Chinese population.37 Our data demonstrated that smoking and alcohol consumption were not significantly associated with ICAS in the adjusted model; smoking has been well established as having a close dose–response correlation, which increases the incidence or recurrence of stroke and atherosclerotic arteries.38 However, Ding and colleagues and Park and colleagues administered surveys in Asian and Korean populations and suggested that smoking was more strongly associated with extracranial arterial stenosis (ECAS) than with ICAS.32,39 In the current study, it was worth noting that a personal history of stroke was a distinct and crucial factor that significantly increased the incidence of ICAS; correspondingly, early neurologic worsening in the phase of ischemic stroke could be determined pre-stroke by the distribution of ICAS subtype.15,40 Consequently, a healthy lifestyle, regular physical examination and effective primary prevention measures are indispensable.22
This study has several limitations. First, our survey was retrospective, and the responses may have been influenced by recall bias. Second, this study was limited to individuals living in northeast China; therefore, it was not based on a nationally representative sample. Third, although TCD is considered a noninvasive and reliable screening tool for evaluating ICAS,41,42 it is possible that ICAS was not detected in some patients. Supplementary diagnostic tools such as digital subtraction angiography, magnetic resonance angiography (MRA), and computed tomography angiography (CTA) should also be used. Furthermore, arterial stenosis assessed by TCD may not be observed in second-level imaging studies (e.g. MRA or CTA); this may affect the accuracy of ICAS diagnosis.
Conclusion
Overall, our study in northeast China reported a high prevalence of ICAS, which increased with the level of stroke risk (low, moderate and high). We further observed that gender, education, residence, hypertension and personal history of stroke are associated with the prevalence of ICAS. The prevalence of ICAS in patients with low and moderate stroke risks should also be noted.
Acknowledgments
We thank the staff associated with the study and all the patients and their families for their cooperation.
Footnotes
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Ying-qi Xing  https://orcid.org/0000-0003-0680-1821
https://orcid.org/0000-0003-0680-1821
Contributor Information
Hong-xiu Chen, Department of Neurology, The First Hospital of Jilin University, Changchun, China.
Li-juan Wang, Department of Neurology, The First Hospital of Jilin University, Changchun, China.
Yi Yang, Department of Neurology, The First Hospital of Jilin University, Changchun, China.
Fei-xue Yue, Department of Neurology, The First Hospital of Jilin University, Changchun, China.
Li-min Chen, Department of Neurology, The First Hospital of Jilin University, Changchun, China.
Ying-qi Xing, Department of Neurology, The First Hospital of Jilin University, Xinmin Street 71, Changchun 130021, China.
References
- 1. Gorelick PB, Wong KS, Bae HJet al. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke 2008; 39: 2396–2399. [DOI] [PubMed] [Google Scholar]
- 2. Qureshi AI, Feldmann E, Gomez CRet al. Intracranial atherosclerotic disease: an update. Ann Neurol 2009; 66: 730–738. [DOI] [PubMed] [Google Scholar]
- 3. Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke 2006; 1: 158–159. [DOI] [PubMed] [Google Scholar]
- 4. Jeng JS, Tang SC, Liu HM. Epidemiology, diagnosis and management of intracranial atherosclerotic disease. Expert Rev Cardiovasc Ther 2010; 8: 1423–1432. [DOI] [PubMed] [Google Scholar]
- 5. Suwanwela NC, Chutinetr A. Risk factors for atherosclerosis of cervicocerebral arteries: intracranial versus extracranial. Neuroepidemiology 2003; 22: 37–40. [DOI] [PubMed] [Google Scholar]
- 6. Sacco RL, Kargman DE, Gu Qet al. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction: the northern Manhattan stroke study. Stroke 1995; 26: 14–20. [DOI] [PubMed] [Google Scholar]
- 7. Arenillas JF. Intracranial atherosclerosis: current concepts. Stroke 2011; 42: S20–S23. [DOI] [PubMed] [Google Scholar]
- 8. Kamal AK, Majeed F, Pasha Oet al. Study protocol: asymptomatic intracranial atherosclerotic disease in Pakistanis. J Vasc Interv Neurol 2015; 8: 27–35. [PMC free article] [PubMed] [Google Scholar]
- 9. Wityk RJ, Lehman D, Klag Met al. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke 1996; 27: 1974–1980. [DOI] [PubMed] [Google Scholar]
- 10. Li H, Wong KS. Racial distribution of intracranial and extracranial atherosclerosis. J Clin Neurosci 2003; 10: 30–34. [DOI] [PubMed] [Google Scholar]
- 11. Liu L, Wang D, Wong KSet al. Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke 2011; 42: 3651–3654. [DOI] [PubMed] [Google Scholar]
- 12. Yang G, Wang Y, Zeng Yet al. Rapid health transition in China, 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 2013; 381: 1987–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong KS, Huang YN, Gao Set al. Intracranial stenosis in Chinese patients with acute stroke. Neurology 1998; 50: 812–813. [DOI] [PubMed] [Google Scholar]
- 14. Wong KS, Li H, Chan YLet al. Use of transcranial Doppler ultrasound to predict outcome in patients with intracranial large-artery occlusive disease. Stroke 2000; 31: 2641–2647. [DOI] [PubMed] [Google Scholar]
- 15. Wang Y, Zhao X, Liu Let al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese intracranial atherosclerosis (CICAS) study. Stroke 2014; 45: 663–669. [DOI] [PubMed] [Google Scholar]
- 16. Wong KS, Huang YN, Yang HBet al. A door-to-door survey of intracranial atherosclerosis in Liangbei County, China. Neurology 2007; 68: 2031–2034. [DOI] [PubMed] [Google Scholar]
- 17. Zhang S, Zhou Y, Zhang Yet al. Prevalence and risk factors of asymptomatic intracranial arterial stenosis in a community-based population of Chinese adults. Eur J Neurol 2013; 20: 1479–1485. [DOI] [PubMed] [Google Scholar]
- 18. Wong KS, Ng PW, Tang Aet al. Prevalence of asymptomatic intracranial atherosclerosis in high-risk patients. Neurology 2007; 68: 2035–2038. [DOI] [PubMed] [Google Scholar]
- 19. Zhang Y, Wu S, Jia Zet al. The relationship of asymptomatic intracranial artery stenosis and Framingham stroke risk profile in a northern Chinese industrial city. Neurol Res 2012; 34:359–365. [DOI] [PubMed] [Google Scholar]
- 20. Stroke Screening and Prevention Program of the National Health and Family Planning Commission. Technical specification of stroke screening and prevention in China. Chin J Front Med Sci 2013; 9: 44–50. [Google Scholar]
- 21. Wen CJ, Ren LJ, Hu SY. Study on reliability and validity of the risk assessment scale of stroke. Chin J Stroke 2016; 11: 202–206. [Google Scholar]
- 22. Zhang FL, Guo ZN, Wu YHet al. Prevalence of stroke and associated risk factors: a population based cross sectional study from northeast China. BMJ Open 2017; 7: e015758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Joint Committee for Revision of Guidelines for the Prevention and Treatment of Dyslipidemia in Adults in China. Guidelines for the prevention and treatment of dyslipidemia in Chinese adults (revised in 2016). Chin Circulation J 2016; 31: 937–953. [Google Scholar]
- 24. Elmore EM, Mosquera A, Weinberger J. The prevalence of asymptomatic intracranial large-vessel occlusive disease: the role of diabetes. J Neuroimaging 2003; 13: 224–227. [PubMed] [Google Scholar]
- 25. Lopez-Cancio E, Dorado L, Millan Met al. The Barcelona-asymptomatic intracranial atherosclerosis (ASIA) study: prevalence and risk factors. Atherosclerosis 2012; 221: 221–225. [DOI] [PubMed] [Google Scholar]
- 26. Bae HJ, Lee J, Park JMet al. Risk factors of intracranial cerebral atherosclerosis among asymptomatics. Cerebrovasc Dis 2007; 24: 355–360. [DOI] [PubMed] [Google Scholar]
- 27. Gorelick PB. Distribution of atherosclerotic cerebrovascular lesions: effects of age, race, and sex. Stroke 1993; 24: I16–I19; Discussion I20–I21. [PubMed] [Google Scholar]
- 28. Suri MF, Georgiadis AL, Tariq Net al. Estimated prevalence of acoustic cranial windows and intracranial stenosis in the US elderly population: ultrasound screening in adults for intracranial disease study. Neuroepidemiology 2011; 37: 64–71. [DOI] [PubMed] [Google Scholar]
- 29. Zhang Q, Jiang R, Wang Yet al. Relation of ideal cardiovascular health metrics to asymptomatic polyvascular disease in a Chinese population. Am J Cardiol 2017; 120: 393–398. [DOI] [PubMed] [Google Scholar]
- 30. Ovesen C, Abild A, Christensen AFet al. Prevalence and long-term clinical significance of intracranial atherosclerosis after ischaemic stroke or transient ischaemic attack: a cohort study. BMJ Open 2013; 3: e003724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang HW, Guo MH, Lin RJet al. Prevalence and risk factors of middle cerebral artery stenosis in asymptomatic residents in Rongqi County, Guangdong. Cerebrovasc Dis 2007; 24: 111–115. [DOI] [PubMed] [Google Scholar]
- 32. Ding X, Li C, Yu Ket al. Different risk factors between intracranial and extracranial atherosclerotic stenosis in Asian population: a systematic review and meta-analysis. Int J Neurosci 2014; 124: 834–840. [DOI] [PubMed] [Google Scholar]
- 33. Kim YS, Hong JW, Jung WSet al. Gender differences in risk factors for intracranial cerebral atherosclerosis among asymptomatic subjects. Gend Med 2011; 8: 14–22. [DOI] [PubMed] [Google Scholar]
- 34. Pu Y, Liu L, Wang Yet al. Geographic and sex difference in the distribution of intracranial atherosclerosis in China. Stroke 2013; 44: 2109–2114. [DOI] [PubMed] [Google Scholar]
- 35. Li Z, Yao Y, Han Wet al. Smoking prevalence and associated factors as well as attitudes and perceptions towards tobacco control in Northeast China. Int J Environ Res Public Health 2015; 12: 8606–8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Uehara T, Tabuchi M, Mori E. Frequency and clinical correlates of occlusive lesions of cerebral arteries in Japanese patients without stroke: evaluation by MR angiography. Cerebrovasc Dis 1998; 8: 267–272. [DOI] [PubMed] [Google Scholar]
- 37. Wang Y, Zhang J, Qian Yet al. Association of inter-arm blood pressure difference with asymptomatic intracranial and extracranial arterial stenosis in hypertension patients. Sci Rep 2016; 6: 29894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meschia JF, Bushnell C, Boden-Albala Bet al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45: 3754–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Park JH, Hong KS, Lee EJet al. High levels of apolipoprotein B/AI ratio are associated with intracranial atherosclerotic stenosis. Stroke 2011; 42: 3040–3046. [DOI] [PubMed] [Google Scholar]
- 40. Lee SJ, Lee DG. Distribution of atherosclerotic stenosis determining early neurologic deterioration in acute ischemic stroke. PLoS One 2017; 12: e0185314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alexandrov AV, Sloan MA, Tegeler CHet al. Practice standards for transcranial Doppler (TCD) ultrasound: part II. Clinical indications and expected outcomes. J Neuroimaging 2012; 22: 215–224. [DOI] [PubMed] [Google Scholar]
- 42. Gao S, Lam WW, Chan YLet al. Optimal values of flow velocity on transcranial Doppler in grading middle cerebral artery stenosis in comparison with magnetic resonance angiography. J Neuroimaging 2002; 12: 213–218. [PubMed] [Google Scholar]


