Abstract
Background:
We aimed to investigate the potential effects of a 4-week motor–cognitive dual-task training on cognitive and motor function as well as exercise motivation in young, healthy, and active adults.
Methods:
A total of 26 participants (age 25 ± 2 years; 10 women) were randomly allocated to either the intervention group or a control group. The intervention group performed a motor–cognitive training (3×/week), while the participants of the control group received no intervention. Before and after the intervention period of 4 weeks, all participants underwent cognitive (d2-test, Trail Making Test) and motor (lower-body choice reaction test and time to stabilization test) assessments. Following each of the 12 workouts, self-reported assessments (rating of perceived exertion, enjoyment and pleasant anticipation of the next training session) were done. Analyses of covariances and 95% confidence intervals plotting for between group and time effects were performed.
Results:
Data from 24 participants were analysed. No pre- to post-intervention improvement nor a between-group difference regarding motor outcomes (choice-reaction: F = 0.5; time to stabilization test: F = 0.7; p > 0.05) occurred. No significant training-induced changes were found in the cognitive tests (D2: F = 0.02; Trail Making Test A: F = 0.24; Trail Making Test B: F = 0.002; p > 0.05). Both enjoyment and anticipation of the next workout were rated as high.
Discussion:
The neuro-motor training appears to have no significant effects on motor and cognitive function in healthy, young and physically active adults. This might be explained in part by the participants’ very high motor and cognitive abilities, the comparably low training intensity or the programme duration. The high degree of exercise enjoyment, however, may qualify the training as a facilitator to initiate and maintain regular physical activity. The moderate to vigorous intensity levels further point towards potential health-enhancing cardiorespiratory effects.
Keywords: Integrated multimodal training, cognition, coordination, dual task
Background
Current research suggests that exercise positively impacts cognitive abilities in all age classes.1,2 A possible underlying mechanism is suggested in exercise-induced neurogenesis and increased interconnections between synapses, as well as in a local increase in blood circulation. Beyond these hypothesized effects of motor training solely, dual tasks may have another effect. As dual tasks of simultaneous movement and cognitive activity require and promote selective attention, training them may improve both cognitive and motor functions.3 Adding a cognitive part to exercise is hence expected to enhance the beneficial effect of physical training on neuroplasticity and cognition.4 The combination of motor whole-body coordination and cognitive dual- and choice-reaction tasks may be such a combined training.
In a recent study, such a combined dual-task training focussing on visual perception, peripheral visual fields, limb–eye coordination and adaption to unfamiliar challenges has been demonstrated to enhance brain plasticity.5 The trainings enhanced functional connectivity, presumably induced by increased brain regions co-activation. The intervention implemented in the Demirakca study is called Life Kinetic, a typical motor–cognitive training of coordination tasks with increasing complexity. The training is combined with additive cognitive tasks assumed to tap the working memory. Although the intervention was invented to be adopted in athletic populations, a study population with unknown physical activity status was included in the Demirakca study.5 First hints indicate effects of the training on cognitive abilities like flexibility, inhibition, working memory, spatial ability, and fluid intelligence, in particular in sedentary young adults.6 In contrast, the training induced no significant effect on cognition in physically active participants within the same study; but an improvement in coordinative performance was found in the same training group, which was larger than the one in the control group.6 In studies on children and adolescent athletes, the training showed systematic improvements elicited by the neurocognitive training on eye–hand coordination7 and football-specific dual task or coordination tests.8 If these findings can be transferred to adults is yet questionable. Both the design of the intervention (motor–cognitive combination and no solely training of one out of these abilities) and the controversial findings in physically active adults highlighted above (benefit on coordination and no benefit on cognition) call for a more detailed investigation on potential effects of the intervention on motor–cognitive coordination abilities.
We thus aimed to investigate the potential effects of a motor–cognitive dual-task programme on a collective of physically active young adults. We hypothesize that the programme increases motor–cognitive coordination abilities like choice reaction and dynamic balance ability when compared to an inactive control group.
Methods
Study design and ethical aspects
We adopted a randomized-controlled, longitudinal study design. The trial was approved by a local ethics committee and conducted in accordance with the ethical standards set by the Declaration of Helsinki (1964) with its modifications (Fortaleza 2013). Each participant signed informed consent prior to study enrollment.
Participants
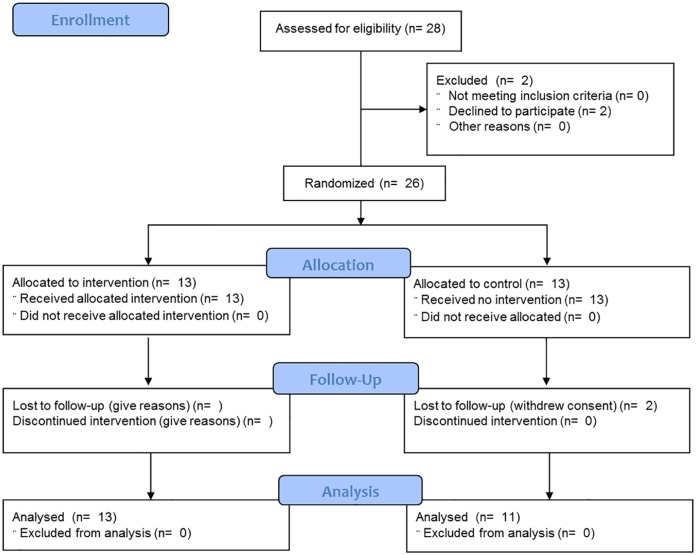
A total of 26 healthy and adult male and female (age = mean 25 ± standard deviation (SD) 2 years; height = 176 ± 6 cm; weight = 71 ± 9 kg; 10 women, 16 men) were recruited. The participants were students in an academic sports science programme and reported regular engagement in physical activity. Exclusion criteria included engagement in other integrated multimodal exercise regimes, acute injuries, or diseases influencing liveability or physical performance, the intake of substances modifying perception (e.g. drugs or medication). The participants flow (Consolidated Standards of Reporting Trials (CONSORT)) is given in Figure 1.
Figure 1.
Study and participant flow (CONSORT). N, number.
Experimental setup
Prior to and following a 4-week-intervention period, all participants performed cognitive and motor tests. A cognitive testing battery (d2 test, Trail Making Test (TMT)) was followed by a motor test battery (choice-reaction tasks and dynamic balance). Following the measurements, the participants were randomly allocated (1:1, complete balanced randomization, BIAS for Windows (version 11.02, 2016, Goethe University of Frankfurt)), either to the intervention group (IV) or to the control group (CG). The IV performed the training intervention for 4 weeks, three units per week. The participants were excluded as dropouts if they completed less than 9 (out of 12) intervention sessions or in the case of incomplete data sets (per protocol analysis). The CG received no treatment.
The intervention
The interventional approach was derived from concepts aiming to connect motion and brain activity evoked by cognitive tasks. The exercises in this study were adapted from the Life Kinetik training concept. Ten different exercises were performed (Table 1), and each subexercise lasted at least 2 min. Each task was divided into levels with increasing exercise intensity; a higher level was reached if the participant was able to adequately perform the task. Several tools, for example, balls, scarfs, and a speed ladder, were used.
Table 1.
The 10 categories of exercises used during intervention with description of the content and examples.
| Number and name | Content | Example |
|---|---|---|
| 1. Parallel ball | Throwing two balls upwards, cross the arms and catch the balls | |
| 2. Speed ladder | Jumping through a speed ladder with cognitive perturbation | Recite the alphabet |
| 3. Go and throw | A trainer calls different commands while walking and throwing a ball | Throwing the ball and stepping forward, backward and sideways |
| 4. Passing a ball | A trainer gives a command while he or she throws a ball, the participant had to catch it in different ways | While stepping with one foot forward |
| 5. Dancing with a scarf | Different scarf movements and stepping combinations | Circle a scarf with one hand and throw a ball with the other hand |
| 6. Finger skills | Upright standing, knees slightly flexed. An examiner gives the respective command, based on a random order | Left and right hand alternating have to show/mimic the letter ‘L’ and ‘O’ |
| 7. Hand skills | A trainer gives commands for moving both or one hand in different directions | Up, down, to the right and to the left |
| 8. Head skills | A trainer calls the direction the participant shall look and simultaneously has to point with his hand into the other direction | |
| 9. March parade | A trainer calls different numbers with different meanings, touching one leg with one hand | #1: right hand touches the right thigh; #14: sidestep left and the leaving the right hand |
| 10. Imitation | A presentation is shown where triangles are placed at the body parts the participant should lift | Hands, arms, feet and legs |
Cognitive assessment
The d2 test assesses concentration and attention. It is an internally consistent and valid measurement.9 The test consists of a paper with 14 rows filled with ‘d’ and ‘p’ letters. Over and/or beneath each letter, various numbers of strokes are placed (stroke no to two strokes both over and below each letter). The participant’s task is to figure out and mark all ‘d’ letters with (in total) two strokes. For each line, test duration is 20 seconds. Concentration, computed as discovered targets minus committed mistakes, was used for further calculations.
The TMT captures visual search velocity and processing speed.10 We used a digital version which exhibits moderate to high correlation with the paper-based version.11 The reliability of the TMT has been found to be high.12 The test is divided into parts A and B. In part A, the participants, as quickly as possible, had to connect 25 numbers in ascending order. In part B, participants had to connect numbers and letters in an alternating manner (1 – A – 2 – B etc., until 13). Time to complete each TMT was employed for further analysis.
Motor assessment
Choice reaction test (intraclass correlation coefficient (ICC): 0.89) was performed on the Quick Board (The Quick Board, LCC), a panel (100 cm × 76 cm) equipped with five sensor pads (upper right and left, lower right and left, and centre) linked to a control box, providing visual stimulus and feedback information via five lights corresponding to the sensor pads.13 For testing, participants started upright standing in neutral position, with the right foot placed between upper and lower right sensors and the left foot between upper and lower left sensors. Both feet did not touch the centre sensor. After a 5-s countdown, one of the five lights, representing the foot sensors on the control box, turns on. Participants were asked to tap as fast as possible with their right or left foot on the respective sensor on the board. The upper and lower right sensors had to be tapped with the right foot and the upper and lower left sensor with the left foot. The centre sensor can be tapped with the right or left foot. After tapping the correct sensor, another light turns on randomly. The participants then returned to the neutral position and taped the next indicated sensor. The number of correct foot contacts during task time (10 s) and average reaction time were recorded. Testing procedure was repeated two times after 60 s of rest in upright standing, respectively.
The time to stabilization test (TTS) was used to measure dynamic postural control after a one-legged countermovement jump of self-selected high and single leg horizontal distance. Participants’ leg length was measured from the trochanter major to the malleoli. The participants started standing on the dominant leg in front of the force plate. After landing on the same leg, they were instructed to stand as still as possible, positioning the hands on the hips and looking at a marker ahead of them placed at the wall. The duration of each measurement was 20 s; the participants had to complete five trials. The time to stabilization was computed as the time until ground reaction force returned to stability (mean ground reaction force over trial duration ± 0.25 SD)14 and the median value of the five trials was used for analysis. The TTS has moderate to high reliability.15
Self-reported outcomes
Self-reported ratings of coordination skills were performed before and after the programme. The participants had to judge their hand–eye coordination, reactivity and balance ability with regard to daily living and sporting activities. For each of the six parameters, the participants selected their self-estimated ability on a numerical rating scale spanning from 1 (poor) to 10 (excellent).
In addition to these pre- and post-intervention ratings, self-estimated data were collected following each training session in the intervention group. The participants rated exercise enjoyment regarding the finished workout as well as anticipation of the next session on an ordinal scale from 1 (no fun/anticipation) to 5 (maximal fun/anticipation).16 To assess self-reported exertion, Borg ratings of perceived exertion (RPE) were assessed using a 6–20-point Likert-type scale.
Statistical analysis
Statistical calculations were performed using SPSS Statistics 23 (IBM, 2015), BIAS for Windows (version 11.02, 2016, Goethe University of Frankfurt), or Excel (Microsoft, 2016). The level of significance (Alpha) was set to 5% for all statistical analyses, p-values below were considered significant. Univariate gain story analyses of covariance (ANCOVAs), using the difference between the pre- and post-results of the main parameters, were performed for the detection of group differences (after checking the underlying assumptions). The baseline values were used as co-variates. In the case of significance, absolute or z-transformed (in the case of systematic co-variate influence) post hoc analyses for group differences were calculated.
Results
Two participants (control group) withdrew their consent for participation without stating a reason. No participant had to be excluded. Thus, data from 24 participants (13 intervention and 11 control) were analysed. Overall, training frequency was 2.7/week (= 10.6 trainings during the intervention period). Compliance rate was thus 88 %. On an individual level, n = 5 volunteers participated in each of the scheduled 12 trainings, n = 2 in 11, n = 2 in 10, and n = 4 in 9.
Motor function
The ANCOVAs for motor function demonstrated no between-group difference (choice-reaction: F = 0.5; TTS: F = 0.7; p > 0.05). Baseline values were (mean ± SD): choice-reaction: IV: 12.1 ± 1.2 hits, CG: 12.1 ± 1.4 hits; TTS: IV: 1.51 ± 0.37 s, CG: 1.44 ± 0.27 s. The pre- to post-differences for each group are illustrated in Figure 2. While no pre- to post-difference occurred in the TTS, both groups increased their number of hits in the choice reaction test.
Figure 2.
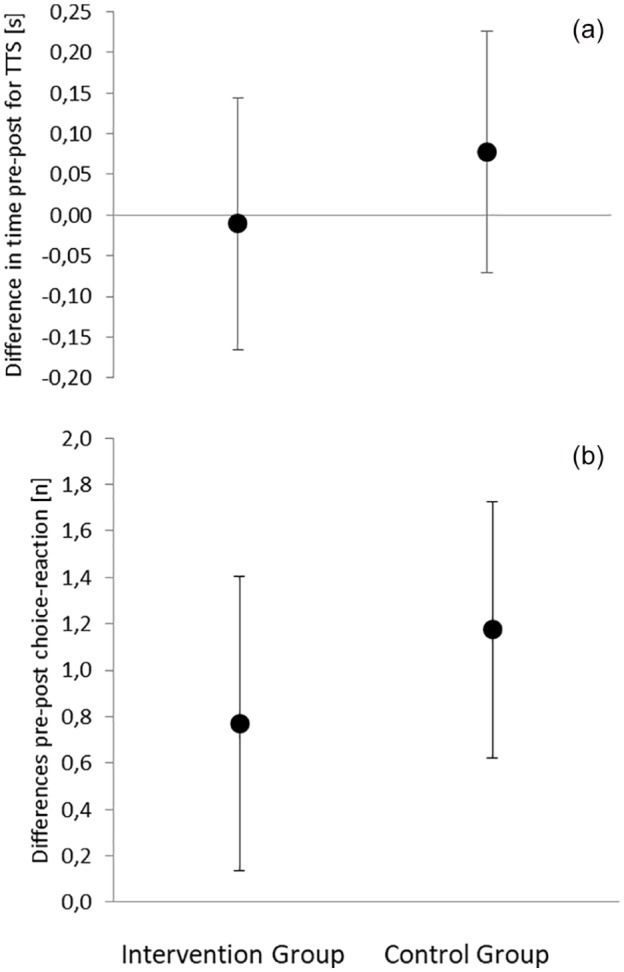
Mean and 95% confidence intervals for the pre- to post-intervention period differences in functional outcomes: (a) time to stabilization test and (b) choice reaction test. Δ = difference in pre-to-post; s = seconds; hits = number of hits in the choice reaction test.
Cognition
Comparable results are shown in the cognitive tests (D2: F = 0.02; TMT A: F = 0.24; TMT B: F = 0.002; p > 0.05). The corresponding pre- to post-differences for each group are illustrated in Figure 3. Baseline values were (mean ± SD): TMT A: IV: 11.6 ± 2.7 s; CG: 14.1 ± 2.3 s; TMT B: IV: 26.6 ± 12 s; CG: 27.2 ± 7.6 s; d2 error frequency: IV: 11 ± 9, CG: 18 ± 11.
Figure 3.
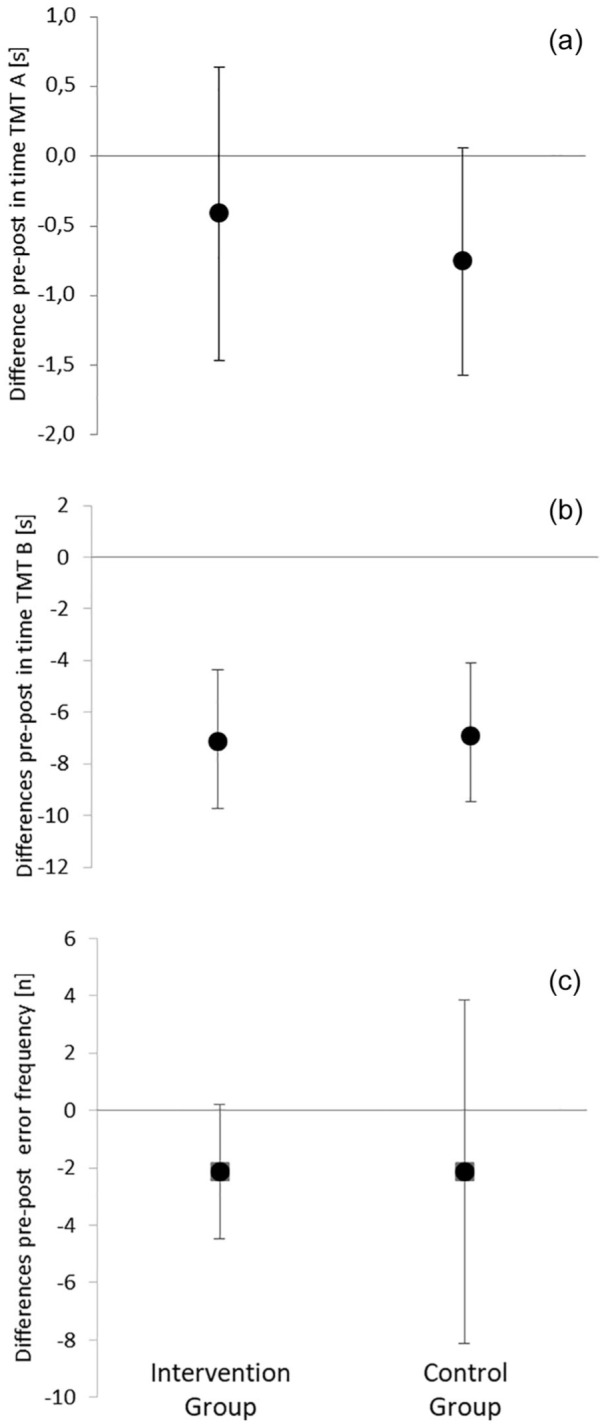
Mean and 95% confidence intervals for the pre- to post-intervention period differences in cognitive outcomes: (a) time for the Trail Making Test A, (b) time for the Trail Making Test B, and (c) D2 frequency of errors F; n = numbers.
Self-reported outcomes
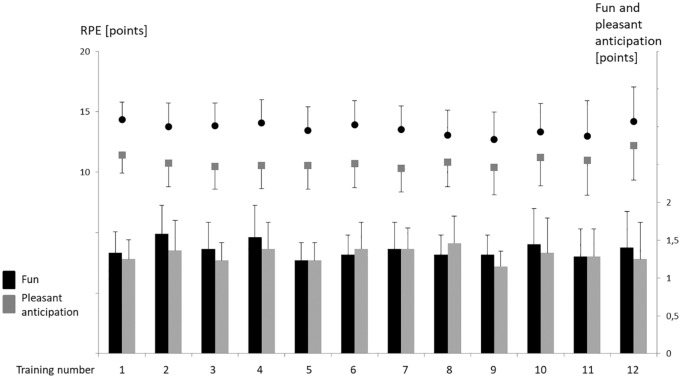
The outcomes of RPE maximum, RPE mean, and fun and pleasant anticipation remained unchanged during the study period (p > 0.05). Figure 4 displays the values for each training session.
Figure 4.
Self-reported outcomes at each training session. Fun and pleasant anticipation (right y-axis) are displayed as bars; maximal (upper dots) and mean (lower dots) RPE values (left y-axis) are displayed as points. Error bars display 95% confidence intervals.
Fun was rated high during the entire intervention. Similarly, most participants looked forward to the next training session. Perceived exertion was constant at an average level between 10.3 and 12.2 and at a maximum level between 12.7 and 14.3.
Discussion
We adopted a motor–cognitive dual-task programme in a collective of physically active, young adults, aiming to increase cognitive and motor abilities. No additional effect of the motor–cognitive multimodal training on motor or cognitive outcomes occurred. The a priori assumed hypotheses are consequently falsified.
Our findings are, partially, in contrast to the results of Demirakca et al.,5 who demonstrated an impact of the intervention on brain co-activity. If such a co-activity, without being pictured in the indirect assessments, occurred in our participants, likewise, is unknown. Having a closer look at the study design,5 the authors included participants with unknown physical activity status and coordinative control ability. Our sample was composed of athletic young participants, and hence, higher baseline performance may explain the lack of effects in this study. Furthermore, the baseline value of the TMT A 11.6/14.1 s is faster than the reference values for highly educated 18–24-year-old persons. The TMT B values of 26.6/27.2 s are even ways below that (mean 11 s faster).17 If not totally attributed to the digital version, the sample of this study may be characterized as high performer in terms of education and physical fitness. Despite that, our sample is comparable to one subsample in the study of Johann et al.6 Herein, no effects of the intervention on physically active young adults were found, likewise. Supporting that finding, a recently published crossover trial found, in comparison to a standard short exercise bout, no superior acute effects of the neuro-motor intervention on visual search, speed of processing, mental flexibility, and executive functions.18 Our findings expand the findings of these working groups by adding motor and coordination outcomes to their solely cognitive assessment. In any case, the initial evidence on the relevance of adding a cognitive part to exercise with the goal to increase beneficial effect of physical training on neuroplasticity and cognition4 is not supported by our data, at least not in young healthy and physically active adults. Again, this is only partially in line with the results of Gabbett et al.19 They recruited young, healthy rugby players. The participants performed a dual-task training, which is comparable to ours (e.g. jumping on the speed ladder while reciting the alphabet or while counting backwards). The authors concluded that ‘the differences in draw and pass proficiency [in high-performance rugby league players] were not statistically significant’,19 but that there was an improvement in draw and pass proficiency under dual-task conditions. Nevertheless, these and our (non-significant, likewise) results highlight the need for further research regarding dual-task motor–cognitive training in athletic populations. On a non-significant level, dual-task training seems to support the ability to perform dual-task draw and pass tasks.
Exercise enjoyment was rated as ‘high’ over the entire intervention period. In addition, most participants looked forward to the next training session. Bauer et al.20 pointed out that ‘fun’ was one of the main reasons for being/staying active in underweight/normal weight compared to overweight/obese women. Although the transferability to young healthy and physical active adults may be limited, exercise enjoyment may be a decisive factor in performing a dual-task training. The RPE ratings corresponded to those of moderate aerobic exercise training. The maximal values were at the level often described as vigorous.21 Hence, and although no superior effects on coordination, choice reaction or cognition were detected, a health-enhancing activity level seems to be given. It thus may be seen as a variety of health-enhancing physical activity schedules. Its potential as a diversified training opportunity in health-enhancing training may be investigated in future studies.
Our small sample size may limit the transferability of the results. It is assumable that the programme may enhance cognitive or motor–cognitive skills in less experienced participants (or even patients); this should be subject to further research. Although a ceiling effect is often seen in trained adults after a certain (i.e. 4 weeks) time,22 a longer intervention period might evoke larger/significant changes in the measured outcomes.
Conclusion
We demonstrated that a 4-week, dual-task intervention does not affect motor and cognitive abilities in healthy, young, and physically active adults. The high scores of exercise enjoyment and the moderate to vigorous intensity levels, however, may qualify the training as a facilitator to initiate and maintain regular exercise and point towards potential health-enhancing cardiorespiratory effects.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from Lokale Ethikkommission des Fachbereich 05 der Goethe-Universität FRankfurt (2016-47).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from all subjects before the study.
Trial registration: This randomized clinical trial was not registered because no clinical trial in its inherent meaning but a training study with healthy participants was performed.
ORCID iD: Daniel Niederer  https://orcid.org/0000-0002-7690-5418
https://orcid.org/0000-0002-7690-5418
References
- 1. Levin O, Netz Y, Ziv G. The beneficial effects of different types of exercise interventions on motor and cognitive functions in older age: a systematic review. Eur Rev Aging Phys Act 2017; 14: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li JW, O’Connor H, O’Dwyer Net al. The effect of acute and chronic exercise on cognitive function and academic performance in adolescents: a systematic review. J Sci Med Sport 2017; 20(9): 841–848. [DOI] [PubMed] [Google Scholar]
- 3. Lauenroth A, Ioannidis AE, Teichmann B. Influence of combined physical and cognitive training on cognition: a systematic review. BMC Geriatr 2016; 16: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hotting K, Roder B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci Biobehav Rev 2013; 37(9 Pt B): 2243–2257. [DOI] [PubMed] [Google Scholar]
- 5. Demirakca T, Cardinale V, Dehn Set al. The exercising brain: changes in functional connectivity induced by an integrated multimodal cognitive and whole-body coordination training. Neural Plast 2016; 2016: 8240894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johann VE, Stenger K, Kersten Set al. Effects of motor-cognitive coordination training and cardiovascular training on motor coordination and cognitive functions. Psychol Sport Exerc 2016; 24: 118127. [Google Scholar]
- 7. Çetin O, Beyleroğlu M, Bağış YEet al. The effect of the exercises brain on boxers’ eye-hand coordination, dynamic balance and visual attention performance. Phys Edu Stud 2018; 22(3): 112–119. [Google Scholar]
- 8. Ozuak A, Çağlayan A. Differential learning as an important factor in training of football technical skills. J Edu Train Stud 2019; 7(6): 68–76. [Google Scholar]
- 9. Bates ME, Lemay EP., Jr. The d2 Test of attention: construct validity and extensions in scoring techniques. J Int Neuropsychol Soc 2004; 10(3): 392–400. [DOI] [PubMed] [Google Scholar]
- 10. Army Individual Test Battery. Manual of directions and scoring. Washington, DC: War Department, Adjutant General’s Office, 1944. [Google Scholar]
- 11. Fellows RP, Dahmen J, Cook Det al. Multicomponent analysis of a digital trail making test. Clin Neuropsychol 2017; 31(1): 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amodio P, Wenin H, Del Piccolo Fet al. Variability of trail making test, symbol digit test and line trait test in normal people: a normative study taking into account age-dependent decline and sociobiological variables. Aging Clin Exp Res 2002; 14(2): 117–131. [DOI] [PubMed] [Google Scholar]
- 13. Galpin AJ, Li Y, Lohnes CAet al. A 4-week choice foot speed and choice reaction training program improves agility in previously non-agility trained, but active men and women. J Strength Cond Res 2008; 22(6): 1901–1907. [DOI] [PubMed] [Google Scholar]
- 14. Fransz DP, Huurnink A, de Boode VAet al. Time to stabilization in single leg drop jump landings: an examination of calculation methods and assessment of differences in sample rate, filter settings and trial length on outcome values. Gait Posture 2015; 41(1): 63–69. [DOI] [PubMed] [Google Scholar]
- 15. Wikstrom EA, Tillman MD, Borsa PA. Detection of dynamic stability deficits in subjects with functional ankle instability. Med Sci Sports Exerc 2005; 37(2): 169–175. [DOI] [PubMed] [Google Scholar]
- 16. Wilke J, Niederer D, Vogt Let al. Is the message getting through? Awareness and use of the 11+ injury prevention programme in amateur level football clubs. PLoS ONE 2018; 13(4): e0195998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tombaugh TN. Trail making test A and B: normative data stratified by age and education. Arch Clin Neuropsychol 2004; 19(2): 203–214. [DOI] [PubMed] [Google Scholar]
- 18. Niederer D, Engeroff T, Wallner Fet al. The acute physical and cognitive effects of a classical workplace physical activity program versus a motor-cognitive coordination workplace program: a randomized crossover trial. J Occup Environ Med 2018; 60: 936–942. [DOI] [PubMed] [Google Scholar]
- 19. Gabbett T, Wake M, Abernethy B. Use of dual-task methodology for skill assessment and development: examples from rugby league. J Sports Sci 2011; 29(1): 7–18. [DOI] [PubMed] [Google Scholar]
- 20. Bauer C, Graf C, Platschek AMet al. Reasons, motivational factors, and perceived personal barriers to engagement in physical activity during pregnancy vary within the BMI classes: the prenatal prevention project Germany. J Phys Act Health 2018; 15(3): 204–211. [DOI] [PubMed] [Google Scholar]
- 21. Eston RG, Davies BL, Williams JG. Use of perceived effort ratings to control exercise intensity in young healthy adults. Eur J Appl Physiol Occup Physiol 1987; 56(2): 222–224. [DOI] [PubMed] [Google Scholar]
- 22. Di Stefano LJ, Clark MA, Padua DA. Evidence supporting balance training in healthy individuals: a systemic review. J Strength Cond Res 2009; 23(9): 2718–2731. [DOI] [PubMed] [Google Scholar]