Abstract
A subpopulation of neurons located within the arcuate nucleus, colocalizing kisspeptin, neurokinin B, and dynorphin (Dyn; termed KNDy neurons), represents key mediators of pulsatile GnRH secretion. The KNDy model of GnRH pulse generation proposes that Dyn terminates each pulse. However, it is unknown where and when during a pulse that Dyn is released to inhibit GnRH secretion. Dyn acts via the κ opioid receptor (KOR), and KOR is present in KNDy and GnRH neurons in sheep. KOR, similar to other G protein–coupled receptors, are internalized after exposure to ligand, and thus internalization can be used as a marker of endogenous Dyn release. Thus, we hypothesized that KOR will be internalized at pulse termination in both KNDy and GnRH neurons. To test this hypothesis, GnRH pulses were induced in gonad-intact anestrous ewes by injection of neurokinin B (NKB) into the third ventricle and animals were euthanized at times of either pulse onset or termination. NKB injections produced increased internalization of KOR within KNDy neurons during both pulse onset and termination. In contrast, KOR internalization into GnRH neurons was seen only during pulse termination, and only in GnRH neurons within the mediobasal hypothalamus (MBH). Overall, our results indicate that Dyn is released onto KNDy cells at the time of pulse onset, and continues to be released during the duration of the pulse. In contrast, Dyn is released onto MBH GnRH neurons only at pulse termination and thus actions of Dyn upon KNDy and GnRH cell bodies may be critical for pulse termination.
In the current study, we investigated the cellular mechanisms responsible for GnRH pulse termination using internalization of the κ opioid receptor as a marker for endogenous release of dynorphin.
GnRH neurons, and their projections to the median eminence, constitute the final common pathway controlling reproduction in mammals. Two modes of GnRH release, pulsatile and surge secretion, are considered to regulate follicular maturation/steroidogenesis and ovulation, respectively, in female mammals (1–3). Whereas surge secretion has been largely studied across several animal species, less is currently known about the cellular and molecular mechanisms that generate the rhythmic discharge of GnRH during pulsatile secretion (4).
The neural mechanism responsible for generating GnRH/LH pulses, collectively called the GnRH pulse generator, has long been proposed to be within the arcuate nucleus of the hypothalamus (ARC) (5–10). It is now generally accepted that a subpopulation of neurons in the ARC colocalizing three neural peptides, kisspeptin, neurokinin B (NKB), and dynorphin (Dyn; termed KNDy neurons), plays a central role in GnRH pulse generation (11). Several lines of evidence led to the hypothesis that KNDy neurons are important for episodic GnRH secretion: (1) both kisspeptin (12, 13) and NKB (14) are critical for normal GnRH secretion in humans; (2) bursts of multiunit electrical activity that correlate with LH pulses are recorded from the vicinity of KNDy neurons (15, 16); and (3) KNDy neurons form an interconnected network (17–19) presumably capable of synchronized firing. Further investigation has since led to the proposal of the KNDy model of GnRH pulse generation (11). The proposed model hypothesizes that each GnRH pulse is initiated by NKB binding to its receptor (NK3R) on cells in the KNDy network stimulating the release of kisspeptin onto GnRH neurons and, following a brief delay, Dyn acting via the κ opioid receptor (KOR) within the KNDy network to inhibit kisspeptin release and thus terminate each pulse (11). There is now strong evidence for this model in ruminants (20), including the inhibition of LH pulses by an antagonist to NK3R (11) and the stimulation of LH pulse frequency, with a burst of multiunit activity associated with each pulse, by an antagonist to KOR (11, 16). Importantly, the KOR antagonist also prolonged the bursts of multiunit activity (16), an action similar to the prolongation of GnRH pulses duration induced by the nonspecific opioid antagonist naloxone (21).
A key unexplained component of the KNDy model for pulse generation is the mechanism responsible for the delay between pulse activation by NKB and pulse termination by Dyn. Evidence from other systems (22–25) suggests that receptor turnover and trafficking may play a key role in delay between pulse onset and termination. Dyn exerts its effects through binding and activation of the KOR, an inhibitory G protein–coupled receptor (26). Similar to most G protein–coupled receptors, KORs are desensitized by receptor phosphorylation and endocytosed after repeated or sustained exposure to agonists, and evidence in vitro has shown that KORs are rapidly activated and internalized (within 5 minutes) following ligand binding (27). Thus, internalization of KORs can be used as a marker of endogenous Dyn release and action on postsynaptic targets. Internalization of other opioid receptors subtypes (e.g., μ receptors) has previously been used as a marker for endogenous release of endogenous opioid peptides in behaving animals (28). We have recently shown that KORs are colocalized in KNDy and GnRH neurons, suggesting that Dyn may act to inhibit GnRH pulses indirectly via the KNDy network and/or directly on GnRH neurons (21). In this study, we used KOR internalization to distinguish between these two possibilities and to assess whether Dyn was released at the beginning and/or end of a pulse.
The ovine model of neuroendocrine function is unique in that sheep are seasonal breeders displaying two distinct modes of reproductive function: breeding season and anestrus (29). During seasonal anestrus, sheep show a marked reduction in GnRH/LH pulse frequency due to enhanced negative feedback responsiveness to estradiol (29). Thus, the low pulse frequency seen in ovary-intact anestrous ewes allows for a baseline upon which to induce individual GnRH/LH pulses. We have previously identified a dose of NKB that consistently induces an LH pulse when injected into the third ventricle of anestrous ewes (30). Thus, in the current study, we induced GnRH/LH pulses in gonad-intact anestrous ewes via intracerebroventricular (icv) injection of NKB and tested the hypothesis that endocytosis of KOR in KNDy and GnRH neurons occurs at times corresponding to pulse termination. Finally, to confirm that the observed KOR endocytosis was the result of endogenous Dyn ligand binding to the receptor, we tested whether pulse termination–associated KOR endocytosis was prevented by treatment with the specific KOR antagonist norbinaltorphimine (nor-BNI).
Materials and Methods
Animals
Adult mixed-breed blackface ewes (n = 26) were obtained from local producers and maintained in an open paddock with free access to shelter and water and daily rations of timothy pellets (Triple Crown Nutrition, Wayzata, MN) and pelleted lamb and sheep feed (Southern States, Clarksburg, WV) at West Virginia University. Ewes were moved indoors 3 to 7 days before surgeries and remained there for the duration of the experiments. All experiments were done during the anestrous season, May through July. While indoors, ewes were fed twice daily to maintain weight and given water and supplemental minerals ad libitum under artificial lighting that was automatically adjusted to match the natural day length of environmental photoperiod.
Surgical and blood collection procedures
All neurosurgeries were performed under aseptic conditions using 2% to 4% isoflurane (Patterson Veterinary, Columbus, OH) in oxygen for anesthesia as previously described (31). Briefly, an 18-gauge stainless steel needle (BD PrecisionGlide; Fisher Scientific, Hampton, NH) was stereotaxically placed into the third ventricle, cemented in place with dental acrylic (Dentsply International, York, PA), protected with a plastic cap, and the hub was plugged to prevent backflow of cerebrospinal fluid. All ewes were treated with daily decreasing doses (from 20 mg to 2 mg IM) of dexamethasone (Vedco, Saint Joseph, MO) and antibiotics twice daily [3.3 mg/kg gentamicin IM (Phoenix, Burlingame, CA) and 100 mg/kg ampicillin (Boehringer Ingelheim, Ingelheim am Rhein, Germany)] from 1 day before to 4 days after surgery. Carprofen (4 mg/kg; Putney, Portland, ME) and butorphanol (0.05 mg/kg; Zoetis, Parsippany-Troy Hills, NJ) were given IV just before and shortly after surgery, respectively, and then gabapentin (4 mg/kg; McCracken Pharmacy, Waynesburg, PA) was given orally twice per day for the next 4 days to minimize pain. Animals were allowed to recover from surgical procedures for at least 7 days before any experimental treatments.
Jugular blood samples (3 to 4 mL each) were taken by venipuncture, placed in tubes containing heparin (50,000 U/mL; Fresenius Kabi USA, Lake Zurich, IL), and plasma was collected and stored at −20°C until assayed for LH. We chose to monitor LH concentrations because they provide a reliable index of episodic GnRH secretion without the additional surgery and stress of hypophysial portal blood collection (32). All procedures were approved by the West Virginia University Animal Care and Use Committee and conducted in accordance with National Institutes of Health guidelines on the care and use of animals in research.
Experiment 1: GnRH/LH pulse induction via icv injection of NKB
For all NKB injections, a stock solution of NKB (catalog no. 1582; Tocris Bioscience, UK) was prepared 1 day prior to the initial treatments as previously described (30). Briefly a stock solution (1 nmol/μL) of NKB in 0.1 N NaOH (Fisher Scientific) was prepared and aliquots were stored at −20°C and then thawed and diluted with sterile saline (33) on the day of treatments to produce a concentration of 0.2 nmol/100 μL. This dose was based on a previous study designed to determine a dose of NKB that would reliably induce a physiological LH pulse (30). NKB, saline (control), and nor-BNI injections into the third ventricle were given as 100-μL injections into unanesthetized ewes during 40 to 60 seconds between collection of individual blood samples.
Discovery study
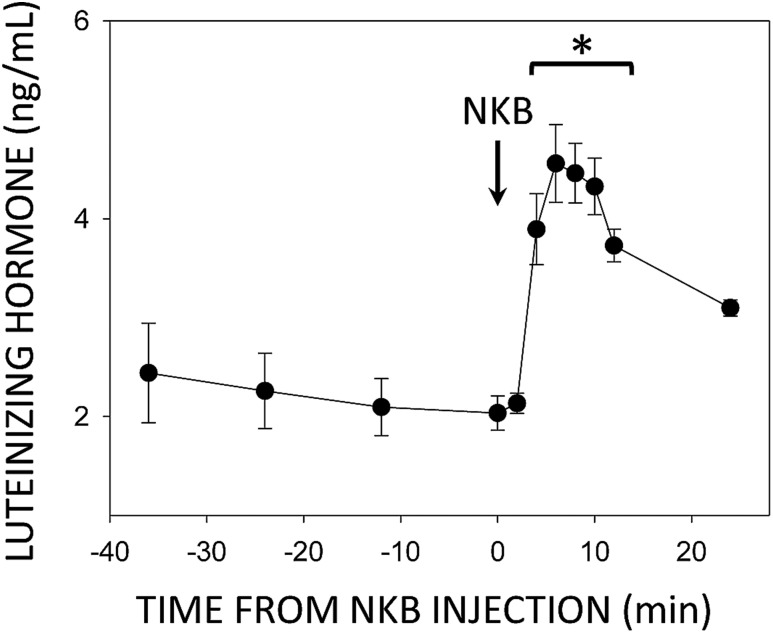
A small discovery study was conducted to determine times of pulse onset and termination upon NKB injection. Approximately 2 weeks after neurosurgery, blood samples were collected from four ewes every 12 minutes from 36 to 12 minutes before and every 2 minutes from 6 minutes before to 12 minutes after icv injection of 0.2 nmol NKB; a final sample was collected 24 minutes after injection. This pilot experiment revealed a reliable LH pulse time course with pulse onset and the rising phase beginning between 2 and 4 minutes after NKB injection (Fig. 1). Mean LH values began to fall 12 minutes after injection, but this was not statistically significant by one-way ANOVA with repeated measures until the 24 minute sample.
Figure 1.
Mean (±SEM) LH concentrations before and after injection (arrow) of 0.2 nmol NKB into the third ventricle of ovary-intact anestrous ewes. *P < 0.05 vs pretreatment controls by one-way ANOVA with repeated measures (F = 12.6, P < 0.001, 10 degrees of freedom).
Experimental design
Approximately 2 weeks after surgery, unanesthetized ewes (n = 19) received a single icv injection of 0.2 nmol/100 μL of NKB or 100 μL of saline [vehicle (Veh)]. Blood samples were collected prior to injection every 12 minutes from 36 to 12 minutes before the icv injection for determination of baseline. Following injection of NKB (n = 13) or Veh (n = 6), ewes were further randomly assigned to two groups that differed in time of euthanasia: 4 minutes or 12 minutes postinjection. Blood samples were collected every 2 minutes from 6 minutes prior to injection until the time when animals were euthanized. This resulted in the following four groups: Veh 4 min (n = 3); Veh 12 min (n = 3); NKB 4 min (n = 6); and NKB 12 min (n = 7). Tissue was collected following euthanasia for analysis of KOR endocytosis and confirmation that cannulae were in the third ventricle.
Experiment 2: pretreatment with nor-BNI
To validate the assumption that any observed internalization of KOR at times corresponding with pulse termination (12 minutes after NKB injection) was a direct result of activation of the KOR by endogenous Dyn binding, an additional set of animals (n = 7) was pretreated with nor-BNI prior to NKB injection. Ewes received a single icv injection of 10 nmol nor-BNI 30 minutes prior to the injection of 0.2 nmol of NKB (as detailed above). Blood sampling and tissue collection were identical to the groups in which tissue was collected 12 minutes after NKB injection in experiment 1. Hypothalamic sections from experiments 1 and 2 were immunoprocessed simultaneously.
LH radioimmunoassay
Ovine LH concentrations in plasma (200 μL) were measured in technical duplicates with an RIA using reagents provided by the National Hormone and Peptide Program (Harbor–University of California Los Angeles Medical Center, CA) as previously described (34). Values are expressed in terms of the reference NIH-LH-S12. The sensitivity limit of the LH radioimmunoassay averaged 0.05 ng per tube (equivalent to 0.1 ng/mL) and the interassay and intra-assay CVs were 5.3% and 5.8%, respectively. Statistical differences in LH concentrations were determine by one-way ANOVA with repeated measures or two-way ANOVA with repeated measures depending on the specific experiment. Post hoc comparisons were made using the Holm–Sidak method with significance levels at P < 0.05.
Tissue collection
Ewes were administered heparin (20,000 U heparin) IV for 10 minutes and, just before, IV injection of sodium pentobarbital (Euthasol; Webster Veterinary, MA). Once they had stopped breathing, the carotids were cut and then the head was removed and perfused via both internal carotids with 6 L of 4% paraformaldehyde (catalog no. 441244; Sigma-Aldrich, St. Louis, MO) in 0.1 M phosphate buffer (PB) (pH 7.3) mixed with 0.1% sodium nitrite (catalog no. S2252; Sigma-Aldrich). After perfusion, the brain was removed and a tissue block containing the septal region, preoptic area (POA), and hypothalamus was dissected. Blocks were incubated in 4% paraformaldehyde at 4°C overnight for postfixation, and then transferred into 30% sucrose (catalog no. S-5-3; Sigma-Aldrich) in 0.1 M PB for cryoprotection. A sliding freezing microtome (SM 200R; Leica Biosystems, Germany) was used to cut coronal sections (45 µm) into 10 parallel series. Sections were stored in cryoprotectant solution (30% ethylene glycol, 1% polyvinylpyrrolidone (catalog no. PVP40-500G; Sigma-Aldrich), 30% sucrose in sodium PB) at −20°C until immunoprocessing.
Immunohistochemistry
Tissues from all experimental groups were immunoprocessed simultaneously. All incubations were performed at room temperature with gentle agitation. Free-floating sections were washed extensively in 0.1 M sodium PB (0.1 M PB containing 0.9% sodium chloride; PBS) containing 0.01% sodium azide (catalog no. S2002-500G; Sigma-Aldrich) overnight at 4°C prior to the start of the protocol and washed extensively with PBS between incubations (4× 5-minute washes between all steps). After completion of immunoprocessing, sections were mounted onto Superfrost slides (Fisher Scientific), dried, and coverslipped with an aqueous mounting medium (Gelvatol) containing an antifading agent (1,4-diazabicyclo[2,2,2]octane; 50 mg/mL).
Immunofluorescent detection of KOR
Series of hypothalamic sections were processed for multilabel immunofluorescence using a biotinylated tyramide amplification procedure and analyzed by confocal microscopy. A heat-mediated antigen retrieval protocol was used as described previously (21). In brief, sections were incubated in 1% H2O2 for 10 minutes, then blocked in incubation solution [PBS containing 0.4% Triton X-100 (Fisher Scientific) and 20% normal goat serum (catalog no. 005-000-121; Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 hour, followed by incubation with monoclonal mouse anti-KOR [dilution 1:250; catalog no. SC- 374479 (KOR-1; D-8); Santa Cruz Biotechnology, Dallas, TX; RRID: AB_10989571] (35) in 0.4% Triton X-100 and 4% normal goat serum made in PBS (PBS+) for 17 hours. Sections were sequentially incubated in biotinylated goat anti-mouse secondary antibody (1:500, 1 hour; catalog no. BA-9200; Vector Laboratories, Burlingame, CA) and Vectastain Elite ABC (1:500, 1 hour; catalog no. ZE0103; Vector Laboratories), biotinylated tyramide (TSA; dilution 1:250 in PBS containing 0.003% H2O2, 10 minutes; catalog NEL760001KT; PerkinElmer, Waltham, MA), and Alexa Fluor 555–streptavidin (dilution 1:100 in PBS, 30 minutes; catalog no. S32355; Molecular Probes, Grand Island, NY). Tissue sections were then incubated in either rabbit anti-Dyn (1:1000; catalog no. H-021-03; Phoenix Pharmaceuticals; RRID: AB_2687416) (36), as the marker for KNDy neurons, or rabbit anti-GnRH (1:400; catalog no. 20075; ImmunoStar, Hudson, WI; RRID: AB_572248) (37) for 17 hours in incubation solution containing 0.4% Triton X-100 and 4% normal goat serum. Dyn was used as a marker for KNDy neurons in anestrous ewes, as it is more reliable than kisspeptin or NKB because each of these peptides is strongly inhibited in anestrus, whereas Dyn expression is not (38). Subsequently, sections were incubated in DyLight 488 goat anti-rabbit (1:100 in PBS, 30 minutes; catalog no. S35553; Thermo Scientific). Finally, sections were counterstained with NeuroTrace 640/660 deep-red fluorescent Nissl stain (1:100 in PBS, 20 minutes; catalog no. N21483; Thermo Scientific) to visualize the cytoplasm and nucleus.
Specificity of the KOR antibody was previously confirmed using recommended controls for immunostaining (39), including peptide blocking controls, primary antibody omissions controls, Western blot analysis, and comparison with the pattern of mRNA expression (40).
Immunofluorescent detection of KOR and EEA1
One series of sections containing the ARC was first stained for KOR immunoreactivity as described above, and next incubated with the early endosomal marker rabbit anti-EEA1 (1:1000 in incubation solution, 17 hours; catalog no. NBP1-05962; Novus Biologicals, Cambridge, UK; RRID: AB_1556168) (41) and DyLight 488 goat anti-rabbit (1:100, 30 minutes; catalog no. S35553; Thermo Scientific). Subsequently, sections were incubated with guinea pig anti-NKB (1:1000 in incubation solution, 17 hours; gift from Prof. Cioffi, IS-3/63, bleed 210493; RRID: AB_2732894) (42) and goat anti–guinea pig DyLight 647 (1:100 in 0.1 M PBS, 30 minutes; catalog no. A-21450; Thermo Scientific). NKB was used as the KNDy marker in this experiment because primary antibodies for Dyn raised in host species other than rabbit have not been validated or are not available for use in sheep.
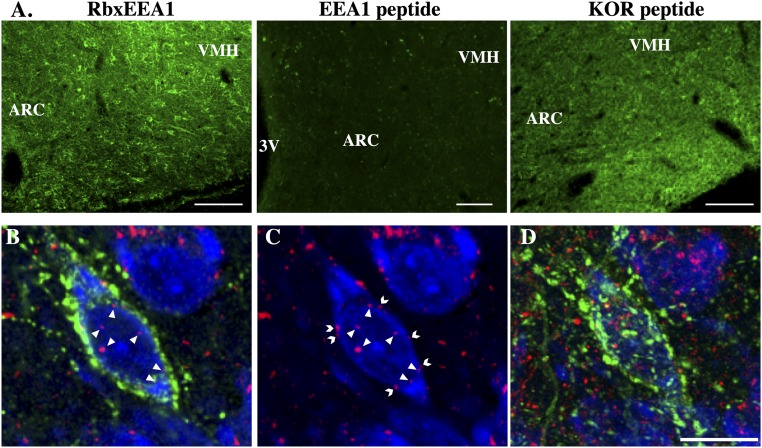
Controls for the immunofluorescence procedures included omission of each of the primary antibodies from the immunostaining protocol, the absence of which completely eliminated staining for the corresponding antigen. Additionally, preabsorption of diluted antibody against EEA1 (1:1000) for 24 hours at 4°C with 500 μg/mL nanomolar concentrations of corresponding purified peptide against which antibody was raised eliminated all staining for that antigen, whereas preabsorption with another peptide [corresponding to the peptide sequence that Ms-anti-KOR (Santa Cruz D8) was raised against] had no effect on immunostaining (Fig. 2A).
Figure 2.
(A) Images for immunohistochemical controls of EEA1 antibody in ARC sections incubated with either rabbit anti-EEA1 without any preabsorption (positive control; RbxEEA1), with the same rabbit anti-EEA1 but preabsorbed with EEA1 peptide (EEA1 peptide), or preabsorbed with KOR peptide used to generate the Ms-anti-KOR antibody used (KOR peptide). Preabsorbtion with the corresponding EEA1 but not KOR peptide eliminated all immunostaining. Scale bars, 100 μm. (B–D) Representative images showing the localization of KOR immunoreactivity in an ARC Dyn-ir neuron 12 min after Veh injection. (B) High magnification (original magnification, ×100) confocal images of 1-μm-thick optical sections showing internalized KOR (red) particles (arrows) in an ARC Dyn (green) neuron counterstained with fluorescent Nissl (blue). Note Dyn (green) immunoreactivity throughout the cytoplasm as well as in axon terminals/boutons surrounding the Dyn-ir neuron. (C) Same image of Dyn cell as in panel (A) but showing only KOR (red) and fluorescent Nissl (blue) for easier visualization of internalized (arrows) and membrane-bound (chevrons) KOR particles. (D) Maximal composite projection of the confocal Z-stack containing the Dyn cell shown in (B) and (C). Scale bar, 20 μm. 3V, third ventricle; VMH, ventromedial hypothalamus.
Immunofluorescent detection of NK3R
As an additional control for the paradigm used to generate GnRH pulses, a separate series of tissue sections was analyzed for internalization of NK3R in KNDy neurons. Internalization was not examined in GnRH neurons because they do not contain NK3R in sheep (43). Tissue was incubated in polyclonal rabbit anti-NK3R (1:20,000; catalog no. NB 300-102; Novus Biologicals, Littleton, CO; RRID: AB_350431) (44) in PBS+ at room temperature, goat anti-rabbit secondary antibody (1:500, 1 hour, Vector Laboratories), ABC Elite (1:500; 1 hour; Vector Laboratories), biotinylated tyramine (TSA; 1:250 in PBS containing 3% H2O2, 10 minutes; PerkinElmer), and Alexa Fluor 555–streptavidin (1:100, 30 minutes; Molecular Probes). Sections were then incubated in rabbit anti-Dyn (1:1000; Phoenix) for 17 h and visualized with DyLight 488 goat anti-rabbit (1:100, 1 hour; Thermo Scientific) and counterstained with NeuroTrace 640/660 deep-red fluorescent Nissl stain (1:100, 20 minutes; Thermo Scientific). Specificity of the NK3R antibody used in this study has been previously shown using standard omission and preabsorption controls (39).
Confocal microscopy and statistical analyses
For all analyses, experimenters were blinded to experimental treatment. To demonstrate the location of KOR immunoreactivity within ARC Dyn, MBH GnRH and POA GnRH neurons were created by projecting (stacking) several optical sections obtained at 1-μm intervals through a section in the z-axis using a Nikon D-Eclipse C1 laser-scanning confocal system (Nikon Corporation) attached to a Nikon Eclipse E800 microscope (Nikon Corporation). Fluorophores are detected by three lasers at wavelengths of 488, 543, and 633 nm, filtered by two dichroic mirrors filtering at 530 and 625 nm, respectively. Confocal Z-stacks of optical sections (1-µm optical sections, at ×60 magnification) were captured through KNDy- and GnRH-immunoreactive (-ir) neurons using EZ-C1 Gold version 3.80 software (Nikon Corporation) with settings for gain and exposure that were identical for all images of all animals. For each Z-stack, only those KNDy- or GnRH-ir neurons with two consecutive optical sections through the middle of the neuron (containing the nucleus) were used for analysis. Internalization of KOR in neurons was evident as immunoreactive intracellular particles ranging in size from 0.5 to 1 μm, consistent with the size of endosomes (45). Only those particles clearly visible within the cytoplasm and not associated with the cell membrane were counted (Fig. 2B and 2C). Numbers of KOR-ir intracellular particles were averaged over the two optical sections containing the nucleus for each cell; for each experiment, 25 to 30 cells (in three to four sections) per animal were analyzed. Data were expressed as means ± SEM. Comparisons of the number of internalized particles between and within groups were analyzed using two-way ANOVA (experiment 1) with a critical level of 0.05. Post hoc comparisons were made using the Holm–Sidak method. Confocal and statistical analysis for EEA1 and NK3R were conducted similar to that of KOR internalization described above. For EEA1 analysis, colocalization of EEA1 and KOR was determined by direct overlap of the fluorophores resulting in a yellow color shift.
Results
KOR internalization in ARC KNDy cells
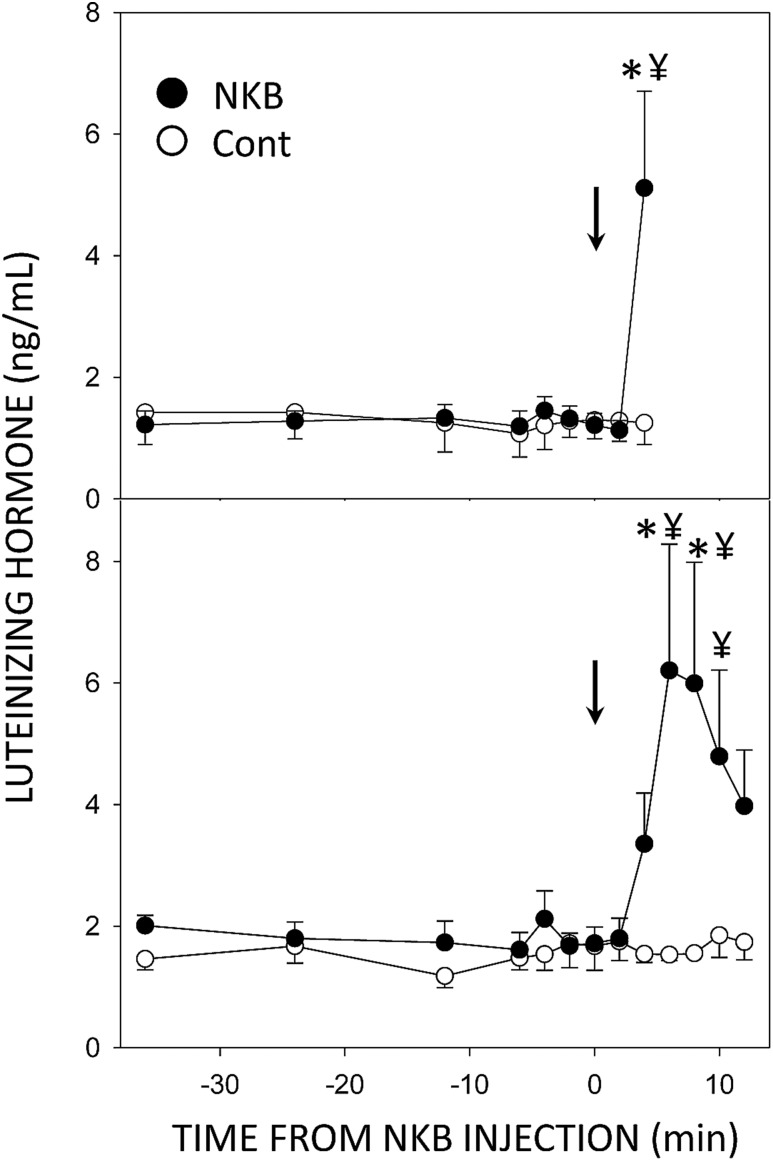
None of the ewes injected icv with Veh had an increase in LH concentrations within the 12 minutes after injection (Fig. 3), whereas there was a significant increase in LH concentrations in all ewes given NKB during this period (Fig. 3). All NKB-treated ewes from which tissue was collected at 4 minutes had an increment in LH concentration in the last sample collected (range, 2.3 to 8 ng/mL) that was significantly different from both pretreatment values in the ewes given NKB and the 4-minute sample in ewes given saline [effect of time, F = 3.81, P = 0.002, 8 degrees of freedom (df); treatment × time, F = 3.93, P = 0.002, 8 df]. LH concentrations were also increased at 4 minutes in most ewes in the 12 minute collection group (the increase occurred at 6 minutes in one ewe), but only concentrations at 6 and 8 minutes postinjection were significantly different from pretreatment values (effect of time, F = 3.15, P = 0.002, 12 df; treatment × time, F = 2.95, P = 0.003, 12 df). LH concentrations at 10 and 12 minutes declined but were not significantly different from peak concentrations. However, they were also no longer significantly different from preinjection values, but the values in the NKB-treated group at 6, 8, and 10 minutes were significantly greater than the corresponding values in the saline-treated group (Fig. 3).
Figure 3.
Mean (±SEM) LH concentrations in ovary-intact anestrous ewes that received injections (arrows) of 100 µL of saline (○) or 0.2 nmol NKB (●). Top panel illustrates values from ewes in which tissue was collected 4 min after injection, and the bottom panel presents data from ewes in which tissue was collected 12 min after injection. *P < 0.05 vs concentrations prior to NKB treatment; ¥P < 0.05 vs saline control within time by two-way ANOVA with repeated measures.
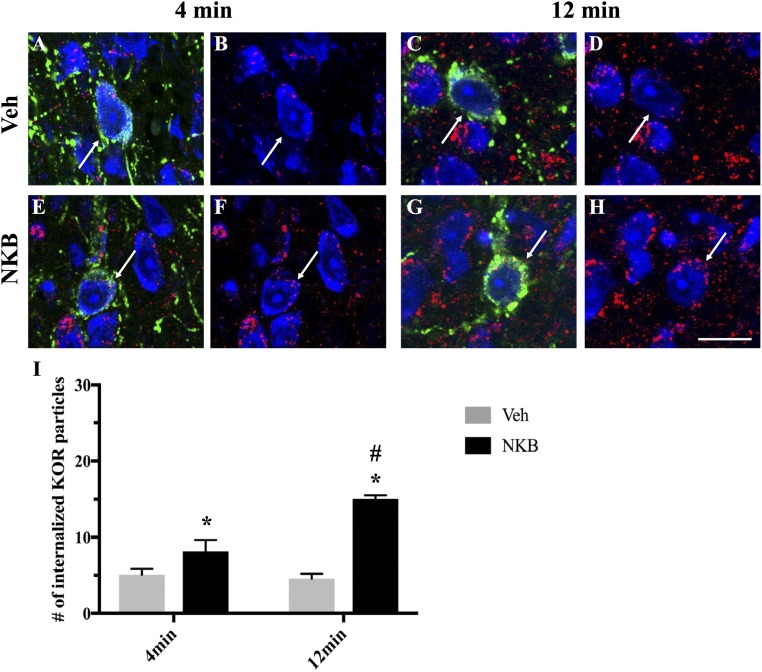
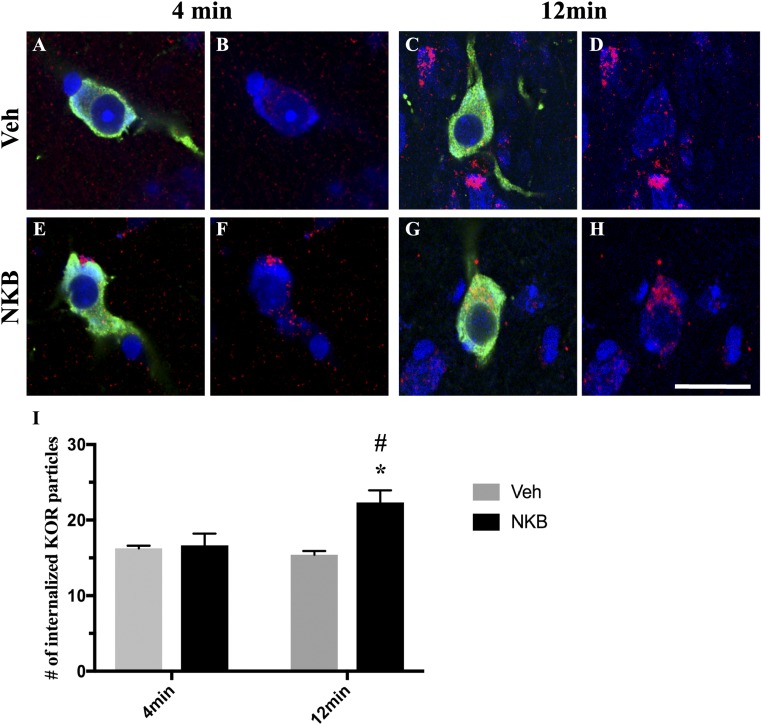
Confocal microscopy was used to analyze alterations in KOR internalization in ARC Dyn neurons in ewes euthanized at 4 minutes and 12 minutes after NKB or Veh injection (Fig. 4A–4H). Quantitative analysis (Fig. 4I) revealed significantly increased numbers of endosome-like particles immunoreactive for KOR within the cytoplasm of ARC Dyn cells in NKB-injected ewes compared with Veh controls regardless of time point [F1,15 = 9.975, P = 0.008 (4 minutes); P < 0.001 (12 minutes)]. Moreover, NKB-injected ewes showed a significant increase in the number of internalized KOR particles per cell at 12 minutes compared with 4 minutes (F1,15 = 7.387, P < 0.001). As expected, no significant changes were found between time points in Veh controls (F1,15 = 7.387, P = 0.796).
Figure 4.
Confocal images of 1-μm-thick optical sections showing internalized KOR (red) particles in ARC Dyn (green) neurons (arrows) counterstained with fluorescent Nissl (blue) (A and E) 4 min and (C and G) 12 min after (A and C) Veh or (E and G) NKB injection. (B, D, F, and H) Same images of Dyn cells as in (A), (C), (E), and (G) but showing only KOR (red) and fluorescent Nissl (blue) to enhance visualization of internalized particles. Scale bar, 20 μm. (I) Mean numbers (±SEM) of internalized KOR particles in Dyn-positive cells in the ARC in ewes 4 min and 12 min postinjection of Veh (gray bars) and NKB (black bars). *P < 0.05, Veh vs NKB within time points; #P < 0.05, 4 min vs 12 min within treatment group.
KOR internalization in MBH GnRH neurons
To determine whether changes in endocytosis of KOR also occurs in MBH GnRH neurons during times of GnRH pulse onset or termination, sections immunofluorescent for KOR and GnRH were analyzed via confocal microscopy (Fig. 5A–5H). Quantitative counts (Fig. 5I) of endosome-like particles immunoreactive for KOR in the cytoplasm of GnRH neurons within the MBH revealed no significant changes between NKB-injected ewes compared with controls at the 4-minute time point (P = 0.606). However, animals that received NKB injection showed significantly higher numbers of internalized particles per cell at 12 minutes postinjection compared with both NKB-infused ewes at the 4-minute time point (F1,15 = 13.643, P < 0.001) and Veh controls at the 12-minute time point (F1,15 = 8.32, P < 0.001). Control animals showed no differences in the numbers of internalized particles per cell between 4 minutes and 12 minutes (P = 0.733).
Figure 5.
Confocal images of 1-μm-thick optical sections showing internalized KOR (red) particles in MBH GnRH (green) neurons counterstained with fluorescent Nissl (blue) (A and E) 4 min and (C and G) 12 min after (A and C) Veh or (E and G) NKB injection. (B, D, F, and H) Same images of GnRH cells as in panels (A), (C), (E), and (G) but showing only KOR (red) and fluorescent Nissl (blue) to enhance visualization of internalized particles. Scale bar, 20 μm. (I) Mean numbers (±SEM) of internalized KOR particles in GnRH-positive cells in the MBH in ewes 4 min and 12 min postinjection of Veh (gray bars) and NKB (black bars). *P < 0.05, Veh vs NKB within time points; #P < 0.05, 4 min vs 12 min within treatment group.
KOR internalization in POA GnRH neurons
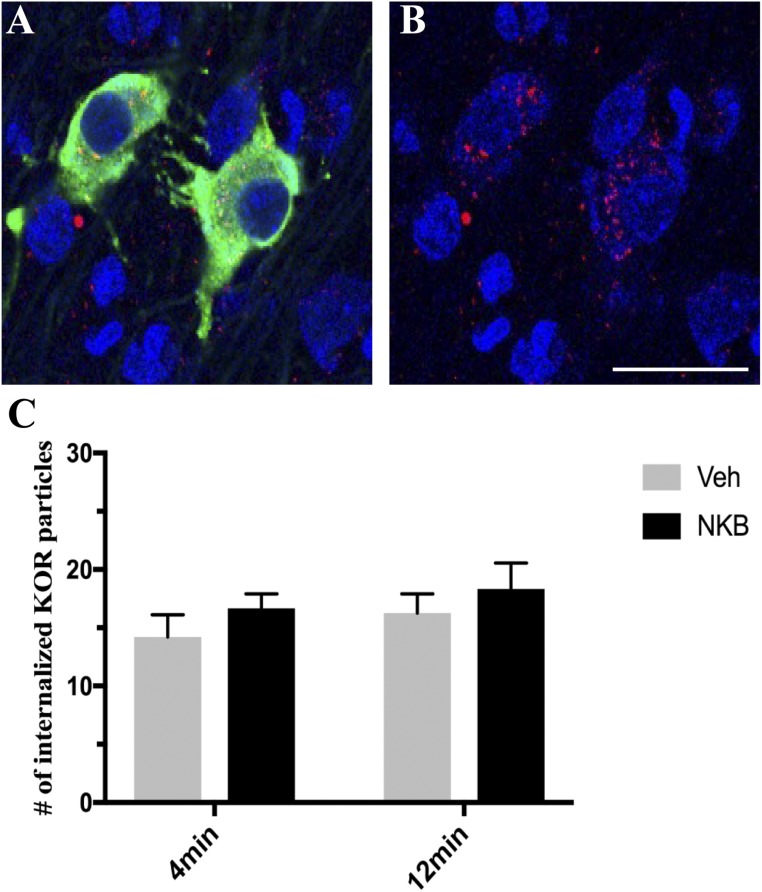
Quantitative analysis revealed no significant differences in the numbers of internalized KOR-ir particles per cell in GnRH neurons within the POA (Fig. 6) in NKB ewes compared with Veh controls regardless of time point (4 minutes, P = 0.821; 12 minutes, P = 0.625). Similarly, no differences were found between time points in either group (NKB, P = 0.875; Veh, P = 0.959).
Figure 6.
(A) Representative confocal image of 1-μm-thick optical sections showing internalized KOR (red) particles in GnRH (green) neurons within the POA counterstained with Fluoro Nissl (blue) 12 min post NKB injection. (B) Same image of GnRH cells as in (A) but showing only KOR (red) and fluorescent Nissl (blue) to enhance visualization of internalized particles. Scale bar, 20 μm. (C) Mean numbers (±SEM) of internalized KOR particles in GnRH-positive cells in the POA in ewes 4 min and 12 min postinjection of Veh (gray bars) and NKB (black bars).
KOR colocalization With EEA1 in ARC KNDy neurons
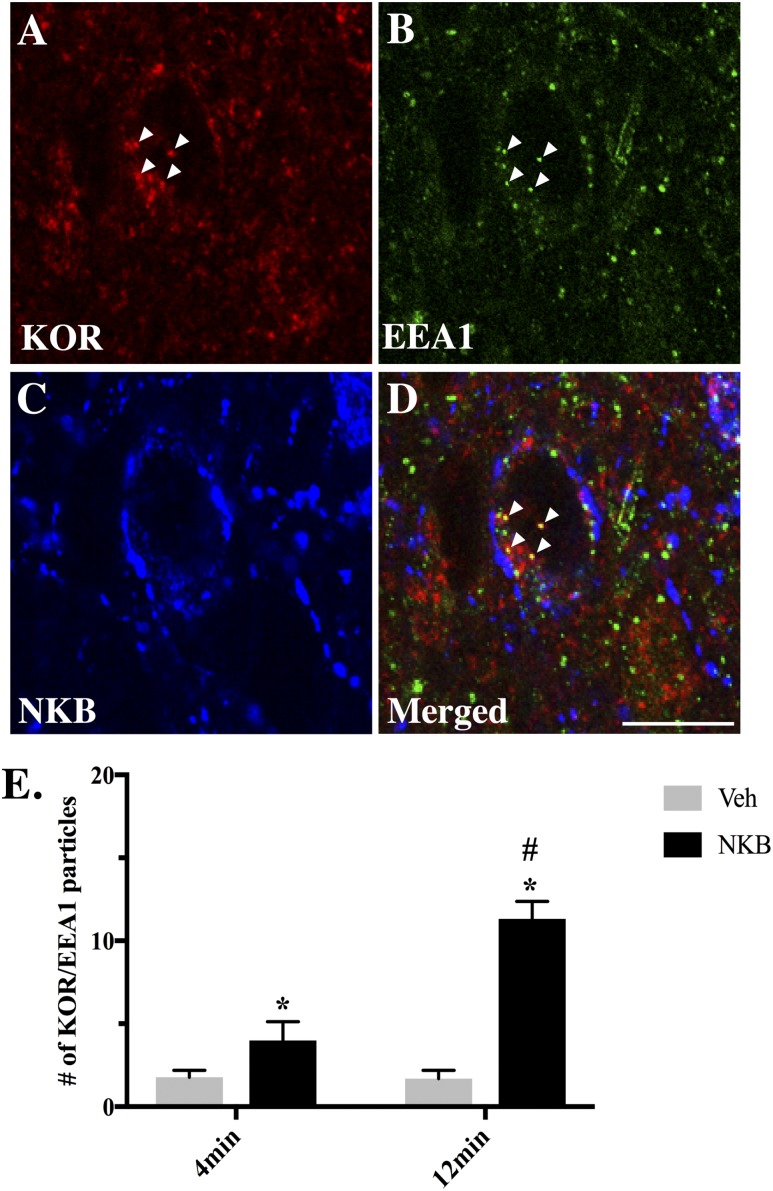
To ensure that internalized KOR-ir particles represent endosomes and not newly synthesized protein, an additional series of tissue was analyzed for immunofluorescent detection of KOR and EEA1 colocalization in ARC NKB cells (Fig. 7A–7D). Quantitative analysis (Fig. 7E) revealed significant increases in the numbers of internalized KOR-ir particles per cell colocalizing EEA1 in NKB-treated ewes regardless of time point [F1,15 = 16.04, P = 0.016 (4 minutes); P < 0.001 (12 minutes)]. Furthermore, NKB-infused ewes showed a significantly higher percentage of KOR/EEA1 colocalization at 12 minutes compared with 4 minutes (F1,15 = 7.85, P < 0.001) that was not seen in Veh controls (P = 0.425).
Figure 7.
(A–C) Representative multichannel image of a 1-μm-thick optical section showing internalized KOR (red) particles and the early endosomal marker EEA1 (green) in a NKB (blue) neuron within the ARC 4 min after NKB injection. (D) Merged 1-µm-thick optical section from single-channel images (A–C) showing colocalization (arrowheads) of internalized KOR particles and EEA1. Scale bar, 20 μm. (E) Mean numbers (±SEM) of internalized KOR particles colocalizing EEA1 in NKB-positive cells in the ARC 4 min and 12 min postinjection of Veh (gray bars) and NKB (black bars). *P < 0.05, Veh vs NKB within time points; #P < 0.05, 4 min vs 12 min within treatment group.
Internalization of NK3R in ARC KNDy neurons
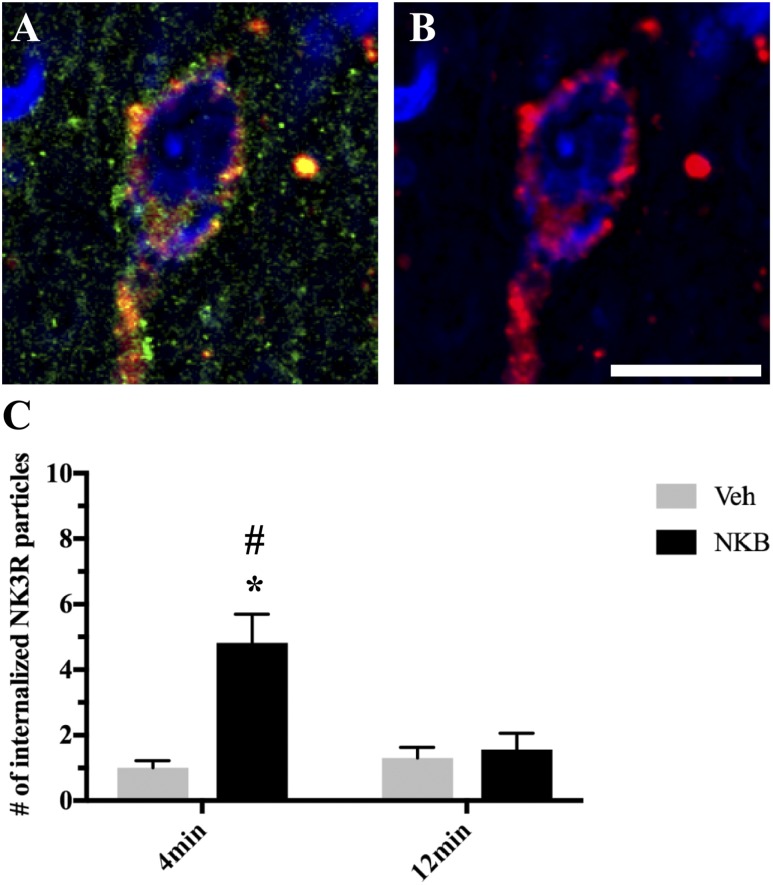
As expected, NKB-injected ewes showed significantly higher numbers of internalized NK3R-ir particles per cell at 4 minutes postinjection compared with control animals (F1,15 = 16.81, P = 0.004) (Fig. 8). However, there was no difference between NKB-injected and control animals in the numbers of internalized NK3R-ir particles per cell at pulse termination (P = 0.978). Additionally, NKB-injected ewes showed significantly higher numbers of NK3R-ir particles per cell at 4 minutes compared with 12 minutes postinjection (F1,15 = 16.60, P = 0.004). Again, no differences were observed between time points in control animals (P = 0.986)
Figure 8.
(A) Representative confocal image of 1-μm-thick optical sections showing internalized NK3R (red) particles in Dyn (green) neurons within the ARC counterstained with fluorescent Nissl (blue) 4 min after NKB injection. (B) Same image of Dyn cell as in (A) showing only NK3R (red) and fluorescent Nissl (blue) to enhance visualization of internalized particles. Scale bar, 20 μm. (C) Mean numbers (±SEM) of internalized NK3R particles in Dyn-positive cells in the ARC in ewes 4 min and 12 min postinjection of Veh (gray bars) and NKB (black bars). *P < 0.05, Veh vs NKB within time points; #P < 0.05, 4 min vs 12 min within treatment group.
Experiment 2: Nor-BNI pretreatment
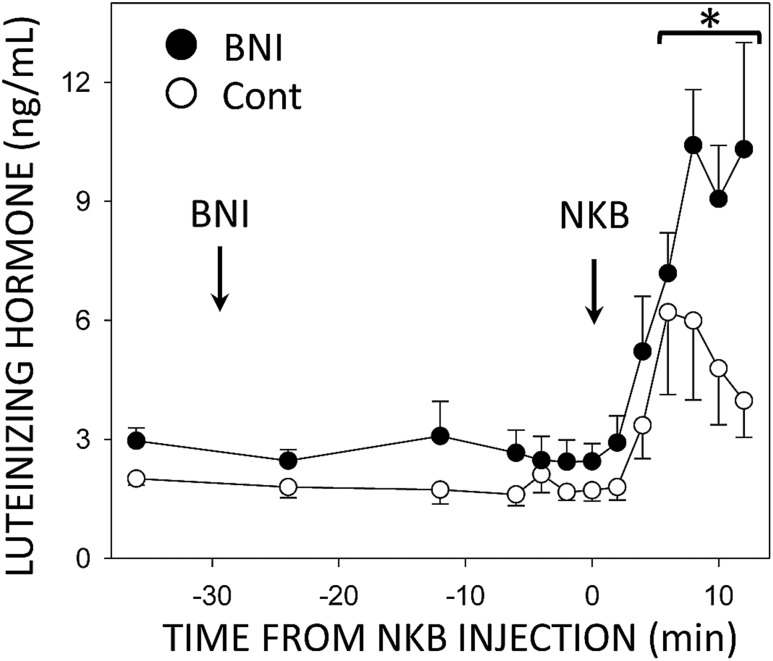
To confirm that changes in KOR internalization were due to endogenous Dyn binding to receptors, additional animals were administered the KOR-specific antagonist nor-BNI prior to NKB injection and tissues were collected at times corresponding with pulse termination (12 minutes). LH concentrations again began to increase at 4 minutes after NKB injections (Fig. 9), with concentrations at 6 minutes significantly different from preinjection controls (F = 15.02, P < 0.001, 12 df). LH concentrations continued to increase with no obvious decline before tissue was collected, and peak LH values were higher than those in the ewes treated only with NKB (Fig. 9). In the latter group, LH concentrations declined after peak values with a half-life of 8.0 ± 1.8 minute, a value similar to the half-life of the rapid phase of ovine LH metabolism (46); the rate of decline could not be determined in the ewes given nor-BNI and NKB because LH concentrations did not fall before tissue collection in most animals. As in experiment 1, confocal images were captured and the total number of endosome-like KOR-ir particles were counted. Animals pretreated with nor-BNI showed numbers of internalized KOR-ir particles per cell in both ARC KNDy (1.51 ± 0.62) and MBH GnRH neurons (8.23 ± 1.18) similar to those seen in control ewes in experiment 1 (Table 1).
Figure 9.
Mean (±SEM) LH concentrations in ovary-intact ewes injected with both nor-BNI and NKB (●) into the third ventricle (arrows). Nor-BNI was injected 30 min before NKB injection and tissue was collected 12 min after the NKB injection. Data from ewes receiving only NKB (○) are duplicated from the bottom panel of Fig. 2 for comparison purposes. *P < 0.05 vs pretreatment LH concentrations and LH concentrations in ewes treated only with NKB by two-way ANOVA with repeated measures.
Table 1.
KOR Internalization in GnRH and KNDy Neurons After nor-BNI Pretreatment
|
No. of Internalized KOR Particles at 12 min
|
|||
|---|---|---|---|
| Veh | NKB | NKB Plus nor-BNI | |
| MBH GnRH | 15.41 ± 0.49 | 22.34 ± 1.55 | 8.23 ± 1.18 |
| KNDy | 4.60 ± 0.79 | 15.03 ± 1.32 | 1.51 ± 0.62 |
Discussion
The “KNDy hypothesis” of pulse generation proposes that Dyn released by reciprocally connected KNDy neurons serves as a stop signal responsible to terminating each individual GnRH pulse (11). This action of Dyn depends on binding to its high-affinity receptor, the KOR, because local microimplants of the KOR antagonist nor-BNI into the ARC increase LH pulse frequency in OVX ewes (11). In our recent work, we identified both KNDy and GnRH neurons as potential sites of Dyn action, based on their colocalization with KOR (21). In the current study, we have directly investigated the question of when and where Dyn is acting during an individual pulse, using a pulse induction model and KOR internalization as a marker of Dyn action during times corresponding to pulse onset and/or termination.
As expected, icv injection of 0.2 nmol NKB successfully and reliably induced a single GnRH/LH pulse in anestrous ewes. This ability of NKB to induce GnRH secretion confirms previous studies (30) and strengthens this approach for use in future investigations. However, this model does have some limitations. First, kisspeptin and NKB are significantly reduced in the ARC of anestrous ewes compared with breeding season animals (38). Owing to this limitation, we chose to use Dyn as a marker for KNDy neurons because Dyn expression does not change seasonally (30); it thus more completely labels the entire population when used as a marker for these neurons. Moreover, because NKB is used to trigger each GnRH pulse in this model, it is difficult to ascertain whether additional endogenous NKB release was also occurring at the time of pulse onset. Thus, investigation of the stimulatory effect of these KNDy peptides utilizing the model in the present study may not fully represent their natural physiological actions, but it still represents a useful model of an LH pulse that can be used for collection of tissue at defined times after the onset of an LH pulse (38).
Note that previous studies in mice (47, 48) have reported a smaller percentage of ARC KNDy cells colocalizing KOR than reported in this study in sheep. This lower degree of KOR colocalization in mouse KNDy cells may be due to technical reasons, as the reports in mice used isotopic in situ hybridization techniques, whereas in this study and our previous study we used a more sensitive antigen-retrieval protocol for immunofluorescent staining combined with tyramide-based signal amplification to allow localization of KOR at a cellular level as well as phenotypic identification. Using this method, we were also able to detect KOR in female rat KNDy and GnRH cells (21). Moreover, the ability of nor-BNI to block KOR internalization in the current study further confirms the specificity of the KOR antibody used here and supports our previous findings (21). However, another possible explanation for differences in the percentage KOR colocalization between species may be related to differences in the functional roles of Dyn in the control of GnRH pulses. In contrast to sheep and goats, administration of nor-BNI has no effect of episodic LH secretion in OVX rats (49), suggesting that, in rodents, other transmitters besides or in addition to Dyn may be responsible for GnRH pulse termination.
In the current study, we first examined the intracellular location of KOR at times that approximate pulse onset and termination (4 minutes and 12 minutes, respectively), after injection of NKB into the third ventricle. We observed that animals receiving NKB injections showed significantly higher numbers of internalized KOR-ir particles per cell compared with Veh controls at both pulse onset and termination. We also observed significant increases in the number of internalized KOR-ir particles during pulse termination compared with onset. Internalization of KOR-ir particles was demonstrated in endosomes via colocalization with the early endosomal marker EAA1. Moreover, receptor antagonism prevented internalization of KOR-ir particles, demonstrating that internalization was dependent on binding of the endogenous ligand Dyn. Hence, internalization of KOR-ir is indicative of Dyn action at the receptor and presumably related to increased presynaptic release of Dyn. Taken together, these results suggest that Dyn is released onto the KNDy network shortly after GnRH pulse onset and bind to KORs, which are then internalized. Dyn then continues to be released during the plateau phase of the pulse, resulting in the additional increase in KOR internalization seen at pulse termination. These data provide a mechanistic explanation for the ability of naloxone to increase the amplitude and duration of GnRH secretion during a pulse (21). The unexpected observation of Dyn release during pulse onset runs contrary to previous evidence in sheep that found no change in the duration of the rising phase of GnRH secretion when Dyn action was blocked by naloxone administration (50). Although this has been interpreted as a lack of action of Dyn during this phase, note that the slope of the increase was probably greater in the presence of naloxone because a higher secretion rate was obtained in the same amount of time. Alternatively, because naloxone is a nonspecific endogenous opioid peptide antagonist binding to all three subtypes of opioid receptors (μ, δ, and κ), its effects on GnRH secretion may reflect more than just an inhibition of Dyn action. Indirect evidence in rodents has shown that Dyn mediates the inhibitory effects of the NK3R agonist senktide (49) when LH secretion is high, suggesting that NKB may act to stimulate Dyn release which may help explain why the KOR internalization seen during pulse onset. A similar inhibitory effect of NKB on LH secretion was seen at high doses in goats (51), but this effect was not observed in sheep (11) and thus may not be applicable to our model. However, further investigation is required to determine the role that Dyn may play during the beginning of the pulse.
Although we previously showed that hypothalamic GnRH neurons contain KORs (21), it was unclear whether Dyn acts directly upon GnRH neurons to inhibit pulsatile secretion. In the current study, we found no differences in KOR internalization in MBH GnRH neurons during pulse onset between NKB- and Veh-injected ewes, suggesting that Dyn is not acting upon GnRH neurons at this time. However, animals that received NKB injections showed significantly higher numbers of internalized KOR-ir particles during pulse termination compared with onset. Thus, it appears that, unlike KNDy cells, Dyn is released onto GnRH neurons only at the time of pulse termination, activating and internalizing KORs in these cells. This is consistent with previous findings suggesting that Dyn acts directly to inhibit MBH GnRH neurons. Studies in sheep have shown that: (1) Dyn-ir fibers synapse directly onto GnRH neuron cell bodies and dendrites in the POA and MBH (34), some of which arise from KNDy neurons (52); (2) KOR is present in GnRH cell bodies but is not colocalized in GnRH terminals in the median eminence (21); and (3) local administration of nor-BNI in the MBH blocked the inhibitory influence of progesterone on LH pulse frequency during the luteal phase (34). Thus, the actions of Dyn at the level of GnRH cell bodies may be critical for pulse termination, but not limiting to GnRH secretion during a pulse. If Dyn release onto GnRH cells arises from KNDy neurons, one would infer that there is differential release of kisspeptin (to drive GnRH secretion during a pulse) and Dyn from KNDy terminals. This is consistent with the report that these peptides are found in different secretory vesicles (53), but it is inconsistent with the generally held view that selective release of neuropeptides from the same terminal seldom occurs. Alternatively, Dyn inputs to GnRH neurons may arise from non-KNDy as well as KNDy neurons (46), so it is possible that release of Dyn from non-KNDy neurons is responsible for KOR internalization at the end of a pulse (54). Additionally, note that the current model is limited to just two time points and therefore does not give a full description of Dyn release during the entire duration of the pulse. It is possible that Dyn may be continuously released onto GnRH cells starting sometime after pulse onset, occurring between the time points used in the present study. Therefore, future studies utilizing this model should include additional time points (between pulse onset and termination) allowing for a more complete description of peptide release/ binding throughout the pulse.
The lack of observed changes in KOR internalization in GnRH neurons within the POA suggest that, unlike MBH GnRH neurons, Dyn does not act upon this population during either pulse onset or termination. The differences between the two GnRH populations reported in the present study are consistent with early work in the sheep (55, 56) using knife cuts between the POA and the MBH, which led to the proposal that GnRH perikarya in the POA are required for the LH surge, whereas those in the MBH are sufficient to generate GnRH/LH pulses. This difference has also been shown pharmacologically; acute stimulation of GnRH pulses via administration of the endogenous opioid peptide antagonist WIN 44,441-3 significantly increased activation of GnRH neurons within the MBH but not in the POA or other regions (57).
One limitation to the use of KOR internalization as a marker for receptor activation is the assumption that KOR-ir particles localized within the cytoplasm are the result of ligand-induced activation and internalization of the receptor, not a new receptor being transported to the cell membrane. Importantly, we observed high rates of colocalization of internalized KOR-ir particles and the early endosomal marker EEA1 in NKB-injected ewes, as well as changes in the percentage of colocalization that paralleled changes in KOR internalization alone. This strongly suggests that internalized KOR particles actually represent endosomes and not newly synthesized protein or lysosomes. Moreover, the presence of early endosomes during both pulse onset and termination suggests recent activation of KOR by Dyn and further supports a continuous release of Dyn following pulse induction. However, not all internalized particles were colocalized with EEA1 (∼40% of KORs did not colocalize with EEA1). Previous in vitro studies have suggested that KORs are either recycled or degraded within 60 minutes of internalization (27). Prior to lysosomal degradation, early endosomes mature into “late endosomes” (58) in a process referred to as receptor downregulation. If we assume the same timeline in vivo it is possible that some of the internalized particles observed may be “late endosomes” and/or lysosomes and could reflect the role of Dyn in the inhibition of GnRH secretion between each pulse. Thus, further investigation is necessary to determine whether KORs in KNDy and/or GnRH neurons are similarly downregulated.
As expected, NKB-injected ewes showed significantly higher numbers of internalized NK3R particles during pulse onset compared with control animals. However, there was no difference between NKB-injected and control animals in the numbers of internalized NK3R particles per cell at pulse termination. These results suggest that following NKB injections, there was subsequent binding and internalization of NK3R in KNDy cells at pulse onset. Whether additional endogenous NKB release was also occurring at the time of pulse onset is not known, but if so, it did not persist to the time of pulse termination. Thus, there may also be selective release of NKB and Dyn onto KNDy neurons, with the latter persisting longer than the former; alternatively, postsynaptic actions of NKB may be terminated before those of Dyn. Regardless, these data further strengthen the model used in the present study and support the putative role of NKB as a start signal for GnRH/LH pulses (11).
Finally, we demonstrated that KOR internalization during pulse termination can be blocked with the KOR antagonist nor-BNI. Therefore, we can conclude that internalization of the receptor is reflective of receptor activation by endogenous Dyn, released during a GnRH pulse. Importantly, pretreatment with nor-BNI lengthened the duration of the individual LH pulse, consistent with previous studies (51), further confirming the inhibitory action of Dyn on GnRH secretion during a pulse. It is also interesting that nor-BNI also increases LH pulse frequency in luteal phase ewes (34). This observation led to the hypothesis that progesterone inhibits GnRH pulse frequency by increasing Dyn release, and there is considerable evidence in sheep that this Dyn arises from KNDy neurons (20). Although this action is closely related to the role of Dyn in pulse termination, it must reflect release of Dyn between pulses. In this regard, animals pretreated with nor-BNI appeared to have lower numbers of internalized KOR-ir particles in both ARC KNDy and MBH GnRH neurons than those seen in Veh-treated control ewes in experiment 1, which is consistent with endogenous Dyn release between pulses. However, differences between the experimental designs precluded the ability to statistically compare the groups. Thus, further investigation is needed to confirm this observation.
In summary, these results suggest that Dyn is released presynaptically onto KNDy cells shortly after pulse onset, and that release continues during the plateau of the pulse, resulting in increased internalization at the time of pulse termination. In contrast, Dyn is released onto MBH GnRH neurons only at pulse termination and not pulse onset. Changes in KOR internalization were only seen in GnRH neurons in the MBH and not the POA, consistent with previous work suggesting that MBH GnRH neurons are critical for pulsatile secretion in the ewe. Thus, actions of Dyn at the level of GnRH cell bodies may be critical for pulse termination. Based on the time course of internalization, these observations suggest that there is little or no delay between NKB and Dyn release onto KNDy neurons at the start of a pulse, and that GnRH pulse termination likely occurs in response to the combined inhibitory actions of Dyn on both KNDy and GnRH neurons. Because KNDy and MBH GnRH neurons are located in close proximity to each other in the sheep brain, the precise contribution of KORs in each cell type to pulse dynamics will await the ability in future experiments to selectively modify KOR expression in a cell-specific manner.
Acknowledgments
We thank Miroslav Valent and Gail Sager for technical assistance with radioimmunoassay and animal surgeries, respectively. We also thank Dr. Jennifer Fridley and Mary Ann Grell for veterinary care, Heather Bungard for routine animal care, and Dr. Al Parlow and the National Hormone and Peptide program for reagents used to measure LH.
Financial Support: This work was supported by National Institutes of Health Grants NIH R01 HD039916 and R01 HD082135 to M.N.L. and R.L.G.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ARC
arcuate nucleus of the hypothalamus
- df
degrees of freedom
- Dyn
dynorphin
- icv
intracerebroventricular
- -ir
-immunoreactive
- KNDy
kisspeptin, neurokinin B, and dynorphin
- KOR
κ opioid receptor
- MBH
mediobasal hypothalamus
- NKB
neurokinin B
- NK3R
neurokinin B receptor
- nor-BNI
norbinaltorphimine
- PB
phosphate buffer
- POA
preoptic area
- Veh
vehicle
References
- 1. Goodman RL. Neuroendocrine control of gonadotropin secretion: comparative aspects In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction. Vol 2 4th ed.Amsterdam, Netherlands: Elsevier; 2015:1537–1574. [Google Scholar]
- 2. Caraty A, Locatelli A, Martin GB. Biphasic response in the secretion of gonadotrophin-releasing hormone in ovariectomized ewes injected with oestradiol. J Endocrinol. 1989;123(3):375–382. [DOI] [PubMed] [Google Scholar]
- 3. Moenter SM, Caraty A, Karsch FJ. The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology. 1990;127(3):1375–1384. [DOI] [PubMed] [Google Scholar]
- 4. Goodman RL, Inskeep EK. Control of the ovarian cycle of the sheep In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction. Vol 2 4th ed.Amsterdam, Netherlands: Elsevier; 2015:1259–1305. [Google Scholar]
- 5. Plant TM, Krey LC, Moossy J, McCormack JT, Hess DL, Knobil E. The arcuate nucleus and the control of gonadotropin and prolactin secretion in the female rhesus monkey (Macaca mulatta). Endocrinology. 1978;102(1):52–62. [DOI] [PubMed] [Google Scholar]
- 6. Soper BD, Weick RF. Hypothalamic and extrahypothalamic mediation of pulsatile discharges of luteinizing hormone in the ovariectomized rat. Endocrinology. 1980;106(1):348–355. [DOI] [PubMed] [Google Scholar]
- 7. Wilson RC, Kesner JS, Kaufman JM, Uemura T, Akema T, Knobil E. Central electrophysiologic correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. Neuroendocrinology. 1984;39(3):256–260. [DOI] [PubMed] [Google Scholar]
- 8. Thiéry JC, Pelletier J. Multiunit activity in the anterior median eminence and adjacent areas of the hypothalamus of the ewe in relation to LH secretion. Neuroendocrinology. 1981;32(4):217–224. [DOI] [PubMed] [Google Scholar]
- 9. Kawakami M, Uemura T, Hayashi R. Electrophysiological correlates of pulsatile gonadotropin release in rats. Neuroendocrinology. 1982;35(1):63–67. [DOI] [PubMed] [Google Scholar]
- 10. Mori Y, Nishihara M, Tanaka T, Shimizu T, Yamaguchi M, Takeuchi Y, Hoshino K. Chronic recording of electrophysiological manifestation of the hypothalamic gonadotropin-releasing hormone pulse generator activity in the goat. Neuroendocrinology. 1991;53(4):392–395. [DOI] [PubMed] [Google Scholar]
- 11. Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy SL, Millar RP, Cernea M, Coolen LM, Lehman MN. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154(11):4259–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100(19):10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. [DOI] [PubMed] [Google Scholar]
- 14. Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda KI, Okamura H. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol. 2009;21(10):813–821. [DOI] [PubMed] [Google Scholar]
- 16. Wakabayashi Y, Yamamura T, Sakamoto K, Mori Y, Okamura H. Electrophysiological and morphological evidence for synchronized GnRH pulse generator activity among kisspeptin/neurokinin B/dynorphin A (KNDy) neurons in goats. J Reprod Dev. 2013;59(1):40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology. 2002;143(11):4366–4374. [DOI] [PubMed] [Google Scholar]
- 18. Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498(5):712–726. [DOI] [PubMed] [Google Scholar]
- 19. Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166(2):680–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weems PW, Lehman MN, Coolen LM, Goodman RL. The roles of neurokinins and endogenous opioid peptides in control of pulsatile LH secretion. Vitam Horm. 2018;107:89–135. [DOI] [PubMed] [Google Scholar]
- 21. Weems PW, Witty CF, Amstalden M, Coolen LM, Goodman RL, Lehman MN. κ-Opioid receptor is colocalized in GnRH and KNDy cells in the female ovine and rat brain. Endocrinology. 2016;157(6):2367–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shuster SJ, Riedl M, Li X, Vulchanova L, Elde R. Stimulus-dependent translocation of κ opioid receptors to the plasma membrane. J Neurosci. 1999;19(7):2658–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shuster SJ, Riedl M, Li X, Vulchanova L, Elde R. The kappa opioid receptor and dynorphin co-localize in vasopressin magnocellular neurosecretory neurons in guinea-pig hypothalamus. Neuroscience. 2000;96(2):373–383. [DOI] [PubMed] [Google Scholar]
- 24. Brown CH. Rhythmogenesis in vasopressin cells. J Neuroendocrinol. 2004;16(9):727–739. [DOI] [PubMed] [Google Scholar]
- 25. Brown CH, Leng G, Ludwig M, Bourque CW. Endogenous activation of supraoptic nucleus κ-opioid receptors terminates spontaneous phasic bursts in rat magnocellular neurosecretory cells. J Neurophysiol. 2006;95(5):3235–3244. [DOI] [PubMed] [Google Scholar]
- 26. Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215(4531):413–415. [DOI] [PubMed] [Google Scholar]
- 27. Li JG, Luo LY, Krupnick JG, Benovic JL, Liu-Chen LY. U50,488H-induced internalization of the human κ opioid receptor involves a β-arrestin- and dynamin-dependent mechanism. κ Receptor internalization is not required for mitogen-activated protein kinase activation. J Biol Chem. 1999;274(17):12087–12094. [DOI] [PubMed] [Google Scholar]
- 28. Coolen LM, Fitzgerald ME, Yu L, Lehman MN. Activation of μ opioid receptors in the medial preoptic area following copulation in male rats. Neuroscience. 2004;124(1):11–21. [DOI] [PubMed] [Google Scholar]
- 29. Weems PW, Goodman RL, Lehman MN. Neural mechanisms controlling seasonal reproduction: principles derived from the sheep model and its comparison with hamsters. Front Neuroendocrinol. 2015;37:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fergani C, Mazzella L, Coolen LM, McCosh RB, Hardy SL, Newcomb N, Grachev P, Lehman MN, Goodman RL. Do substance P and neurokinin A play important roles in the control of LH secretion in ewes? Endocrinology. 2016;157(12):4829–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodman RL, Okhura S, Okamura H, Coolen LM, Lehman MN. KNDy hypothesis for generation of GnRH pulses: evidence from sheep and goats. In: Herbison AE, Plant TM, eds. The GnRH Neuron and Its Control. Hoboken, NJ: Wiley; 2018:289–324.
- 32. Hamernik DL, Nett TM. Gonadotropin-releasing hormone increases the amount of messenger ribonucleic acid for gonadotropins in ovariectomized ewes after hypothalamic-pituitary disconnection. Endocrinology. 1988;122(3):959–966. [DOI] [PubMed] [Google Scholar]
- 33. Grachev P, Li XF, Hu MH, Li SY, Millar RP, Lightman SL, O’Byrne KT. Neurokinin B signaling in the female rat: a novel link between stress and reproduction. Endocrinology. 2014;155(7):2589–2601. [DOI] [PubMed] [Google Scholar]
- 34. Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145(6):2959–2967. [DOI] [PubMed] [Google Scholar]
- 35.RRID:AB_10989571.
- 36.RRID:AB_2687416.
- 37.RRID:AB_572248.
- 38. Weems P, Smith J, Clarke IJ, Coolen LM, Goodman RL, Lehman MN. Effects of season and estradiol on KNDy neuron peptides, colocalization with D2 dopamine receptors, and dopaminergic inputs in the ewe. Endocrinology. 2017;158(4):831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saper CB. An open letter to our readers on the use of antibodies. J Comp Neurol. 2005;493(4):477–478. [DOI] [PubMed] [Google Scholar]
- 40. Weems PW, Coolen LM, Hileman SM, Hardy SL, McCosh RB, Goodman RL, Lehman MN Kappa opioid receptors are internalized in arcuate KNDy cells during GnRH pulse termination in the ewe. In: Proceedings of Annual Meeting of Society for Neuroscience; 12–16 November 2016; San Diego, CA. Abstract 60.04. [Google Scholar]
- 41.RRID:AB_1556168.
- 42.RRID:AB_2732894.
- 43. Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2010;22(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.RRID:AB_350431.
- 45. Trafton JA, Abbadie C, Marek K, Basbaum AI. Postsynaptic signaling via the μ-opioid receptor: responses of dorsal horn neurons to exogenous opioids and noxious stimulation. J Neurosci. 2000;20(23):8578–8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Navarro VM, Gottsch ML, Wu M, García-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152(11):4265–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Grachev P, Li XF, Kinsey-Jones JS, di Domenico AL, Millar RP, Lightman SL, O’Byrne KT. Suppression of the GnRH pulse generator by neurokinin B involves a κ-opioid receptor-dependent mechanism. Endocrinology. 2012;153(10):4894–4904. [DOI] [PubMed] [Google Scholar]
- 50. Goodman RL, Parfitt DB, Evans NP, Dahl GE, Karsch FJ. Endogenous opioid peptides control the amplitude and shape of gonadotropin-releasing hormone pulses in the ewe. Endocrinology. 1995;136(6):2412–2420. [DOI] [PubMed] [Google Scholar]
- 51. Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Merkley CM, Coolen LM, Goodman RL, Lehman MN. Evidence for changes in numbers of synaptic inputs onto KNDy and GnRH neurones during the preovulatory LH surge in the ewe. J Neuroendocrinol. 2015;27(7):624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Murakawa H, Iwata K, Takeshita T, Ozawa H. Immunoelectron microscopic observation of the subcellular localization of kisspeptin, neurokinin B and dynorphin A in KNDy neurons in the arcuate nucleus of the female rat. Neurosci Lett. 2016;612:161–166. [DOI] [PubMed] [Google Scholar]
- 54. Foradori CD, Goodman RL, Lehman MN. Distribution of preprodynorphin mRNA and dynorphin-a immunoreactivity in the sheep preoptic area and hypothalamus. Neuroscience. 2005;130(2):409–418. [DOI] [PubMed] [Google Scholar]
- 55. Jackson GL, Kuehl D, McDowell K, Zaleski A. Effect of hypothalamic deafferentation on secretion of luteinizing hormone in the ewe. Biol Reprod. 1978;18(5):808–819. [DOI] [PubMed] [Google Scholar]
- 56. Whisnant CS, Goodman RL. Effect of anterior hypothalamic deafferentation on the negative feedback of gonadal steroids on luteinizing hormone pulse frequency in the ewe. Domest Anim Endocrinol. 1994;11(2):151–159. [DOI] [PubMed] [Google Scholar]
- 57. Boukhliq R, Goodman RL, Berriman SJ, Adrian B, Lehman MN. A subset of gonadotropin-releasing hormone neurons in the ovine medial basal hypothalamus is activated during increased pulsatile luteinizing hormone secretion. Endocrinology. 1999;140(12):5929–5936. [DOI] [PubMed] [Google Scholar]
- 58. Skieterska K, Rondou P, Van Craenenbroeck K. Regulation of G protein-coupled receptors by ubiquitination. Int J Mol Sci. 2017;18(5):E923. [DOI] [PMC free article] [PubMed] [Google Scholar]