Abstract
The prevalence of vitamin D deficiency, as determined by circulating levels of 25-hydroxycalciferol [25(OH)D], is greater in older individuals compared with the young. To examine the hypothesis that altered production or inactivation of 25(OH)D contributes to lower circulating levels of 25(OH)D, we measured the serum levels of parent vitamin D3 (cholecalciferol) and 25(OH)D. We also determined the relative abundance of transcripts encoding hepatic CYP2R1 and CYP27B1, the principal 25-hydroxylases, transcripts encoding enzymes that degrade 25(OH)D in the liver (Cyp3A11) and kidney (Cyp24A1) and transcripts encoding megalin and cubilin, proteins critical to vitamin D resorption in the kidney in mice at three different ages. We observed a significant decline in the relative abundance of Cyp2R1 in the liver with aging (one-way ANOVA, P = 0.0077). Concurrent with the decrease in mRNA, a significant decline in hepatic CYP2R1 protein (one-way ANOVA for trend, P = 0.007) and 25(OH)D (one-way ANOVA for trend, P = 0.002) and in the ratio of 25(OH)D3 to cholecalciferol (one-way ANOVA, P = 0.0003). By contrast, levels of the transcripts encoding Cyp3a11, Cyp24a1, and Cyp27b1 megalin and cubilin were unchanged with aging. A significant positive correlation was found between Cyp2r1 mRNA and 25(OH)D, and a stronger correlation was found between Cyp2r1 mRNA and the ratio of 25(OH)D3 to cholecalciferol. These results indicate that decreased expression of CYP2R1 contributes to the reduced serum levels of 25(OH)D in aging.
Cyp2r1 mRNA and 25(OH)D decrease with aging, although the mRNA of other vitamin D proteins involved in vitamin D metabolism remains unchanged. The change in Cyp2r1 mRNA abundance correlates with the 25(OH)D to cholecalciferol ratio.
Optimal bone and mineral metabolism require normal vitamin D metabolism. The first step in activation of vitamin D is 25-hydroxylation of parent calciferols to generate 25-hydroxycalciferol [25(OH)D], a process that is mediated principally by the hepatic p450 enzymes CYP2R1 and CYP27A1 (1–5). Circulating 25(OH)D is further modified in the kidney, where CYP27B1-dependent 1-hydroxlation yields calcitriol [1,25(OH)D]. Vitamin D metabolites are bound by the vitamin D-binding protein (GC protein), this protein is freely filtered and its resorption depends on megalin (Lrp2) and cubilin (Cubn). Although 1,25(OH)2D is the most active form of vitamin D, serum concentrations of calcifediol [25(OH)D], the most abundant form of vitamin D, are measured to determine vitamin D status (6). Individuals with haploinsufficiency of CYP2R1 have decreased serum 25(OH)D and decreased responsiveness to supplementation with ergocalciferol or cholecalciferol (2).
Serum concentrations of 25(OH)D decrease with aging (7–10). The initial reports attributed this alteration in vitamin D metabolism to decreased gastrointestinal absorption of vitamin D (11) and/or decreased cutaneous generation of cholecalciferol in response to sunlight (12). However, later studies showed that serum levels of 25(OH)D increase less and decline more rapidly in older subjects than in younger subjects after administration of oral or injectable calciferols (13). Several mechanisms have been hypothesized to contribute to this effect, such as sequestration of vitamin D in adipose tissue, increased catabolism of vitamin D due to CYP24A1 in the kidney and adipose tissue, or diminished synthesis of 25(OH)D by the liver enzyme CYP2R1 (14). In the present work, we hypothesized that decreased serum 25(OH)D in the elderly and decreased responsiveness of the elderly to supplementation with calciferols might be due to age-related alterations in the generation or inactivation of 25(OH)D. We, therefore, analyzed the relationship between circulating levels of cholecalciferol and 25(OH)D and determined the relative abundance of mRNA for the hepatic 25 hydroxylases (Cyp2r1 and Cyp27a1), transcripts encoding enzymes that inactivate 25(OH)D in liver (Cyp3a11) and kidney (Cyp24a1), and megalin and cubilin transcripts in the kidney in male mice at 26 weeks (adult), 39 weeks (late-middle age), and 49 weeks (elderly). Our results indicated that alterations in the expression of the 25-hydroxylase Cyp2R1 affect vitamin D homeostasis and suggest that decreased expression of CYP2R1 contributes to the pathophysiology of low circulating levels of 25(OH)D and decreased responsiveness to vitamin D supplementation in the elderly.
Materials and Methods
Ethics statement
All procedures were performed in accordance with the National Institutes of Health (NIH) Guidelines on the Care and Use of Animals and approved by the Children’s Hospital of Philadelphia institutional animal care and use committee (protocol no. 2011-17-988).
Experimental animals and genotyping
We used male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME). Male mice were used to avoid any effects of the estrus cycle variation on P450 enzyme expression. The mice received a standard chow diet (NIH no. 31M) containing 4180 IU/kg cholecalciferol and were estimated to ingest 20 IU (400 to 600 IU/kg body weight) of cholecalciferol daily. At the appropriate ages, the mice were killed between 8:00 am and 10:00 am at the start of their daylight cycle.
Digital droplet PCR analysis of transcripts encoding vitamin D metabolizing enzymes
We performed digital droplet PCR using the QX100™ Droplet Digital™ PCR system (Bio-Rad, Hercules, CA) to determine the abundance of Cyp2r1 transcripts and to determine the abundance of Nono, Hprt, and 18s RNAs, which were used as our control reference gene after using Bio-Rad M96 mouse reference gene plates (100-29130) to determine which reference genes varied the least between samples. First-strand cDNA was generated from 1 µg of total RNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Waltham, MA) per the manufacturer’s instructions. The following Applied Biosystems probe-primer sets were used for digital droplet quantitative PCR: for Cyp2r1, Mm01159413_m1 (VIC dye); for Cyp27a1, Mm00470430_m1 (FAM dye); for Cyp24a1, Mm00487244_m1; for Cyp3a11, Mm00731567_m1 (VIC dye); for 18s, Mm03928990_g1 (FAM dye); for Nono, Mm00834875_m1 (VIC dye); for cubilin, Mm01325077_m1 (FAM dye); for megalin, Mm01328171_m1 (FAM dye); and for Hprt, Mm03024075_m1 (FAM dye). All probe-primer sets were tested to ensure that the cDNA concentrations were within the linear range of each assay. Cyp2r1, Cyp27a1, Cyp24a1, Cyp3a11, Cubn, and Lrp2 mRNA abundance was normalized to 18s RNA abundance, Nono mRNA abundance, and Hprt mRNA abundance.
Measurement of serum vitamin D metabolites
Serum concentrations of vitamin D2, vitamin D3, 25(OH)D2, and 25(OH)D3 were determined using liquid chromatography–tandem mass spectrometry (Heartland Laboratories, Ames, IA) All assays were performed by researchers who were unaware of the identity of each sample.
Immunoblot for murine CYP2R1 protein
We were unable to detect a specific band for the Cyp2r1 protein from mouse liver or from overexpressed protein using any of the commercially available antibodies. We contracted with Li International (Denver, CO) to generate a rabbit antibody to residues 271-305 (AVNRKP HLPHHFVDAY LDEMDQGQND PLSTFSKE; RRID: AB_2732819) (15). All liver samples were homogenized with ice-cold homogenization buffer (radioimmunoprecipitation assay with Halt protease and phosphatase inhibitors; 1:100) and then evaluated for total protein content using a BCA protein assay kit (Thermo Scientific, Rockford, IL). Next, 15 µg of protein from each liver sample was separated on a 4% to 12% graded SDS polyacrylamide gel and then transferred to a nitrocellulose membrane. Membranes were incubated in SuperBlock plus Tween20 (Thermo Scientific, Waltham, MA) blocking buffer for 1 hour and then incubated with rabbit anti-cyp2r1 (Dr. Li, Li International, Denver, CO; 1:1000; RRID: AB_2732819) (15) overnight. The blots were then washed three times with PBS with 0.1% Tween-20, and then incubated with goat anti-rabbit Alexa Fluor 680 (Rockland, Pottstown, PA; 1:50,000; RRID: AB_1660962) (16) secondary antibody for 1 hour. The blots were rinsed with PBS with 0.1% Tween-20 three times. Blots were scanned on an Odyssey (LI-COR, Lincoln, NE) imaging system. The blots were then reblotted with mouse anti–β-actin (LifeSpan, Seattle, WA; 1:500; RRID: AB_10944987) (17) and then incubated with goat anti-mouse Alexa Fluor 680 (Rockland; 1:20,000; RRID: AB_1057546) (18) and rinsed as above. Using Image Studio Lite, version 5.2.5, software, band densitometry was assessed for Cyp2r1 and β-actin, and the band intensity was normalized to β-actin.
Statistical analysis
All values are presented as the mean ± SEM and represent data from four mice at each time point. The data were analyzed using Prism software, version 6 (GraphPad, San Diego, CA), or JMP, version 13 (SAS Institute, Cary, NC). The mean values were compared using one-way ANOVA with posttests for trend, as appropriate. Differences were considered statistically significant at P < 0.05.
Results
Serum 25(OH)D decreases with aging in mice
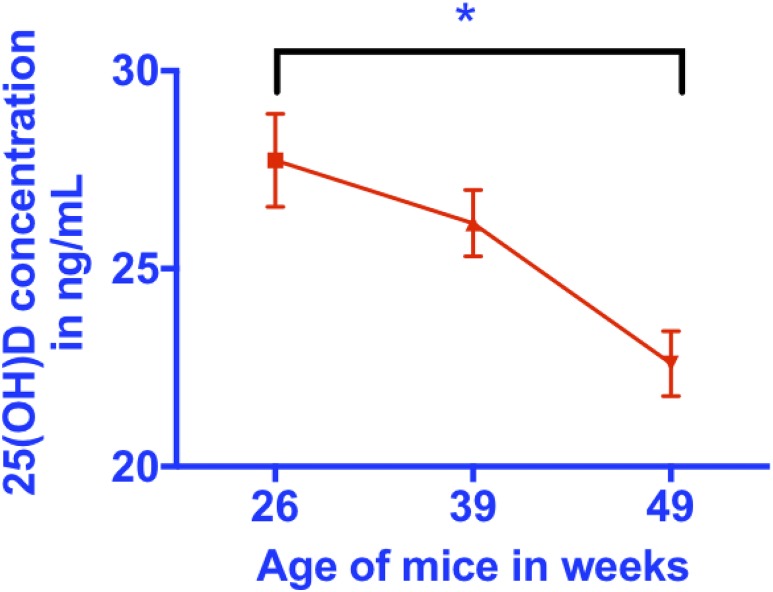
We found that the serum levels of 25(OH)D decreased in mice with aging between 26, 39 and 49 weeks (one-way ANOVA, P = 0.027 for differences between groups; P = 0.008 for trend of decrease with aging; and P = 0.021 for a difference between 26 and 49 weeks using the Tukey test for multiple posttest comparisons; Fig. 1; Table 1). By contrast, no significant differences were found in the serum levels of cholecalciferol in the three groups of mice (Table 1). These ages are approximately equivalent to the life stages of the mid 30s and 50 and 60 years in humans and confirm that aging mice replicate the effects of aging on vitamin D metabolism in humans. This decrease was not associated with a consistent increase in weight over time (Table 1).
Figure 1.
Serum 25(OH)D decreases with aging. Serum total 25(OH)D measured by HPLC-MS decreases with aging. *Indicates significance: P = 0.027 for differences between groups; P = 0.0082 for a trend of a decrease with aging; and P = 0.021 for a difference between 26 and 49 weeks using the Tukey test for multiple posttest comparisons.
Table 1.
Serum 25(OH)D3 and the Ratio of 25(OH)D3 to D3 Decreases With Aging
| 26 wk | 39 wk | 49 wk | P Value | |
|---|---|---|---|---|
| Weight, g | 28.8 ± 1.0 | 32.9 ± 1.2 | 29.7 ± 2.2 | NS |
| Serum 25(OH)D3, ng/mL | 27.7 ± 1.2a | 26.1 ± 0.8 | 22.6 ± 0.8a | P < 0.05 for differences between groups; P < 0.01 for trend toward change over time |
| Serum D3, ng/mL | 12.7 ± 0.9 | 13.0 ± 0.7 | 15.2 ± 0.9 | NS |
| Ratio of 25(OH)D3 to D3 | 2.4 ± 0.1a | 2.0 ± 0.1 | 1.7 ± 0.1a | P < 0.05 for differences between groups; P < 0.01 for trend toward change over time |
Abbreviation: NS, not significant.
P < 0.05 for differences between groups adjusted for multiple comparisons using Tukey test.
Aging is associated with a specific decrease in the abundance of Cyp2r1 mRNA
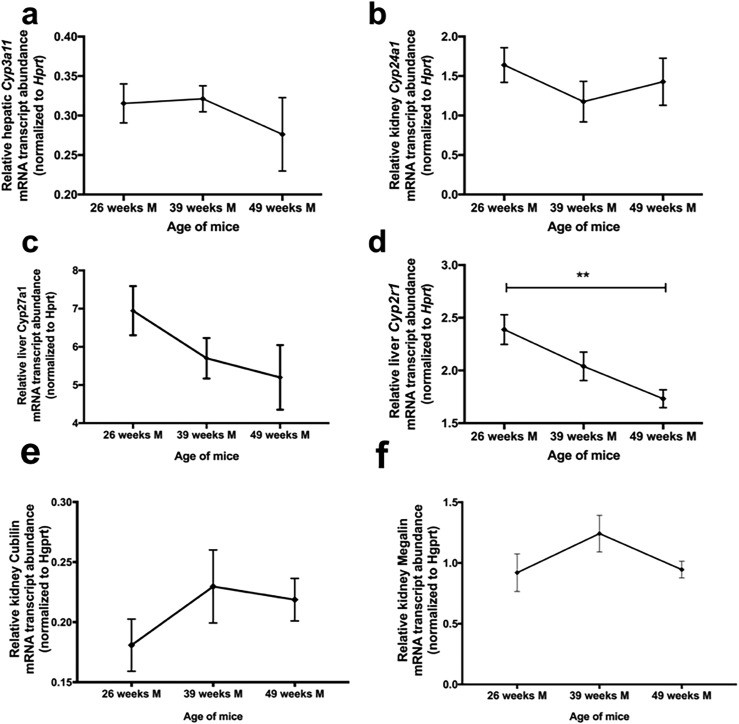
To examine the possibility that alterations in the relative abundance of vitamin D metabolizing enzymes were the cause of decreasing serum 25(OH)D with aging, we isolated total RNA from liver and kidneys from mice at 26, 39, and 49 weeks and used digital droplet PCR to quantitate the relative abundance of mRNAs corresponding to the enzymes involved in metabolism of vitamin D in mice (Cyp27a1, Cyp3a11, Cyp24a1, and Cyp2r1). No significant differences were found in the relative abundance of liver Cyp3a11 or Cyp27a1 mRNA (Fig. 2a and 2c) or kidney Cyp24a1 mRNA (Fig. 2b) with aging. A mild insignificant trend was seen toward a decrease in Cyp27a1 (P = 0.23). However, we observed a significant decline in the relative abundance in Cyp2R1 mRNA in the liver with aging (one-way ANOVA, P = 0.006 for differences between groups; P < 0.0084 for a trend of a decrease with aging; and P = 0.005 for a difference between 26 and 49 weeks using the Tukey test for multiple posttest comparisons; Fig. 2d). This result is consistent with the possibility that Cyp2R1 mRNA alteration might contribute to decreased serum levels of 25(OH)D in aged mice. Additionally, kidney megalin and cubilin mRNA abundance was measured to determine whether decreased serum levels of 25(OH)D resulted from an increased rate of clearance. The relative abundance of cubilin and megalin mRNA was not significant between age groups (Fig. 2e and 2f). For each of these transcripts, our sample number provided enough power to see a significant decrease with aging ~80% of the time (Supplemental Power Calculations).
Figure 2.
No significant change or trend toward a change in liver Cyp3a11 mRNA abundance or kidney Cyp24a1 mRNA abundance with aging was observed. However, liver Cyp2r1 mRNA abundance significantly decreased with aging. (a) No significant differences in the relative abundance of liver Cyp3a11 mRNA with aging. (b) No significant differences in the relative abundance of kidney Cyp24a11 mRNA with aging. (c) No significant differences in the relative abundance of liver Cyp27a1 mRNA with aging. (d) Cyp2R1 mRNA in the liver decreased significantly with aging (one-way ANOVA, ** Indicates significance: P = 0.006 for differences between groups; P < 0.0084 for trend of decrease with aging; and P = 0.005 for a difference between 26 and 49 weeks by Tukey test for multiple posttest comparisons). (e, f) No significant differences were found in the relative abundance of kidney megalin or cubilin mRNA with aging.
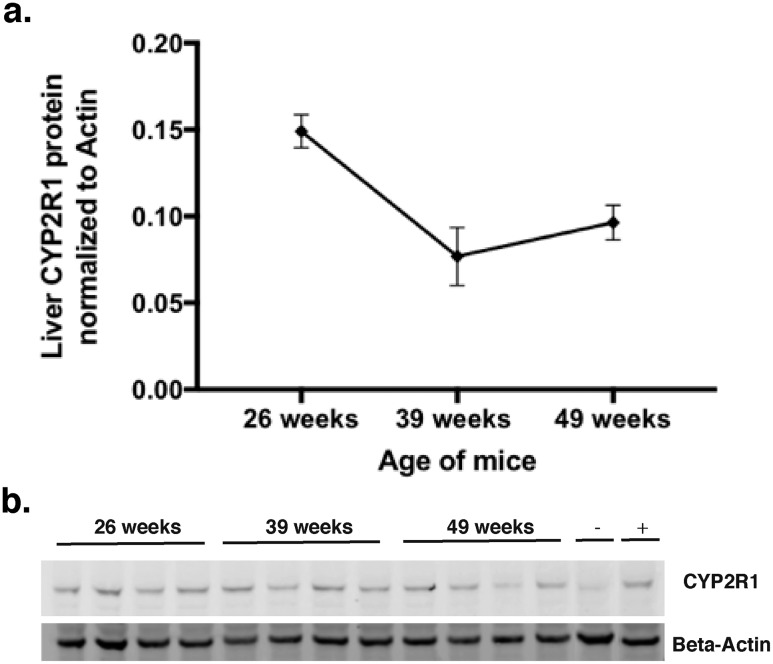
CYP2R1 protein abundance decreases with age
Using our anti-CYP2R1 antibody, we were able to compare CYP2R1 abundance in the liver of mice of difference ages. Similar to mRNA, we observed a significance decrease in CYP2R1 abundance over time (Fig. 3a and 3b). To further examine the relationship between CYP2R1 and 25(OH)D, we attempted to use other in vivo measures of 25-hydroxylase activity.
Figure 3.
Liver Cyp2r1 protein abundance decreased with age. (a) Representative Western blot of aged groups [n = 4; quantified in panel (b)]. (b) Quantitation of Cyp2r1 liver protein reveals Cyp2r1 expression decreases with aging (one-way ANOVA, P = 0.0074 for a trend of decrease over time, for differences between group; posttests revealed P = 0.0083 between 26 and 39 weeks and P = 0.0461 between 26 and 49 weeks by Tukey multiple comparison tests).
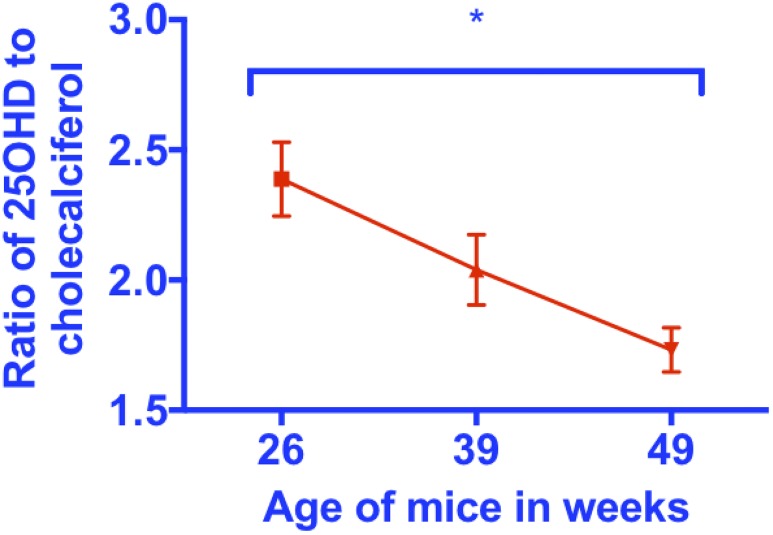
Serum 25(OH)D3-to-cholecalciferol ratio decreases in aging mice
Other investigators have shown that the product to substrate ratio for other enzymes involved in vitamin D metabolism correlates with the activity or expression level of the enzyme responsible for conversion of substrate to product (19, 20). Thus, if decreased expression of Cyp2r1 contributes to decreased levels of 25(OH)D, we would expect to observe a decrease in the serum 25(OH)D3-to-cholecalciferol ratio. To examine this possibility we determined the serum 25(OH)D3-to-cholecalciferol ratios in mice at three ages, 26, 39, and 49 weeks. We found a significant decline in this ratio with aging (Fig. 4; one-way ANOVA, P = 0.014 for differences between group; posttests revealed P = 0.0045 for a trend of a decrease over time and P = 0.011 for differences between 26 and 49 weeks using the Tukey multiple comparison tests).
Figure 4.
A significant decrease in the ratio of 25(OH)D3 to cholecalciferol with aging. Serum 25(OH)D3-to-cholecalciferol ratio decreases with aging (one-way ANOVA, *P = 0.014 for differences between groups; posttests revealed P = 0.0045 for trend of decrease over time and P = 0.011 for differences between 26 and 49 weeks by the Tukey multiple comparison tests).
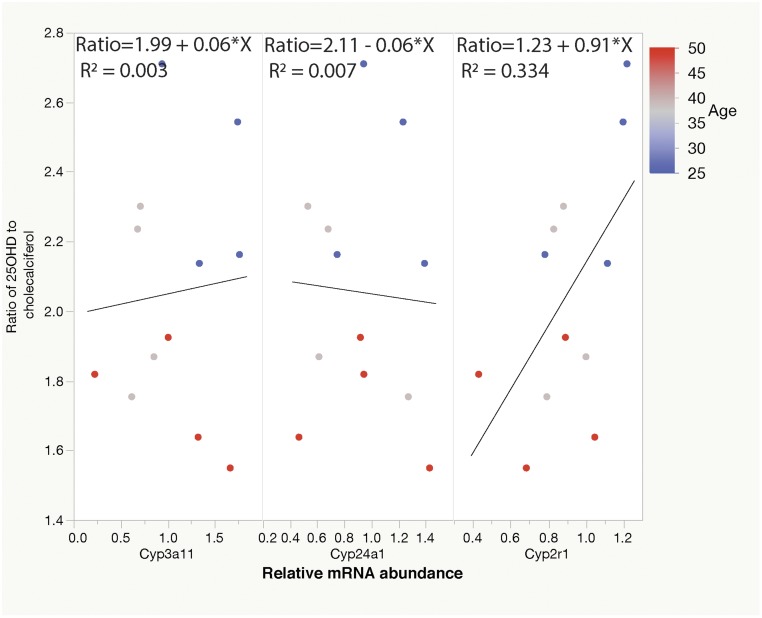
25(OH)D3-to-cholecalciferol ratio correlates with abundance of liver Cyp2r1 mRNA
To further examine the possibility that decreased Cyp2r1 activity might be responsible for the decreased serum 25(OH)D in aged mice, we examined the abundance of the encoding mRNA. Using linear regression, we found a significant relationship between the 25(OH)D3-to-cholecalciferol ratio of aging mice and normalized Cyp2r1 abundance (Figure 5; Table 2; P = 0.049; R2 = 0.334). By contrast, no significant relationships between the serum 25(OH)D3-to-cholecalciferol ratio and the relative abundance of liver Cyp3a11 mRNA or kidney Cyp24a1 mRNA. For each of these transcripts for an R2 value of 0.33, our sample number provided enough power to see a significant decrease with aging 50% of the time (details provided in the Supplemental Power Calculations).
Figure 5.
Conversion of calciferols to 25(OH)D3 decreases with age, and this conversion [as measured by the 25(OH)D3-to-cholecalciferol ratio] correlates with liver Cyp2r1 mRNA abundance. (a) No significant relationship was found between the serum 25(OH)D3-to-cholecalciferol ratio and the relative abundance of liver Cyp3a11 mRNA. (b) No significant relationship was observed between the serum 25(OH)D3-to-cholecalciferol ratio and the relative abundance of kidney Cyp24a1 mRNA. (c) The 25(OH)D3-to-cholecalciferol ratio correlates significantly with liver Cyp2r1 mRNA abundance (R2 = 0.334).
Table 2.
Relative Cyp2r1 mRNA Abundance Correlates With the Ratio of 25(OH)D3 to D3
| Predictor mRNA | R 2 | Estimate of Effect | Significance |
|---|---|---|---|
| Cyp3a11 | 0.003 | 0.06 ± 0.23 | F > 0.80 |
| Cyp24A1 | 0.007 | −0.06 ± 0.34 | F > 0.86 |
| Cyp2r1 | 0.334 | 0.91 ± 0.40 | F > 0.0491 |
Discussion
The roles of inadequate dietary intake of vitamin D and impaired cutaneous synthesis of vitamin D3 in the genesis of reduced circulating levels of 25(OH)D in aging subjects are well established. However, these factors cannot completely explain the smaller increases in 25(OH)D that occur in older compared with younger subjects after vitamin D supplementation. Recent genome-wide association studies have identified the CYP2R1 gene locus as an important determinant of serum levels of 25(OH)D (1, 4, 21), thus raising the possibility that variations in CYP2R1 enzyme activity might also contribute to interindividual variation in vitamin D homeostasis. Studies of individuals with CYP2R1 haploinsufficiency have confirmed this possibility, as individuals who are heterozygous for null CYP2R1 alleles have lower serum 25(OH)D levels and show blunted increases in serum 25(OH)D after administration of ergocalciferol or cholecalciferol (2).
We have extended the role of CYP2R1 in vitamin D pathobiology by describing reduced expression of hepatic Cyp2r1 as the likely basis for decreased conversion of cholecalciferol to 25(OH)D in aging mice. The effect of aging on 25(OH)D homeostasis was specific to CYP2R1, because expression of CYP3A11 and CYP24A1, both of which can oxidize 25(OH)D to inactive metabolites, was not affected. Not only do our results provide insights into vitamin D metabolism in the aging, they have also demonstrated that expression of CYP2R1 can be regulated. In the context of previous studies of rats in which uremia led to decreased expression of CYP2R1 and reduced 25-hydroxylase (22), our results have refuted the notion that the expression of CYP2R1 is entirely constitutive (23, 24).
One limitation of our study was that we only used male mice. Thus, although we expect the results to be generalizable to females, such studies will need to be performed in the future. However, our study had several important strengths. First, we used the expression of several different housekeeping genes to normalize the abundance of target transcripts to ensure that we were not measuring a global effect of aging on mouse tissues. Second, we have developed and validated the 25(OH)D3-to-cholecalciferol ratio as a methods of assessing organismal conversion of substrate cholecalciferol to 25(OH)D3 and showed that this ratio correlated with expression of Cyp2r1 mRNA in the liver. Finally, we found that both the ratio and Cyp2r1 abundance declined pari passu with aging.
In conclusion, older mice had significantly lower serum levels of 25(OH)D3 and lower liver Cyp2r1 protein expression than younger mice overall and at all time points. Our studies have provided a potential mechanism to explain why vitamin D supplementation is less effective in aged subjects than in young subjects and are consistent with recent work that has proposed that it might be more effective to treat vitamin D insufficiency in the aged with calcifediol than with calciferols (25).
Supplementary Material
Acknowledgments
Financial Support: The project described was supported by NIH Grant K08-HD087964-01 (to J.D.R.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 1,25(OH)D
calcitriol
- 25(OH)D
25-hydroxycalciferol
- NIH
National Institutes of Health
References
- 1. Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O’Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Järvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hyppönen E, Spector TD. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thacher TD, Fischer PR, Singh RJ, Roizen J, Levine MA. CYP2R1 mutations impair generation of 25-hydroxyvitamin D and cause an atypical form of vitamin D deficiency. J Clin Endocrinol Metab. 2015;100(7):E1005–E1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci USA. 2004;101(20):7711–7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, Jacobs EJ, Ascherio A, Helzlsouer K, Jacobs KB, Li Q, Weinstein SJ, Purdue M, Virtamo J, Horst R, Wheeler W, Chanock S, Hunter DJ, Hayes RB, Kraft P, Albanes D. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19(13):2739–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hysinger EB, Roizen JD, Mentch FD, Vazquez L, Connolly JJ, Bradfield JP, Almoguera B, Sleiman PM, Allen JL, Levine MA, Hakonarson H. Mendelian randomization analysis demonstrates that low vitamin D is unlikely causative for pediatric asthma. J Allergy Clin Immunol. 2016;138(6):1747–1749.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barnes MS, Horigan G, Cashman KD, Hill TR, Forsythe LK, Lucey AJ, McSorley EM, Kiely M, Bonham MP, Magee PJ, Strain JJ, Wallace JM. Maintenance of wintertime vitamin D status with cholecalciferol supplementation is not associated with alterations in serum cytokine concentrations among apparently healthy younger or older adults. J Nutr. 2011;141(3):476–481. [DOI] [PubMed] [Google Scholar]
- 8. Pérez-López FR, Chedraui P, Fernández-Alonso AM. Vitamin D and aging: beyond calcium and bone metabolism. Maturitas. 2011;69(1):27–36. [DOI] [PubMed] [Google Scholar]
- 9. Mazahery H, von Hurst PR. Factors affecting 25-hydroxyvitamin D concentration in response to vitamin D supplementation. Nutrients. 2015;7(7):5111–5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baker MR, Peacock M, Nordin BE. The decline in vitamin D status with age. Age Ageing. 1980;9(4):249–252. [DOI] [PubMed] [Google Scholar]
- 11. Holt PR, Dominguez AA. Intestinal absorption of triglyceride and vitamin D3 in aged and young rats. Dig Dis Sci. 1981;26(12):1109–1115. [DOI] [PubMed] [Google Scholar]
- 12. MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76(4):1536–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ilahi M, Armas LA, Heaney RP. Pharmacokinetics of a single, large dose of cholecalciferol. Am J Clin Nutr. 2008;87(3):688–691. [DOI] [PubMed] [Google Scholar]
- 14. Forsythe LK, Livingstone MB, Barnes MS, Horigan G, McSorley EM, Bonham MP, Magee PJ, Hill TR, Lucey AJ, Cashman KD, Kiely M, Strain JJ, Wallace JM. Effect of adiposity on vitamin D status and the 25-hydroxycholecalciferol response to supplementation in healthy young and older Irish adults. Br J Nutr. 2012;107(1):126–134. [DOI] [PubMed] [Google Scholar]
- 15. RRID:AB_2732819.
- 16. RRID:AB_1660962.
- 17. RRID:AB_10944987.
- 18. RRID:AB_1057546.
- 19. Pasquali M, Tartaglione L, Rotondi S, Muci ML, Mandanici G, Farcomeni A, Marangella M, Mazzaferro S. Calcitriol/calcifediol ratio: an indicator of vitamin D hydroxylation efficiency? BBA Clin. 2015;3:251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stubbs JR, Zhang S, Friedman PA, Nolin TD. Decreased conversion of 25-hydroxyvitamin D3 to 24,25-dihydroxyvitamin D3 following cholecalciferol therapy in patients with CKD. Clin J Am Soc Nephrol. 2014;9(11):1965–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anderson D, Holt BJ, Pennell CE, Holt PG, Hart PH, Blackwell JM. Genome-wide association study of vitamin D levels in children: replication in the Western Australian Pregnancy Cohort (Raine) study. Genes Immun. 2014;15(8):578–583. [DOI] [PubMed] [Google Scholar]
- 22. Michaud J, Naud J, Ouimet D, Demers C, Petit JL, Leblond FA, Bonnardeaux A, Gascon-Barré M, Pichette V. Reduced hepatic synthesis of calcidiol in uremia. J Am Soc Nephrol. 2010;21(9):1488–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dreyfus M, Wion D. Investigating the relationship between vitamin D and cancer requires dosing the bioavailable nonhydroxylated vitamin D storage in cancer tissues. Cancer. 2015;121(18):3362–3363. [DOI] [PubMed] [Google Scholar]
- 24. Andersson S, Holmberg I, Wikvall K. 25-Hydroxylation of C27-steroids and vitamin D3 by a constitutive cytochrome P-450 from rat liver microsomes. J Biol Chem. 1983;258(11):6777–6781. [PubMed] [Google Scholar]
- 25. Vaes AMM, Tieland M, de Regt MF, Wittwer J, van Loon LJC, de Groot LCPGM. Dose-response effects of supplementation with calcifediol on serum 25-hydroxyvitamin D status and its metabolites: a randomized controlled trial in older adults. Clin Nutr. 2018;37(3):808–814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.