Abstract
Pelvic endometriosis is a complex syndrome characterized by an estrogen-dependent chronic inflammatory process that affects primarily pelvic tissues, including the ovaries. It is caused when shed endometrial tissue travels retrograde into the lower abdominal cavity. Endometriosis is the most common cause of chronic pelvic pain in women and is associated with infertility. The underlying pathologic mechanisms in the intracavitary endometrium and extrauterine endometriotic tissue involve defectively programmed endometrial mesenchymal progenitor/stem cells. Although endometriotic stromal cells, which compose the bulk of endometriotic lesions, do not carry somatic mutations, they demonstrate specific epigenetic abnormalities that alter expression of key transcription factors. For example, GATA-binding factor-6 overexpression transforms an endometrial stromal cell to an endometriotic phenotype, and steroidogenic factor-1 overexpression causes excessive production of estrogen, which drives inflammation via pathologically high levels of estrogen receptor-β. Progesterone receptor deficiency causes progesterone resistance. Populations of endometrial and endometriotic epithelial cells also harbor multiple cancer driver mutations, such as KRAS, which may be associated with the establishment of pelvic endometriosis or ovarian cancer. It is not known how interactions between epigenomically defective stromal cells and the mutated genes in epithelial cells contribute to the pathogenesis of endometriosis. Endometriosis-associated pelvic pain is managed by suppression of ovulatory menses and estrogen production, cyclooxygenase inhibitors, and surgical removal of pelvic lesions, and in vitro fertilization is frequently used to overcome infertility. Although novel targeted treatments are becoming available, as endometriosis pathophysiology is better understood, preventive approaches such as long-term ovulation suppression may play a critical role in the future.
Essential Points
Pelvic endometriosis, manifested by chronic pelvic pain and infertility, is a complex syndrome characterized by an estrogen-dependent chronic inflammatory process that affects primarily pelvic tissues, including the ovaries, caused by repeated retrograde travel and survival of shed endometrial tissue in the lower abdominal cavity
The underlying pathologic mechanisms in the intracavitary endometrium and extrauterine endometriotic tissue involve defectively programmed endometrial mesenchymal progenitor/stem cells
Although endometriotic stromal cells, which compose the bulk of endometriotic lesions, do not carry somatic mutations, they demonstrate specific epigenetic abnormalities that alter expression of key transcription factors such as excessive production of GATA-binding factor-6, steroidogenic factor-1, and estrogen receptor-β, which collectively cause estrogen-dependent inflammation, and deficient expression of progesterone receptor, which causes progesterone resistance
Populations of endometrial and endometriotic epithelial cells harbor multiple cancer driver mutations, such as KRAS, which may be associated with the establishment of pelvic endometriosis or ovarian cancer
It is not known how interactions between epigenetically defective stromal cells and the mutated genes in epithelial cells contribute to the pathogenesis of endometriosis
Endometriosis-associated pelvic pain is currently managed by suppression of ovulatory menses and estrogen production, cyclooxygenase inhibitors, and surgical removal of pelvic lesions, whereas in vitro fertilization is frequently used to overcome infertility
Although novel targeted treatments are becoming available, as endometriosis pathophysiology is better understood, simple preventive approaches such as long-term ovulation suppression are currently underused
Definition of Endometriosis
Advances made during the last two decades have revealed endometriosis as a complex clinical syndrome characterized by an estrogen-dependent chronic inflammatory process that affects primarily pelvic tissues, including the ovaries (1, 2). Endometriosis is the most common cause of chronic pelvic pain in reproductive-age women and is strongly linked to persistent episodes of ovulation, menstruation, and cycling steroid hormones (1, 2). Its multifactorial etiology and high prevalence resemble other chronic inflammatory disorders associated with pain, such as inflammatory bowel disease and gastroesophageal reflux disorder (1, 2). Its dependence on estrogen as the key biologic driver of inflammation, however, makes endometriosis unique (3–5).
The classical definition of endometriosis is the surgical detection of endometrial tissue outside of the uterine cavity (6); however, this narrow anatomic definition has proven insufficient to explain the natural history of endometriosis, the full spectrum of its clinical features, the frequent recurrence of its symptoms, the underlying molecular pathophysiology, or its responsiveness to currently available management modalities (1, 2, 7, 8). Recently, the definition of endometriosis has evolved to one that is more patient-focused and takes into account the cellular and molecular origins of the disease; its natural history from teenage years to the menopause; its complex, chronic, and systemic nature; the variety of tissues involved, including the central nervous system; and the need for treatments that address long-term suppression of ovulation (2, 9).
Pelvic endometriosis, which may involve pelvic peritoneal surfaces, subperitoneal fat, rectovaginal space, or ovaries, occurs primarily via retrograde menstruation and comprises the vast majority of all cases of endometriosis (Fig. 1). The disease may also affect the bladder, bowel (most commonly the rectum and appendix), deep pelvic nerves, ureters, anterior abdominal wall, abdominal skin, diaphragm, pleura, lungs, pericardium, and brain (10). The symptoms of pelvic endometriosis—painful periods, painful intercourse, and chronic pelvic pain and infertility—often disrupt the social, professional, academic, and economic potential of young women. Living with severe cyclic or continuous pelvic pain or the threat of its return, often for decades, can also lead to anxiety and depression (11). Another key source of stress associated with endometriosis is the potential compromise of current or future fertility (11). Herein, we review the clinical, biological, and genetic advances that have been made in the area of endometriosis during the past two decades, which may inform the development of treatment and prevention approaches for this debilitating disease.
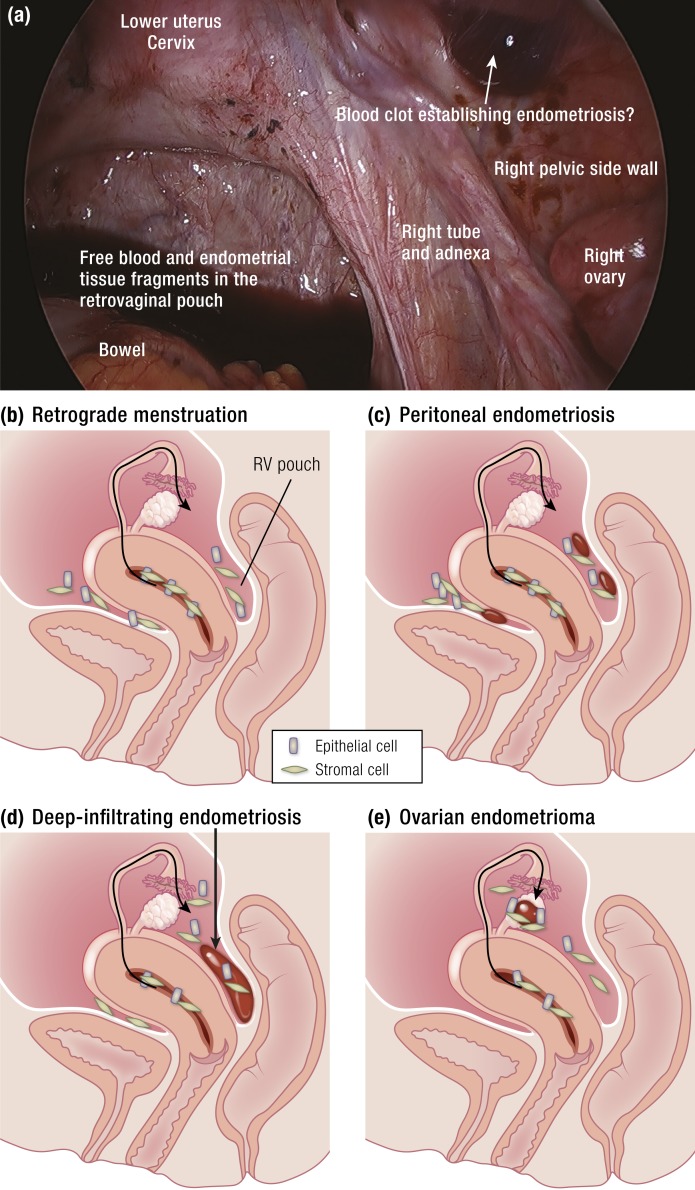
Figure 1.
(a) Laparoscopy of the pelvis performed at the time of menstruation. Predictable cyclic ovulatory menses giving rise to repetitious episodes of retrograde travel of endometrial tissue and blood into the dependent portions of the pelvic cavity is the main cause of pelvic endometriosis. Not all women who experience retrograde menstruation, however, develop endometriosis. This suggests that a number of differences between the patients with endometriosis and disease-free women may account for this condition. These include increased quantities of menstrual tissue that reach the abdominal cavity because of outflow track obstruction or deeper separation of the functionalis layer from the basalis layer (see Fig. 6) and cellular and molecular defects in eutopic endometrial or peritoneal tissues of women with endometriosis. (b) Graphic depiction of retrograde flow of endometrial tissue fragments made of spindly stromal and cuboidal epithelial cells. (c and d) Menstrual tissue fragments may survive and grow on peritoneal or subperitoneal locations (peritoneal endometriosis) or may get deposited into the rectovaginal (RV) pouch during repetitious episodes of menstruation and remodel the neighboring vaginal, rectal, and cervical tissues via a chronic inflammatory process to give rise to a deep-infiltrating RV nodule. (e) The endometrial tissue fragments may populate the exposed lining of a follicular or corpus luteum cyst to eventually evolve into an endometrioma. [Adapted with permission from Bulun SE. Endometriosis. In: Strauss J, Barbieri R, eds. Yen & Jaffe’s Reproductive Endocrinology. 8th ed. Philadelphia, PA: Elsevier; 2019:609–642. Copyright © 2019 by Elsevier.]
Salient medical features
Taking a broad perspective of endometriosis—from the molecular mechanisms to its impact on quality of life—is essential for optimizing management that will benefit patients suffering from this disease (2). The following are considered key features of endometriosis. (i) Intense primary dysmenorrhea (painful periods), uninterrupted and repetitious episodes of ovulation giving rise to heavy periods associated with retrograde menstruation, and pelvic endometriosis visualized by laparoscopy are linked and likely represent progressive stages of a common disorder (Fig. 1) (12). (ii) Debilitating menstrual pelvic pain first experienced during or soon after menarche gradually increases in severity as the inflammatory stimuli from pelvic disease persist and the peripheral and central nervous system are recurrently conditioned, leading to the phenomenon of central sensitization (9, 13). Patients with endometriosis experience chronic pain continually or intermittently until the menopause. (iii) All three forms of pelvic endometriosis, namely, peritoneal endometriotic implants, rectovaginal nodules, and ovarian endometriomas, are intimately linked with ovulatory menses and probably originate primarily from retrograde menstruation that follow ovulation (Fig. 2) (14). (iv) The current diagnostic standard, gross laparoscopic visualization of pelvic endometriosis, does not take into account the presence of microscopic inflammatory disease in the pelvic peritoneum or eutopic endometrial tissue, both of which may be the source of chronic pain that responds to suppression of ovulation or estrogen formation (15, 16). Thus, endometriosis should be a clinical diagnosis of exclusion and based on a detailed history and symptoms, as clinicians in practice primarily manage the symptoms of the disease. (v) Treatment-naive patients with endometriosis significantly benefit from surgical or medical management, which leads to substantial pain relief for practically all of these patients (17); however, the response rate progressively and sharply decreases with each treatment attempt (18, 19). Therefore, management of recurrent disease or its symptoms is the key clinical challenge in endometriosis (20). (vi) Long-term suppression of ovulatory menses is the mainstay of any successful management strategy for endometriosis-associated pelvic pain (2, 21). (vii) Epidemiologic studies have found significant associations between the prevalence of endometriosis and ovarian cancer (22). The direct observation of malignant transformation of endometriosis to an adenocarcinoma, however, has been reported only rarely (23). Currently, the potential for malignant transformation of endometriosis is not considered a significant factor in standard clinical decision-making, which should be revisited and updated in light of recently published data (24, 25).
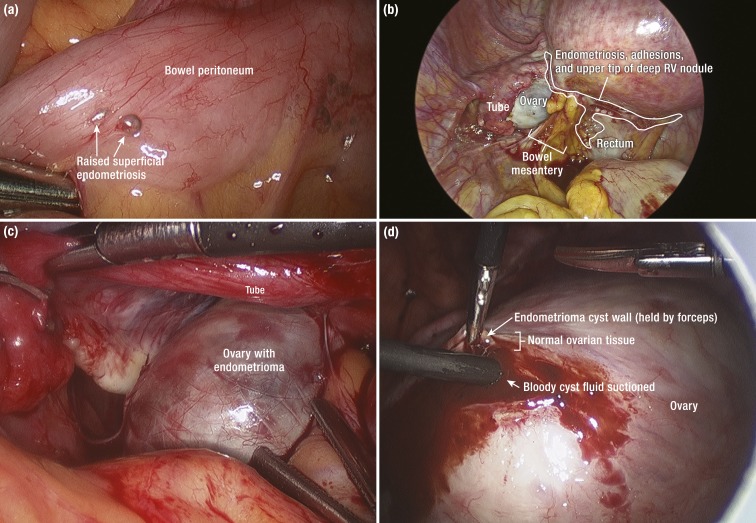
Figure 2.
Laparoscopic views of pelvic endometriosis. (a) A raised superficial endometriotic implant on bowel serosa [visceral peritoneum]. (b) Deep-infiltrating endometriosis. A laparoscopic image sometimes described as “frozen pelvis” because of extensive endometriosis and diffuse tissue remodeling causing dense adhesions between the ovary, bowel (rectum), and the uterine peritoneum. White vesicular endometriotic lesions are visible in the delineated area that represents the upper tip of diffuse adhesions caused by endometriosis. A challenging dissection into this plane will eventually take the surgeon into the previously existing rectovaginal (RV) space now harboring a nodule composed of endometriotic tissue and surrounding fibrosis and allow the removal of this RV nodule [see Fig. 4b]. (c) Enlarged left ovary because of a large endometrioma buried in the normal tissue [see (d) and Fig. 4c]. (d) Dissection into the overly stretched normal white-tan ovarian cortical tissue. The fibrotic endometrioma cyst wall is grasped by a forceps. The suction apparatus was inserted into the cyst lumen to remove the thick chocolaty fluid composed of blood products. The surgeon will develop a plane between the normal ovarian tissue and cyst wall in an attempt to remove the cyst in its entirety.
Clinically useful research findings
The following key observations and cellular and molecular characteristics of endometriosis are helpful starting points for interpreting more in-depth and complex mechanistic studies of the disease. (i) Menstruation that follows an ovulatory cycle is indispensably linked to the development of endometriosis, at least in its early stages (Fig. 3). Species that do not menstruate do not develop endometriosis, and women who never had ovulatory menses do not develop endometriosis. Interruption of ovulatory menses in women with endometriosis is usually therapeutic (Fig. 3) (1, 2). (ii) Pathology in various tissue types may contribute to the overall clinical picture of endometriosis. Distinct cellular and molecular abnormalities involving inflammation and steroid responsiveness have been well described at least in two types of tissues: eutopic (intrauterine) endometrium and ectopic endometriotic tissue (1). It is also plausible that defects in uterine spiral arteries and arterioles or pelvic peritoneal tissue may predispose certain women to endometriosis (26, 27). The list of affected tissues may grow as we understand more about endometriosis (28, 29). It follows that surgical removal of pelvic endometriotic tissue, even by the most capable surgeons, will not be curative. (iii) Most endometriotic implants are composed primarily of stromal cells and contain a small epithelial component that lacks the deep invaginations (glands) typically observed in the eutopic endometrium (Fig. 4). The endometriotic stromal cell is epigenetically misprogrammed and displays partial phenotypes of ovarian theca/granulosa cells and tissue macrophages. For example, endometriotic stromal cells express the full cascade of steroidogenic proteins and enzymes such as steroidogenic acute regulatory protein (STAR) and aromatase and convert the precursor molecule cholesterol to substantial quantities of progesterone and estradiol (1). The endometriotic stromal cell also expresses and secretes large amounts of immune molecules such as IL-1β, IL-6, TNF, regulated upon activation normal T cell expressed and secreted (RANTES), and monocyte chemoattractant protein-1 (MCP-1) (1, 30, 31). (iv) The importance of estrogen excess in endometriosis is somewhat similar to the role of insulin deficiency in diabetes or the metabolic syndrome (32–34). Estradiol is essential for endometrial tissue attachment to peritoneum; lesion survival; production of inflammatory substances such as metalloproteinases, cytokines, or prostaglandins and growth factors; and angiogenesis (Fig. 3) (35–38). Estrogen receptor (ER)β mediates the effects of estradiol in endometriosis and triggers pathways that enhance lesion survival, remodel pelvic peritoneal tissue, and produce inflammatory substances, which stimulate nociceptors in pelvic tissues, leading to pain (Fig. 3) (32, 34). Blocking estradiol production either naturally (menopause) or pharmacologically (ovarian suppression or aromatase inhibition) causes regression of the disease and its symptoms, including pain (Fig. 3) (36, 37, 39). (v) Endometriosis is a unique condition whereby progesterone resistance occurs owing to a deficiency of progesterone receptor (PR) in endometriotic stromal cells (Fig. 3) (40–42). Endometriotic stromal cells produce large amounts of progesterone locally; this is consistent with progesterone resistance resulting from PR deficiency (43). This characteristic also explains, in part, why endometriotic tissue responds poorly to progesterone or its agonists. Alternatively, synthetic antiprogestin compounds such as mifepristone and ulipristal acetate (UPA), which bind to low levels of PR with higher affinity, suppress endometriosis and reduce pain more effectively (44–46).
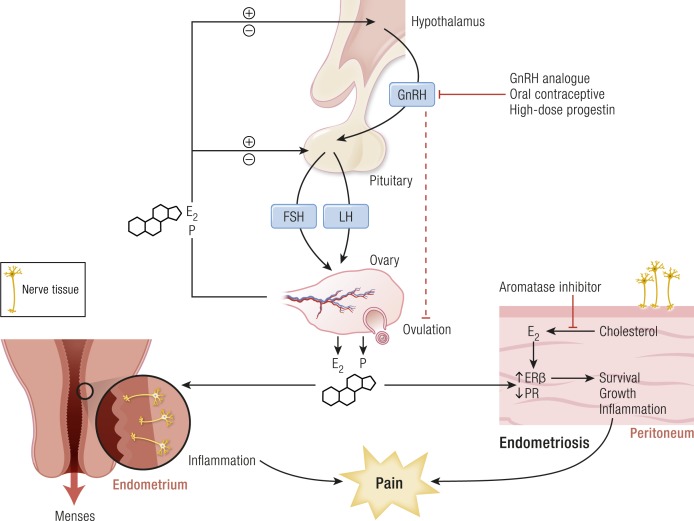
Figure 3.
The indispensable roles of ovulation and estrogen in endometriosis, highlighting the mechanisms of menstruation, tissue survival, inflammation and pain, and the targets of treatment in endometriosis. E2, estradiol; P, progesterone. [Adapted with permission from Bulun SE. Endometriosis. In: Strauss J, Barbieri R, eds. Yen & Jaffe’s Reproductive Endocrinology. 8th ed. Philadelphia, PA: Elsevier; 2019:609–642. Copyright © 2019 by Elsevier.]
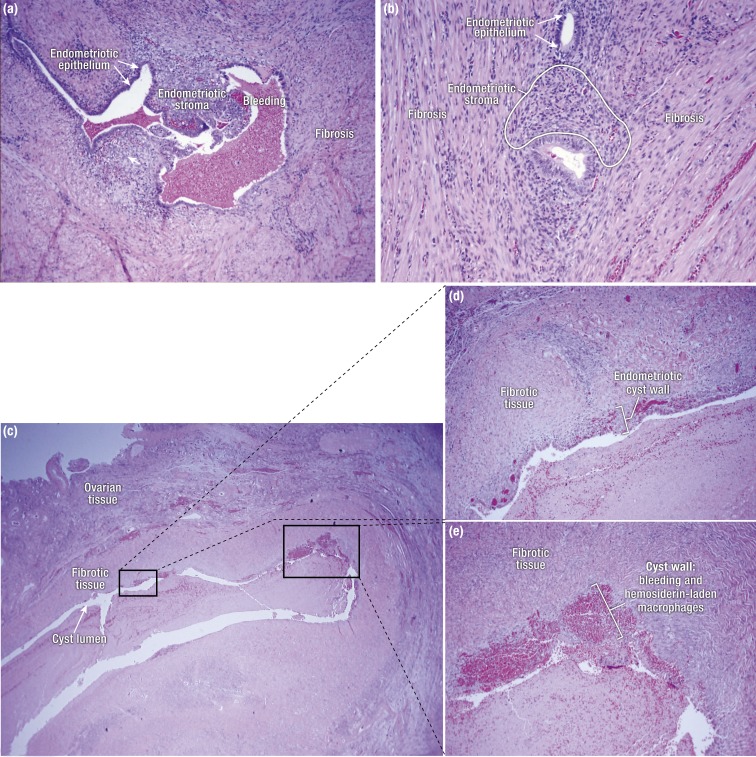
Figure 4.
(a) Peritoneal endometriosis with fibrosis. (b) Rectovaginal nodule with extensive fibrosis and tissue remodeling surrounding islands of endometriotic stroma and occasional epithelial cells. (c) Sections of an ovarian endometrioma cyst. Note that the thickness of the endometrial lining (stroma and luminal epithelium) varies throughout the cyst wall, with foci of bleeding and macrophages containing blood pigment. The cyst wall is primarily composed of fibrotic tissue. (d and e) Higher-magnification pictures showing details of the ovarian endometrioma cyst wall from (c).
Recently, three groups independently described mutations affecting PIK3CA, KRAS, ARID1A, and other cancer driver genes in the epithelial components of benign ovarian and extraovarian pelvic endometriosis tissue (24, 25, 47). Stunningly, the epithelial cells of clinically and histologically normal endometrial samples also contained mutations in mutant allele frequencies similar to those observed in endometriotic epithelium and some cancer types (25, 47). These well-characterized driver mutations have also been reported in clear cell and endometrioid-type ovarian cancers uniquely associated with endometriosis, as well as in other epithelial ovarian adenocarcinomas (48, 49). It appears that fragments of inflamed endometriotic stroma, together with mutated epithelium, may localize into ovarian inclusion cysts and transform to cancer, possibly because the unique microenvironment within the ovary becomes permissive to carcinogenesis (25, 47). Although the same phenomena and mutations are observed in extraovarian deep-infiltrating endometriosis, the risk of malignancy in such sites is negligible, as the extraovarian location is probably not supportive of malignant transformation (24). The significance of these epithelial cancer driver mutations in histologically normal-appearing and clinically disease-free eutopic endometrial tissue remains enigmatic.
Prevalence and distribution of endometriosis
Clinically, it is more meaningful to discuss the prevalence of endometriosis rather than its incidence because an average patient may suffer from the disease for prolonged periods of time—up to decades (12). The disease process can begin as early as at the time of first menses, and it may persist until after the menopause (12, 50). This natural history expands the pool of patients affected by endometriosis at any given time. An estimated 1 in 10 reproductive-age women—or ∼200 million women worldwide—may be suffering from endometriosis (51). In women undergoing abdominal surgery for chronic pelvic pain or infertility, endometriosis represents one of the most common pelvic pathologies (52, 53).
The most significant risk factor and cellular mechanism underlying pelvic endometriosis is retrograde menstruation (Fig. 1) (54–58). The risk of endometriosis appears to increase with a greater lifetime number of ovulatory cycles (Fig. 3) (59). The gradual but consistent trend toward more menstruations due to earlier ages of menarche and a progressively decreasing number of pregnancies per woman (which naturally disrupt ovulatory cycles) has increased the risk of implantation of retrograde-traveled endometrium on pelvic tissues (60–63). Repeated cycles of ovulation and the genesis of ovarian endometriosis have been linked, as endometriomas seem to develop from ovarian follicles (64) and by direct transition from hemorrhagic corpus luteum cysts, based on serial ultrasound examinations (Figs. 1 and 2) (14). It is quite likely that retrograde menstrual endometrial material trapped in a recently ruptured ovarian follicle gives rise to ovarian endometriomas (14). It was also suggested that endometrial fragments on the ovarian surface epithelium may get trapped in cortical invagination cysts and give rise to endometriomas; this possibility, however, was solely based on histopathological examinations but not on temporal evidence as in the case of the ovarian follicle hypothesis (14, 65).
Histopathology
As discussed above, the term endometriosis initially referred to a histologic diagnosis, but as the clinical picture has become clearer with recent clinical and molecular discoveries, the definition has evolved to describe a symptom complex that affects the pelvic tissues. Anatomically, pelvic endometriosis refers to the presence of endometrium-like tissue outside of the uterine cavity, usually on the pelvic peritoneal surfaces, in the ovary or on other pelvic tissues such as bowel (Fig. 1). Surgical pathologic diagnosis is made upon the identification of at least two of three key elements: (i) endometrial stromal cells, (ii) endometrial epithelial cells, and (iii) signs of chronic bleeding in or adjacent to endometrium-like tissue, including collections of red blood cells and hemosiderin-laden macrophages (immune cells that engulf blood pigment, Fig. 4). Fibrosis comprised of fibroblasts and extracellular matrix is commonly observed surrounding endometriotic implants and possibly represents extensive inflammation and tissue remodeling (Fig. 4). From a surgical perspective, there are three major forms of pelvic endometriosis: (i) peritoneal endometriosis found on uterine serosa or peritoneal or subperitoneal tissue in pelvic side walls (Fig. 4a), (ii) deep infiltrating endometriosis that involves substantial fibrosis and surrounding tissue remodeling and is usually found in the pouch between the vagina and the rectum, that is, rectovaginal nodule (Fig. 4b), and (iii) ovarian endometrioma, an ovarian cyst with a wall that is lined up with endometrial tissue and contains substantial amounts of clotted and unclotted blood products in its lumen (Fig. 4c–4e).
Mechanisms of Endometriosis
Tissues that contribute to endometriosis
Several mechanisms have been proposed regarding the histologic origins of endometriosis. Sampson (6) and others postulated that fragments of menstrual endometrium pass retrograde through the fallopian tubes, then implant and persist on peritoneal surfaces (Fig. 2). This has been demonstrated in primate models and observed naturally in humans; it is also supported by the observation that spontaneous endometriosis occurs exclusively in species that menstruate, that is, female primates, including humans (66). Young women with a transverse vaginal septum or imperforate hymen that blocks the expulsion of menstrual tissue from the vagina almost invariably develop pelvic endometriosis (1). An alternative hypothesis is that the peritoneum, derived from the coelomic epithelium, undergoes metaplasia to differentiate into islands of endometriotic lesions within the peritoneal cavity (67). The defenders of yet another hypothesis suggest that menstrual tissue from the endometrial cavity reaches pelvic or other distant body sites via veins or lymphatic vessels (68). Additionally, it has been proposed that circulating blood cells originating from bone marrow differentiate into endometriotic tissue at various body sites (69). Distant organ endometriosis such as lung endometriosis is very rarely encountered and may be explained by vascular spread. However, it is highly challenging to construct clinically relevant models to support or refute the mechanisms proposed as alternatives to Sampson’s retrograde menstruation theory. Moreover, most molecular and clinical data accumulated during the past two decades support Sampson’s postulate as the main mechanism for pelvic endometriosis (1, 70). Thus, this review centers on Sampson’s retrograde menstruation hypothesis and considers the eutopic endometrium as the key tissue source for the genesis of pelvic endometriosis.
Intrauterine (eutopic) endometrium
Retrograde flow followed by implantation of intrauterine endometrial tissue is the most plausible mechanism for most pelvic endometriotic lesions (Fig. 2) (71). Reflux menstruation of endometrial tissue mixed with blood is routinely observed in almost all ovulatory women, but only ∼10% of these women develop the symptom complex of endometriosis. Several explanations have been offered for how refluxed endometrium to the peritoneal surface successfully implants and survives in the long term in a limited population of women (71). (i) The mucosal surfaces of the uterus, that is, eutopic endometrium and the tubal mucosa, in women with endometriosis display a number of cellular and molecular abnormalities. Molecular defects have been reported in eutopic endometrial tissues of women with endometriosis, such as activation of oncogenic pathways or biosynthetic cascades that favor increased production of estrogen, cytokines, prostaglandins, and metalloproteinases (Fig. 5) (28, 30, 72–75). Upon the attachment of these defective tissues to the peritoneum or the ovary, the magnitude of these abnormalities is amplified drastically to enhance their implantation and longevity (76). (ii) Compared with endometriosis-free women, women with endometriosis may simply have overwhelmingly higher numbers of molecularly unremarkable endometrial tissue, including somatic stem cells, that arrive at the pelvic abdominal cavity during each menstrual episode (Fig. 5). Several groups proposed that endometrial somatic stem cells may be involved in the pathogenesis of premenarchal and adolescent endometriosis through retrograde neonatal uterine bleeding due to maternal progesterone withdrawal at birth (77–79). These endometrial somatic stem cells would remain dormant beneath the peritoneum until increasing estradiol levels before menarche activate them to initiate clonal growths of ectopic endometrium in the peritoneal cavity (8).
Figure 5.
Overview of the complex roles of retrograde menstruation, epigenetically defective endometrial stromal cells, the epithelial cells carrying mutations, DNA methylation, nuclear receptors, and inflammation in endometriosis. E2, estradiol. Endometrial tissue fragments may implant on pelvic peritoneal tissue surfaces or may get trapped in an ovarian inclusion cyst such as a hemorrhagic corpus luteum cyst.
It has also been suggested that a defective immune system might fail to clear implants off the peritoneal surface (72, 80, 81). One can further imagine that defective spiral arterioles may lead to hypermenorrhea or other menstrual abnormalities and contribute to a phenotype that favors endometriosis (27). It is quite likely that a whole variety of cellular and molecular abnormalities in the eutopic endometrium or elsewhere may lead to the common phenotype of endometriosis together with its symptom complex.
Cellular and molecular aberrations in eutopic endometrium.
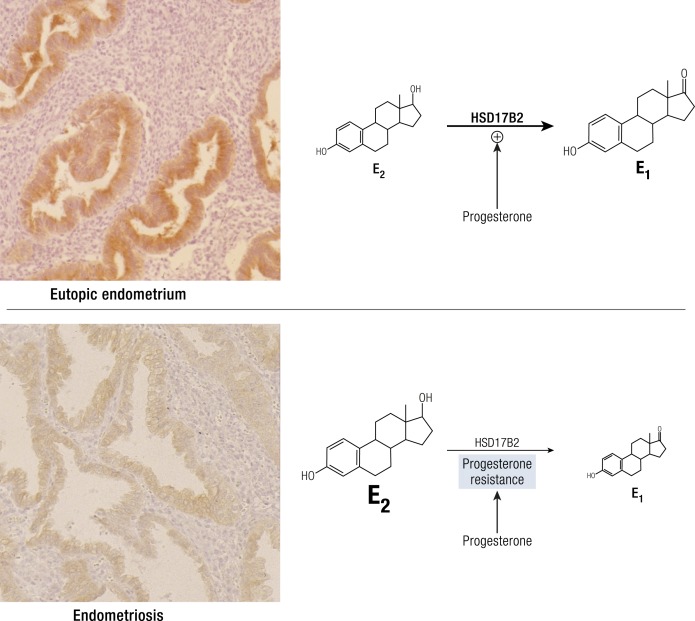
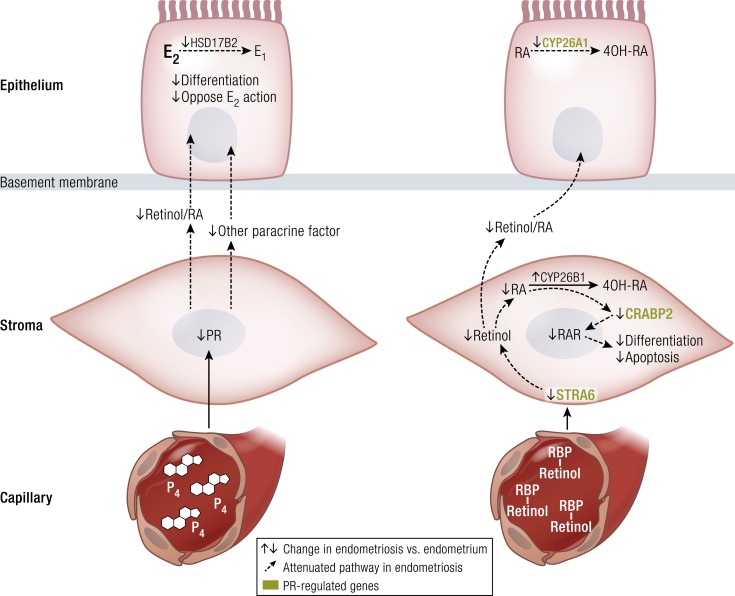
Eutopic endometrium of women with endometriosis looks identical to that of disease-free controls under routine microscopic examination via hematoxylin and eosin staining. However, substantial clinical and molecular data support the existence of cellular and molecular differences in eutopic endometrial tissue of patients with endometriosis (Fig. 5) (28). Stromal cells of eutopic endometrium of women with endometriosis express strikingly higher levels of aromatase, cyclooxygenase (COX)2, and IL-6 mRNA and protein and display readily detectable levels of aromatase enzyme activity that catalyzes the conversion of androgens to estrogens (Fig. 5) (3, 4, 30, 82). Transcriptomic analysis of eutopic endometrium from women with or without endometriosis confirmed these earlier observations and found dysregulation of selected genes that contribute to implantation, including embryonic attachment, embryo toxicity, immune dysfunction, and apoptotic responses, as well as genes that likely contribute to the pathogenesis of endometriosis, including aromatase, PR, and angiogenic factors (Fig. 5) (28).
Nerve tissue has been immunohistochemically identified in the functional layer of eutopic endometrial tissue in all women with endometriosis but not in the eutopic endometrium of disease-free women (Fig. 3) (83). The pathologic presence of nerve fibers points to a general defect in the differentiation of somatic stem cells in the endometrium of patients with endometriosis. This may have important implications for understanding the generation of pain in these patients. For example, this may explain why patients with endometriosis continue to experience pain after surgical resection of ectopic implants (83). These nerve fibers may be directly involved in transmitting pain both in eutopic or ectopic locations (83).
Progenitor/stem cells
In ovulatory women, the progenitor/stem cell populations in the basalis layer of endometrium regenerates the full thickness mucosa every month within days under the influence of estrogen (Fig. 6). Thus, stem cell activity is extraordinarily important for endometrial function. Mesenchymal (stromal) stem cells isolated from endometrial tissue have similar properties to bone marrow mesenchymal stem cells (8). Selective markers for mesenchymal stem cell enrichment, CD146, β-type platelet-derived growth factor receptor (PDGFRB), and sushi domain containing-2 (SUSD2), reveal a perivascular location of mesenchymal stem cells and suggest a pericyte identity of these cells in blood vessels of the endometrium (8). Molecular analyses of SUSD2-positive cells demonstrate their phenotype as perivascular cells. Endometrial stromal cells or stromal cells isolated from menstrual blood also display mesenchymal stem cell characteristics and demonstrate broad differentiation potential for mesodermal, endodermal, and ectodermal lineages, indicating their plasticity and multipotency (8). A recent study showed that, in contrast to endometrial mesenchymal stem cells from disease-free patients, eutopic endometrial mesenchymal stem cells isolated from patients with endometriosis did not differentiate in vitro to stromal cells that decidualize properly (84). This suggested that the observed proinflammatory and progesterone-resistant gene expression signature in eutopic endometrial stromal tissue from patients with endometriosis originates from defective endometrial mesenchymal stem cells (Figs. 5 and 6). Immunohistochemical analyses of endometrial tissues of patients undergoing bone marrow transplants has also suggested that bone marrow cells may contribute small populations of endometrial cell types (85). It is conceivable that these circulating stem cells may be involved in rare cases of extraperitoneal lesions (86).
Figure 6.
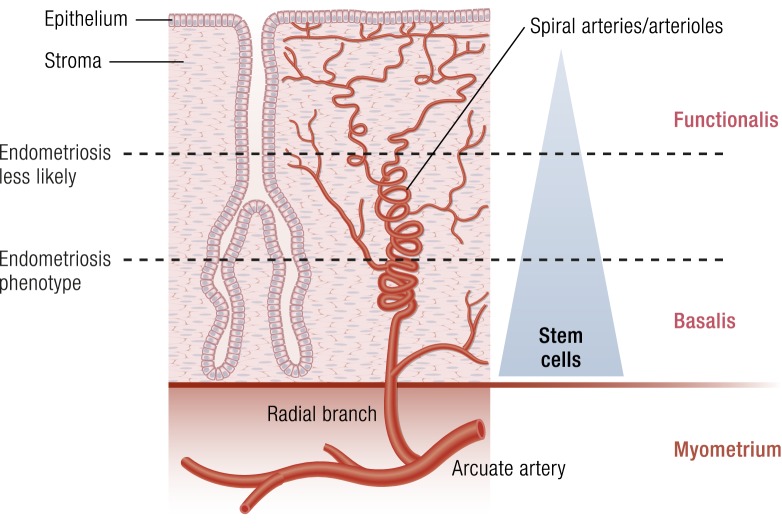
Possible interactions between spiral arterioles, somatic stem cells, and menstruation and the risk of endometriosis. Menstruation occurs after the functionalis layer of endometrium separates from the basalis and is expelled through the cervix or uterine tubes. This separation occurs in association with vasoconstriction and coagulation in the spiral arterioles giving rise to degradation of the extracellular matrix, hypoxia, and necrosis in the separated segment. The basalis layer may contain higher numbers of functional progenitor cells that will regenerate the functionalis layer during the next proliferative phase under the influence of estrogen. It is plausible to hypothesize that the separation between the two layers at a relatively superficial plane may lessen the likelihood of heavy bleeding or travel of stem cells into the pelvic cavity. Separation at a deeper plane, however, may favor the endometriosis phenotype. One can envision, therefore, that possible epigenetic abnormalities in the vascular system may affect the process of separation between the functionalis and basalis and thus affect the risk for endometriosis. The somatic stem cells per se may also be defective, or simply larger numbers of otherwise normal stem cells that travel into the abdominal cavity may increase the risk for endometriosis. (Adapted with permission from Bulun SE. Endometriosis. In: Strauss J, Barbieri R, eds. Yen & Jaffe’s Reproductive Endocrinology. 8th ed. Philadelphia, PA: Elsevier; 2019:609–642. Copyright © 2019 by Elsevier.)
The discovery of markers for endometrial stem/progenitor cells has enabled the examination of their role in endometriosis, adenomyosis, and healthy endometrial development for implantation (87). However, we are still far from establishing clinically relevant experimental models of human endometrial stem cells that regenerate endometrial tissue in response to estrogen as during the menstrual cycle (88). Together with the DNA methylation defects observed in eutopic endometrium of patients with endometriosis, these findings substantiate the extraordinarily important and interactive roles of epigenomic mechanisms and stem cells in the pathophysiology of endometriosis (Figs. 5 and 6) (89).
Uterine vasculature
Endometrial vasculature, in particular, the spiral arterioles play a critical role in the process of menstruation (27). Upon the withdrawal of progesterone and estrogen during the late luteal phase of a nonpregnant woman, these vessels undergo acute vasoconstriction and intravascular coagulation accompanied by inflammatory cell infiltration and degradation of extracellular matrix in adjacent endometrial tissue. This leads to shedding and expulsion of the top endometrial layer named functionalis along with blood. It was proposed that the spiral arteries and arterioles may be molecularly different in the uteri of women with endometriosis compared with those in disease-free women (Fig. 6) (27). Owing to these differences, it is plausible that the process of separation of the functionalis layer from the basalis layer may occur at a deeper plane in women with endometriosis and heavy menses (Fig. 6). Because endometrial somatic stem cells may likely be concentrated in the deeper (basalis) portion of this tissue, this may lead to an higher influx of stem-like cells into the lower abdominal cavity via retrograde menstruation in women with endometriosis.
Peritoneum
Theoretically, the formation of peritoneal endometriosis requires the initial adhesion of shed endometrial cells to peritoneal and subperitoneal surfaces. From a mechanistic perspective, this involves the expression of extracellular matrix adhesion molecules and their coreceptors (90). Circumstantial and experimental observations suggest that the process of implant attachment to the peritoneal surface and its eventual establishment into the subperitoneal space through breaks in the mesothelial cell layer happens over a short period of time (Fig. 5) (90). Thus, a mesothelial surface with physical and temporal “windows” that facilitate the implantation and survival of endometrial tissue fragments may be essential for the formation of peritoneal endometriosis.
In contrast to the implantation theory, the metaplasia theory suggests that the coelomic epithelium pathologically differentiates into endometrium-like tissue to give rise to peritoneal endometriosis (91). The idea of metaplasia is plausible, because cells from both the peritoneum and endometrium derive from a common embryologic precursor called the coelomic epithelium. However, it is challenging to demonstrate this potential mechanism experimentally. Although overwhelming experimental data that support the implantation of retrograde menstrual material have accumulated, this is not the case for the metaplasia theory (1). Even so, the pelvic peritoneum and its mesothelial surface seem to contribute critically to the establishment of endometriosis significantly (1).
Key biological processes in endometriosis
Cell fate in endometriotic tissue has been studied primarily at the level of the endometriotic stromal cell, which dominates the lesion in terms of quantity and exhibits most of the key and therapeutically targetable molecular abnormalities of endometriosis, such as estradiol production, progesterone resistance, cytokine production, and prostaglandin production (Fig. 5) (1). Endometrial or endometriotic stromal cells can be maintained in primary culture to study their biology, whereas this is more challenging with epithelial cells (4, 30). This technical limitation has led to a disproportionate number of mechanistic molecular studies that employ endometrial or endometriotic stromal cells, which may create a scientific bias against the potential roles of other cell types. Until very recently, relatively little has been known about the endometriotic epithelial cell (24, 25, 47). Although no somatic mutations have been found in endometriotic stromal cells (24), a stunningly high number of somatic mutations in cancer driver genes have been recently discovered in the epithelial cells of both clinically and histologically normal eutopic endometrium as well as in benign pelvic endometriotic lesions (24, 25, 47). The effects of these mutations on epithelial cell fate, the neighboring stromal cells, and endometriotic tissue as a whole remain to be determined. Somatic epithelial mutations in endometriosis are discussed in greater detail below. The following biologic processes pertain to endometriotic stromal and epithelial cells.
Reduced apoptosis
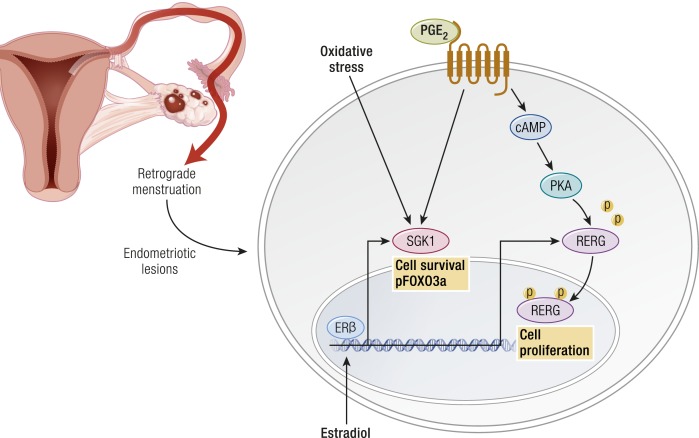
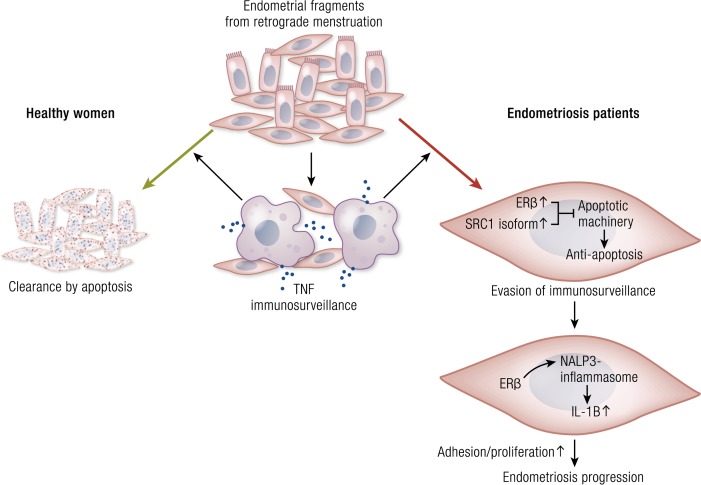
Apoptosis is significantly decreased in endometriotic stromal and epithelial cells compared with eutopic endometrial tissues (92, 93). This may be linked to pathologic levels of local estradiol biosynthesis (1). ERβ mediates the antiapoptotic effects of estradiol in endometriotic stromal cells (33).
Defective differentiation
A critical observation in endometriosis is deficient differentiation (decidualization) of the stromal cell (29, 94, 95), which has been linked to progesterone resistance (40, 42). Although the first molecular evidence of progesterone resistance was observed in the endometriotic epithelial cell, deficient stromal decidualization was eventually found to be the primary mechanism (41, 42). Epigenetically misprogrammed mesenchymal stem cells in endometriosis also drives deficient differentiation (8, 84).
Inflammation
Inflammation is the central process in endometriosis. It leads to pain, remodeling of neighboring tissues, fibrosis, adhesion formation, and infertility. The production of cytokines and prostaglandins and infiltration of immune cells are some of the hallmarks of inflammation (96). The endometriotic stromal cell is one of the major sources of cytokines and prostaglandins (30, 82). Recurrent episodes of bleeding and an attempt by macrophages to remove blood pigments also contribute to the inflammatory process and adhesion formation (Fig. 1b). Inflammatory processes are thought to be induced by estradiol and mediated by ERβ in endometriosis, as denial of ovarian or local estradiol to endometriotic tissue using a GnRH analog and/or an aromatase inhibitor stops or decreases pain (Fig. 3) (33, 34, 36, 39, 97).
Proliferation
In eutopic endometrial tissue, the epithelial cell proliferates under the influence of estrogen (41). The strikingly rapid rate of proliferation possibly contributes to the acquisition of somatic epithelial mutations in eutopic endometrial tissue (25). Most endometriotic implants, however, contain scant epithelium and are comprised primarily of stromal cells (Fig. 4). In contrast to an epithelial cell, human mesenchymal cells are not epigenetically programmed to proliferate extensively, nor are they prone to accumulate mutations. Therefore, endometrial or endometriotic stromal cells show limited proliferative activity. In fact, significantly less proliferative activity was reported in endometriotic tissue compared with eutopic endometrial tissue (93). One exception is in abdominal wall endometriosis. Endometriotic lesions seem to grow quite extensively in the subcutaneous adipose tissue in the abdominal wall (10), likely driven by significant proliferative activity. It is tempting to hypothesize that aromatase activity in adipose fibroblasts in subcutaneous fat tissue may contribute to this process (98). Importantly, note that aromatase activity in human subcutaneous adipose tissue is significantly higher than that in the omentum or visceral (e.g., subperitoneal) adipose tissue (99).
Angiogenesis
Formation of blood vessels and their steroid hormone–dependent function are routinely observed in endometriotic lesions. In fact, recurrent and chronic bleeding evident by hemosiderin-laden macrophages is a unique histologic feature of endometriosis (Fig. 4). Cytokines such as IL-17A may enhance angiogenesis in the peritoneal cavity for the establishment and growth of endometriotic lesions (100). Moreover, an ERβ-selective estradiol agonist stimulated the expression of SLIT3 that may play a key role in enhancing angiogenesis in endometriosis (101).
Tissue remodeling
Some survival capability seems to be necessary for the initial attachment of endometrial tissue fragments to pelvic peritoneum and the establishment of subperitoneal endometriotic lesions (90). Once the initial implantation of the endometrial tissue fragment occurs, limited proliferation and tissue growth may be necessary for the long-term survival of the tissue. Under the influence of estrogen, inflammation ensues and causes significant remodeling of the peritoneal and subperitoneal tissues, such as adipose tissue (Fig. 1B and Fig. 4). One of the best studied pathways that mediates surrounding tissue remodeling involves high metalloproteinase activity in endometriotic implants (35). In fact, fibrosis of the surrounding tissue is a hallmark of peritoneal or ovarian endometriosis (Fig. 4) (102). Invasion and proliferation, however, are much less important to the pathology of endometriosis than they are to the pathology of malignant (e.g., endometrial cancer) or benign (e.g., uterine leiomyoma) neoplasms (41). Estrogen-driven inflammation seems to be the central process that shapes the pathology of endometriosis.
Genomic alterations
Genomic alterations affect the structure and function of the complete set of DNA including all of its genes and noncoding intergenic regions. Genomic mechanisms affect the regulation of all genes, which direct the production of proteins with the assistance of epigenomic modifications (see below). Proteins make up cells and tissues, regulate chemical reactions, and carry signals between cells. Recent advances in genomics have revolutionized how we approach complex systems in health and disease. Intensive efforts have been made to understand genome-wide mechanisms in endometriosis. Selected studies are highlighted below.
Inheritance
Endometriosis as a phenotype seems to be transmitted in families in a polygenic manner (103). In one study, the mothers and sisters of women with severe endometriosis had a sevenfold higher likelihood of suffering from endometriosis compared with primary female relatives of their partners (103). Consistent with this observation is the finding that familial cases of endometriosis tend to be more severe and have an earlier onset of symptoms than do sporadic cases (104). Based on a study of 3096 twins, the heritability of endometriosis, or the proportion of disease variance due to genetic factors, has been estimated at ∼52% (105). These studies of familial endometriosis point to genetic transmission of the disease. The mode of transmission, however, seems to be complex, as in other chronic inflammatory conditions such as inflammatory bowel disease.
Candidate gene studies
Since the 1990s, numerous large-scale international studies have tried to identify candidate genes that lead to the endometriosis phenotype. A number of “off-the-shelf” candidate gene loci have been interrogated based on their biological plausibility and possible connection with endometriosis, but these studies were negative (106, 107). Case-control genetic association studies, performed primarily to find polymorphisms, also failed to discover any function-altering genetic variants in familial endometriosis (108–110). Geneticists have cited the following factors for the failure of candidate gene studies to ascertain the genetic basis of complex inflammatory diseases such as endometriosis. (i) The underlying biological hypothesis may not be valid. (ii) These studies typically investigate a limited number of genes in a potentially important biological pathway. (iii) A limited number of variants in a gene are tested. (iv) Cases (endometriosis) and controls (endometriosis-free) may not be optimally defined. (v) Sample sizes may not be sufficient to detect the effect sizes that are expected for variants influencing a complex trait (111). The key reason for the lack of meaningful results, however, could be as simple as the actual absence of any classically defined germ cell mutations that cause the endometriosis phenotype.
Genetic linkage analysis
Using genetic linkage analysis, an international study group analyzed 1176 affected sister-pair families and found evidence of significant linkage of endometriosis to chromosome 10q26 (112). Subsequent fine-mapping association analyses of chromosome 10q26 suggested a possible association of common variants near the CYP2C19 gene, but mutations that could explain the linkage signal could not be demonstrated (113, 114). Note that CYP2C19 encodes an important drug-metabolizing enzyme involved in the biotransformation of proton pump inhibitors and antidepressants (115). Estradiol via ERα inhibits CYP2C19 expression (115). The same group later identified significant linkage to chromosome 7p13–15 in a subanalysis of 248 families with more than three affected members, suggesting that the presence of one or more rare variants confers high risk; however, no mutations could be identified (110, 116). To date, family-based or case-control genetic association studies have not led to any biologically meaningful results.
Genome-wide association studies in endometriosis
Genome-wide association studies (GWASs) typically focus on associations between single-nucleotide polymorphisms (SNPs) and traits such as major human diseases and do not require the use of pedigrees. Since 2010, a number of widely acknowledged endometriosis GWASs have been published; these studies employed data sets involving thousands of endometriosis cases and controls, primarily women of Japanese or of European ancestry (113, 114, 117). Meta-analyses of these GWAS data sets confirmed their findings and revealed additional loci (118). Many GWAS-identified loci were reported in women with endometriosis. A subset of these is discussed below.
Endometriosis GWASs collectively provided significant evidence for association of loci near: cyclin-dependent kinase inhibitor 2B antisense RNA (CDKN2B-AS1), an intergenic region on chromosome 7p15.2, wingless-type MMTV integration site family member-4 (WNT4), vezatin (VEZT), an intergenic region on 2p14, and inhibitor of DNA binding-4 (ID4) (119). Some of these loci had stronger effect sizes among American Society of Reproductive Medicine advanced stage (III to IV) cases, implying that they are likely to be implicated in the development of moderate to severe, or ovarian, disease (119). Results from follow-up papers have either confirmed or questioned these findings and highlight the heterogeneity in the genetic loci that underlie endometriosis in different populations, even when sampled from women with similar ethnic ancestry (119). Despite these large-scale and expensive studies, no mutations affecting these genes in familial cases of endometriosis were reported. To date, these studies have not resulted in the identification of any therapeutically targetable molecules or gene products.
Meta-analyses of endometriosis GWASs also identified loci containing genes involved in ovarian steroid hormone pathways: growth regulation by estrogen in breast cancer-1 (GREB1), coiled-coil domain containing 170 (CCDC170), ERα (ESR1), spectrin repeat containing, nuclear envelope-1 (SYNE1), and FSH β-subunit (FSHB) (118, 120). The roles of FSHB and ERα in ovarian function and estrogen action are well known (121). The product of the estradiol/ERα target gene GREB1 increases proliferation and extracellular matrix formation by ovarian cancer cells (122). GREB1 is expressed in 75% to 85% of serous, endometrioid, mucinous, and clear cell carcinomas. Serous, endometrioid, and mucinous ovarian cancers are almost always positive for either ESR1 or GREB1, suggesting a possible reliance on signaling through ESR1 and/or GREB1 (122). In breast cancer, recurrent rearrangements are seen between ESR1 and its neighbor CCDC170, which are enriched in the more aggressive and endocrine-resistant luminal-B tumors (123). GWASs also demonstrated a critical missense SNP located 19 kb downstream of ESR1 in another neighboring gene, SYNE1 (124). SYNE1 is involved in nuclear organization and structural integrity, function of the Golgi apparatus, and cytokinesis (124). An isoform encoded by SYNE1 is downregulated in ovarian and other cancers; this SNP was genotyped in patients with serous and mucinous ovarian cancers (124).
Some of these loci have stronger effect sizes among advanced stage (III to IV) endometriosis, implying that they are likely to be implicated in the development of moderate to severe ovarian disease (119). Despite these large-scale and expensive genetic linkage studies or GWASs, no germline mutations affecting these genes in familial cases of endometriosis have been reported. In summary, endometriosis GWASs identified genes associated with uterine development and stem cell function (WNT4), ovulatory function (FSHB, ESR1), and estrogen action (ESR1, GREB1, CCDC170, SYNE1, CYP2C19) (115, 122, 123); notably, most of these genes also have associations with breast or ovarian cancer (122–124). As the mechanistic link between endometriosis and ovarian cancer becomes more clear, the roles of these genes with SNPs may become more apparent.
Somatic epithelial mutations in endometrium and endometriosis
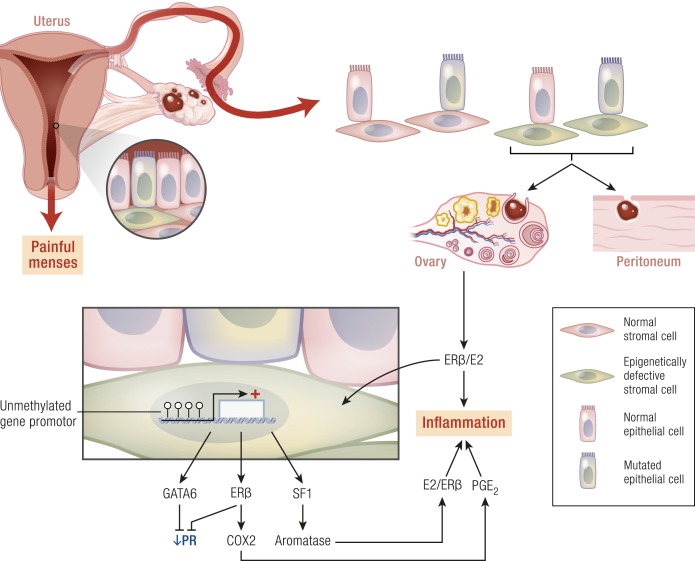
Recently, a number of exome-wide sequencing studies reported somatic epithelial mutations in ovarian endometriomas and nonovarian deep-infiltrating endometriosis as well as in eutopic endometrium (Figs. 5 and 7) (24, 25, 47). In 2014, a group of investigators from China was the first to report a large number (thousands) of single nucleotide variants in laser-microdissected epithelial cells from ovarian endometriosis and matched eutopic endometrial epithelial cells and in endometrial epithelium from endometriosis-free women (47). Most nonsynonymous and synonymous single-nucleotide variants were commonly present in both ectopic and matched eutopic endometrial cells and associated with genes involved in chromatin remodeling and cell adhesion (47). These authors did not particularly emphasize any ovarian cancer driver genes, but they focused on the possible patterns of mutagenesis at the base pair level (47). Surprisingly, they observed a large number of nonsynonymous single-nucleotide variants predicted to alter protein structure in endometrial epithelial cells from endometriosis-free controls (Fig. 5) (47). The numbers of mutations were comparable to those found in common human malignancies (47).
Figure 7.
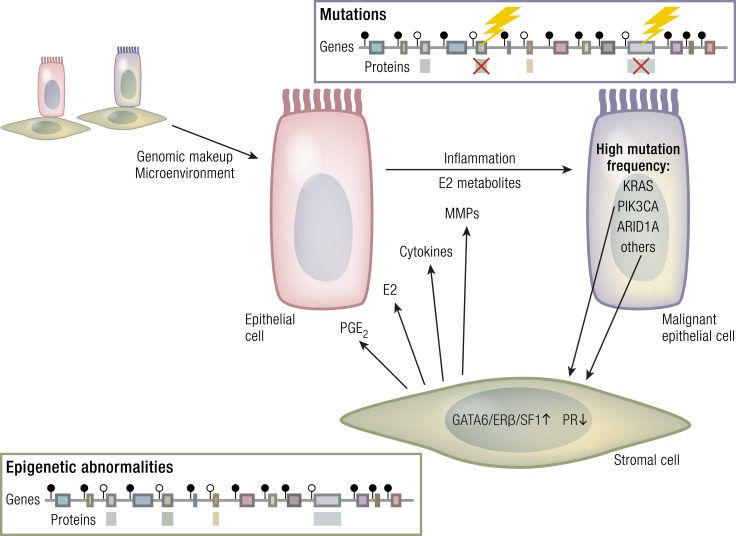
Roles of endometriotic cell types with distinct abnormalities. Endometriotic stromal cells do not contain nonsynonymous base pair alterations (mutations) but display extensive epigenetic defects that regulate the expression or silencing of genes. Specific patterns of DNA methylation (●) or demethylation (○) give rise to the suppression or overproduction of specific proteins. In endometriotic stromal cells, GATA6, ERβ, and SF1 proteins are overproduced, whereas PR is suppressed, leading to progesterone resistance. These changes collectively cause the accumulation of inflammatory and tissue-remodeling substances, including PGE2, E2, cytokines, and matrix metalloproteinases (MMPs). In contrast, portions of the eutopic endometrial or endometriotic epithelial cells accumulate tumor driver mutations  that disrupt or change the function of critical proteins, including Kirsten rat sarcoma homolog (KRAS), phosphatidylinositol-3-kinase, catalytic α polypeptide (PIK3CA), AT-rich interactive domain-containing protein 1A (ARID1A), and numerous other oncogenes or tumor suppressors. Extraordinarily high concentrations of E2 and its metabolites in the ovary and stroma-derived inflammation may contribute to the accumulation of epithelial mutations. [Chronic inflammation is suspected to cause mutagenesis and carcinogenesis in other tissues such as Barrett’s esophagus and esophageal cancer (208).] The relative contributions of endometriotic stromal vs epithelial cells to the development of pelvic endometriosis are currently unknown. The effects of each cell type on the acquisition of genome-wide epigenetic defects or mutagenesis are also not known.
that disrupt or change the function of critical proteins, including Kirsten rat sarcoma homolog (KRAS), phosphatidylinositol-3-kinase, catalytic α polypeptide (PIK3CA), AT-rich interactive domain-containing protein 1A (ARID1A), and numerous other oncogenes or tumor suppressors. Extraordinarily high concentrations of E2 and its metabolites in the ovary and stroma-derived inflammation may contribute to the accumulation of epithelial mutations. [Chronic inflammation is suspected to cause mutagenesis and carcinogenesis in other tissues such as Barrett’s esophagus and esophageal cancer (208).] The relative contributions of endometriotic stromal vs epithelial cells to the development of pelvic endometriosis are currently unknown. The effects of each cell type on the acquisition of genome-wide epigenetic defects or mutagenesis are also not known.
A Japanese study published in 2018 examined laser-microdissected cells from >100 ovarian endometriomas and a large number of control eutopic endometria (Fig. 7) (25). In endometriotic and normal endometrial epithelial cells, numerous somatic mutations were identified within genes frequently mutated in endometriosis-associated epithelial ovarian cancers. PIK3CA and KRAS were found to be the most frequently mutated genes in ovarian endometriotic epithelial cells (Fig. 7). In particular, there was a significantly higher mutant allele frequency for KRAS (Fig. 7) (25). An exception was ARID1A, a gene frequently mutated in ovarian clear cell cancer, a malignancy epidemiologically associated with pelvic endometriosis (Fig. 7) (48). Although ARID1A mutations were frequently detected in endometriotic epithelium, they were absent or very rare in endometrial epithelium (25). A detailed sequencing analysis of >100 single intrauterine endometrial glands (deeply invaginated epithelial cells) demonstrated that each gland carried distinct cancer-associated mutations, suggesting the heterogeneity of the genomic architecture of endometrial epithelial cells (25). It was proposed that significant increases in mutant allele frequency in cancer-associated genes in endometriotic epithelium are consistent with retrograde flow of endometrial cells already harboring cancer-associated mutations. If these tissue fragments implant at ectopic anatomic locations with selective survival-supporting advantages, this may increase the risk for the development of endometriosis and also malignant transformation (Figs. 5 and 7) (25). It follows that the ovary, particularly a hemorrhagic corpus luteum cyst, is fertile ground for the development of endometriosis and its malignant transformation. The ovarian microenvironment, with massive levels of estrogen and possibly other mitogens of cancer cells, may contribute to this phenomenon.
“Despite these large-scale and expensive studies, no mutations affecting these genes in familial cases of endometriosis were reported.”
Another recent American exome-sequencing study, published in 2017, targeted extraovarian deep-infiltrating endometriotic tissue and examined both epithelial and stromal components of endometriotic tissues (24). No mutations were detected in endometriotic or endometrial stromal cells (Fig. 7) (24). Whole-exome sequencing of epithelial cells of extraovarian endometriosis demonstrated somatic mutations in ∼80% of patients (Fig. 7). The mutant allele frequencies of somatic mutations were quite low, however, at <20%. This suggests that that only a subgroup of endometriotic epithelial cells had mutations (24). Although 26% of endometriotic tissues carried known ovarian cancer driver mutations in ARID1A, PIK3CA, KRAS, or PPP2R1A, it was highly improbable that these driver mutations occurred at this rate incidentally (P = 0.001; Fig. 7) (24). A KRAS mutation was detected in 4% to 38% of epithelial cells in each endometriotic lesion, as shown by droplet digital PCR analysis (24). KRAS polymorphisms reported in patients with endometriosis further support a role of this gene (125). Because the risk of cancer in extraovarian endometriosis is negligible, the clinical significance of these sporadic epithelial mutations in deep-infiltrating endometriosis remains to be determined (24). Similar driver mutations in the epithelial cells of ovarian endometriomas, however, may play key roles in initiating clear cell, endometrioid cell, or possibly other types of ovarian adenocarcinomas (Fig. 7) (24). Additionally, the epithelial mutations in extraovarian deep-infiltrating endometriosis may play critical roles in the implantation and establishment of endometriotic lesions in these locations, although they may not become malignant.
Epigenomic alterations
Epigenomic mechanisms affect all genes and intergenic regions in DNA, which are packaged together with proteins (e.g., histones) into a structure known as chromatin. Epigenetic modifications are reversible alterations of a cell’s DNA or histones that regulate gene expression without altering the DNA sequence, as opposed to genomic or genetic alterations that involve base pair changes that may change the structure and function of a protein encoded by a particular gene (Fig. 7). Two of the most characterized epigenetic (single genes) or epigenomic (genome-wide) modifications are DNA (cytosine) methylation and histone modification (methylation or acetylation of specific histones in the chromatin). Studies of endometriotic stromal cells provided clues about epigenomic changes that lead to inheritable traits, without altering the DNA sequence (Fig. 7). Some of these studies are reviewed below and link epigenetic modifications to functional consequences such as steroid production and action.
Genome-wide defective DNA programming in endometriotic and endometrial stromal cells
Unlike endometriotic epithelial cells, endometriotic stromal cells do not carry any somatic mutations, but the expression levels of many genes in endometriotic stromal cells are strikingly different than those in eutopic endometrial stromal cells of women with or without endometriosis (1, 25). This prompted investigators to examine potential epigenomic alterations in endometriosis. Although genome-wide alterations in histone modifications have not been well defined in endometriosis, differential DNA methylation in single genes as well as genome-wide has been studied extensively (Fig. 8) (94).
Figure 8.
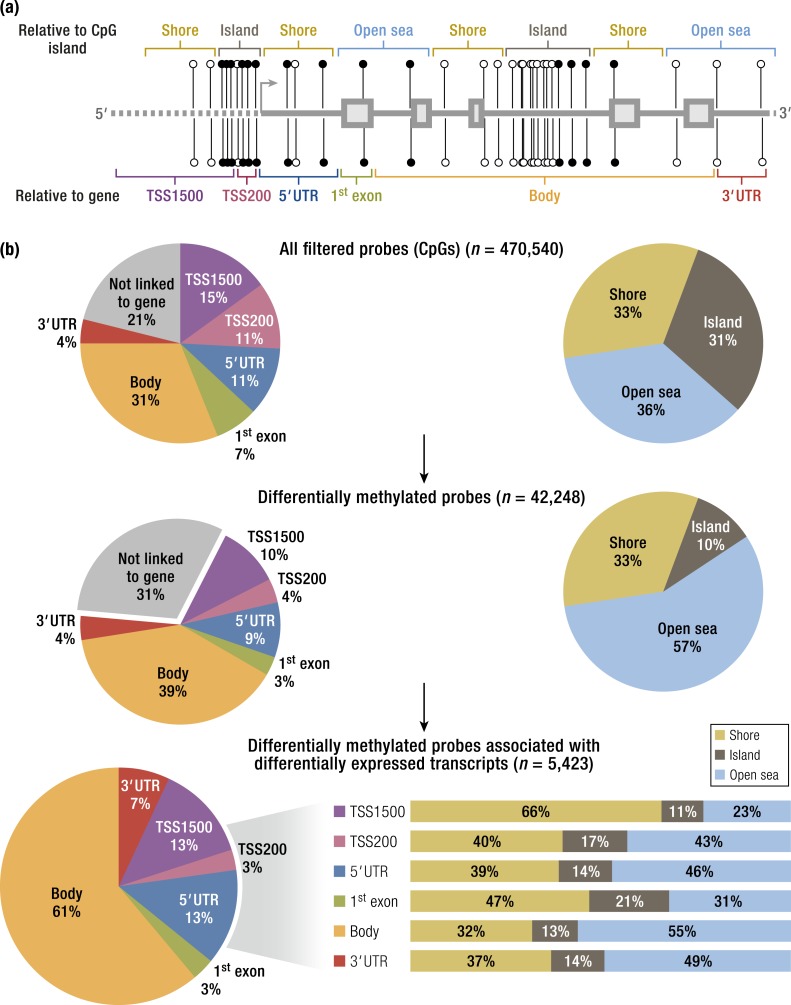
Genome-wide DNA methylation differences between stromal cells isolated from eutopic endometrium and endometriosis. The methylated (●) or unmethylated (○) state of a cytosine nucleotide in the CpG (or CG) sequence may in part regulate how genes are expressed in the DNA of a cell. (a) Schematic diagram of CpGs depicts their genomic context relative to the nearest CpG island (top) or gene (bottom). Gene contexts of CpGs were described as within an “island” (brown); on the “shore,” defined as within 4 kb around the island (yellow); or in “open sea,” defined as at least 4 kb distal from an island, and relative to the nearest open reading frame within 1500 bp (TSS1500; purple) or 200 bp (TSS200; pink) of a transcription start site (TSS); in the 5′ untranslated region (UTR), the first exon of a transcript (green); in the body of the gene (orange) or the 3′UTR (red). (b) Pie charts show the distribution of the CpGs examined based on their genomic context for all probes retained from the original array (top), for all the probes identified as differentially methylated between endometriosis and endometrium (middle), and for all the differentially methylated probes that were matched to differentially expressed mRNAs (bottom; note that stacked bar graphs show the CpG island context broken down for each of the gene contexts). A large number (5423) of differentially methylated CpG islands in this array were linked to a differentially expressed gene. A further integrative analysis demonstrated that 403 genes associated with one or more differentially methylated CpGs are differentially expressed with possible functional consequences. “Transcription factors” as a category comprised the most important gene category, whereas the top biologic pathway was “blood vessel development.” [Reproduced from Dyson MT, Roqueiro D, Monsivais D, et al. Genome-wide DNA methylation analysis predicts an epigenetic switch for GATA factor expression in endometriosis. PLoS Genet. 2014;10(3):e1004158. Copyright © 2014 by Dyson et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.]
Methylation of DNA is initiated and maintained by three DNA methyltransferases (DNMTs)—DNMT1, DNMT3A, and DNMT3B—which catalyze the addition of methyl groups to the 5′ carbon of cytosine in targeted cytosine-phosphate-guanine dinucleotides (CpGs) (126). Among these, DNMT3B seems to be differentially expressed in eutopic endometrial and endometriotic stromal cells during the decidualization process (126). Additionally, DNMT3B also binds to the promoter regions of key genes, steroidogenic factor-1 (SF1) and ESR1, in endometrial vs endometriotic stromal cells (127). Thus, differential DNMT3B expression and binding to critical gene promoters in endometriotic stromal cells may contribute to an aberrant DNA methylome that misdirects gene expression in endometriosis and contributes to these cells’ altered response to steroid hormones (127).
Genome-wide differences in DNA methylation between endometriotic and normal endometrial stromal cells have been reported and were correlated with gene expression using an interaction analysis strategy (94). Out of some 470,000 methylation sites, >42,000 differentially methylated CpGs were identified in endometriotic stromal cells compared with normal endometrial stromal cells (Fig. 8) (94). Differential CpG methylation patterns were seen most often in intragenic regions and distal to classic CpG islands, the methylation of which is typically negatively correlated with gene expression (Fig. 8) (94). A total of 403 genes showed significant differences in methylation; a large proportion of these genes encode transcription factors (Fig. 8) (94). Many of these genes are implicated in the pathology of endometriosis and decidualization. Differential methylation affected the HOX gene clusters, nuclear receptor genes, and the GATA family of transcription factors (94). It is now clear that DNA methylation differentially regulates the expression of hundreds of genes in endometriotic and endometrial stromal cells (Fig. 8).
The eutopic endometrium of women with endometriosis also displays DNA methylation abnormalities giving rise to altered gene expression and progesterone resistance (84, 128). In fact, this phenomenon was demonstrated in a mouse model of endometriosis (128, 129). These findings support the hypothesis that, during the process of retrograde menstruation, epigenetically abnormal populations of stromal cells in eutopic endometrium may be selected to survive as endometriotic implants in the pelvis (Fig. 5).
GATA-binding factor-2/GATA-binding factor-6: opposing master regulators of physiology and pathology
Differential DNA methylation and expression.
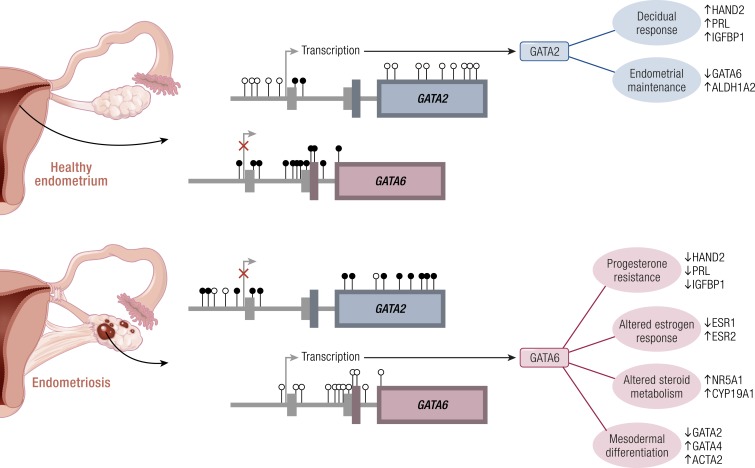
Numerous CpGs throughout the promoter and coding region of GATA-binding factor-2 (GATA2) showed higher methylation in endometriotic stromal cells relative to endometrial stromal cells, whereas GATA-binding factor-6 (GATA6) had less methylation across ranges of intronic CpGs flanking CpG islands in endometriotic stromal cells (Fig. 9) (94). Detailed mapping showed complete unmethylation of the GATA2 exon 4 in endometrium; this region was predominantly methylated in endometriosis (Fig. 9) (94). Likewise, exon 2 of GATA6 showed full methylation in endometrium and full unmethylation in endometriosis (Fig. 9) (94). GATA2 protein was highly abundant in endometrial stromal cells with a strong nuclear signal, but it was scarcely detectable in endometriotic stromal cells. In contrast, GATA6 was robustly expressed and localized to the nuclei in all endometriotic stromal cells but barely detectable in endometrial stromal cells (94).
Figure 9.
An epigenetic switch from GATA2 to GATA6 in endometriosis. In endometrium, the GATA2 gene is characterized by a lack of DNA methylation (○). In contrast, endometriosis has hypermethylated CpGs throughout a CpG island and proximal sequences within the GATA2 promoter and throughout the gene. Several regions of the GATA6 gene are completely methylated in endometrium, whereas methylation was absent within three CpG islands and their shores within the body of the gene. As a result, GATA2 is expressed in healthy cells and upregulates several genes involved in decidualization; GATA2 may also maintain the expression of aldehyde dehydrogenase 1 family, member A2 (ALDH1A2), which is a key enzyme in retinoid metabolism. In endometriotic cells, aberrant methylation permits expression of GATA6. GATA6 regulates the expression of several genes involved in steroid metabolism, the nuclear steroid hormone receptors, and other GATA family members. Ectopic expression of GATA6 drives a pattern of gene expression similar to that seen in endometriotic tissues, essentially transforming healthy endometrium away from spontaneous decidualization and toward the disease phenotype. [Reproduced from Dyson MT, Roqueiro D, Monsivais D, et al. Genome-wide DNA methylation analysis predicts an epigenetic switch for GATA factor expression in endometriosis. PLoS Genet. 2014;10(3):e1004158. Copyright © 2014 by Dyson et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.]
Physiologic functions of GATA2 in endometrium.
In mice, conditional GATA2 depletion in uterine tissue resulted in diminished PR expression in uterine epithelial cells and attenuated progesterone signaling. Moreover, GATA2 depletion in mouse uterine tissue gave rise to infertility due to implantation failure (130). In human endometrial stromal cells, however, GATA2 depletion did not alter PR, ERα, or ERβ levels (94), or the expression of some other critical endometriosis-related genes such as aromatase. In contrast, it was found that silencing GATA2 significantly reduced the established markers of decidualization (Fig. 9) (94). In the absence of GATA2, the induction of HAND2 and prolactin in response to an in vitro decidualization (IVD) cocktail including a progestin was reduced approximately by half, and IGFBP1 induction was strikingly reduced by 88% (Fig. 9) (94). These findings collectively suggest that GATA2 acts as a mediator and partner of progesterone signaling in normal endometrial stromal cells and may enhance the decidual response (Fig. 9) (94, 130).
Pathologic roles of GATA6 in endometriosis.
The ectopic expression of GATA6 in otherwise normal eutopic endometrial stromal cells shifted gene expression to reflect GATA6 expression patterns seen in endometriosis (Fig. 9) (94). All three of the critical steroid receptors showed significant changes in expression (Fig. 9). ERα mRNA was reduced by 1.6-fold when GATA6 was overexpressed without IVD, and by 3-fold after IVD (94). The overexpression of GATA6 also reduced PR transcript levels an average of 2.6-fold. In contrast, the expression of ERβ increased an average of 2.5-fold; IVD did not significantly affect these genes (94). The effects of GATA6 on the protein levels of these steroid receptors have not been reported. Matrix metallopeptidase 11 (MMP11) and aromatase mRNA levels were also strikingly altered by GATA6 overexpression (Fig. 9). MMP11 was repressed by IVD, but overexpression of GATA6 further reduced its transcript levels by 30-fold relative to untreated controls. Aromatase was expressed at very low levels basally, but was strikingly increased after GATA6 overexpression (Fig. 9) (94). Overexpression of GATA6 profoundly restricted the ability of endometrial stromal cells to decidualize, effectively blocking all four genes expected to increase with IVD (FOXO1, HAND2, PRL, and IGFBP1; Fig. 9) (94).
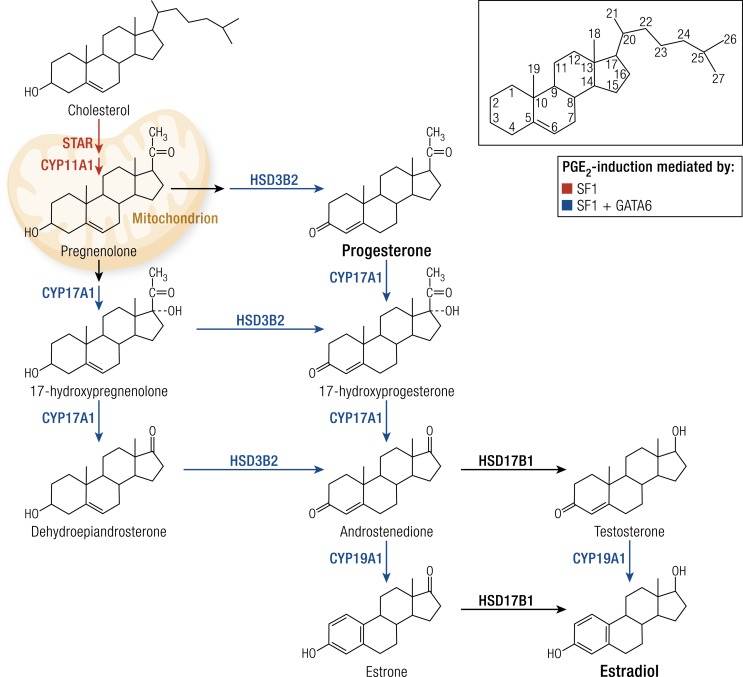
Endometriotic stromal cells synthesize estradiol via the steroidogenic pathway, whereas normal endometrial stromal cells do not produce steroid hormones (1). SF1 (also known as nuclear receptor subfamily 5, group A, member 1, or NR5A1) is critical, but not sufficient, for activating the cascade that involves at least five steroidogenic proteins or enzymes (Fig. 10) (127). Silencing of GATA6 in endometriotic stromal cells showed that GATA6 was necessary for catalyzing the conversion of progesterone to androstenedione by 17-hydroxylase/17,20-lyase (CYP17A1) (Fig. 10). Ectopic expression of GATA6 alone or with SF1 was essential for converting pregnenolone to estrogen [3β-hydroxysteroid dehydrogenase-2 (HSD3B2), CYP17A1, and aromatase (CYP19A1)] (127). However, simultaneous ectopic expression of both GATA6 and SF1 was required and sufficient to confer induction of all five genes and their encoded proteins that convert cholesterol to estradiol (Fig. 10) (127). Functionally, only simultaneous knockdown of GATA6 and SF1 blocked estradiol formation in endometriotic stromal cells (127). The presence of both transcription factors was required and sufficient to transform endometrial stromal cells into endometriotic-like cells that produced estradiol in large quantities (Fig. 10) (127). Thus, expression of both GATA6 and SF1 in endometrial stromal cells is sufficient for transformation into endometriotic-like cells capable of converting cholesterol to estradiol (Fig. 10) (127).
Figure 10.
Estradiol production in endometriosis: roles of SF1 and GATA6. The endometriotic stromal cell is capable of converting cholesterol to estradiol via a number of enzymatic steps. Both transcription factors, SF1 and GATA6, are essential for the production of estradiol and mediate the effects of PGE2 in stimulating these steroidogenic genes. SF1 is essential and sufficient for the expression of at least five steroidogenic genes, whereas SF1 and GATA6 together are essential and sufficient for the expression of three of these genes. The effects of SF1 or GATA6 on HSD17B1 in endometriosis are not known. The addition (ectopic expression) of both SF1 and GATA6 is required for a normal endometrial stromal cell to make estradiol from cholesterol. CYP11A1, side-chain cleavage enzyme; HSD3B2, 3β-hydroxysteroid dehydrogenase-2; HSD17B1, 17β-hydroxysteroid dehydrogenase type 1.
SF1: coordinator of estrogen formation in endometriosis
Epigenetic regulation of the SF1 gene.
SF1 is an orphan nuclear receptor (without a known ligand) that was first described as a regulator of adrenal and gonadal steroid production (131). It was the first nuclear receptor whose function was defined in detail in endometriosis (132). The practical absence of the orphan nuclear receptor SF1 in endometrial stromal cells and its 12,000-fold higher presence in endometriotic stromal cells is, in part, determined by a classic CpG island within its promoter. This CpG island is heavily methylated in endometrial stromal cells, which allows recruitment of the silencer-type transcription factor methyl-CpG–binding domain protein 2 that interferes with the binding of transcriptional activators to the SF1 promoter (133). In endometriotic cells, the transcription factor upstream stimulatory factor-2 (USF2) is able to bind to the unmethylated SF1 promoter to activate it (134). In vivo, USF2 levels are strikingly higher in endometriosis compared with endometrium. Thus, differential SF1 expression in endometriosis vs endometrium is primarily controlled by an epigenetic mechanism that permits binding of activator vs inhibitor complexes to its promoter (133, 134).
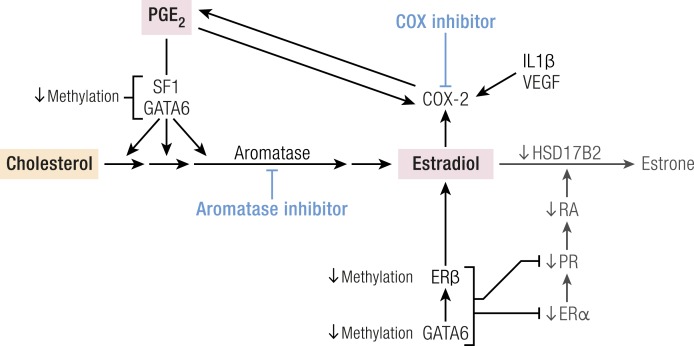
As detailed above, SF1 and GATA6 play a key role in the conversion of cholesterol to estradiol in endometriotic stromal cells (Fig. 10) (43). Estrogen supports endometriotic cell survival, whereas prostaglandins and cytokines lead to inflammation, chronic pelvic pain, and infertility (35, 135). A positive feedback loop links inflammation and estrogen production in endometriotic tissue, favoring overexpression of key steroidogenic genes, notably aromatase, overexpression of COX2, and continuous local production of estradiol and prostaglandin E2 (PGE2) (Fig. 5) (4, 136–138).
Tissue sources of estrogen in endometriosis.
Inhibition of estrogen action by GnRH analogs, oral contraceptives, progestins, and aromatase inhibitors significantly reduces pelvic disease and pain (Fig. 3) (139). Estrogen production starts with the entry of cytosolic cholesterol into the mitochondrion facilitated by STAR. Six enzymes [side chain cleavage enzyme (CYP11A1), HSD3B2, CYP17A1, aromatase (CYP19A1), and 17β-hydroxysteroid dehydrogenase-1 (HSD17B1)] catalyze the conversion of cholesterol to biologically active estradiol (Fig. 10) (99). Inhibition of aromatase (CYP19A1) effectively eliminates all estrogen production (Fig. 10) (99). SF1 coordinately binds to the promoters of the genes that encode STAR, CYP11A1, HSD3B2, CYP17A1, and aromatase (CYP19A1), making it an essential transcription factor regulating steroidogenesis in the ovary and endometriosis (Fig. 10) (1).
Estrogen is produced at three major body sites in women with endometriosis. First, estradiol is secreted by the ovary and reaches endometriotic tissue via the circulation. Follicular rupture at each ovulation causes extraordinarily large amounts of estradiol to be released directly onto pelvic implants. Second, aromatase produced by peripheral adipose and skin tissue converts circulating androstenedione to estrone, which may reach endometriotic tissue via the circulation. Endometriotic tissue expresses the complete set of steroidogenic genes, including aromatase, allowing local conversion of cholesterol to estradiol (1). Local aromatase activity in endometriotic tissues may underlie the observed superior effect of treatment with a GnRH analog plus an aromatase inhibitor compared with GnRH analog alone for providing long-lasting pain relief in women with endometriosis (Fig. 3) (17).
PGE2 stimulates estradiol formation.
PGE2 stimulates the expression of all steroidogenic genes in endometrial stromal cells, leading to local de novo synthesis of estradiol (43). PGE2 regulation of STAR and aromatase has been characterized extensively (Fig. 10) (43).
The PGE2 receptor subtypes EP1, EP2, EP3, and EP4 are expressed at similar levels in both endometriotic and endometrial stromal cells (140). Activation of the EP2 receptor increases the levels of intracellular cAMP, which induces STAR and aromatase expression in endometriotic stromal cells (99, 137, 140). STAR and aromatase levels and activity are stimulated by PGE2 or a cAMP analog in endometriotic cells but not in endometrial stromal cells (Fig. 10) (4, 99, 137). The transcription factor SF1, which is present in endometriosis and absent in endometrium, mediates the PGE2/cAMP-dependent induction of STAR, aromatase, and other steroidogenic genes in endometriotic stromal cells (Fig. 10) (127). In PGE2-treated endometriotic cells, SF1 assembles into an enhancer transcriptional complex on the STAR and aromatase promoters to induce gene expression (99).
The absence of SF1 in endometrial cells underlies the lack of responsiveness of steroidogenic genes to PGE2. Additionally, a number of transcriptional inhibitors of STAR and aromatase provides a fail-safe system for silencing these steroidogenic genes in endometrial cells. These repressors are chicken ovalbumin upstream transcription factor (COUP-TF), Wilms’ tumor-1 (WT1), and CAAT/enhancer binding protein-β (C/EBPβ). Normal endometrial tissue expresses much higher levels of WT1 and C/EBPβ compared with endometriotic tissue. In the absence of SF1, these repressors form a transcriptional complex on the steroidogenic promoters that suppresses expression in endometrial cells (99).
In summary, upon PGE2 induction, SF1 is recruited to the promoters of the essential steroidogenic genes to drive local estradiol synthesis in endometriotic stromal cells (Fig. 10). Aromatase inhibitors completely block estradiol synthesis, making it an attractive target for endometriosis therapeutics. Aromatase inhibitors have been shown to diminish or eradicate treatment-refractory endometriotic implants and associated pain (141).
ERβ: mediator of estrogen-induced inflammation
Epigenetic regulation of the ESR2 and ESR1 genes.
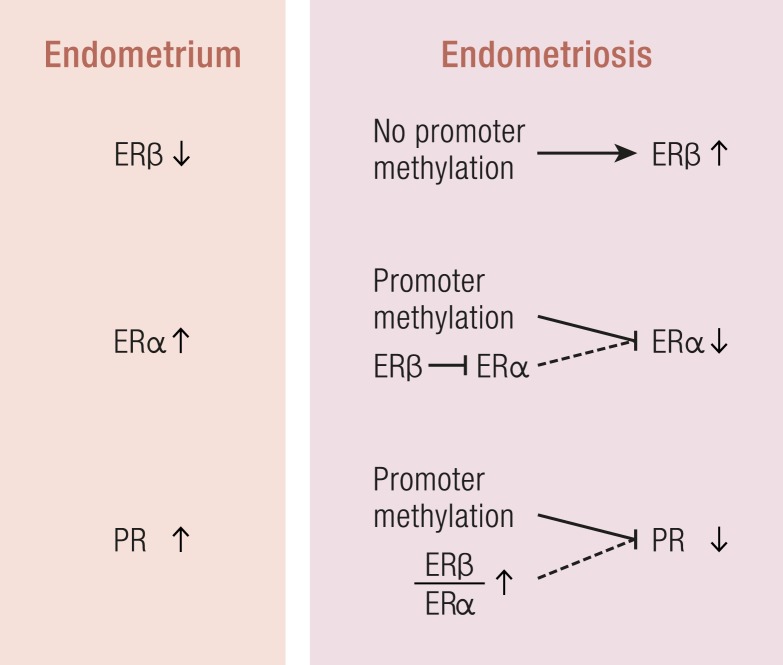
In endometriotic stromal cells, ERβ (ESR2) levels are 142-fold higher and ERα (ESR1) levels are 9-fold lower compared with normal endometrium (Fig. 11) (142). Hypomethylation of a CpG island at the promoter region of the ESR2 gene leads to high levels of expression in endometriotic stromal cells, and hypermethylation silences the ESR2 gene in endometrial stromal cells (Fig. 11). In contrast, the ESR1 promoter is unmethylated in eutopic endometrium and heavily methylated in endometriosis (Fig. 11) (94), leading to lower ERα levels in endometriotic vs endometrial stromal cells (142). Consequently, the abnormally high ERβ/ERα ratio in endometriotic stromal cells may perturb estradiol induction of the PGR (PR) gene, which in turn may in part be the basis for low PR expression in endometriosis (Fig. 11). In endometrial vs endometriotic stromal cells, the PGR gene is also differentially methylated, which may contribute to its suppressed expression in endometriotic cells (Fig. 11) (94).
Figure 11.
Estrogen and PRs in endometriosis. Compared with endometrial stromal cells, ERβ levels are higher and ERα levels are lower in endometriotic stromal cells because of altered DNA methylation. The PR gene is also differentially methylated between these two cells types. It is likely that the severely elevated ratio of ERβ/ERα is in part responsible for the observed inhibition of ERα and PR expression in endometriotic stromal cells.
ERβ is the key mediator of estrogen action in endometriotic stromal cells. Endometriotic stromal cells show severely suppressed ERα and significantly higher ERβ levels compared with eutopic endometrial stromal cells (Fig. 11) (142). A hypomethylated promoter region in the ESR2 gene results in remarkably higher ERβ mRNA and protein expression relative to the normal endometrium (Fig. 11) (34, 142). The eutopic endometrium of women with endometriosis also shows higher ERβ expression compared with the endometrium of healthy women, suggesting that abnormally high levels of ERβ in the endometrium may predispose women to develop endometriosis (143, 144). In endometriotic stromal cells, ERβ was found to transcriptionally repress ERα, indicating that elevated ERβ confers a unique estrogen response mechanism that may contribute to disease progression (Fig. 11) (145).
ERβ action in endometriosis and uterine tissue.
Genome-wide comparative analysis of ERβ binding and gene expression in human endometriosis and endometrial tissues identified Ras-like, estrogen-regulated, growth inhibitor (RERG) and serum and glucocorticoid–regulated kinase (SGK1) as key ERβ targets (Fig. 12) (33, 34). Estradiol induces RERG mRNA and protein levels in human endometriotic stromal cells, whereas PGE2 phosphorylates RERG and enhances its nuclear translocation. RERG induces ribosome biogenesis and the proliferation of primary endometriotic cells (Fig. 12); thus, the integration of ERβ and PGE2 signals at RERG leads to endometriotic cell proliferation. The ERβ/PGE2/RERG pathway represents a novel candidate for therapeutic intervention (Fig. 12) (34). Estradiol/ERβ also stimulates SGK1 expression and enzyme activity, leading to increased human endometriotic cell survival (Fig. 12) (33). In uterine microvascular endothelial cells, ERβ mediates estradiol-stimulated COX2 expression and PGE2 production (146).
Figure 12.
ERβ action in endometriosis. ERβ induces RERG expression, whereas PGE2, via protein kinase A (PKA), phosphorylates RERG. RERG phosphorylation is associated with its nuclear translocation. Estradiol- and PGE2-mediated activation of RERG regulates endometriotic cell proliferation. Estradiol via ERβ also induces expression of serum and glucocorticoid–regulated kinase (SGK1), which is also activated by PGE2 and oxidative stress. This leads to phosphorylation of FOXO3a, a proapoptotic factor, and enhanced cell survival. [Adapted from Monsivais D, Dyson MT, Yin P, et al. ER beta- and prostaglandin E2-regulated pathways integrate cell proliferation via Ras-like and estrogen-regulated growth inhibitor in endometriosis. Mol Endocrinol. 2014;28(8):1304–1315.]
In a mouse model of endometriosis that expresses high ERβ levels, treatment with an ERβ-selective estradiol antagonist suppressed mouse ectopic lesion growth (Fig. 13) (97). ERβ interaction with apoptotic machinery in the cytoplasm to inhibit TNF-induced apoptosis may evade endogenous immune surveillance and contribute to cell survival (Fig. 13) (97). ERβ also enhances cellular adhesion and proliferation properties by interacting with components of the cytoplasmic inflammasome to increase IL-1β (Fig. 13) (97).
Figure 13.
Interactions of ERβ, TNF, SRC1, and IL-1β in endometriosis. ERβ plays a unique role in endometriotic tissue, where it interacts with the cytoplasmic apoptotic machinery and inflammasome complex to prevent TNF-induced cell death and enhance adhesion and proliferative activities of endometriotic tissues via SRC1 and IL-1β in this broad mechanistic pathway. This nongenomic action of ERβ has a predominant role in endometriosis progression. [Reproduced from Han SJ, Jung SY, Wu SP, Hawkins SM, Park MJ, Kyo S, Qin J, Lydon JP, Tsai SY, Tsai MJ, DeMayo FJ, O’Malley BW. Estrogen receptor β modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell. 2015;163(4):960–974. doi: 10.1016/j.cell.2015.10.034. Copyright © 2015 by Elsevier, Inc.]
ERβ and PGE2 connect estrogen and inflammation.
Pelvic pain and infertility in endometriosis are directly related to endometriotic cell survival and inflammation. Estrogen enhances the survival or persistence of ectopic endometriotic tissue, whereas prostaglandins and cytokines mediate pain, inflammation, and infertility (35, 135). Inflammation and estrogen production in endometriosis are linked by a positive feedback cycle in which chronic overexpression of aromatase and COX2 support sustained production of estradiol and PGE2 in endometriotic tissue (Fig. 5) (4, 136–138). Estradiol/ERβ stimulates PGE2 formation, whereas PGE2 stimulates estradiol synthesis (3, 146). Selective or nonselective COX inhibitors that disrupt PGE2 synthesis effectively reduce pelvic pain in endometriosis (147). Likewise, aromatase inhibitors that disrupt estradiol biosynthesis significantly reduce endometriosis-associated pain and cause regression of pelvic lesions (39, 141).
Taken together, ERβ plays a central role in the development and pathophysiology of endometriosis. The overproduction of estradiol in endometriosis drives ERβ signaling to support endometriotic tissue survival and inflammation (39). Additionally, ERβ may have estradiol-independent pathologic actions.
PR: progesterone resistance
Role of progesterone in endometriosis.
Compared with the clearly pathologic effect of estrogen in endometriosis, the role of progesterone has remained ambiguous for several reasons. First, although progesterone has a protective role in endometrial cancer (characterized by epithelial proliferation), it does not have the same effect in endometriosis, in which benign lesions are comprised of primarily stromal cells with diminished apoptosis or differentiation (41). Paradoxically, progesterone induces a transient proliferation of stromal cells in normal endometrium during the secretory phase (41). Second, progestin-based treatments are at best variably effective at reducing endometriosis-related pain, especially in patients who received other forms of treatment (148, 149). Progestins likely reduce pain via suppressing or attenuating ovulation (41), although a direct effect on endometriotic tissue cannot be excluded. Third, antiprogestins with mixed agonist and antagonist properties (also known as selective PR modulators) reduce endometriosis-associated pelvic pain, possibly more effectively than progestins (41, 46, 150). Furthermore, endometriotic tissues produce significant quantities of progesterone and express significantly lower levels of PR compared with endometrium (43, 137). These conflicting observations have prevented the formation of a unified hypothesis describing progesterone’s role in endometriosis.
PR deficiency in endometriosis.
Progesterone resistance in endometriotic tissue is readily explained by the extremely low PR levels (40). In normal endometrium, levels of the PR isoforms, PR-B and PR-A, increase during the proliferative phase, peak just before ovulation, and then diminish; this pattern suggests that estradiol stimulates PR expression (40). In simultaneously collected tissues of endometriosis, PR-B is undetectable and PR-A is markedly lower compared with normal endometrium. Moreover, the endometrium of baboons with endometriosis shows deficiency of the co-chaperone FKBP52, which is necessary for PR function, and this may contribute to progesterone resistance (151).
17β-Hydroxysteroid dehydrogenase-2 enzyme deficiency in endometriosis.
Progesterone has an antiestrogenic effect in the healthy endometrium, but a hallmark of endometriosis is progesterone resistance and elevated local estradiol levels (42). 17β-Hydroxysteroid dehydrogenase type (2HSD17B2) catalyzes the conversion of biologically potent estradiol to less potent estrone (Fig. 14) (152–155). Progesterone via PR increases formation of retinoic acid (RA) in endometrial stromal cells, which induces HSD17B2 expression in neighboring endometrial epithelial cells (Fig. 15) (156–158). In contrast, endometriotic stromal cells with progesterone resistance do not produce RA (Fig. 15) (159), which leads to a loss of paracrine signaling to induce HSD17B2 expression in the epithelial cells and therefore failure to inactivate estradiol in endometriosis (Figs. 14 and 15) (42, 159). Combined with high estradiol production due to aberrant aromatase activity, this additional defect further contributes to the abnormally high estradiol activity in endometriosis (Figs. 14 and 15) (160).
Figure 14.
Progesterone resistance: HSD17B2 deficiency in endometriotic epithelium. HSD17B2 is specifically expressed in eutopic endometrial epithelial cells during the secretory phase. This expression coincides with progesterone secretion by the corpus luteum. HSD17B2 converts the biologically potent estrogen, estradiol (E2), to estrogenically weak estrone (E1). In endometriotic tissue, however, this epithelial HSD17B2 is severely deficient, giving rise to E2 excess. These findings are suggestive that progesterone stimulates HSD17B2 in eutopic endometrium and that its deficiency in endometriosis is a consequence of progesterone resistance. [Reproduced from Zeitoun K, Takayama K, Sasano H, Suzuki T, Moghrabi N, Andersson S, Johns A, Meng L, Putman M, Carr B, Bulun SE. Deficient 17β-hydroxysteroid dehydrogenase type 2 expression in endometriosis: failure to metabolize 17β-estradiol. J Clin Endocrinol Metab. 1998;83(12):4474–4480.]
Figure 15.
Paracrine stromal–epithelial interactions for progesterone and retinoid action in endometrium and endometriosis. Deficient genes and pathways in endometriosis are indicated by arrows and dotted lines. Blood vessels deliver progesterone (P4) to endometrial stromal cells, which express PR; activation leads to the production of paracrine factors, including RA. RA acts in a paracrine manner to stimulate differentiation and oppose estradiol (E2)-dependent proliferation in endometrial epithelial cells. Moreover, RA stimulates HSD17B2, which converts biologically active E2 to estrogenically weak estrone (E1). Endometriotic stromal cells express lower levels of PR, which leads to lower RA formation. As a result, paracrine signaling to neighboring epithelial cells is lost, and these cells do not differentiate or express HSD17B2, leading to excess E2. The mechanisms supporting retinoid transport between endometrial stromal and epithelial cells are not fully understood. The retinoid cell surface receptor stimulated by RA-6 (STRA6) in endometrial stromal cells binds retinol binding protein (RBP)–bound retinol in the circulation for cellular uptake of retinol. Retinol is converted to RA and transported to nuclear RARs by the shuttling protein cellular RA binding protein-2 (CRABP2). RA-bound RAR stimulates endometrial stromal cell differentiation and apoptosis. PR induces stromal STRA6 and CRABP2 expression. In endometriosis, this pathway is disrupted by a deficiency of PR, STRA6, and CRABP2 as abnormal expression of the RA-metabolizing enzymes CYP26B1 and CYP26A1 in stromal and epithelial endometrial cells. 4OH-RA, 4-hydroxy-RA. [Reproduced from Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocrine Rev. 2013;34(1):130–162.]
Retinoid deficiency in endometriosis.
Retinol binding protein binds retinol (vitamin A) and delivers it to tissues via the circulation (Fig. 15) (161, 162). Retinol binds its cell surface receptor STRA6 and enters the cell, where retinol and retinal dehydrogenase enzymes catalyze its conversion to transcriptionally active RA. RA acts through various RA receptors (RARs), including RAR-α, RAR-β, and RAR-γ and peroxisome proliferator-activated receptor (PPAR)-β/δ, to regulate target gene transcription (Fig. 15) (163). Because STRA6 is strongly induced by PR signaling, PR-deficient endometriotic stromal cells are unable to produce sufficient RA (Fig. 15) (161, 162).
Transcriptional activation of the nuclear receptor RAR by RA often leads to cell growth inhibition. However, in some tissues, RA acting through PPARβ/δ induces the expression of prosurvival genes to promote cell survival and hyperplasia (163). RA activation of these two receptors is regulated by the intracellular lipid-binding proteins CRABP2 and FABP5, which specifically deliver RA from the cytosol to nuclear RAR and PPARβ/δ, respectively (163). Thus, RA signals through RAR to drive apoptosis in cells with a high CRABP2/FABP5 ratio, but signals through PPARβ/δ to promote survival in cells with a low CRABP2/FABP5 ratio (163).
In normal endometrial stromal cells, RA induces apoptosis via the CRABP2/RARα pathway; in CRABP2/RARα-deficient endometriotic cells, RA does not have the same effect (Fig. 15) (161, 162). This favors survival of endometriotic stromal cells and may support the development and persistence of pelvic endometriotic implants. Because PR induces CRABP2 gene expression (Fig. 15) (161), PR resistance in endometriotic stromal cells leads to deficiency of CRABP2; this supports the overall model of deficient PR action in endometriosis (Fig. 15) (40).
General deficiency of progesterone action in endometriosis.
Estrogen and progesterone are essential and sufficient for endometrial function by regulating expression of multiple genes during the menstrual cycle (164). Indeed, administration of estradiol and progesterone is sufficient to prepare the endometrium for implantation in postmenopausal women undergoing donor embryo transfer (165). Progesterone exposure induces differentiation of both endometrial stromal cells (decidualization) and epithelial cells (secretory phenotype). Increased epithelial glycodelin and stromal prolactin levels signal progesterone action in the endometrium (164, 166, 167). Progesterone induces lower levels of prolactin expression in endometriotic vs endometrial stromal cells, again supporting the progesterone resistance model in endometriosis (160).
Gene expression profiles in the endometrium of women with or without endometriosis revealed that a number of progesterone target genes are dysregulated during the window of implantation, the time when progesterone levels in the endometrium reach their peak (28, 29). As an example, the progesterone-responsive gene glycodelin is downregulated in the endometrium of women with endometriosis compared with women without endometriosis (28). Thus, the eutopic endometrium of women with endometriosis also shows evidence of progesterone resistance (28, 168).
To summarize, progesterone resistance in endometriosis is associated with PR deficiency in poorly differentiated stromal cells (Fig. 15). Defective progesterone action has tangible downstream consequences. Upon exposure to progesterone in endometrial capillaries, poorly differentiated stromal cells with deficient PR fail to send physiologic paracrine signals to adjacent epithelial cells (40). RA is an essential paracrine factor, and its deficiency due to progesterone resistance is associated with the deficiency of the enzyme HDS17B2 in endometrial epithelial cells (158, 161, 162). HSD17B2 is essential for effectively inactivating local estradiol at the endometrial epithelium–embryo interface during the implantation window (42). 17HSDB2 deficiency because of progesterone resistance gives rise to local estradiol excess in endometriotic tissue (42). This possibly causes enhances estradiol-driven inflammation and also implantation failure in eutopic endometrium because progesterone resistance was also demonstrated in eutopic endometrial tissue of patients with endometriosis (41). Interestingly, compounds with mixed progesterone antagonist/agonist properties more effectively reduce uterine bleeding and pelvic pain associated with endometriosis compared with progestins (44, 46, 150). This may be due to their ability to bind and act via lower levels of PR in endometriotic tissue and/or by affecting the endometrium (44, 46, 150).
Nuclear receptor coregulators in endometriosis
Steroid receptor coactivators (SRCs) dynamically modulate nuclear receptor–mediated cellular processes in a tissue-selective manner to precisely respond to external hormonal stimuli (144). Alterations in SRC concentrations or modifications of SRCs in steroid target tissues may underlie nuclear receptor–dependent human disease progression (144). An SRC1-null mouse model revealed that the SRC1 gene plays an essential role in endometriosis progression (144). Compared with normal endometrium, a 70-kDa proteolytic C-terminal isoform of SRC1 is highly elevated in both the endometriotic tissue of mice with surgically induced endometriosis and in eutopic endometrial stromal cells biopsied from patients with endometriosis (144). TNF-induced MMP9 activity mediates formation of the 70-kDa SRC1 isoform in endometriotic mouse tissue. In contrast to full-length SRC1, the endometriotic SRC1 fragment prevents TNF-mediated apoptosis in human endometrial epithelial cells and causes the epithelial–mesenchymal transition and the invasion of human endometrial cells (144). Thus, the TNF/MMP9/SRC1 pathway promotes key pathologic functions in endometriotic tissue (Fig. 13) (144).
The nuclear actions of the SRC1 fragment are currently not known (169). It is possible that SRC1 or other nuclear receptor coregulators modify the activities of key nuclear receptors, such as ERβ, SF1, and PR, in endometriotic stromal cells (Fig. 13).
Summary of key mechanisms in endometriosis
Women who develop the clinical symptoms of endometriosis may have an inheritable predisposition to this disease, including epigenomic abnormalities in tissue progenitor/stem cells in the coelomic epithelium (peritoneum), endometrium, endometrial vasculature, and other pelvic tissues. Continual and frequent episodes of ovulatory menses that expose the lower abdominal cavity to large quantities of blood and shed endometrial tissue increase the risk for developing pelvic endometriosis. Exposure of pelvic tissues to high levels of estradiol also increases the risk and severity of endometriosis, driving the persistence, tissue remodeling, and inflammation in endometriotic lesions (Fig. 16).
Figure 16.
Summary of key estrogen-dependent mechanisms in endometriosis. Pathologically decreased methylation of the SF1, GATA6, and ERβ genes causes extremely high levels of their proteins in endometriotic stromal cells. SF1 together with GATA6 coordinately mediate the PGE2-induced expression of many genes in the estrogen synthetic cascade to produce estradiol from cholesterol. In addition to decreased methylation of its gene promoter, ERβ expression is high also due to a stimulatory effect from GATA6. Estradiol and ERβ induce COX2 expression and PGE2 production in endometrial cells. Moreover, PGE2per se and IL-1β also induce COX2 expression and PGE2 production in endometrial cells. ERβ and GATA6 suppress ERα and PR, leading deficiencies of RA production and the HSD17B2 enzyme. The end result is extremely high local concentrations of estradiol and PGE2 in endometriotic tissue; both of these molecules are key inducers of inflammation and pain in endometriosis. This pathway is clinically relevant because its disruption by an aromatase inhibitor or a COX inhibitor (e.g., a nonsteroidal anti-inflammatory drug) reduces the extent of disease or pelvic pain.
Repeated exposure of lower abdominal tissues to poorly differentiated endometrial mesenchymal stem cells gives rise to the formation of symptomatic endometriosis in the peritoneum, ovarian surface and hemorrhagic cysts, and the area between the rectum and vagina. The endometriotic stromal cell displays critical abnormalities in genome-wide DNA methylation, regulation of gene expression, and signaling pathways. Most of these perturbations occur in stromal cells exhibiting stem cell properties and partial ovarian granulosa cell–like and immune cell–like characteristics such as estradiol, prostaglandin, and cytokine production. These cells originate from the eutopic endometrium and exhibit abnormal expression of key transcription factors, including high levels of GATA6, SF1, and ERβ and deficiencies in GATA2, ERα, and PR in both eutopic and ectopic stromal cells (Figs. 9 and 16).
GATA2 and ERα regulate key genes necessary for progesterone-driven differentiation of healthy endometriotic stromal cells. The promoters of these genes are hypermethylated and thus transcriptionally repressed in endometriotic cells, whereas GATA6 is hypomethylated and abundant in endometriotic cells. GATA6 blocks hormone sensitivity, represses GATA2, and induces markers of endometriosis when expressed in healthy endometrial cells. Ectopic expression of GATA6 in a normal endometrial stromal cell effectively transforms it to an endometriotic stromal cell, recapitulates key molecular defects such as aromatase and ERβ expression, and induces estrogen biosynthesis and progesterone resistance (Figs. 9 and 16).
The sustained cell survival and inflammation characteristic of endometriosis have been linked to a stromal cell defect involving excessive formation of estrogen and prostaglandin and progesterone resistance, all of which originate from two transcription factors, SF1 and ERβ. Exposure of endometriotic cells to the local hormone PGE2 leads to coordinated binding of SF1 to the promoters of multiple steroidogenic genes, including aromatase, causing formation of large quantities of estradiol (Fig. 16).
ERβ mediates estrogen action in endometriosis. Estradiol acts via ERβ to stimulate COX2, leading to overproduction of PGE2. Thus, inflammation and estrogen are linked via a positive feedback cycle involving overexpression of genes encoding the aromatase and COX2 enzymes and continuous formation of their products, estradiol and PGE2, in endometriotic tissue. Moreover, a strikingly low ERα/ERβ ratio is associated with deficient induction of PR levels, which leads to progesterone resistance and disruption of a paracrine pathway that inactivates estradiol. Enhanced estradiol formation and deficient inactivation in endometriosis results in accumulation of estradiol. Finally, via genomic and nongenomic pathways, ERβ modulates the activities of RERG, TNF, and IL-1β to promote survival, proliferation, and inflammation in poorly differentiated endometrial stromal cells. This model is clinically relevant because therapeutic targeting of aromatase, COX2, ERβ, or PR reduces pelvic pain or ablates visible endometriotic lesions (Figs. 12, 13, and 16).
It is now clear that the epithelial cells of normally functioning endometrium contain numerous mutations, including driver mutations of ovarian cancer. Some of these mutations (e.g., in KRAS, PIK3CA, and ARID1A) are enriched in endometriotic epithelial cells from ovarian endometriomas or extraovarian deep-infiltrating endometriosis and may play roles in the pelvic implantation of endometriotic tissue fragments with survival and growth advantages. Although epithelial driver mutations in extraovarian sites do not cause malignant transformation, the same mutations in ovarian endometriomas may be essential for initiating endometriosis-associated epithelial ovarian cancers. The intense paracrine inflammatory signals, together with estrogen from epigenomically misprogrammed endometriotic cells, may enhance epithelial mutagenesis or carcinogenesis [Fig. 7 (208)].
Some intriguing questions remain unanswered. What are the relative roles of the environment vs the genome in the widespread epigenomic abnormalities in endometriotic stromal cells? Are these epigenomic abnormalities transmissible through the generations? What happens to the epithelial cells carrying cancer driver mutations in eutopic endometrium? What determines the malignant transformation of some but not all ovarian endometriomas with similar epithelial mutations? How do epigenomically abnormal stromal cells and mutated epithelial cells influence each other in a paracrine fashion in benign and malignant settings?
Innovations in Clinical Management
The goals of medical therapy for endometriosis are pain control, improvement of the quality of life, prevention of disease recurrence, fertility preservation, and reduction of operative intervention (170). Endometriosis-associated pain involves the stimulation of sensory nerve tissue in the eutopic endometrium, pelvic peritoneum, and other pelvic tissues by inflammatory substances produced as a consequence of the complex disease process. Inflammation is closely linked to repeated episodes of ovulatory menses and retrograde travel of blood and other menstrual tissue into the pelvic cavity. Estrogen is the most hierarchically upstream and potent stimulus of survival and inflammation in eutopic and ectopic endometrial tissues.
Following from this mechanistic understanding of disease pathology, successful treatments of endometriosis-associated pain focus on suppression of ovulation and estrogen production (170). The rationale for hormone therapy is to induce amenorrhea, thereby creating a relatively hypoestrogenic environment that will inhibit the inflammatory process and prevent disease progression (171). Traditionally, ovulatory cycles associated with estrogen secretion and retrograde menstruation have been suppressed at the level of hypothalamus/pituitary using oral contraceptives, progestins, or GnRH agonists (1). Pain and other abdominal symptoms usually recur upon treatment discontinuation, however (Fig. 3), and these treatments do not address pathogenic factors in endometriosis tissue such as local aromatase activity, nuclear receptor activity, or cytokine production. Some recently approved, experimental, or emerging treatments are discussed below.
Oral GnRH antagonists
Although GnRH agonists have been used to suppress ovulation in the treatment of endometriosis, GnRH antagonists have not been available until recently. Oral nonpeptide forms of GnRH antagonists (e.g., elagolix, relugolix) have been developed, and elagolix has been approved by the Food and Drug Administration to treat endometriosis (172, 173). Elagolix is an oral GnRH antagonist that rapidly suppresses the pituitary-ovarian hormones in a dose-dependent manner (174). However, ovulation may still occur during treatment, particularly at lower doses that partially suppress ovarian estrogen production (174). Oral GnRH antagonists produce a hypoestrogenic environment; however, at low doses they can maintain sufficient circulating estradiol levels to avoid severe vasomotor symptoms, vaginal atrophy, and bone demineralization (174, 175). The efficacy, safety, and tolerability of elagolix for the management of endometriosis-associated pain have been demonstrated in phase 1 and phase 2 trials (176). During a 24-week period, elagolix demonstrated similar efficacy on endometriosis-associated pain to subcutaneous medroxyprogesterone acetate but with a minimal effect on bone mineral density (172).
Phase 3 trials showed that higher (200 mg twice a day) and lower (150 mg daily) doses of elagolix were effective in improving dysmenorrhea and nonmenstrual pelvic pain during a 6-month period in women with endometriosis-associated pain (173). Both doses of elagolix were, however, associated with hypoestrogenic adverse effects (173). Extension studies evaluated 12 continuous months of treatment with both doses of elagolix (175). Endometriosis patients benefited from sustained reductions in dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia (175). The higher dose was associated with greater improvement of dysmenorrhea (175). The side effects were consistent with reduced estrogen levels and no new concerns were associated with long-term use of elagolix (175). Nonhormonal contraception is needed, as neither dose of elagolix reliably suppresses ovulation (175).
Aromatase inhibitors
Most hormonal treatments for endometriosis are focused on inhibiting ovarian estrogen production rather than blocking estrogen produced locally in endometriotic lesions. Aromatase is encoded by a single gene and is the final enzyme in the estrogen biosynthesis pathway. Its inhibition effectively eliminates all estrogen production. Recently introduced highly specific aromatase inhibitors have successfully treated pelvic pain and significantly reduced lesion size (141). In premenopausal women, an aromatase inhibitor alone may induce ovarian folliculogenesis, and thus aromatase inhibitors are combined with a progestin, a combination oral contraceptive, or a GnRH agonist (Fig. 3) (141). Although combining an aromatase inhibitor with a GnRH agonist may lead to extremely severe hypoestrogenic side effects, the side-effect profile of aromatase inhibitors administered in combination with an oral contraceptive or a progestin is remarkably tolerable and consists of mild hot flashes and decreased sexual desire (141). In premenopausal patients, the combination of an oral contraceptive or progestin (norethindrone acetate, 2.5 to 5 mg/d) with an aromatase inhibitor reduces visible lesions and pelvic pain refractory to other available medical and conservative surgical treatments (18–20, 177).
Endometriosis that persists after surgically induced or natural menopause is rather uncommon but continues to be a serious clinical challenge (39). For postmenopausal endometriosis, the medical treatment of choice is an aromatase inhibitor, either anastrozole (1 mg/d) or letrozole (2.5 mg/d) (39). Hot flashes and bone loss may be mitigated by adding norethindrone acetate (2.5 to 5 mg/d) and/or a bisphosphonate (zoledronic acid, 5 mg IV infusion/yr). Long-term use of a progestin may increase breast cancer risk in a postmenopausal woman (178). In contrast, in postmenopausal women with breast cancer, zoledronic acid decreases the recurrence of invasive breast cancer but slightly increases the risk of jaw necrosis associated with a dental procedure (179). In advanced stage (American Society for Reproductive Medicine stage III to IV) endometriosis, the combination of anastrozole and a GnRH agonist during postsurgical therapy decreased pain recurrence in approximately half of patients at the 2-year follow-up (17).
Antiprogestins
Selective PR modulators have primarily antiprogestogenic effects and include mifepristone (RU486), asoprisnil, and UPA (46). Mifepristone alleviates pain symptoms and induces amenorrhea without causing hypoestrogenism in patients with endometriosis (44, 180). UPA (5 to 10 mg) is approved for the treatment of endometriosis in Europe and Canada (181). In clinical trials, anovulation was observed in 81.8% women in the 5 mg group and 80% in the 10 mg group, and amenorrhea occurred in 81.2% and 90% of women in the 5 mg and 10 mg groups, respectively (182). Because UPA induces amenorrhea and possibly reduces pain, it can be used in cases of endometriosis with pain refractory to existing treatments (182). After this drug was approved in Europe and Canada, sporadic cases of liver injury and hepatic failure were recently reported in association with its use (183). UPA was not taken off the market, but the European Medicines Agency has advised periodic liver monitoring before, during, and after treatment with UPA in all patients who consider using it for uterine fibroids (183).
Targeting prostaglandin production or action
Prostaglandins are locally produced hormones linked to inflammation and pain in endometriosis. PGE2 and prostaglandin F (PGF)2α are produced excessively in uterine and endometriotic tissues (184). PGF2α induces vasoconstriction and uterine contractions that may contribute to dysmenorrhea, whereas PGE2 may induce pain directly (184). Reduction of prostaglandin formation by nonselective COX inhibitors has been shown to significantly decrease endometriosis associated pelvic pain (139, 185). Chronic administration of nonselective COX inhibitors (e.g., ibuprofen, naproxen) is limited due to their gastrointestinal side effects. Long-term administration of a selective COX2 inhibitor with milder side effects significantly reduces chronic pelvic pain, but its use has been limited by increased cardiovascular risk (147). In fact, the COX2-selective nonsteroidal anti-inflammatory drug celexocib was approved by the Food and Drug Administration for primary dysmenorrhea that is part of the symptom complex of endometriosis (147).
Arachidonic acid released by phospholipase A2 is presented to COX, which catalyzes its conversion to prostaglandin H2 (PGH2) in most cells, including in myometrium, endometrium, and endometriotic tissue (186). Among the two isoforms, COX1 is generally responsible for basal prostaglandin synthesis, whereas COX2 is important in various inflammatory and induced settings. The coupling of COX-dependent synthesis of PGH2 to its metabolism by downstream enzymes is orchestrated in a cell-specific fashion. Uterine cells are rich in PGF synthase and microsomal PGE synthase (mPGES)2, which catalyze the conversion of PGH2 to PGF2α and PGE2, respectively (184).
The contribution of excessive PGE2 production via induction of multiple enzymes, in particular COX2 and mPGES, in inflammatory settings such as endometriosis is a developing concept (187). Large quantities of PGE2 produced by endometriotic stromal cells in turn induce local estrogen biosynthesis and pelvic pain (4, 132, 136, 137). COX2 is upregulated in endometriotic stromal cells compared with endometrial stromal cells, and its expression is also higher in endometrium of women with endometriosis compared with endometrium of disease-free women (82, 188). Expression of mPGES has been observed in both benign and malignant endometrium (189–191). Thus, increased synthesis of PGE2 observed in vivo in endometriosis may be due to coordinate hyperactivity of COX2 and mPGES.
COX2 expression and PGE2 production are stimulated in endometriotic and uterine cells (Fig. 15). The cytokine IL-1β and PGE2 itself (in an autocrine manner) induce COX2 expression in endometriotic and endometrial stromal cells (192, 193). The angiogenic factor VEGF and estradiol via ERβ rapidly induce COX2 expression in uterine endothelial and endometriotic stromal cells (192–194). These redundant mechanisms maintain large quantities of PGE2 in endometriotic tissue (Fig. 15).
The biological actions of PGE2 are mediated via the G protein receptors EP1, EP2, EP3, and EP4, which integrate multiple cell signaling pathways (195). Using a xenograft mouse model of endometriosis that utilizes human endometriotic cell lines, pharmacological inhibition of the EP2 and EP4 receptors demonstrated a potential clinical benefit (195). Specifically, selective inhibition of EP2/EP4: (i) decreased growth and survival of endometriosis lesions; (ii) decreased angiogenesis and innervation of endometriosis lesions; (iii) suppressed the proinflammatory state of dorsal root ganglia neurons to decrease pelvic pain; (iv) blunted the proinflammatory, estrogen-dominant, and progesterone-resistant molecular environment of the endometrium and endometriotic lesions; and (v) restored endometrial functional receptivity (195). These data suggest that long-term inhibition of PGE2 action may be an alternative treatment of endometriosis (195).
In summary, cytokines, angiogenic factors, PGE2, and estradiol all induce COX2 expression in endometriosis to ensure large quantities of PGE2 production. Estradiol induces COX2 via ERβ in endometriosis. Disruption of PGE2 synthesis via selective or nonselective COX inhibitors effectively reduces pelvic pain in endometriosis (147).
Cytokines and ERβ
Estrogen and progesterone have variable effects on the production of different cytokines in endometrial or endometriotic cells in vitro and in vivo (196, 197). Recently, an intriguing link was noted between ERβ, TNF, and IL-1β in endometriotic tissue (97). ERβ acts as a master regulator that interacts with the cytoplasmic apoptotic machinery and inflammasome complex to prevent TNF-induced cell death and IL-1β–enhanced adhesion and proliferative activities in mouse and human endometriotic tissues (97). This nongenomic action of ERβ has a predominant role in endometriosis progression (Figs. 12 and 13), and ERβ-selective compounds that act as estradiol antagonists in endometriotic tissue are potential therapeutics. The potential role for such a compound was recently demonstrated in a mouse model of endometriosis (97). Likewise, the blockage of TNF action using systemically administered recombinant TNF receptor type-1 or a monoclonal antibody against TNF prevented the establishment of endometriosis or reduced the lesion size in a baboon model of endometriosis (198, 199).
Management of endometriosis-related infertility
Infertility management in women with endometriosis is challenging and controversial. Clinicians and patients have moved away from laparoscopic surgical treatment of all stages of endometriosis, and many clinicians no longer operate on ovarian endometriomas until fertility treatments are completed (200). In women with minimal to mild endometriosis (American Society for Reproductive Medicine stage I to II), all of the fertility outcomes were comparable to disease-free women; this included live birth rate (201). Women with more severe disease (American Society of Reproductive Medicine stage III to IV), however, had a lower live birth rate, lower clinical pregnancy rate, and lower mean number of oocytes retrieved per in vitro fertilization (IVF) cycle, when compared with women with no endometriosis (201). Ovulation induction combined with intrauterine insemination seems to robustly increase the risk of recurrent endometriosis-associated pain or disease progression (202). Therefore, IVF is the preferred method for treating infertility associated with endometriosis (201). In general, live birth rates associated with IVF are not different in infertility patients with or without endometriosis (203), and pregnancy rates are not seemingly reduced in oocyte donation recipients affected by endometriosis (204). However, ovarian suppression with a GnRH agonist or oral contraceptive significantly improved implantation rates in patients with endometriosis (204). Ovarian suppression before IVF should be considered in patients with advanced-stage endometriosis or adenomyosis. Importantly, note that the clinical impact of endometrial factor infertility in endometriosis is still debated and not well understood.
Future treatment considerations
Theoretically, replacement of abnormal eutopic endometrial stromal cells with normal ones is a potential novel therapeutic approach for endometriosis. As detailed in previous sections, epigenetically defective and progesterone-resistant eutopic endometrial stromal cells with stem-like properties may reach the pelvic cavity via retrograde menstruation and contribute to the establishment and persistence of endometriosis (1, 8, 95). During the past decade, induced pluripotent stem (iPS) cells derived from bone marrow or skin biopsies provided a patient-specific source that can be differentiated to various cells types and used as a cell-based treatment (205). It was also suggested that normal endometrial stromal cells themselves could serve as a source of adult stem cells for therapeutic applications (206).
A recent breakthrough opened up the possibility of iPS cell–based treatment of endometriosis (Fig. 17). A group directed the differentiation of human iPS cells through intermediate mesoderm, coelomic epithelium followed by the Müllerian duct to endometrial stromal fibroblasts under molecularly defined embryoid body culture conditions using specific hormonal treatments (207). Activation of β-catenin was essential for expression of PR that mediated the final differentiation step of endometrial stromal cells (207). Thus, it is possible to use skin fibroblasts of an endometriosis patient to generate iPS cells and then epigenetically reprogram these iPS cells under defined hormonal and molecular conditions to produce healthy and progesterone-responsive endometrial stromal cells. These healthy iPS-derived cells can be used for endometrial regeneration for future cell-based treatments. Multiple major technical challenges need to be overcome before it may be possible to replace diseased endometrial stromal cells with the normal ones regenerated from iPS cells.
Figure 17.
A vision of the future of endometriosis treatment. Human iPS cells are currently being tested for the treatment of a number of human conditions. The genomes of the biopsied skin or bone marrow cells are reprogrammed via the addition of four stem cell factors (OCT4, SOX2, KLF4, and MYC) to generate iPS cells. It was recently demonstrated that these pluripotent stem cells can be differentiated to endometrial stromal cells (ESCs) under defined molecular conditions (207). In the future, the differentiation protocol may be optimized to produce appropriately progesterone-responsive and healthy ESCs, which can be used to replace epigenetically defective and progesterone-resistant stromal cells in the intrauterine endometrial tissue of a woman with endometriosis.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health/National Institute of Child Health and Human Development Grant R37-HD38691 (to S.E.B.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- CCDC170
coiled-coil domain containing 170
- COX
cyclooxygenase
- CpG, cytosine-phosphate-guanine dinucleotide
CYP17A1
- 17-hydroxylase/17,20-lyase
CYP19A1
- aromatase
DNMT
- DNA methyltransferase
ER
- estrogen receptor
ESR1
- ERα
ESR2
- ERβ
FSHB
- FSH β-subunit
GATA2
- GATA-binding factor-2
GATA6
- GATA-binding factor-6
GREB1
- growth regulation by estrogen in breast cancer-1
GWAS
- genome-wide association study
HSD3B2
- 3β-hydroxysteroid dehydrogenase-2
HSD17B2
- 17β-hydroxysteroid dehydrogenase type 2
iPS
- induced pluripotent stem
IVD
- in vitro decidualization
IVF
- in vitro fertilization
mPGES
- microsomal PGE synthase
PGE2
- prostaglandin E2
PGF
- prostaglandin F
PGH2
- prostaglandin H2
PPAR
- peroxisome proliferator-activated receptor
PR
- progesterone receptor
RA
- retinoic acid
RAR
- RA receptor
RERG
- Ras-like
estrogen-regulated
- growth inhibitor
SF1
- steroidogenic factor-1
SNP
- single-nucleotide polymorphism
SRC
- steroid receptor coactivator
STAR
- steroidogenic acute regulatory protein
SYNE1
- spectrin repeat containing
nuclear envelope-1
- UPA
ulipristal acetate
References and Notes
- 1. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279. [DOI] [PubMed] [Google Scholar]
- 2. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10(5):261–275. [DOI] [PubMed] [Google Scholar]
- 3. Noble LS, Simpson ER, Johns A, Bulun SE. Aromatase expression in endometriosis. J Clin Endocrinol Metab. 1996;81(1):174–179. [DOI] [PubMed] [Google Scholar]
- 4. Noble LS, Takayama K, Zeitoun KM, Putman JM, Johns DA, Hinshelwood MM, Agarwal VR, Zhao Y, Carr BR, Bulun SE. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab. 1997;82(2):600–606. [DOI] [PubMed] [Google Scholar]
- 5. Han SJ, Jung SY, Wu SP, Hawkins SM, Park MJ, Kyo S, Qin J, Lydon JP, Tsai SY, Tsai MJ, DeMayo FJ, O’Malley BW. Estrogen receptor β modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell. 2015;163(4):960–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14(4):422–469. [Google Scholar]
- 7. Giudice LC. Endometriosis. N Engl J Med. 2010;362(25):2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gargett CE, Schwab KE, Deane JA. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update. 2016;22(2):137–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brawn J, Morotti M, Zondervan KT, Becker CM, Vincent K. Central changes associated with chronic pelvic pain and endometriosis. Hum Reprod Update. 2014;20(5):737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davis AC, Goldberg JM. Extrapelvic endometriosis. Semin Reprod Med. 2017;35(1):98–101. [DOI] [PubMed] [Google Scholar]
- 11. Chen LC, Hsu JW, Huang KL, Bai YM, Su TP, Li CT, Yang AC, Chang WH, Chen TJ, Tsai SJ, Chen MH. Risk of developing major depression and anxiety disorders among women with endometriosis: a longitudinal follow-up study. J Affect Disord. 2016;190:282–285. [DOI] [PubMed] [Google Scholar]
- 12. Brosens I, Gordts S, Benagiano G. Endometriosis in adolescents is a hidden, progressive and severe disease that deserves attention, not just compassion. Hum Reprod. 2013;28(8):2026–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aredo JV, Heyrana KJ, Karp BI, Shah JP, Stratton P. Relating chronic pelvic pain and endometriosis to signs of sensitization and myofascial pain and dysfunction. Semin Reprod Med. 2017;35(1):88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vercellini P, Somigliana E, Vigano P, Abbiati A, Barbara G, Fedele L. “Blood on the Tracks” from corpora lutea to endometriomas. BJOG. 2009;116(3):366–371. [DOI] [PubMed] [Google Scholar]
- 15. Khan KN, Fujishita A, Kitajima M, Hiraki K, Nakashima M, Masuzaki H. Occult microscopic endometriosis: undetectable by laparoscopy in normal peritoneum. Hum Reprod. 2014;29(3):462–472. [DOI] [PubMed] [Google Scholar]
- 16. Matsuzaki S, Canis M, Pouly JL, Wattiez A, Okamura K, Mage G. Cyclooxygenase-2 expression in deep endometriosis and matched eutopic endometrium. Fertil Steril. 2004;82(5):1309–1315. [DOI] [PubMed] [Google Scholar]
- 17. Soysal S, Soysal ME, Ozer S, Gul N, Gezgin T. The effects of post-surgical administration of goserelin plus anastrozole compared to goserelin alone in patients with severe endometriosis: a prospective randomized trial. Hum Reprod. 2004;19(1):160–167. [DOI] [PubMed] [Google Scholar]
- 18. Ailawadi RK, Jobanputra S, Kataria M, Gurates B, Bulun SE. Treatment of endometriosis and chronic pelvic pain with letrozole and norethindrone acetate: a pilot study. Fertil Steril. 2004;81(2):290–296. [DOI] [PubMed] [Google Scholar]
- 19. Amsterdam LL, Gentry W, Jobanputra S, Wolf M, Rubin SD, Bulun SE. Anastrazole and oral contraceptives: a novel treatment for endometriosis. Fertil Steril. 2005;84(2):300–304. [DOI] [PubMed] [Google Scholar]
- 20. Abushahin F, Goldman KN, Barbieri E, Milad M, Rademaker A, Bulun SE. Aromatase inhibition for refractory endometriosis-related chronic pelvic pain. Fertil Steril. 2011;96(4):939–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vercellini P, Eskenazi B, Consonni D, Somigliana E, Parazzini F, Abbiati A, Fedele L. Oral contraceptives and risk of endometriosis: a systematic review and meta-analysis. Hum Reprod Update. 2011;17(2):159–170. [DOI] [PubMed] [Google Scholar]
- 22. Heidemann LN, Hartwell D, Heidemann CH, Jochumsen KM. The relation between endometriosis and ovarian cancer—a review. Acta Obstet Gynecol Scand. 2014;93(1):20–31. [DOI] [PubMed] [Google Scholar]
- 23. Wilbur MA, Shih IM, Segars JH, Fader AN. Cancer implications for patients with endometriosis. Semin Reprod Med. 2017;35(1):110–116. [DOI] [PubMed] [Google Scholar]
- 24. Anglesio MS, Papadopoulos N, Ayhan A, Nazeran TM, Noë M, Horlings HM, Lum A, Jones S, Senz J, Seckin T, Ho J, Wu RC, Lac V, Ogawa H, Tessier-Cloutier B, Alhassan R, Wang A, Wang Y, Cohen JD, Wong F, Hasanovic A, Orr N, Zhang M, Popoli M, McMahon W, Wood LD, Mattox A, Allaire C, Segars J, Williams C, Tomasetti C, Boyd N, Kinzler KW, Gilks CB, Diaz L, Wang TL, Vogelstein B, Yong PJ, Huntsman DG, Shih IM. Cancer-associated mutations in endometriosis without cancer. N Engl J Med. 2017;376(19):1835–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suda K, Nakaoka H, Yoshihara K, Ishiguro T, Tamura R, Mori Y, Yamawaki K, Adachi S, Takahashi T, Kase H, Tanaka K, Yamamoto T, Motoyama T, Inoue I, Enomoto T. Clonal expansion and diversification of cancer-associated mutations in endometriosis and normal endometrium. Cell Reports. 2018;24(7):1777–1789. [DOI] [PubMed] [Google Scholar]
- 26. Nezhat FR, Mahmoud MS. Allen masters peritoneal defect: a potential pathway to deep infiltrating rectovaginal endometriosis? J Minim Invasive Gynecol. 2014;21(3):321–322. [DOI] [PubMed] [Google Scholar]
- 27. Brosens I, Pijnenborg R, Benagiano G. Defective myometrial spiral artery remodelling as a cause of major obstetrical syndromes in endometriosis and adenomyosis. Placenta. 2013;34(2):100–105. [DOI] [PubMed] [Google Scholar]
- 28. Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144(7):2870–2881. [DOI] [PubMed] [Google Scholar]
- 29. Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148(8):3814–3826. [DOI] [PubMed] [Google Scholar]
- 30. Tseng JF, Ryan IP, Milam TD, Murai JT, Schriock ED, Landers DV, Taylor RN. Interleukin-6 secretion in vitro is up-regulated in ectopic and eutopic endometrial stromal cells from women with endometriosis. J Clin Endocrinol Metab. 1996;81(3):1118–1122. [DOI] [PubMed] [Google Scholar]
- 31. Hornung D, Klingel K, Dohrn K, Kandolf R, Wallwiener D, Taylor RN. Regulated on activation, normal T-cell-expressed and -secreted mRNA expression in normal endometrium and endometriotic implants: assessment of autocrine/paracrine regulation by in situ hybridization. Am J Pathol. 2001;158(6):1949–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bulun SE, Monsavais D, Pavone ME, Dyson M, Xue Q, Attar E, Tokunaga H, Su EJ. Role of estrogen receptor-β in endometriosis. Semin Reprod Med. 2012;30(1):39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Monsivais D, Dyson MT, Yin P, Navarro A, Coon JS, Pavone ME, Bulun SE. Estrogen receptor β regulates endometriotic cell survival through serum and glucocorticoid-regulated kinase activation. Fertil Steril. 2016;105(5):1266–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Monsivais D, Dyson MT, Yin P, Coon JS, Navarro A, Feng G, Malpani SS, Ono M, Ercan CM, Wei JJ, Pavone ME, Su E, Bulun SE. ERβ- and prostaglandin E2-regulated pathways integrate cell proliferation via Ras-like and estrogen-regulated growth inhibitor in endometriosis. Mol Endocrinol. 2014;28(8):1304–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bruner KL, Matrisian LM, Rodgers WH, Gorstein F, Osteen KG. Suppression of matrix metalloproteinases inhibits establishment of ectopic lesions by human endometrium in nude mice. J Clin Invest. 1997;99(12):2851–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Langoi D, Pavone ME, Gurates B, Chai D, Fazleabas A, Bulun SE. Aromatase inhibitor treatment limits progression of peritoneal endometriosis in baboons. Fertil Steril. 2013;99(3):656–662.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fang Z, Yang S, Gurates B, Tamura M, Simpson E, Evans D, Bulun SE. Genetic or enzymatic disruption of aromatase inhibits the growth of ectopic uterine tissue. J Clin Endocrinol Metab. 2002;87(7):3460–3466. [DOI] [PubMed] [Google Scholar]
- 38. McLaren J, Prentice A, Charnock-Jones DS, Millican SA, Müller KH, Sharkey AM, Smith SK. Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J Clin Invest. 1996;98(2):482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takayama K, Zeitoun K, Gunby RT, Sasano H, Carr BR, Bulun SE. Treatment of severe postmenopausal endometriosis with an aromatase inhibitor. Fertil Steril. 1998;69(4):709–713. [DOI] [PubMed] [Google Scholar]
- 40. Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85(8):2897–2902. [DOI] [PubMed] [Google Scholar]
- 41. Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34(1):130–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zeitoun K, Takayama K, Sasano H, Suzuki T, Moghrabi N, Andersson S, Johns A, Meng L, Putman M, Carr B, Bulun SE. Deficient 17β-hydroxysteroid dehydrogenase type 2 expression in endometriosis: failure to metabolize 17β-estradiol. J Clin Endocrinol Metab. 1998;83(12):4474–4480. [DOI] [PubMed] [Google Scholar]
- 43. Attar E, Tokunaga H, Imir G, Yilmaz MB, Redwine D, Putman M, Gurates B, Attar R, Yaegashi N, Hales DB, Bulun SE. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J Clin Endocrinol Metab. 2009;94(2):623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kettel LM, Murphy AA, Morales AJ, Ulmann A, Baulieu EE, Yen SS. Treatment of endometriosis with the antiprogesterone mifepristone (RU486). Fertil Steril. 1996;65(1):23–28. [DOI] [PubMed] [Google Scholar]
- 45. Gainer EE, Ulmann A. Pharmacologic properties of CDB(VA)-2914. Steroids. 2003;68(10–13):1005–1011. [DOI] [PubMed] [Google Scholar]
- 46. Chwalisz K, Perez MC, Demanno D, Winkel C, Schubert G, Elger W. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr Rev. 2005;26(3):423–438. [DOI] [PubMed] [Google Scholar]
- 47. Li X, Zhang Y, Zhao L, Wang L, Wu Z, Mei Q, Nie J, Li X, Li Y, Fu X, Wang X, Meng Y, Han W. Whole-exome sequencing of endometriosis identifies frequent alterations in genes involved in cell adhesion and chromatin-remodeling complexes. Hum Mol Genet. 2014;23(22):6008–6021. [DOI] [PubMed] [Google Scholar]
- 48. Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, Yang W, Heravi-Moussavi A, Giuliany R, Chow C, Fee J, Zayed A, Prentice L, Melnyk N, Turashvili G, Delaney AD, Madore J, Yip S, McPherson AW, Ha G, Bell L, Fereday S, Tam A, Galletta L, Tonin PN, Provencher D, Miller D, Jones SJ, Moore RA, Morin GB, Oloumi A, Boyd N, Aparicio SA, Shih IeM, Mes-Masson AM, Bowtell DD, Hirst M, Gilks B, Marra MA, Huntsman DG. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363(16):1532–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kuo KT, Mao TL, Jones S, Veras E, Ayhan A, Wang TL, Glas R, Slamon D, Velculescu VE, Kuman RJ, Shih IeM. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am J Pathol. 2009;174(5):1597–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Suvitie PA, Hallamaa MK, Matomäki JM, Mäkinen JI, Perheentupa AH. Prevalence of Pain Symptoms Suggestive of Endometriosis Among Finnish Adolescent Girls (TEENMAPS Study). J Pediatr Adolesc Gynecol. 2016;29(2):97–103. [DOI] [PubMed] [Google Scholar]
- 51. Rogers PA, D’Hooghe TM, Fazleabas A, Gargett CE, Giudice LC, Montgomery GW, Rombauts L, Salamonsen LA, Zondervan KT. Priorities for endometriosis research: recommendations from an international consensus workshop. Reprod Sci. 2009;16(4):335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schliep KC, Mumford SL, Peterson CM, Chen Z, Johnstone EB, Sharp HT, Stanford JB, Hammoud AO, Sun L, Buck Louis GM. Pain typology and incident endometriosis. Hum Reprod. 2015;30(10):2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Apostolopoulos NV, Alexandraki KI, Gorry A, Coker A. Association between chronic pelvic pain symptoms and the presence of endometriosis. Arch Gynecol Obstet. 2016;293(2):439–445. [DOI] [PubMed] [Google Scholar]
- 54. Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24(2):235–258. [DOI] [PubMed] [Google Scholar]
- 55. Missmer SA, Cramer DW. The epidemiology of endometriosis. Obstet Gynecol Clin North Am. 2003;30(1):1–19. [DOI] [PubMed] [Google Scholar]
- 56. Viganò P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):177–200. [DOI] [PubMed] [Google Scholar]
- 57. Chapron C, Chopin N, Borghese B, Foulot H, Dousset B, Vacher-Lavenu MC, Vieira M, Hasan W, Bricou A. Deeply infiltrating endometriosis: pathogenetic implications of the anatomical distribution. Hum Reprod. 2006;21(7):1839–1845. [DOI] [PubMed] [Google Scholar]
- 58. Farquhar C. Endometriosis. BMJ. 2007;334(7587):249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Malspeis S, Willett WC, Hunter DJ. Reproductive history and endometriosis among premenopausal women. Obstet Gynecol. 2004;104(5 Pt 1):965–974. [DOI] [PubMed] [Google Scholar]
- 60. Thomas EJ. Endometriosis. BMJ. 1993;306(6871):158–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Eaton SB, Pike MC, Short RV, Lee NC, Trussell J, Hatcher RA, Wood JW, Worthman CM, Jones NG, Konner MJ, Hill KR, Bailey R, Hurtado AM. Women’s reproductive cancers in evolutionary context. Q Rev Biol. 1994;69(3):353–367. [DOI] [PubMed] [Google Scholar]
- 62. Evers J, Dunselman G, Land J, Bouckaert P, Van Der Linden P. Management of recurrent endometriosis. In: Coutinho EM, Spinola P, Hanson de Moura L, eds. Progress in the Management of Endometriosis. London, UK: Parthenon Publishing Group; 1995:291–297. [Google Scholar]
- 63. Vercellini P, Somigliana E, Viganò P, De Matteis S, Barbara G, Fedele L. Post-operative endometriosis recurrence: a plea for prevention based on pathogenetic, epidemiological and clinical evidence. Reprod Biomed Online. 2010;21(2):259–265. [DOI] [PubMed] [Google Scholar]
- 64. Jain S, Dalton ME. Chocolate cysts from ovarian follicles. Fertil Steril. 1999;72(5):852–856. [DOI] [PubMed] [Google Scholar]
- 65. Scurry J, Whitehead J, Healey M. Classification of ovarian endometriotic cysts. Int J Gynecol Pathol. 2001;20(2):147–154. [DOI] [PubMed] [Google Scholar]
- 66. D’Hooghe TM, Debrock S. Endometriosis, retrograde menstruation and peritoneal inflammation in women and in baboons. Hum Reprod Update. 2002;8(1):84–88. [DOI] [PubMed] [Google Scholar]
- 67. Ferguson BR, Bennington JL, Haber SL. Histochemistry of mucosubstances and histology of mixed Müllerian pelvic lymph node glandular inclusions. Evidence for histogenesis by Müllerian metaplasia of coelomic epithelium. Obstet Gynecol. 1969;33(5):617–625. [PubMed] [Google Scholar]
- 68. Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3(2): 93–110.43. [PMC free article] [PubMed] [Google Scholar]
- 69. Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci. 2008;1127(1):106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bulun SE, Monsivais D, Kakinuma T, Furukawa Y, Bernardi L, Pavone ME, Dyson M. Molecular biology of endometriosis: from aromatase to genomic abnormalities. Semin Reprod Med. 2015;33(3):220–224. [DOI] [PubMed] [Google Scholar]
- 71. Lucidi RS, Witz CA, Chrisco M, Binkley PA, Shain SA, Schenken RS. A novel in vitro model of the early endometriotic lesion demonstrates that attachment of endometrial cells to mesothelial cells is dependent on the source of endometrial cells. Fertil Steril. 2005;84(1):16–21. [DOI] [PubMed] [Google Scholar]
- 72. Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–1799. [DOI] [PubMed] [Google Scholar]
- 73. Hornung D, Ryan IP, Chao VA, Vigne JL, Schriock ED, Taylor RN. Immunolocalization and regulation of the chemokine RANTES in human endometrial and endometriosis tissues and cells. J Clin Endocrinol Metab. 1997;82(5):1621–1628. [DOI] [PubMed] [Google Scholar]
- 74. Wu Y, Kajdacsy-Balla A, Strawn E, Basir Z, Halverson G, Jailwala P, Wang Y, Wang X, Ghosh S, Guo SW. Transcriptional characterizations of differences between eutopic and ectopic endometrium. Endocrinology. 2006;147(1):232–246. [DOI] [PubMed] [Google Scholar]
- 75. Osteen KG, Bruner KL, Sharpe-Timms KL. Steroid and growth factor regulation of matrix metalloproteinase expression and endometriosis. Semin Reprod Endocrinol. 1996;14(3):247–255. [DOI] [PubMed] [Google Scholar]
- 76. Nair AS, Nair HB, Lucidi RS, Kirchner AJ, Schenken RS, Tekmal RR, Witz CA. Modeling the early endometriotic lesion: mesothelium-endometrial cell co-culture increases endometrial invasion and alters mesothelial and endometrial gene transcription. Fertil Steril. 2008;90(4Suppl):1487–1495. [DOI] [PubMed] [Google Scholar]
- 77. Brosens I, Benagiano G. Is neonatal uterine bleeding involved in the pathogenesis of endometriosis as a source of stem cells? Fertil Steril. 2013;100(3):622–623. [DOI] [PubMed] [Google Scholar]
- 78. Brosens I, Brosens J, Benagiano G. Neonatal uterine bleeding as antecedent of pelvic endometriosis. Hum Reprod. 2013;28(11):2893–2897. [DOI] [PubMed] [Google Scholar]
- 79. Gargett CE, Schwab KE, Brosens JJ, Puttemans P, Benagiano G, Brosens I. Potential role of endometrial stem/progenitor cells in the pathogenesis of early-onset endometriosis. Mol Hum Reprod. 2014;20(7):591–598. [DOI] [PubMed] [Google Scholar]
- 80. Dmowski WP, Gebel HM, Rawlins RG. Immunologic aspects of endometriosis. Obstet Gynecol Clin North Am. 1989;16(1):93–103. [PubMed] [Google Scholar]
- 81. Osteen KG, Sierra-Rivera E. Does disruption of immune and endocrine systems by environmental toxins contribute to development of endometriosis? Semin Reprod Endocrinol. 1997;15(3):301–308. [DOI] [PubMed] [Google Scholar]
- 82. Ota H, Igarashi S, Sasaki M, Tanaka T. Distribution of cyclooxygenase-2 in eutopic and ectopic endometrium in endometriosis and adenomyosis. Hum Reprod. 2001;16(3):561–566. [DOI] [PubMed] [Google Scholar]
- 83. Tokushige N, Markham R, Russell P, Fraser IS. High density of small nerve fibres in the functional layer of the endometrium in women with endometriosis. Hum Reprod. 2006;21(3):782–787. [DOI] [PubMed] [Google Scholar]
- 84. Barragan F, Irwin JC, Balayan S, Erikson DW, Chen JC, Houshdaran S, Piltonen TT, Spitzer TL, George A, Rabban JT, Nezhat C, Giudice LC. Human endometrial fibroblasts derived from mesenchymal progenitors inherit progesterone resistance and acquire an inflammatory phenotype in the endometrial niche in endometriosis. Biol Reprod. 2016;94(5):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292(1):81–85. [DOI] [PubMed] [Google Scholar]
- 86. Hufnagel D, Li F, Cosar E, Krikun G, Taylor HS. The role of stem cells in the etiology and pathophysiology of endometriosis. Semin Reprod Med. 2015;33(5):333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hapangama DK, Drury J, Da Silva L, Al-Lamee H, Earp A, Valentijn AJ, Edirisinghe DP, Murray PA, Fazleabas AT, Gargett CE. Abnormally located SSEA1+/SOX9+ endometrial epithelial cells with a basalis-like phenotype in the eutopic functionalis layer may play a role in the pathogenesis of endometriosis. Hum Reprod. 2019;34(1):56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li D, Li H, Wang Y, Eldomany A, Wu J, Yuan C, Xue J, Shi J, Jia Y, Ha C, Han S, Liu X, Yang J, Liu D. Development and characterization of a polarized human endometrial cell epithelia in an air-liquid interface state. Stem Cell Res Ther. 2018;9(1):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Houshdaran S, Nezhat CR, Vo KC, Zelenko Z, Irwin JC, Giudice LC. Aberrant endometrial DNA methylome and associated gene expression in women with endometriosis. Biol Reprod. 2016;95(5):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Witz CA, Thomas MR, Montoya-Rodriguez IA, Nair AS, Centonze VE, Schenken RS. Short-term culture of peritoneum explants confirms attachment of endometrium to intact peritoneal mesothelium. Fertil Steril. 2001;75(2):385–390. [DOI] [PubMed] [Google Scholar]
- 91. Meyer R. Zur Frage der heterotopen Epithelwucherung, insbesondere des Peritonealepithels und in den Ovarien. Virchows Arch. 1924;250(3):595–610. [Google Scholar]
- 92. Dmowski WP, Ding J, Shen J, Rana N, Fernandez BB, Braun DP. Apoptosis in endometrial glandular and stromal cells in women with and without endometriosis. Hum Reprod. 2001;16(9):1802–1808. [DOI] [PubMed] [Google Scholar]
- 93. Béliard A, Noël A, Foidart JM. Reduction of apoptosis and proliferation in endometriosis. Fertil Steril. 2004;82(1):80–85. [DOI] [PubMed] [Google Scholar]
- 94. Dyson MT, Roqueiro D, Monsivais D, Ercan CM, Pavone ME, Brooks DC, Kakinuma T, Ono M, Jafari N, Dai Y, Bulun SE. Genome-wide DNA methylation analysis predicts an epigenetic switch for GATA factor expression in endometriosis. PLoS Genet. 2014;10(3):e1004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Aghajanova L, Tatsumi K, Horcajadas JA, Zamah AM, Esteban FJ, Herndon CN, Conti M, Giudice LC. Unique transcriptome, pathways, and networks in the human endometrial fibroblast response to progesterone in endometriosis. Biol Reprod. 2011;84(4):801–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75(1):1–10. [DOI] [PubMed] [Google Scholar]
- 97. Chandler RL, Damrauer JS, Raab JR, Schisler JC, Wilkerson MD, Didion JP, Starmer J, Serber D, Yee D, Xiong J, Darr DB, Pardo-Manuel de Villena F, Kim WY, Magnuson T. Coexistent ARID1A–PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat Commun. 2015;6(1):6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bulun SE, Simpson ER. Competitive reverse transcription-polymerase chain reaction analysis indicates that levels of aromatase cytochrome P450 transcripts in adipose tissue of buttocks, thighs, and abdomen of women increase with advancing age. J Clin Endocrinol Metab. 1994;78(2):428–432. [DOI] [PubMed] [Google Scholar]
- 99. Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, Tamura M, Langoi D, Deb S. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57(3):359–383. [DOI] [PubMed] [Google Scholar]
- 100. Ahn SH, Edwards AK, Singh SS, Young SL, Lessey BA, Tayade C. IL-17A contributes to the pathogenesis of endometriosis by triggering proinflammatory cytokines and angiogenic growth factors. J Immunol. 2015;195(6):2591–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Greaves E, Collins F, Esnal-Zufiaurre A, Giakoumelou S, Horne AW, Saunders PT. Estrogen receptor (ER) agonists differentially regulate neuroangiogenesis in peritoneal endometriosis via the repellent factor SLIT3. Endocrinology. 2014;155(10):4015–4026. [DOI] [PubMed] [Google Scholar]
- 102. Bulun SE. Endometriosis. In: Strauss J, Barbieri R, eds. Yen & Jaffe’s Reproductive Endocrinology. 8th ed. Philadelphia, PA: Elsevier; 2019:609–642. [Google Scholar]
- 103. Simpson JL, Elias S, Malinak LR, Buttram VC Jr. Heritable aspects of endometriosis. I. Genetic studies. Am J Obstet Gynecol. 1980;137(3):327–331. [DOI] [PubMed] [Google Scholar]
- 104. Kennedy S, Mardon H, Barlow D. Familial endometriosis. J Assist Reprod Genet. 1995;12(1):32–34. [DOI] [PubMed] [Google Scholar]
- 105. Treloar SA, O’Connor DT, O’Connor VM, Martin NG. Genetic influences on endometriosis in an Australian twin sample. Fertil Steril. 1999;71(4):701–710. [DOI] [PubMed] [Google Scholar]
- 106. Montgomery GW, Nyholt DR, Zhao ZZ, Treloar SA, Painter JN, Missmer SA, Kennedy SH, Zondervan KT. The search for genes contributing to endometriosis risk. Hum Reprod Update. 2008;14(5):447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rahmioglu N, Missmer SA, Montgomery GW, Zondervan KT. Insights into assessing the genetics of endometriosis. Curr Obstet Gynecol Rep. 2012;1(3):124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Treloar SA, Zhao ZZ, Le L, Zondervan KT, Martin NG, Kennedy S, Nyholt DR, Montgomery GW. Variants in EMX2 and PTEN do not contribute to risk of endometriosis. Mol Hum Reprod. 2007;13(8):587–594. [DOI] [PubMed] [Google Scholar]
- 109. Zhao ZZ, Nyholt DR, Le L, Treloar SA, Montgomery GW. Common variation in the CYP17A1 and IFIT1 genes on chromosome 10 does not contribute to the risk of endometriosis. Open Reprod Sci J. 2008;1(1):35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lin J, Zong L, Kennedy SH, Zondervan KT. Coding regions of INHBA, SFRP4 and HOXA10 are not implicated in familial endometriosis linked to chromosome 7p13–15. Mol Hum Reprod. 2011;17(10):605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zondervan KT, Cardon LR, Kennedy SH. What makes a good case-control study? Design issues for complex traits such as endometriosis. Hum Reprod. 2002;17(6):1415–1423. [DOI] [PubMed] [Google Scholar]
- 112. Treloar SA, Wicks J, Nyholt DR, Montgomery GW, Bahlo M, Smith V, Dawson G, Mackay IJ, Weeks DE, Bennett ST, Carey A, Ewen-White KR, Duffy DL, O’connor DT, Barlow DH, Martin NG, Kennedy SH. Genomewide linkage study in 1,176 affected sister pair families identifies a significant susceptibility locus for endometriosis on chromosome 10q26. Am J Hum Genet. 2005;77(3):365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Painter JN, Anderson CA, Nyholt DR, Macgregor S, Lin J, Lee SH, Lambert A, Zhao ZZ, Roseman F, Guo Q, Gordon SD, Wallace L, Henders AK, Visscher PM, Kraft P, Martin NG, Morris AP, Treloar SA, Kennedy SH, Missmer SA, Montgomery GW, Zondervan KT. Genome-wide association study identifies a locus at 7p15.2 associated with endometriosis. Nat Genet. 2011;43(1):51–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Painter JN, Nyholt DR, Morris A, Zhao ZZ, Henders AK, Lambert A, Wallace L, Martin NG, Kennedy SH, Treloar SA, Zondervan KT, Montgomery GW. High-density fine-mapping of a chromosome 10q26 linkage peak suggests association between endometriosis and variants close to CYP2C19. Fertil Steril. 2011;95(7):2236–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mwinyi J, Cavaco I, Pedersen RS, Persson A, Burkhardt S, Mkrtchian S, Ingelman-Sundberg M. Regulation of CYP2C19 expression by estrogen receptor α: implications for estrogen-dependent inhibition of drug metabolism. Mol Pharmacol. 2010;78(5):886–894. [DOI] [PubMed] [Google Scholar]
- 116. Zondervan KT, Treloar SA, Lin J, Weeks DE, Nyholt DR, Mangion J, MacKay IJ, Cardon LR, Martin NG, Kennedy SH, Montgomery GW. Significant evidence of one or more susceptibility loci for endometriosis with near-Mendelian inheritance on chromosome 7p13–15. Hum Reprod. 2007;22(3):717–728. [DOI] [PubMed] [Google Scholar]
- 117. Uno S, Zembutsu H, Hirasawa A, Takahashi A, Kubo M, Akahane T, Aoki D, Kamatani N, Hirata K, Nakamura Y. A genome-wide association study identifies genetic variants in the CDKN2BAS locus associated with endometriosis in Japanese. Nat Genet. 2010;42(8):707–710. [DOI] [PubMed] [Google Scholar]
- 118. Nyholt DR, Low SK, Anderson CA, Painter JN, Uno S, Morris AP, MacGregor S, Gordon SD, Henders AK, Martin NG, Attia J, Holliday EG, McEvoy M, Scott RJ, Kennedy SH, Treloar SA, Missmer SA, Adachi S, Tanaka K, Nakamura Y, Zondervan KT, Zembutsu H, Montgomery GW. Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat Genet. 2012;44(12):1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Rahmioglu N, Nyholt DR, Morris AP, Missmer SA, Montgomery GW, Zondervan KT. Genetic variants underlying risk of endometriosis: insights from meta-analysis of eight genome-wide association and replication datasets. Hum Reprod Update. 2014;20(5):702–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sapkota Y, Steinthorsdottir V, Morris AP, Fassbender A, Rahmioglu N, De Vivo I, Buring JE, Zhang F, Edwards TL, Jones S, O D, Peterse D, Rexrode KM, Ridker PM, Schork AJ, MacGregor S, Martin NG, Becker CM, Adachi S, Yoshihara K, Enomoto T, Takahashi A, Kamatani Y, Matsuda K, Kubo M, Thorleifsson G, Geirsson RT, Thorsteinsdottir U, Wallace LM, Yang J, Velez Edwards DR, Nyegaard M, Low SK, Zondervan KT, Missmer SA, D’Hooghe T, Montgomery GW, Chasman DI, Stefansson K, Tung JY, Nyholt DR; iPSYCH-SSI-Broad Group. Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat Commun. 2017;8:15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bulun SE. Physiology and pathology of the female reproductive axis. In: Melmed S, Polonsky K, Larsen P, Kronenberg H, eds. Williams Textbook of Endocrinology. 13th ed.Philadelphia, PA: Elsevier; 2016:627–663. [Google Scholar]
- 122. Hodgkinson K, Forrest LA, Vuong N, Garson K, Djordjevic B, Vanderhyden BC. GREB1 is an estrogen receptor-regulated tumour promoter that is frequently expressed in ovarian cancer. Oncogene. 2018;37(44):5873–5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Veeraraghavan J, Tan Y, Cao XX, Kim JA, Wang X, Chamness GC, Maiti SN, Cooper LJ, Edwards DP, Contreras A, Hilsenbeck SG, Chang EC, Schiff R, Wang XS. Recurrent ESR1–CCDC170 rearrangements in an aggressive subset of oestrogen receptor-positive breast cancers. Nat Commun. 2014;5(1):4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Doherty JA, Rossing MA, Cushing-Haugen KL, Chen C, Van Den Berg DJ, Wu AH, Pike MC, Ness RB, Moysich K, Chenevix-Trench G, Beesley J, Webb PM, Chang-Claude J, Wang-Gohrke S, Goodman MT, Lurie G, Thompson PJ, Carney ME, Hogdall E, Kjaer SK, Hogdall C, Goode EL, Cunningham JM, Fridley BL, Vierkant RA, Berchuck A, Moorman PG, Schildkraut JM, Palmieri RT, Cramer DW, Terry KL, Yang HP, Garcia-Closas M, Chanock S, Lissowska J, Song H, Pharoah PD, Shah M, Perkins B, McGuire V, Whittemore AS, Di Cioccio RA, Gentry-Maharaj A, Menon U, Gayther SA, Ramus SJ, Ziogas A, Brewster W, Anton-Culver H, Pearce CL; Australian Ovarian Cancer Study Management Group; Australian Cancer Study (Ovarian Cancer); Ovarian Cancer Association Consortium (OCAC). ESR1/SYNE1 polymorphism and invasive epithelial ovarian cancer risk: an Ovarian Cancer Association Consortium study. Cancer Epidemiol Biomarkers Prev. 2010;19(1):245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Grechukhina O, Petracco R, Popkhadze S, Massasa E, Paranjape T, Chan E, Flores I, Weidhaas JB, Taylor HS. A polymorphism in a let-7 microRNA binding site of KRAS in women with endometriosis. EMBO Mol Med. 2012;4(3):206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Dyson MT, Kakinuma T, Pavone ME, Monsivais D, Navarro A, Malpani SS, Ono M, Bulun SE. Aberrant expression and localization of deoxyribonucleic acid methyltransferase 3B in endometriotic stromal cells. Fertil Steril. 2015;104(4):953–963.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bernardi LA, Dyson MT, Tokunaga H, Sison C, Oral M, Robins JC, Bulun SE. The essential role of GATA6 in the activation of estrogen synthesis in endometriosis. Reprod Sci. 2019;26(1):60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Naqvi H, Ilagan Y, Krikun G, Taylor HS. Altered genome-wide methylation in endometriosis. Reprod Sci. 2014;21(10):1237–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lee B, Du H, Taylor HS. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol Reprod. 2009;80(1):79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Rubel CA, Wu SP, Lin L, Wang T, Lanz RB, Li X, Kommagani R, Franco HL, Camper SA, Tong Q, Jeong JW, Lydon JP, DeMayo FJ. A Gata2-dependent transcription network regulates uterine progesterone responsiveness and endometrial function. Cell Reports. 2016;17(5):1414–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lala DS, Rice DA, Parker KL. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol Endocrinol. 1992;6(8):1249–1258. [DOI] [PubMed] [Google Scholar]
- 132. Zeitoun K, Takayama K, Michael MD, Bulun SE. Stimulation of aromatase P450 promoter (II) activity in endometriosis and its inhibition in endometrium are regulated by competitive binding of steroidogenic factor-1 and chicken ovalbumin upstream promoter transcription factor to the same cis-acting element. Mol Endocrinol. 1999;13(2):239–253. [DOI] [PubMed] [Google Scholar]
- 133. Xue Q, Lin Z, Yin P, Milad MP, Cheng YH, Confino E, Reierstad S, Bulun SE. Transcriptional activation of steroidogenic factor-1 by hypomethylation of the 5′ CpG island in endometriosis. J Clin Endocrinol Metab. 2007;92(8):3261–3267. [DOI] [PubMed] [Google Scholar]
- 134. Utsunomiya H, Cheng YH, Lin Z, Reierstad S, Yin P, Attar E, Xue Q, Imir G, Thung S, Trukhacheva E, Suzuki T, Sasano H, Kim JJ, Yaegashi N, Bulun SE. Upstream stimulatory factor-2 regulates steroidogenic factor-1 expression in endometriosis. Mol Endocrinol. 2008;22(4):904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ryan IP, Taylor RN. Endometriosis and infertility: new concepts. Obstet Gynecol Surv. 1997;52(6):365–371. [DOI] [PubMed] [Google Scholar]
- 136. Tsai SJ, Wu MH, Lin CC, Sun HS, Chen HM. Regulation of steroidogenic acute regulatory protein expression and progesterone production in endometriotic stromal cells. J Clin Endocrinol Metab. 2001;86(12):5765–5773. [DOI] [PubMed] [Google Scholar]
- 137. Sun HS, Hsiao KY, Hsu CC, Wu MH, Tsai SJ. Transactivation of steroidogenic acute regulatory protein in human endometriotic stromalcells is mediated by the prostaglandin EP2 receptor. Endocrinology. 2003;144(9):3934–3942. [DOI] [PubMed] [Google Scholar]
- 138. Bulun SE, Yang S, Fang Z, Gurates B, Tamura M, Zhou J, Sebastian S. Role of aromatase in endometrial disease. J Steroid Biochem Mol Biol. 2001;79(1-5):19–25. [DOI] [PubMed] [Google Scholar]
- 139. Olive DL, Pritts EA. Treatment of endometriosis. N Engl J Med. 2001;345(4):266–275. [DOI] [PubMed] [Google Scholar]
- 140. Zeitoun KM, Bulun SE. Aromatase: a key molecule in the pathophysiology of endometriosis and a therapeutic target. Fertil Steril. 1999;72(6):961–969. [DOI] [PubMed] [Google Scholar]
- 141. Attar E, Bulun SE. Aromatase inhibitors: the next generation of therapeutics for endometriosis? Fertil Steril. 2006;85(5):1307–1318. [DOI] [PubMed] [Google Scholar]
- 142. Xue Q, Lin Z, Cheng YH, Huang CC, Marsh E, Yin P, Milad MP, Confino E, Reierstad S, Innes J, Bulun SE. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod. 2007;77(4):681–687. [DOI] [PubMed] [Google Scholar]
- 143. Juhasz-Böss I, Fischer C, Lattrich C, Skrzypczak M, Malik E, Ortmann O, Treeck O. Endometrial expression of estrogen receptor β and its splice variants in patients with and without endometriosis. Arch Gynecol Obstet. 2011;284(4):885–891. [DOI] [PubMed] [Google Scholar]
- 144. Han SJ, Hawkins SM, Begum K, Jung SY, Kovanci E, Qin J, Lydon JP, DeMayo FJ, O’Malley BW. A new isoform of steroid receptor coactivator-1 is crucial for pathogenic progression of endometriosis. Nat Med. 2012;18(7):1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Trukhacheva E, Lin Z, Reierstad S, Cheng YH, Milad M, Bulun SE. Estrogen receptor (ER) β regulates ERα expression in stromal cells derived from ovarian endometriosis. J Clin Endocrinol Metab. 2009;94(2):615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Tamura M, Deb S, Sebastian S, Okamura K, Bulun SE. Estrogen up-regulates cyclooxygenase-2 via estrogen receptor in human uterine microvascular endothelial cells. Fertil Steril. 2004;81(5):1351–1356. [DOI] [PubMed] [Google Scholar]
- 147. Daniels S, Robbins J, West CR, Nemeth MA. Celecoxib in the treatment of primary dysmenorrhea: results from two randomized, double-blind, active- and placebo-controlled, crossover studies. Clin Ther. 2009;31(6):1192–1208. [DOI] [PubMed] [Google Scholar]
- 148. Vercellini P, Cortesi I, Crosignani PG. Progestins for symptomatic endometriosis: a critical analysis of the evidence. Fertil Steril. 1997;68(3):393–401. [DOI] [PubMed] [Google Scholar]
- 149. Surrey ES. The role of progestins in treating the pain of endometriosis. J Minim Invasive Gynecol. 2006;13(6):528–534. [DOI] [PubMed] [Google Scholar]
- 150. Madauss KP, Grygielko ET, Deng SJ, Sulpizio AC, Stanley TB, Wu C, Short SA, Thompson SK, Stewart EL, Laping NJ, Williams SP, Bray JD. A structural and in vitro characterization of asoprisnil: a selective progesterone receptor modulator. Mol Endocrinol. 2007;21(5):1066–1081. [DOI] [PubMed] [Google Scholar]
- 151. Jackson KS, Brudney A, Hastings JM, Mavrogianis PA, Kim JJ, Fazleabas AT. The altered distribution of the steroid hormone receptors and the chaperone immunophilin FKBP52 in a baboon model of endometriosis is associated with progesterone resistance during the window of uterine receptivity. Reprod Sci. 2007;14(2):137–150. [DOI] [PubMed] [Google Scholar]
- 152. Tseng L, Gurpide E. Estradiol and 20α-dihydroprogesterone dehydrogenase activities in human endometrium during the menstrual cycle. Endocrinology. 1974;94(2):419–423. [DOI] [PubMed] [Google Scholar]
- 153. Tseng L, Gurpide E. Induction of human endometrial estradiol dehydrogenase by progestins. Endocrinology. 1975;97(4):825–833. [DOI] [PubMed] [Google Scholar]
- 154. Satyaswaroop PG, Wartell DJ, Mortel R. Distribution of progesterone receptor, estradiol dehydrogenase, and 20α-dihydroprogesterone dehydrogenase activities in human endometrial glands and stroma: progestin induction of steroid dehydrogenase activities in vitro is restricted to the glandular epithelium. Endocrinology. 1982;111(3):743–749. [DOI] [PubMed] [Google Scholar]
- 155. Yang S, Fang Z, Gurates B, Tamura M, Miller J, Ferrer K, Bulun SE. Stromal PRs mediate induction of 17β-hydroxysteroid dehydrogenase type 2 expression in human endometrial epithelium: a paracrine mechanism for inactivation of E2. Mol Endocrinol. 2001;15(12):2093–2105. [DOI] [PubMed] [Google Scholar]
- 156. Casey ML, MacDonald PC, Andersson S. 17 beta-Hydroxysteroid dehydrogenase type 2: chromosomal assignment and progestin regulation of gene expression in human endometrium. J Clin Invest. 1994;94(5):2135–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Mustonen MV, Isomaa VV, Vaskivuo T, Tapanainen J, Poutanen MH, Stenbäck F, Vihko RK, Vihko PT. Human 17β-hydroxysteroid dehydrogenase type 2 messenger ribonucleic acid expression and localization in term placenta and in endometrium during the menstrual cycle. J Clin Endocrinol Metab. 1998;83(4):1319–1324. [DOI] [PubMed] [Google Scholar]
- 158. Cheng YH, Yin P, Xue Q, Yilmaz B, Dawson MI, Bulun SE. Retinoic acid (RA) regulates 17β-hydroxysteroid dehydrogenase type 2 expression in endometrium: interaction of RA receptors with specificity protein (SP) 1/SP3 for estradiol metabolism. J Clin Endocrinol Metab. 2008;93(5):1915–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Cheng YH, Imir A, Fenkci V, Yilmaz MB, Bulun SE. Stromal cells of endometriosis fail to produce paracrine factors that induce epithelial 17β-hydroxysteroid dehydrogenase type 2 gene and its transcriptional regulator Sp1: a mechanism for defective estradiol metabolism. Am J Obstet Gynecol. 2007;196(4):391.e1–7. [DOI] [PubMed] [Google Scholar]
- 160. Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, Innes J, Julie Kim J. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248(1–2):94–103. [DOI] [PubMed] [Google Scholar]
- 161. Pavone ME, Reierstad S, Sun H, Milad M, Bulun SE, Cheng YH. Altered retinoid uptake and action contributes to cell survival in endometriosis. J Clin Endocrinol Metab. 2010;95(11):E300–E309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Pavone ME, Dyson M, Reirstad S, Pearson E, Ishikawa H, Cheng YH, Bulun SE. Endometriosis expresses a molecular pattern consistent with decreased retinoid uptake, metabolism and action. Hum Reprod. 2011;26(8):2157–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129(4):723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002;143(6):2119–2138. [DOI] [PubMed] [Google Scholar]
- 165. Bulun S, Adashi E. The physiology and pathology of the female reproductive axis. In: Kronenberg HM, Melmed S, Polonsky KS, Larsen PR, eds. Williams Textbook of Endocrinology, 10th ed. Philadelphia, PA: Elsevier Health Sciences; 2003:587–663. [Google Scholar]
- 166. Brosens JJ, Hayashi N, White JO. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology. 1999;140(10):4809–4820. [DOI] [PubMed] [Google Scholar]
- 167. Fazleabas AT, Brudney A, Chai D, Langoi D, Bulun SE. Steroid receptor and aromatase expression in baboon endometriotic lesions. Fertil Steril. 2003;80(Suppl 2):820–827. [DOI] [PubMed] [Google Scholar]
- 168. Osteen KG, Bruner-Tran KL, Eisenberg E. Reduced progesterone action during endometrial maturation: a potential risk factor for the development of endometriosis. Fertil Steril. 2005;83(3):529–537. [DOI] [PubMed] [Google Scholar]
- 169. Dyson MT, Bulun SE. Cutting SRC-1 down to size in endometriosis. Nat Med. 2012;18(7):1016–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Bedaiwy MAA, Allaire C, Yong P, Alfaraj S. Medical management of endometriosis in patients with chronic pelvic pain. Semin Reprod Med. 2017;35(1):38–53. [DOI] [PubMed] [Google Scholar]
- 171. Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166(2):740–745. [DOI] [PubMed] [Google Scholar]
- 172. Carr B, Dmowski WP, O’Brien C, Jiang P, Burke J, Jimenez R, Garner E, Chwalisz K. Elagolix, an oral GnRH antagonist, versus subcutaneous depot medroxyprogesterone acetate for the treatment of endometriosis: effects on bone mineral density. Reprod Sci. 2014;21(11):1341–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Taylor HS, Giudice LC, Lessey BA, Abrao MS, Kotarski J, Archer DF, Diamond MP, Surrey E, Johnson NP, Watts NB, Gallagher JC, Simon JA, Carr BR, Dmowski WP, Leyland N, Rowan JP, Duan WR, Ng J, Schwefel B, Thomas JW, Jain RI, Chwalisz K. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377(1):28–40. [DOI] [PubMed] [Google Scholar]
- 174. Struthers RS, Nicholls AJ, Grundy J, Chen T, Jimenez R, Yen SS, Bozigian HP. Suppression of gonadotropins and estradiol in premenopausal women by oral administration of the nonpeptide gonadotropin-releasing hormone antagonist elagolix. J Clin Endocrinol Metab. 2009;94(2):545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Surrey E, Taylor HS, Giudice L, Lessey BA, Abrao MS, Archer DF, Diamond MP, Johnson NP, Watts NB, Gallagher JC, Simon JA, Carr BR, Dmowski WP, Leyland N, Singh SS, Rechberger T, Agarwal SK, Duan WR, Schwefel B, Thomas JW, Peloso PM, Ng J, Soliman AM, Chwalisz K. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132(1):147–160. [DOI] [PubMed] [Google Scholar]
- 176. Melis GB, Neri M, Corda V, Malune ME, Piras B, Pirarba S, Guerriero S, Orrù M, D’Alterio MN, Angioni S, Paoletti AM. Overview of elagolix for the treatment of endometriosis. Expert Opin Drug Metab Toxicol. 2016;12(5):581–588. [DOI] [PubMed] [Google Scholar]
- 177. Committee opinion no. 663: aromatase inhibitors in gynecologic practice. Obstet Gynecol. 2016;127(6):e170–e174. [DOI] [PubMed] [Google Scholar]
- 178. Chlebowski RT, Rohan TE, Manson JE, Aragaki AK, Kaunitz A, Stefanick ML, Simon MS, Johnson KC, Wactawski-Wende J, O’Sullivan MJ, Adams-Campbell LL, Nassir R, Lessin LS, Prentice RL. Breast cancer after use of estrogen plus progestin and estrogen alone: analyses of data from 2 Women’s Health Initiative randomized clinical trials. JAMA Oncol. 2015;1(3):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Coleman RE, Marshall H, Cameron D, Dodwell D, Burkinshaw R, Keane M, Gil M, Houston SJ, Grieve RJ, Barrett-Lee PJ, Ritchie D, Pugh J, Gaunt C, Rea U, Peterson J, Davies C, Hiley V, Gregory W, Bell R; AZURE Investigators. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med. 2011;365(15):1396–1405. [DOI] [PubMed] [Google Scholar]
- 180. Kettel LM, Murphy AA, Morales AJ, Yen SS. Preliminary report on the treatment of endometriosis with low-dose mifepristone (RU 486). Am J Obstet Gynecol. 1998;178(6):1151–1156. [DOI] [PubMed] [Google Scholar]
- 181. Donnez J, Tomaszewski J, Vázquez F, Bouchard P, Lemieszczuk B, Baró F, Nouri K, Selvaggi L, Sodowski K, Bestel E, Terrill P, Osterloh I, Loumaye E; PEARL II Study Group. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366(5):421–432. [DOI] [PubMed] [Google Scholar]
- 182. Chabbert-Buffet N, Pintiaux-Kairis A, Bouchard P; VA2914 Study Group. Effects of the progesterone receptor modulator VA2914 in a continuous low dose on the hypothalamic-pituitary-ovarian axis and endometrium in normal women: a prospective, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2007;92(9):3582–3589. [DOI] [PubMed] [Google Scholar]
- 183. Donnez J, Arriagada P, Marciniak M, Larrey D. Liver safety parameters of ulipristal acetate for the treatment of uterine fibroids: a comprehensive review of the clinical development program. Expert Opin Drug Saf. 2018;17(12):1225–1232. [DOI] [PubMed] [Google Scholar]
- 184. Jabbour HN, Sales KJ, Smith OP, Battersby S, Boddy SC. Prostaglandin receptors are mediators of vascular function in endometrial pathologies. Mol Cell Endocrinol. 2006;252(1–2):191–200. [DOI] [PubMed] [Google Scholar]
- 185. Hayes EC, Rock JA. COX-2 inhibitors and their role in gynecology. Obstet Gynecol Surv. 2002;57(11):768–780. [DOI] [PubMed] [Google Scholar]
- 186. Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–1875. [DOI] [PubMed] [Google Scholar]
- 187. Mancini JA, Blood K, Guay J, Gordon R, Claveau D, Chan CC, Riendeau D. Cloning, expression, and up-regulation of inducible rat prostaglandin e synthase during lipopolysaccharide-induced pyresis and adjuvant-induced arthritis. J Biol Chem. 2001;276(6):4469–4475. [DOI] [PubMed] [Google Scholar]
- 188. Wu MH, Wang CA, Lin CC, Chen LC, Chang WC, Tsai SJ. Distinct regulation of cyclooxygenase-2 by interleukin-1β in normal and endometriotic stromal cells. J Clin Endocrinol Metab. 2005;90(1):286–295. [DOI] [PubMed] [Google Scholar]
- 189. Ni H, Sun T, Ding NZ, Ma XH, Yang ZM. Differential expression of microsomal prostaglandin e synthase at implantation sites and in decidual cells of mouse uterus. Biol Reprod. 2002;67(1):351–358. [DOI] [PubMed] [Google Scholar]
- 190. Sun T, Li SJ, Diao HL, Teng CB, Wang HB, Yang ZM. Cyclooxygenases and prostaglandin E synthases in the endometrium of the rhesus monkey during the menstrual cycle. Reproduction. 2004;127(4):465–473. [DOI] [PubMed] [Google Scholar]
- 191. Jabbour HN, Milne SA, Williams AR, Anderson RA, Boddy SC. Expression of COX-2 and PGE synthase and synthesis of PGE2 in endometrial adenocarcinoma: a possible autocrine/paracrine regulation of neoplastic cell function via EP2/EP4 receptors. Br J Cancer. 2001;85(7):1023–1031. [DOI] [PubMed] [Google Scholar]
- 192. Tamura M, Sebastian S, Yang S, Gurates B, Fang Z, Bulun SE. Interleukin-1beta elevates cyclooxygenase-2 protein level and enzyme activity via increasing its mRNA stability in human endometrial stromal cells: an effect mediated by extracellularly regulated kinases 1 and 2. J Clin Endocrinol Metab. 2002;87(7):3263–3273. [DOI] [PubMed] [Google Scholar]
- 193. Tamura M, Sebastian S, Yang S, Gurates B, Ferrer K, Sasano H, Okamura K, Bulun SE. Up-regulation of cyclooxygenase-2 expression and prostaglandin synthesis in endometrial stromal cells by malignant endometrial epithelial cells. A paracrine effect mediated by prostaglandin E2 and nuclear factor-κB. J Biol Chem. 2002;277(29):26208–26216. [DOI] [PubMed] [Google Scholar]
- 194. Tamura M, Sebastian S, Gurates B, Yang S, Fang Z, Bulun SE. Vascular endothelial growth factor up-regulates cyclooxygenase-2 expression in human endothelial cells. J Clin Endocrinol Metab. 2002;87(7):3504–3507. [DOI] [PubMed] [Google Scholar]
- 195. Arosh JA, Lee J, Balasubbramanian D, Stanley JA, Long CR, Meagher MW, Osteen KG, Bruner-Tran KL, Burghardt RC, Starzinski-Powitz A, Banu SK. Molecular and preclinical basis to inhibit PGE2 receptors EP2 and EP4 as a novel nonsteroidal therapy for endometriosis. Proc Natl Acad Sci USA. 2015;112(31):9716–9721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Reis FM, Petraglia F, Taylor RN. Endometriosis: hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum Reprod Update. 2013;19(4):406–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Akoum A, Lemay A, Maheux R. Estradiol and interleukin-1β exert a synergistic stimulatory effect on the expression of the chemokine regulated upon activation, normal T cell expressed, and secreted in endometriotic cells. J Clin Endocrinol Metab. 2002;87(12):5785–5792. [DOI] [PubMed] [Google Scholar]
- 198. D’Hooghe TM, Nugent NP, Cuneo S, Chai DC, Deer F, Debrock S, Kyama CM, Mihalyi A, Mwenda JM. Recombinant human TNFRSF1A (r-hTBP1) inhibits the development of endometriosis in baboons: a prospective, randomized, placebo- and drug-controlled study. Biol Reprod. 2006;74(1):131–136. [DOI] [PubMed] [Google Scholar]
- 199. Falconer H, Mwenda JM, Chai DC, Wagner C, Song XY, Mihalyi A, Simsa P, Kyama C, Cornillie FJ, Bergqvist A, Fried G, D’Hooghe TM. Treatment with anti-TNF monoclonal antibody (c5N) reduces the extent of induced endometriosis in the baboon. Hum Reprod. 2006;21(7):1856–1862. [DOI] [PubMed] [Google Scholar]
- 200. Benaglia L, Somigliana E, Vighi V, Ragni G, Vercellini P, Fedele L. Rate of severe ovarian damage following surgery for endometriomas. Hum Reprod. 2010;25(3):678–682. [DOI] [PubMed] [Google Scholar]
- 201. Hamdan M, Omar SZ, Dunselman G, Cheong Y. Influence of endometriosis on assisted reproductive technology outcomes: a systematic review and meta-analysis. Obstet Gynecol. 2015;125(1):79–88. [DOI] [PubMed] [Google Scholar]
- 202. D’Hooghe TM, Denys B, Spiessens C, Meuleman C, Debrock S. Is the endometriosis recurrence rate increased after ovarian hyperstimulation? Fertil Steril. 2006;86(2):283–290. [DOI] [PubMed] [Google Scholar]
- 203. Harb HM, Gallos ID, Chu J, Harb M, Coomarasamy A. The effect of endometriosis on in vitro fertilisation outcome: a systematic review and meta-analysis. BJOG. 2013;120(11):1308–1320. [DOI] [PubMed] [Google Scholar]
- 204. de Ziegler D, Pirtea P, Galliano D, Cicinelli E, Meldrum D. Optimal uterine anatomy and physiology necessary for normal implantation and placentation. Fertil Steril. 2016;105(4):844–854. [DOI] [PubMed] [Google Scholar]
- 205. Daley GQ. The promise and perils of stem cell therapeutics. Cell Stem Cell. 2012;10(6):740–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Mutlu L, Hufnagel D, Taylor HS. The endometrium as a source of mesenchymal stem cells for regenerative medicine. Biol Reprod. 2015;92(6):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Miyazaki K, Dyson MT, Coon V JS, Furukawa Y, Yilmaz BD, Maruyama T, Bulun SE. Generation of progesterone-responsive endometrial stromal fibroblasts from human induced pluripotent stem cells: role of the WNT/CTNNB1 pathway. Stem Cell Reports. 2018;11(5):1136–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Jin RU, Mills JC. Are gastric and esophageal metaplasia relatives? The case for Barrett’s stemming from SPEM. Dig Dis Sci. 2018;63(8):2028–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]