Abstract
Study Design:
Retrospective cohort study.
Objective:
To assess both implant performance and the amount of correction that can be achieved using multilevel anterior lumbar interbody fusion (ALIF).
Methods:
Retrospective cohort study (n = 178) performed over a 4-year period. Surgical variables examined included blood loss, operative time, perioperative complications, and secondary/revision procedures. Follow-up radiographic assessment was performed to record implant-related problems. Radiographic parameters were examined pre- and postoperatively. Health-related quality of life (HRQOL) outcome measures were collected preoperatively and at 6 weeks, 6 months, 1 year, and 2 years postoperatively. Descriptive and comparative statistical analysis, using paired-sample t test and repeated-measures analysis of variance (rANOVA), was performed.
Results:
Lumbar lordosis increased from 42° ± 17° preoperatively to 55° ± 11° postoperatively (P < .001). The visual analog scale back pain mean score improved from 8.3 ± 1.5 preoperatively to 2.6 ± 2.4 at 2 years (P < .001). The mean Oswestry Disability Index improved from 69.5 ± 21.5 preoperatively to 19.9 ± 15.2 at 2 years (P < .001). The EQ-5D mean score improved from 0.2 ± 0.2 preoperatively to 0.8 ± 0.1 at 2 years (P = .02). There were no neurological, vascular, or visceral approach–related injuries reported. No rod breakages and no symptomatic nonunions occurred. There was one revision procedure performed for fracture.
Conclusions:
The use of porous tantalum cages as part of a 360-degree fusion to treat adult degenerative spinal deformity has been demonstrated to be a safe and effective strategy, leading to good clinical, functional, and radiographic outcomes in the short term.
Keywords: degenerative, lumbar, deformity, interbody cage, ALIF, tantalum, trabecular metal
Introduction
Degenerative lumbar spondylosis involves a spectrum of pathology, including disc degeneration, lumbar stenosis, facet hypertrophy, and degenerative spondylolisthesis, often resulting in low back pain and/or radiculopathy. Surgical intervention is generally reserved for cases of significant disc space degeneration, mechanical instability, nerve root impingement, and/or failure of nonoperative treatment.1 Asymmetrical disc degeneration in conjunction with multilevel loss of disc height leads to the combined coronal and sagittal plane deformity, often exhibited in de novo adult scoliosis. Patients with adult spinal deformity often have combined coronal and sagittal plane abnormalities and present a significant surgical challenge.
Over the past number of decades, a wide variety of surgical techniques have been popularized to facilitate lumbar interbody fusion, including posterior lumbar interbody fusion (PLIF), transforaminal lumbar interbody fusion (TLIF), oblique lumbar interbody fusion/anterior to psoas (OLIF/ATP), anterior lumbar interbody fusion (ALIF), and lateral lumbar interbody fusion (LLIF). The ALIF approach offers several key advantages. It offers a direct midline view of the disc space with extensive lateral exposure of the vertebral bodies. This facilitates efficient disc space clearance, rapid endplate preparation, and maximizes the potential for fusion.2,3 Furthermore, anterior access to the disc space facilitates maximal implant size and surface area to provide aggressive correction of local foraminal height and restoration of segmental lordosis.4-6
Tantalum trabecular metal has several potential advantages over conventional implant materials, such as its uniformity and structural continuity, strength, low stiffness, high porosity, and high coefficient of friction.7 The development of porous fixation surfaces with a high volumetric porosity for biologic ingrowth and high frictional characteristics provide enhanced implant stability.8
The aims of this study were to assess both implant performance and the amount of correction that can be achieved using multilevel ALIF.
Patients and Methods
This study was approved by the Institutional Review Board of the Royal National Orthopaedic Hospital, Stanmore, UK.
Patient Population
This was a retrospective review of prospectively collected data on a cohort of 178 consecutive patients (n = 178) having 561 porous tantalum cages (Zimmer TM-400 implant) (Figure 1) inserted as part of a 2-stage 360-degree lumbosacral fusion. The study inclusion criteria were patients having multilevel ALIF as part of a primary spinal reconstruction for degenerative lumbar deformity with a minimum 2-year clinical follow-up. This study was performed at a national tertiary referral center for adult spinal deformity between January 1, 2011 and December 1, 2014.
Figure 1.

Zimmer TM-400 implant used for anterior lumbar interbody fusion (ALIF) as part of a 2-stage 360-degree lumbar spondylodesis in this case series.
Surgical Technique
The surgical technique involved a 2-stage procedure. The first stage ALIF was performed in a semilateral position using a left-sided approach, as previously described.9 Freeze-dried cancellous allograft chips were inserted into ALIF cages prior to insertion. The second stage was performed using a traditional open midline approach and a predominantly computer-navigated pedicle screw insertion technique, using the O-arm imaging and StealthStation navigation systems (Medtronic, Minneapolis, MN, USA). This was followed by resection of the posterior elements, including the facet joints, at the levels implanted with ALIF cages to optimize the segmental lordosis achieved with posterior instrumentation. Posterolateral bony fusion was performed using local bone graft and freeze-dried cortical cancellous allograft.
Outcome Measures
Surgical variables examined included blood loss, operative time, perioperative complications, and secondary/revision procedures. Follow-up radiographic assessment was performed to record implant-related problems. The radiographic parameters measured pre- and postoperatively included pelvic incidence (PI), pelvic tilt (PT), sacral slope (SS), lumbar lordosis (LL), thoracic kyphosis (TK), and sagittal vertical axis (SVA). Additionally, health-related quality of life (HRQOL) outcome measures were collected preoperatively and at 6 weeks, 6 months, 1 year, and 2 years postoperatively. These outcome scores included visual analog scale (VAS) back pain, VAS leg pain, EQ-5D, EQ-5D VAS, Oswestry Disability Index (ODI), and Scoliosis Research Society–22 (SRS-22).
Statistical Analysis
The study data was collected prospectively in a database specific to this study. Descriptive and comparative statistical analysis was performed using SPSS version 22.0. All descriptive data was presented as mean ± standard deviation and a paired-sample t test was used to compare the pre- and postoperative radiographic parameters. Paired-sample t test and repeated measures analysis of variance (rANOVA) were used to compare preoperative HRQOL outcome measures with scores at 6 weeks, 6 months, 1 year, and 2 years postoperatively. Statistical significance was considered to be a P value <.05.
Results
Demographics
This patient cohort had a mean age of 63 ± 12 years and had a female preponderance, with a female to male ratio of 5.7:1. The mean body mass index (BMI) was 31 ± 2.1 kg/m2. The mean ASA (American Society of Anesthesiologists) grade of the cohort was 2.1 ± 0.8.
Levels Fused, Operative Time, and Blood Loss
The mean number of spinal levels that had an ALIF performed, as part of a 360-degree fusion, was 3.15 ± 1.1 levels. There were 58/178 patients (32.6%) having 2-level anterior fusion, 63/178 patients (35.4%) having 3-level anterior fusion, 29/178 patients (16.3%) having 4-level anterior fusion, and 28/178 patients (16.3%) having a 5-level anterior fusion. The mean number of spinal levels instrumented posteriorly was 3.81 ± 3.58 levels.
The operative time and blood loss for the anterior approach and fusion of the patients who underwent ALIF of 2 levels was 86 ± 10.1 minutes and 46.7 ± 10.9 mL, respectively; 2 levels was 120 ± 20.4 minutes and 74.2 ± 16.1 mL; 4 levels was 141 ± 22.6 minutes and 98.3 ± 18.9 mL; and 5 levels was 153 ± 23.9 minutes and 126 ± 20.8 mL (Table 1).
Table 1.
Table Demonstrating Operative Time and Blood Loss per Number of Levels Fused.
| Levels (No. of Patients) | Operation Time (min) | Estimated Blood Loss (mL) |
|---|---|---|
| 2 Levels (n = 58) | 86 ± 10.1 | 46.7 ± 10.9 |
| 3 Levels (n = 63) | 120 ± 20.4 | 74.2 ± 16.1 |
| 4 Levels (n = 29) | 141 ± 22.6 | 98.3 ± 18.9 |
| 5 Levels (n = 28) | 153 ± 23.9 | 126 ± 20.8 |
Radiographic Outcomes
The mean PI of this cohort was 58° ± 13°. PT decreased from 12° ± 15° preoperatively to 9° ± 14° postoperatively (P < .001). SS increased from 33° ± 17° preoperatively to 39° ± 10° postoperatively (P < .001). LL increased from 42° ± 17° preoperatively to 55° ± 11° postoperatively (P < .001). TK increased from 38° ± 16° preoperatively to 43° ± 13° postoperatively (P < .001). SVA reduced from 8 ± 6 cm preoperatively to 3 ± 2 cm postoperatively (P < .001).
Clinical Outcomes
The mean VAS back pain score improved from 8.3 ± 1.5 preoperatively to 2.4 ± 2.3 at 6 weeks (P < .001), 2.5 ± 2.1 at 6 months (P < .001), 2.6 ± 2.3 at 1 year (P < .001), and 2.6 ± 2.4 at 2 years (P < .001). The mean VAS leg pain score improved from 5.0 ± 3.8 preoperatively to 2.0 ± 2.8 at 6 weeks (P < .001), 1.6 ± 2.1 at 6 months (P < .001), 1.8 ± 1.9 at 1 year (P < .001), and 1.9 ± 2.5 at 2 years (P = .002). The mean ODI improved from 69.5 ± 21.5 preoperatively to 41.7 ± 18.3 at 6 weeks (P < .001), 31.5 ± 17.8 at 6 months (P < .001), 21.7 ± 16.6 at 1 year (P < .001) and 19.9 ± 15.2 at 2 years (P < .001). The mean EQ-5D score improved from 0.2 ± 0.2 preoperatively to 0.7 ± 0.1 at 6 weeks (P < .001), 0.7 ± 0.1 at 6 months (P < .001), 0.7 ± 0.2 at 1 year (P < .001) and 0.8 ± 0.1 at 2 years (P = .02). The mean EQ-5D VAS score improved from 45.0 ± 19.8 preoperatively to 74.6 ± 10.5 at 6 weeks (P < .001), 82.1 ± 13.5 at 6 months (P < .001), 76.2 ± 16.2 at 1 year (P = .007), and 77.6 ± 19.2 at 2 years (P = .06). The mean SRS-22 score improved from 2.3 ± 0.4 preoperatively to 3.5 ± 0.6 at 6 weeks (P < .001), 3.5 ± 0.5 at 6 months (P < .001), 3.7 ± 0.6 at 1 year (P < .001), and 4.2 ± 0.5 at 2 years (P = .03).
Multilevel Fusions
To examine the subgroup of cases having multilevel fusion (≥2 levels) in greater detail, we outlined a brief summary of the radiographic outcomes (ie, LL correction) and clinical outcomes (ie, EQ-5D and SRS-22 at 2 years) for each fusion group (ie, 2-level, 3-level, 4-level, and 5-level). The results outlined in Table 2 highlight an increasing lordotic correction with multilevel ALIF surgery, along with favorable outcomes at 2 years’ follow-up in Table 3.
Table 2.
Table Summarizing the Radiographic Outcomes (ie, LL Correction, PT Correction, and SVA Correction) Postoperatively From Multilevel Fusions (≥2 Levels).
| Fusion Level | LL Correction | PT Correction | SVA Correction |
|---|---|---|---|
| 2 Levels (n = 58) | 11° ± 8° | 3° ± 3° | 4 ± 2 cm |
| 3 Levels (n = 63) | 13° ± 11° | 3° ± 4° | 5 ± 2 cm |
| 4 Levels (n = 29) | 18° ± 12° | 4° ± 4° | 6 ± 3 cm |
| 5 Levels (n = 28) | 22° ± 13° | 5° ± 6° | 7 ± 4 cm |
Abbreviations: LL, lumbar lordosis; PT, pelvic tilt; SVA, sagittal vertical axis.
Table 3.
Table Summarizing the Clinical Outcomes (ie. EQ-5Q and SRS-22 at 2-Year Follow-up) From Multilevel Fusions (≥2 Levels).
| Fusion Level | Mean EQ-5D | Mean SRS-22 |
|---|---|---|
| 2 Levels (n = 58) | 0.8 ± 0.1 | 4.3 ± 0.4 |
| 3 Levels (n = 63) | 0.8 ± 0.1 | 4.2 ± 0.4 |
| 4 Levels (n = 29) | 0.7 ± 0.2 | 4.0 ± 0.6 |
| 5 Levels (n = 28) | 0.7 ± 0.2 | 4.0 ± 0.7 |
Abbreviations: EQ-5D, instrument for measuring health status developed by the EuroQol Group; SRS-22, Scoliosis Research Society–22.
Complications
No neurological, vascular, or visceral approach–related injuries were reported. All postoperative complications are documented in Table 4. Additionally, there were no cases of symptomic nonunion requiring revision at the levels that had a tantalum cage. This was assessed clinically and radiographically. Clinical features assessed included persistent significant back pain and radiographic features considered included rod breakage, screw breakage, and peri-screw (including iliac bolts) or peri-tantalum cage osteolysis.
Table 4.
Complications Occurring in the Study Group.
| Complication | No. of Patients | % |
|---|---|---|
| Transient MEP deficits | 8/178 | 4.5 |
| Wound complications | 6/178 | 3.3 |
| CSF leaks | 10/178 | 5.6 |
| Ileus | 15/178 | 8.4 |
| UTI | 23/178 | 12.9 |
| Thromboembolic (PE/DVT) | 5/178 | 2.8 |
| Graft migration | 1/178 | 0.6 |
| Proximal junctional failure | 1/178 | 0.6 |
Abbreviations: CSF, cerebrospinal fluid; DVT, deep vein thrombosis; MEP, motor-evoked potential; PE, pulmonary embolism; UTI, urinary tract infection.
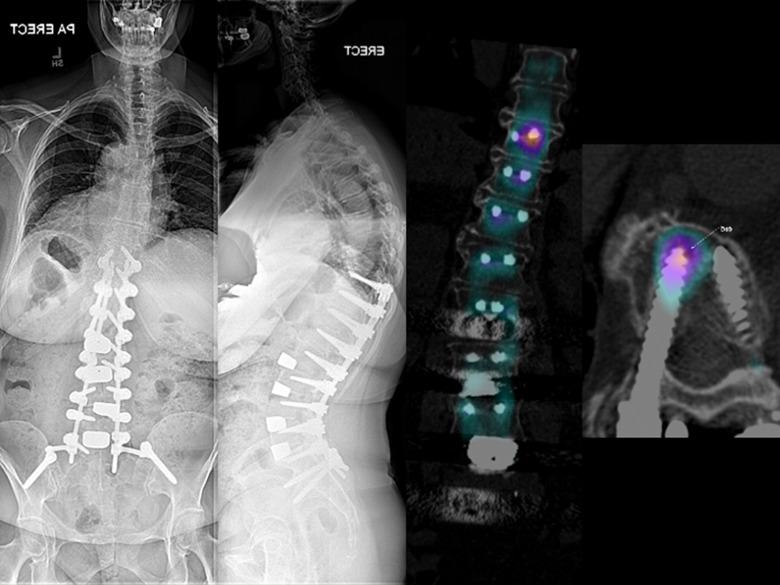
One patient had a complication at a level where there was a tantalum cage. This patient sustained a fractured sacrum following a fall and the tantalum cage became displaced. This patient had rheumatoid arthritis and advanced systemic disease and required long construct fixation at the index procedure due to significant sagittal plane deformity and deteriorating mobility. There was a delay in revising this case and at the revision surgery the tantalum cage had osteointegrated with the sacrum and needed to be removed with an osteotome (Figure 2). There were also 3 patients presenting with significant proximal junctional pain and signs suggestive of proximal junctional failure. Two of these patients displayed stress responses on SPECT/CT (single-photon emission computed tomography/computed tomogrpahy) imaging but no signs of mechanical failure and did not require surgical intervention. However, 1 patient did require extension of instrumentation from the T10 level to T4 as a treatment for significant pain and signs of proximal junctional failure.
Figure 2.
A revision L5/S1 anterior lumbar interbody fusion (ALIF) was performed on a patient with rheumatoid arthritis and advanced systemic disease, who sustained a sacral fracture and tantalum cage subsidence after a fall following her index procedure.
ALIF offers a number of advantages over PLIF and TLIF, particularly when it is part of a 360-degree spondylodesis. ALIF facilitates significant indirect foraminal decompression with restoration of disc height to produce a superior correction of segmental lordosis and total lumbar lordosis.3
A study of 220 consecutive patients with 309 operative levels compared ALIF (184 levels), LLIF (86 levels), and TLIF (39 levels) for sagittal correction and spondylolisthesis reduction, with an average follow-up of 19.2 months.10 The authors demonstrated that lordosis restoration was significant for both the ALIF and LLIF groups, but not the TLIF group, with intergroup analysis demonstrating that ALIF had significantly improved lordosis compared to both LLIF and TLIF. Both ALIF and LLIF were associated with a significantly increased disc height compared with the TLIF. All the 3 techniques led to a significant reduction in spondylolisthesis, with no difference between the groups.
The recent growing body of data appears to support ALIF as the interbody fusion technique providing the greatest degree of lordosis correction, followed by LLIF and then TLIF.2 A recent systematic review compared the degrees of correction of lumbar lordosis achieved by ALIF, LLIF, and TLIF.11 For ALIF, 21 studies were identified with mean correction 4.67° (standard deviation [SD] ±4.24) and median correction 5.20°. Fifteen studies were identified that met criteria for Forest plot analysis with mean correction 4.90° (standard error of the mean [SEM] ±0.40). For LLIF, 17 studies were identified with mean ± SD correction 4.47° ± 4.80° and median correction 4.00°. Nine studies were identified that met criteria for Forest plot analysis with mean ± SEM correction 2.91° ± 0.56°. For TLIF, 31 studies were identified with mean ± SD correction 3.89° ± 4.33° and median correction 3.50°. Twenty-five studies were identified that met criteria for Forest plot analysis with mean ± SEM correction 5.33° ± 0.27°.11
The data from our study supports multilevel ALIF as a safe and effective surgical strategy to achieve restoration of lumbar lordosis and correction of sagittal alignment. This surgical strategy is effective for patients with a moderate-to-severe sagittal deformity, requiring a gradual correction in lumbar lordosis across several segments. Multilevel ALIF avoids the significant morbidity, high blood loss, and neurologic complications associated with 3-column osteotomies, such as pedicle subtraction osteotomy (PSO).12 From a biomechanical perspective, multilevel ALIF provides a more harmonious sagittal plane correction, mimicking the gradual segmental lumbar lordosis of the normal spine.3 This contrasts considerably with the abrupt angular correction achieved at the index level of PSO. Furthermore, biomechanical studies suggest that ALIF may limit the destabilization of axial rotational stability seen with lumbar PSO.13 This may potentially decrease the mechanical demand on posterior instrumentation and limit rod fractures, hardware failure, and pseudarthrosis. Additionally, circumferential fusion of the lumbosacral junction is considered an important strategy to avoid pseudarthrosis and implant failure.12
Although ALIF can provide significant restoration of lumbar lordosis and sagittal plane correction, it is associated with a number of approach-related complications. Intraoperative complications include vascular injury (venous <4%, arterial <1%), visceral injury (urogenital <1%, peritoneal/bowel <1%) or neurologic injury (superior hypogastric plexus 4%-6%, sympathetic chain 6%-10%). Postoperative (1-6 weeks) complications include vascular (venous thromboembolism <2%, arterial thrombus <1.5%, retroperitoneal hematoma <1%), muscular (abdominal wall hernia <1%), visceral (postoperative ileus 0.6%-5.6%), wound (dehiscence <1%) and graft/cage related complications (<1%).14 In order to minimize the associated complication rate we developed a novel surgical approach to significantly reduce the risk of neurological, vascular or visceral injuries.9
Despite the obvious limitations to a retrospective study with no control or comparison group, we report the largest clinical series of tantalum trabecular metal interbody implants used in spinal surgery. This data was collected prospectively but analyzed in a retrospective fashion. We were able to evaluate fusion and assess clinical and radiographic outcomes, when using porous tantalum cages for ALIF in a 360-degree fusion. Our patient series demonstrated excellent radiographic and clinical outcomes.
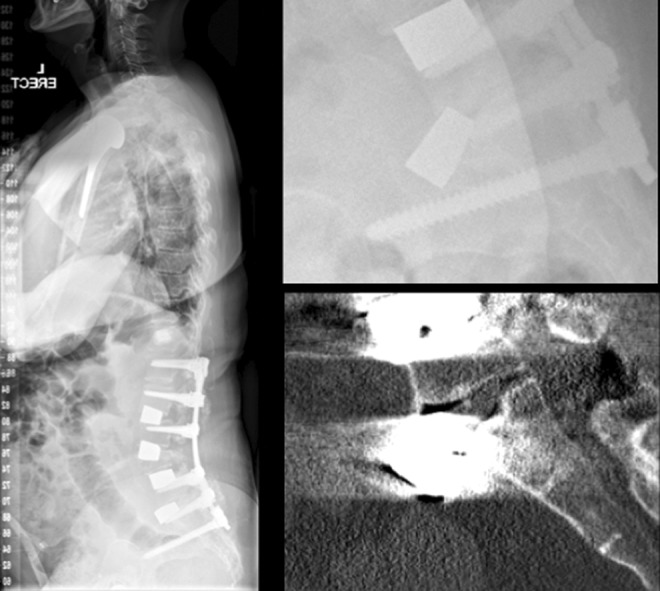
We acknowledge that metal artifact produced from the tantalum trabecular metal implants used in this study precludes the definitive assessment of fusion in our patient cohort by the gold standard of CT scanning. However, when we have had to investigate postoperative patients for pain at the proximal end of a long construct, we have used SPECT/CT fusion imaging. This dual-imaging modality scan can highlight areas of mechanical stress with increased tracer uptake around the proximal screw fixation points in a long construct deformity correction and can also demonstrate quiescent interbody spaces with ALIF tantalum cages indicating stability (Figure 3). Additionally, the lack of an appropriate comparison group, which limits our ability to claim equivalency or superiority with other biomaterials. Finally, although there were no approach-related complications in this series, it must be acknowledged that there is a complication rate associated with this surgical approach, particularly when the need exists to mobilize the great vessels. The authors acknowledge that the lack of approach-related complications is due to the study being performed in a high-volume spinal deformity center with significant anterior exposure expertise to minimize neurological, vascular, or visceral injuries.9
Figure 3.
A 62-year-old woman with prior 2-stage deformity correction, presenting 2 years postoperatively with significant junctional pain and signs of proximal junctional failure. This diagnosis was supported by an obvious stress response on SPECT/CT fusion imaging highlighted by increased radiotracer activity at the proximal junctional region. Note a lack of tracer uptake at prior anterior lumbar interbody fusion (ALIF) levels suggesting stable osseous integration of the porous tantalum cages.
In conclusion, the use of porous tantalum cages as part of a 360-degree fusion to treat adult degenerative spinal deformity has been demonstrated to be a safe and effective strategy, leading to good clinical, functional, and radiographic outcomes in the short term.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded, in part, by an institutional research grant from Zimmer Biomet, who played no role in study design, data collection, data analysis or manuscript preparation.
References
- 1. Madigan L, Vaccaro AR, Spector LR, Milam RA. Management of symptomatic lumbar degenerative disc disease. J Am Acad Orthop Surg. 2009;17:102–111. [DOI] [PubMed] [Google Scholar]
- 2. Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg. 2015;1:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hsieh PC, Koski TR, O’Shaughnessy BA. et al. Anterior lumbar interbody fusion in comparison with transforaminal lumbar interbody fusion: implications for the restoration of foraminal height, local disc angle, lumbar lordosis, and sagittal balance. J Neurosurg Spine. 2007;7:379–386. [DOI] [PubMed] [Google Scholar]
- 4. Phan K, Thayaparan GK, Mobbs RJ. Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion—systematic review and meta-analysis. Br J Neurosurg. 2015;29:705–711. [DOI] [PubMed] [Google Scholar]
- 5. Rao PJ, Loganathan A, Yeung V, Mobbs RJ. Outcomes of anterior lumbar interbody fusion surgery based on indication: a prospective study. Neurosurgery. 2015;76:7–23. [DOI] [PubMed] [Google Scholar]
- 6. Rao PJ, Maharaj MM, Phan K, Lakshan Abeygunasekara M, Mobbs RJ. Indirect foraminal decompression after anterior lumbar interbody fusion: a prospective radiographic study using a new pedicle-to-pedicle technique. Spine J. 2015;15:817–824. [DOI] [PubMed] [Google Scholar]
- 7. Cohen R. A porous tantalum trabecular metal: basic science. Am J Orthop (Belle Mead NJ). 2002;31:216–217. [PubMed] [Google Scholar]
- 8. Hanc M, Fokter SK, Vogrin M, Molicnik A, Recnik G. Porous tantalum in spinal surgery: an overview. Eur J Orthop Surg Traumatol. 2016;26:1–7. [DOI] [PubMed] [Google Scholar]
- 9. Molloy S, Butler JS, Benton A, Malhotra K, Selvadurai S, Agu O. A new extensile anterolateral retroperitoneal approach for lumbar interbody fusion from L1 to S1: a prospective series with clinical outcomes. Spine J. 2016;16:786–791. [DOI] [PubMed] [Google Scholar]
- 10. Watkins RG, 4th, Hanna R, Chang D, Watkins RG., 3rd Sagittal alignment after lumbar interbody fusion: comparing anterior, lateral, and transforaminal approaches. J Spinal Disord Tech. 2014;27:253–256. [DOI] [PubMed] [Google Scholar]
- 11. Rothrock RJ, McNeill IT, Yaeger K, Oermann EK, Cho SK, Caridi JM. Lumbar lordosis correction with interbody fusion: systematic literature review and analysis. World Neurosurg. 2018;118:21–31. [DOI] [PubMed] [Google Scholar]
- 12. Chan AK, Mummaneni PV, Shaffrey CI. Approach selection: multiple anterior lumbar interbody fusion to recreate lumbar lordosis versus pedicle subtraction osteotomy: when, why, how? Neurosurg Clin N Am. 2018;29:341–354. [DOI] [PubMed] [Google Scholar]
- 13. Dahl BT, Harris JA, Gudipally M, Moldavsky M, Khalil S, Bucklen BS. Kinematic efficacy of supplemental anterior lumbar interbody fusion at lumbosacral levels in thoracolumbosacral deformity correction with and without pedicle subtraction osteotomy at L3: an in vitro cadaveric study. Eur Spine J. 2017;26:2773–2781. [DOI] [PubMed] [Google Scholar]
- 14. Czerwein JK, Jr, Thakur N, Migliori SJ, Lucas P, Palumbo M. Complications of anterior lumbar surgery. J Am Acad Orthop Surg. 2011;19:251–258. [DOI] [PubMed] [Google Scholar]