Abstract
Background
Although Hippo/Yes-associated protein (YAP) signaling plays crucial roles in radiation sensitivity and resistance of multiple kinds of cancers, its role in the radiation sensitivity of glioma cells remains unclear. The present study aimed to reveal Hippo/YAP role in the radiation sensitivity of glioma cells.
Methods
Glioma U251 cells were administrated with different doses of irradiation. Cell Counting Kit-8 (CCK-8) and flow cytometry assays were used to assess cell viability and apoptosis. Co-immunoprecipitation (co-IP) assay was used to assess the interactions between proteins.
Results
The results showed that irradiation exposure significantly inhibited cell viability and induced cell apoptosis in a dose-dependent manner, as well as decreased YAP1 expression via enhancing RCHY1-mediated YAP1 protein degradation. In addition, we observed that downregulation of YAP1 or RCHY1 weakened the role of irradiation exposure in cell viability inhibition and apoptosis promotion.
Conclusion
Collectively, this study emphasizes the vital role of Hippo/YAP signaling in radiation sensitivity of glioma, that RCHY1-mediated YAP1 protein downregulation is a main mechanism accounting for radiation-induced glioma cell apoptosis. Our study may enrich the theoretical basis of Hippo/YAP signaling as a new target for improving radiation sensitivity in glioma.
Keywords: radiation, Hippo/YAP signaling, ubiquitination, RCHY1, glioma
Introduction
Glial-derived tumor, or glioma, is the most common brain tumor and accounts for about 74.6% of all malignant tumors occurring in brain and central nervous system.1 Approximately 82% of gliomas cases are diagnosed at high malignant WHO grades III and IV, with a 5-year survival rate of only 10%.2 It is identified that radiotherapy has synergistic role with surgery and chemotherapy and is considered as the most effective therapeutic method except for surgery. However, the high intrinsic radioresistance severely restricts its therapeutic efficacy.3 Therefore, it is needful to clarify the mechanisms of the radioresistance in glioma, aiming to relieve the pain of glioma patients and improve their life quality.
The Hippo signaling pathway, first identified in Drosophila, was originally discovered to play a crucial role in regulating tissue growth; gradually it was found to be closely implicated in the modulation of multiple biological processes such as cell proliferation and apoptosis.4–7 Mst1/2 (Mammalian Ste20-like 1 and 2), LATS1/2 (large tumor suppressor kinase 1/2), MOB1A/1B (MOB kinase activator 1A/1B) and SAV1 (salvador family WW domain-containing protein 1) are core components of Hippo signaling pathway, and their main role is to prevent the transportation of Yes-associated protein (YAP) to nuclear, thereby inhibiting the transcription of its downstream genes.8,9 Notably, studies have confirmed that Hippo/YAP signaling plays a crucial role in chemoradiotherapy resistance of tumors. For example, Song et al7 found that knockdown of YAP1 significantly enhanced the sensitivity of colorectal cancer LoVo-R cells, an acquired 5-fluorouracil resistant cell line. Fernandez et al10 demonstrated that YAP conferred tumor cell radio-resistance and promoted cell ongoing proliferation after radiation in medulloblastoma. Wang et al11 revealed that increased activation and nuclear translocation of YAP protein induced by MST1 and LATS1/2 downregulation obviously promoted the proliferation and chemoresistance of osteosarcoma MG63 cells. All of the findings emphasize the significant role of Hippo/YAP signaling in cancer chemoradiotherapy resistance. However, its effects and underlying mechanisms in glioma radiotherapy remain incompletely clear.
In the present study, we focused on uncovering the role of Hippo/YAP signaling in the radiosensitivity in glioma and its underlying mechanisms, aiming to improve the radiosensitivity of glioma.
Materials and methods
Cell culture and treatment
Human glioma U251 cells obtained from American Type Culture Collection (Manassas, VA, USA) were cultured in DMEM (Thermo Fisher Scientific, Waltham, MA, USA), supplemented with 10% FBS (Thermo Fisher Scientific) and 1% penicillin/streptomycin (Thermo Fisher Scientific). Cells were treated with 100 μg/mL of cycloheximide (CHX) for 1, 2, 4, 8 or 24 hrs to stop protein synthesis.
Irradiation exposure
U251 cells in exponentially growing phase were irradiated at a dose of 2, 4, 6, or 8 Gy at room temperature through using a 6 MV photon beam generated (2100C/D; VARIAN, Carlsbad, CA, USA).
Cell transfection
To delete the expression of YAP1, glioma cells were transfected with the small interfering RNAs (si-YAP1) purchased from OriGene (No. SR323110; Beijing, China). Meanwhile, the plasmid used to overexpress RCHY1 (OE-RCHY1) in U251 cells was also obtained from OriGene (No. SC107968). Cell transfection was executed by using Lipofectamine 3000 reagent (Thermo Fisher Scientific) following the manufacturer’s descriptions.
Real-time quantitative PCR (qPCR) assay
Total RNA was obtained by using Trizol reagent (Thermo Fisher Scientific) referring to the manufacturer’s instructions. After a total of 1 μg RNA being reversely transcripted into cDNA, real-time qPCR was performed using an EasyScript One-Step RT-PCR SuperMix (TransGen Biotech, Beijing, China) on an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). GAPDH mRNA level was used to normalize mRNA expression. Primer sequences were obtained from Sangon Biotech (Shanghai, China) and were listed as follows:
YAP1: sense-5ʹ-CCCTCGTTTTGCCATGAACC-3ʹ,
YAP1: antisense-5ʹ-GTTGCTGCTGGTTGGAGTTG-3ʹ;
Mst1: sense-5ʹ-CAGAGCTGCGGCATCAAATC-3ʹ,
Mst1: antisense-5ʹ-ACCTTGGTCGAGGAACTTGC-3ʹ;
LATS1: sense-5ʹ-GCTGCACCAAAACCCATCTG-3ʹ,
LATS1: antisense-5ʹ-ACACCAAGCAAACAGATGATTAAGT-3ʹ;
MOB1: sense-5ʹ-AGGTTTGCAAAGGCTCGCA-3ʹ,
MOB1: antisense-5ʹ-CTGCTGCGAGGACAAGAGAA-3ʹ;
SAV1: sense-5ʹ-GCGGGGAAAGTTTACGGGAT-3ʹ,
SAV1: antisense-5ʹ-GGGACAGCATCCTTCTCGAC-3ʹ;
GAPDH: sense-5ʹ-CCACTAGGCGCTCACTGTTCT-3ʹ,
GAPDH: antisense-5ʹ-GCATCGCCCCACTTGATTTT-3ʹ.
Western blotting assay
The isolation of total protein from U251 cells was carried out with RIPA lysis buffer (Beyotime, Jiangsu, China). Cytoplasm and nucleoprotein were obtained using the Nuclear and Cytoplasmic Protein Extraction Kit (Weiao Co., LTD, Shanghai, China). After protein concentrations were assessed using BCA kit (Thermo Fisher Scientific) and degenerated at 100°C for 10 mins, 25 μg proteins from each sample were loaded onto 10% SDS-PAGE and separated by it, followed by transfection onto the polyvinylidene fluoride (PVDF) membranes (Thermo Fisher Scientific). Then, the membranes were blocked with 5% non-fat milk for 1 hr and probed with primary antibodies, including YAP1 (No. ab56701; Abcam, Cambridge, MA, USA), p-YAP1 (No. ab76252; Abcam), caspase 3 (No. #9662; Cell Signaling Technology, Danvers, MA, USA), cleaved caspase3 (c-caspase3; No. #9661, Cell Signaling Technology), Bax (No. ab32503; Abcam), TrCP (No. ab71753; Abcam), CDC4 (No. ab64533; Abcam), RCHY1 (No. 5754; Cell Signaling Technology), MDM2 (No. ab38618; Abcam), SKP2 (No. ab68455; Abcam), UBE3A (No. ab126765; Abcam), SMURF1 (No. ab57573; Abcam) and GAPDH (No. TA-08; Zhongshanjinqiao Co., Ltd., Beijing, China) overnight at 4°C. Subsequently, the membranes were incubated with the corresponding secondary antibodies purchased from Zhongshanjinqiao Co., Ltd. for 1 hr. The blot bands were visualized with incubating an enhanced chemiluminescence reagent (Millipore, Billerica, MA, USA) and detected by the gel imaging instrument (Eberhardzell, Germany) and analyzed by ImageJ software (National Institutes of Health, Bethesda, MA, USA).
Co-immunoprecipitation (Co-IP) assay
Co-IP assay was performed to assess the interactions between proteins. In detail, U251 cells were lysed in IP lysis buffer (Thermo Fisher Scientific) in accordance with the manufacturer's instructions. Next, the cell lysate containing 200 μg proteins was incubated with Dynabeads® protein G for 1 hr, and incubated with 2 μg anti-YAP1 antibody (No. ab56701; Abcam) or IgG (negative control) overnight at 4°C, followed by incubation with Dynabeads® protein G for another 1 hr. Then, the immune complex was submitted to western blotting assay with antibodies against Ub (No. 3933), TEA/ATTS domain (TEAD , No. 13295), P73 (No. 14620) and runt-related transcription factor 2 (RUNX2; No. 12556), all purchased from Cell Signaling Technology, and then anti-cAMP responsive element-binding protein 1 (CREB; No. ab31387; Abcam) antibody.
Immunofluorescence technology
Immunofluorescence was performed according to a previous study.12 Cells were incubated with theprimary antibody against YAP1 (No. ab52771; Abcam) overnight at 4°C and then probed with Alexa Fluor 488-conjugated IgG (Santa Cruz, Dallas, Tx, USA) in the dark at room temperature for 1 hr. The staining was recorded by using a laser-scanning confocal microscope (LSM510; Carl Zeiss, Inc., Oberkochen, Germany).
Cell counting kit-8 (CCK-8) assay
Cell viability was detected by using a CCK-8 kit (Merck KGaA, Darmstadt, Germany) according to the manufacturer's instructions. Briefly, U251 cells transfected with si-NC, si-YAP or si-RCHY1 were seeded into 96-well plates at a density of 3000 cells/well. After attachment, the cells were treated with 2, 4, 6 or 8 Gy radiation. After another 24 hrs of culture, cell viability was measured by CCK-8 kit (Sigma-Aldrich, St Louis, MO, USA).
Flow cytometry assay
Cell apoptosis was detected by flow cytometry assay with Annexin V Apoptosis Detection kit (BD Biosciences, San Diego, CA, USA). First, U251 cells were seeded in 12-well plates and transfected with si-NC, si-YAP1 or si-RCHY1. After 24 hrs of cell transfection, cells were treated with 2, 4, 6 or 8 Gy radiation. After another 24 hrs incubation at 37°C, cells were harvested and washed with PBS, followed by incubation with 5 μL of Annexin V and 5 μL propidium iodide (PI) solution diluted in 100 μL 1X binding buffer for 15 mins in the dark. Then, cells were washed with 1X binding buffer and resuspended with 500 μL of 1X binding buffer and submitted to flow cytometry assay on a Beckman FC500 flow cytometer (Beckman Coulter, Inc., Brea, CA, USA) and analyzed by FlowJo 7.6 software (Tree Star, Lnc, Ashland, OR, USA).
Statistical analysis
Every experiment in this study was performed ≥3 times. Data were indicated as mean ± SD. Statistical analyses were performed by GraphPad (version 6.0; GraphPad Software, Inc., La Jolla, CA, USA) with one-way ANOVA followed by the Tukey’s test for multiple groups and Student’s t-test for two groups. P<0.05 was thought as statistically significant difference.
Results
Radiation promotes cell apoptosis and deceases YAP1 mRNA level in glioma cells
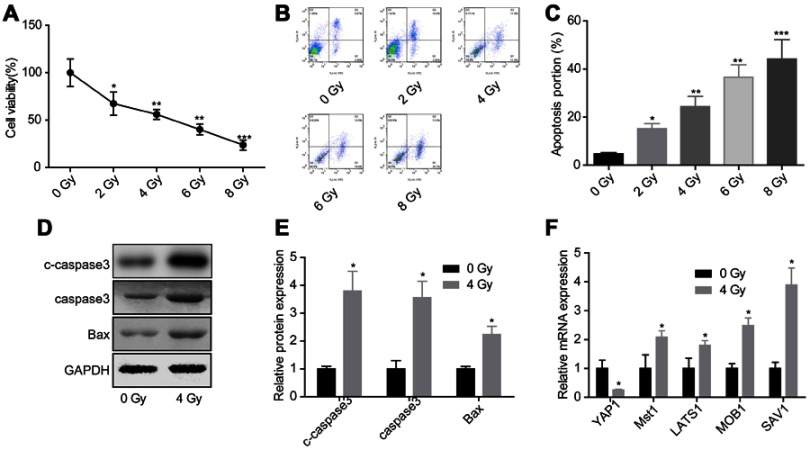
To evaluate the effects of Hippo/YAP signaling in cell apoptosis induced by radiation, we first assessed the effects of radiation on the activation of Hippo/YAP signaling. Compared with control group, irradiation exposure obviously inhibited glioma U251 cell growth (Figure 1A) and induced cell apoptosis (Figure 1B and C) in a dose-dependent manner, hence we chose the average dose of 4 Gy for the following experiments. Compared with the control group, 4 Gy exposure increased the expression of c-caspase3, caspase3 and Bax in U251 cells (Figure 1D and E). Besides, the mRNA expression of YAP1 was decreased, while Mst1, LATS1, MOB1 and SAV1 expressions were increased when U251 cells were treated with 4 Gy irradiation (Figure 1F). These results suggested that irradiation could promote glioma cell apoptosis and decease YAP1 mRNA level.
Figure 1.
Evaluation of the activation of Hippo/YAP signaling under irradiation exposure. U251 cells were treated with 0, 2, 4, 6 or 8 Gy irradiation, then the cells were collected 24 hrs after irradiation and submitted for the following assays. (A) CCK-8 assay to detect cell viability. (B, C) Flow cytometry assay with Annexin V/PI double staining was used to assess cell apoptosis. (D, E) Western blotting assay was performed to test the protein expression of caspase 3 and Bax. (F) Real-time qPCR was carried out the mRNA levels of Hippo/YAP1 signaling-related genes (n=3, *P<0.05, **P<0.01, ***P<0.001).
Abbreviations: CCK-8, Cell counting kit-8; YAP, Yes-associated protein.
Radiation exposure represses the activation of YAP1 signaling in glioma
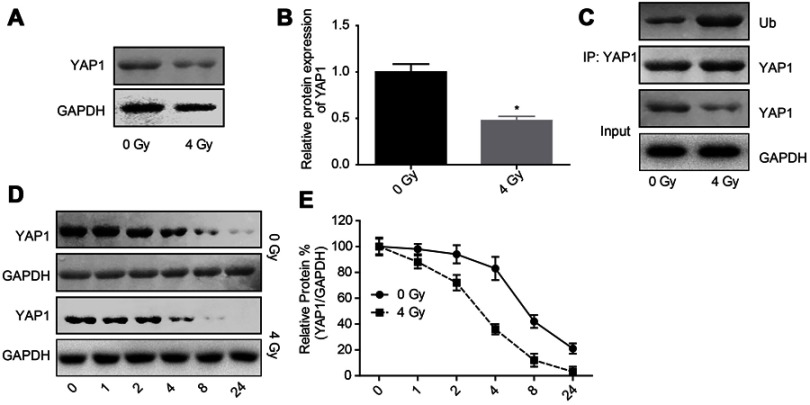
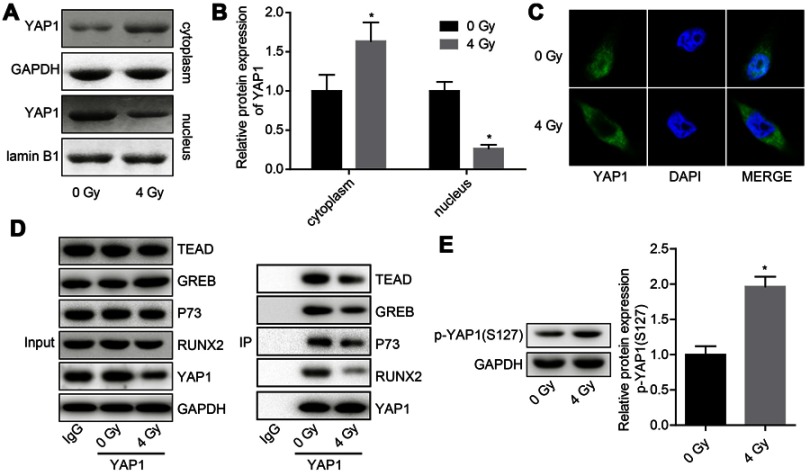
Next, we explored the effects of radiation exposure on the expression, stability and ubiquitination of YAP1 protein. Western blotting result showed that the expression of YAP1 protein was reduced when U251 cells were treated with 4 Gy irradiation (Figure 2A and B). Besides, 4 Gy irradiation increased the ubiquitination of YAP1 (Figure 2C) and crippled its protein stability (Figure 2D and E). Moreover, 4 Gy irradiation promoted the transportation of YAP1 protein from nuclear to cytoplasm (Figure 3A and C) and significantly impaired YAP1 protein interaction with the nuclear transcription factors TEAD, CREB, P73 and RUNX2 (Figure 3D). In addition, 4 Gy irradiation significantly increased the phosphorylation level of YAP1 (Figure 4E). These results demonstrated that radiation stimulation could repress the activation of YAP1 signaling.
Figure 2.
Irradiation decreased YAP1 expression by increasing its ubiquitination degradation. (A, B) The protein expression of YAP1 was determined by Western blotting assay after 24 hrs of U251 cells treatment with 4 Gy irradiation. (C) The interaction between Ub and YAP1 proteins was detected by Co-IP assay. (D, E) After 24 hrs of 4 Gy exposure, U251 cells were incubated with 100 μg/mL CHX for 0, 1, 2, 4, 8 and 24 hrs, then the cells were collected for Western blotting assay to detect YAP1 expression (n=3, *P<0.05).
Abbreviations: YAP, Yes-associated protein; CHX, cycloheximide; Co-IP, co-immunoprecipitation.
Figure 3.
Evaluation of the effects of irradiation on the subcellular location of YAP1 protein and the interactions of YAP1 protein with nuclear transcription factors. U251 cells were exposed to 4 Gy irradiation, then the cells were collected after 24 hr and subjected to the following assays. (A, B) Western blotting assay was used to detect the expression of YAP1 in nuclear and cytoplasm. (C) Immunofluorescence technology was used to assess the effect of 4 Gy irradiation on the subcellular location of YAP1 protein in U251 cells. (D) Co-IP assay was used to detect the interaction between YAP1 protein and TEAD, CREB, P73 and RUNX2 proteins, IgG was used as negative controls. (E.) Western blotting analysis of the expression of p-YAP1 after cells was treated with 4 Gy irradiation (n=3, *P<0.05).
Abbreviations: CREB, cAMP responsive element binding protein 1; RUNX2, runt-related transcription factor 2; TEAD, TEA/ATTS domain; YAP, Yes-associated protein; Co-IP, co-immunoprecipitation.
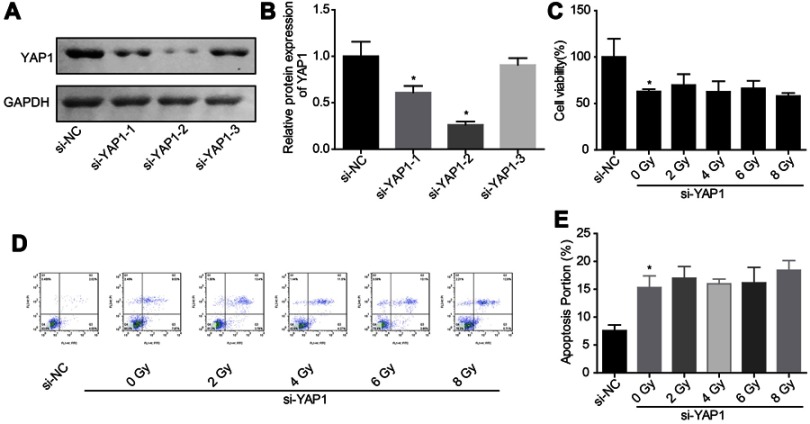
Figure 4.
Downregulation of YAP1 weakened the effect of irradiation on cell apoptosis induction. (A, B) The knockdown efficiency of si-YAP1 was assessed by Western blotting assay after 48 hrs of cell transfection. (C) Cell proliferation was detected by CCK-8 assay after 24 hrs of irradiation exposure. (D, E) Cell apoptosis was tested by flow cytometry assay after 24 hrs of irradiation exposure (n=3, *P<0.05).
Abbreviations: YAP, Yes-associated protein; CCK-8, Cell counting kit-8; Co-IP, co-immunoprecipitation.
Downregulation of YAP1 weakens the role of irradiation on cell apoptosis promotion in glioma
Then, we explored the effects of YAP1 on cell apoptosis under irradiation stimulation through loss-of-function assays. As shown in Figure 4A and B, si-2 targeting YAP1 gene showed the best knockdown efficiency between the 3 siRNAs, hence we chose it for the further study. Downregulation of YAP1 decreased U251 cell growth (Figure 4C) and induced cell apoptosis (Figure 4D and E), but abolished the effects of high dose of irradiation on cell apoptosis promotion. These results confirmed the vital role of Hippo/YAP signaling on irradiation-induced cell apoptosis in glioma.
Downregulation of RCHY1 increases YAP1 expression and inhibits glioma cell apoptosis
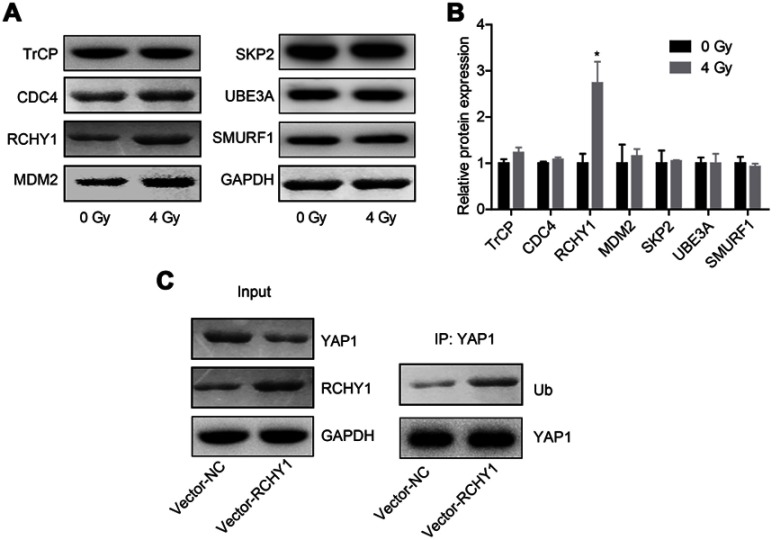
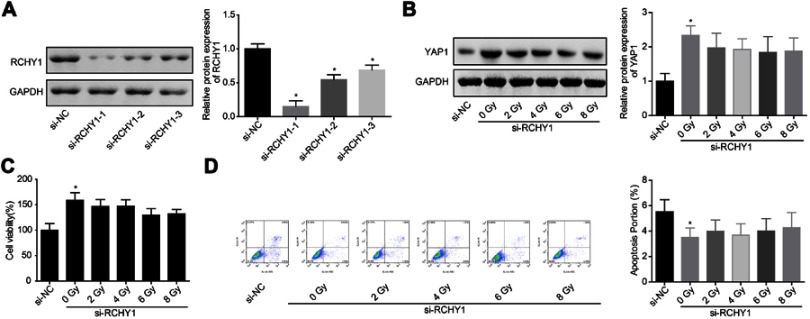
Subsequently, we probed the molecular mechanism of YAP1 ubiquitination. Among 6 ubiquitination-related proteins, TrCP, CDC4, RCHY1, MDM2, SKP2, UBE3A and SMURF1, irradiation treatment only increased RCHY1 expression, with no obvious change in the expression levels of TrCP, CDC4, MDM2, SKP2, UBE3A and SMURF1 (Figure 5A and B). And, RCHY1 upregulation significantly enhanced the ubiquitination of YAP1 protein (Figure 5C), suggesting that RCHY1 induced the ubiquitination of YAP1 protein. Then, we used si-RCHY1 to downregulate RCHY1 expression and then explored its role in irradiation-induced cell apoptosis in glioma. As shown in Figure 6A, si-1 significantly decreased RCHY1 expression in U251 cells. Downregulation of RCHY1 increased YAP1 expression in a dose of irradiation-independent manner (Figure 6B). Additionally, RCHY1 downregulation increased cell viability (Figure 6C) and reduced cell apoptosis (Figure 6D) in a dose of irradiation-independent manner. These above findings illustrated that the role of irradiation-induced cell apoptosis in glioma was closely related to RCHY1-regulated YAP1 expression.
Figure 5.
The ubiquitination of YAP1 protein was regulated by RCHY1. U251 cells were treated with 4 Gy irradiation, then the following assays were carried out after 24 hrs of irradiation exposure. (A, B) The expression of TrCP, CDC4, RCHY1, MDM2, SKP2, UBE3A and SMURF1 was detected by Western blotting assay. (C) Co-IP assay was used to detect the effects of RCHY1 overexpression on the ubiquitination of YAP1 protein (n=3, *P<0.05).
Abbreviation: YAP, Yes-associated protein.
Figure 6.
Downregulation of RCHY1 weakened the effect of irradiation on YAP1 downregulation and cell apoptosis induction. (A) The knockdown efficiency of si-RCHY1 was assessed by Western blotting assay after 48 hrs of cell transfection. Next, cells were transfected with si-RCHY1, following by irradiation exposure, then the following experiments were carried out. (B) The expression of YAP1 protein was assessed by Western blotting assay after 24 hrs of irradiation exposure. (C) Cell viability was detected by CCK-8 assay after 24 hrs of irradiation exposure. (D) Cell apoptosis was tested by flow cytometry assay after 24 hrs of irradiation exposure (n=3, *P<0.05).
Abbreviations: YAP, Yes-associated protein; CCK-8, Cell counting kit-8.
Discussion
Radiotherapy is one of the main therapeutical means for both low- and high-grade gliomas. However, radioresistance causes poor radiotherapeutic responses especially in glioblastoma patients; hence, overcoming radioresistance remains a pressing challenge for clinicians and researchers.13 It is well documented that pathways induced tumor cell excessive proliferation and defective apoptosis account for the major mechanisms of radioresistance in cancer cells.14–16 Therefore, here, we explored the roles of Hippo/YAP signaling in glioma cell proliferation and apoptosis under irradiation exposure. We report a new effective manner that downregulation of YAP can significantly enhance the radiosensitivity of glioma cells.
It is identified that Hippo pathway exerts a tumor suppressive role, while YAP exerts an oncogenic role in carcinogenesis via modulating several biological processes, including cell proliferation, apoptosis, survival and tumorigenesis.9,17,18 Physiologically, when Hippo signaling is active, YAP and transcriptional co-activator with PDZ-binding motif (TAZ) are phosphorylated and controlled in cytoplasm by forming core complexes with LATS1/2, Mst1/2, MOB1 and SAV1.18 However, the inactivation of Hippo pathway will redistribute YAP/TAZ to nuclear, leading to the transactivation of the downstream target genes via interacting with transcription factors, such as TEAD, p73, CREB and RUNX2.19–21 In the current study, to reveal the role of Hippo/YAP signaling in irradiation-mediated cell apoptosis, we first used real-time qPCR assay to assess the expressions of key genes of Hippo/YAP pathway under the treatment of irradiation. We found that the expressions of LATS1, Mst1, MOB1 and SAV1 mRNA were all increased when U251 cells were treated with irradiation, whereas YAP1 mRNA expression was reduced, as well as YAP1 protein expression. Moreover, we observed that irradiation promoted the nuclear export of YAP1 protein and weakened its interaction with TEAD, p73, CREB and RUNX2 proteins. All of the results suggest that the activation of Hippo and inactivation of YAP1 might play an important role in the radiation resistance of glioma.
YAP is known to possess oncogenic properties, including the ability to suppress cell apoptosis and promote cell growth.22 However, YAP role in carcinogenesis is highly context-dependent. For example, it acts as an oncogene in liver,23 pancreas24 cancers, whereas it exerts as a tumor suppressor in some breast cancers.25 In glioma, YAP1 and their target genes, CRY61, CTGF, and BIRC5 were identified to be significantly amplified in glioma tissues, and upregulation of YPA1 enhanced cell proliferation ability and conferred glioma cells cisplatin-resistance,26–28 illustrating that YAP1 plays a role in glioma chemosensitivity. Moreover, Zhang et al29 reported that inhibition of TAZ, an effector of Hippo signaling significantly promoted radiation-induced senescence and growth inhibition in glioma cells, suggesting a vital role of Hippo signaling plays in glioma radiosensitivity. In the present study, we recruited siRNA to silence YAP1 in U251 cells and thereby evaluated its function in irradiation-induced apoptosis. The results showed that YAP1 downregulation reduced cell viability and induced cell apoptosis, and impaired irradiation roles in apoptosis promotion, suggesting that irradiation promoted glioma cell apoptosis via downregulating YAP1.
Furthermore, we found that irradiation exposure significantly reduced YAP1 expression via accelerating its ubiquitination degradation in an RCHY1-dependent way. RCHY1 as an E3 ubiquitin ligase regulates proteasomal degradation of its target proteins such as p53, p63, and p73.30–32 And, the current study illustrated, for the first time, that the E3 ubiquitin ligase RCH1 but not CDC4, MDM2, SKP2, UBE3A or SMURF1 could interact with YAP1 and promoted its ubiquitination degradation. Besides, we observed that knockdown of RCHY1 abolished the inhibitory role of irradiation in YAP1 expression, thereby enhancing cell viability and inhibiting cell apoptosis regardless of high/low dose of irradiation imposition. This result indicated that irradiation treatment induced cell apoptosis through downregulating YAP1 in an RCH1-dependent manner.
In conclusion, the present study uncovers a vital role of Hippo/YAP signaling in radiation-induced cell apoptosis in glioma. Deregulation of YAP1 induced by RCHY1-mediated ubiquitination degradation can obviously weaken radiation-mediated cell apoptosis promotion, which then contributes to the improvement of radiosensitivity. Our study provides a potential of Hippo/YAP signaling as a target for overcoming radiation resistance in glioma.
Acknowledgments
This study was supported by grants from the Department of Education of Jilin Province (No. JJKH20170852KJ) and Department of Finance of Jilin Province.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19:v1–v88. doi: 10.1093/neuonc/nox158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(Suppl 5):v1–v49. doi: 10.1093/neuonc/nos218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naidu MD, Mason JM, Pica RV, Fung H, Pena LA. Radiation resistance in glioma cells determined by DNA damage repair activity of Ape1/Ref-1. J Radiat Res. 2010;51:393–404. doi: 10.1269/jrr.09077 [DOI] [PubMed] [Google Scholar]
- 4.Tapon N, Harvey KF, Bell DW, et al. Salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–478. doi: 10.1016/s0092-8674(02)00824-3 [DOI] [PubMed] [Google Scholar]
- 5.He C, Mao D, Hua G, et al. Davis JS and Wang C. The Hippo/YAP pathway interacts with EGFR signaling and HPV oncoproteins to regulate cervical cancer progression. EMBO Mol Med. 2015;7:1426–1449. doi: 10.15252/emmm.201404976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yimlamai D, Fowl BH, Camargo FD. Emerging evidence on the role of the Hippo/YAP pathway in liver physiology and cancer. J Hepatol. 2015;63:1491–1501. doi: 10.1016/j.jhep.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song R, Gu D, Zhang L, et al. Functional significance of Hippo/YAP signaling for drug resistance in colorectal cancer. Mol Carcinog. 2018;57:1608–1615. doi: 10.1002/mc.22883 [DOI] [PubMed] [Google Scholar]
- 8.Liu AM, Xu MZ, Chen J, Poon RT, Luk JM. Targeting YAP and Hippo signaling pathway in liver cancer. Expert Opin Ther Targets. 2010;14:855–868. doi: 10.1517/14728222.2010.499361 [DOI] [PubMed] [Google Scholar]
- 9.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez LA, Squatrito M, Northcott P, et al. Oncogenic YAP promotes radioresistance and genomic instability in medulloblastoma through IGF2-mediated Akt activation. Oncogene. 2012;31:1923–1937. doi: 10.1038/onc.2011.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang DY, Wu YN, Huang JQ, et al. Hippo/YAP signaling pathway is involved in osteosarcoma chemoresistance. Chin J Cancer. 2016;35:47. doi: 10.1186/s40880-016-0109-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xing Y, Liu Y, Liu T, et al. TNFAIP8 promotes the proliferation and cisplatin chemoresistance of non-small cell lung cancer through MDM2/p53 pathway. Cell Commun Signal. 2018;16:43. doi: 10.1186/s12964-018-0254-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han X, Xue X, Zhou H, Zhang G. A molecular view of the radioresistance of gliomas. Oncotarget. 2017;8:100931–100941. doi: 10.18632/oncotarget.21753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bussink J, van der Kogel AJ, Kaanders JH. Activation of the PI3-K/AKT pathway and implications for radioresistance mechanisms in head and neck cancer. Lancet Oncol. 2008;9:288–296. doi: 10.1016/S1470-2045(08)70073-1 [DOI] [PubMed] [Google Scholar]
- 15.Alhajala HS, Nguyen HS, Shabani S, et al. Irradiation of pediatric glioblastoma cells promotes radioresistance and enhances glioma malignancy via genome-wide transcriptome changes. Oncotarget. 2018;9:34122–34131. doi: 10.18632/oncotarget.26137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qu W, Li D, Wang Y, Wu Q, Hao D. Activation of sonic hedgehog signaling is associated with human osteosarcoma cells radioresistance characterized by increased proliferation, migration, and invasion. Med Sci Monit. 2018;24:3764–3771. doi: 10.12659/MSM.908278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiang L, Gilkes DM, Hu H, et al. Hypoxia-inducible factor 1 mediates TAZ expression and nuclear localization to induce the breast cancer stem cell phenotype. Oncotarget. 2014;5:12509–12527. doi: 10.18632/oncotarget.2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mo J-S, Park HW, Guan K-L. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15:642–656. doi: 10.15252/embr.201438638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebio A, Lenz HJ. Molecular pathways: hippo signaling, a critical tumor suppressor. Clin Cancer Res. 2015;21:5002–5007. doi: 10.1158/1078-0432.CCR-15-0411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zanconato F, Battilana G, Cordenonsi M, Piccolo S. YAP/TAZ as therapeutic targets in cancer. Curr Opin Pharmacol. 2016;29:26–33. doi: 10.1016/j.coph.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu AM, Poon RT, Luk JM. MicroRNA-375 targets Hippo-signaling effector YAP in liver cancer and inhibits tumor properties. Biochem Biophys Res Commun. 2010;394:623–627. doi: 10.1016/j.bbrc.2010.03.036 [DOI] [PubMed] [Google Scholar]
- 24.Jiang Z, Zhou C, Cheng L, et al. Inhibiting YAP expression suppresses pancreatic cancer progression by disrupting tumor-stromal interactions. J Exp Clin Cancer Res. 2018;37:69. doi: 10.1186/s13046-018-0740-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan M, Tomlinson V, Lara R, et al. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ. 2008;15:1752–1759. doi: 10.1038/cdd.2008.108 [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Geng D, Gao J, et al. Expression and significance of Hippo/YAP signaling in glioma progression. Tumour Biol. 2016. doi: 10.1007/s13277-016-5318-1 [DOI] [PubMed] [Google Scholar]
- 27.Baia GS, Caballero OL, Orr BA, et al. Yes-associated protein 1 is activated and functions as an oncogene in meningiomas. Mol Cancer Res. 2012;10:904–913. doi: 10.1158/1541-7786.MCR-12-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orr BA, Bai H, Odia Y, Jain D, Anders RA, Eberhart CG. Yes-associated protein 1 is widely expressed in human brain tumors and promotes glioblastoma growth. J Neuropathol Exp Neurol. 2011;70:568–577. doi: 10.1097/NEN.0b013e31821ff8d8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Cheng F, Wei Y, Guo D, Wang B, Li W. Inhibition of TAZ contributes radiation-induced senescence and growth arrest in glioma cells. Oncogene. 2019;38:2788–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leng RP, Lin Y, Ma W, et al. Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell. 2003;112:779–791. doi: 10.1016/s0092-8674(03)00193-4 [DOI] [PubMed] [Google Scholar]
- 31.Jung YS, Qian Y, Chen X. The p73 tumor suppressor is targeted by Pirh2 RING finger E3 ubiquitin ligase for the proteasome-dependent degradation. J Biol Chem. 2011;286:35388–35395. doi: 10.1074/jbc.M111.261537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung YS, Qian Y, Yan W, Chen X. Pirh2 E3 ubiquitin ligase modulates keratinocyte differentiation through p63. J Invest Dermatol. 2013;133:1178–1187. doi: 10.1038/jid.2012.466 [DOI] [PMC free article] [PubMed] [Google Scholar]