Abstract
Background
Egg production is the most economically-important trait in layers as it directly influences benefits of the poultry industry. To better understand the genetic architecture of egg production, we measured traits including age at first egg (AFE), weekly egg number (EN) from onset of laying eggs to 80 weeks which was divided into five stage (EN1: from onset of laying eggs to 23 weeks, EN2: from 23 to 37 weeks, EN3: from 37 to 50 weeks, EN4: from 50 to 61 weeks, EN5: from 61 to 80 weeks) based on egg production curve and total egg number across the whole laying period (Total-EN). Then we performed genome-wide association studies (GWAS) in 1078 Rhode Island Red hens using a linear mixed model.
Results
Estimates of pedigree and SNP-based genetic parameter showed that AFE and EN1 exhibited high heritability (0.51 ± 0.09, 0.53 ± 0.08), while the h2 for EN in other stages varied from low (0.07 ± 0.04) to moderate (0.24 ± 0.07) magnitude. Subsequently, seven univariate GWAS for AFE and ENs were carried out independently, from which a total of 161 candidate SNPs located on GGA1, GGA2, GGA5, GGA6, GGA9 and GGA24 were identified. Thirteen SNP located on GGA6 were associated with AFE and an interesting gene PRLHR that may affect AFE through regulating oxytocin secretion in chickens. Sixteen genome-wide significant SNPs associated with EN3 were in a strong linkage disequilibrium (LD) region spanning from 117.87 Mb to 118.36 Mb on GGA1 and the most significant SNP (rs315777735) accounted for 3.57% of phenotypic variance. Genes POLA1, PDK3, PRDX4 and APOO identified by annotating sixteen genome-wide significant SNPs can be considered as candidates associated with EN3. Unfortunately, our study did not find any candidate gene for the total egg number.
Conclusions
Findings in our study could provide promising genes and SNP markers to improve egg production performance based on marker-assisted breeding selection, while further functional validation is still needed in other populations.
Electronic supplementary material
The online version of this article (10.1186/s12863-019-0771-7) contains supplementary material, which is available to authorized users.
Keywords: Egg production, Genome-wide association study, Candidate genes, Heritability, Chicken
Background
Egg production is the most important trait in layers as it directly affects economic benefits of poultry farmers [1]. Therefore, improving egg production is one of the main goals in a chicken breeding program. The egg production can be evaluated using different measurements, such as egg number (EN), hen-housed egg production (HHP) and egg production rate. Egg number is of great significance to select hens with higher capacity of egg laying in modern poultry breeding, especially for the country where the eggs are sold by quantity, since it can efficiently evaluate the individual egg production in a certain period. Different from EN, HHP is a good estimation of group egg production. The age at first egg (AFE) is also a very important trait for egg production as it is a partial determination of laying period.
Until now, egg production has acquired considerable improvement by the conventional selection method. However, the conventional breeding approaches could not completely eliminate the environmental effects which unavoidably results in inaccuracy of heritability estimates [2, 3]. Anang et al.(2000) reported the estimates of heritability and genetic correlation of monthly egg production in a population of White Leghorn [4]. They showed that the estimates of heritability for cumulative records were higher than monthly records, and phenotypic and genetic correlations among monthly production were high for contiguous periods. With the advances in technologies of molecular genetics and availability of single nucleotide polymorphism (SNP) markers, many studies had been performed to elucidate the genetic basis of egg production trait [5–7]. Currently, over 185 quantitative trait loci (QTL) on 24 different chromosomes (https://www.animalgenome.org/cgi-bin/QTLdb/GG/index) have been reported to be associated with age at first egg, egg number and egg production rate in chickens [8–14]. Although many QTLs were identified to exert main effect on egg production trait, some of them had wide confident intervals for position and were rarely replicated [15, 16]. A few useful QTLs can be utilized to improve breeding programs based on marker-assisted selection and best linear unbiased prediction (BLUP) [17]. A new research era began with subsequent advances in sequencing technologies and SNP chips, when genome-wide association analysis has become one of the most efficient methods to detect genetic variation in livestock. In previous researches, Liu et al. (2011), Wolc et al. (2012) and Yuan et al. (2015) reported some candidate genes for egg production, such as ODZ2, GRB14, GTF2A1 and CLSPN etc. [18–22]. Most of these candidates were detected based on an F2 cross population or cross-sectional in a specific laying period, while no studies were focused on pure line population across the whole laying cycle.
In our research, we employed the commercial 600 K SNP array to identify the genomic regions and candidate genes associated with age at first egg and egg numbers in a pure line population derived from Rhode Island Red using genome-wide association study (GWAS) that could potentially accelerate the genetic improvement of egg production.
Results
Phenotype and genetic parameter statistics
Descriptive statistics of AFE and ENs across the whole laying period are shown in Table 1. The mean value of AFE in this population was 137 days, which meant that hens started laying eggs at about 20 weeks of age. Moreover, EN1 (egg numbers from onset of laying to 23 weeks) and EN5 (egg numbers from 61 to 80 weeks) had higher phenotypic coefficient of variation (22.67, 25.89%) than the other traits (2.27% ~ 17.27%). The pedigree-based heritability was high for AFE and EN1 (0.51 ± 0.09, 0.53 ± 0.08) and relatively low (0.09) to moderate (0.24) for EN at other stages.
Table 1.
Descriptive statistics for AFE and egg number in different stage
| Traits | N | Mean | SD | CV(%) | h 2 (SE) |
|---|---|---|---|---|---|
| AFE | 1063 | 137.37d | 5.7 | 4.15 | 0.51 (0.09) |
| EN1 | 1063 | 24.15 | 5.48 | 22.67 | 0.53 (0.08) |
| EN2 | 1063 | 95.16 | 2.16 | 2.27 | 0.16 (0.06) |
| EN3 | 1063 | 84.58 | 6.07 | 7.17 | 0.24 (0.07) |
| EN4 | 1060 | 64.71 | 11.17 | 17.27 | 0.23 (0.07) |
| EN5 | 1004 | 105.57 | 27.33 | 25.89 | 0.14 (0.06) |
| Total-EN | 1063 | 368.13 | 47.84 | 13.00 | 0.09 (0.05) |
Abbreviations: AFE: age at first egg; EN1, EN2, EN3, EN4, EN5: total egg numbers in each of five stages (from onset of laying eggs to 23 weeks, from 23 to 37 weeks, from 37 to 50 weeks, from 50 to 61 weeks and from 61 to 80 weeks); Total-EN: total egg number from onset of laying eggs to 80 weeks; N number of samples, SD standard deviation, CV coefficient of variance; h2(SE), pedigree-based heritability (standard error)
Estimates of SNP-based heritability as well as genetic and phenotypic correlations between AFE and ENs are displayed in Table 2. The pedigree-based heritability estimates were larger than those due to SNP information for all traits except for EN5 and Total-EN (0.14 vs 0.18, 0.09 vs 0.14). Genetic correlation analyses revealed that ENs in different laying period were most positively interrelated and ENs at late laying period (EN3, EN4 and EN5) had high correlation with the Total-EN.
Table 2.
Estimates of SNP based heritabilities (on the diagonal) and of genetic (above the diagonal) and phenotypic correlations between egg production traits
| Traits | AFE | EN1 | EN2 | EN3 | EN4 | EN5 | Total-EN |
|---|---|---|---|---|---|---|---|
| AFE | 0.40 (0.05) | – | 0.14 (0.21) | −0.02 (0.15) | −0.13 (0.15) | − 0.15 (0.15) | −0.53 (0.14) |
| EN1 | −0.95 | 0.39 (0.05) | −0.19 (0.21) | 0.03 (0.15) | 0.12 (0.15) | 0.10 (0.15) | 0.51 (0.14) |
| EN2 | 0.19 | −0.19 | 0.07 (0.04) | 0.64 (0.25) | 0.35 (0.26) | −0.16 (0.29) | 0.10 (0.29) |
| EN3 | 0.08 | −0.08 | 0.24 | 0.19 (0.05) | 0.81 (0.11) | 0.38 (0.17) | 0.58 (0.15) |
| EN4 | 0.03 | −0.04 | 0.14 | 0.47 | 0.18 (0.05) | 0.77 (0.11) | 0.87 (0.07) |
| EN5 | 0.06 | −0.06 | 0.12 | 0.32 | 0.53 | 0.18 (0.05) | 0.88 (0.05) |
| Total-EN | −0.15 | 0.16 | 0.18 | 0.51 | 0.78 | 0.87 | 0.14 (0.05) |
AFE: age at first egg; EN1, EN2, EN3, EN4, EN5: total egg numbers in each of five stages (from onset of laying eggs to 23 weeks, from 23 to 37 weeks, from 37 to 50 weeks, from 50 to 61 weeks and from 61 to 80 weeks); Total-EN: total egg number from onset of laying eggs to 80 weeks
Genome-wide association study
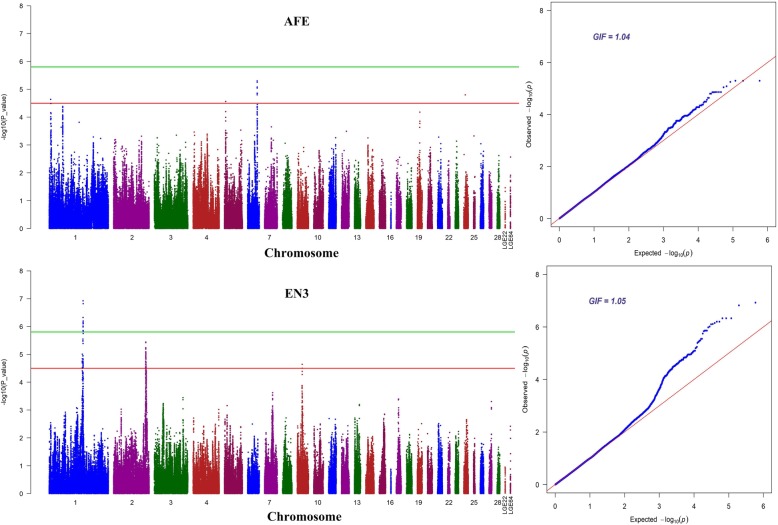
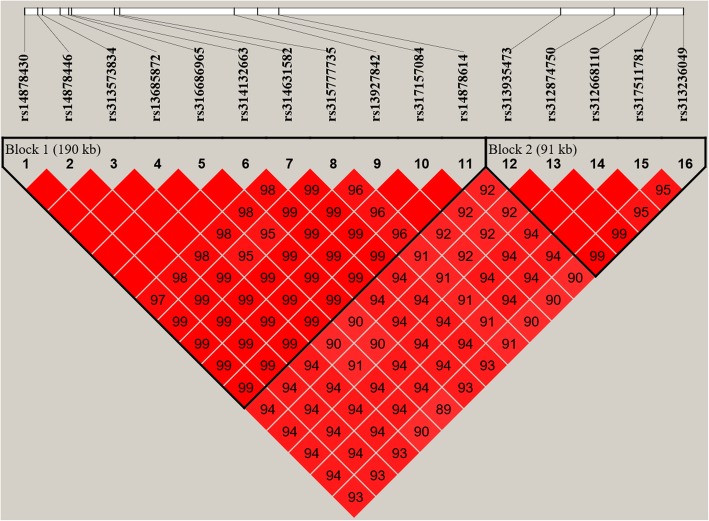
Association tests for AFE and ENs were performed using a univariate linear mixed model. A total of 161 unique candidate SNPs (P value < 3.17E-05) located on GGA1, GGA2, GGA5, GGA6, GGA9 and GGA24 were identified (Additional file 1: Table S1). Seventeen of them scattering on four different chromosomes were suggestively associated with AFE (Table 3), while the rest containing 16 significant and 139 suggestive SNPs were related to ENs. The Manhattan and Q-Q plots for AFE and EN3 GWAS are presented in Fig. 1, which also show the genomic inflation factor (GIF) were 1.04 and 1.05, respectively. And the results of other traits are in Additional file 2: Figure. S1. For EN3, sixteen of those candidate SNPs reached genome-wide significant level (P value < 1.58E-06) and were located within a 0.49 Mb region that spans from 117.87 Mb to 118.36 Mb on GGA1 (Table 4). LD analysis revealed that all genome-wide significant SNPs located in above 0.49 Mb region were in strong LD status and were clustered into two blocks (Block 1: 190Kb and Block 2: 91Kb) (Fig. 2). Unfortunately, there was no genome-wide significant hits associated with EN at other stages.
Table 3.
GWAS and annotations of suggestive SNPs (P value < 3.17E-05) for AFE
| GGAa | Regions | N_SNPsb | Candidate/Nearst genec |
|---|---|---|---|
| 1 | 4.61 Mb ~ 4.62 Mb | 2 | GATA3 |
| 5 | 6,928,284 | 1 | HIPK3 |
| 6 | 29.53 Mb ~ 29.73 Mb | 13 | RAB11FIP2; FAM204A; PRLHR |
| 24 | 1,994,941 | 1 | NTM |
a Chicken chromosome; b the number of SNPs in the regions; cgene name (Gallus_gallus-5.0 source)
Fig. 1.
Manhattan and Q-Q plots derived from GWASs for AFE and EN3. Each dot on this figure corresponds to a SNP within the dataset, while the horizontal red and black lines denote the genome-wide significance (1.58e-6) and suggestive significance thresholds (3.17e-5), respectively. The Manhattan plot contains -log10 observed P-values for genome-wide SNPs (y-axis) plotted against their corresponding position on each chromosome (x-axis), while the Q-Q plot contains expected -log10-transformed P-values plotted against observed -log10-transformed P-values. GIF denotes the genomic inflation factor indicating the degree of population stratification
Table 4.
Annotation of genome-wide significant SNPs (P value < 1.58E-06) associated with EN3
| SNP | GGAa | Positionb | ALT/REFc | MAFd | Beta (SE)e | P value | Consequence | Candidate/ Nearest gene |
|---|---|---|---|---|---|---|---|---|
| rs14878430 | 1 | 117,871,938 | C/T | 0.407 | 0.25 (0.05) | 7.96E-07 | Intron | POLA1 |
| rs14878446 | 1 | 117,881,774 | T/C | 0.408 | 0.25 (0.05) | 1.38E-06 | Intron | POLA1 |
| rs313573834 | 1 | 117,885,299 | A/G | 0.408 | 0.25 (0.05) | 1.38E-06 | Intron | POLA1 |
| rs13685872 | 1 | 117,898,459 | A/G | 0.417 | 0.25 (0.05) | 1.04E-06 | Intron | POLA1 |
| rs316686965 | 1 | 117,904,908 | T/A | 0.417 | 0.25 (0.05) | 9.65E-07 | Intron | POLA1 |
| rs314132663 | 1 | 117,907,038 | A/G | 0.408 | 0.25 (0.05) | 1.38E-06 | Intron | POLA1 |
| rs314631582 | 1 | 117,939,090 | C/T | 0.406 | 0.26 (0.05) | 7.05E-07 | Intron | POLA1 |
| rs315777735 | 1 | 117,943,070 | A/G | 0.418 | 0.27 (0.05) | 1.19E-07 | Intron | POLA1 |
| rs13927842 | 1 | 118,028,676 | C/A | 0.426 | 0.26 (0.05) | 4.70E-07 | Intron | POLA1 |
| rs317157084 | 1 | 118,046,119 | C/T | 0.426 | 0.26 (0.05) | 4.70E-07 | Downstream_43.27Kb | PDK3 |
| rs14878614 | 1 | 118,062,286 | C/A | 0.426 | 0.26 (0.05) | 4.70E-07 | Downstream_27.35Kb | PDK3 |
| rs313935473 | 1 | 118,272,993 | A/G | 0.423 | 0.24 (0.05) | 1.45E-06 | Upstream_3.672Kb | APOO |
| rs312874750 | 1 | 118,313,008 | G/A | 0.424 | 0.25 (0.05) | 7.67E-07 | Downstream_5.72Kb | APOO |
| rs312668110 | 1 | 118,340,009 | G/C | 0.433 | 0.25 (0.05) | 6.31E-07 | Intron | ACOT9 |
| rs317511781 | 1 | 118,344,902 | T/G | 0.433 | 0.25 (0.05) | 6.31E-07 | Downstream_86bp | ACOT9 |
| rs313236049 | 1 | 118,364,746 | C/T | 0.451 | 0.26 (0.05) | 1.51E-07 | Upstream_5.44Kb | PRDX4 |
aChicken chromosome; bGallus_gallus-5.0 source; cALT/REF: alternative allele/reference allele; dMinor allele frequency; eEstimated allelic substitution effect per copy of the effect allele based on an inverse-normal transformed scale under an additive model, expressed in SD unit/allele
Fig. 2.
Linkage Disequilibrium (LD) analyses of SNPs in the significant region (0.49 Mb) for egg number in period 3 (EN3). LD plot of significant SNPs on GGA1 from 117.87 Mb to 118.36 Mb
Annotation of candidate SNPs
All the candidate genes associated with AFE were displayed in Table 3, including GATA binding protein 3 (GATA3), homeodomain interacting protein kinase 3 (HIPK3), RAB11 family interacting protein 2 (RAB11FIP2), Family with sequence similarity 204 member A (FAM204A), prolactin releasing hormone receptor (PRLHR) and Neurotrimin (NTM).
The phenotypic variance of EN3 explained by the most genome-wide significant SNP (rs315777735) were estimated as 3.57% using the GCTA software. To detect promising EN3-associated genes, detailed information of sixteen genome-wide significant SNPs were annotated using the online VEP tool (Table 4). The SNP (rs315777735) was located within the intron of the DNA polymerase alpha 1, catalytic subunit (POLA1), while the other SNPs were near to the candidate genes Pyruvate dehydrogenase kinase 3 (PDK3), Peroxiredoxin 4 (PRDX4), apolipoprotein O (APOO) and acyl-CoA thioesterase 9 (ACOT9).
Discussion
Egg production is an important economic trait. So far, many studies have focused on the genetic determinants of AFE and EN in chicken, and most of them were based on an F2 crossed population or cross-section in a specific laying period. In earlier work, Goraga et al. (2011) and Wolc et al. (2014) reported that some QTLs on chromosomes 4, 5, 7 and 17 had an influence on EN [14, 19]. Yuan et al. (2015) subsequently carried out a GWAS in an F2 population across the whole laying period (from 21 to 72 week) [20], from which, nine candidate loci on GGA5 and two promising genes were annotated to be associated with EN. However, most of above regions or significant SNPs were population-specific and only a few candidates were found by multiple populations. In our study, we performed GWAS for AFE and EN in different stage of production using a univariate linear mixed model. This is the first GWAS using the 600 K SNP array in a Rhode Island Red pure line population across the whole laying period.
The descriptive statistics (Table 1) reveal that EN5 had the largest phenotypic variation than the other traits due to the reason that some birds died or were too old to lay eggs in this stage because of low rate of follicle development [23]. Besides, genetic parameter estimates show that AFE is a high heritable trait, which is approximately coincided with the previous reports [20, 24]. In addition, we divided the whole laying period (from onset of laying eggs to 80 weeks) into five stages according to the egg production curve and counted the total egg number in each stage, which was similar to Yuan et al. (2015). Estimate of pedigree-based heritability for EN5 was smaller than EN at the early laying stages (EN1, EN2, EN3 and EN4) probably due to the increased environmental or phenotypic variation in the late laying stage as demonstrated by Engstrom et al. [25]. Results also show that the pedigree-based heritability estimates were different with the SNP-based estimates, which were likely caused by the difference of addictive variance estimates using the pedigree or common 600 K SNPs in the animal model. Moreover, genetic and phenotypic correlations among ENs were mostly positive, especially between two neighboring stages. The egg numbers at late laying stages (EN3, EN4 and EN5) had higher correlation with the total egg number in the whole laying period than those at earlier laying stage (EN1 and EN2). These results were consistent with previous studies in White Leghorn hens [4, 26].
We carried out seven univariate genome-wide association analysis for AFE and ENs independently. Previous QTL mapping and genome-wide association studies reported some QTLs or SNPs on chromosome 1, 2, 3, 4, 5, 7, 11, 13, 20, 24 and Z were significantly associated with AFE (https://www.animalgenome.org/cgi-bin/QTLdb/GG/index) [12, 18, 19, 27]. Our GWAS for AFE did not detect any genome-wide significant SNPs, while two chromosomal regions (GGA1: 4.61 Mb ~ 4.62 Mb; GGA6: 29.53 Mb ~ 29.73 Mb) and two SNPs (located on GGA5 and GGA24) were suggestively associated to AFE. These candidate QTLs were firstly reported and six genes around these SNPs were annotated using the VEP tool, especially the Prolactin releasing hormone receptor (PRLHR) gene also known as PrRPR in chickens. Previous studies reported that PrRP and its structurally related peptide (C-RFa) may play distinct roles in controlling feed intake and pituitary functions in chicks [28]. In addition, PrRP participates in many important physiological processes to influence sexual maturity, including gonadotropin-releasing hormone, vasopressin and oxytocin secretion [29–31]. We speculate that the PRLHR gene has an indirect effect on AFE and further validation is required in multiple populations.
Univariate tests in egg numbers at different stages detected a total of 155 SNPs located on GGA1, GGA2, GGA6 and GGA9, only sixteen of which associated with EN3 were genome-wide significant (P value < 1.58E-06) and were located in a strong LD region (0.49 Mb) on GGA1 (Table 4). Furthermore, several promising genes around the significant regions were obtained. POLA1 gene encodes the catalytic subunit of DNA polymerase alpha 1 and is an essential component of the DNA replication machinery [32, 33]. Another gene pyruvate dehydrogenase kinase isoenzyme 3 (PDK3) is one of the four PDK isoenzymes, which negatively regulates the activity of pyruvate dehydrogenase complex (PDC) by reversible phosphorylation [34]. The PDK3 exhibits tissue-specific expression in testes, brain, kidney and pancreatic islets of adult [35, 36]. And the PRDX4 is a 2-cysteine peroxiredoxin that is a major component of the endoplasmic reticulum (ER) oxidative protein folding pathway [37, 38]. Thus, PRDX4 oxidative activity acts as a sensor to directly couple neuronal differentiation with redox environments in the ER [39]. As there are not in-depth functional researches about above genes in chickens, we speculate that they may regulate egg production via interacting with neuronal system based on studies in human. In addition, an interesting gene the apolipoprotein O (APOO) around the significant SNPs was also detected, which was a new member of the apolipoprotein family. Previous studies reported that APOO participated in fatty acid and lipid metabolism and regulated the production of lipoprotein through hormones in the chicken [40, 41]. We therefore suggest that the APOO is a pleiotropic gene, which affects not only the fatty deposition, but also the egg number at a specific stage in chickens. However, our study did not find a gene that affecting the egg number all the time.
Conclusion
In conclusion, the GWAS performed in this study demonstrates that AFE is highly heritable and negatively correlated with ENs which have relatively low heritability. PRLHR gene may affect AFE through regulating oxytocin secretion in chickens. Moreover, four additional genes (POLA1, PDK3, PRDX4 and APOO) identified by annotating sixteen genome-wide significant SNPs can be considered as candidates associated with EN. Findings in our research could better understand the genetic basis of egg production, while further functional validation is still needed in other populations.
Methods
Animals and phenotypes
A total of 1078 Rhode Island Red hens from Beijing Huadu Yukou Poultry Breeding Co., Ltd. were used in our study. The egg production and quality of this pure line has been artificially selected over ten generations. We only utilized the data from the last generation (G11) in the study, and the population was produced by crossing 92 sires and 801 dams (One sire mating 8–12 hens). Blood samples were collected from brachial veins using the standard procedure of the breeding program, which was approved by the Animal Welfare Committee of China Agricultural University.
All birds had pedigree and were housed in individual cages of the same condition. The age at first egg (AFE) and weekly egg production from onset of laying eggs to 80 weeks of age for each bird were recorded. We divided the whole laying period into 5 stages based on the egg production curve (Additional file 3: Figure. S2) and counted the egg numbers (EN) in each individual phase: the period of rapidly ascending egg production (the rate < 95% from onset of laying eggs to 23 weeks) (EN1); the period whose laying rate was over 95% was called peak of laying, which last from 23 to 37 weeks of age (EN2); while the period whose laying rate is between 90% and 95% including 37 to 50 weeks of age was set as the third phase (EN3); the fourth period with 80% to 90% laying rate was the decline phase lasting from 50 to 61 weeks (EN4); the laying rate of final phase including 61 to 80 weeks is lower than 80% (EN5). We also calculated the total egg number from onset of laying eggs to 80 weeks (Total-EN). The values used in the following analyses were derived from the rank-based inverse normal transformation of phenotypic records using the GenABEL package in R software (https://www.r-project.org/). Because the transformed data has the lower standard deviation.
Genotyping and quality control
Genomic DNA was extracted from 1.5-mL blood samples using DNeasy 96 Blood & Tissue Kits (QIAGEN, Germany). A total of 1078 hens were genotyped with the Chicken 600 K SNP array [42] (Affymetrix, Inc. Santa Clara, CA, USA) which contained 580,961 SNPs across 28 autosomes and two sex chromosomes. We first discarded 6593 SNPs with unknown physical position and repeated genomic coordinates. The Affymetrix Power Tools v1.19.0 (APT) software was then implemented to control the quality of sample call rate (> 97%) and dish quality (> 0.82). After the quality control, 1063 individuals and 517,856 SNPs remained. In addition, the low quality of SNPs (SNP call rate < 95%, minor allele frequency < 0.01, Hardy-Weinberg equilibrium P < 1 × 10–6) were filtered out through the PLINK package [43]. The remaining SNPs with missing genotypes were imputed using the Beagle v4.0 procedure [44]. Finally, a total of 1063 individuals and 294,705 SNPs located on autosomes (Additional file 4: Table S2) were deemed eligible for the following analyses.
Genome-wide association studies
Prior to the GWAS, a principal component analysis (PCA) was implemented to evaluate the population stratification using the PLINK package [43]. We pruned all SNPs to obtain independent SNPs and blocks via the option of --indep-pairwise 25 5 0.2 and –blocks-max-kb 500, respectively. A total of 14,878 independent SNPs and 16,711 linkage disequilibrium blocks were detected. The relationship matrix was built through the independent SNPs. And the principal components were calculated by the eigenvectors of the relationship matrix. Then, GWAS were carried out independently for AFE and ENs. Seven univariate association analyses which models AFE and egg numbers in different stages were performed in GEMMA software [45]. The univariate linear mixed model was as follows:
In this expression, Y denotes the vector of phenotypic values of AFE and ENs for n samples; W is a matrix of covariates (including a column of 1 s and fixed effects, such as batch, top five principal components which contributing to majority of population structure variations were included in the model to control population structure); α is the corresponding coefficients of the fixed effects; x is a vector of SNP genotypes (coded as 0, 1, 2); β is the effect sizes of SNPs for the phenotypes; u is a vector of random polygenic effects with u ~ MVNn(0, λτ−1K) where λ represents the ratio between the two variance components and τ−1 denotes the variance of the residual errors, K is a known kinship relatedness matrix; ε is a matrix of errors. We applied the Wald statistical test to evaluate the null hypothesis that the SNP effect sizes for all phenotypes are zero, H0: β = 0 for each SNP versus H1: β ≠ 0. For each SNP in turn, GEMMA acquires either the maximum likelihood estimate or restricted maximum likelihood estimate of λ, and outputs the corresponding P value.
Considering the over-conservation of 5% Bonferroni correction method, we adjusted the threshold of genome-wide significant P-values based on the number of linkage disequilibrium blocks and the independent SNP markers [46]. Therefore, the threshold P values of suggestive and genome-wide significance were calculated as 3.17E-05 (1.00/31,589) and 1.58E-06 (0.05/31,589), respectively.
The Manhattan and quantile-quantile (Q-Q) plots for AFE and EN were performed using the “gap” package in the R (https://cran.r-project.org/web/packages/gap/). Genomic inflation factor (GIF) was calculated using the “GenABEL” package in the R software to judge the extent of false positive signals [47].
Linkage disequilibrium analysis and gene annotation
We performed linkage disequilibrium (LD) analysis to further characterize causative regions associated with AFE and ENs traits by applying the solid spine algorithm in the Haploview v4.2 software [48]. Annotation of genes adjacent to candidate SNPs were obtained using the variant effect predictor (VEP) supplied by Ensembl genome browser 91(http://www.ensembl.org/Tools/VEP) [49].
Genetic parameter and SNP effect
The pedigree-based hereditability values for AFE and ENs were estimated using a multi-trait general animal model method in the DMU v6.0 software [50]. The model was as follows:
where y denotes the vector of the phenotypic values, 1 is the n × 1 vector of all 1’s,μis population means (fixed effect), Z is a incidence matrix of random effect (n × 1 vector), and a and e denote the additive effect and random residual error, respectively.
Moreover, the SNP-based heritability (h2snp) [51] and pairwise genetic correlations of AFE and EN were calculated using a restricted maximum likelihood (REML) approach implemented in the software GCTA v1.24 [52]. Besides, this procedure was used to estimate the phenotypic variance contribution of significant SNPs based on a genetic matrix constructed from all eligible SNPs. The models of SNP-based heritability and the phenotypic variance contribution were the same as our previously described by Yi et al. (2015) [53].
Additional files
Table S1. The whole information of genome-wide significant and suggestively significant SNPs for AFE and ENs using genome-wide association studies. (XLSX 21 kb)
Figure S1. Manhattan plots for egg numbers at different stages. (PDF 537 kb)
Figure S2. The plot of egg production curve in this population. (JPG 109 kb)
Table S2. Basic information of SNP markers on a physical map after quality control. (XLSX 11 kb)
Abbreviations
- AFE
Age at first egg
- EN
Egg number
- GWAS
Genome-wide association study
- LD
Linkage disequilibrium
- QTL
Quantitative trait loci
- REML
Restricted maximum likelihood
Authors’ contributions
CS, GW and NY conceived the study and designed the project. ZL performed the genetic and bioinformatic analyses. ZL, YY, GL, GW, and AL all contributed by collecting samples and measuring phenotypic data. ZL wrote the manuscript, which was then revised by NY and CS. All authors read and approved the final draft.
Funding
This work was funded in part by grants from the China Agriculture Research Systems (CARS-40), Chinese Universities Scientific Fund (2018QC030), Breeding and Distribution of Jing Pink Layer Variety (D171100007817001) and Programs for Changjiang Scholars and Innovative Research in University (IRT_15R62). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Ethics approval
All the blood sample collections were performed in accordance with the Guidelines for Experimental Animals established by the Ministry of Agriculture of China (Beijing, China). The whole study was approved by the Animal Welfare Committee of China Agricultural University (permit number: SYXK 2007–0023).
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhuang Liu, Email: zliu0106@126.com.
Ning Yang, Email: nyang@cau.edu.cn.
Yiyuan Yan, Email: yiyuan1112@126.com.
Guangqi Li, Email: Lgq717535@126.com.
Aiqiao Liu, Email: liuaq@hdyk.com.cn.
Guiqin Wu, Email: wugq@hdyk.com.cn.
Congjiao Sun, Email: cjsun@cau.edu.cn.
References
- 1.Wolc A, Arango J, Settar P, O'Sullivan NP, Dekkers JC. Evaluation of egg production in layers using random regression models. Poult Sci. 2011;90(1):30–34. doi: 10.3382/ps.2010-01118. [DOI] [PubMed] [Google Scholar]
- 2.Meuwissen THE, Hayes BJ, Goddard ME. Prediction of Total Genetic Value Using Genome-Wide Dense Marker Maps. 2001;157:1819–1829. doi: 10.1093/genetics/157.4.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muir WM. Comparison of genomic and traditional BLUP-estimated breeding value accuracy and selection response under alternative trait and genomic parameters. J Anim Breed Genet. 2007;124:342–355. doi: 10.1111/j.1439-0388.2007.00700.x. [DOI] [PubMed] [Google Scholar]
- 4.Anang A, Mielenz N, Schuler L. Genetic and phenotypic parameters for monthly egg production in white Leghorn hens. J Anim Breed Genet. 2000;117:407–415. [Google Scholar]
- 5.Mohammadifar A, Mohammadabadi M. Melanocortin-3 receptor (MC3R) gene association with growth and egg production traits in Fars indigenous chicken. Malays Appl Biol. 2018;47(3):85–90. [Google Scholar]
- 6.Moazeni SM, Mohammadabadi M, Sadeghi M, Shahrbabak HM, Koshkoieh AE, Bordbar F. Association between UCP gene polymorphisms and growth, breeding value of growth and reproductive traits in Mazandaran indigenous chicken. Open Journal of Animal Sciences. 2016;06(01):1–8. [Google Scholar]
- 7.Mohammadabadi MR, Nikbakhti M, Mirzaee HR, Shandi A, Saghi DA, Romanov MN, Moiseyeva IG. Genetic variability in three native Iranian chicken populations of the Khorasan province based on microsatellite markers. Russ J Genet. 2010;46(4):505–509. [PubMed] [Google Scholar]
- 8.Tuiskula-Haavisto M, Honkatukia M, Vilkki J, de Koning DJ, Schulman NF, Maki-Tanila A. Mapping of quantitative trait loci affecting quality and production traits in egg layers. Poult Sci. 2002;81:919–927. doi: 10.1093/ps/81.7.919. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki O, Odawara S, Takahashi H, Nirasawa K, Oyamada Y, Yamamoto R, Ishii K, Nagamine Y, Takeda H, Kobayashi E, et al. Genetic mapping of quantitative trait loci affecting body weight, egg character and egg production in F2 intercross chickens. Anim Genet. 2004;35(3):188–194. doi: 10.1111/j.1365-2052.2004.01133.x. [DOI] [PubMed] [Google Scholar]
- 10.Schreiweis MA, Hester PY, Settar P, Moody DE. Identification of quantitative trait loci associated with egg quality, egg production, and body weight in an F2 resource population of chickens. Anim Genet. 2006;37(2):106–112. doi: 10.1111/j.1365-2052.2005.01394.x. [DOI] [PubMed] [Google Scholar]
- 11.Atzmon G, Blum S, Feldman M, Cahaner A, Lavi U, Hillel J. QTLs detected in a multigenerational resource chicken population. J Hered. 2008;99(5):528–538. doi: 10.1093/jhered/esn030. [DOI] [PubMed] [Google Scholar]
- 12.Goto T, Ishikawa A, Onitsuka S, Goto N, Fujikawa Y, Umino T, Nishibori M, Tsudzuki M. Mapping quantitative trait loci for egg production traits in an F2 intercross of oh-Shamo and white Leghorn chickens. Anim Genet. 2011;42(6):634–641. doi: 10.1111/j.1365-2052.2011.02190.x. [DOI] [PubMed] [Google Scholar]
- 13.Xu H, Zeng H, Luo C, Zhang D, Wang Q, Sun L, Yang L, Zhou M, Nie Q, Zhang X. Genetic effects of polymorphisms in candidate genes and the QTL region on chicken age at first egg. BMC Genet. 2011;12:33. doi: 10.1186/1471-2156-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goraga ZS, Nassar MK, Brockmann GA. Quantitative trait loci segregating in crosses between New Hampshire and white Leghorn chicken lines: I. egg production traits. Anim Genet. 2012;43(2):183–189. doi: 10.1111/j.1365-2052.2011.02233.x. [DOI] [PubMed] [Google Scholar]
- 15.Ball AD, Stapley J, Dawson DA, Birkhead TR, Burke T, Slate J. A comparison of SNPs and microsatellites as linkage mapping markers: lessons from the zebra finch (Taeniopygia guttata) BMC Genomics. 2010;11:218. doi: 10.1186/1471-2164-11-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernández ME, Goszczynski DE, Lirón JP, Villegas-Castagnasso EE, Carino MH, Ripoli MV, Rogberg-Muñoz A, Posik DM, Peral-García P, Giovambattista G. Comparison of the effectiveness of microsatellites and SNP panels for genetic identification, traceability and assessment of parentage in an inbred Angus herd. Genet Mol Biol. 2013;36(2):185–191. doi: 10.1590/S1415-47572013000200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Totir LR, Fernando RL, Dekkers JC, Fernandez SA, Guldbrandtsen B. The effect of using approximate gametic variance covariance matrices on marker assisted selection by BLUP. Genetics, selection, evolution : GSE. 2004;36(1):29–48. doi: 10.1186/1297-9686-36-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu W, Li D, Liu J, Chen S, Qu L, Zheng J, Xu G, Yang N. A genome-wide SNP scan reveals novel loci for egg production and quality traits in white leghorn and brown-egg dwarf layers. PLoS One. 2011;6(12):e28600. doi: 10.1371/journal.pone.0028600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolc A, Arango J, Jankowski T, Dunn I, Settar P, Fulton JE, O'Sullivan NP, Preisinger R, Fernando RL, Garrick DJ, et al. Genome-wide association study for egg production and quality in layer chickens. J Anim Breed Genet=Zeitschrift fur Tierzuchtung und Zuchtungsbiologie. 2014;131(3):173–182. doi: 10.1111/jbg.12086. [DOI] [PubMed] [Google Scholar]
- 20.Yuan J, Sun C, Dou T, Yi G, Qu L, Qu L, Wang K, Yang N. Identification of promising mutants associated with egg production traits revealed by genome-wide association study. PLoS One. 2015;10(10):e0140615. doi: 10.1371/journal.pone.0140615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang GX, Fan QC, Wang JY, Zhang T, Xue Q, Shi HQ. Genome-wide association study on reproductive traits in Jinghai yellow chicken. Anim Reprod Sci. 2015;163:30–34. doi: 10.1016/j.anireprosci.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Liao R, Zhang X, Chen Q, Wang Z, Wang Q, Yang C, Pan Y. Genome-wide association study reveals novel variants for growth and egg traits in Dongxiang blue-shelled and white Leghorn chickens. Anim Genet. 2016;47(5):588–596. doi: 10.1111/age.12456. [DOI] [PubMed] [Google Scholar]
- 23.Johnson PA, Stephens CS, Giles JR. The domestic chicken: causes and consequences of an egg a day. Poult Sci. 2015;94(4):816–820. doi: 10.3382/ps/peu083. [DOI] [PubMed] [Google Scholar]
- 24.Niknafs S, Nejati-Javaremi A, Mehrabani-Yeganeh H, Fatemi SA. Estimation of genetic parameters for body weight and egg production traits in Mazandaran native chicken. Trop Anim Health Prod. 2012;44(7):1437–1443. doi: 10.1007/s11250-012-0084-6. [DOI] [PubMed] [Google Scholar]
- 25.Engström G, Liljedahl L, Wilhelmson M, Johansson K. The pattern of genetic and environmental variation in relation to ageing in laying hens. Genetics, selection, evolution : GSE. 1992;24:265–275. [Google Scholar]
- 26.Venturini GC, Savegnago RP, Nunes BN, Ledur MC, Schmidt GS, El Faro L, Munari DP. Genetic parameters and principal component analysis for egg production from white Leghorn hens. Poult Sci. 2013;92(9):2283–2289. doi: 10.3382/ps.2013-03123. [DOI] [PubMed] [Google Scholar]
- 27.Hu ZL, Park CA, Reecy JM. Developmental progress and current status of the animal QTLdb. Nucleic Acids Res. 2016;44(D1):D827–D833. doi: 10.1093/nar/gkv1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tachibana T, Tsukada A, Fujimoto M, Takahashi H, Ohkubo T, Boswell T, Furuse M. Comparison of mammalian prolactin-releasing peptide and Carassius RFamide for feeding behavior and prolactin secretion in chicks. Gen Comp Endocrinol. 2005;144(3):264–269. doi: 10.1016/j.ygcen.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 29.RUBINEK T, HADANI M, BARKAI G, MELMED S, SHIMON I. Prolactin (PRL)-releasing peptide stimulates PRL secretion from human fetal pituitary cultures and growth hormone release from cultured pituitary adenomas. J Clin Endocrinol Metabol. 2001;86:6. doi: 10.1210/jcem.86.6.7591. [DOI] [PubMed] [Google Scholar]
- 30.Iijima N, Matsumoto Y, Yano T, Tanaka M, Yamamto T, Kakihara K, Kataoka Y, Tamada Y, Matsumoto H, SuzukI N, et al. A novel function of prolactin-releasing peptide in the control of growth hormone via secretion of somatostatin from the hypothalamus. Endocrinology. 2000;142:7. doi: 10.1210/endo.142.7.8257. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Wang CY, Wu Y, Huang G, Li J, Leung FC. Identification of the receptors for prolactin-releasing peptide (PrRP) and Carassius RFamide peptide (C-RFa) in chickens. Endocrinology. 2012;153(4):1861–1874. doi: 10.1210/en.2011-1719. [DOI] [PubMed] [Google Scholar]
- 32.Starokadomskyy P, Gemelli T, Rios JJ, Xing C, Wang RC, Li H, Pokatayev V, Dozmorov I, Khan S, Miyata N, et al. DNA polymerase-alpha regulates the activation of type I interferons through cytosolic RNA:DNA synthesis. Nat Immunol. 2016;17(5):495–504. doi: 10.1038/ni.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Baranovskiy AG, Tahirov ET, Tahirov TH, Pavlov YI. Divalent ions attenuate DNA synthesis by human DNA polymerase alpha by changing the structure of the template/primer or by perturbing the polymerase reaction. DNA Repair (Amst) 2016;43:24–33. doi: 10.1016/j.dnarep.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennerson ML, Yiu EM, Chuang DT, Kidambi A, Tso SC, Ly C, Chaudhry R, Drew AP, Rance G, Delatycki MB, et al. A new locus for X-linked dominant Charcot-Marie-tooth disease (CMTX6) is caused by mutations in the pyruvate dehydrogenase kinase isoenzyme 3 (PDK3) gene. Hum Mol Genet. 2013;22(7):1404–1416. doi: 10.1093/hmg/dds557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.BOWKER-KINLEY MM, DAVIS WI, WU P, HARRIS RA, POPOV KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J. 1998;329:191–196. doi: 10.1042/bj3290191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Degenhardt T, Saramaki A, Malinen M, Rieck M, Vaisanen S, Huotari A, Herzig KH, Muller R, Carlberg C. Three members of the human pyruvate dehydrogenase kinase gene family are direct targets of the peroxisome proliferator-activated receptor beta/delta. J Mol Biol. 2007;372(2):341–355. doi: 10.1016/j.jmb.2007.06.091. [DOI] [PubMed] [Google Scholar]
- 37.Rhee SG, Woo HA, Kil IS, Bae SH. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J Biol Chem. 2012;287(7):4403–4410. doi: 10.1074/jbc.R111.283432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato Y, Kojima R, Okumura M, Hagiwara M, Masui S, Maegawa K, Saiki M, Horibe T, Suzuki M, Inaba K. Synergistic cooperation of PDI family members in peroxiredoxin 4-driven oxidative protein folding. Sci Rep. 2013;3:2456. doi: 10.1038/srep02456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan Y, Wladyka C, Fujii J, Sockanathan S. Prdx4 is a compartment-specific H2O2 sensor that regulates neurogenesis by controlling surface expression of GDE2. Nat Commun. 2015;6:7006. doi: 10.1038/ncomms8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidinger B, Weijler AM, Schneider WJ, Hermann M. Hepatosteatosis and estrogen increase apolipoprotein O production in the chicken. Biochimie. 2016;127:37–43. doi: 10.1016/j.biochi.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Wu C-L, Zhao S-P, Yu B-L. Microarray analysis provides new insights into the function of apolipoprotein O in HepG2 cell line. Lipids Health Dis. 2013;12:186. doi: 10.1186/1476-511X-12-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kranis A, Gheyas AA, Boschiero C, Turner F, Yu L, Smith S, Talbot R, Pirani A, Brew F, Kaiser P, et al. Development of a high density 600K SNP genotyping array for chicken. BMC Genomics. 2013;14:59. doi: 10.1186/1471-2164-14-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Browning BL, Browning SR. A unified approach to genotype imputation and haplotype-phase inference for large data sets of trios and unrelated individuals. Am J Hum Genet. 2009;84(2):210–223. doi: 10.1016/j.ajhg.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou X, Stephens M. Genome-wide efficient mixed-model analysis for association studies. Nat Genet. 2012;44(7):821–824. doi: 10.1038/ng.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao X, Becker LC, Becker DM, Starmer JD, Province MA. Avoiding the high Bonferroni penalty in genome-wide association studies. Genet Epidemiol. 2010;34(1):100–105. doi: 10.1002/gepi.20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Devlin B. Genomic control for association studies. Biometrics. 1999;55:997–1004. doi: 10.1111/j.0006-341x.1999.00997.x. [DOI] [PubMed] [Google Scholar]
- 48.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 49.McLaren W, Pritchard B, Rios D, Chen Y, Flicek P, Cunningham F. Deriving the consequences of genomic variants with the Ensembl API and SNP effect predictor. Bioinformatics. 2010;26(16):2069–2070. doi: 10.1093/bioinformatics/btq330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madsen P, Jensen J. A User's guide to DMU:a package for Analysing multivariate mixed models. 2013. [Google Scholar]
- 51.Lee SH, Yang J, Goddard ME, Visscher PM, Wray NR. Estimation of pleiotropy between complex diseases using single-nucleotide polymorphism-derived genomic relationships and restricted maximum likelihood. Bioinformatics. 2012;28(19):2540–2542. doi: 10.1093/bioinformatics/bts474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88(1):76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yi G, Shen M, Yuan J, Sun C, Duan Z, Qu L, Dou T, Ma M, Lu J, Guo J, et al. Genome-wide association study dissects genetic architecture underlying longitudinal egg weights in chickens. BMC Genomics. 2015;16(1):746. doi: 10.1186/s12864-015-1945-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The whole information of genome-wide significant and suggestively significant SNPs for AFE and ENs using genome-wide association studies. (XLSX 21 kb)
Figure S1. Manhattan plots for egg numbers at different stages. (PDF 537 kb)
Figure S2. The plot of egg production curve in this population. (JPG 109 kb)
Table S2. Basic information of SNP markers on a physical map after quality control. (XLSX 11 kb)
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.