Introduction
Metaplastic breast cancer (MBC) is a rare histological subtype that represents <1% of all breast cancers and is defined by the differentiation of neoplastic epithelium into squamous and/or mesenchymal components.1 The histology can be comprised of squamous, fibromatosis-like, spindle cell, or mesenchymal elements which include chondroid and osseous differentiation. It is unclear at this time what significance the various subtypes represent. MBC may be either entirely composed of metaplastic elements or a mixture of invasive ductal carcinoma (IDC) and metaplastic areas. Due to this heterogeneity, the diagnosis of MBC is frequently missed on routine core biopsies and therefore accurate diagnosis is delayed until the time of tumor resection.2
Most cases of MBC are triple negative (TN) in that they do not express the estrogen receptor (ER), progesterone receptor (PR) or human epidermal growth factor receptor 2 (HER2/ErbB2).3–6 In contrast to other types of triple negative breast cancer (TNBC), it is unusual that these tumors involve regional lymph nodes. Metastatic spread to lung and bone is the most common pattern of dissemination.7 Overall, MBC does not respond well to conventional chemotherapy and, despite the usual absence of nodal involvement, has a worse prognosis compared to other TNBCs.8–12 A recent study of SEER data on patients with early and locally advanced MBC reported the 3-year overall survival of patients with TN-MBC at 75.4%. For stage I TN-MBC 3-year overall survival was 91.4%, stage II was 76.4% and stage III was 47.1%.13
In this report, we discuss the characteristics, treatment and clinical outcomes of all patients who received adjuvant or neoadjuvant treatment for MBC over 15 years at our institution. The study was reviewed and approved by the Institutional Review Board. Pathology records between 2000 and 2015 identified 48 breast cancer reports which included the terms metaplastic, squamous, spindle, chondroid or osseous. Each of the cases was reviewed by an independent pathologist to confirm the diagnosis of MBC and to classify each tumor by subtype. Of the cases identified 18 were reviews of pathology slides from other institutions. Five patients received adjuvant chemotherapy and follow-up at other institutions and two were octogenarians who elected not to receive adjuvant chemotherapy. The remaining 23 patients are the subject of this review including three cases that initially were diagnosed as IDC but subsequently recognized as MBC. None had distant metastatic disease at presentation.
Due to the lack of an established standard of care for adjuvant treatment of MBC and because of poor outcomes reported with conventional adjuvant regimens, a clinical decision was made at this institution to offer patients whose tumor was TN and predominantly (> 50%) metaplastic an alternative platinum and taxane based regimen that has known activity and toxicity in treatment of breast cancer.14–16 Although there is no standard for the extent of metaplastic change required to be classified as MBC, treating outside of a clinical trial and for ethical reasons, the patients whose tumors were predominantly adenocarcinomas with lesser areas of metaplastic histology received standard of care treatment for IDC. Seven patients with predominantly TN MBC elected to receive the alternative regimen (carboplatin dosed at AUC=2 plus paclitaxel 80mg/m2 weekly for 12 weeks) as their adjuvant treatment and 2 other patients received a taxane/carboplatin combination, one after local recurrence and one who had a synchronous contralateral HER2-positive IDC. Of the 23 patients in this series, nine were treated with a combination of platinum and taxane therapy and all nine patients are alive and without evidence of disease with more than 5 years follow-up (median 8.58 years, range 5.08-14.42). Of the 14 MBC patients treated with conventional adjuvant therapy six have died and of these five died as a result of their disease.
Discussion
The median age at diagnosis was 56 years (Table 1). Most patients were non-Hispanic Caucasian, 3 identified as Hispanic and 3 African American. Tumors were subtyped according to the WHO classification.1 Twelve tumors were identified as chondroid, three mesenchymal (not otherwise specified), two fibromatosis-like, one osseous, one spindle cell, two squamous cell and one with mixed squamous and chondroid histology. Median follow-up is 102 months, ranging from 29 to 177 months. All but two patients presented with high-grade malignancy. Fourteen patients had T2 and four had T3 tumors. Two patients in our series had involvement of axillary nodes associated with their MBCs, both identified prior to neoadjuvant chemotherapy. Two additional patients had synchronous bilateral breast cancer and both had axillary nodal involvement on the side that did not have MBC. Only one of the tumors showing MBC exhibited expression of hormone receptors in the non-metaplastic portion of the tumor and one exhibited HER2 amplification following neoadjuvant treatment which was not seen on diagnostic biopsies. All other metaplastic tumors showed no expression of hormone receptors or HER2. Patients were treated with surgery and radiation according to standard treatment guidelines.
Table 1.
Clinical and Pathological Characteristics
| Patient | Age at DX | R/E | Classification | Grade | Size of 1° (cm) | Adjuvant Chemo | Recurrence | Follow-up (mo) | Current Status |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | C | chondroid | 3 | 9 | AC | lung, bone, brain in 7 mo | 12 | deceased |
| 2 | 31 | AA | chondroid | 3 | 4.9 | AC | no | 182 | NED |
| 3 | 60 | C | fibromatosis | 2 | 3.1 | C/T | lung at 6 years | 168 | NED |
| 4 | 64 | C | mesenchymal NOS | 3 | 5 | C/T | no | 173 | NED |
| 5 | 57 | C | chondroid | 3 | 9 | AC | bone in 26 mo | 27 | deceased |
| 6 | 68 | C | fibromatosis | 1 | 1.5 | none | local at 2 years | 102 | NED |
| 7 | 64 | C | squamous* | 3 | 1.4 | none | no | 41 | deceased |
| 8 | 47 | C | chondroid | 3 | 3.5 | XT-AC(neo); C/T | local at 4 years | 117 | NED |
| 9 | 69 | H | osseous | 3 | 2.5 | C/T | no | 120 | NED |
| 10 | 56 | AA | chondroid | 3 | 3 | AC | no | 111 | NED |
| 11 | 50 | C | chondroid | 3 | 5.5 | AC-T | no | 113 | NED |
| 12 | 40 | H | mesenchymal NOS | 3 | 2.3 | C/T | no | 103 | NED |
| 13 | 67 | C | spindle cell | 3 | 1.2 | AC | no | 100 | NED |
| 14 | 44 | C | mesenchymal NOS | 3 | 3 | C/T | no | 85 | NED |
| 15 | 34 | H | chondroid | 3 | 2.5 | TC | no | 86 | NED |
| 16 | 61 | C | chondroid | 3 | 1.1 | C/T | no | 88 | NED |
| 17 | 56 | C | chondroid | 3 | 2.3 | C/T | no | 91 | NED |
| 18 | 64 | C | chondroid | 3 | 0.7 | TC | no | 69 | NED |
| 19 | 46 | C | chondroid | 3 | 5.3 | TCH | no | 61 | NED |
| 20 | 68 | C | squamous | 3 | 3.1 | TC | no | 70 | NED |
| 21 | 57 | C | squamous+chondroid | 2 | 4.1 | AC-T(neo) | lung in 6 mo | 10 | deceased |
| 22 | 51 | C | metaplastic NOS | 3 | 3.3 | AC-T(neo) | brain in 19 mo | 23 | deceased |
| 23 | 40 | AA | Chondroid** | 3 | 3 | AC-T(neo) | lung, bone, brain in 15 mo | 23 | deceased |
non-metaplastic portion of tumor was estrogen receptor positive
Post-neoadjuvant treatment tumor was erbB-2(HER2) positive
Abbreviations: Age at Dx = age at breast cancer diagnosis; R/E = race/ethnicity; C = Caucasian; H = Hispanic; AA = African American; Size of 1° = size of primary tumor at presentation. Chemo = adjuvant chemotherapy; neo = neoadjuvant chemotherapy; C/T = carboplatin and paclitaxel; TCH = docetaxel, carboplatin and trastuzumab; AC = doxorubicin and cyclophosphamide; TC = docetaxel and cyclophosphamide; XT=capecitabine and docetaxel; AC-T = doxorubicin and cyclophosphamide followed by paclitaxel; NED = no evidence of disease; NOS = not otherwise specified
Patients 4, 9, 12, 14, 16 and 17 were treated with adjuvant carboplatin and paclitaxel and have remained disease free since their initial treatment. Of note, patient 9 had a reaction to carboplatin after week 7 and completed the regimen with cisplatin at 75mg/m2 q3 weeks for 2 doses along with continued weekly paclitaxel. Patient 3 had a 3.1 cm. moderately differentiated tumor showing fibromatosis-like histology and received the weekly carboplatin/paclitaxel adjuvant regimen. She experienced an isolated pulmonary recurrence 6 years later when she underwent imaging for evaluation of chest pain. She was treated by wedge resection and pathology confirmed recurrence of MBC. She did not receive further systemic therapy and remains in remission after 8 years of additional follow-up.
One patient (#8) was initially diagnosed with MBC by core biopsy and enrolled in a neoadjuvant clinical trial on which she received docetaxel and capecitabine followed by doxorubicin and cyclophosphamide. She subsequently underwent a lumpectomy and sentinel lymph node biopsy followed by local radiation. Pathology revealed a single microscopic focus (<1mm) of residual disease in the breast. Four years later she developed an ipsilateral breast recurrence showing MBC and had a completion mastectomy with reconstruction. She then was treated with weekly carboplatin and paclitaxel for 12 weeks and has had no further disease recurrence in more than 5 years of additional follow-up.
Patient 19 was diagnosed with two independent primary breast cancers, on the left a 5.3cm MBC with no nodal involvement and on the right a multifocal, HER2 positive cancer involving one out of nine axillary nodes. After bilateral mastectomy, she received adjuvant therapy with docetaxel, carboplatin and trastuzumab (TCH) as standard therapy for HER2 positive disease, thus providing platinum and taxane chemotherapy for her MBC as well. She has been disease free for more than five years.
Of the remaining 14 patients in this series eight received adjuvant treatment with doxorubicin and cyclophosphamide (AC) or docetaxel and cyclophosphamide (TC), two of whom had T1 tumors. Four patients received doxorubicin and cyclophosphamide followed by paclitaxel (ACT), three of whom were treated pre-operatively. An additional patient (#6) had a 1.5 cm grade 1 tumor and received no systemic treatment. She experienced a local recurrence treated with surgery and radiation only and has remained in remission after 6 additional years of follow-up. One other patient (#7) had a 1.4 cm hormone receptor positive ductal carcinoma with 25% of the tumor showing hormone receptor negative squamous carcinoma. She received adjuvant therapy with tamoxifen and died later without evidence of recurrent cancer. Eight of these 14 patients survive, all without evidence of disease.
All surviving patients continue follow-up in our breast cancer survivors’ clinic. No patient experienced unexpected side effects from chemotherapy. Of the 8 patients who were treated with weekly carboplatin and paclitaxel two experienced significant neutropenia and continued treatment on schedule with the support of weekly doses of filgastrim. As mentioned above, one patient had a reaction to carboplatin on week 7 and her treatment was completed using cisplatin.
Figure 1 graphically illustrates the Kaplan-Meier plot of overall survival for our patients treated with a platinum/taxane adjuvant therapy and that of our patients treated with conventional adjuvant therapy. Because this is a retrospective case series, patients were not randomly assigned to platinum-based or conventional therapy and the stage distribution is different in the two cohorts. In light of these considerations a formal statistical comparison of the two cohorts is not appropriate. Instead, we chose to calculate the probability that the nine platinum/taxane treated patients would have survived irrespective of treatment modality. We noted that seven of the nine patients in our series treated with platinum/taxane therapy had T2N0 TN disease at diagnosis, one had T3N0 and one T1N0. Accordingly, we queried the national Surveillance, Epidemiology, and End Results (SEER) registry to determine the expected 5-year survival probability of patients with T2N0M0 TN MBC. We used the SEER*Stat software and the International Classification of Diseases for Oncology, version 3 (OCD-O-3) morphology codes 8052, 8070, 8071, 8072, 8074, 8560, 8571, 8572, 8575, and 8980 to identify T2N0M0 TN female malignant MBC cases for the years 2010 through 2014 including 18 SEER registry data sets.13,17 Cases without follow-up information were excluded from the analysis. Using the Kaplan-Meier method we determined that the expected 5-year survival for T2N0M0 TN MBC is 75%. Because of the small number of patients in this platinum/taxane cohort and assuming an expected five-year survival of approximately 75% based on SEER data, using a simple binomial probability calculation, the likelihood that all nine of our patients treated with platinum/taxane therapy would survive more than 5 years is 0.75^9=7.5%, which is unlikely to have occurred by chance.
Figure 1:
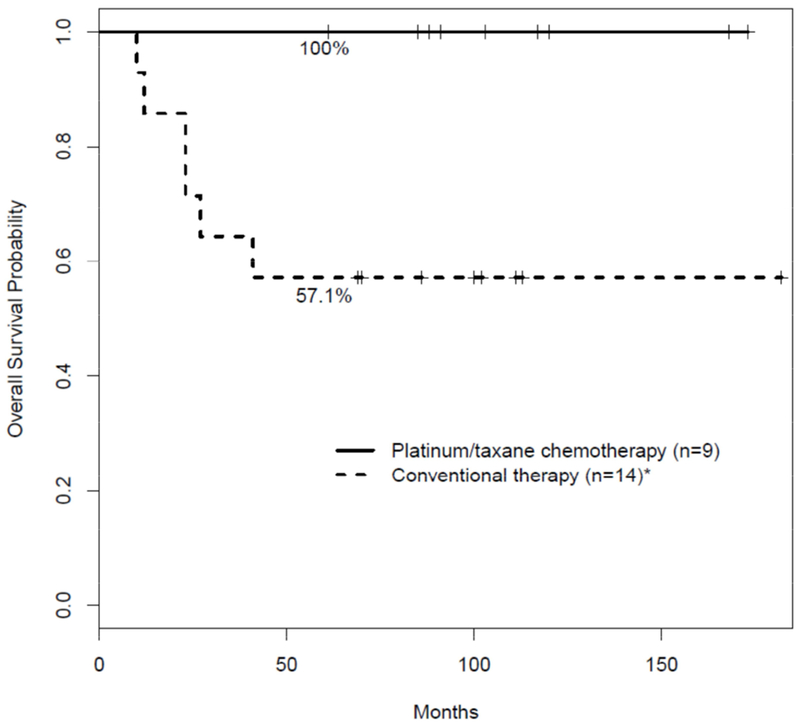
Overall survival for metaplastic breast cancer.
Overall survival is shown for patients treated with conventional adjuvant therapy or platinum/taxane chemotherapy. Two patients treated with conventional therapy did not receive chemotherapy.
*One patient who received conventional therapy died without cancer recurrence.
Of the 14 patients in our series treated with conventional therapy, eight remain alive and currently have no evidence of disease. The clinical outcome of these patients treated with conventional adjuvant therapy is similar to that reported by other investigators. In a recently reported single institution study of MBCs diagnosed over a 10 year period in which tumor pathology was carefully reviewed, although 72% of patients had T1 or T2 lesions, 44% of the patients died of their disease.18 Another single institution study with thorough pathology review showed a 5-year distant recurrence rate of 35%.2 In contrast, all nine of our patients who received a platinum/taxane doublet as adjuvant therapy are currently without evidence of cancer, all with more than 5 years of follow-up.
Our patients’ clinical outcome suggests that their tumors were sensitive to platinum-based chemotherapy. In this regard, an intriguing feature of MBC is the high degree of BRCA dysfunction found in these tumors. One study showed that 63% of MBCs exhibit methylation-induced BRCA1 silencing compared to 12% of control specimens.19 BRCA1 dysfunction, including gene silencing, has been proposed as a feature of tumors with potential sensitivity to platinum based chemotherapy.20 A study using BRCA-deficient TNBC patient-derived xenographs showed that BRCA1 promoter methylation that resulted in loss of BRCA1 expression in tumors yielded sensitivity to platinum agents and PARP inhibitors similar to BRCA1 mutant tumors. They further demonstrated that in patients receiving neoadjuvant chemotherapy residual (therapy resistant) disease resected at the time of definitive surgery displayed reduced BRCA1 methylation and increased BRCA1 mRNA levels compared to their pre-chemotherapy control values.21 Thus, the degree of BRCA dysfunction found in untreated MBCs, and hence responsiveness to platinum-based therapies, may be significantly greater in tumors without prior exposure to conventional chemotherapy. These observations suggest that in a disease such as MBC the ideal setting to test the efficacy of platinum-based therapy may be in patients without prior exposure to other conventional cytotoxic agents. This was the case with all but one of the platinum treated patients in our series.
New possibilities are continually emerging for the treatment of breast cancer. Although a proportion of MBCs have been shown to express potential therapeutic targets such as PIK3CA mutations and expression of PD-L1, it is important to recognize that MBCs differ in fundamental and significant ways from other TNBCs.22–24 For example, although most MBCs exhibit a basal-like genomic profile, a distinctive biologic feature shown by transcriptome analysis is that, compared with basal-like IDC, MBCs exhibit significant down regulation of PTEN and TOP2A which may contribute to a degree of chemoresistance to anthracycline based therapies.25 Other studies have shown that MBCs are frequently enriched in markers of epithelial-to-mesenchymal transition (EMT) and stem cell-like features that distinguish them from other basal-like or luminal cancers.26,27 Consistent with these findings, a high percentage of MBCs express the p53 homolog nuclear transcription factor p63 which is necessary for mammary development but only rarely expressed in IDCs.28,29 In summary, although MBCs represent a heterogeneous cluster of rare breast tumors, they are also distinct in several ways from IDCs and specifically from triple negative IDCs. These distinctive features of MBC suggest that results of clinical studies involving broader populations of patients with TNBC may not be applicable to this subgroup and may require independent validation for patients whose tumors have metaplastic histology. Moreover, whether patients’ tumors express specific targets or not, an optimal chemotherapy backbone remains important and the results of our limited case series suggest that, for adjuvant therapy of MBC, initiation of treatment with a platinum/taxane doublet may be valuable.
Patients with TNBC whose tumors show a pathologic complete response (pCR) following neoadjuvant chemotherapy have improved overall survival compared with those who have residual disease at surgery.30 As a result, there has been interest in the results of clinical trials that improve pCR. Two such studies, CALGB 40603 and the German GeparSixto, have shown that the addition of carboplatin to anthracycline and taxane chemotherapy improved the pCR rate in both studies by approximately 15%.31,32 Although not powered to assess overall survival benefit, these trials have resulted in an increased use of carboplatin in the neoadjuvant setting for TNBC to maximize the number of patients in a good prognosis group. In both trials carboplatin was incorporated in the treatment regimen starting with the first cycle of chemotherapy. Consistent with the results of our patients’ experience, a chemotherapy regimen such as used in CALGB 40603 of carboplatin plus weekly paclitaxel followed by dose dense doxorubicin/cyclophosphamide may be appropriate for treatment of MBC. Our patients with TN node negative MBC, however, received a platinum/taxane doublet in the adjuvant setting but without anthracycline chemotherapy. This raises the question to what degree the addition of anthracycline treatment can improve clinical outcome in this rare and biologically unique subset of TNBC. Such a question is appropriate to address in a multi-institutional effort to optimize treatment with minimal toxicity for these patients. Our small series cannot provide a definitive recommendation about routine clinical management. However, for adjuvant treatment of metaplastic node negative breast cancer, if patients receive initial therapy with carboplatin and paclitaxel, we believe the subsequent addition of anthracycline chemotherapy should be at the discretion of the treating physician after a thorough discussion with the patient taking into account the patient’s performance status and wishes and potential anthracycline toxicities.
Conclusions
Pure metaplastic breast cancer is a rare subtype of breast cancer that is often high grade, hormone-receptor negative and poorly responsive to conventional chemotherapy. We describe the treatment and outcomes of 23 patients treated over 15 years at a single institution. The 14 patients who received conventional adjuvant therapy experienced similar outcome to patients reported by other institutions. In contrast, of the nine patients treated with a platinum/taxane combination, which was well tolerated, all are alive and only one has had a distant recurrence which was successfully treated by metastectomy. Data from SEER indicate that the expected 5-year survival for T2N0M0 TN MBC irrespective of treatment is 75%. Using this estimate, we calculated the probability that all nine patients in our series treated with a platinum/taxane doublet would survive more than 5 years is just 7.5%, thus suggesting the platinum-taxane regimen is likely effective and responsible for the improved survival of these patients. To our knowledge a regimen of weekly paclitaxel and carboplatin has not been reported for adjuvant treatment of MBC. A limitation of our study is that, because our patients were treated prior to the recommendation to expand germline testing for BRCA mutations to all TNBC patients up to age 60, data on germline mutation status of these patients are incomplete at this time. Also, similar to other reports in MBC, we acknowledge the limitations of the small size of this series. Despite these considerations, the results from our small study suggest that the role of a low-dose weekly platinum/taxane doublet as initial therapy for adjuvant treatment of MBC deserves further clinical and laboratory investigation.
Clinical Practice Points.
Metaplastic breast cancer (MBC) represents a cluster of rare malignancies. When the metaplastic histology is the predominant feature of the tumor, MBCs exhibit a basal-like gene expression profile and typically do not express receptors for estrogen, progesterone or HER2/ErbB2 (triple negative). Despite a low frequency of axillary nodal involvement, the prognosis of MBC is worse than that of triple negative ductal carcinomas. These tumors show a high rate of distant recurrence after patients are treated with conventional adjuvant cytotoxic regimens. Because of the recognized poor clinical outcome and given the lack of a standard of care for MBC, a clinical decision was made at our institution to offer patients whose tumors were triple negative and predominantly metaplastic an alternative to conventional anthracycline and/or taxane based adjuvant chemotherapy using a regimen of weekly carboplatin and paclitaxel. This case series summarizes the treatment and outcome of 23 patients with MBC who received adjuvant therapy at our institution over 15 years, nine of whom were treated with a platinum/taxane combination therapy. With a median follow-up of 8.5 years for the series, all nine of the patients treated with this combination are alive without evidence of disease. We also discuss biologic factors that distinguish these tumors from other basal-like and triple negative breast cancers and provide a rationale for further basic and clinical exploration of this regimen for MBC patients.
Acknowledgements:
The authors wish to thank Dana Napier of the Markey Cancer Center for her assistance with this project.
Funding: This research was supported by the Biospecimen Procurement and Translational Pathology Shared Resource Facility and the Biostatistics and Bioinformatics Shared Resource Facility of the University of Kentucky Markey Cancer Center (P30CA177558). RLS is supported by the NIH National Center for Advancing Translational Sciences through grant number KL2 TR001996.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest declarations:
Natalie W. Harper – none
Kurt B. Hodges – none
Rachel L. Stewart – none
Jianrong Wu – none
Bin Huang – none
Kathleen L. O’Connor – none
Edward H. Romond – none
References
- 1.Lakhani S, Ellis I, Schnitt S, et al. 4th. Lyon: IARC Press; 2012. WHO Classification of Tumours of the Breast. [Google Scholar]
- 2.Leyrer CM, Berriochoa CA, Agrawal S, et al. Predictive factors on outcomes in metaplastic breast cancer. Breast Cancer Res Treat 2017; 165:499–504. [DOI] [PubMed] [Google Scholar]
- 3.Weigelt B, Eberle C, Cowell CF, et al. Metaplastic breast carcinoma: more than a special type. Nature Reviews Cancer 2014: doi: 10.1038/nrc3637. [DOI] [PubMed] [Google Scholar]
- 4.Al Sayed AD, El Weshi AM, Tulbah AM,et al. , Metaplastic carcinoma of the breast clinical presentation, treatment results and prognostic factors. Acta Oncol 2006; 45:188–95. [DOI] [PubMed] [Google Scholar]
- 5.Tse GM, Tan PH, Putti TC, et al. , Metaplastic carcinoma of the breast: a clinicopathological review. J Clin Pathol 2006; 59:1079–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKinnon E and Xiao P, Metaplastic carcinoma of the breast. Arch Pathol Lab Med 2015; 139:819–22. [DOI] [PubMed] [Google Scholar]
- 7.Tzanninis I-G, Kotteas EA, Ntanasis-Stathopoulos I, et al. , Management and Outcomes in Metaplastic Breast Cancer. Clin Breast Cancer 2016; 16:437–43. [DOI] [PubMed] [Google Scholar]
- 8.Lester TR, Humt KK, Nayeemuddin KM, et al. Metaplastic sarcomatoid carcinoma of the breast appears more aggressive than other triple negative breast cancers. Breast Cancer Res Treat 2012; 131:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen IC, Lin CH, Huang CS, et al. Lack of efficacy to systemic chemotherapy for treatment of metaplastic carcinoma of the breast in the modern era. Breast Cancer Res Treat 2011; 130:345–351. [DOI] [PubMed] [Google Scholar]
- 10.Nelson RA, Guye ML, Luu T, et al. , Survival outcomes of metaplastic breast cancer patients: results from a US population-based analysis. Ann Surg Oncol 2015; 22:24–31. [DOI] [PubMed] [Google Scholar]
- 11.Hennessy BT, Giordano S, Broglio K, et al. , Biphasic metaplastic sarcomatoid carcinoma of the breast. Ann Oncol 2006; 17:605–13. [DOI] [PubMed] [Google Scholar]
- 12.Bae SY, Lee SK, Koo MY, et al. , The prognoses of metaplastic breast cancer patients compared to those of triple-negative breast cancer patients. Breast Cancer Res Treat 2011; 126:471–78. [DOI] [PubMed] [Google Scholar]
- 13.Schroeder MC, Rastogi P, Geyer CE, et al. Early and locally advanced metaplastic breast cancer: presentation and survival by receptor status in Surveillance, Epidemiology, and End Results (SEER) 2010–2014. The Oncol 2018; 23:481–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loesch D, et al. , Phase II multicenter trial of a weekly paclitaxel and carboplatin regimen in patients with advanced breast cancer. J Clin Oncol 2002; 20:3857–64. [DOI] [PubMed] [Google Scholar]
- 15.Burris H 3rd, et al. , Phase II trial of trastuzumab followed by weekly paclitaxel/carboplatin as first-line treatment for patients with metastatic breast cancer. J Clin Oncol 2004; 22:1621–29. [DOI] [PubMed] [Google Scholar]
- 16.Perez EA, et al. , Two concurrent phase II trials of paclitaxel/carboplatin/trastuzumab (weekly or every-3-week schedule) as first-line therapy in women with HER2-overexpressing metastatic breast cancer: NCCTG study 983252. Clin Breast Cancer 2005; 6:425–32. [DOI] [PubMed] [Google Scholar]
- 17.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2017 Sub (1973–2015 varying) - Linked To County Attributes - Total U.S., 1969–2016 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017 submission. [Google Scholar]
- 18.Edenfield J, Schammel C, Collins J, et al. Metaplastic breast cancer: molecular typing and identification of potential targeted therapies at a single institution. Clin Breast Cancer 2017; 17:e1–10. [DOI] [PubMed] [Google Scholar]
- 19.Turner NC, Reis-Filho JS, Russell AM, et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene 2007; 26:2126–32. [DOI] [PubMed] [Google Scholar]
- 20.Tutt A, Tovey H, Cheang MC, et al. Carboplatin in BRCA1/2-mutated and triple negative BRCAness subgroups: the TNT trial. Nature Medicine 2018. 24:628–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ter Brugge P, Kristel P, van der Burg E, et al. Mechanisms of therapy resistance in patient-derived xenograft models of BRCA1-deficient breast cancer. J Natl Cancer Inst 2016; 108:djw 148. [DOI] [PubMed] [Google Scholar]
- 22.Hennessy BT, Gonzalez-Angulo A-M, Stemke-Hale K, et al. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Ca Res 2009; 69:4116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piscuoglio S, Ng C, Geyer FC, et al. Genomic and transcriptomic heterogeneity in metaplastic carcinomas of the breast. npj Breast Cancer 2017; 3:48; doi: 10.1038/s41523-017-0048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joneja U, Vranic S, Swensen J, et al. Comprehensive profiling of metaplastic breast carcinomas reveals frequent overexpression of programmed death-ligand 1. J Clin Path 2017; 70:255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weigelt B, Kreike B, and Reis-Filho JS, et al. Metaplastic breast carcinomas are basal-like breast cancers: a genomic profiling analysis. Breast Cancer Res Treat 2009; 117:273–80. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Toy KA, Kleer CG. Metaplastic breast carcinomas are enriched in markers of tumor initiating cells and epithelial to mesenchymal transition. Mod Pathol 2012; 25:178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Creighton CJ, Chang JC, Rosen JM. Epithelial-mesenchymal transition (EMT) in tumor initiating cells and its clinical implications in breast cancer. J Mammary Gland Biol Neoplasia 2010; 15:253–60. [DOI] [PubMed] [Google Scholar]
- 28.Cooper CL, Karim RZ, Selinger C, et al. Molecular alterations in metaplastic breast cancer. J Clin Pathol 2013; 66:522–28. [DOI] [PubMed] [Google Scholar]
- 29.Reis-Filho JS, Milanezi F, Paredes J, et al. Novel and classic myoepithelial/stem cell markers in metaplastic carcinomas of the breast. Appl Immunohistochem MolMorph 2003; 11:1–8. [DOI] [PubMed] [Google Scholar]
- 30.Cortazar P, Zhang L, Untch M, et al. Pathologic complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384:164–72. [DOI] [PubMed] [Google Scholar]
- 31.Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followedby dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III Triple-Negative Breast Cancer: CALGB 40603 (Alliance). J Clin Oncol 2015; 33:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 2014; 15:747–756. [DOI] [PubMed] [Google Scholar]


