Abstract
Copper sulfide (CuS) nanoparticles have been considered one of the most clinical relevant nanosystems because of their straightforward chemistry, small particle size, low toxicity, and intrinsic theranostic characteristics. In our previous studies, radioactive [64Cu]CuS nanoparticles were successfully developed to be used as efficient radiotracers for positron emission tomography and for photothermal ablation therapy of cancer cells using near-infrared laser irradiation. However, the major challenge of CuS nanoparticles as a theranostic platform is the lack of a means for effective targeted delivery to the tumor site. To overcome this challenge, we designed and synthesized angiogenesis-targeting [64Cu]CuS nanoparticles, which are coupled with cyclic RGDfK peptide [c(RGDfK)] through polyethylene glycol (PEG) linkers using click chemistry. In assessing their tumor-targeting efficacy, we found that the tumor uptakes of [64Cu]CuS-PEG-c(RGDfK) nanoparticles at 24 h after intravenous injection were significantly greater (8.6%±1.4% injected dose/gram of tissue) than those of nontargeted [64Cu]CuS-PEG nanoparticles (4.3%±1.2% injected dose/gram of tissue, p < 0.05). Irradiation of tumors in mice administered [64Cu]CuS-PEG-c(RGDfK) nanoparticles induced 98.7% necrotic areas. In contrast, irradiation of tumors in mice administered non-targeted CuS-PEG nanoparticles induced 59% necrotic areas (p < 0.05). The angiogenesis-targeting [64Cu]CuS nanoparticles may serve as a promising platform for image-guided ablation therapy with high efficacy and minimal side effects in future clinical translation of this novel class of multifunctional nanomaterials.
Graphical Abstract

Because of their distinctive physicochemical properties, nanoparticles offer a unique opportunity for combining cancer diagnosis and therapy in a single system, i.e., theranostics.1 Copper sulfide (CuS) nanoparticles are synthesized in one straightforward step, displaying small particle size (5–20 nm in diameter), low toxicity, and intrinsic theranostic properties. Moreover, CuS nanoparticles are amenable without surface chemistry changes to 64Cu labeling for positron emission tomography (PET) imaging and radiotherapy; they also show promise as contrast agents for photoacoustic imaging and as photothermal coupling agents for photothermal ablation (PTA) therapy owing to their strong absorption in the near-infrared (NIR) region.2–7 For example, CuS nanoparticles have been intrinsically labeled with 64Cu for PET imaging and employed for combined radiotherapy and PTA, taking advantage of the ionizing radiation from β particles emitted from 64Cu and the photothermal conversion property of the CuS nanoparticles.8, 9 The quantitative imaging provided by these nanoparticles was excellent, while the synergistic radiotherapy/PTA completely eliminated the primary tumor and prevented potential tumor metastasis without any apparent toxicity.
Although CuS nanoparticles were proven to be an excellent platform for accurate imaging and effective therapy, the major challenge is the lack of a means for efficient delivery to the tumor site.10 In some cases, intratumoral injection has been used to ensure sufficient tumor retention of 64Cu-labeled CuS ([64Cu]CuS) nanoparticles for effective radiotherapy. However, intratumoral injection is not suitable for treating metastatic lesions or tumors located in sites inaccessible to minimally invasive interventions. While systemic administration can lead to passive targeting and tumor accumulation of CuS nanoparticles through the enhanced permeability and retention effect, the efficiency of passive targeting is highly variable and is influenced by a number of factors, such as particle size, surface characteristics, and degree of angiogenesis and permeability of tumor blood vessels.11
It is highly desirable that CuS nanoparticles are selectively delivered to tumor sites through active targeting mechanisms to improve therapeutic outcome.1 Among the strategies for active targeting, targeting angiogenic blood vessels is especially attractive because this approach does not require nanoparticles entering the extravascular fluid space, a rate-limiting, inefficient process for nanoparticle delivery. Angiogenesis is required for supporting tumor growth by ensuring continuous supply of nutrients and oxygen.12 As the cell adhesion molecule integrin αvβ3 is overexpressed in angiogenic blood vessels.13. It specifically binds to stroma proteins containing the tripeptide motif arginine-glycine-aspartic acid (RGD), such as fibrinogen, vitronectin, thrombospondin, and osteopontin.14, 15 Cyclic pentapeptides that have a relatively high and specific affinity for the RGD sequence of the αvβ3 integrin have been used to improve imaging contrast with imaging probes and to enhance delivery efficiency of nanoparticles.16–18
In previous studies, chelator-free [64Cu]CuS nanoparticles synthesized rapidly by a simple process were shown to possess high radiolabeling stability and high specific activity. Chemically, 64Cu is the same as its naturally abundant natCu used to build CuS nanoparticles. As such, no radiometal chelator is required for radiolabeling of CuS nanoparticles; [64Cu]CuS nanoparticles synthesized by doping CuS with tracer amount of 64Cu were shown to be stable and used in both PET u and a PTA therapy.5 We hypothesized, therefore, that c(RGDfK)-tagged [64Cu]CuS nanoparticles can increase the tumor-targeting efficiency over untagged nanoparticles and mediate an effective antitumor effect. In this work, we report the first click-chemistry-assisted synthesis for the introduction of peptides to the surface to the class of CuS nanoparticles, their physicochemical characterization and specific cellular binding in vitro, and in vivo uptake and therapeutic effect in tumor xenograft models.
RESULTS
Synthesis and characterization of CuS-PEG-c(RGDfK) nanoparticles.
The reaction scheme for the introduction of c(RGDfK) peptide to CuS-polyethylene glycol (PEG) nanoparticles is shown in Figure 1. The c(RGDfK) peptide was synthesized by standard Fmoc solid-phase strategy. The structures of the starting, intermediate, and final products were confirmed by 1H NMR (500 MHz) (Figure S1–S4). The side chain amine group on Lys was then reacted with 4-pentynoic acid to introduce the alkyne functional group, which was confirmed by liquid chromatography/mass spectrometry (LC/MS; mass calculated for c(RGDfK): 603.68, found: [M+H+] = 604.33; mass calculated for 4-pentynoic acid–modified c(RGDfK): 683.69, found: [M+H+] = 684.34).19, 20
Figure 1.
Schematic illustration of the synthesis of [64Cu]CuS-PEG-c(RGDFK) nanoparticles.
CuS-PEG-N3 nanoparticles were rapidly synthesized from CuCl2 and Na2S in the presence of thiol-PEG-azide in water. The react proceeded at 90°C for 15 min. 1H NMR spectra showed a peak at 3.6 ppm characteristic of CH2CH2O repeating units in PEG, suggesting that the CuS nanoparticles were coated by thiol-PEG-N3 (Figure S5a). The alkyne-modified c(RGDfK) was attached to the CuS-PEG-N3 nanoparticles through azide-alkyne click chemistry in aqueous phase in the presence of copper catalyst.21 Analysis of amino acid composition in the resulting CuS-PEG-c(RGDfK) nanoparticles showed that the molar ration of RGD peptide to PEG chains on the surface of the nanoparticles could be readily controlled by controlling the feeding ratio of alkyne-modified c(RGDfK) and PEG-N3 (Table S1).
The maximum absorption wavelengths for both CuS-PEG and CuS-PEG-c(RGDfK) nanoparticles were ~970 nm (Figure S5b), confirming that the modification with c(RGDfK) peptide did not significantly change the optical property of PEG-CuS nanoparticles.5 The hydrodynamic sizes of the CuS-PEG and CuS-PEG-c(RGDfK) nanoparticles were 42±0.6 nm and 49.7±0.4 nm, respectively. The zeta potential of CuS-PEG-c(RGDfK) nanoparticles (−11 mV) was less negative than that of PEG-CuS nanoparticles (−24 mV) because of the positive charge in the guanidinium group of the arginine in c(RGDfK) (Figure S5c and S5d), suggesting successful peptide conjugation. TEM images confirmed that the diameters of both CuS-PEG and CuS-PEG-c(RGDfK) were less than 50 nm, and were spherical and irregular in shape (Fig. S5e). The yields of peptide molecules conjugated to the surface of CuS-PEG nanoparticles by click chemistry was 87% when the molar ratio of peptide-to-PEG was 0.08:1. The yield decreased to 60% when the peptide-to-PEG molar radio was increased to 0.6:1 (Table S1).
To examine the photothermal effect in aqueous solution after peptide modification of the nanoparticles, we compared the photothermal effect of CuS-PEG-c(RGDfK) nanoparticles with that of CuS-PEG nanoparticles using two different laser power levels. The temperature was elevated to 46.1°C and 45.2°C at 2 OD (optical density) for CuS-PEG and CuS-PEG-c(RGDfK) nanoparticles, respectively, at a laser power density of 1 W/cm2. The maximum temperature increased to 36.9°C and 35.6°C for CuS-PEG and CuS-PEG-c(RGDfK) nanoparticles, respectively, at a laser power density of 0.5 W/cm2 (Figure 2a). The photothermal effect in aqueous solution did not differ significantly for the two nanoparticles. To assess the photothermal effect at different concentrations, serial dilutions of the CuS-PEG-c(RGDfK) nanoparticles were irradiated with NIR laser at 3 W/cm2. As shown in Figure 2b, the temperature increased linearly with the concentration of the nanoparticles. The temperature was elevated to 94.3°C at a nanoparticle concentration of 1 mM. Furthermore, the photothermal effect of CuS-PEG-c(RGDfK) nanoparticles was dependent on the laser power. The temperature increased linearly with the increasing laser power from 0.5 to 2 W/cm2 (Figure 2c).
Figure 2. CuS-PEG-c(RGDfK) nanoparticles mediated efficient photothermal effects.
(a) Comparison of the photothermal effects of CuS-PEG and CuS-PEG-c(RGDfK) nanoparticles in aqueous solution. (b) Photothermal effects of CuS-PEG-c(RGDfK) at different concentrations. (c) Photothermal effects of CuS-PEG-c(RGDfK) nanoparticles with different levels of laser power.
Cytotoxicity study.
The effect of the nanoparticles on cell viability was evaluated by MTS assay. In HEK 293 cells, the effects of CuS-PEG nanoparticles, CuS-c(RGDfK) nanoparticles, and gold nanorods on cell viability did not differ significantly (Figure S6a); cell viability was around 100% at lower nanoparticle concentrations and decreased to ~70% at the highest concentration (3 OD, 1.5 mM). In HepG2 cells, no significant difference was observed at the lower nanoparticle concentrations, whereas CuS-PEG nanoparticles lowered cell viability at Cu concentrations 0.5 mM and higher (Figure S6b). In U87 cells, the viability was around 100% after treatment with all concentrations of CuS-PEG nanoparticles, CuS-c(RGDfK) nanoparticles, or gold nanorods (Figure S6c). In all three cell lines, the cell viability was significantly lower than in the other groups after treatment with cetyltrimethylammonium bromide (CTAB)-stabilized gold nanoparticles starting from 0.005 mM.
Radiolabeling efficiency and stability.
The radiolabeling efficiency and stability of [64Cu]CuS-PEG-c(RGDfK) nanoparticles is shown in Figure S7. The radiochemical yield was >98%. After suspension in phosphate-buffered saline solution (PBS) or human plasma at room temperature for 24 h, CuS-c(RGDfK nanoparticles lost ~0.6% and ~1.7 % of their radioactivity, respectively. [64Cu]CuS-c(RGDfK) nanoparticles lost about 2.7% and 3.3% radioactivity after 48 h incubation in PBS or human plasma, respectively.
In vitro αvβ3 integrin targeting.
The specific binding of CuS-PEG-c(RGDfK) nanoparticles to αvβ3 integrin was investigated using a sandwich ELISA binding assay.22 Figure 3a shows that CuS-PEG nanoparticles had minimal detectable binding to the αvβ3 integrin, while the binding to αvβ3 integrin of CuS-c(RGDfK) nanoparticles increased with increasing peptide-to-PEG ratio. Furthermore, the binding of CuS-PEG-c(RGDfK) (peptide:PEG = 0.13:1 and higher ratios) was significantly higher than that of CuS-PEG nanoparticles without peptide. To study the binding specificity of the CuS-PEG-c(RGDfK) nanoparticles, large excess amounts of c(RGDfK) peptides (50 μM, 100 μL) were pre-incubated with αvβ3 protein, followed by addition of different concentrations (1 to 5 OD at 930 nm) of CuS-PEG-c(RGDfK) nanoparticles (peptide:PEG 0.6:1). Binding of CuS-PEG-c(RGDfK) to αvβ3 was completed blocked at 3 different nanoparticle concentrations tested (1, 2.5 and 5 OD) (Figure 3b), demonstrating the excellent binding specificity to αvβ3 integrin.
Figure 3. CuS-PEG-c(RGDfK) nanoparticles selectively bound to integrin αvβ3 in vitro.
(a) The binding affinity of CuS-PEG-c(RGDfK) nanoparticles at different concentrations on an integrin-coated plate using a sandwich ELISA (n=3); *p<0.05, **p<0.01. (b) Comparison of the binding affinities of CuS-PEG-c(RGDfK) nanoparticles and CuS-PEG-c(RGDfK) nanoparticles blocked with an excess amount of free c(RGDfK) peptide on an integrin-coated plate using a sandwich ELISA (n=3). (c) Cellular uptake of [64Cu]CuS-PEG and [64Cu]CuS-PEG-c(RGDfK) nanoparticles in U87 cells (n=6/group).
We then evaluated cellular uptake of [64Cu]CuS-PEG-c(RGDfK) nanoparticles by U87 glioblastoma cells, which express high levels of integrin αvβ3 receptors. In this study, [64Cu]CuS-PEG-c(RGDfK) nanoparticles exhibited a significant binding to the integrin-overexpressing cells, about three-fold higher than the control nanoparticles, whereas the two controls, [64Cu]CuS-PEG and CuS-PEG-scrambled peptide, were taken up by these cells to a similar extent (Figure 3c). Moreover, exposure to the free c(RGDfK) peptide blocked this specific binding effect of the [64Cu]CuS-PEG-c(RGDfK) nanoparticles, and the cellular uptake was decreased to levels equivalent to those of the control nanoparticles (i.e., CuS-PEG, CuS-PEG-scrambled peptide) (Figure 3c). Overall, these results highlighted the high specific affinity of the CuS-PEG-c(RGDfK) nanoparticles to the integrin αvβ3 receptors in vitro.
In vitro photothermal ablation.
To demonstrate the targeting effect of the CuS-PEG-c(RGDfK) nanoparticles for photothermal treatment, U87 cells in culture were incubated with CuS-PEG or CuS-PEG-c(RGDfK) nanoparticles at the same concentration (3 OD) for 2 h. After the removal of free nanoparticles, the cells were irradiated with the laser at 3 W/cm2 for 2 min. As shown in Figure 4a, U87 cells within the laser region were completely depleted after laser treatment via CuS-PEG-c(RGDfK) nanoparticles, while some U87 cells in the irradiated region were still alive for U87 cells exposed to CuS-PEG nanoparticles. For both treatments, the cells in the region outside the laser footprint remained viable. Moreover, this destructive effect was not observed in U87 cells treated by NIR laser alone. For quantitative analysis of cell viability, a similar experiment was performed in which U87 cells were treated with CuS-PEG nanoparticles or different concentrations of CuS-PEG-c(RGDfK) nanoparticles. The results (Figure 4b and c) reveal that CuS-PEG-c(RGDfK) nanoparticles mediated a more effective photothermal effect against U87 cells. Furthermore, the cell killing effect of CuS-PEG-c(RGDfK) nanoparticles + laser exhibited a concentration-dependent profile. These results indicate that CuS-PEG-c(RGDfK), with their higher uptake in U87 cells, led to more effective photothermal destruction tumor cells within the zone of laser irradiation.
Figure 4. Visualization and quantification of U87 cell ablation after NIR laser treatment in vitro.
(a) Photothermal ablation of U87 cells using NIR laser mediated by CuS-PEG-c(RGDfK) or CuS-PEG nanoparticles (NPs). Representative images are shown. Viable U87 cells were stained green. (b) Cell viability after treatment with the same concentration of CuS-PEG-c(RGDfK) or CuS-PEG nanoparticles (1.5 mM) and the same laser power (n=5/group). (c) Cell viability after treatment with different concentrations (0.25–1.5 mM) of CuS-PEG-c(RGDfK) nanoparticles (n=5/group; *p<0.05, **p<0.01 compared to blank control).
µPET/CT imaging and biodistribution.
In vivo PET/CT imaging with [64Cu]CuS-PEG-c(RGDfK) or [64Cu]CuS-PEG nanoparticles was acquired 24 h after intravenous injection of one of these nanoparticle formulations into U87 tumor-bearing mice. The results revealed significantly higher tumor uptake of [64Cu]CuS-PEG-c(RGDfK) nanoparticles than of [64Cu]CuS-PEG nanoparticles (Figure 5a). On the basis of quantitative region of interest analysis for the representative PET/CT images, the tumor uptake of targeted [64Cu]CuS-PEG-c(RGDfK) nanoparticles at 24 h was 10.3%ID/g, whereas the tumor uptake of non-targeted [64Cu]CuS-PEG nanoparticles was only 4.9%ID/g. The localization of the [64Cu]CuS-PEG-c(RGDfK) and the [64Cu]CuS-PEG nanoparticles was determined by a biodistribution study in the same groups of mice (Figure 5b). No significant differences in liver, spleen, and kidney uptake were observed between [64Cu]CuS-PEG-c(RGDfK) and [64Cu]CuS-PEG. The tumor uptake for [64Cu]CuS-PEG-c(RGDfK) (9.44 ± 1.18%ID/g) was significantly higher than that of [64Cu]CuS-PEG (4.31 ± 1.36%ID/g) (p<0.05). These results confirmed the observation of higher tumor cell uptake by [64Cu]CuS-PEG-c(RGDfK) nanoparticles, suggesting the superb targeting efficiency and specificity of RGD-conjugated CuS nanoparticles in integrin αvβ3–overexpressing tumors.
Figure 5. c(RGDfK)-tagged CuS nanoparticles increased the tumor-targeting efficiency over untagged nanoparticles.
(a) PET/CT images of representative nude mice bearing a U87 xenograft tumor. Images were acquired 24 h after intravenous injection of [64Cu]CuS-PEG-c(RGDfK) or [64Cu]CuS-PEG nanoparticles. The arrows point to the tumor. (b) Biodistribution of [64Cu]CuS-PEG and [64Cu]CuS-PEG-c(RGDfK) nanoparticles (NPs) 24 h after intravenous administration of nanoparticles (n=4/group; p<0.05).
In vivo photothermal ablation therapy.
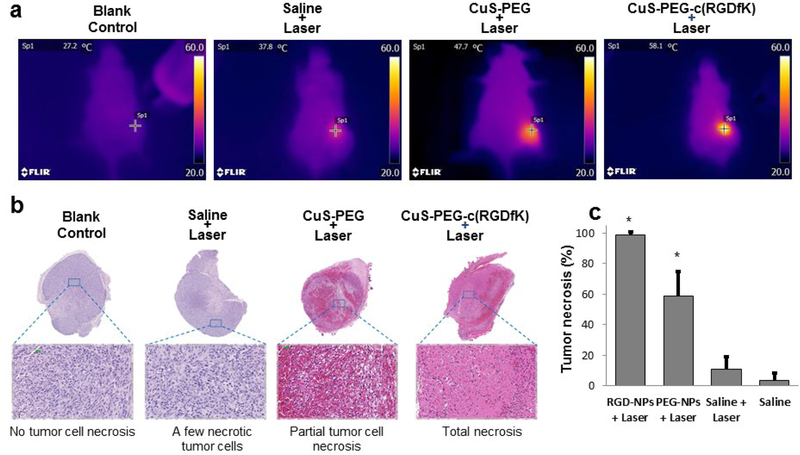
The therapeutic effects of CuS-PEG-c(RGDfK) and CuS-PEG were examined with an 808-nm continuous-wave laser in U87 tumor-bearing mice. The tumor temperature increased from 38°C in the mouse that received saline solution and NIR laser irradiation to 47.7°C in the mouse treated with CuS-PEG nanoparticles and NIR laser, and further increased to 58.1°C in the mouse administered CuS-c(RGDfK) nanoparticles and received NIR laser (Figure 6a). Histological analysis of the tumors, removed 24 h after laser treatment, confirmed that the treatment with CuS-PEG-c(RGDfK) nanoparticles and NIR laser led to a greater necrotic response than treatment with CuS-PEG nanoparticles and NIR laser (Figure 6b and c); about 98% of tumor tissue was necrotic in the tumors treated with CuS-PEG-c(RGDfK) nanoparticles, whereas the fraction of necrotized tissue was ~60% in the tumors treated with CuS-PEG nanoparticles and laser. For the tumors treated with saline solution and laser, the fraction of necrosis was at the baseline level of <5%.
Figure 6. c(RGDfK)-tagged CuS nanoparticles mediated effective photothermal ablation of tumors in U87 xenograft–bearing mice.
(a) Temperature at the tumor site during photothermal ablation study. (b) Representative microphotographs of histologic sections of tumors removed 24 h after photothermal ablation. (c) Quantitative analysis of the percentage of necrosis induced by different treatments; NPs, nanoparticles. NIR laser treatment was instituted for 3 min at a power density of 4 W/cm2.
Discussion
Attaching targeting ligands to the surface of nanoparticles for targeting nanoparticle delivery to solid tumor have been attempted in numerous preclinical studies, with limited success.23 Targeting surface receptors expressed in both angiogenic blood vessel and tumor cells is attractive because even if binding of nanoparticles to tumor cells may be impeded by limited extravasation into the extravascular fluid space and inefficient convection towards tumor cells, selective binding to tumor vasculature should still be possible. In this study, we report robust conjugation of cyclic c(RGDfK) peptide to PEG-coated CuS nanoparticles using click chemistry, and successful demonstration of selective binding of c(RGDfK)-tagged CuS nanoparticles to αvβ3 integrin both in vitro and in vivo.
Conjugation of c(RGDfK) to the terminus of PEG coating on the surface of CuS nanoparticles did not affect their optical and photothermal conversion properties. In vitro binding to αvβ3, evaluated using a modified ELISA assay, showed an increased binding of CuS-PEG-c(RGDfK) nanoparticles to αvβ3-coated plates with increasing peptide density on the surface of nanoparticles. Such binding could be completely blocked by free c(RGDfK). In addition, cellular uptake of CuS-PEG-c(RGDfK) nanoparticles in αvβ3-expressing U87 cells increased with increasing nanoparticle concentration, which again, could be completely blocked by free c(RGDfK). These data, together with the finding that CuS-PEG-scrambled peptide did not bind to αvβ3 integrin, indicate that c(RGDfK)-tagged CuS nanoparticles selectively bind to αvβ3 receptor.
In vitro cytotoxicity studies revealed that CuS-PEG-c(RGDfK) nanoparticles and gold nanoparticles, which are thought to be biocompatible, exhibited similar cytotoxicities in all three cell lines tested. The viability of HepG2 and U87 cells treated with either of these nanoparticles was ~100% at all the concentrations tested, from 0.00015 to 1.5 mM, while the viability of HEK 293 cells was decreased slightly, to ~80%, at higher nanoparticle concentrations. In contrast, CTAB-stabilized gold nanorods that are commonly used in photothermal ablation applications exhibited significant cytotoxicity in all three cell lines.
To facilitate quantitative analysis of the biodistribution of nanoparticles in vivo, we labeled both CuS-PEG and CuS-PEG-c(RGDfK) nanoparticles with the positron emitter 64Cu without the use of any radiometal chelator as 64Cu was efficiently incorporated into the matrix of CuS nanoparticles. µPET/CT imaging was applied to assess the in vivo biodistribution of the CuS-PEG-c(RGDfK) nanoparticles. Almost all the 64Cu (>98%) was successfully integrated into the CuS nanoparticles at the end of the synthesis. 64Cu-labeled nanoparticles displayed high labeling stability: the [64Cu]CuS-PEG-c(RGDfK) nanoparticles lost only ~3% of radioactivity after incubation in PBS or human plasma at room temperature for 48 h. The µPET/CT and biodistribution studies showed that both [64Cu]CuS-PEG and [64Cu]CuS-PEG-c(RGDfK) nanoparticles were almost completely cleared from blood circulation 24 h after injection. [64Cu]CuS-PEG-c(RGDfK) (9.44 ± 1.18%ID/g) was more than twice that of [64Cu]CuS-PEG (4.31 ± 1.3%ID/g in the U87 tumor, indicating successful tumor targeting by the [64Cu]CuS-PEG-c(RGDfK) nanoparticles. Both [64Cu]CuS-PEG and [64Cu]CuS-PEG-c(RGDfK) showed high uptake values in the liver and the spleen, consisting with a general observation that even with PEG modification, most nanoparticles are eventually removed by the cells of the mononuclear phagocytic system. One possible solution to reduce uptake of CuS nanoparticles in the liver and the spleen is to make CuS nanodots with average size of less than 6 nm, as have been shown recently.3 These ultrasmall CuS nanodots were cleared via the renal-urinary system and exhibited low uptake in the liver and the spleen.
Higher uptake of CuS-PEG-c(RGDfK) nanoparticles in tumor cells than CuS-PEG nanoparticles led to more efficient photothermal killing of U87 tumor cells in vitro with αvβ3-targeting CuS nanoparticles. Higher tumor deposition of CuS-PEG-c(RGDfK) than CuS-PEG in vivo resulted in more than 10°C higher temperature elevation of laser-irradiated tumors under the same laser treatment conditions: a tumor temperature of 47.7ºC was achieved with CuS-PEG nanoparticles and NIR laser, whereas a much higher temperature of 58.1ºC was achieved with CuS-PEG-c(RGDfK) nanoparticles and NIR laser instituted for 3 min at a power density of 4 W/cm2. Under the laser treatment conditions used, almost the entire tumor became necrotic with CuS-PEG-c(RGDfK), whereas only partial tumor ablation was achieved with the CuS-PEG nanoparticles. These data indicate that successful targeting of CuS-PEG-c(RGDfK) nanoparticles to tumor vasculature and αvβ3 integrin-expressing tumor cells mediated efficient photothermal ablation of tumors in vivo.
CONCLUSIONS
We have developed a robust procedure for the introduction of targeting ligand to the surface of CuS and [64Cu]CuS nanoparticles via PEG linkers using click chemistry. [64Cu]CuS-PEG-c(RGDfK) demonstrated selective uptake in U87 tumors. PTA ablation of U87 tumors was also significantly more efficient with CuS-PEG-c(RGDfK) nanoparticles than with non-targeted CuS-PEG nanoparticles. These results indicate that [64Cu]CuS-PEG-c(RGDfK) can be used not only for molecular imaging of angiogenic blood vessels, but also for PET/CT image-guided PTA therapy.
MATERIALS AND METHODS
Materials.
1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrocholoride (EDAC), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 4-pentynoic acid, acetonitrile, ammonia hydroxide, benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (Py-BOP), bovine serum albumin (BSA), calcein AM, copper chloride, copper sulfate pentahydrate, DMEM cell culture medium, DMEM cell culture medium without phenol red, dimethyl sulfoxide (DMSO), ethylenediaminetetraacetic acid (EDTA), ethyl 4-bromobutyrate, ethyl ether, fetal bovine serum (FBS), hydrochloric acid (HCl), hydroxybenzotriazole (HOBt), hydrazine, methylene chloride (DCM), N,N-diisopropylethylamine (DIPCDI), 1,3 diisopropylcarbodiimide (DIPCDI), N-hydroxyl succinimide (NHS), PBS, PBS without Mg and Ca, 0.9% saline solution (sodium chloride injection), sodium azide, sodium chloride, sodium ascorbate, sodium hydroxide, sodium sulfate, sodium sulfide nonahydrate, trypsin-EDTA solution 0.25%, triethylsilane (TES), and trifluoroacetic acid (TFA) were purchased from Sigma-Aldrich (St. Louis, MO). Avidin-horseradish peroxidase (HRP), dimethylfromamide (DMF), methanol, and piperidine were purchased from Fisher Scientific (Hampton, NH). The biotin-anti-PEG conjugation kit was purchased from Abcam (Cambridge, UK). Fmoc-Arg(Pbf)-OH, Fmoc-Asp(Wang resin LL)-ODmab, Fmoc-Gly-OH, Fmoc-Lys)Mtt)-OH, and Fmoc-Phe-OH were purchased from Novabiochem (Billerica, MA). Thiol-PEG-amine (MW 5000 Da) was purchased from Nanocs Inc. (New York, NY). 64CuCl2 was produced by the cyclotron research facility at The University of Texas MD Anderson Cancer Center (Houston, TX). Tetramethylbenzidine (TMB) ELISA substrate solution and stop solution were purchased from Life Technologies (Carlsbad, CA). Wash buffer (1 PBS with 0.05% Tween 20) was purchased from AbD Serotec (Kidlington, UK). Integrin αvβ3 protein was purchased from Chemicon International (Temecula, CA).
Synthesis of thiol-PEG-azide.
Azide-modified PEG was synthesized as previously described in the literature.24 Briefly, the starting reactants ethyl 4-bromobutyrate (5.85 g, 30 mmol) and sodium azide (2.925 g, 45 mmol) were added into DMSO (20 mL) with stirring. The reaction mixture was stirred for 22 h at 55°C and cooled to room temperature. Water was added to the reaction mixture and then extracted three times, each with 30 mL of ethyl ether. The combined organic layer was washed with water and brine, and a rotary evaporator was used to remove the organic solvent to yield 3.67 g of ethyl 4-azidobtyrate. Ethyl 4-azidobutyrate (3.14 g, 20 mmol) was dissolved in sodium hydroxide aqueous solution (1 N, 24 mL) with a minimum amount of methanol to make the solution homogeneous. The reaction mixture was stirred for 3 h at room temperature. Methanol was removed using a rotary evaporator and then the aqueous solution was acidified to pH 0 with HCl. After extraction with 50 mL ethyl ether three times, the ether layer was dried with an excess amount of sodium sulfate and filtered. The ethyl ether was then removed with a rotary evaporator to afford 2.11 g of 4-azidobutyric acid. N-hydroxyl succinimide (1.65 g, 14.3 mmol) was added into methylene chloride (100 mL), followed by 4-azidobutyric acid (1.68 g, 13 mmol) and EDAC (2.74 g, 14.3 mmol). After stirring for 4 h at room temperature, the reaction mixture was washed with water and brine, dried with an excess amount of sodium sulfate, and filtered. The organic solvent was then removed with a rotary evaporator to yield 0.61 g of succinimidyl 4-azidobutyrate. Succinimidyl 4-azidobutyrate was reacted with thiol-PEG-amine in 0.1 M HEPES with 0.15 M NaCl pH 8.0 for 2 h at a 2:1 molar ratio. The final product was purified by dialysis with a filtration membrane with molecular cut-off of 5,000 Da. Thiol-PEG-azide (SH-PEG-N3) was collected after lyophilization.
Synthesis of alkyne-modified c(RGDfK) peptide.
Alkyne-modified cyclic Arg-Gly-Asp-Phe-Lys peptide was synthesized using Fmoc solid-phase strategy as described in the literature.18 Amino acids (3 eq) were coupled stepwise on the resin in the presence of DIPCDI (3 eq) and HOBt (3 eq) as coupling reagents. After removal of the Dmab- and Fmoc-protecting groups using 2% hydrazine (3 eq) in DMF and 20% piperidine in DMF, respectively, head and tail cyclization on the resin was carried out in DMF using Py-BOP (3 eq), HOBt (3 eq), and DIPCDI (6 eq) as coupling agents. After cyclization, Mtt-protecting group was selectively removed by treatment with TFA/TES/DCM (1/2/97) to yield cyclo- [Lys-Arg(Pbf)-Gly-Asp(resin)-phe]. Then 4-pentynoic acid (3 eq) and DIPCDI (6 eq) were added in DCM for 30 min, followed by addition of HOBt (6 eq). After stirring for 2 h at room temperature, the resin with cyclo- [Lys-Arg(Pbf)-Gly-Asp(resin)-phe]. peptides was added into the 4-pentynoic/DIPCDI/HOBt reaction mixture. After mixing overnight at room temperature, the resin was washed with 5 mL of DMF and DCM three times. Final deprotection and cleavage of the peptides from the resin were simultaneously achieved by treatment with TFA/H2O/TES (95/1/4, v/v/v) to yield alkyne-modified c(RGDfK) peptide. The product was purified by high-performance liquid chromatography, using the following eluting conditions: solvent: A, 0.1% TFA in water; B, 0.1% TFA in acetonitrile; gradient: B, 0–10% over 0–10 min; B, 10–50% over 10–12 min; B, 50–80% over 12–20 min; B, 80% to 10% over 20–21 min. The flow rate was 1 mL/min. The final product was validated using LC/MS time-of-flight mass spectrometer equipped with a Vydac C-18 column (4.6×250.0 mm, 7-μm particle size, 300-Å pore size).
Synthesis of N3-PEG-CuS nanoparticles.
The procedure for the synthesis of N3-PEG-CuS nanoparticles in water was similar to a general procedure reported previously.5 In brief, 20 mL of aqueous CuCl2 (1 mM) solution was added to 1 mg of thiol-PEG-N3 and stirred for 5 min. Then, 20 µL of sodium sulfide solution (Na2S, 1 M) was added to the reaction mixture with stirring at room temperature. The mixture solution turned dark-brown immediately once sodium sulfide was added. After stirring for 5 min, the mixture was heated to 90°C and stirred for another 15 min until the color changed to dark green. The mixture was cooled in an ice-water bath.
Synthesis of CuS-PEG-c(RGDfK) nanoparticles.
N3-PEG-CuS nanoparticles (20 mL) were reacted with alkyne-modified c(RGDfK) peptide (PEG:peptide ratio 1:1) at 90°C in the presence of copper sulfate and sodium ascorbate (0.05 eq and 0.1 eq, respectively) as the catalysts. The reaction mixture was stirred for 2 h, then purified through a dialysis membrane with MW CO 6,000–8,000. The overall synthesis scheme for targeted CuS nanoparticles is shown in Figure 1.
Characterization of CuS-PEG-c(RGDfK) nanoparticles.
UV-vis spectra of CuS-PEG and CuS-PEG-c(RGDfK) nanoparticles were examined with a UV-vis DU-800 spectrometer (Beckman-Coulter, Brea, CA) with a 1.0-cm optical path length quartz cuvette. Under aqueous conditions, the particle size, size distribution, and surface charge were investigated by dynamic light scattering (DLS) (Brookhaven Instruments Corporation, Holtsville, NY). Transmission electron microscopy (TEM) images were acquired using a transmission electron microscope (JEM 2010, JEOL Japan) at an accelerating voltage of 200 kV. Aqueous solution of CuS NPs was deposited on carbon-enhanced copper grids without negative staining. Amino acid analysis was used to quantify the amount of peptide attached to the surface of the nanoparticles. For sample preparation, the nanoparticles were dissolved in ammonia hydroxide and then dried in a hydrolysis tube. Hydrochloric acid (6 N) was added to the tube. After the peptide was hydrolyzed at 110°C for 24 h, the mixture was dried and dissolved in water for amino acid analysis.
Sandwich ELISA binding study.
Ninety-six–well plates were coated with purified αvβ3 integrin protein at 0.1 μg/well and kept at 4°C overnight. The plates were washed in a washing buffer (1 PBS with 0.05% Tween 20) after incubation and then blocked with blocking buffer (1 PBS with 2% BSA) at room temperature for 2 h. The blocking buffer was removed, and the plates were inoculated in triplicate with CuS-PEG (control) or CuS-PEG-c(RGDfK) at a series of PEG:c(RGDfK) peptide ratios. A solution of nanoparticles (1 OD) was prepared using blocking buffer containing Ca2+ and Mg2+ and then incubated at room temperature for 1 h. The detection antibody biotin-anti-PEG, which recognizes the backbone of the PEG molecule, was added at 0.05 μg/well. The plates were incubated at room temperature for 1 h, then washed, and the bound biotin– anti–PEG was detected using avidin–HRP conjugate at 0.005 μg/well. After washing, 100 μL of substrate solution was added to each well and the luminescence was read. The resulting luminescence signal intensity was plotted against PEG:c(RGDfK) peptide ratios. To validate the binding specificity, a blocking study was performed with incubation of excess c(RGDfK) peptide similar to the ELISA binding study. The concentration of c(RGDfK) was 50 μM (100 μL/well).
Cell culture.
Human hepatocellular carcinoma cells (HepG2), human kidney epithelial cells (HEK 293), and human glioblastoma cells (U87) were purchased from ATCC (Manassas, VA) and were cultured in Eagle minimum essential medium. The cell lines were maintained at 37°C in a humidified atmosphere containing 5% CO2. The U87 cells expressed high levels of αvβ3 integrin on the surface of cells.16 HepG2 and HEK 293 cells were used as models for testing the cytotoxicity of the nanoparticles for liver and kidney cells, respectively. Citrate-stabilized gold nanoparticles and CTAB-stabilized gold nanorods were used as controls.
Cytotoxicity study.
HepG2, HEK 293, and U87 cells were seeded separately at a density of 1 104 cells/well in a 96-well plate and incubated for at least 24 h prior to treatment. The cells were treated with culture medium alone, or gold NPs, gold nanorods, PEG-CuS nanoparticles, and c(RGDfK)-CuS nanoparticles in culture media, respectively, at different concentrations (1.5, 0.5, 0.15, 0.05, 0.015, 0.005, 0.0015, 0.0005 and 0.00015 mM equivalent [Cu] or [Au]) for 24 h in an incubator at 37°C in an atmosphere containing 5% CO2. MTS working solution was then pipetted into each well and the cells were incubated at 37°C for an additional 3 h. The absorption was measured at 490 nm using an EL 800 microplate reader (BioTek Instruments, Winooski, VT). Each sample was prepared in sextuplet, and the data are reported as means ± standard deviation.
Cellular uptake studies.
U87 cells were seeded in a 24-well plate at a density of 6 104/well and were incubated for at least 24 h before treatment. [64Cu]CuS-PEG or [64Cu]CuS-PEG-c(RGDfK) nanoparticles at different concentrations (0.1, 0.17, 0.33 and 0.67 mM equivalent [Cu]) were added to the wells and incubated at 37 C for 2 h. For the blocking assay, the cells were incubated with a large excess of c(RGDfK) peptide (500 mM) for 2 h, followed by addition of [64Cu]CuS-PEG-c(RGDfK) nanoparticles at different concentrations. The mixture was incubated at 37 C for 2 h. The cells were then washed three times with PBS and collected after treatment with 200 μL of trypsin. The cell-associated radioactivity was measured with a Packard Cobra gamma counter (GMI Inc, Ramsey, MN). Each sample was prepared in sextuplet, and the data are reported as means ± standard deviation.
Photothermal effect in aqueous solution.
To compare the photothermal effects of the CuS-PEG and CuS-PEG-c(RGDfK) nanoparticles, a 15PLUS laser (Diomed, Andover, MA) with a continuous-wave, center wavelength of 808 ± 10 nm, was used. To transfer the laser power from the laser unit to the aqueous solution of nanoparticles, a 5 m, core LCM-001 optical fiber (BioTex Inc., Houston, TX) was attached to a retort stand via a clamp. The end of the fiber was placed directly above the tube containing the aqueous solution. The temperature changes mediated by nanoparticles under NIR laser light were measured through an i7 thermal camera (Flir Systems Inc., Portland, OR). Water was used as the control.
In vitro photothermal ablation study.
U87 cells were seeded onto a 24-well plate at a density of 6 104 per well and were incubated for at least 24 h prior to treatment. Cells were washed three times with PBS and then incubated with CuS-PEG nanoparticles or CuS-PEG-c(RGDfK) nanoparticles (1.5 mM of [Cu]), n=3) in DMEM at 37°C for 2 h. Cells not incubated with nanoparticles and not laser irradiated were used as negative controls. After treatment with nanoparticles, the culture medium was replaced with fresh DMEM without phenol red, and the cells were irradiated with a diode NIR laser centered at 808 nm at an output power of 3 W/cm2 for 2 min. After the treatment, cells were re-supplied with DMEM containing 10% FBS. After 24 h, the cells were washed with PBS and stained with 1 µM calcein AM solution (5 µL calcein AM stock solution added to 10 mL of PBS); viable cells were visualized under a Zeiss Axio Observer.Z1 fluorescence microscope. To quantify the dead cells after photothermal ablation, U87 cells were treated with nanoparticles and NIR laser irradiation as just described. After the cells were washed with PBS, MTS working solution was added to the cells, and the absorption was measured 3 h later at 490 nm using a microplate reader. The data were reported as means ± standard deviation.
Radiolabeling of CuS-PEG-c(RGDfK) nanoparticles.
64CuCl2 (20 μL, 1000 μCi) was added to 20 mL of CuCl2 solution (1 mM), followed by 1 mg thiol-PEG-N3. After addition of 2 μL of sodium sulfide solution (1 M) into the dark CuCl2 solution, the mixture was heated to 90°C for 15 min until the color of the solution turned green. The reaction mixture was transferred to ice-cold water to obtain [64Cu]CuS-PEG-N3 nanoparticles. Then alkyne-modified c(RGDfK) peptide in water (1:1 molar ratio) was added into an aqueous solution of [64Cu]CuS-PEG-N3 nanoparticles with copper sulfate (0.05 eq.) and sodium ascorbate (0.1 eq.) to catalyze the reaction. The reaction mixture was stirred at 90°C for 2 h and was purified using a disposable PD 10 column.
The radiolabeling efficiency and stability of labeled nanoparticles were analyzed using instant thin-layer chromatography (ITLC). The ITLC strips were developed with PBS (pH 7.4) containing 4 mM EDTA and radioactivity quantified using an IAR-2000 TLC imaging scanner (Eckert & Ziegler Radiopharma, Berlin, Germany). To study the labeling stability, [64Cu]CuS-PEG-c(RGDfK) nanoparticles were suspended in PBS or human plasma and incubated at room temperature for 24 h or 48 h. Free 64Cu2+ ions moved to the top of the ITLC strip, while the nanoparticles remained at the original spot. The radioactivity at the original spot was recorded as a percentage of the total radioactivity applied to the ITLC strip.
µPET/CT imaging and biodistribution.
All animal studies followed protocols and guidelines approved by the Institutional Animal Care and Use Committee at The University of Texas MD Anderson Cancer Center. Eight- to 10-week-old female nude mice (Harlan-Sprague-Dawley, Indianapolis, IN) were inoculated subcutaneously with human U87 glioblastoma cells (7×106 cells/mouse) in the right thigh. When tumors had grown to 5–8 mm in diameter, mice were randomly allocated into two groups (n=3/group). Mice in Group A received an intravenous injection of [64Cu]CuS-PEG-c(RGDfK) nanoparticles (200 µCi/mouse; 0.2 mL). Mice in Group B received an intravenous injection of [64Cu]CuS-PEG nanoparticles (200 µCi/mouse; 0.2 mL). Twenty-four hours after injection of the radiolabeled nanoparticles, the animals were anesthetized with a mixture of isoflurane and oxygen gas (2% of isoflurane) and placed in a prone position, and images were acquired using an Inveon µPET/CT scanner (Siemens Medical Solutions, Erlangen, Germany). The µPET and CT images were generated separately and then fused using Inveon Research Workplace software. After µPET/CT imaging, the mice were euthanized by CO2 inhalation, and their blood, heart, liver, spleen, kidneys, lungs, stomach, intestine, muscle, bone, brain, and tumor tissues were harvested and weighed; the radioactivity of each tissue was measured with a Packard Cobra gamma counter (GMI, Inc., Ramsey, MN). Uptake of 64Cu-labeled CuS nanoparticles in various organs was expressed as percentage of injected dose per gram of tissue (%ID/g). The data were expressed as means ± standard deviation.
In vivo photothermal ablation therapy.
When the tumors had grown to 7–10 mm in diameter, mice were randomly allocated into four groups (n=4 each). Mice in group A were injected intravenously with CuS-PEG-c(RGDfK) nanoparticles (200 µL, 4 mM/mouse). Mice in group B were injected intravenously with CuS-PEG nanoparticles (200 µL, 4 mM/mouse). Mice in group C were injected intravenously with saline solution. Mice in group D were not treated and were used as a control. Mice in groups A-C were irradiated with NIR laser at 3 W/cm2 for 2 min 24 h after injection. The mice were euthanized at 24 h after laser treatment, and tumors were removed, snap frozen, and cryosectioned into 1,000-µm sections. The slides were stained with hematoxylin-eosin and examined under a Zeiss Axio Observer.Z1 fluorescence microscope (Carl Zeiss AG, Oberkochen, Germany). The images were captured by a Zeiss AxioCam MRc5 color camera, and the extent of tumor necrosis, expressed as a percentage of the entire tumor area, was analyzed with Zeiss AxioVision software (version 4.6.3).
Statistical analysis.
All data were analyzed using a two-tailed Student t-test. The cytotoxicity of the nanoparticles was analyzed by using one-way ANOVA, followed by the post-hoc Tukey test (Graphpad Prism version 5.02; GraphPad Software, La Jolla, CA).
Supplementary Material
ACKNOWLEDGMENTS
We thank Kathryn Hale at the Department of Scientific Publications, MD Anderson Cancer Center for editing the manuscript. This work was supported in part by the John S. Dunn Foundation, by John S. Dunn, Sr. Distinguished Chair in Diagnostic Imaging to Dr. William A. Murphy Jr., and by the University Cancer Foundation via the Institutional Research Grant program at the University of Texas MD Anderson Cancer Center. This research was also conducted at the MD Anderson Center for Advanced Biomedical Imaging in-part with equipment support from General Electric Healthcare. The Research Animal Support Facility, Research Cyclotron Facility, NMR Core Facility, and Small Animal Imaging Facility are supported by a Cancer Center Support Grant from the National Institutes of Health (P30CA016672).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website. Supplementary tables and figures are provided.
The authors declare no conflicts of interest.
REFERENCES
- (1).Li C (2014) A targeted approach to cancer imaging and therapy. Nat. Mater 13, 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Zhou M, Ku G, Pageon L, and Li C (2014) Theranostic probe for simultaneous in vivo photoacoustic imaging and confined photothermolysis by pulsed laser at 1064 nm in 4T1 breast cancer model. Nanoscale 6, 15228–15235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Zhou M, Li J, Liang S, Sood AK, Liang D, and Li C (2015) CuS nanodots with ultrahigh efficient renal clearance for positron emission tomography imaging and image-guided photothermal therapy. ACS Nano 9, 7085–7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Zhou M, Tian M, and Li C (2016) Copper-based nanomaterials for cancer imaging and therapy. Bioconjug. Chem 27, 1188–1199. [DOI] [PubMed] [Google Scholar]
- (5).Zhou M, Zhang R, Huang M, Lu W, Song S, Melancon MP, Tian M, Liang D, and Li C (2010) A chelator-free multifunctional [64Cu]CuS nanoparticle platform for simultaneous micro-PET/CT imaging and photothermal ablation therapy. J. Am. Chem. Soc 132, 15351–15358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Huang J, Zhou J, Zhuang J, Gao H, Huang D, Wang L, Wu W, Li Q, Yang D-P, and Han M-Y (2017) Strong near-infrared absorbing and biocompatible cus nanoparticles for rapid and efficient photothermal ablation of gram-positive and -negative bacteria. ACS Appl. Mater. Interfaces 9, 36606–36614. [DOI] [PubMed] [Google Scholar]
- (7).Zhang W, Xiao J, Cao Q, Wang W, Peng X, Guan G, Cui Z, Zhang Y, Wang S, Zou R, Wan X, Qiu H, and Hu J (2018) An easy-to-fabricate clearable CuS-superstructure-based multifunctional theranostic platform for efficient imaging guided chemo-photothermal therapy. Nanoscale 10, 11430–11440. [DOI] [PubMed] [Google Scholar]
- (8).Zhou M, Chen Y, Adachi M, Wen X, Erwin B, Mawlawi O, Lai SY, and Li C (2015) Single agent nanoparticle for radiotherapy and radio-photothermal therapy in anaplastic thyroid cancer. Biomaterials 57, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Zhou M, Zhao J, Tian M, Song S, Zhang R, Gupta S, Tan D, Shen H, Ferrari M, and Li C (2015) Radio-photothermal therapy mediated by a single compartment nanoplatform depletes tumor initiating cells and reduces lung metastasis in the orthotopic 4T1 breast tumor model. Nanoscale 7, 19438–19447. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- (10).Goel S, Chen F, and Cai W (2014) Synthesis and biomedical applications of copper sulfide nanoparticles: from sensors to theranostics. Small 10, 631–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Blanco E, Shen H, and Ferrari M (2015) Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol 33, 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Davis CD, Emenaker NJ, and Milner JA (2010) Cellular proliferation, apoptosis and angiogenesis: molecular targets for nutritional preemption of cancer. Semin. Oncol 37, 243–257. [DOI] [PubMed] [Google Scholar]
- (13).Hood JD, and Cheresh DA (2002) Role of integrins in cell invasion and migration. Nat. Rev. Cancer 2, 91–100. [DOI] [PubMed] [Google Scholar]
- (14).Li L, Wartchow CA, Danthi SN, Shen Z, Dechene N, Pease J, Choi HS, Doede T, Chu P, Ning S, Lee DY, Bednarski MD, and Knox SJ (2004) A novel antiangiogenesis therapy using an integrin antagonist or anti-Flk-1 antibody coated 90Y-labeled nanoparticles. Int. J. Radiat. Oncol. Biol. Phys 58, 1215–1227. [DOI] [PubMed] [Google Scholar]
- (15).Ruoslahti E (1996) RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol 12, 697–715. [DOI] [PubMed] [Google Scholar]
- (16).Lu W, Melancon MP, Xiong CY, Huang Q, Elliott A, Song SL, Zhang R, Flores LG, Gelovani JG, Wang LHV, Ku G, Stafford RJ, and Li C (2011) Effects of photoacoustic imaging and photothermal ablation therapy mediated by targeted hollow gold nanospheres in an orthotopic mouse xenograft model of glioma. Cancer Res 71, 6116–6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Wang W, Ke S, Wu Q-P, Charnsangavej C, Gurfinkel M, Gelovani JG, Abbruzzese JL, Sevick-Muraca EM, and Li C (2004) Near-infrared optical imaging of integrin avb3 in human tumor xenografts. Mol. Imaging 3, 343–351. [DOI] [PubMed] [Google Scholar]
- (18).Wang W, McMurray JS, Wu Q, Campbell ML, and Li C (2005) Convenient solid-phase synthesis of diethylenetriaminepenta-acetic acid (DTPA)- conjugated cyclic RGD peptide analogues. Cancer Biother. Radiopharm 20, 547–556. [DOI] [PubMed] [Google Scholar]
- (19).Lu J, Shi M, and Shoichet MS (2009) Click chemistry functionalized polymeric nanoparticles target corneal epithelial cells through RGD-cell surface receptors. Bioconjug. Chem 20, 87–94. [DOI] [PubMed] [Google Scholar]
- (20).McCusker CF, Kocienski PJ, Boyle FT, and Schatzlein AG (2002) Solid-phase synthesis of c(RGDfK) derivatives: on-resin cyclisation and lysine functionalisation. Bioorg. Med. Chem. Lett 12, 547–549. [DOI] [PubMed] [Google Scholar]
- (21).Mindt TL, Muller C, Stuker F, Salazar JF, Hohn A, Mueggler T, Rudin M, and Schibli R (2009) A “click chemistry” approach to the efficient synthesis of multiple imaging probes derived from a single precursor. Bioconjug. Chem 20, 1940–1949. [DOI] [PubMed] [Google Scholar]
- (22).Xie H, Diagaradjane P, Deorukhkar AA, Goins B, Bao A, Phillips WT, Wang Z, Schwartz J, and Krishnan S (2011) Integrin alphavbeta3-targeted gold nanoshells augment tumor vasculature-specific imaging and therapy. Int. J. Nanomedicine 6, 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, and Chan WCW (2016) Analysis of nanoparticle delivery to tumours. Nature Rev. Mater 1, 16014. [Google Scholar]
- (24).von Maltzahn G, Ren Y, Park JH, Min DH, Kotamraju VR, Jayakumar J, Fogal V, Sailor MJ, Ruoslahti E, and Bhatia SN (2008) In vivo tumor cell targeting with “click” nanoparticles. Bioconjug. Chem 19, 1570–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.