Abstract
IMPORTANCE
An observational study found an increased risk of febrile seizure on the day of or 1 day after vaccination (days 0–1) with trivalent inactivated influenza vaccine (TIV) in the 2010–2011 season; risk was highest with simultaneous vaccination with TIV and 13-valent pneumococcal vaccine (PCV13) in children who were 6 to 23 months old. Text messaging is a novel method for surveillance of adverse events after immunization that has not been used for hypothesis-driven vaccine safety research.
OBJECTIVE
To prospectively evaluate whether children receiving TIV and PCV13 simultaneously had higher rates of fever on days 0 to 1 than those receiving either product without the other.
DESIGN, SETTING, AND PARTICIPANTS
Prospective observational cohort study of parents of children 6 to 23 months old recruited from 3 medical center–affiliated clinics in New York City from November 1, 2011, through April 5, 2012. A total of 530 of 614 eligible participants (86.3%) were enrolled. Parents were texted on the night of vaccination (day 0) and the 7 subsequent nights (days 1–7) to report their child’s temperature. We used log-binomial regression to calculate adjusted relative risks (aRRs) and excess risk for fever on days 0 to 1, adjusted for age group, past influenza vaccination and simultaneous receipt of selected inactivated vaccines.
EXPOSURES
Receipt of TIV and/or PCV13.
MAIN OUTCOME(S) AND MEASURE(S)
Temperature of 38°C or higher on days 0 to 1 after vaccination.
RESULTS
On days 0 to 1, children receiving TIV and PCV13 simultaneously had higher rates (37.6%) of fever (temperature ≥38°C) than those receiving TIV (7.5%; aRR, 2.69; 95% CI, 1.30–5.60) or PCV13 (9.5%; aRR, 2.67; 95% CI, 1.25–5.66). The excess risk of fever after TIV and PCV13 was 20 and 23 per 100 vaccinations compared with TIV without PCV13 and PCV13 without TIV, respectively. Fever rates for days 2 to 7 were similar across groups. For days 0 to 1, 74.8% of the text messages were confirmed delivered; for another 9.0%, delivery status was unknown. Response rates were 95.1% and 90.9% for days 0 and 1 for confirmed delivered messages, respectively.
CONCLUSIONS AND RELEVANCE
Simultaneous TIV and PCV13 administration was associated with higher transient increased fever risk than administration of either vaccine without the other product. Text messaging to prospectively assess a specific vaccine adverse event has potential for enhancing prelicensure and postlicensure monitoring of adverse events after immunization and deserves further study.
TRIAL REGISTRATION
clinicaltrials.gov Identifier:
During the 2010–2011 influenza season, an epidemiologic study conducted in the Centers for Disease Control and Prevention–supported Vaccine Safety Datalink (VSD) found that trivalent inactivated influenza vaccine (TIV) was associated with an increased risk of febrile seizure during the day of and 1 day after vaccination (days 0–1) in US children who were 6 to 59 months old. Risk was highest among those 6 to 23 months old who received TIV and 13-valent pneumococcal conjugate vaccine (PCV13) simultaneously.1,2 Fever rates were not assessed.
Fever after pediatric vaccination is relatively common and therefore more amenable to study than febrile seizure, which occurs in 2% to 5% of children.3 Furthermore, fever can lead to parental concern and health care visits.4 We sought to study rates of fever in children receiving the 2011–2012 TIV formulation (which was the same TIV formulation used in 2010–2011)5,6 and PCV13. We hypothesized that fever rates would be significantly higher during days 0 to 1 after simultaneous vaccination with TIV and PCV13 compared with TIV or PCV13 without the other product.
In addition, we sought to use a novel method, text messaging, to assess postvaccination fever. Most US adults (91%) have cell phones.7 Latino adults are most likely to use text messaging.8 Although text messaging has been piloted for vaccine safety surveillance9,10 and vaccination reminders,11–13 it has not been used to prospectively assess a specific vaccine safety question. We sought to assess the utility and acceptability of text messaging to monitor a vaccine adverse event. We hypothesized that parents would use text messaging to report postvaccination fever and report high satisfaction with its use.
Methods
We conducted a prospective observational cohort study during the 2011–2012 influenza season in 3 community-based clinics affiliated with New York–Presbyterian Hospital/Columbia University Medical Center in New York City, serving a primarily Latino and publicly insured population. The clinics use a common electronic health record linked to a hospital immunization registry. All decisions regarding which vaccinations patients received were made by their health care professionals. It was not routine practice to provide antipyretics at vaccination.
Study Population
Families were eligible to enroll if they (1) had a child 6 to 23 months old receiving TIV and/or PCV13 from November 1, 2011, through April 5, 2012; (2) had a cell phone with the ability to receive text messages; and (3) spoke English or Spanish. Exclusion criteria included (1) child’s temperature of 38°C or higher at enrollment; (2) antipyretic administered within 6 hours before vaccination; (3) intent to use prophylactic antipyretics; (4) intent to move from New York City within 6 months; (5) child not with guardian; (6) parental inability to read text messages; or (7) child received TIV or PCV13 within 7 days before enrollment or live attenuated influenza vaccine on vaccination day. Receipt of other vaccines was permitted as was enrollment for more than one vaccination event.
Study Enrollment
Columbia University Medical Center’s institutional review board approved the study. After consent, families were verbally administered an intake form. Text messaging procedures were explained. Parents were trained to use a temporal artery thermometer14 and were instructed to take the temperature when their child felt febrile or nightly if the child did not feel febrile. Participant compensation included the thermometer (retail price, $30-$35) and a round-trip New York City Transit Authority Metrocard ($4.50).
Follow-up
Families were sent interactive text messages nightly on days 0 to 7 and reported the highest temperature since the last text, time taken, antipyretic use, and, for those with fever, care sought. Messages were sent in English or Spanish based on participant preference. Study staff called parents not responding to text messages in full or in part. Starting in February 2012, families were also given a card and a preaddressed stamped envelope to complete with the same information as the texts to add to reporting. Using a medical record abstraction tool, all health care visits after vaccination on days 0 to 7 were recorded from the electronic health record. From February through May 2012, families enrolled after January 1 were contacted to complete a telephone survey about satisfaction and future participation in vaccine safety studies.
Outcomes
The primary outcome was fever (temperature ≥38°C) on days 0 to 1 after vaccination. Main text messaging–related outcomes included response to delivered texts on days 0 to 1 and day 7 and parental satisfaction (very satisfied, somewhat satisfied, somewhat dissatisfied, or very dissatisfied) with reporting by text messaging.
Statistical Analysis
Fever After Vaccination
Children were included in the primary fever analysis if they had a (1) valid temperature measurement (defined as temperature ≥35°C) reported on both day 0 and day 1 or (2) had a fever (temperature ≥38°C) reported on either day 0 or day 1 even if the response was invalid or missing on the other day. We compared the presence of a temperature of 38°C or higher on days 0 to 1 using the Pearson χ2 test in children receiving TIV and PCV13 vs TIV without PCV13 or PCV13 without TIV. Children with and without antipyretic use were classified as having a fever based on the same cutoff values.
On the basis of fever in the first week of vaccination in prior studies of TIV (11%)15 and PCV13 (30%),16 with a total sample size of at least 461, we were powered to detect a 2-fold increase in fever rates comparing TIV and PCV13 vs TIV without PCV13 and a 1.7-fold increase vs PCV13 without TIV, assuming an 80% power and 5% type I error.
We also assessed associations between day 0 to 1 fever and potential covariates, including demographic factors (child age group, sex, and race/ethnicity), history (medical problem associated with high risk of influenza complications5 and reported family history of vaccine reaction), and enrollment month. Interaction between covariates and vaccine type (TIV and PCV13, TIV, or PCV13) was assessed at P < .05. Race/ethnicity was based on self-report by the caregiver. Pairwise correlation was tested via Pearson correlation coefficients, whereas multicollinearity was assessed using conditional indexes. We used log-binomial regression to calculate relative risks adjusted for the significant covariates at the level of P < .05 anda priori selected covariates that could affect day0 to1 fever: age group (6–11 and 12–23 months), history of prior influenza vaccination, and coadministration of common inactivated vaccines (combination diphtheria and tetanus toxoids and acellular pertussis, Haemophilus influenzae type b, and 4 inactivated poliovirus [DTaP-Hib-IPV], Hepatitis B and Hepatitis A) (eTable 1 in the Supplement). All 3 vaccination types (TIV and PCV13, TIV, and PCV13) were analyzed in the same model, with TIV and PCV13 as the referent, to allow creation of one model. Data are presented as the reciprocal value illustrating the risk of simultaneous vaccination vs vaccination of one product without the other. Using Mantel-Haenszel standardized risk estimates, we also determined the risk difference (excess risk) by calculating the adjusted fever rate in children receiving TIV and PCV13 minus the rate in those receiving PCV13 or TIV without the other product. This analysis estimates the number of additional fevers seen per 100 children vaccinated simultaneously with TIV and PCV13.
Secondary analyses assessed differences for temperatures of 39°C or higher to determine relationships with moderate fever, as well as differences stratified by TIV dose: first (TIV-1) or second (TIV-2) that season. In addition fever rates on days 2 to 7 were assessed to verify the risk window of days 0 to 1. Children were included if they had a valid temperature measurement reported on all 6 days or reported fever.
Three sensitivity analyses were conducted. First, children with reported antipyretic use on days 0 to 1 were excluded. Second, data only for first enrollments was analyzed. Third, generalized estimation equations were used to account for the children with multiple enrollments.
Use of Text Messaging
The percentage of messages confirmed delivered and response rates on days 0 to 1 are described. Bivariate analyses assessed the association between demographic factors and response to delivered texts on days 0 to 1 and day 7. The percentages of participants who returned cards and parental satisfaction information are described.
Analyses were performed using SAS statistical software, version 9.3 (SAS Institute Inc) and SPSS statistical software, version 19 (SPSS Inc).
Results
Five hundred thirty of 614 eligible participants (86.3%) enrolled, representing 484 children. A total of 39.2% received TIV without PCV13, 20.8% PCV13 without TIV, and 40.0% simultaneous TIV and PCV13 (Figure 1). Children were primarily Latino and publicly insured; 54.2% were 6 to 11 months old (Table 1). Approximately half (56.2%) of caregivers had a high school education or less. Nearly all (95.2%) had unlimited text messaging plans and texted at least weekly (91.7%).
Figure 1. Study Flow Diagram.
In patients receiving TIV-1, 68% of TIV-1 doses are the first influenza dose the patient received in the 2011–2012 season but not necessarily the first dose that patient ever received. In patients receiving TIV-1 and PCV13, 96% of TIV-1 and PVC13 doses are the first influenza dose the patient ever received. In patients receiving PCV13, 0.9% were receiving their first dose, 2.7% their second dose, 16.4% their third dose, and 80.0% their fourth dose. In patients receiving TIV and PVC13, 0.9% were receiving their first dose of PCV13, 5.7% their second dose, 65.6% their third dose, and 35.8% at least their fourth dose. In patients receiving TIV-1 and PCV13, 1.3% were receiving their first dose of PCV13, 6.4% their second dose, 81.5% their third dose, and 10.8% their fourth dose. In patients receiving TIV-2 and PCV13, 0% were receiving their first dose, 3.6% their second dose, 20.0% their third dose, and 76.3% at least their fourth dose. PCV13 indicates 13-valent pneumococcal conjugate vaccine; TIV, trivalent inactivated influenza vaccine; TIV-1, first influenza dose that season; and TIV-2, second influenza dose that season.
Table 1.
Demographic and Health Characteristics of the Study Population
| No. (%) of Participants |
|||||
|---|---|---|---|---|---|
| Characteristic | Total a | TIV | TIV and PCV13 | PCV13 | P Value |
| Total children | 530 | 208 | 212 | 110 | |
| Age, mob | |||||
| 6–11 | 287 (54.2) | 124 (59.6) | 145 (68.4) | 18 (16.4) | <.001 |
| 12–23 | 243 (45.8) | 84 (40.4) | 67 (31.6) | 92 (83.6) | |
| Sex | |||||
| Female | 263 (49.6) | 101 (48.6) | 98 (46.2) | 64 (58.2) | .12 |
| Male | 267 (50.4) | 107 (51.4) | 114 (53.8) | 46 (41.8) | |
| Race/ethnicity | |||||
| Latino | 458 (86.4) | 171 (82.2) | 187 (88.2) | 100 (90.9) | .38 |
| Black non-Latino | 60 (11.3) | 30 (14.4) | 21 (9.9) | 9 (8.2) | |
| White non-Latino | 7 (1.3) | 4 (1.9) | 2 (0.9) | 1 (0.9) | |
| Other non-Latino | 5 (0.9) | 3 (1.4) | 2 (0.9) | 0 (0.0) | |
| Insurance | |||||
| Private | 9 (1.7) | 5 (2.4) | 3 (1.4) | 1 (0.9) | .80 |
| Medicaid/SCHIP | 519 (97.9) | 202 (97.1) | 208 (98.1) | 109 (99.1) | |
| Uninsured | 2 (0.4) | 1 (0.5) | 1 (0.5) | 0 (0.0) | |
| High risk of complication from influenza4 | 34 (6.4) | 15 (7.2) | 8 (3.8) | 11 (10.0) | .08 |
| Total caregivers | 484 | 179 | 208 | 97 | |
| Age, y | |||||
| 16–29 | 307 (64.0) | 113 (64.2) | 135 (65.2) | 59 (60.8) | .76 |
| ≥30 | 173 (36.0) | 63 (35.8) | 72 (34.8) | 38 (39.2) | |
| English proficiency | |||||
| Excellent-good | 318 (65.7) | 115 (64.2) | 141 (67.8) | 62 (63.9) | .46 |
| Fair-poor | 156 (32.2) | 60 (33.5) | 65 (31.3) | 31 (32.0) | |
| Not at all | 10 (2.1) | 4 (2.2) | 2 (1.0) | 4 (4.1) | |
| Language in which caregiver prefers to receive text messages | .80 | ||||
| Spanish | 232 (47.9) | 83 (46.4) | 100 (48.1) | 49 (50.5) | |
| English | 252 (52.1) | 96 (53.6) | 108 (51.9) | 48 (49.5) | |
| Educational level | |||||
| Less than high school | 72 (14.9) | 26 (14.5) | 27 (13.0) | 19 (19.6) | .03 |
| High school only, GED, or trade school | 200 (41.3) | 88 (49.2) | 75 (36.1) | 37 (38.1) | |
| Some college or more | 212 (43.8) | 65 (36.3) | 106 (51.0) | 41 (42.3) | |
| Caregiver text message plan | |||||
| Unlimited | 461 (95.2) | 171 (95.5) | 199 (95.7) | 91 (93.8) | .76 |
| Limited or pay as goes | 23 (4.8) | 8 (4.5) | 9 (4.3) | 6 (6.2) | |
| Frequency of text messages use by caregiver at baseline | |||||
| At least weekly | 444 (91.7) | 168 (93.9) | 194 (93.3) | 82 (84.5) | .06 |
| Every few weeks to months | 31 (6.4) | 8 (4.5) | 12 (5.8) | 11 (11.3) | |
| Never receives texts | 9 (1.9) | 3 (1.7) | 2 (1.0) | 4 (4.1) | |
Abbreviations: GED, general education development; PCV13, 13-valent pneumococcal conjugate vaccine; SCHIP, State Children’s Health Insurance Program; TIV, trivalent inactivated influenza vaccine.
For children, analytic sample for fever analyses; for caregivers, analytic sample for response rate analyses
The percentages for age do not total because of missing age data on 4 caregivers.
In adjusted analyses, children who received simultaneous TIV and PCV13 were 2.7 times more likely to have a day 0 to 1 temperature of 38°C or higher than those receiving TIV without PCV13; the same adjusted relative risk (aRR) was found vs PCV13 without TIV (Table 2; eTable 2 and eTable 3 in the Supplement). Significantly higher rates of temperature of 39°C or higher during days 0 to 1 after TIV and PCV13 were also observed vs TIV but not vs PCV13 (Table 2). The adjusted risk difference for temperatures of 38°C or higher were 0.20 (95% CI, 0.06–0.35) for TIV and PCV13 vs TIV and 0.23 (95% CI, 0.11–0.34) vs PCV13, indicating an additional 20 cases of fever per 100 children vaccinated with TIV and PCV13 vs TIV and an additional 23 cases with TIV and PCV13 vs PCV13. The adjusted risk difference for temperatures of 39°C and higher for simultaneous TIV and PCV13 vs TIV was 0.15 (95% CI, 0.035–0.26).
Table 2.
Rates of Fever After Vaccination in Children Receiving Simultaneous TIV and PCV13 vs TIV or PCV13 Administration Without the Other Vaccine
| Vaccine Type and Dose | Temperature ≥38°C on Days 0–1 |
Temperature ≥39°C on Days 0–1 |
||||
|---|---|---|---|---|---|---|
| Patients, No. (%) | RRa,b (95% CI) | Adjusted RRa,b,c (95% CI) | Patients, No. (%) | RRa,b (95% CI) | Adjusted RRa,b,c (95% CI) | |
| TIV any dose analysis | ||||||
| TIV, TIV-1, and TIV-2 with PCV13 (n = 170) | 64 (37.6) | Reference | Reference | 19 (11.2) | Reference | Reference |
| TIV (n = 159) | 12 (7.5) | 4.99 (2.80–8.89) | 2.69 (1.30–5.60) | 4 (2.5) | 4.44 (1.54–12.77) | 3.92 (1.09–14.14) |
| PCV13 (n = 84) | 8 (9.5) | 3.95 (1.99–7.86) | 2.67 (1.25–5.66) | 4 (4.8) | 2.35 (0.82–6.68) | 2.53 (0.76–8.42) |
| TIV-1 analysis | ||||||
| TIV-1 and PCV13 (n = 126) | 54 (42.9) | Reference | Reference | 14 (11.1) | Reference | Reference |
| TIV-1 (n = 39) | 4 (10.3) | 4.18 (1.62–10.80) | 3.47 (1.02–11.85) | 1 (2.6) | 4.33 (0.59–31.95) | 7.50 (0.54–103.09) |
| PCV13 (n = 84) | 8 (9.5) | 4.50 (2.26–8.97) | 3.71 (1.42–9.70) | 4 (4.8) | 2.33 (0.80–6.84) | 4.77 (0.73–31.25) |
| TIV-2 analysis | ||||||
| TIV-2 and PCV13 (n = 44) | 10 (22.7) | Reference | Reference | 5 (11.4) | Reference | Reference |
| TIV-2 (n = 120) | 8 (6.7) | 3.41 (1.44–8.08) | 2.22 (0.89–5.51) | 3 (2.5) | 4.55 (1.13–18.25) | 3.09 (0.66–14.41) |
| PCV13 (n = 84) | 8 (9.5) | 2.39 (1.01–5.61) | 2.05 (0.85–4.94) | 4 (4.8) | 2.39 (0.67–8.44) | 2.09 (0.53–8.21) |
Abbreviations: PCV13, 13-valent pneumococcal conjugate vaccine; RR, relative risk; TIV, trivalent inactivated influenza; TIV-1, first influenza dose that season; TIV-2, second influenza dose that season.
TIV and PCV13, TIV-1 and PCV13, and TIV-2 and PCV13 were assessed using 3 separate models.
Calculated reciprocal relative risk is 1/RR, representing the RR of fever with simultaneous vaccination with TIV and PCV vs TIV and vs PCV.
Adjusted for age group (6–11 and 12–23 months), coadministration of inactivated vaccines (hepatitis A; hepatitis B; and combination diphtheria and tetanus toxoids and acellular pertussis, Haemophilus influenzae type b, and 4 inactivated poliovirus [DTaP-Hib-IPV]), and previous influenza vaccination (not in TIV-2 model).
Receipt of hepatitis B vaccine and previous receipt of influenza vaccine were correlated with each other (Pearson correlation coefficient, −0.79), yet there was no evidence of multicollinearity in our full model. No significant interaction was present between vaccine type and covariates for temperatures of 38°C or higher on days 0 to 1; interaction could not be assessed on days 0 to 1 for temperatures of 39°C or higher because of zero cell counts.
The aRR for fever was significantly higher after TIV-1 and PCV13 vs TIV-1 or PCV13 for temperatures of 38°C or higher but not for temperatures of 39°C or higher; no significant differences were observed for TIV-2 (Table 2). No between-group differences were found in fever rates on days 2 to 7 on bivariate or multivariable analyses for temperatures of 38°C or higher and 39°C or higher.
When children whose families reported antipyretic use on days 0 to 1 (n = 50) were excluded or when analyses were limited to first enrollments (n = 484), findings were similar. Generalized estimation equation models were similar to the original model assuming independent correlation except that the comparison of TIV-2 and PCV13 to TIV-2 became significant for temperatures of 38°C or higher (aRR, 2.22; 95% CI, 1.02–4.86). Of the 84 children with a day 0 to 1 fever, 6 had a medical visit that included fever (3 after TIV and PCV13, 2 after TIV, and 1 after PCV13); 4 went to the emergency department and 2 to a primary care clinic. Four of the visits occurred on days 1 to 3. There were no hospitalizations or febrile seizures noted on days 0 to 7 for any study child.
Use of Text Messaging
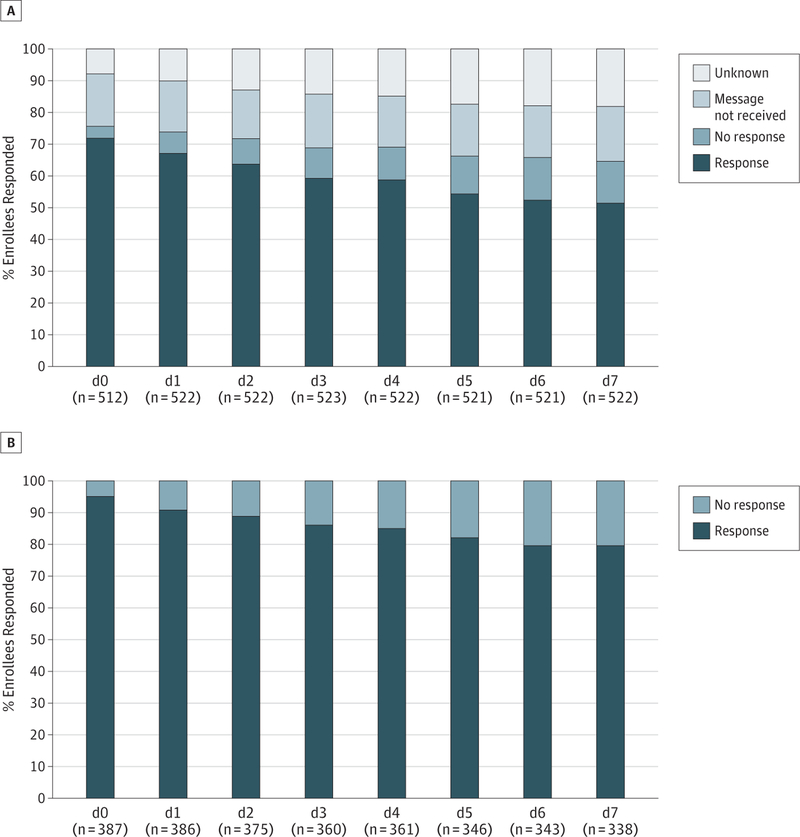
On days 0 to 1, 74.8% of messages were confirmed delivered (773 of 1034 sent); for another 9.0%, delivery status was unknown. For all days, 69.6% of messages were confirmed delivered; for 14.1%, delivery status was unknown. For families for whom delivery was confirmed, 95.1% replied on day 0; reply rates slowly decreased to 79.6% on day 7 (Figure 2). Only caregiver age (day 7) and level of reported text message use at baseline before enrollment (days 0–1 and day 7) affected likeliness to respond to messages (Table 3).
Figure 2. Response Rates to TextMessages Monitoring for Fever on Day 0 Through Day 7.
A, Response rates to all messages regardless of delivery status.
B, Response rates only to messages with confirmed delivery.
Table 3.
Response Rates to Text Messages Confirmed Delivered by Study Population and Caregiver Characteristics
| Responded Days 0–1 |
Responded Day 7 |
|||
|---|---|---|---|---|
| Characteristic | No. (%) of 374 Participants |
P Value | No. (%) of 312 Participants |
P Value |
| Child age, mo | ||||
| 6–11 | 184/201 (91.5) | .94 | 133/169 (78.7) | .31 |
| 12–23 | 158/173 (91.3) | 119/143 (83.2) | ||
| Child race/ethnicity | ||||
| Latino | 309/337 (91.7) | .48 | 228/280 (81.4) | .63 |
| Black non-Latino | 30/33 (90.9) | 22/29 (75.9) | ||
| White non-Latino | 2/3 (66.7) | 2/3 (66.7) | ||
| Other non-Latino | 1/1 (100) | … | ||
| Child insurance | ||||
| Private | 8/8 (100) | .62 | 5/6 (83.3) | .78 |
| Medicaid/SCHIP | 332/364 (91.2) | 245/304 (80.6) | ||
| Uninsured | 2/2 (100) | 2/2 (100) | ||
| Child high risk for complications from influenza5 | ||||
| Yes | 324/354 (91.5) | .81 | 238/294 (81.0) | .74 |
| No | 18/20 (90.0) | 14/18 (77.8) | ||
| Caregiver age, y | ||||
| 16–29 | 218/244 (89.3) | .056 | 158/206 (76.7) | .015 |
| ≥30 | 120/126 (95.2) | 91/103 (83.3) | ||
| Caregiver English proficiency | ||||
| Excellent-good | 232/253 (91.7) | .77 | 166/209 (79.4) | .15 |
| Fair-poor | 105/115 (91.3) | 84/99 (84.8) | ||
| Not at all | 5/6 (83.3) | 2/4 (50.0) | ||
| Language in which caregiver prefers to receive text messages | ||||
| Spanish | 157/176 (89.2) | .14 | 116/143 (81.1) | .89 |
| English | 185/198 (93.4) | 136/169 (80.5) | ||
| Caregiver educational level | ||||
| Less than high school | 44/51 (86.3) | .37 | 26/36 (72.2) | .37 |
| High school only, GED, or trade school | 144/156 (92.3) | 104/128 (81.2) | ||
| Some college or more | 154/167 (92.2) | 122/148 (82.4) | ||
| Caregiver text message plan | ||||
| Unlimited | 329/360 (91.4) | .85 | 241/299 (80.6) | .72 |
| Limited or pay as goes | 13/14 (92.9) | 11/13 (84.6) | ||
| Frequency of text messages use by caregiver at baseline | <.001 | |||
| At least weekly | 328/356 (92.1) | .001 | 243/294 (82.7) | |
| Every few weeks to months | 13/15 (86.7) | 9/16 (56.2) | ||
| Never receives texts | 1/3 (33.3) | 0 | ||
Abbreviations: GED, general education development; SCHIP, State Children’s Health Insurance Program.
For days 0 to 1 temperature data, 75.8% was via text, 8.7% via card, and 15.4% via telephone follow-up. Only 43.4% of those given cards returned them, and 39.1% returned cards with usable day 0 to 1 temperature data; on average, cards arrived on postvaccination day 19.
Among families completing the survey (325 of 418 [77.8%]), nearly all were very satisfied (84.9%), 12.9% were somewhat satisfied, and 94.1% were willing to re-enroll. Most either preferred text to paper reporting (65.7%) or had no preference (20.0%). Most (83.1%) indicated they would be willing to have their child’s blood drawn as part of a future study.
Discussion
This study demonstrated that young children who received TIV and PCV13 simultaneously had an increased risk of fever in the day 0 to 1 postvaccination period compared with those who received TIV or PCV13 without the other product (with or without other vaccines). It also indicates the novel and potential use of text messaging to prospectively assess a vaccine safety question. Although this was the first postlicensure study in the United States to assess fever risk after simultaneous TIV and PCV13 administration, these findings are consistent with 2 observational studies conducted during the 2010–2011 season.2,17 Our study identified increased risk of fever after TIV and PCV13 on days 0 to 1 but not on days 2 to 7, validating the VSD findings of increased febrile seizure risk on days 0 to 1 in children receiving TIV and PCV13.2 Our findings using text message reporting were also similar to a TIV safety study17 using paper reporting in Canada, which found that children 6 to 59 months old receiving TIV and PCV13 were more likely to have an axillary fever on days 0 to 3 after vaccination than those receiving TIV without PCV13. Validation of these paper-reported findings lends credibility to the use of text messaging as a method for surveillance and research of adverse events after immunization. This corroboration, along with high cell phone use and enrollment rates and minimal differences in response rates among demographic groups, also illustrates the potential utility of text messaging to enhance prelicensure and postlicensure monitoring of adverse events after immunization.
In our adjusted models, there were an additional 20 to 23 cases of temperature of 38°C or higher per 100 children with simultaneous vaccination vs TIV or PCV13 without the other product and 15 additional cases of temperature of 39°C or higher for TIV and PCV13 vs TIV. Our data suggest that simultaneous administration of TIV and PCV13 confers an overall transient higher risk of fever; however, this finding should be interpreted with caution because we did not assess the risk of fever in children receiving both TIV and PCV13 simultaneously compared with receiving both vaccines but on different days. This risk should also be viewed in the context of overall benefits of both vaccines,5,18,19 the currently low influenza vaccination coverage,20 and the desire to decrease missed opportunities to vaccinate. Both in this study and the Canadian study,17 few medical visits resulted from fevers, supporting the Advisory Committee on Immunization Practices’ recommendation to administer TIV and PCV13 according to the routine schedule, including simultaneous vaccination.19 Health care professionals could use this information to provide anticipatory guidance for families regarding fever.21 Understanding this increased fever risk may be particularly useful in caring for children for whom postvaccination fever could be associated with increased morbidity, such as those with a febrile seizure history.22
The pathogenesis of higher fever rates associated with simultaneous administration of TIV and PCV13 is unclear. It is well known that bacterial and viral antigens provoke fever.23 Therefore, the increased fever rate observed could be due to the increased antigen load of multiple vaccine epitopes. Alternatively, the balance between proinflammatory cytokines, such as interleukin 1, and the anti-inflammatory cytokines, such as interleukin 1 receptor antagonist and interleukin 10, could influence the degree of febrile response.24 It is possible that this specific vaccine combination results in higher levels of proinflammatory cytokines. A better understanding of the pathways that lead to fever is needed.25,26 To successfully conduct such cytokine studies immediately after vaccination, near real-time reporting of fever and collection of biological specimens are needed. Unlike conventional methods for postlicensure surveillance and research of adverse events after immunization in which reporting may be delayed,27 text message data are received in near real time and in an electronic form available for immediate review, thereby making such rapid collection of specimens possible on a larger scale.
Text messaging could be an important additional component to the current US vaccine safety monitoring effort. Spontaneous reporting systems, such as the Vaccine Adverse Event Reporting System, are useful to identify safety signals but not to test vaccine safety hypotheses.28 Although large linked databases, such as those used in VSD, are robust for studying rare serious adverse events after immunization, they have limited ability to capture nonmedically attended events.29 Prospective clinical studies are well suited to assess adverse events after immunization that occur outside medical settings but often use paper-based data collection, which can be slow and time consuming. In addition to complementing these systems, text messaging allows for standardization of surveillance across wide geographical areas through centralized deployment and monitoring. Although Internet surveillance may be similar, text messages are sent to the person’s own telephone and response can take seconds. In addition, in lower-income populations that may generally be underrepresented in studies,30 cell phone use is more common than computer-based Internet use.8,31,32 Although identifying adverse events that do not require medical attention could help with the underreporting of adverse events after immunization,28 the increase in reports may detract from identifying more clinically important adverse events after immunization; however, this may be offset by identifying a patient-centered outcome important to families. In addition, the methods could be adjusted to specifically capture more serious outcomes that may otherwise not be reported. Rapid monitoring of vaccine safety is an important component of national and international pandemic influenza plans.33
This study had several limitations. Children were not randomized to which vaccine they received; their own health care practitioners made all vaccine decisions. Regardless, no baseline difference was found between groups other than age. Although trends for higher fever risk after TIV and PCV13 vaccination with TIV-1 and TIV-2 in a given season were noted, this study was not powered to adequately make that distinction. Similarly, the study was not powered to assess differences between children receiving different doses of PCV13. Although the study controlled for receipt of combination diphtheria and tetanus toxoids and acellular pertussis, Haemophilus influenzae type b, and 4 inactivated poliovirus (DTaP-Hib-IPV), we were unable to assess the potential effect of other diphtheria and tetanus toxoids and acellular pertussis products. This study was conducted during a single influenza season, and influenza vaccine strains change year to year5; documenting fever patterns with different formulations may be helpful. This study took place in a primarily Latino, urban population and may not be representative of the general population. Text messaging behaviors could differ, and limited data suggest that race/ethnicity may affect fever risk after influenza vaccination.34 Older caregivers were slightly more likely to continue responding at day 7. Not all text messages were delivered; patients used their own cell phones, some of which routinely block messages from a 5-digit short code. Use of a 10-digit long code (normal telephone number) would likely increase delivery rates. In addition, some data were received by card and telephone follow-up; inclusion of these adjunct methods when using text message collection may be helpful. Finally, the main outcome of the study was to assess fever rates, and we did not conduct a randomized trial comparing text message reporting and paper reporting. Future studies could directly compare these modalities for vaccine safety surveillance.
Conclusions
Simultaneous TIV and PCV13 administration was associated with a higher transient increased fever risk than administration of either vaccine without the other product. Future studies could address the potential benefits and risks of administering TIV and PCV13 on different days or the effect of prophylactic antipyretics on vaccine-specific immune responses35 in patients for whom fever should be avoided for medical reasons. In addition, the use of text messaging to prospectively assess a specific vaccine adverse event has potential for enhancing prelicensure and postlicensure monitoring of adverse events after immunization and deserves further study.
Supplementary Material
Acknowledgments
Funding/Support: This study was funded by the Clinical Immunization Safety Assessment Network through a subcontract with America’s Health Insurance Plans under contract 200-2002-00732 from the Centers for Disease Control and Prevention.
Footnotes
Conflict of Interest Disclosures: None reported.
Role of the Sponsor: Investigators from the Centers for Disease Control and Prevention took part in the design and conduction of the study; analysis and interpretation of the data; and review of the manuscript as described below.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Previous Presentations: This study was presented as a platform presentation at the 2013 Pediatric Academic Societies’ Annual Meeting; May 4–7, 2013; Washington, DC; and the 2012 mHealth Summit; December 3–5, 2012; National Harbor, Maryland.
Contributor Information
Melissa S. Stockwell, Department of Pediatrics, Columbia University, New York, New York; Department of Population and Family Health, Mailman School of Public Health, Columbia University, New York, New York; NewYork–Presbyterian Hospital, New York.
Karen Broder, Immunization Safety Office, Centers for Disease Control and Prevention, Atlanta, Georgia.
Philip LaRussa, Department of Pediatrics, Columbia University, New York, New York.
Paige Lewis, Immunization Safety Office, Centers for Disease Control and Prevention, Atlanta, Georgia.
Nadira Fernandez, Department of Pediatrics, Columbia University, New York, New York.
Devindra Sharma, Immunization Safety Office, Centers for Disease Control and Prevention, Atlanta, Georgia.
Angela Barrett, Department of Pediatrics, Columbia University, New York, New York.
Jose Sosa, Department of Pediatrics, Columbia University, New York, New York.
Claudia Vellozzi, Immunization Safety Office, Centers for Disease Control and Prevention, Atlanta, Georgia.
REFERENCES
- 1.Broder KR, Martin DB, Vellozzi C. In the heat of a signal: responding to a vaccine safety signal for febrile seizures after 2010–11 influenza vaccine in young children, United States. Vaccine 2012;30(11):2032–2034. [DOI] [PubMed] [Google Scholar]
- 2.Tse A, Tseng HF, Greene SK, Vellozzi C, Lee GM; VSD Rapid Cycle Analysis Influenza Working Group. Signal identification and evaluation for risk of febrile seizures in children following trivalent inactivated influenza vaccine in the Vaccine Safety Datalink Project, 2010–2011. Vaccine 2012;30(11):2024–2031. [DOI] [PubMed] [Google Scholar]
- 3.Subcommittee on Febrile Seizures; American Academy of Pediatrics. Neurodiagnostic evaluation of the child with a simple febrile seizure. Pediatrics 2011;127(2):389–394. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan JE, Farrar HC; Section on Clinical Pharmacology and Therapeutics; Committee on Drugs. Fever and antipyretic use in children. Pediatrics 2011;127(3):580–587. [DOI] [PubMed] [Google Scholar]
- 5.Fiore AE, Uyeki TM, Broder K, et al. ; Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010;59(RR-8):1–62. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011;60(33):1128–1132. [PubMed] [Google Scholar]
- 7.Brenner J Pew Internet: Mobile, Pew Internet and American Life Project, Pew Research Center, 2013. http://pewinternet.org/Commentary/2012/February/Pew-Internet-Mobile.aspx. Accessed July 17, 2013.
- 8.Zichuhr K, Smith A. Digital differences. Pew Internet & American Life Project http://www.pewinternet.org/~/media//Files/Reports/2012/PIP_Digital_differences_041312.pdf. Accessed July 17, 2013.
- 9.Stockwell M, Andres R, Fernandez N, Vargas C, Lara M. FluNet: Real-time influenza vaccine surveillance. Poster presented at: Infectious Disease Society of America Annual Conference; October 20–23, 2011; Boston, MA; and mHealth Summit; December 5–7, 2011; National Harbor, MD. [Google Scholar]
- 10.Baron S, Goutard F, Nguon K, Tarantola A. Use of a text message-based pharmacovigilance tool in Cambodia: pilot study. J Med Internet Res 2013;15(4):e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stockwell MS, Kharbanda EO, Martinez RA, Vargas CY, Vawdrey DK, Camargo S. Effect of a text messaging intervention on influenza vaccination in an urban, low-income pediatric and adolescent population: a randomized controlled trial. JAMA 2012;307(16):1702–1708. [DOI] [PubMed] [Google Scholar]
- 12.Stockwell MS, Kharbanda EO, Martinez RA, et al. Text4Health: impact of text message reminder-recalls for pediatric and adolescent immunizations. Am J Public Health 2012;102(2):e15–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kharbanda EO, Stockwell MS, Fox HW, Andres R, Lara M, Rickert VI. Text message reminders to promote human papillomavirus vaccination. Vaccine 2011;29(14):2537–2541. [DOI] [PubMed] [Google Scholar]
- 14.Greenes DS, Fleisher GR. Accuracy of a noninvasive temporal artery thermometer for use in infants. Arch Pediatr Adolesc Med 2001;155(3):376–381. [DOI] [PubMed] [Google Scholar]
- 15.Fluzone package insert http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM305089.pdf. Accessed July 18, 2013.
- 16.PCV13 package insert http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM201669.pdf. Accessed July 18, 2013.
- 17.Van Buynder PG, Frosst G, Van Buynder JL, et al. Increased reactions to pediatric influenza vaccination following concomitant pneumococcal vaccination. Influenza Other Respi Viruses 2013;7(2):184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nuorti JP, Whitney CG; Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children: use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2010;59(RR-11):1–18. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2012–13 influenza season. MMWR Morb Mortal Wkly Rep 2012;61(32):613–618. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Flu vaccination coverage, United States, 2011–12 Influenza Season http://www.cdc.gov/flu/professionals/vaccination/coverage_1112estimates.htm. Accessed July 18, 2013.
- 21.Vaccine information statements. http://www.cdc.gov/vaccines/pubs/vis/-flu. Accessed July 18, 2013.
- 22.Berg AT, Shinnar S, Shapiro ED, Salomon ME, Crain EF, Hauser WA. Risk factors for a first febrile seizure: a matched case-control study. Epilepsia 1995;36(4):334–341. [DOI] [PubMed] [Google Scholar]
- 23.Barberà-Cremades M, Baroja-Mazo A, Gomez AI, Machado F, Di Virgilio F, Pelegrín P. P2X7 receptor-stimulation causes fever via PGE2 and IL-1β release. FASEB J 2012;26(7):2951–2962. [DOI] [PubMed] [Google Scholar]
- 24.Virta M, Hurme M, Helminen M. Increased plasma levels of pro- and anti-inflammatory cytokines in patients with febrile seizures. Epilepsia 2002;43(8):920–923. [DOI] [PubMed] [Google Scholar]
- 25.Choi J, Min HJ, Shin JS. Increased levels of HMGB1 and pro-inflammatory cytokines in children with febrile seizures. J Neuroinflammation 2011;8:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishizaki Y, Kira R, Fukuda M, et al. Interleukin-10 is associated with resistance to febrile seizures: genetic association and experimental animal studies. Epilepsia 2009;50(4):761–767. [DOI] [PubMed] [Google Scholar]
- 27.Salmon DA, Pavia A, Gellin B. Editors’ introduction: vaccine safety throughout the product life cycle. Pediatrics 2011;127 (suppl 1):S1–S4. [DOI] [PubMed] [Google Scholar]
- 28.Varricchio F, Iskander J, Destefano F, et al. Understanding vaccine safety information from the Vaccine Adverse Event Reporting System. Pediatr Infect Dis J 2004;23(4):287–294. [DOI] [PubMed] [Google Scholar]
- 29.Baggs J, Gee J, Lewis E, et al. The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics 2011;127(suppl 1):S45–S53. [DOI] [PubMed] [Google Scholar]
- 30.Blumberg SJ, Luke JV. Reevaluating the need for concern regarding noncoverage bias in landline surveys. Am J Public Health 2009;99(10):1806–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox S Mobile health 2010. Pew Internet and American Life Project, 19 October 2010. http://www.pewinternet.org/~/media//Files/Reports/2010/PIP_Mobile_Health_2010.pdf. Accessed July 17, 2013.
- 32.Smith A Mobile access 2010. Pew Internet and American Life Project, 7 July 2010. http://www.pewinternet.org/~/media//Files/Reports/2010/PIP_Mobile_Access_2010.pdf. Accessed July 17, 2013.
- 33.US Department of Health and Human Services (HHS) Text4Health Task Force. Health text messaging recommendations to the Secretary http://www.hhs.gov/open/initiatives/mhealth/recommendations.html. Accessed July 18, 2013.
- 34.Petousis-Harris H, Poole T, Turner N, Reynolds G. Febrile events including convulsions following the administration of four brands of 2010 and 2011 inactivated seasonal influenza vaccine in NZ infants and children: the importance of routine active safety surveillance. Vaccine 2012;30(33):4945–4952. [DOI] [PubMed] [Google Scholar]
- 35.Prymula R, Siegrist CA, Chlibek R, et al. Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: two open-label, randomised controlled trials. Lancet 2009;374(9698):1339–1350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.