Abstract
Objectives:
Novel cardiac biomarkers serum ST2 and Galectin-3 may be associated with increased likelihood of important events after cardiac surgery. Our objective was to explore the association between pre- and post-operative serum biomarker levels and 30-day readmission or mortality for pediatric patients.
Methods:
We prospectively enrolled pediatric patients <18 years of age who underwent at least one cardiac surgical operation at Johns Hopkins Children’s Center from 2010 to 2014 (N=162). Blood samples were collected immediately prior to surgery and at the end of bypass. We evaluated the association between pre- and post-operative Galectin-3 and ST2 with 30-day readmission or mortality, using backwards stepwise logistic regression, adjusting for covariates based on the Society of Thoracic Surgeons (STS) Congenital Heart Surgery Mortality Risk Model.
Results:
In our cohort, 21 (12.9%) patients experienced readmission or mortality 30-days from discharge. Before adjustment, preoperative ST2 terciles demonstrated a strong association with readmission and/or mortality after surgery (OR: 2.58; 95 % CI: 1.17 – 3.66 and OR: 4.37; 95% CI: 1.31 – 14.57). After adjustment for covariates based on the STS congenital risk model, Galectin-3 postoperative mid-tercile was significantly associated with 30-day readmission or mortality (OR: 6.17; 95% CI: 1.50 – 25.43) as was the highest tercile of postoperative ST2 (OR: 4.98; 95% CI: 1.06 – 23.32).
Conclusions:
Elevated pre-and postoperative levels of ST2 and Galectin-3 are associated with increased risk of readmission or mortality after pediatric heart surgery. These clinically available biomarkers can be used for improved risk stratification and may guide improved patient care management.
Keywords: pediatric congenital heart disease, biomarkers, prediction, readmission
Introduction
Congenital heart defects affect nearly 40,000 births per year in the United States.1,2 Among children undergoing pediatric congenital heart surgery, up to 20% experience 30-day hospital readmission and 4.2% of surgeries result in death.3,4 There are limited data regarding the primary causes of early mortality in children undergoing heart surgery. A recent study suggested that cardiovascular disease was the most common cause of death, with pulmonary and infectious diseases also being common causes.5,6 Reasons for readmission following congenital heart surgery have not been studied extensively. A single-center study suggested that pleural effusions, inflammation and infection, and cardiac related problems are among the major causes of readmission after congenital heart surgery.7
Beyond these clinical risk factors, novel biomarkers may also play a role in predicting 30-day readmission or death after congenital heart surgery. While multiple studies have shown that soluble suppression of tumorigenicity (ST2) and Galectin-3 are predictive of hospitalization and death in adults with heart failure, remarkably little is known about clinical predictors of readmission or mortality in pediatric cardiac care. Although studies have established their roles in immune system regulation and the inflammatory response, it is unknown if these biomarkers are associated with hospital readmission or mortality following congenital heart surgery. Therefore we sought to evaluate the ability of these biomarkers to identify children with an increased risk of 30-day readmission or mortality after pediatric congenital heart surgery.
Methods
Patients and Methods
This is a single center prospective longitudinal cohort of 244 consecutive patients who underwent at least one congenital cardiac operation, with or without cardiopulmonary bypass, at Johns Hopkins Children’s Center from 2010 to 2014. Patient, procedural, and outcome data were collected. The cohort was limited to patients with biomarker information collected in association with the initial cardiac surgery operation for each admission (index operation; N = 174). We excluded patients with unknown prematurity status, patients weighing 2.5 kg or less, and patients that were aged > 18 years old (N=12). We restricted our cohort to patients who had at least one preoperative and one postoperative biomarker measurement (N=162). The Committee for the Protection of Human Subjects at Dartmouth College and Johns Hopkins University (Institutional Review Board) approved this study for the prospective cohort with parental consent.
Biomarker Sample Collection
Perioperative blood samples (heparinized plasma) were collected immediately prior to skin incision and at the end of bypass. Samples were processed and stored at −80°C until assayed. Biomarkers were measured by ELISA using a custom printed multiplex assay (Meso Scale Discovery) using commercial antibodies and calibrators (R&D Systems).
Main Outcome
The composite study endpoint is unplanned readmission within 30 days following discharge or mortality either in-hospital during the surgical admission or at any location within 30 days following discharge from the surgical admission. Readmissions were defined as the first unplanned admission within 30 days of an index hospitalization where the congenital heart surgery occurred.
A composite endpoint was used due to the limited number of events. Readmission status and all-cause mortality data were obtained through chart abstraction.
Statistical Analysis
Patient, clinical and procedural characteristics were compared by our composite endpoint using descriptive statistics. Differences in risk factors were compared using Pearson’s chi-square tests or Fisher’s exact test; continuous variables were compared with two-sample t-test or Wilcoxon ranksum test. We evaluated the biomarkers in log continuous, above and below the population median, and tercile forms. Indicator variables were created for the tercile and median values.
Multivariate logistic regression analysis was used to assess the association between preoperative and postoperative log continuous, median and tercile cut-points. These analyses were adjusted using covariates based on the Society of Thoracic Surgeons (STS) Congenital Heart Surgery Database (STS-CHSD) Mortality Risk model.8 The procedural stratification for the STAT category was defined by the Society of Thoracic Surgeons European Association for Cardio-Thoracic Surgery (STAT) Mortality Category. The STAT scoring system assigns operations to categories on the basis of a similar risk of in-hospital mortality, where category 1 has the lowest risk of death and category 5 has the highest. Details of the STAT category assignment are previously described.8
Backwards stepwise logistic regression was used to determine the variables in our final model. Variables included in the initial backwards stepwise regression included: STAT mortality category,9 cardiopulmonary bypass time, any preoperative factor, acute kidney injury, ICU length of stay, hospital length of stay and major complication. Risk factors in the final adjusted model (p-values < 0.1) include: cardiopulmonary bypass time, any major complication (yes/no), and any preoperative factor (yes/no).
Results
Patient and pre-procedural characteristics are described in Table 1. In our cohort, 21 (12.9%) patients experienced the composite endpoint of mortality (N=3) or readmission (N=18) within 30-days from discharge. Among those in our cohort, the median age was 281 days (range 2 days to 17.3 years) and the median weight was 8.4kg (range 2.7 to 106). There was a slight male predominance (59.9% to 40.1%). Of the patients in our cohort, the most common procedure was ventricular septal defect repair (18, 11.1%). Among those who were readmitted, the mean days to readmission was 11.1 days (range 1 to 30 days). Pre-and postoperative biomarker levels and the association with readmission or mortality are listed in Table 2.
Table 1.
Patient characteristics
| Risk Factors | N = (162) | No readmission or mortality N = 141 (%) |
Readmission or Mortality N = 21 (%) |
p value |
|---|---|---|---|---|
| Age group | ||||
| Neonates | 16 | 13 (9.2) | 3 (14.3) | |
| Infants | 68 | 60 (42.6) | 8 (38.1) | |
| Children | 78 | 68 (48.2) | 10 (47.6) | 0.755 |
| Age, by days (median, IQR) | 287 (139, 2137) | 230 (98, 3726) | 0.270 | |
| Gender | ||||
| Female | 65 | 55 (39.0) | 10 (47.6) | |
| Male | 97 | 86 (61.0) | 11 (52.4) | 0.453 |
| Weight | ||||
| >10th percentile | 148 | 130 (92.2) | 18 (85.7) | |
| <10th percentile | 14 | 11 (7.8) | 3 (14.3) | 0.324 |
| Weight, by kg (median, IQR) | 8.3 (5.8, 19.6) | 12.9 (4.2, 44.5) | 0.059 | |
| Prematurity among neonates and infants | ||||
| No | 141 | 121 (85.8) | 20 (95.2) | |
| Yes | 21 | 20 (14.2) | 1 (4.8) | 0.230 |
| STAT Level | ||||
| 1 | 69 | 65 (46.1) | 4 (19.1) | |
| 2 | 43 | 33 (23.4) | 10 (47.6) | |
| 3 | 18 | 17 (12.1) | 1 (4.8) | |
| 4 | 15 | 12 (8.5) | 3 (14.3) | |
| 5 | 10 | 7 (5.0) | 3 (14.3) | |
| Missing | 7 | 7 (4.9) | 0 (0.0) | 0.031 |
| Prior cardiothoracic operation | ||||
| No | 129 | 118 (83.7) | 11 (52.4) | |
| Yes | 33 | 23 (16.3) | 10 (47.6) | 0.001 |
| Any non cardiac congenital anatomic abnormality | ||||
| No | 141 | 123 (87.2) | 18 (85.7) | |
| Yes | 21 | 18 (12.8) | 3 (14.3) | 0.847 |
| Chromosomal abnormality or syndrome | ||||
| No | 118 | 105 (74.5) | 13 (61.9) | |
| Yes | 44 | 36 (25.5) | 8 (38.1) | 0.227 |
| Major complication* | ||||
| No | 156 | 138 (97.9) | 18 (85.7) | |
| Yes | 6 | 3 (2.1) | 3 (14.3) | 0.006 |
| Cardiopulmonary Bypass Time* | ||||
| ≥120 | 91 | 74 (53.6) | 17 (81.0) | |
| <120 | 68 | 64 (46.4) | 4 (19.0) | 0.018 |
| Preoperative variables | ||||
| Mechanical circulatory support | ||||
| No | 162 | 162 (100.0) | 0 (0.0) | |
| Renal dysfunction requiring dialysis | ||||
| No | 161 | 140 (99.3) | 21 (100.0) | |
| Yes | 1 | 1 (0.7) | 0 (0.0) | 0.699 |
| Persistent shock at time of operation | ||||
| No | 161 | 140 (99.3) | 21 (100.0) | |
| Yes | 1 | 1 (0.7) | 0 (0.00) | 0.699 |
| Mechanical ventilation to treat cardiorespiratory failure | ||||
| No | 160 | 140 (99.3) | 20 (95.2) | |
| Yes | 2 | 1 (0.7) | 1 (4.8) | 0.117 |
| Preoperative neurological deficit | ||||
| No | 161 | 140 (99.3) | 21 (100.0) | |
| Yes | 1 | 1 (0.7) | 0 (0.0) | 0.699 |
| Any other preoperative factor | ||||
| No | 146 | 130 (92.2) | 16 (76.2) | |
| Yes | 16 | 11 (7.8) | 5 (23.8) | 0.022 |
| Any preoperative factor* | ||||
| No | 136 | 123 (87.2) | 13 (61.9) | |
| Yes | 26 | 18 (12.8) | 8 (38.1) | 0.003 |
Notes variables included in the final model
IQR: Interquartile range
Table 2.
Pre-and postoperative biomarkers levels and the association with readmission or mortality 30-days from surgery
| ST2 and Gal3 (ng/mL) by Readmission or Mortality at 30-days Median (IQR) |
|||||||
|---|---|---|---|---|---|---|---|
| ST2 | Gal3 | ||||||
| ALL (n=162) |
No (n=141) |
Yes (n=21) |
All (n=162) |
No (n=141) |
Yes (n=21) |
||
| PREOPERATIVE | Immediately prior to skin incision | 2.1 (1.2, 3.9) |
1.9 (1.2, 3.1) |
2.6 (2.0, 7.0) |
15.9 (9.0, 20.8) |
14.8 (8.8, 19.9) |
18.2 (14.6, 30.7) |
| POSTOPERATIVE | End of bypass | 3.3 (2.0, 6.0) |
3.2 (2.0, 5.4) |
6.8 (3.3, 10.0) |
24.5 (15.4, 37.0) |
23.0 (14.5, 35.8) |
29.9 (22.9, 44.6) |
Preoperative and Post-operative ST2 and 30-day readmission or mortality
Unadjusted preoperative ST2 median and highest terciles, compared to the lowest tercile, were significantly associated with 30-day readmission or mortality (OR: 2.58; 95% CI: 1.17 – 3.66; p= 0.012; OR: 4.37; 95% CI: 1.31 – 14.57; p = 0.016, respectively). After adjusting for covariates based on the STS CHSD mortality risk model, there were increased odds of readmission and mortality within 30-days, but the association was not statistically significant.
Before adjustment, postoperative ST2 values above the median and the highest tercile were significantly associated with readmission and mortality after heart surgery (OR:3.96; 95% CI: 1.52 – 10.31; p=0.005; OR: 7.01; 95% CI: 2.10 – 23.45; p=0.002, respectively). After adjustment, postoperative ST2 levels in the highest tercile showed a strong and significant association with readmission and mortality (OR: 4.98; 95% CI: 1.06 – 23.32 p = 0.042). (Table 3).
Table 3.
Biomarker associations with readmission or mortality within 30-days from discharge
| Biomarker | Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | ||
| GAL PRE | Log Continuous | 1.69 | 1.08 – 2.64 | 0.022 | 1.60 | 0.92 – 2.80 | 0.096 |
| Above Median vs. Below | 1.94 | 0.77 – 4.90 | 0.160 | 2.00 | 0.74 – 5.42 | 0.173 | |
| Tercile 2 vs. 1 | 2.23 | 0.70 – 7.11 | 0.174 | 2.84 | 0.82 – 9.81 | 0.100 | |
| Tercile 3 vs. 1 | 2.99 | 0.87 – 10.27 | 0.081 | 2.82 | 0.74 – 10.76 | 0.127 | |
| ST2 PRE | Log Continuous | 1.66 | 1.16 – 2.37 | 0.006 | 1.48 | 0.87 – 2.51 | 0.152 |
| Above Median vs. Below | 2.07 | 0.82–5.26 | 0.125 | 1.10 | 0.33 – 3.67 | 0.878 | |
| Tercile 2 vs. 1 | 2.58 | 1.17 – 3.66 | 0.012 | 2.59 | 0.76 – 8.81 | 0.128 | |
| Tercile 3 vs. 1 | 4.37 | 1.31 – 14.57 | 0.016 | 2.57 | 0.52 – 12.56 | 0.245 | |
| GAL POST | Log Continuous | 2.12 | 1.11 – 4.02 | 0.022 | 2.11 | 0.99 – 4.55 | 0.054 |
| Above Median vs. Below | 1.90 | 0.74 – 4.88 | 0.183 | 1.98 | 0.73 – 5.38 | 0.179 | |
| Tercile 2 vs. 1 | 6.27 | 1.61 – 24.44 | 0.008 | 6.17 | 1.50 – 25.43 | 0.012 | |
| Tercile 3 vs. 1 | 3.97 | 0.99 – 15.88 | 0.051 | 4.07 | 0.96 – 17.17 | 0.056 | |
| ST2 POST | Log Continuous | 2.26 | 1.37 – 3.72 | 0.001 | 1.99 | 1.03 – 3.86 | 0.042 |
| Above Median vs. Below | 3.96 | 1.52 – 10.31 | 0.005 | 2.84 | 0.90 – 8.91 | 0.074 | |
| Tercile 2 vs. 1 | 2.16 | 0.65 – 7.22 | 0.212 | 1.69 | 0.48 – 6.03 | 0.412 | |
| Tercile 3 vs. 1 | 7.01 | 2.10 – 23.45 | 0.002 | 4.98 | 1.06 – 23.32 | 0.042 | |
Preoperative and Post-operative Galectin-3 and 30-day readmission or mortality
Unadjusted preoperative Galectin-3 values above the median and terciles conferred increased risk of readmission and mortality, however these associations were not significant. Preoperative Galectin-3 values in the log continuous form were found to be statistically significant before adjustment (OR: 1.69; 95% CI: 1.02 – 2.64; p=0.022), however after adjustment, the associations were not significant.
Before adjustment, postoperative Galectin-3 values for the median tercile were significantly associated with increased risk of readmission and mortality (OR: 6.27; 95% CI: 1.61 – 24.44; p=0.008). The highest tercile for preoperative Galectin-3, before adjustment, also conferred increased risk but was not statistically significant (OR: 3.97; 95% CI: 0.99 – 15.88; p=0.051).
After adjustment, postoperative Galectin-3 values for the median tercile demonstrated significantly higher odds of readmission and mortality (OR: 6.17; 95% CI: 1.50 – 25.43; p value: 0.012). The other associations were not statistically significant (Table 3).
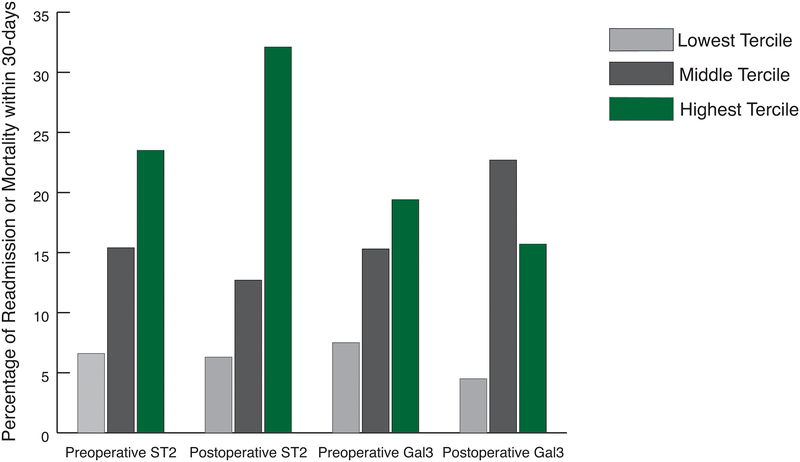
There is a significant association between unadjusted elevated preoperative ST2 levels and risk of 30-day readmission or mortality following pediatric congenital heart surgery (p<0.041). For patients in the lowest tercile of preoperative ST2, 6.6% experienced readmission or mortality compared to 23.5% in the highest biomarker tercile. Similarly, for patients in the lowest tercile of postoperative ST2, 6.3% were readmitted or died within 30-days from discharge, compared to 32.1% in the highest tercile (p=0.002). We notice a similar pattern with preoperative and post-operative Galectin-3. For those in the lowest preoperative tercile, 7.5% experience readmission and/or mortality compared to 19.4% in the highest tercile (p=0.182); patients in the lowest post-operative tercile of Galectin-3, 4.5% were readmitted or died compared to 15.7% in the highest tercile (p=0.016). The association of elevated cardiac biomarkers and increased risk of readmission or mortality after pediatric cardiac surgery is represented in Figure 1.
Figure 1.
There is a significant association between unadjusted pre-and post-operative ST2 and Galectin-3 terciles with increased risk of readmission or mortality. We observe a stepwise association of readmission or mortality with elevated ST2 and Galectin-3 biomarkers after pediatric cardiac surgery.
Discussion
This is the first study to examine the relationship between pre- and post-operative levels of these novel biomarkers and 30-day readmission or mortality after pediatric congenital heart surgery. In this single-center, prospective study of pediatric patients undergoing cardiac surgery, we found that post-operative ST2 and Galectin-3 were strong and significant predictors of readmission or mortality. With each unit increase in log-transformed values of post-operative ST2 and Galectin-3, the risk of 30-day readmission and/or mortality increases by 2 fold. Evaluated by terciles, post-operative ST2 and Galectin-3 had 4 to 7-times greater odds of readmission and/or mortality at the median and highest tercile, compared to the lowest tercile. Our findings strongly suggest that perioperative ST2 and Galectin-3 are objective indicators of children at high risk of early death or readmission.
While ST2 and Galectin-3 are well established biomarkers of hospitalization and death in adults with heart failure, remarkably little is known about clinical predictors of readmission or mortality in pediatric cardiac care.10–15 Research has demonstrated that ST2 and Galectin-3 are associated with worsening heart failure (HF) severity and that they are independent predictors of mortality in HF patients. In the pediatric population, Meeusen et al found no relationship between elevated levels of Galectin-3 and pediatric patients without heart failure.16 Hauser et al did not find a significant association between elevated levels of ST2 and pediatric patients with heart failure.17
Novel cardiac biomarkers ST2 and Galectin-3 are signals of myocardial fibrosis and remodeling that may provide additional benefit for the management of congenital heart disease.18 ST2 is known to play a critical role in the regulation of the inflammatory and autoimmune response in the body.13,16,19 Specifically in the heart, ST2 is expressed in both cardiomyocytes and cardiac fibroblasts that induced either by biomechanical strain injury or angiotensin. Therefore, elevated levels of ST2 present as a response to myocardial stress, such as myocardial ischemia, neurohormonal activation or mechanical overload.18,19, 50
Galectin-3 is involved in numerous physiological and pathological processes, including fibrosis and inflammation, which are critical contributing pathophysiological mechanisms to the development and progression of heart failure.10,20–22 Elevated Galectin-3 stimulates the release of various mediators and promotes cardiac fibroblast proliferation and ventricular dysfunction.23 Both ST2 and Galectin-3 were included in the AHA guidelines as additive risk stratification biomarkers for acute and chronic heart failure in adults.24
Novel biomarkers ST2 and Galectin-3 offer an advantage over the traditional biomarker B-type natriuretic peptide (BNP). BNP is a cardiac hormone and sensitive measure of cardiac stretch and has been used in heart failure treatment and to predict outcomes in patients with congenital heart disease and pulmonary hypertension. However ST2 and Galectin-3, unlike BNP, are not developmentally regulated and therefore not confounded by age.25 Furthermore, the biologic and analytic variability of ST2 is much lower than BNP, suggesting that ST2 levels may be better in risk stratification and prognositication.24,26
Limitations
This study has a few limitations. First, risk adjustment was initially performed using the covariates from the contemporary version of the STS-CHSD mortality risk model, which is a method of adjusting for clinical and procedural case mix. Due to the low event rate, we elected to perform a backwards stepwise regression model informed by covariates in the STS-CHSD model, thereby limiting the number of clinical covariates included in the model. Second, the relatively small proportion of patients with our combined endpoint after congenital heart surgery may have obfuscated some of the statistical associations between the biomarkers and our model covariates. In addition, a larger sample size would have improved our power to assess the performance of ST2 and Galectin-3 in the preoperative setting. Third, we included only patients for whom both pre-and post-operative samples had been collected. This approach may lead to sampling bias if biomarker collection was associated with congenital heart disease severity.
Conclusion
This study is the first to demonstrate that elevated pre-and post-operative levels of ST2 and Galectin-3 are significantly associated with increased risk of readmission or mortality after pediatric cardiac surgery. The ability to predict postoperative adverse outcomes from preoperative clinical data has significant implications for determining appropriate timing of surgery, assessing surgical alternatives and readiness for discharge after cardiac surgery. As a result, these cardiac biomarkers can be useful tools for risk stratification and improved patient care management. Future studies should further investigate the role of these cardiac biomarkers as well as well as other novel cardiac, renal and inflammatory biomarkers to improve prediction of adverse events following cardiac surgery.
Acknowledgements:
No authors have any conflicts of interest to disclose.
Funding Statement: This research is supported by the National Heart Lung and Blood Institute R01HL119664 (PI: Brown). All authors are research staff or investigators on the grant.
References
- 1.Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. The Journal of pediatrics. 2008;153(6):807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–1900. [DOI] [PubMed] [Google Scholar]
- 3.Feudtner C, Pati S, Goodman DM, et al. State-level child health system performance and the likelihood of readmission to children’s hospitals. The Journal of pediatrics. 2010;157(1):98–102.e101. [DOI] [PubMed] [Google Scholar]
- 4.Mildh LH, Pettila V, Sairanen HI, Rautiainen PH. Cardiac troponin T levels for risk stratification in pediatric open heart surgery. The Annals of thoracic surgery. 2006;82(5):1643–1648. [DOI] [PubMed] [Google Scholar]
- 5.Ohye RG, Schonbeck JV, Eghtesady P, et al. Cause, timing, and location of death in the Single Ventricle Reconstruction trial. The Journal of thoracic and cardiovascular surgery. 2012;144(4):907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlingmann TR, Thiagarajan RR, Gauvreau K, et al. Cardiac medical conditions have become the leading cause of death in children with heart disease. Congenital heart disease. 2012;7(6):551–558. [DOI] [PubMed] [Google Scholar]
- 7.Kogon B, Jain A, Oster M, Woodall K, Kanter K, Kirshbom P. Risk factors associated with readmission after pediatric cardiothoracic surgery. The Annals of thoracic surgery. 2012;94(3):865–873. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien SM, Jacobs JP, Pasquali SK, et al. The Society of Thoracic Surgeons Congenital Heart Surgery Database Mortality Risk Model: Part 1-Statistical Methodology. The Annals of thoracic surgery. 2015;100(3):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs JP, Jacobs ML, Maruszewski B, et al. Initial application in the EACTS and STS Congenital Heart Surgery Databases of an empirically derived methodology of complexity adjustment to evaluate surgical case mix and results. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2012;42(5):775–779; discussion 779–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bansal N, Katz R, Seliger S, et al. Galectin-3 and Soluble ST2 and Kidney Function Decline in Older Adults: The Cardiovascular Health Study (CHS). American journal of kidney diseases: the official journal of the National Kidney Foundation. 2016;67(6):994–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayes-Genis A, de Antonio M, Galan A, et al. Combined use of high-sensitivity ST2 and NTproBNP to improve the prediction of death in heart failure. Euro J Heart Fail. 2012;14(1):32–38. [DOI] [PubMed] [Google Scholar]
- 12.Henry-Okafor Q, Collins SP, Jenkins CA, et al. Soluble ST2 as a Diagnostic and Prognostic Marker for Acute Heart Failure Syndromes. The open biomarkers journal. 2012;2012(5):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Januzzi JL Jr., Peacock WF, Maisel AS, et al. Measurement of the interleukin family member ST2 in patients with acute dyspnea: results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. Journal of the American College of Cardiology. 2007;50(7):607–613. [DOI] [PubMed] [Google Scholar]
- 14.Pascual-Figal DA, Ordonez-Llanos J, Tornel PL, et al. Soluble ST2 for predicting sudden cardiac death in patients with chronic heart failure and left ventricular systolic dysfunction. Journal of the American College of Cardiology. 2009;54(23):2174–2179. [DOI] [PubMed] [Google Scholar]
- 15.Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RR, Januzzi JL. Serum levels of the interleukin-1 receptor family member ST2, cardiac structure and function, and long-term mortality in patients with acute dyspnea. Circulation Heart failure. 2009;2(4):311–319. [DOI] [PubMed] [Google Scholar]
- 16.Meeusen JW, Johnson JN, Gray A, et al. Soluble ST2 and galectin-3 in pediatric patients without heart failure. Clinical biochemistry. 2015;48(18):1337–1340. [DOI] [PubMed] [Google Scholar]
- 17.Hauser JA, Demyanets S, Rusai K, et al. Diagnostic performance and reference values of novel biomarkers of paediatric heart failure. Heart (British Cardiac Society). 2016;102(20):1633–1639. [DOI] [PubMed] [Google Scholar]
- 18.Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RR, Januzzi JL. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail. 2010;12(8):826–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chida A, Sato H, Shintani M, et al. Soluble ST2 and N-terminal pro-brain natriuretic peptide combination. Useful biomarker for predicting outcome of childhoodpulmonary arterial hypertension. Circulation journal: official journal of the Japanese Circulation Society. 2014;78(2):436–442. [DOI] [PubMed] [Google Scholar]
- 20.Amin HZ, Amin LZ, Wijaya IP. Galectin-3: a novel biomarker for the prognosis of heart failure. Clujul medical (1957). 2017;90(2):129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Boer RA, Voors AA, Muntendam P, van Gilst WH, van Veldhuisen DJ. Galectin-3: a novel mediator of heart failure development and progression. Eur J Heart Fail. 2009;11(9):811–817. [DOI] [PubMed] [Google Scholar]
- 22.Dieplinger B, Egger M, Leitner I, et al. Interleukin 6, galectin 3, growth differentiation factor 15, and soluble ST2 for mortality prediction in critically ill patients. Journal of critical care. 2016;34:38–45. [DOI] [PubMed] [Google Scholar]
- 23.Lok DJ, Lok SI, Bruggink-Andre de la Porte PW, et al. Galectin-3 is an independent marker for ventricular remodeling and mortality in patients with chronic heart failure. Clinical research in cardiology: official journal of the German Cardiac Society. 2013;102(2):103–110. [DOI] [PubMed] [Google Scholar]
- 24.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810–1852. [DOI] [PubMed] [Google Scholar]
- 25.Hammerer-Lercher A, Geiger R, Mair J, et al. Utility of N-terminal pro-B-type natriuretic peptide to differentiate cardiac diseases from noncardiac diseases in young pediatric patients. Clinical chemistry. 2006;52(7):1415–1419. [DOI] [PubMed] [Google Scholar]
- 26.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. The Journal of clinical investigation. 2007;117(6):1538–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]