Abstract
Background:
Activation of Akt is a marker of decreased event-free or overall survival in neuroblastoma (NB) patients. MK-2206, a novel allosteric Akt inhibitor, is now tested in clinical trials in adult cancers. In this study, effect of MK-2206 on tumor growth and murine survival, alone or in combination with etoposide or rapamycin was evaluated.
Methods:
The anti-cell proliferation effect of MK-2206 was tested in eight NB cell lines by MTS assay. Caspase 3/7 activity, cell cycle analysis and reactive oxygen species (ROS) production were determined. Effect of MK-2206 combined with etoposide or rapamycin was evaluated in vitro and in vivo. Akt phosphorylation was measured by Western blotting in NB cells and tumors.
Results:
In vitro, MK-2206 treatment inhibited NB cell proliferation which was accompanied by a cell line selective G1 arrest of cell cycle or production of ROS. A synergistic effect between MK-2206 and etoposide was detected in 4 tested NB cell lines via caspase-dependent apoptosis, while increased inhibition of cell growth induced by combination of MK-2206 and rapamycin was mediated by ROS production. In vivo, MK-2206 alone decreased tumor growth and increased murine survival at dose which inhibited Akt phosphorylation in tumors. MK-2206, in combination with etoposide or rapamycin, caused a significant decrease in tumor growth and increase of murine survival compared to MK-2206 alone.
Conclusion:
Akt inhibition by MK-2206 increased the efficacy of etoposide or rapamycin. Our study supports future clinical evaluation of MK-2206 in combination with conventional cytotoxic therapy or with rapamycin in high-risk NB patients.
Keywords: Neuroblastoma, MK-2206, etoposide, rapamycin
Introduction:
Neuroblastoma (NB) is the most common extracranial solid tumor in childhood (1). It accounts for more than 7% of malignancies in patients younger than 15 years and causes 10% of all pediatric oncology deaths (2). Approximately half of all NB patients are diagnosed with high-risk disease, and despite aggressive treatment strategies, such as high-dose chemotherapy, radiotherapy and bone marrow transplantation, the overall survival rate of these patients is dismal (3). The recent inclusion of anti-GD2 immunotherapy significantly increased the survival of high-risk patients compared to standard therapy (4). In spite of these improvements, therapies are still inadequate for some 40% of patients with high-risk disease. Therefore, development of new treatment approaches is still a challenge to improve treatment efficacy in high–risk NB patients.
Targeting key mediators of tumor survival signaling pathways is one approach to improve therapies and we have shown that activated Akt mediates resistance to cytotoxic agents used in NB treatment (5). Activated Akt is a downstream target of BDNF/TrkB signaling pathway (5, 6) and ALK pathway (7–9) in NB cells. Both pathways have been implicated in pathogenesis of neuroblastoma tumors (6–10). Cellular processes regulated by Akt include cell proliferation, growth and survival, metabolism, angiogenesis, and tissue invasion. All these processes represent the hallmarks of cancer, and a burgeoning literature has defined the importance of alterations of Akt activity in human cancer and experimental models of tumorigenesis (11).
Recently we showed that an Akt inhibitor perifosine had anti-tumor growth effect alone or in combination with chemotherapy in NB both in vitro and in vivo (12, 13). As perifosine works not only on Akt, but also on other molecular targets or pathways (14, 15), we sought to evaluate how a specific Akt inhibitor affects the sensitivity of NB cells to cytotoxic agents. MK-2206, an allosteric Akt inhibitor, binds to the Akt protein at a site in the pleckstrin-homology (PH) domain which causes a conformational change in the protein that prevents its localization to the plasma membrane thus inhibiting its subsequent activation (16). As an anticancer agent, MK-2206 is being tested both in vitro and in vivo in adult tumors (17–23) and in a spectrum of pediatric tumors (24). Recently, in a clinical trial stable disease was observed in patients with advanced solid tumors (25). The activation of Akt in NB and its association with biological characteristics of poor prognosis makes MK-2206 a potential approach to improve therapeutic efficacy for NB tumors. In the present study we evaluated the anti-tumor growth effect of MK-2206 as a single agent, and in combination with a cytotoxic drug, etoposide, or the mTOR inhibitor rapamycin in vitro and in vivo.
Materials and Methods:
Cell lines and Cell Culture
Eight human NB cell lines----SK-N-AS (AS), SK-N-BE2(BE2), NGP, SY5Y, SMS-KCNR(KCNR), SKN-FI, SKN-DZ, LAN-5 and a normal human retinal pigment epithelial cell line (ARPE-19) (6, 12, 26–28)(Table 1) were used in this study. All tumor cell lines have been authenticated by single nucleotide polymorphism analyses and are genetically homogeneous (S. J. Chanock, Division of Cancer Genetics and Epidemiology, National Cancer Insistute). Cells were cultured in RPMI-1640 medium (Mediatech Inc, Manassas, VA) containing 10% fetal bovine serum (FBS) (Gemini Bio-Products, West Sacramento, CA) as described before (4).
Table 1.
Specific mutations in neuroblastoma (NB) cell lines
| Cell line | Ref. | P53 | ALK | MNA | 1pLOH | MK-2206 (IC50 μM) |
|---|---|---|---|---|---|---|
| SKN-AS | 12 | Δexon9 | Wt | − | + | 16.5 |
| SKN-FI | 26 | M246R | S1136S | − | − | 8.7 |
| SKN-DZ | 6 | R110L | Wt | + | − | 8.1 |
| SKN-BE2 | 12 | C135F | Wt | + | + | 3.6 |
| SY5Y | 6 | Wt | F1174L | − | − | 1.7 |
| LAN5 | 27 | Wt | R1275Q | + | + | 1.3 |
| SMS-KCNR | 12 | Wt | R1275Q | + | + | 1.1 |
| NGP | 12 | Wt | Wt | + | − | 0.6 |
P53 = a tumor suppressor gene
ALK = anaplastic lymphoma kinase; MNA = MYCN gene amplification
MNA = MYCN gene amplification
1pLOH = loss of heterozygosity at chromosome 1p
Wt = wild type.
Reagents and antibodies:
MK-2206 was obtained from Merk & Co., Inc. (Whitehouse Station, NJ, USA) through Cancer Therapy Evaluation Program (CTEP). Etoposide was obtained from Bedford Laboratories (Bedford, OH). Z-VAD-fmk was purchased from R&D Systems, Inc, (Minneapolis, MN). N-actetyl-L-cysteine (NAC) and α-Tocopherol were obtained from Sigma-Aldrich (St. Louis, MO). Chloromethyl-dichlorodihydrofluorescein diacetate (CM-H2DCF-DA) was purchased from Invitrogen (Carlsbad, CA). Anti-Akt antibody, anti-phospho-Akt (P-Akt, Ser473) antibody, anti-S6 antibody (T-S6), anti-phospho-S6 (P-S6, Ser235/236) antibody, anti-GAPDH antibody, anti-cleaved caspase 3 antibody as well as rapamycin were purchased from Cell Signaling Technology (Beverly, MA, USA).
Cell Treatment and Cell Proliferation Assay:
To detect cell proliferation, NB cells were treated with MK-2206 at concentrations ranging from 0.001μm to 20μm, or etoposide ranging from 0.05μg/ml to 50μg/ml, or rapamycin ranging from 0.1nM to 100nM for 48 hrs. For combination of MK-2206 and etoposide, MK-2206 was administrated 2 hrs before etoposide. For combination of MK-2206 and rapamycin, rapamycin was given 2 hrs before MK-2206. To assess mechanisms of cell death, cells were pretreated with Z-VAD-fmk or NAC for 2 hrs before other drugs were administrated. At end of the experiment, cell proliferation was measured using 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt assay (MTS assay) according to manufacturer’s specification (Promega Corporation, Madison, WI). The percentage of cell proliferation was calculated by dividing absorbance value of treated NB cells by absorbance value of control cells within each group. All experiments were performed 2–3 times.
Reactive oxygen species measurements:
NB cells were treated with MK-2206 for 24 hrs, cultured with CM-H2DCF-DA (5μg/ml) at 37°C for 30 minutes (min), harvested and washed with PBS twice. The fluorescence intensity of DCF was determined by flow cytometry.
Assay of Caspase-3 and Caspase-7 Activity:
NB cells were treated with reagents either alone or in a combination for 24 hrs. The activity of caspase-3/7 was evaluated using Caspase-Glo 3/7 Assay Kit (Promega Corporation) according to manufacturer’s instruction. Luminescence was detected by a luminometer, Victor 3 (PerkinElmer Life and Analytical Sciences, Shelton, CT).
Western Blotting:
To evaluate Akt and mTOR signaling pathways after drug treatment, protein lysates were extracted from NB cells (5minutes on ice) or NB tumor tissues (sonicating tumor tissues for 15 minutes) using a Mammalian Protein Prep Kit (Qiagen, Valencia, CA). Immunoblotting was performed to detect P-Akt, P-S6, T-Akt and T-S6 as described previously (4).
Cell Cycle Analysis:
NB cells were treated with MK-2206 for 48 hrs, washed with PBS, incubated with RNase A (Roche, Indianapolis, IN) at 100μg/mL and propidium iodide (Sigma-Aldrich Corp, St Louis, MO) at 50μg/mL for 30 minutes at room temperature. The stained cells were analyzed for DNA content by Flow cytometry (Becton Dickinson & Co., Franklin Lakes, NJ). FlowJo software (BD Biosciences) was used to quantify the percentage of cells in different stages of the cell cycle.
In vivo Studies:
NB cells (AS, NGP, BE2, and SY5Y) were harvested, washed with Hanks balanced salt solution (HBSS) (Invitrogen), and re-suspended in HBSS and Matrigel (Trevigen, Gaithersburg, MD). AS, NGP or BE2 (2 ×106 cells) or SY5Y (4 ×106 cells) cells in 100μl were inoculated into subcutaneous tissue of right flank of 5- to 6-week-old female athymic nude mice (Frederick, MD). When tumors reached 100–200 mm3, treatment began. MK-2206 (100 or 200mg/kg) was administered by oral gavage, etoposide (100mg/kg) and rapamycin (5mg/kg) were given by intraperitoneal (IP) injection. MK-2206 and etoposide were given three times a week on Monday, Wednesday and Friday. Rapamycin was give 5 days a week from Monday to Friday. Dimensions of tumors were measured three times a week and tumor volume (mm3) was calculated as (L× W2)/4, in which L indicates length in mm and W indicates width in mm. To determine mice survival, we counted the days from the date treatment was administered to the time when tumors reached a length of 20mm, or to the end of the experiment in treatment groups. Mice bearing AS, NGP, BE2 and SY5Y tumors were treated with MK-2206 at 200mg/kg for 3 times to evaluate P-Akt levels in tumors; Mice bearing NGP tumors were treated with rapamycin at 2.5 or 5mg/kg for 2 weeks to evaluate P-S6 levels.
All xenograft studies were approved by the Animal Care and Use Committee of the National Cancer Institute, and all mouse treatments, including their housing, were in accordance with the institutional guidelines (PB-023).
Statistical Analysis:
The synergistic effects between two agents in in vitro experiments were evaluated through combination index (CI) values obtained with the CompuSyn software bought from ComboSyn, Inc (www.ComboSyn.com) (29). Other statistical analysis was performed with the Graphpad Prism software (GraphPad Software, Inc, version 3.0, La Jolla, CA). Comparisons between 2 groups were performed using unpaired Student t test; comparisons among ≥3 groups were performed using one way analysis of variance (ANOVA) with Bonferroni correction. Mice survival was evaluated by Kaplan-Meier method. All P values less than 0.05 were considered statistically significant.
Results:
1. MK-2206 inhibits cell proliferation and Akt phosphorylation in NB cells.
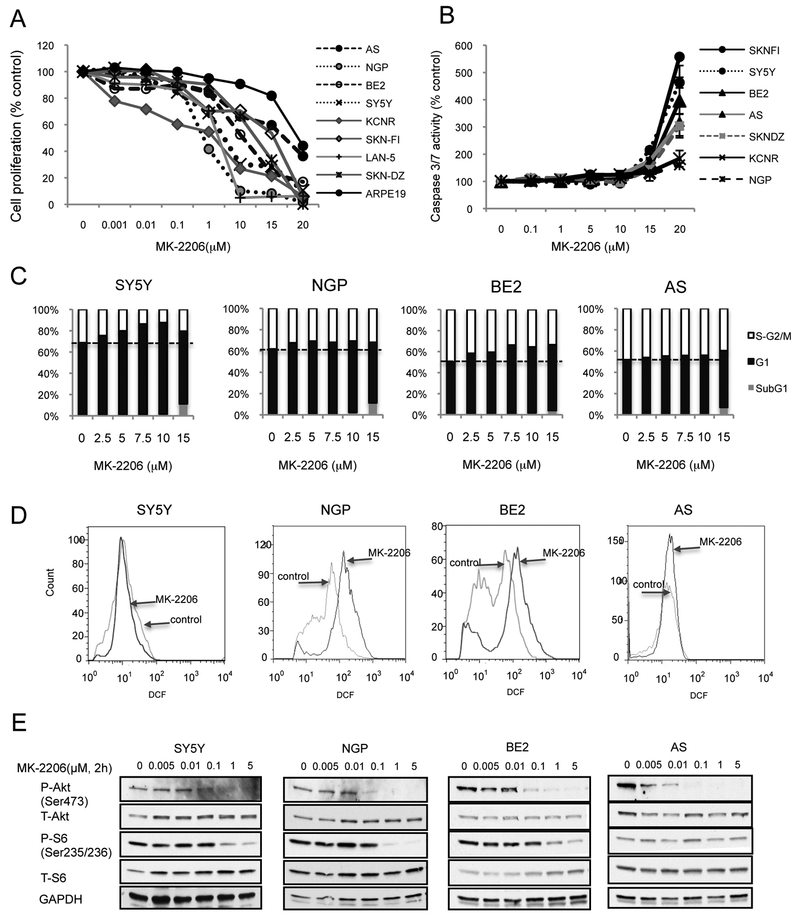
To study the effect of MK-2206 on NB cell proliferation, 8 NB cell lines (AS, NPG, BE2, SY5Y, KCNR, SKN-FI, LAN-5, SKN-DZ) and ARPE-19 cells were selected and treated with MK-2206 for 48hrs. MK-2206 treatment induced a concentration-dependent inhibition of cell proliferation in these NB cell lines, with the IC50 ranged from 0.6μM in NGP cells to 16.5μM in AS cells (Table 1). However, high concentrations of MK-2206 (15μM and 20μM) also inhibited proliferation of the non-transformed cell line ARPE-19, which had an IC50 of 59μM (Figure 1A).
Figure 1: MK-2206 inhibits NB cell proliferation and Akt phosphorylation.
A: NB cell lines and control ARPE-19 cells were treated with MK-2206 for 48 hrs. Cell proliferation was evaluated. B: NB cell lines were treated with MK-2206 for 16 hrs, caspase 3/7 activity was evaluated. Bar, SD. C: SY5Y, NGP, BE2 and AS cells were treated with MK-2206 for 48 hrs, stained with propidium iodide. The percentage of cells in S-G2/M, G1 and SubG1 phases is shown. D: SY5Y, NGP, BE2 and AS cells were treated with 7.5μM of MK-2206 for 24 hrs, stained with DCF-DA. E: SY5Y, NGP, BE2 and AS cells were treated with MK-2206 for 2 hrs, total protein was extracted for analysis of phosphorylated-Akt (P-Akt), total Akt, phosphorylated S6 (P-S6) and total S6.
To assess whether MK-2206-induced inhibition of cell proliferation was mediated via a caspase-dependent apoptotic pathway, caspase-3/7 activity was evaluated after MK-2206 treatment. There was not a statistically significant increase in caspase 3/7 activity in NB cells treated with MK-2206 at concentrations of ≤10μM. Only cells treated with high (15μM or 20μM) concentrations of MK-2206 showed increased caspase 3/7 activity (Fig. 1B). Pretreatment with pan caspase inhibitor Z-VAD-fmk before MK-2206 didn’t protected cells from MK-2206-induced inhibition of cell proliferation at concentrations of ≤10μM, and only partially protected cells treated with MK-2206 at 15μM or 20μM (data not shown).
To evaluate whether MK-2206 inhibits cell proliferation via regulation of cell cycle arrest, cell cycle analysis was performed by flow cytometry in SY5Y, NGP, BE2 and AS cells exposed to MK-2206 for 48hrs (Fig. 1C). Increased concentrations of MK-2206 (from 2.5 to 10μM) caused an initial accumulation of cells in G1 phase of the cell cycle in SY5Y and BE2 cells (up to 18% increase), followed by a reduced percentage of cells in S-G2/M phase. Increase of cells in G1 phase was not significant in NGP and AS cells. At concentration of 15μM, an increase of cells in SubG1 phase was detected in all the tested 4 NB cell lines indicating the presence of apoptosis (Fig. 1C).
As it is reported that ROS induction inhibits cell growth without an increase of caspase activity (30), we assessed whether there is any change of ROS production during MK-2206 treatment. In Fig. 1D, MK-2206 treatment induced a right shift of histogram at FL-1(DCF) in BE2 and NGP cells, an up-shift in AS cells while SY5Y is not affected. This indicates that MK-2206 induces an increase in ROS in BE2 and NGP cells, but not in AS and SY5Y cells. Pretreatment with ROS inhibitor NAC partially blocked the MK-2206-induced inhibition of cell proliferation in BE2 and NGP cells (data not shown).
To study the inhibition effect of MK-2206 on Akt signaling pathway, NB cells were treated with MK-2206 for 2hrs. A decrease of phosphorylated Akt (P-Akt) levels (from 40% to 70%) was detected in SY5Y, BE2 and NGP cells at 0.1μM, while a 60% decrease of P-Akt was observed at 0.01μM in AS cells. The levels of phosphorylated S6 (P-S6), a downstream target of Akt/mammalian target of rapamycin (mTOR), were reduced by MK-2206 at 1μM or higher in SY5Y, NGP and BE2 cells, but no reduction of P-S6 was detected in AS cells. Total Akt and total S6 were not influenced by MK-2206 treatment (Fig. 1E).
These data indicate that MK-2206 treatment induced inhibition of NB cell proliferation and Akt phosphorylation in NB cells. Cell cycle arrest and ROS production may mediate the reduced proliferation seen in cells treated with MK-2206. However, at concentrations necessary to inhibit Akt, caspase dependent apoptosis is not induced.
2. In vivo effects of MK-2206 as a single agent on tumor growth and mice survival.
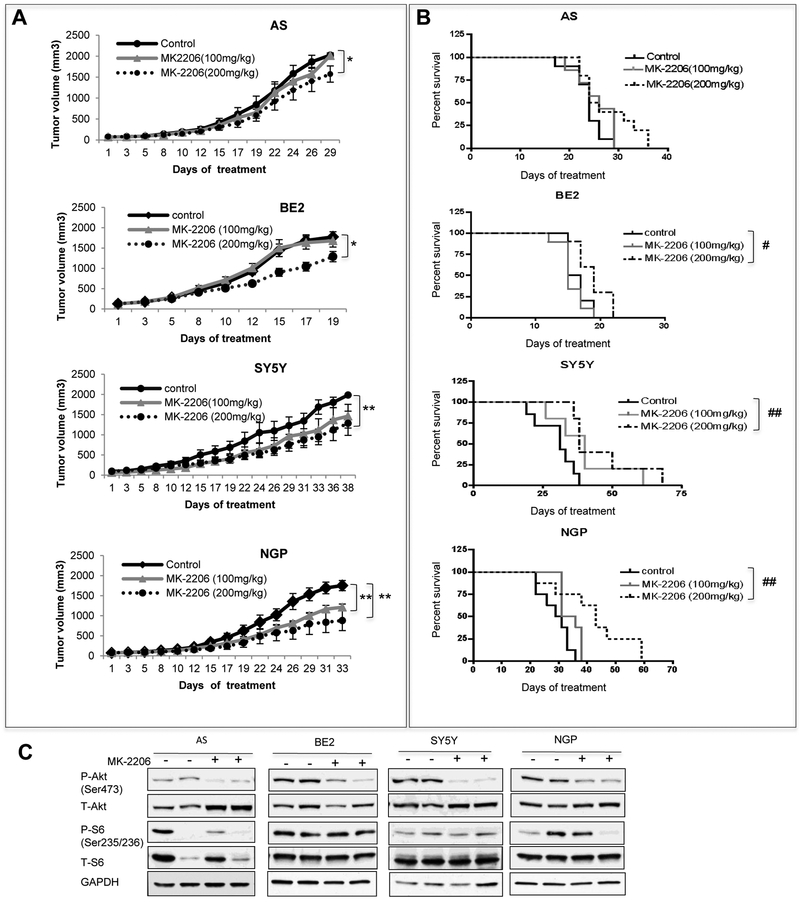
To determine whether MK-2206 affects NB tumor growth in vivo, mice bearing AS, BE2, SY5Y and NGP tumors were treated with MK-2206 at 100mg/kg or 200mg/kg dose. High dose MK-2206 (200mg/kg) showed anti-tumor growth effect in all the 4 NB tumors with varying efficacy (Fig. 2A). The tumor growth inhibition was 22% in AS tumors (P=0.037), 30% in BE2 tumors (P=0.014), 44% in SY5Y tumors (P=0.004) and 48% in NGP tumors (P=0.007). While low dose MK-2206 (100mg/kg) only significantly inhibited the growth of NGP tumors (P=0.002). There was a 27% inhibition of tumor growth in SY5Y tumors at the low dose, although this didn’t reach statistical significance (P=0.06).
Figure 2: In vivo effects of MK-2206 as a single agent on tumor growth and mice survival.
AS, BE2, SY5Y and NGP cells were injected into mice. Mice with tumors were treated with vehicle or MK-2206 (100mg/kg/day or 200mg/kg/day). A: Tumor volumes from control and treated groups were compared when the last mouse from each control group was euthanized. Bar, SE. * P<0.05, ** P<0.01, treated group versus control group. B: Survival curve was plotted by Kaplan-Meier analysis. # P<0.05, ## P<0.01, treated group versus control group. C: NB tumors were treated with 200mg/kg/day MK-2206 for 3 times, total protein was extracted for analysis of P-Akt, T-Akt, P-S6, T-S6 and GAPDH.
There was a statistically significant survival advantage in MK-2206 treated mice bearing BE2, SY5Y and NGP xenograft tumors at 200mg/kg but not 100mg/kg (Fig. 2B). The median survival time for control vs MK-2206 was 16 days vs 19 days (P=0.018) in mice bearing BE2 tumors, 31 days vs 44 days (P=0.009) in mice bearing SY5Y tumors, 30 days vs 43 days (P=0.005) in mice bearing NGP tumors. MK-2206 didn’t significantly affect the mice survival bearing AS xenograft tumors (Fig. 2B).
To determine whether MK-2206 treatments inhibited the target, we assessed the level of P-Akt and P-S6 in tumors from control and MK-2206-treated mice (3 doses at 200mg/kg/dose). The level of P-Akt was inhibited in MK-2206-treated tumors in all the tested 4 NB cell lines yet inhibition of P-S6 was not detected in NB tumor xenografts (Fig. 2C). These data indicated that, as a single agent, MK-2206 showed a significant inhibition of tumor growth in mice bearing AS, NGP, BE2 and SY5Y xenograft tumors (4/4) and a statistically significant increase in the survival of mice bearing NGP, BE2 and SY5Y tumors (3/4). These effects were accompanied with inhibition of P-Akt in tumor tissues.
3. The synergistic inhibition of cell proliferation with combination treatments of MK-2206 and Etoposide.
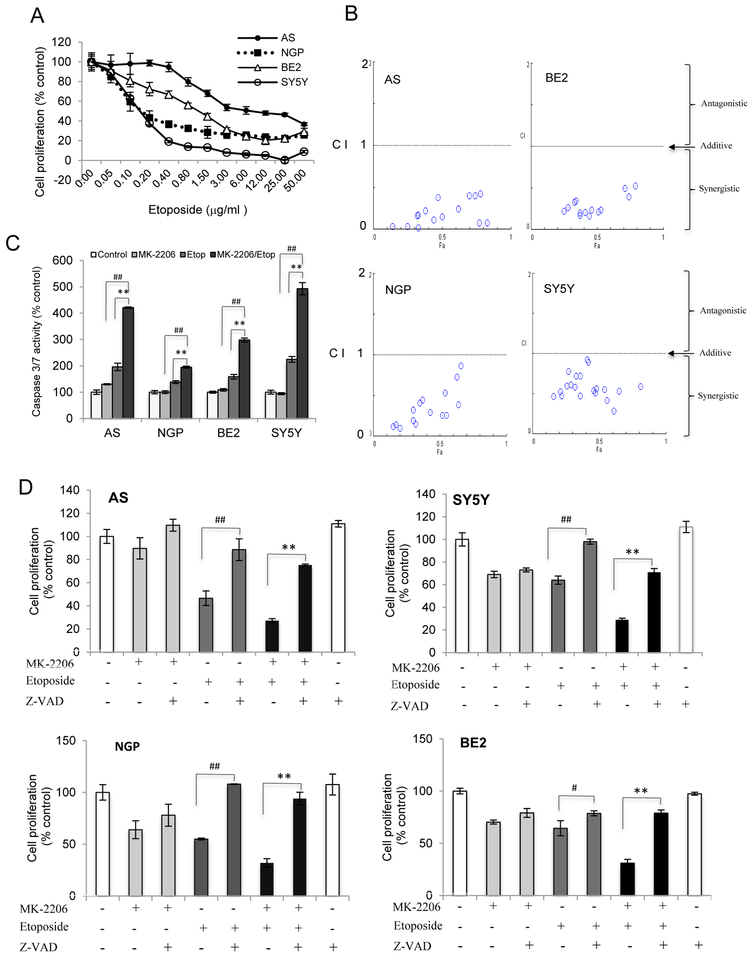
Etoposide is an active chemotherapeutic drug used in NB treatment. To evaluate whether MK-2206 increases the sensitivity of NB cells to etoposide, we first assessed the responses of NB cells to etoposide. Etoposide treatment induced a dose-dependent decrease in cell proliferation in all 4 NB cell lines tested (Fig. 3A). At an unfixed ratio, concentrations of MK-2206 and etoposide spanning the IC50 for each cell line were selected for combination study by calculating Combination Index (CI) value with ComboSyn software (Fig.3B). A CI<1 indicates synergism, a CI=1 reflects an additive effect and a CI>1 indicates drug antagonism. A synergistic effect between MK-2206 and etoposide was observed in the 4 NB cell lines as the CI values were smaller than 1 (Fig.3B). Similar results were found using a combination of cisplatin and MK-2206 (Fig. S1).
Figure 3: The synergistic inhibition of cell proliferation with combination treatment of MK-2206 and etoposide.
A: AS, BE2, NGP, and SY5Y cells were treated with etoposide for 48 hrs, cell proliferation was evaluated. B: AS, BE2, NGP, and SY5Y cells were treated with MK-2206 and etoposide either alone or in combination for 48 hrs. The concentrations of each agent spanned the IC50 dose in each cell line. Cell proliferation and combination index were evaluated. C: Caspase 3/7 activity was detected in AS, BE2, NGP, and SY5Y cells treated with MK-2206 (NGP and SY5Y (5μM), AS and BE2 (7.5μM)) and etoposide (AS (20 μg/ml), BE2 (1μg/ml), NGP (0.2 μg/ml) and SY5Y (0.1μg/ml)) either alone or in combination for 24 hrs, Bar, SD. ## P<0.01: MK-2206/etoposide-treated cells versus MK-2206-treated cells; **P<0.01: MK-2206/etoposide-treated cells versus etoposide-treated cells. D: AS, SY5Y, NGP and BE2 cells were pretreated with Z-VAD-fmk (100μM) for 2 hrs followed by treatment of MK-2206 (7.5μM in AS, BE2 cells, 5μM in SY5Y and NGP cells) and etoposide (AS (20μg/ml), SY5Y (0.1μg/ml), NGP (0.1μg/ml), BE2 (1μg/ml)) either alone or in combination. Cell proliferation was evaluated 48 hrs later. Bar, SD, # P<0.05, ## P<0.01: Z-VAD-fmk /etoposide-treated cells versus etoposide-treated cells; **P<0.01: Z-VAD-fmk /etoposide/MK-2206-treated cells versus etoposide/MK-2206-treated cells.
To study whether the synergistic inhibition of cell proliferation caused by MK-2206 and etoposide was modulated via enhanced caspase-dependent apoptosis, we evaluated caspase 3/7 activity. MK-2206 didn’t increase the caspase 3/7 activity in NGP, BE2 and SY5Y cells although there was a (30%) increased in AS cells. However the addition of MK-2206 to etoposide induced higher caspase 3/7 activity than etoposide alone. The activation of caspase 3/7 activity for etoposide vs etoposide+MK-2206 was 1.9 fold vs 4.2 fold in AS cells, 1.4 fold vs 2 fold in NGP cells, 1.6 fold vs 3.0 fold in BE2 cells and 2.2 fold vs 5.0 fold in SY5Y cells (Fig. 3C). Pretreatment of NB cells with pan-caspase inhibitor Z-VAD-fmk before administration of MK-2206 and/or etoposide showed that Z-VAD-fmk blocked the inhibition of cell proliferation induced by etoposide or etoposide+MK-2206 in AS, SY5Y, NGP and BE2 cells (Fig.3D). Consistent with the lack of caspase3/7 activity induced by MK-2206 alone, Z-VAD-fmk didn’t alter MK-2206 effects. These data indicated that MK-2206 increased the sensitivity of NB cells to etoposide by enhancing the caspase-dependent apoptosis.
4. The Combination effects of MK-2206 and etoposide on tumor growth and mice survival in vivo.
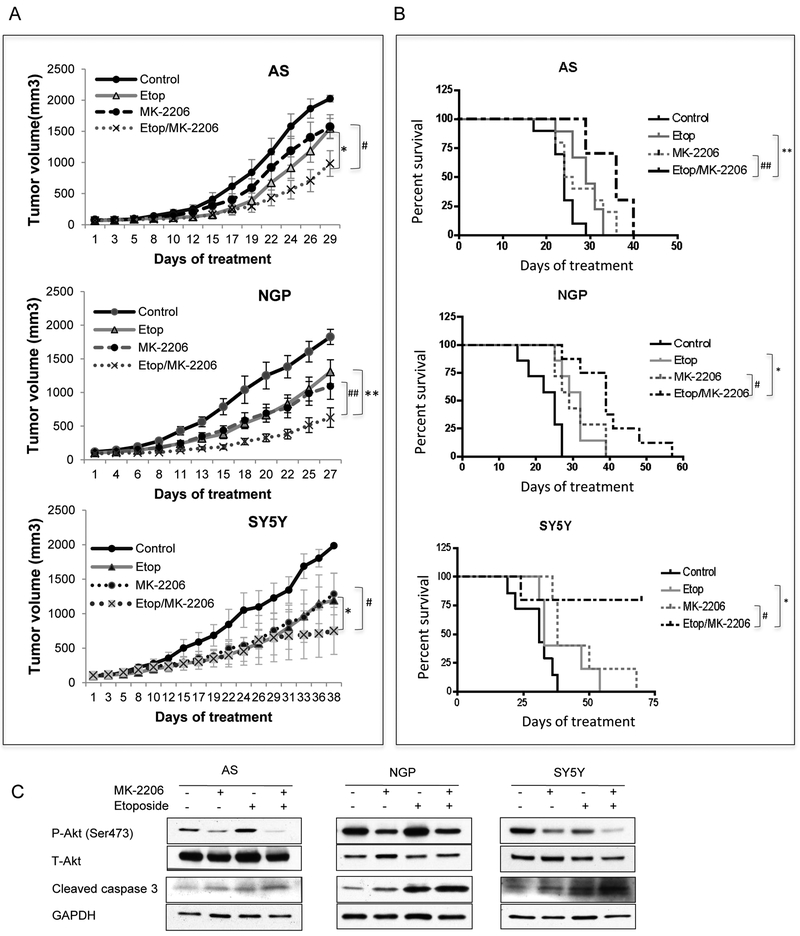
To evaluate the efficacy of MK-2206 and etoposide combination against the tumor growth and mice survival in vivo, mice bearing AS, SY5Y and NGP tumors were treated with etoposide (10mg/kg) and MK-2206 (200mg/kg) alone or in combination. In mice bearing AS tumors, there was a 52% inhibition of tumor growth in etoposide+MK-2206 combination group, which was significantly higher than 24% inhibition of growth in etoposide-treated group (P=0.05) or 22% growth inhibition in MK-2206-treated group (P=0.046) (Fig. 4A). The survival of mice was also significantly increased in combination group with median survival time of 36 days, compared to 29 days in etoposide-treated group (P=0.0024) or 25 days in MK-2206-treated group (P=0.0064) (Fig. 4B). In mice bearing NGP tumors, inhibition of tumor growth in etoposide+MK-2206 combination group was 66%, which was significantly higher than 28% in etoposide-treated group (P=0.01) or 40% in MK-2206-treated group (P=0.008) (Fig. 4A). The survival of mice was also significantly increased in combination group with median survival time of 39 days, compared to 32 days in etoposide-treated group (P=0.035) or 29 days in MK-2206-treated group (P=0.035) (Fig. 4B). In mice bearing SY5Y tumors, inhibition of tumor growth in etoposide+MK-2206 combination group was 62%, which was higher than 40% in etoposide-treated group (P=0.045) or 44% in MK-2206-treated group (P=0.05) (Fig. 4A). The survival of mice was also significantly increased in combination group with an unreached median survival time during the experiment, compared to 40 days in the etoposide-treated group (P=0.048) or 44 days in MK-2206-treated group (P=0.049) (Fig. 4B). Furthermore, we evaluated the effect of the combination of MK-2206 and etoposide on P-Akt levels and cell death. Inhibition of P-Akt was detected in MK-2206 and MK-2206/etoposide treated AS, NGP and SY5Y tumors. P-Akt levels decreased in SY5Y xenografts from mice treated with etoposide but not in etoposide treated mice bearing AS and NGP tumors. An increase in cleaved caspase 3 was detected in AS, NGP and SY5Y xenografts from mice treated with etoposide alone or with the combination of MK-2206 and etoposide (Fig. 4C). These data indicated that by combining MK-2206 and etoposide the anti-tumor growth effect and mice survival advantage were increased compared to either agent alone.
Figure 4: In vivo effects of MK-2206 and etoposide on tumor growth and mice survival.
Mice bearing AS, NGP and SY5Y tumors were treated with MK-2206 (200mg/kg) and etoposide (10mg/kg) either alone or in combination. A: Tumor volumes from control and treated groups were compared when the last mouse in each control group was euthanized. Bar, SE. B: Mice survival was compared through Kaplan Meier curves. #P<0.05, ##P<0.01: MK-2206/etoposide-treated group versus MK-2206-treated group. * P<0.05, **P<0.01: MK-2206/etoposide-treated group versus etoposide-treated group. C: NB tumors were harvested at end points, total protein was extracted for analysis of P-Akt, T-Akt, cleaved caspase 3 and GAPDH.
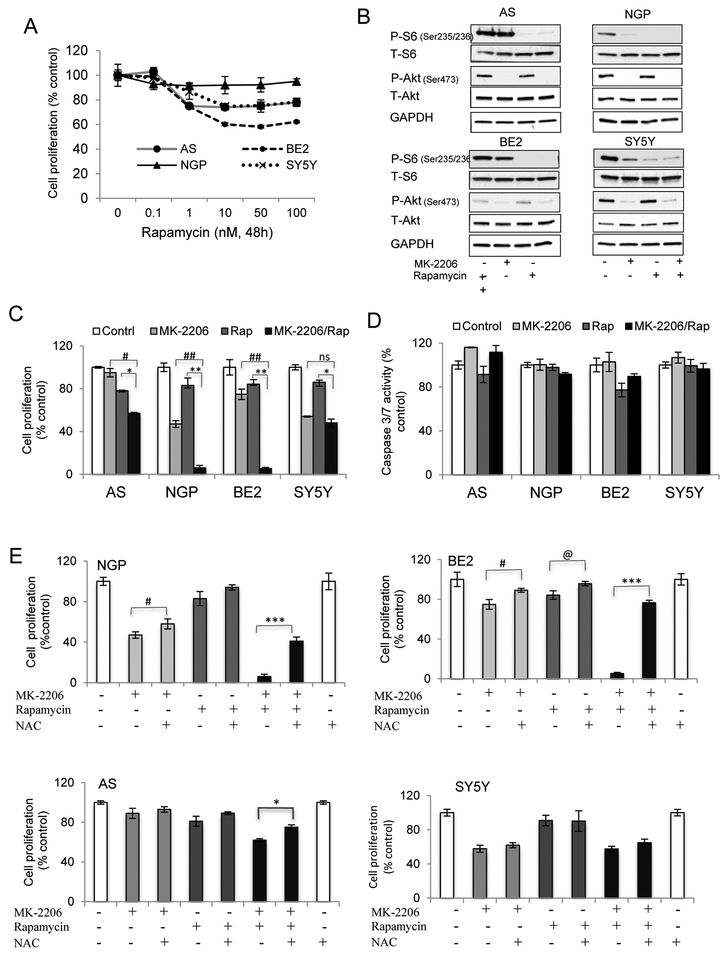
5. Combination of MK-2206 and rapamycin enhanced the inhibition effect on cell proliferation via ROS.
To study whether cell growth inhibition could be enhanced by targeting one more node in the Akt signaling pathway, the response of NB cells to rapamycin, a mTOR inhibitor, was evaluated. NB cells were treated with rapamycin for 48hrs. Rapamycin-induced inhibition of cell proliferation was observed at concentrations ≥1nM, while no further inhibition was observed by increasing concentrations of rapamycin from 10nM to 100nM (Fig. 5A). To confirm the inhibition of mTOR pathway by rapamycin, NB cells were treated with MK-2206 and rapamycin alone or in combination. The levels of P-Akt and the downstream target of mTOR, P-S6, were detected. Rapamycin alone or in combination with MK-2206 completely inhibited P-S6 in the 4 NB cell lines. Within the time pointes we tested, rapamycin didn’t affect P-Akt levels (Fig. 5B). NB cells were treated with the same concentrations of MK-2206 and rapamycin for 48 hrs. A combination of MK-2206 and rapamycin significantly enhanced inhibition of cell proliferation in BE2 and NGP cells compared to either alone. There was little to no effect of the combination in AS or SY5Y cells (Fig. 5C).
Figure 5. The combination effects of MK-2206 and rapamycin in vitro and in vivo.
AS, BE2, NGP and SY5Y cells were used. A. NB cells were treated with rapamycin for 48 hrs and cell proliferation was evaluated. Bar, SD. B: NB cells were treated with rapamycin (10nM) for 2 hrs followed by MK-2206 (7.5μM) treatment for another 2 hrs, either alone or in combination. Total protein was extracted to detect P-S6, T-S6, P-Akt, T-Akt and GAPDH levels. C and D: NB cells were treated with rapamycin (10nM) for 2 hrs followed by MK-2206 (7.5μM) treatment and cell proliferation was evaluated after 48 hrs (C) or caspase 3/7 activity was detected after 24hrs (D). Bar, SD. # P<0.05, ##P<0.01: MK-2206/rapamycin-treated cells versus MK-2206-treated cells; ** P<0.05, **P<0.01: MK-2206/rapamycin-treated cells versus rapamycin-treated cells. NS: no statistical significance. E: NGP, BE2, AS and SY5Y cells were pretreated with NAC (100mM) for 2 hrs followed by MK-2206 (7.5μM) and Rapamycin (10nM) treatment, either alone or in combination for 48 hrs. Cell proliferation was detected. Bar, SD. # P<0.05: NAC/MK-2206-treated cells versus MK-2206-treated cells; @ P<0.05: NAC /rapamycin-treated cells versus rapamycin-treated cells, *P<0.05, ***P<0.001: NAC /rapamycin/MK-2206-treated cells versus rapamycin/MK-2206-treated cells. F: Mice bearing NGP tumors were treated with rapamycin at 2.5mg/kg/day or 5mg/kg/day for 2 weeks by IP injection. Total proteins from tumor tissues were extracted for evaluation of P-S6, T-S6, P-Akt and T-Akt. G and H: Mice bearing NGP tumors were treated with MK-2206 (200mg/kg) and rapamycin (5mg/kg) either alone or in combination. Tumor volumes from control and treated groups were compared when the last mouse in control group was euthanized (G). Bar, SE. Mice survivals were compared with the Kaplan-Meier curves (H). ## P<0.01: rapamycin/MK-2206-treated group versus MK-2206-treated group. *P<0.05: rapamycin/MK-2206-treated group versus rapamycin-treated group. I: NB tumors were harvested at end points, total protein was extracted for analysis of P-Akt, T-Akt, P-S6, T-S6 and GAPDH.
To study if the enhanced effect in BE2 and NGP cells was through caspase-dependent apoptosis, caspase 3/7 activity was evaluated. No significant increase in caspase 3/7 activity was observed in MK-2206 and rapamycin-treated cells, alone or in combination in any of the 4 NB cell lines tested (Fig. 5D). As MK-2206 treatment induced an increase of ROS in BE2 and NGP cells (Fig. 1D), we pretreated the cells with ROS inhibitor NAC before MK-2206 and rapamycin treatment to study if ROS is involved in the combination effect of MK-2206 and rapamycin. Fig. 5E showed that pretreatment with NAC blocked the enhanced inhibition of cell proliferation induced by combination of MK-2206 and rapamycin in NGP and BE2 cells, although it had much less effect or no effect on either of the individual agents. Compared to NGP and BE2 cells, NAC had much less effect in AS cells or no effect in SY5Y cells (Fig. 5E). Another ROS inhibitor α-Tocopherol showed a similar effect in NGP and BE2 cells (Fig. S2). Also an increase in ROS production was detected in the combination of MK-2206 and rapamycin compared to MK-2206 treatment alone (Fig. S3). These data indicated that the anti-cell proliferation effect induced by the combination of MK-2206 and rapamycin was mediated in large part through the generation of ROS.
6. The combination effect of MK-2206 and rapamycin on tumor growth and mice survival in vivo.
To assess the efficacy of MK-2206 and rapamycin combination against tumor growth and mice survival in vivo, mice bearing NGP tumors were first treated with rapamycin at either 2.5 or 5 mg/kg for 2 weeks to determine a dose that effectively inhibited mTOR pathway in vivo. While either the 2.5mg/kg or 5mg/kg dosing of rapamycin significantly inhibited P-S6 levels in tumor xenografts, P-Akt levels were elevated at 2.5mg/kg but not 5mg/kg dosing schedule (Fig. 5F). Thus to test the combination therapy, we treated mice with 5mg/kg rapamycin and 200mg/kg MK-2206.
There was a 76% inhibition of NGP tumor xenograft growth in the cohort receiving MK-2206+rapamycin, which is significantly higher than 40% inhibition of tumor growth in MK-2206-treated group (P=0.0001) or 56% in rapamycin-treated group (P=0.03) (Fig. 5G). The survival of mice was also significantly increased in combination group with median survival time of 44.5 days, compared to 29 days in MK-2206-treated group (P=0.002) or 37.5 days in rapamycin cohort (P=0.025) (Fig. 5H). Furthermore, we evaluated the combination effect of MK-2206 and rapamycin on P-Akt and P-S6 at end points. Although rapamycin at 5mg/kg didn’t affect P-Akt at 2 weeks (Fig 5F), a longer time treatment increased P-Akt levels (Fig. 5I). Also a longer time treatment of MK-2206 increased P-S6 level; while a combination of MK-2206 and rapamycin induced an inhibition of both P-Akt and P-S6 (Fig. 5I). These data demonstrated that combination of MK-2206 and rapamycin increased the anti-tumor growth effect and enhanced the mice survival compared to either agent alone.
Discussion:
In this study an enhanced anti-cell proliferation effect was found in combination of MK-2206 and etoposide via apoptosis, or in combination of MK-2206 with rapamycin via ROS production in vitro. In vivo, the anti-tumor growth effect and mice survival were increased when MK-2206 was combined with etoposide or rapamycin. Inhibition of Akt phosphorylation was detected in NB cells in vitro and in vivo.
MK-2206 works as an allosteric Akt inhibitor and demonstrates greater than 100-fold selectivity for Akt compared to 256 other kinases (25). So it is considered as a Akt inhibitor with high specificity compared to other Akt inhibitors (31) and few off-target effects have been reported (25). As an anti-cancer agent, inhibition of MK-2206 on tumor cell growth in vitro or in vivo has been found in adult cancers (17, 18, 22, 23). Recently, a clinical trial of MK-2206 showed stable disease in 6/33 patients with advanced solid tumors and it was well tolerated at biologically active doses that inhibit Akt signaling (25). Reducing cytotoxicity and increasing treatment efficacy is the ideal goal for tumor treatment, so rational combination of different agents is a commonly used strategy. In vitro studies found that MK-2206 has synergistic anti-tumor cell growth effect when combined with different molecules targeted inhibitors (18, 22, 23, 32), or cytotoxic agents (17). Similar results were found in in vivo studies (17, 23). Clinical trials assessing combination of MK-2206 with molecules targeted inhibitors or with chemotherapy are ongoing. A recent study in pediatric cancer patients reported the effect of MK-2206 as a single agent. ALL is the most sensitive to MK-2206, while NB is less sensitive. But the biological inhibition of Akt by MK-2206 and the combination of MK-2206 with other therapies, which is important for cancer treatment especially when one agent is not effective, were not investigated (24). In contrast, we addressed these issues in our study. To our knowledge, the evaluation of MK-2206 effect on pediatric tumors in combination with either chemotherapeutic drugs or mTOR inhibitor rapamycin was not reported before. Our data will provide evidence for clinical trials in NB patients and for evaluation of MK-2206 in other pediatric tumors that have activation of Akt.
The mechanisms underlying anti-tumor cell growth effect of MK-2206 were investigated. As MK-2206 at concentrations ≥15μM inhibited cell growth not only in NB cells but also in ARPE-19 control cells, we focused on concentrations ≤ 15μM for our analysis. Our data indicated that caspase-dependent apoptosis is not substantially involved in MK-2206 effect which is consistent with results in lung cancer cells (17). We observed ROS production in NGP and BE2 cells which was blocked by NAC and a G1 phase arrest in SY5Y and BE2 cells. Cell cycle arrest or ROS production has not been reported as mechanisms of MK-2206-induced inhibition of cell proliferation before. But they do not seem to be the common mechanisms in the 4 NB cells lines tested. So it is possible that cell type or cell line-dependent mechanisms are involved. Given the heterogeneity of NB cell lines this is not unusual. We did find that NB cell lines with p53 mutations were less sensitive to MK-2206 than those with wild type p53. In contrast, ALK mutation, MYCN amplification or 1pLOH could not distinguish NB cell lines with different sensitivity to MK-2206. This suggests that P53 mutations maybe a potential indicator of MK-2206 resistance.
Further investigation on the mechanisms underlying combination effect of MK-2206 and etoposide showed that etoposide-induced caspase-dependent apoptosis in NB cells was enhanced when combined with MK-2206, implying that NB cells with low level of activated Akt are sensitive to etoposide treatment. Similar results were found when MK-2206 was combined with EGFR inhibitor erlotinib or the dual EGFR/her2 inhibitor lapatinib (17).
In contrast to the increased apoptosis seen in combination of MK-2206 with etoposide, cell death induced by MK-2206 and rapamycin combination was blocked by pretreatment with NAC but not Z-VAD-fmk, indicating a role for generation of ROS. The ROS-mediated cell growth inhibition without changes in caspase activity has been detected in thymoquinone-treated prostate cancer cells (30). Recently, it is reported that acute increases in intracellular concentrations of ROS induced cell death in lung cancer cells via inhibiting pyruvate kinase M2 (PKM2) (33, 34), so it may possible that MK-2206 may inhibit PKM2 by inducing ROS, then inhibit glucose metabolism to induce cell growth inhibition or cell death.
In conclusion, our data provide in vitro and preclinical evidence for clinical trials of MK-2206 in combination with conventional therapy to improve treatment efficacy in patients with high-risk NB. The potential mechanisms of MK-2206 will help for selection of different combination agents.
Supplementary Material
Translational relevance:
Recently, aberrant Akt activation was identified as a novel indicator of poor prognosis in neuroblastoma (NB). In this study, we investigated whether a novel allosteric Akt inhibitor MK-2206 increased the sensitivity of NB to etoposide or rapamycin. Here we showed that in vitro, a synergistic effect was detected in combination of MK-2206 with etoposide through apoptosis, and MK-2206 enhanced the sensitivity to rapamycin via reactive oxygen species; in vivo, a significant increased anti-tumor growth effect and murine survival advantage were observed in the combination of MK-2206 with etoposide or rapamycin. This study provides the basis for the combination use of molecular targeted drug of Akt with other treatment regimens in NB or other cancers with aberrant Akt activation and has important clinical implications.
Acknowledgements:
We thank Merck & Co., Inc for providing MK-2206. We thank Dr. Bozena A Zietara (from Merck & Co., Inc) and Dr. Sherry Ansher (from Cancer Therapy Evaluation Program) for coordinating the supply of MK-2206. We thank Renee Chen and Stephen J, Chanock (Laboratory of Translational Genomics, National Cancer Institute) for genotyping cell lines used in the study. We thank the members of Cellular & Molecular Biology Section, Center for Cancer Research, National Cancer Institute, for their thoughtful review of the study.
Grant support: This work is supported by the Intramural Research Program, Center for Cancer Research, National Cancer Institute, National Institute of Health and an American Pediatric Society Student Research Program Award to Sridevi Ramalingam.
References:
- 1.Brodeur GM Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 1993;11: 1466–77. [DOI] [PubMed] [Google Scholar]
- 2.Maris JM. Recent advances in neuroblastoma. N Engl J Med 2010;362: 2202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet 2007;369: 2106–20. [DOI] [PubMed] [Google Scholar]
- 4.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010; 363:1324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Jaboin J, Dennis PA, Thiele CJ. Genetic and pharmacologic identification of Akt as a mediator of brain-derived neurotrophic factor/TrkB rescue of neuroblastoma cells from chemotherapy-induced cell death. Cancer Res 2005;65: 2070–5. [DOI] [PubMed] [Google Scholar]
- 6.Jaboin J, Kim CJ, Kaplan DR, Thiele CJ. Brain-derived neurotrophic factor activation of TrkB protects neuroblastoma cells from chemotherapy-induced apoptosis via phosphatidylinositol 3’-kinase pathway. Cancer Res 2002;62: 6756–63. [PubMed] [Google Scholar]
- 7.George RE, Sanda T, Hanna M, Frohling S, Luther W 2nd, Zhang J, et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature 2008;455: 975–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Takita J, Choi YL, Kato M, Ohira M, Sanada M, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature 2008;455: 971–4. [DOI] [PubMed] [Google Scholar]
- 9.Mosse YP A Wood, JM Maris. Inhibition of ALK signaling for cancer therapy. Clin Cancer Res 2009;15: 5609–14. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawara A, Azar CG, Scavarda NJ, Brodeur GM. Expression and function of TRK-B and BDNF in human neuroblastomas. Mol Cell Biol 1994;14: 759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellacosa A, Testa JR, Moore R, Larue L. A portrait of AKT kinases: human cancer and animal models depict a family with strong individualities. Cancer Biol Ther 2004;3: 268–75. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Tan F, Liewehr DJ, Steinberg SM, Thiele CJ. In vitro and in vivo inhibition of neuroblastoma tumor cell growth by AKT inhibitor perifosine. J Natl Cancer Inst 2010;102: 758–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z, Oh DY, Nakamura K, Thiele CJ. Perifosine-induced inhibition of akt attenuates brain-derived neurotrophic factor/TrkB-induced chemoresistance in neuroblastoma in vivo. Cancer 2011;117: 5412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiarini F, Del Sole M, Mongiorgi S, Gaboardi GC, Cappellini A, Mantovani I, et al. The novel Akt inhibitor, perifosine, induces caspase-dependent apoptosis and downregulates P-glycoprotein expression in multidrug-resistant human T-acute leukemia cells by a JNK-dependent mechanism. Leukemia 2008;22: 1106–16. [DOI] [PubMed] [Google Scholar]
- 15.van Blitterswijk WJ, Verheij M. Anticancer alkylphospholipids: mechanisms of action, cellular sensitivity and resistance, and clinical prospects. Curr Pharm Des 2008;14: 2061–74. [DOI] [PubMed] [Google Scholar]
- 16.Lindsley CW Barnett SF, Yaroschak M, Bilodeau MT, Layton ME. Recent progress in the development of ATP-competitive and allosteric Akt kinase inhibitors. Curr Top Med Chem 2007;7: 1349–63. [DOI] [PubMed] [Google Scholar]
- 17.Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther 2010;9: 1956–67. [DOI] [PubMed] [Google Scholar]
- 18.Cheng Y, Zhang Y, Zhang L, Ren X, Huber-Keener KJ, Liu X, et al. MK-2206, a novel allosteric inhibitor of Akt, synergizes with gefitinib against malignant glioma via modulating both autophagy and apoptosis. Mol Cancer Ther 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng Y, Yan L, Ren X, Yang JM. eEF-2 kinase, another meddler in the “yin and yang” of Akt-mediated cell fate? Autophagy 2011;7: 660–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balasis ME, Forinash KD, Chen YA, Fulp WJ, Coppola D, Hamilton AD, et al. Combination of farnesyltransferase and Akt inhibitors is synergistic in breast cancer cells and causes significant breast tumor regression in ErbB2 transgenic mice. Clin Cancer Res 2011;17: 2852–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knowles JA, Golden B, Yan L, Carroll WR, Helman EE, Rosenthal EL. Disruption of the AKT pathway inhibits metastasis in an orthotopic model of head and neck squamous cell carcinoma. Laryngoscope 2011;121: 2359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu R, Liu D, Trink E, Bojdani E, Ning G, Xing M. The Akt-specific inhibitor MK2206 selectively inhibits thyroid cancer cells harboring mutations that can activate the PI3K/Akt pathway. J Clin Endocrinol Metab 2011;96: E577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng J, Dai B, Fang B, Bekele BN, Bornmann WG, Sun D, et al. Combination treatment with MEK and AKT inhibitors is more effective than each drug alone in human non-small cell lung cancer in vitro and in vivo. PLoS One 2010;5: e14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorlick R, Maris JM, Houghton PJ, Lock R, Carol H, Kurmasheva RT, et al. Testing of the Akt/PKB inhibitor MK-2206 by the pediatric preclinical testing program. Pediatr Blood Cancer 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, et al. First-in-Man Clinical Trial of the Oral Pan-AKT Inhibitor MK-2206 in Patients With Advanced Solid Tumors. J Clin Oncol 2011. [DOI] [PubMed] [Google Scholar]
- 26.Goillot E, Combaret V, Ladenstein R, Baubet D, Blay JY, Philip T, et al. Tumor necrosis factor as an autocrine growth factor for neuroblastoma. Cancer Res 1992;52: 3194–200. [PubMed] [Google Scholar]
- 27.Goldschneider D, Horvilleur E, Plassa LF, Guillaud-Bataille M, Million K, Wittmer-Dupret E, et al. Expression of C-terminal deleted p53 isoforms in neuroblastoma. Nucleic Acids Res 2006;34: 5603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res 1996;62: 155–69. [DOI] [PubMed] [Google Scholar]
- 29.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 2006;58: 621–81. [DOI] [PubMed] [Google Scholar]
- 30.Koka PS, Mondal D, Schultz M, Abdel-Mageed AB, Agrawal KC. Studies on molecular mechanisms of growth inhibitory effects of thymoquinone against prostate cancer cells: role of reactive oxygen species. Exp Biol Med (Maywood) 2010;235: 751–60. [DOI] [PubMed] [Google Scholar]
- 31.Lindsley CW. The Akt/PKB family of protein kinases: a review of small molecule inhibitors and progress towards target validation: a 2009 update. Curr Top Med Chem 2010;10: 458–77. [DOI] [PubMed] [Google Scholar]
- 32.Chen KF, Chen HL, Tai WT, Feng WC, Hsu CH, Chen PJ, et al. Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. J Pharmacol Exp Ther 2011;337: 155–61. [DOI] [PubMed] [Google Scholar]
- 33.Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, et al. Inhibition of Pyruvate Kinase M2 by Reactive Oxygen Species Contributes to Antioxidant Responses. Science 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo W, Semenza GL. Pyruvate kinase M2 regulates glucose metabolism by functioning as a coactivator for hypoxia-inducible factor 1 in cancer cells. Oncotarget 2011;2: 551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.