Abstract
The rat is a favored model organism to study physiological function in vivo. This is largely due to the fact it has been used for decades and is often more comparable to corresponding human conditions (both normal and pathologic) than mice. Although the development of genetic manipulations in rats have been slower than in mice, recent advances of new genomic editing tools allows for the generation of targeted global and specific cell type mutations in different rat strains. The rat is an ideal model for advancing imaging techniques like intravital multi-photon microscopy or IVMPM. Multi-photon excitation microscopy can be applied to visualize real-time physiologic events in multiple organs including the kidney. This imaging modality can generate 4-dimensional high resolution images that are inherently confocal due to the fact that the photon density needed to excite fluorescence only occurs at the objective focal plane; not above or below. Additionally, longer excitation wavelengths allow for deeper penetration into tissue, improved excitation, and are inherently less photo-toxic than shorter excitation wavelengths. Applying imaging tools to study physiology in rats has become a valuable scientific technique due to the relatively simple surgical procedures, improved quality of reagents, and reproducibility of established assays. In this chapter, the authors provide an example of the application of fluorescent techniques to study cardio-renal functions in rat models. Use of experimental procedures described here, together with multiple available genetic modified animal models, provide new prospective for the further application of multi-photon microscopy in basic and translational research.
Keywords: intravital imaging, fluorescence microscopy, multi-photon, kidney, rat, cardio-renal, rat models, vascular, albumin
1. Introduction
Breakthroughs in biomedical fields often follow discoveries in technological advances that afford researchers opportunities to probe physiologic processes where mechanisms were previously deduced using more indirect methods. Intravital multi-photon microscopy (IVMPM), as applied to the study of renal anatomy and physiology, represents such a disruptive technology. The technique allows for the direct visualization of dynamic pathways and labeled structured in vivo at a level of spatial resolution to discriminate small, individual structures such as endosomes [1] with temporal resolution approaching real time through the use of resonant scanners and smaller viewing regions. IVMPM was first used to study neuronal calcium dynamics in a living rat by Svoboda and colleagues in 1997 [2]. The brain represents an ideal organ to study, having a lower opacity than the kidney thus minimizing photon scatter allowing for deep tissue imaging. Also, the stereotactic skull apparatus allows for greater stability and minimal affect from breathing to induce motion artifacts. In 2002 Peti-Peterdi et al. brought IVMPM technology to the renal field and utilized two-photon excitation to study the isolated, perfused juxtaglomerular apparatus [3]. Our group was the first who applied multi-photon excitation to study the intact, attached rat kidney showing three dimensional data at subcellular resolution while also capturing dynamic physiological processes in vivo [1].
The rat as a research model provides an excellent platform in which to study complex processes simultaneously in conjunction with IVMPM. Rat organ size allows for ease of use on most standard inverted microscope stages. Surgical procedures and disease models, some complex such the 5/6 nephrectomy model [4], although initially challenging can be mastered with practice. Similar procedures in mice would be more challenging and require highly specialized tools for manipulation. Furthermore, blood volumes in rats allow for infusion of more feasible quantities of fluorescent compounds than in mice, which may require the undesirable need to mix compounds to minimize the total volume delivered to avoid hypervolemia.
In this chapter, we will provide a basis for the experienced surgeon and research microscopist with which to expand their methods “toolbox” and incorporate IVMPM to augment their research. We will discuss microscope settings and the importance of keeping detector settings constant between experiments to produce consistent data that allow the best comparison even though acquisition occurs on different days. We will briefly touch upon the advantages of using multi-photon excitation over single-photon excitation for intravital studies. One important consideration relating to microscope settings is the type of data that will be collected and the subsequent analysis that will be conducted. Simplified data analysis such as morphological changes or regions a structure occupies allow for more flexibility in acquisition parameters. In contrast, intensity based comparisons demand more exacting attention be placed on acquisition settings since the microscope’s ability to consistently detect fluorescence emanating from a region of interest will vary depending on these settings. Detector gain and offset, and laser power transmissivity used for fluorophore excitation must be kept consistent. We will also cover the necessary materials needed to anesthetize the rat and perform the surgery to expose the kidney for imaging with an inverted microscope. The final sections of the chapter will expand on the list of fluorescent dyes and compounds needed to study a host of dynamic processes occurring within the renal cortex, and methods by which to quantify them. Scoring renal injury arising from disease models should be done imposing stringent parameters and careful selection of the fluorophores which is imperative to provide meaningful data. Finally utilizing a strain of rat with surface glomeruli, glomerular permeability of large and small molecular weight compounds can be determined using ratiometric intensities in the capillary loops and Bowman’s space.
The described techniques represent a small sample of those available to study and quantify kidney processes. Great strides have been made in advancing this technique since this group first used it in the descriptive publication in 2002 [1]. They have occurred through the use of novel fluorescent compounds and advances in the development of transgenic models expressing fluorescent proteins conjugates allowing visualization of morphological structures and dynamic processes. There are, however, still challenges ahead such as the limited ability of current technology to penetrate opaque tissues like the kidney. The ability to image through an entire glomerular volume using higher magnification objectives represents an elusive but tangible goal that may be realized through the use of adaptive optics which employs deformable mirrors capable of compensating for scatter associated with more opaque tissues. Newer lasers generating longer excitation wavelengths with better tissue penetration may enhance the ability to efficiently excite fluorescence from deeper regions in the kidney to minimize the scattering of excitation photons.
To summarize, IVMPM can serve as an invaluable research tool to enhance the goals of laboratories studying dynamic physiologic processes. One of the key benefits of technological advances is the ability to produce more robust hardware components, while lowering cost; making systems such as multi-photon confocal microscopes turnkey and affordable. When utilized correctly this disruptive technology can create novel insights of complex physiologic processes via their direct visualization in vivo.
2. Materials
2.1. Rat Strains
A variety of rat strains are available to study specific disease processes. The Sprague Dawley strain is the most widely used outbred rat used in research; with study applications ranging from aging to behavior and reproductive/developmental biology. The one key aspect this strain lacks with regard to the kidney is superficial glomeruli. The Frömter and Simonsen strains of Munich Wistar rats possess superficial glomeruli immediately adjacent to the surface which allow for direct visualization of renal filtration and the study of glomerular structures through the use of fluorescent dyes or transgenic animals expressing fluorescent proteins. The structural density and opacity of the kidney do not allow for the deep imaging attainable in other more transparent organs such as the brain [5,2]. A publication by Castrop et al. [6] surveyed the distance of glomeruli to the renal surface in mice, and none were found to be within range for IVMPM as with the two Munich Wistar strains of rats. Consequently, although some structural information may be visualized in mice with near surface glomeruli, photon scatter of light emanating from those focal planes equates to less robust quantitative information and images with reduced resolution.
Despite improved therapy, the increased prevalence of metabolic and physiologic disorders, such as hypertension and diabetes in the general population, makes rat models with similar diseases highly desirable to study mechanisms and test therapeutics [7]. A widely used, since the derivation in 1962, research model of salt-induced hypertension - the Dahl SS rat provides numerous phenotyping studies demonstrating the importance of the kidney in the regulation of blood pressure. The Dahl SS rat is a naturally occurring model of salt-sensitive hypertension that recapitulates many aspects of progressive human hypertension and has provided key insights into the mechanisms underlying salt-sensitivity [8,9]. Moreover, with the injection of streptozotocin, Dahl SS rats can be used as the model of diabetes-induced renal disease and display renal histological lesions characteristic of those seen in patients with diabetic nephropathy [10–12]. Our recent studies confirmed that the Dahl SS rat kidneys contain superficial glomeruli suitable for IVMPM application [13] and corresponding direct measurements of albumin filtration at the glomerulus and reuptake at the proximal tubule during the development of salt-sensitive hypertension. Importantly, this is an inbred rat strain commonly used to manipulate specific genes to test their function and recently utilized for the creation of tissue specific Podocin-Cre (glomeruli podocyte specific) line which we believe can be used for the future IVMPM studies.
2.2. Microscope settings/Renal morphology
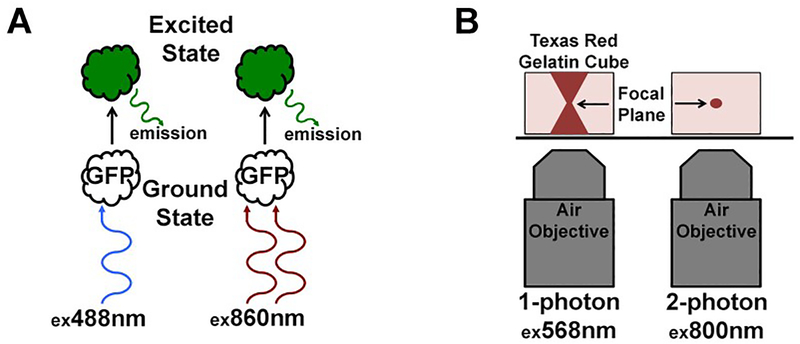
Fluorescence microscopy of thick tissue presents a unique problem in that unlike cultured cells which are relatively flat and have fluorescence emanating from a confined focal area, images from tissue also contain out of focus fluorescence from above and below the focal plane generating images with drastically reduced resolution and contrast. Confocal microscopy addresses this issue through the use of a pinhole aperture which rejects out of focus light and unfortunately, scattered light from the focal plane. This leads to a reduction in the ability to capture information from deeper regions of the tissue since scattering increases at deeper depths. Multi-photon excitation of fluorophores is performed through the use of two or three longer wavelengths, with lower energy photons, absorbed simultaneously (see Fig. 1). For example, Fluorescein, which is typically stimulated by a 488 nm photon, can also be excited by two 800 nm photons. This phenomenon of multi-photon excitation is an incredibly low probability event; occurring only at the focal plane where photon density is sufficient to assure this occurs. Extended wavelength photons passing above and below the focal plane, lacking the requisite energy to excite the fluorophore, go undetected; hence fluorescence is generated only from the focal plane. This allows for the collection of all emitted photons, which in turn produces enhanced quality and higher contrast images; see figure one for details. Three-dimensional imaging of live tissue is an application ideally suited for multi-photon imaging. Over a given volume, photo-excitation and possible photo-toxicity occur only at the focal plane with multi-photon excitation. This aids in the prevention of photo-bleaching of fluorophores above and below focal planes; allowing for the repeated collection of the same volume with reduced phototoxicity.
Fig. 1.
Multi-photon excitation of a fluorophore. The schematic in panel A shows two GFP molecules (clear, no color) in the ground state. These two fluorophores can be excited by either single photon excitation (left) or multi-photon excitation (right) at their respective peak excitation wavelengths. After absorption of the photon or photons, the fluorophore jumps to the excited state where it remains there for a duration lasting in the single digit to tens of nanoseconds. Upon decay from the excited state, a small amount of heat is given off along with a photon of lower energy than the peak single photon excitation wavelength; in this case, emission is typically around 520 nm. The schematic in panel B illustrates the extremely low probability event of multi-photon excitation. The objective and gelatin cube on the left demonstrates how single photon excitation of a fluorophore occurs at any photon density since absorption occurs at that specific wavelength, in this case, 568 nm for Texas Red. Note how the column of illumination widens above and below the focal plane, where even though the photon density drops off, excitation nonetheless occurs via single photon excitation. In contrast, the objective and cube on the right illustrate multi-photon excitation; where only the focal plane condenses the photon density to such a high degree that this extremely low probability event becomes a certainty. Above and below the focal plane where the requisite photon density is not established, excitation does not occur making the image inherently confocal.
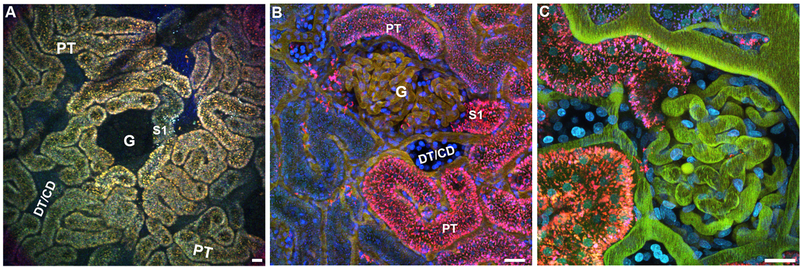
When viewing the unlabeled renal cortex through the microscope eyepieces, using standard epifluorescence, distinct tubule types and other structures can be readily distinguished (see Fig. 2A). It is recommended to equip the multi-photon microscope with a fluorescein/rhodamine dual pass cube to simultaneously view these commonly used fluorophores as well as the autofluorescence associated with the kidney. The orange/yellow autofluorescence associated in lysosomes of proximal tubules quickly distinguishes them from the surrounding distal tubules, collecting ducts and surface glomeruli (in Munich Wistar rats) which lack any lysosomal fluorescence. The peritubular vasculature appears as dark tracts surrounding the proximal tubules, distal tubules and collecting ducts which have a faint blue tint. The glomeruli in both strains of Munich Wistar rats, typically have the appearance of a circular void, are distinguishable from distal tubules and collecting ducts whose space generally is more tube-like and linear; see figure two for a detailed example and technical specs on the dual pass cube.
Fig. 2.
Autofluorescence of the renal outer cortex. Autofluorescence associated within the lysosomes of the proximal tubule epithelia help demarcate landmarks and localize other non-fluorescent structures. Panel A is a low power 20× image showing the prevalence of proximal tubule cells (PT). A glomerulus is seen in the center (G) with the corresponding S1 segment (S1). Distal tubules and collecting ducts (DT/CD) lack autofluorescence and their presence are noted by a tubular-shaped empty space. Surrounding the PTs, the peritubular microvasculature can be seen meandering between the tubules. Panels B and C represent the outer cortex having been pre-labeled 24hrs prior with a small Cascade Blue dextrans (blue) and given a Texas Red labeled and Fluorescein labeled albumin, successively; filling up the plasma. Panel B is a 40× image showing a glomerulus (G) in the center with an S1 segment adjacent, as well as a distal tubule/collecting duct (DT/CD) below. Early proximal tubule segments (PT) can be seen having taken up the filtered albumins. Due to fluorescence quenching of Fluorescein at the lower pH of the endosomes/lysosomes, the localized albumin appears mainly red/magenta. Panel C shows a 60× high magnification image with the glomerulus and open S1 segment at the center. Note the clearly delineated peritubular vasculature with flowing red blood cells. Distal tubules/collecting ducts can be identified by their brighter staining with Hoechst (cyan), and lack of albumin accumulation. A dual pass FITC/Rhodamine cube is recommended to see epifluorescence images similar to those seen in panel A. The technical specs for the cube is FITC exBP490/15, emBP560/25; Rhodamine exPB560/25, emBP605/30. (Bar= 20 μm).
The majority of data acquired in our studies is accomplished using an excitation wavelength of 800 nm. This wavelength efficiently excited many common fluorophores in the blue, green, and red spectra such as Cascade Blue, Fluorescein and Rhodamine; respectively. To produce images that closely match what is seen through the eyepieces the detector gain settings for our systems are kept at a ratio of 750/630 for the green and red emission detectors; respectively. These values may not be applicable across all systems and the operator should determine what proportions work best on their individual system. It is then important to keep those numbers consistent for day to day reproducibility; understanding that certain situations will require some deviation. In our system laser power transmissivity is kept low, between 12–15% at 800 nm. The use of longer wavelengths causes a reduction in power output from the laser and transmissivity can be increased accordingly (for more details and examples see Fig. 2).
3. Methods
The experimental procedures described below were approved by the Institutional Animal Care and Use Committee at the Indiana University School of Medicine and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
3.1. Anesthesia and Exposure of the kidney
The type of anesthesia utilized for any study should correspond to the desired length of anesthesia and whether or not recovery will be the eventual outcome after imaging. The two forms of anesthesia used in our laboratory are the long acting thiobutabarbital derivative Inactin, used in terminal studies, and isoflurane used in serial imaging studies. For detailed information see the chapter by Rhodes in the journal Methods [14].
3.2. Isoflurane
Initial induction of anesthesia occurs inside an induction chamber with manifold set at 5% with an oxygen flow rate of 0.5 L/minute.
Assure the rat is adequately sedated and carefully shave the left flank between the left arm and leg as well as the neck or inner thigh where a venous or arterial access line will be placed. It may be necessary to re-sedate the rat to assure complete shaving of the areas mentioned above.
After shaving is complete switch the anesthetic feed line from the induction chamber to the closed rodent circuit, which will provide a constant administration of isoflurane to the closed circuit (see Note 1).
Once the rat is sedated on the closed circuit, reduce the manifold to 1.5%−2% at 0.5 L/minute oxygen flow rate and proceed with the necessary surgical procedures.
3.3. Inactin
This anesthetic is made up at a concentration of 130 mg/mL in 0.9% saline and administered at a dose of ~130 mg/Kg, intraperitoneally and the surgeon must access the efficacy after about 10 minutes by toe pinch to check for reflexes; assuring complete anesthesia (see Note 2). In our recent experiences, it is advantageous to utilize isoflurane as described above in the preparation of the rat. Care must be taken to assure the rat is taken off the isoflurane circuit once the inactin takes effect. Typically it takes less than one minute, and reintroduction of isoflurane can be administered as needed.
3.4. Surgery to expose the kidney (see Note 3)
Place the rat on the right side. The shaved left flank faces up with the legs pointed to the left. Assure the rat is perfectly flat and straight with the right and left front paws touching and the rear paws touching each other. Any shift in the rat’s body plane from being perfectly flat will lead to a less than ideal initial incision.
With your thumb, fore and middle finger gently palpate to feel the kidney in the peritoneal cavity and determine the natural position therein (see Note 4).
At the center where the kidney naturally lays, carefully pick up the skin with a pair of toothed forces and pinch the skin with a pair of hemostats along the line and hold for approximately five to ten seconds; this will crush the tissue and almost wholly prevent bleeding.
Cut along the incision using a pair of surgical scissors. Crushing the outer skin and the muscle layers before cutting will dramatically reduce and typically eliminate bleeding.
Carefully repeat previous step for the thin outer muscle layer (see Note 5). Using the two handling forceps widen the incision to prepare for cutting into the inner muscle layer.
Cutting into the inner, final muscle layer will expose the peritoneum and care must be taken to assure the incision will be made in the optimal location so re-palpate to feel for the kidney and assess the size. Pinch a smaller section of the kidney with the hemostats directly over the center of the organ and make the incision; you should now see the peritoneal space. If you are using isoflurane and wish to switch over to inactin, now is the time to administer the correct dose into the peritoneal space. This initial incision was kept small since it is easier to widen the incision than suturing it if the size is too large (see Note 6).
Peering into the peritoneal cavity should reveal a white/tan collection of fat surrounding the kidney, which should have a light reddish/purple appearance, other organs in proximity such as the spleen or liver will be much darker.
Using a pair of handling forceps grab the fat and lift it close to the incision so that you can grip the region below the first pair of forceps with the second pair of forceps. The goal here is to carefully work your way down the fat in a hand-over-hand fashion until you have reached the lower pole of the kidney while nicely, but firmly gripping the fat so that it does not tear.
Once you have reached the lower pole, put down the forceps in your free hand and gently place the thumb and forefinger around the incision. In a fluid motion very gently push down an abdominal wall while delicately squeezing below the medial plane of the kidney. Gently pull the kidney out of the peritoneal cavity with the forceps (see Note 7).
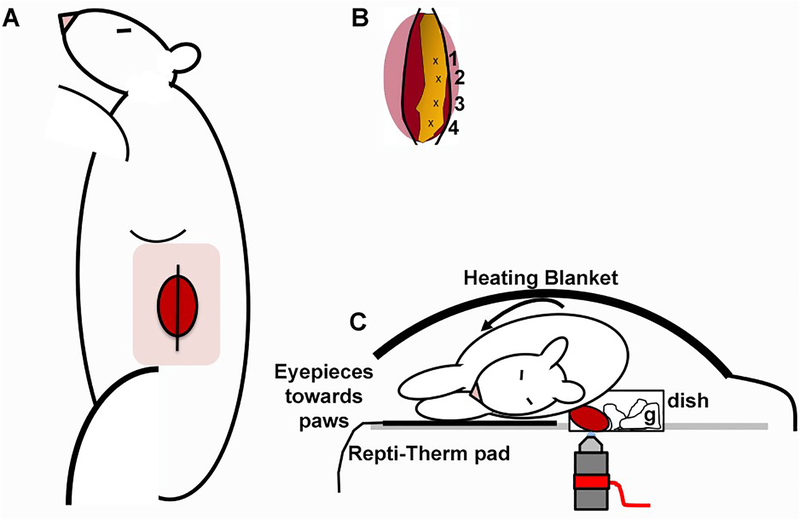
Moisten a 2×2 gauze with warmed 0.9% saline and place over the exposed kidney to keep it moist during transport and preparation of the microscope stage (for schematic details see Fig. 3). For a video on this procedure see our publication in JoVE [15].
Fig. 3.
Exteriorization of kidney and placement on the microscope stage. Panel A shows an anesthetized rat, placed with its right side down. The pink square indicates the shaved area, with the position of the kidney underneath the skin and muscle layers. Once sequential incisions are made to penetrate successive muscle layers, the kidney, and surrounding fat should appear as the schematic in panel B. Carefully grip the fat (in yellow) in positions 1–4, successively moving down in a hand-over-hand fashion until the lower pole is reached (position 4). Gently grip the side of the incision and pop the kidney out. Placement of the kidney inside the coverslip bottom dish shown in panel C, with the kidney as far forward to the edge of the dish as to minimize motion artifact. Three to four pieces of 2”x2” gauze generously moistened with warm sterile 0.9% saline and should be placed behind the kidney, to further stabilize movement and keep the organ wet.
3.5. Placing the rat on the inverted microscope stage.
Carefully transport the rat with the kidney draped in the moistened gauze to the microscope and place the organ in the 50 mm coverslip bottom dish (see Note 8). The rat will be placed with the exposed kidney inside the 50 mm dish; the head should be positioned towards the right as you face the microscope stage (see Note 9).
To assure the thorax lies far enough away from the kidney and minimize motion artifact from breathing, place the kidney up against the edge closest to you with the ventral side touching the coverslip and the dorsal rotated side up against the body. Here, the goal is to place the most considerable distance between the thorax and the kidney.
If a motion is detected while looking through the eyepiece, stretch the rat along the sagittal axis to assure it is not hunched up (see Note 10).
3.6. Synthesizing Fluorescent Albumin
The application of IVMPM to an initial study to follow renal handling of fluorescent albumin lead to the observation that a far greater amount is filtered across the glomerular capillary loops [10,16]. IVMPM can also be used to quantify the amount of albumin that is efficiently reclaimed from the urinary space by the proximal tubules [17]. Previous methods used to study renal albumin handling such as fractional clearance [18] and micropuncture have reported very low values [19]. In the case of micropuncture, sampling of the filtered fluid is done further downstream of the glomerulus along the nephron; while fractional clearance samples concentrations of albumin in the plasma and urine. Both of these techniques, however, do not account for the avid uptake of albumin that occurs in the S1 segment immediately adjacent to the glomerulus, which we have shown in numerous studies [10,16,15,20,17]. This would deplete the detectable level of albumin collected from tubule segments more distal to the S1 or the urine, producing a permeability value that is artificially low. Here, fluorescent albumin will be used to demonstrate quantitative analysis of glomerular permeability and uptake by proximal tubules within the same set of images.
Dissolve 96 mg of Rat Serum Albumin (RSA) in 6.4 mL of 100 mM Sodium Bicarbonate pH 8.3; final concentration 15 mg/ml in a 15 ml conical tube.
The reaction will be carried out at room temperature.
Add 100 μL of high quality anhydrous Dimethyl Formamide (DMF) to a 2 mg vial of Texas Red-X- Succinimidyl Ester, single isomer (TR-X); vortex on medium for 15 sec.
While vortexing the uncapped 15 mL conical with the RSA solution on medium (vortex at a speed that keeps the solution from spilling out), add the 100uL solution of DMF with the dissolved TR-X.
Cap the 15 mL conical tube, cover with in foil (Parafilm can be wrapped around the cap to assure a tight seal), and place on a rocker at low speed to gently agitate the reaction for 1 Hr at room temperature.
Add 45 grams of NaCl to 5 L of ddH20 to make a significant volume of normal saline to remove unconjugated TR-X fluorophore via dialysis. Wet/reconstitute per instructions a 50 kDa molecular weight cut off membrane of choice; sufficient to hold the ~7 mL of a reaction solution.
Carefully inject/pipette the reaction into the dialysis membrane/chamber and place in the 5 L container of normal saline, dialyze overnight at 4°C with gentle agitation using a stir bar.
Change the dialysis solution with 5 more liters of normal saline in the morning and again in the afternoon with a 5 more liters of normal saline and allow to dialyze again overnight; all dialysis should occur at 4°C.
In the morning, the final 5 L volume should be clear of any free TR-X. Carefully remove the solution from the dialysis membrane/chamber and measure the volume. Divide the initial 96 mg of RSA by the volume to give you concentration; which will be approximately 12 mg/mL due to swelling (see Note 11).
3.7. Glomerular Permeability.
Using a lower powered objective, such as a 20× water immersion (NA 0.7), prior to infusion of TR-RSA, scan the kidney surface in a raster pattern and mark the positions where glomeruli are suspected to be present in a Munich Wistar rat of either strain (see Note 12).
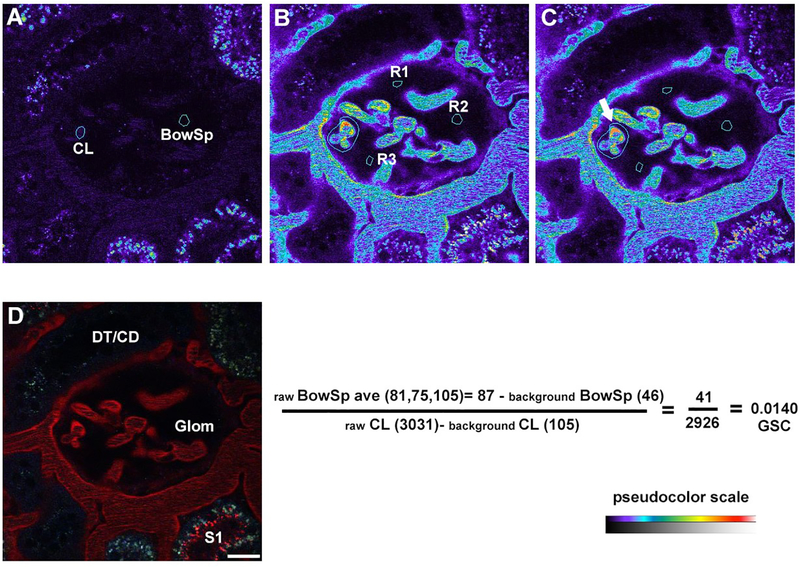
Acquire a 3D volume of the glomeruli before delivery of TR-RSA to be used as a background subtraction image during quantitation (see Fig. 4). Carefully adjust the detector offset or black level (see Note 13). The Bowman’s space in the background images should have a few random pixels displaying values of zero (typically less than 10 or so total pixels) when the offset/black level is correctly set (see Note 14).
For the infusion of TR-RSA, a glomerulus showing capillary loops or a larger superficial vessel can be selected to monitor plasma concentration, be sure to use a LUT or palette that will inform you if saturation occurs. Set the desired number of frames to collect for the infusion time series, 50–200 frames is typical. In one hand take the syringe containing the TR-RSA, connected to an indwelling IV line, it is best to anchor it by resting it near the eyepieces or some other stable feature of the microscope. Take the free hand and start a timer or stopwatch to mark the start of the albumin infusion, click the microscope software to start acquiring the time series and finally start the slow infusion of the TR-RSA. You will notice there will be fluctuations in plasma intensities characterized by peaks as material is pushed in, followed by ebbs as systemic vascular distribution occurs. In total, approximately 3.0–5.0 mg of TR-RSA will be infused (see Note 15).
Wait approximately 5 to 10 minutes before starting the collection of images from the list of glomeruli marked in step 1. This will assure complete distribution of the TR-RSA. To collect images, focus on the area approximately 5 to 15 μm below the Bowman’s capsule where a few capillary loops are visible, surrounded by an abundant amount of empty Bowman’s space; taking either a single image or a 3D volume of the glomerulus (see Note 16).
Save and transfer the images for analysis using image processing software such as Metamorph (see Fig. 4–6) or Image J (see Fig. 7); the analysis below will be conducted using Metamorph.
To analyze the images, match the focal plane of the background image taken before TR-RSA infusion to the corresponding image after infusion (see Note 17). In your background image, draw a region of interest closely around one the capillary loops (which will be very faint) and a small region in the Bowman’s space away from the Bowman’s capsule and capillary loop and note the average intensity value for each. For the image with circulating TR-RSA, select three regions in the Bowman’s space and mark the average of the three average intensity values. For the capillary loop select the one with the brightest intensity and draw a region to loosely encompass all of the brightest region. Use the threshold tool to select the very bright plasma intensities along the capillary loop edge, avoiding the red blood cells appearing as streaks, or the regions between the streaks (see Note 18).
-
The glomerular sieving coefficient (GSC) is calculated below using the average intensity values obtained above (see Note 19):Using GSC values obtained by IVMPM and known GFR values for the experimental rat model total filtered albumin can be determined. By determining PT uptake total 24 hours urinary albumin can be calculated by following equation (for more details see [17]):
Fig. 4.
Glomerular permeability in Munich Wistar Fömter rats. Calculating the degree to which a fluorescence compound can filter across the glomerular capillary loops, glomerular sieving coefficient (GSC), is done by rationing the intensity in the Bowman’s space to fluorescence within the capillary loops. Panel A shows a background image with a glomerulus in the center and two outlined regions, the capillary loops (CL) and Bowman’s space (BowSp) selected to determine background fluorescence. In panel B, an image taken approximately 15 minutes after infusion of Texas Red rat serum albumin (TR-RSA) shows the albumin filling the plasma along a large vessel surrounding the glomerulus and capillary loops. Three regions (R1-R3) have been selected within the Bowman’s space, and the fluorescence values noted. Panel C shows the same image, highlighting the brightest capillary loops (arrow), with thresholding applied to select regions within the plasma to note the fluorescence intensity. The formula below panels B and C show the calculation to determine the GSC for TR-RSA (for this particular glomerulus is 0.014). Panel D shows a color image of the same region, note the S1 segment (lower right) with an accumulation of filtered TR-RSA at the apical surface. (Bar = 20 μm).
Fig. 6.
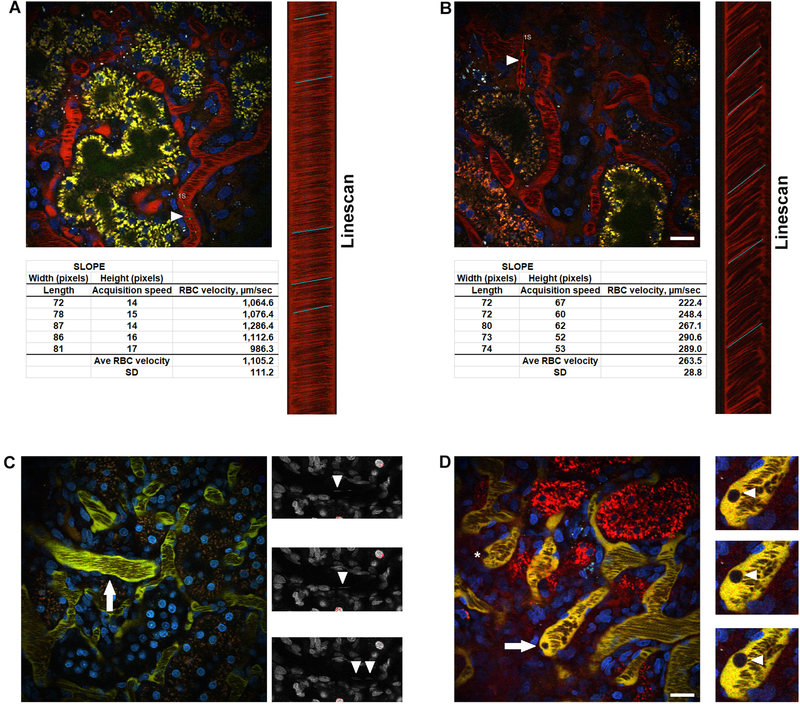
Determining red blood cell (RBCs) velocity utilizing the linescan function and quantifying white blood cell (WBCs) dynamics. The velocity of RBCs can be determined by utilizing the motion artifact generated whereas the RBCs appear as streaks within peritubular and glomerular capillary loops. The slope of the streak is dependent on speed; RBCs with higher velocities will have shallower slopes while cells with slower velocities will have steeper slopes. Panel A shows the reference image from an untreated rat with a highlighted vessel (arrowhead, “1S”) and the corresponding linescan to the right. The linescan corresponds to 1,000 scans placed on top of each other to form a column generating the slopes associated with the flowing RBCs. In the linescan, the Y-axis related to time while the X-axis relates to distance. Five slopes are drawn along the column to generate an average RBC velocity for the vessel. In panel B, RBC velocity is appreciably slower following ischemia and reperfusion. The highlighted vessel (arrowhead, “1S”) and associated linescan show steeper slopes and slower RBC velocity. Panel C shows a photomicrograph of an untreated rat with a series of images of the large vessel (arrow). Hoechst 33342 stains the nuclei of WBCs, which produce streaks within the vessels seen here (arrowheads) in the blue channel lacking any vascular marker to highlight the streaks better. Counting the number of streaks and factoring in the length of time the temporal acquisition occurred gives a rate of occurrence per minute. In panel D, activated WBCs can adhere to the inner vessel wall and show no movement during a time series (asterisk). Activated WBCs can also roll along the vessel wall. The highlighted WBC in panel D (arrow and cropped regions, arrowheads) show the motion of a WBC as it rolls along the vessel wall. (Bar= 20 μm).
Fig. 7.
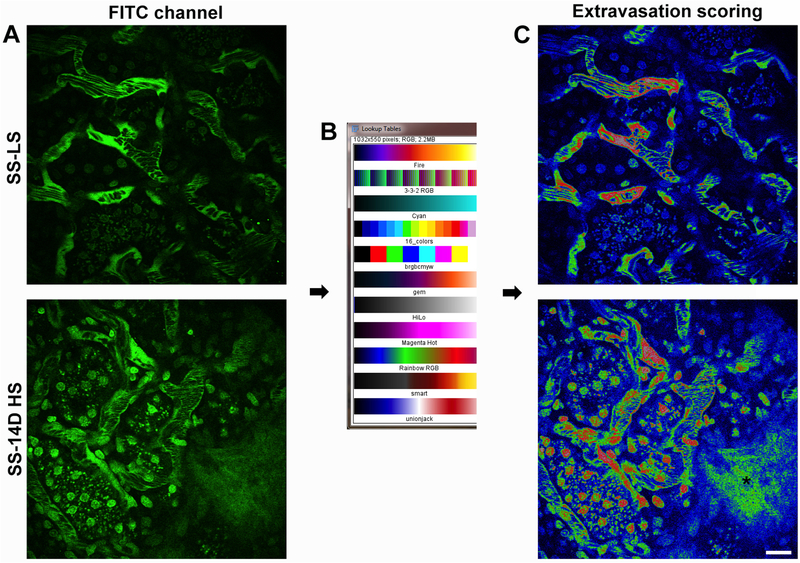
Quantifying vascular permeability in Dahl salt sensitive rats. Extravasation of large compounds normally retained within the vasculature into the interstitial space can be quantified using semi-quantitative scoring. Photomicrographs were given a score from 0–3, where increasing values indicated more severe damage. A score of Zero indicates an image was having no leakage of the large molecular weight compound into the interstitial space. A score of one showed some leakage into the interstitial space, while a score of two indicated leakage into the majority of the interstitial space within an image. A score of three was reserved for the most severe damage, where the intensity of the compound within the interstitial space was very near or equal the intensity of the plasma. In panel A, a photomicrograph of the kidney cortex from the salt sensitive Dahl S rats on a low salt (SS LS, upper image) and after 14D on high salt (8% NaCl; SS 14D HS, lower image). Note the lack of fluorescence in areas adjacent to the peritubular microvasculature as compared to the 14D high salt image below showing a high degree of extravasation at the lower left. Panel B shows the “Rainbow RGB look up table (LUT)” available using ImageJ software (National Institute of Health; 1.47v) and the corresponding images in panel A now displayed using the LUT in panel C. This LUT display allows for better visualization of subtle differences in intensities that are not readily discernible in images using single color or B/W display LUTs. (Bar= 20 μm).
3.8. Proximal tubule endocytosis
Accumulation of fluorescent albumin or other compounds via endocytosis, when studied over a protracted period, can lead to fluorescence saturation within the endosomal pool, in particular, the lysosomes. To correct for this, collect a series of identical background reference images at different laser power transmissivity (see Note 20). It is also essential to design the experiment beforehand to plan for the collection of images at the appropriate time points after delivery of the fluorescent probes.
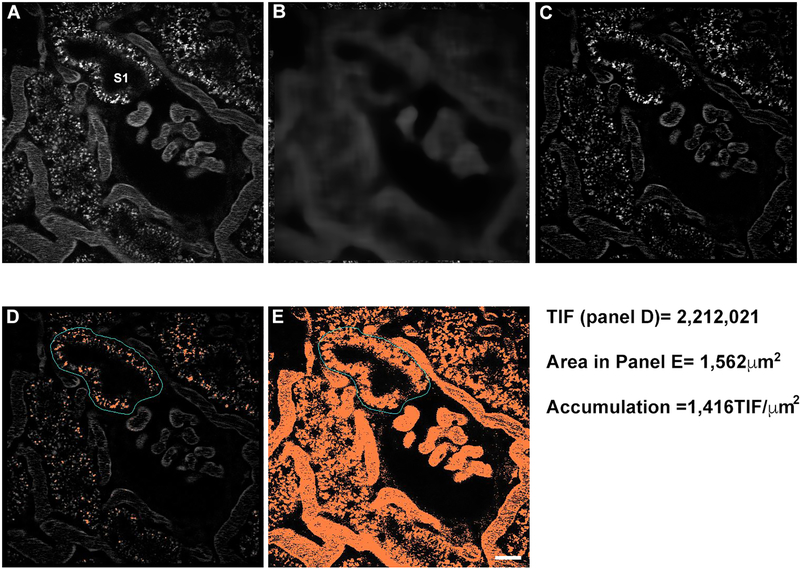
Select an image to analyze and apply a 32×32 (pixel width × pixel height) Median filter with the sub-sample ratio set to 1; this will be used as a background image (see Fig. 5).
Take the original image and subtract out the background image (see Fig. 5A–C).
Draw a region around the proximal tubule you wish to analyze (see Fig. 5D); it can be done by tracing around the basolateral side of the proximal tubule (see Note 21).
Threshold the image to select the bright endosomes/lysosomes and mark the total integrated fluorescence (TIF, expressed as fluorescence units FU) (see Fig. 5E), which is the product of the total number of pixels × of the average intensity (see Note 22).
Metamorph software allows for the transfer of the outline of a drawn region of interest (ROI) from one image to another in the corresponding position; transfer the ROI from the background subtracted image to the original.
In the original image, a threshold the proximal tubule inside the ROI until all of the area occupied by the proximal tubule is highlighted, not just the endosomes/lysosomes (see Note 23). Make a note of the total number of pixels in the thresholded region, not the entire region.
Multiply the number of pixels by size dimensions associated with the microscope (see Note 24).
Proximal tubule accumulation is therefore calculated by taking the TIF/area with the value expressed as TIF/μm2. This method is preferred since it normalizes uptake regardless of the size of the region analyzed (for more details see Fig. 5).
Fig. 5.
Quantifying uptake of albumin in proximal tubules. Measuring uptake of albumin or any fluorescent compound can be used to gauge temporal accumulation, derive a rate or stratify differences between untreated or disease models. This process starts by applying to a 32 × 32, subset = 1 median filter to generate a background image (panel B) from the original (panel A). The background image is subtracted from the original image (A-B) to generate a clean background corrected image (C). For this study, a region of interest (cyan) was around the S1 segment of the proximal tubule, and the remaining albumin signal was thresholded to encompass of the associated pixels (in orange). The total integrated fluorescence (TIF) for this S1 segment (the product of average fluorescence intensity × number of highlighted pixels) = 2,212,021. Since this value can vary widely based on the area highlighted, it is essential to normalize this value to the area such as μm2. The region of interest from panel D is transferred to the original images (panel A), and the region is thresholded to encompass all of the proximal tubule area (shown in panel E, orange). The total number of pixels in panel E = 9,114. The area of an individual pixel is determined by multiplying the X/Y pixel size (0.414 μm × 0.414 μm) to arrive at 0.171 μm2. Multiplying the number of pixels (9,114) by the area (0.171) = 1,562 μm2. The value for this image region = 1,416 TIF/μm2. (Bar= 20 μm).
3.9. Renal blood flow dynamics
Injury, either related to ischemia, exposure to a toxin, or arising from progression associated with a disease model, is often characterized by alteration in blood flow dynamics within the peritubular microvasculature or glomerular capillary loops; and on occasion the degradation of vascular integrity. Through the use of a large molecular weight dextran (more than 150 kDa) which is retained in the bloodstream, the velocity of red blood cells (RBC’s) can be calculated by exploiting a motion artifact that occurs during image acquisition [21]. The typical multi-photon microscope uses galvo (galvanometer-based) scanner to direct the laser across the sample in a raster pattern to generate an image. The velocity of RBC’s traveling across a peritubular vessel is far higher than the acquisition speed of the microscope; this causes the RBC’s to appear as thin, slanted streaks within the blood vessels. The slope of the streaks is dependent on the speed at which the RBC’s travel across the blood vessel; a shallow slope indicates a rapid RBC velocity while a steeper slope is associated with a slower speed. Changes in RBC velocity are not the only changes in vascular dynamics that can occur in injury and disease models, often white blood cells (WBC’s) become activated leading to localized obstruction of vascular flow [22]. This phenomenon can also be quantified when analyzing a time series of the blood flow and looking for WBC whose nuclei are labeled with Hoechst 33342. The results from these data stratify the WBC’s as freely flowing, rolling or adhered based on motion. Of note, the type of WBC’s cannot be determined through the simple use of a nuclear dye; this would require the use of specific markers or transgenic rats expressing fluorescent proteins in certain WBC populations.
Infuse in approximately 300 μg of Hoechst 33342 to label the systemic nuclei including WBC’s (see Note 25).
After placing the rat on the microscope, slowly infuse in approximately 2.0–5.0 mg of a large 150 kDa dextran (see Note 26).
Red blood cell velocity will be measured on the microscope using the “linescan” setting (see Fig. 6). Briefly, this allows the operator to draw a line down the middle of the blood vessel parallel to the length and adjust the settings to acquire 1,000 lines (see Note 27).
In Metamorph, open the dialog box “Show Region Statistics” along with any linescan image acquired. Using the line tool, draw a line across the linescan across the slope of either the RBC or the plasma in between to best approximate that slope and note the Width and Height values which are displayed in pixels.
-
An excel spreadsheet can be set up to calculate RBC velocity using the following formula:Example:
As previously mentioned the nuclei of WBC’s will stain with Hoechst 33342; to study WBC dynamics disengage the “linescan” function and return to full frame acquisition mode on the microscope, typically 512 × 512 pixels. Set up a time series to capture at least 30 frames (see Note 28), and select a focal plane where the majority of blood vessels are shown in cross section and acquire the time series.
Flowing WBC’s are the most difficult to quantify since they appear as faint streaks inside the microvascular tracts surrounding the tubular epithelia; to aid in counting break up the image into four 256 × 256 quadrants and count the nuclear streaks in all 30 frames for each quadrant and total the tally (see Note 29).
This data can be presented as occurrences per minute after multiplying by the appropriate time factor (for a detailed practical application of protocol 3.9 see Fig. 6).
3.10. Vascular permeability
Assessing vascular dynamics such as RBC velocity and WBC adhesion produces values based on more direct physical parameters captured by the multi-photon microscope; there is little room for error in the determination of the slope of the slant in an image caused by a flowing RBC’s or the visualization of adhered WBC’s obstructing renal blood flow. Scoring, as a quantitative method may initially seem a more arbitrary way of assessing an observed abnormality in physiologic function or morphologic perturbation, however as long as stringent parameters are defined and followed it can produce a tangible method of quantifying an observable alteration. Changes in vascular permeability present a unique challenge in the way in which they can be quantified. These alterations are rarely global in nature and to compound the problem a suitable marker or markers to define these alterations must be carefully selected. In this section, we present a method for scoring vascular permeability that relies on not a single marker but two markers to address the heterogeneity in severity [13,23]. We utilize Texas Red labeled albumin to localize moderate alterations in vascular permeability. The red emitting fluorophore is used because it is downstream of any possible bleed through emissions and will likely be the marker with the highest amount of extravasation into the interstitial space. A large narrowly dispersed 150 kDa Fluorescein dextran is used to localize severe perturbations in vascular integrity (see Note 30).
Using the motorized stage function on your microscope select at least 10 random fields to study. Infuse in 2.0–5.0 mg of a 150 kDa fluorescein dextran and approximately 3.0 to 5.0 mg of TR-RSA (see Note 31).
Acquire images at five, fifteen and thirty minutes post infusion of the selected fields. Since we are quantifying the interstitial space between the vasculature and tubular epithelia, a glancing focal plane just below the surface will provide the largest interstitial area to study.
Permeability is assessed based on a score between 0 and 3 with increasing numbers corresponding to greater vascular leak. Zero indicates no leakage of material into the interstitial space. A score of one indicates a small amount of the material in the interstitial space in a small region of the image. Two indicates material has leaked into the interstitial space throughout a majority of the region. A score of three means enough material has leaked into the interstitial space where the fluorescence intensity closely matches that in the circulating plasma. This indicates severe damage, particularly if this occurs with the large molecular weight dextran (for a detailed practical application of protocol 3.10 see Fig. 7).
4. Notes
Anesthesia must be safely maintained as a closed circuit to provide scavenging of excess isoflurane and to prevent inhalation by laboratory personnel.
On occasion, an unintended delivery of the anesthetic to the guts can significantly increase the time it takes for the drug to take effect or in some cases, re-dosing may be required. In some instances, this can lead to a slow, unintentional overdose on the microscope stage characterized by a reduction in renal physiologic parameters, such as reduced peritubular blood flow and collapsing of the tubular epithelial lumen which will be evident in the acquired images.
It is advantageous to use male rats at approximately 8–12 weeks of age. Older males will have more substantial fat deposits that will make step 9 (protocol 3.4) difficult; females regardless of age will have much more fat surrounding the smaller kidneys with the ovaries attached to the lower pole.
Until you become familiar and comfortable with the procedure, use a sharpie to draw a line down the body from the ribcage to the upper thigh.
The rat may reflexively respond to pinching of the inner muscle layers (this is normal); a toe pinch with toothed forceps will confirm anesthesia is still useful.
Making the incision too close to the ventral side will induce motion artifact from breathing while making it too close to the dorsal side will cause a reduction or cessation of renal blood flow.
Do not force it! If the incision seems too small and the kidney will not “pop out” widen the incision by no more than a few millimeters at a time and try again. With practice, you will learn the incision should be approximately 75% the length of the kidney to prevent it from receding into the peritoneal space once externalized.
For these studies a 50 mm cell culture dish with a 40 mm coverslip bottom allows the largest area for visualization and should be used. Inverted microscope stage that accommodate 50 mm dishes inserts are commercially available.
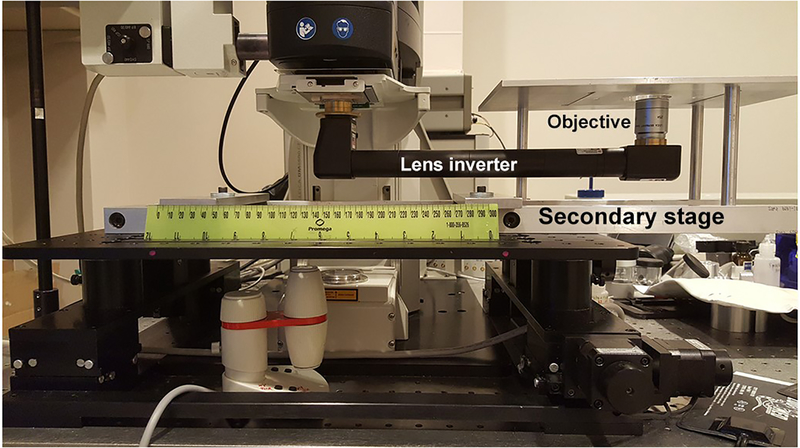
See Fig. 8 for a description of an objective inverter to change the objective orientation on an upright platform.
Try aligning the paws so that the two front and two rear paws touch. If motion persists, roll the rat very slightly, so the ventral/abdominal side is closer to the microscope stage insert.
The reaction with succinimidyl Ester should give a 1:1 dye:protein ratio. In a typical experiment, approximately 4 mg of TR-RSA is used per rat. A single conjugation can provide enough material for over 20 individual tests. For long term storage it is best to lyophilize into individual tubes containing 5–10 mg each.
Most current microscope systems have a motorized stage that allows the operator to mark locations for computerized relocation. If unavailable a rudimentary map can be drawn marking positions in relation to landmarks on the surface such as large blood vessels or fat pockets.
Setting detector sensitivity value incorrectly can cause the detector to collect lower intensity values, especially in regions with lower fluorescence intensities such as the Bowman’s space. Use a look up table (LUT, see Fig. 7B) or palette on the microscope images that informs you if your settings fall above or below the dynamic range. Typically saturated values appear as Red and pixels with values of zero appear either blue or green.
If this region is displaying a large percentage of pixels at zero, the detector will fail to acquire photons efficiently from these regions resulting in an artificially lower permeability value; see our study characterizing this phenomenon [24]. This will result in a low and inaccurate sensitivity.
Introduce the TR-RSA slowly to avoid saturation, should slight saturation occur, it will likely clear once the serial image collection starts approximately 10 minutes later. If saturation persists the experiment is over, lowering the laser power transmissivity to get plasma levels below saturation will invalidate the background images taken at the higher laser transmissivity.
Analysis is much easier at more shallow depths because images taken further into the glomerulus suffer from a greater amount of photon scatter and have decreased resolution and contrast.
For the visualization of TR-RSA it will be easier to use a pseudocolor LUT images; especially in the background.
Thresholded pixels should appear in a different highlight color than the surrounding dimmer pixels. When recording the average intensity value for the capillary loops plasma, make sure you are selecting the average intensity for the thresholded pixels and not the entire region; see figure four for details.
The approach can be used to measure the GSC of fluorescent compounds having a wide range of molecular weights. However, compounds that are freely, or nearly freely filtered should be studied under a continuous IV infusion to maintain constant intensity values in the plasma. The widely fluctuating intensities within the capillary loops produced via a single bolus infusion would produce inaccurate values.
For example, if the initial microscope transmissivity is 15%, once a field is selected showing proximal tubule autofluorescence, take identical images at 15%, 13%, 11% and 9% transmissivity. Generate a correction factor by making the average fluorescence intensity for a small region of the image; keep the region identical for all the images. Next, divide the average intensity of the higher transmissivity image by the average intensity of the lower one; this will give a positive integer greater than one. The obtained correction factor that will be applied when comparing the lower transmissivity images to the higher one to correctly scale up the values.
For the analyses of proximal tubule endocytosis it is not necessary to circle inside the lumen to locate the apical surface since we will threshold the endosomes to determine the integrated fluorescence therein (see Fig 5D).
During threshold procedure be sure you are selecting the data from the thresholded values and only from the region of interest, not the entire image.
The goal is to determine the total area associated with the proximal tubule the TIF file was derived from. During the selection try to highlight the tubule and not the tubular lumen if the proximal tubule is in cross-section.
For example, Olympus FLUOVIEW FV1000, when using a 60× water immersion objective, has pixel dimensions of 0.414 μm × 0.414 μm =0.171 μm2.
Hoechst infusion can be done on the bench after surgery to expose the kidney and prior to placement on the microscope stage.
Similarly to fluorescent albumin injection (described at 3.7), use caution to avoid detector saturation in the plasma. Although, in this case laser transmissivity can be reduced, since current measurement procedures are not intensity based.
Linescan acquisition mode requires complete stability of the rat on the stage to produce a suitable image for analysis. The microscope will scan the length of the line drawn across the vessel 1,000 times, building a tall column as the image builds line-by-line with distance represented in the X-axis and time in the Y-axis (see Fig. 6A, B).
In current configuration at the Olympus imaging system the acquisition rate is approximately 1 frame per second.
Rolling WBC will move along the wall of the peritubular vasculature for at least 4 frames if subsequent dislodging occurs. Cells are counted as being adherent if they stay in place without moving for at least 4 frames if they become dislodged during the time series.
Since 150 kDa Fluorescein dextran produces fluorescent emissions “upstream” from Texas Red, it will not confound determination of moderate alterations of permeability. Moreover, it is essential to understand the nature of the compounds used. Large molecular weight dextrans, despite having a large listed size average, can be a product of large and small sized polymers around the average mean. This means unless a narrowly dispersed or purified compound is used, leakage of the low molecular weight components into the interstitial space will produce erroneous data suggesting severe damage where none exists.
Both compounds (TR-RSA and 150 kDa fluorescein dextran) can be introduced in rapid succession to avoid saturation of one of the compounds. Systemic distribution should occur within five minutes.
Fig. 8.
The example of reconfiguration of upright microscope to the inverted platform by using a commercially available lens inverter (LSM tech). A secondary stage was custom made to anchor the new inverted stage to the automated table equipped initially with the upright multiphoton Leica SP-8 system.
Acknowledgment
The authors acknowledge grant support from the National Institutes of Health (NIH) (DK091623 and DK079312), the Veterans Administration through a Merit Review award (to B.A.M.), and the American Heart Association 17SDG33660149 (to OP).
References
- 1.Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ, Bacallao RL, Molitoris BA (2002) Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am J Physiol Cell Physiol 283 (3):C905–916. doi: 10.1152/ajpcell.00159.2002 [DOI] [PubMed] [Google Scholar]
- 2.Svoboda K, Denk W, Kleinfeld D, Tank DW (1997) In vivo dendritic calcium dynamics in neocortical pyramidal neurons. Nature 385 (6612):161–165. doi: 10.1038/385161a0 [DOI] [PubMed] [Google Scholar]
- 3.Peti-Peterdi J, Morishima S, Bell PD, Okada Y (2002) Two-photon excitation fluorescence imaging of the living juxtaglomerular apparatus. Am J Physiol Renal Physiol 283 (1):F197–201. doi: 10.1152/ajprenal.00356.2001 [DOI] [PubMed] [Google Scholar]
- 4.Ferrell N, Sandoval RM, Bian A, Campos-Bilderback SB, Molitoris BA, Fissell WH (2015) Shear stress is normalized in glomerular capillaries following (5/6) nephrectomy. Am J Physiol Renal Physiol 308 (6):F588–593. doi: 10.1152/ajprenal.00290.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleinfeld D, Mitra PP, Helmchen F, Denk W (1998) Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc Natl Acad Sci U S A 95 (26):15741–15746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiessl IM, Bardehle S, Castrop H (2013) Superficial nephrons in BALB/c and C57BL/6 mice facilitate in vivo multiphoton microscopy of the kidney. PLoS One 8 (1):e52499. doi: 10.1371/journal.pone.0052499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chobanian AV (2009) Shattuck Lecture. The hypertension paradox--more uncontrolled disease despite improved therapy. N Engl J Med 361 (9):878–887. doi: 10.1056/NEJMsa0903829 [DOI] [PubMed] [Google Scholar]
- 8.Cowley AW Jr. (1997) Genetic and nongenetic determinants of salt sensitivity and blood pressure. Am J Clin Nutr 65 (2 Suppl):587S–593S [DOI] [PubMed] [Google Scholar]
- 9.Mattson DL, Dwinell MR, Greene AS, Kwitek AE, Roman RJ, Jacob HJ, Cowley AW Jr. (2008) Chromosome substitution reveals the genetic basis of Dahl salt-sensitive hypertension and renal disease. Am J Physiol Renal Physiol 295 (3):F837–842. doi: 10.1152/ajprenal.90341.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russo LM, Sandoval RM, Campos SB, Molitoris BA, Comper WD, Brown D (2009) Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol 20 (3):489–494. doi: 10.1681/ASN.2008050503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilatovskaya DV, Levchenko V, Lowing A, Shuyskiy LS, Palygin O, Staruschenko A (2015) Podocyte injury in diabetic nephropathy: implications of angiotensin II-dependent activation of TRPC channels. Sci Rep 5:17637. doi: 10.1038/srep17637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slaughter TN, Paige A, Spires D, Kojima N, Kyle PB, Garrett MR, Roman RJ, Williams JM (2013) Characterization of the development of renal injury in Type-1 diabetic Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 305 (7):R727–734. doi: 10.1152/ajpregu.00382.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endres BT, Sandoval RM, Rhodes GJ, Campos-Bilderback SB, Kamocka MM, McDermott-Roe C, Staruschenko A, Molitoris BA, Geurts AM, Palygin O (2017) Intravital imaging of the kidney in a rat model of salt-sensitive hypertension. Am J Physiol Renal Physiol 313 (2):F163–F173. doi: 10.1152/ajprenal.00466.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhodes GJ (2017) Surgical preparation of rats and mice for intravital microscopic imaging of abdominal organs. Methods 128:129–138. doi: 10.1016/j.ymeth.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandoval RM, Molitoris BA (2013) Quantifying glomerular permeability of fluorescent macromolecules using 2-photon microscopy in Munich Wistar rats. J Vis Exp (74). doi: 10.3791/50052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD (2007) The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int 71 (6):504–513. doi: 10.1038/sj.ki.5002041 [DOI] [PubMed] [Google Scholar]
- 17.Wagner MC, Campos-Bilderback SB, Chowdhury M, Flores B, Lai X, Myslinski J, Pandit S, Sandoval RM, Wean SE, Wei Y, Satlin LM, Wiggins RC, Witzmann FA, Molitoris BA (2016) Proximal Tubules Have the Capacity to Regulate Uptake of Albumin. J Am Soc Nephrol 27 (2):482–494. doi: 10.1681/ASN.2014111107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asgeirsson D, Venturoli D, Rippe B, Rippe C (2006) Increased glomerular permeability to negatively charged Ficoll relative to neutral Ficoll in rats. Am J Physiol Renal Physiol 291 (5):F1083–1089. doi: 10.1152/ajprenal.00488.2005 [DOI] [PubMed] [Google Scholar]
- 19.Tojo A, Endou H (1992) Intrarenal handling of proteins in rats using fractional micropuncture technique. Am J Physiol 263 (4 Pt 2):F601–606. doi: 10.1152/ajprenal.1992.263.4.F601 [DOI] [PubMed] [Google Scholar]
- 20.Sandoval RM, Wagner MC, Patel M, Campos-Bilderback SB, Rhodes GJ, Wang E, Wean SE, Clendenon SS, Molitoris BA (2012) Multiple factors influence glomerular albumin permeability in rats. J Am Soc Nephrol 23 (3):447–457. doi: 10.1681/ASN.2011070666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto T, Tada T, Brodsky SV, Tanaka H, Noiri E, Kajiya F, Goligorsky MS (2002) Intravital videomicroscopy of peritubular capillaries in renal ischemia. Am J Physiol Renal Physiol 282 (6):F1150–1155. doi: 10.1152/ajprenal.00310.2001 [DOI] [PubMed] [Google Scholar]
- 22.Sharfuddin AA, Sandoval RM, Berg DT, McDougal GE, Campos SB, Phillips CL, Jones BE, Gupta A, Grinnell BW, Molitoris BA (2009) Soluble thrombomodulin protects ischemic kidneys. J Am Soc Nephrol 20 (3):524–534. doi: 10.1681/ASN.2008060593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCurley A, Alimperti S, Campos-Bilderback SB, Sandoval RM, Calvino JE, Reynolds TL, Quigley C, Mugford JW, Polacheck WJ, Gomez IG, Dovey J, Marsh G, Huang A, Qian F, Weinreb PH, Dolinski BM, Moore S, Duffield JS, Chen CS, Molitoris BA, Violette SM, Crackower MA (2017) Inhibition of alphavbeta5 Integrin Attenuates Vascular Permeability and Protects against Renal Ischemia-Reperfusion Injury. J Am Soc Nephrol 28 (6):1741–1752. doi: 10.1681/ASN.2016020200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandoval RM, Wang E, Molitoris BA (2014) Finding the bottom and using it: Offsets and sensitivity in the detection of low intensity values in vivo with 2-photon microscopy. Intravital 2 (1). doi: 10.4161/intv.23674 [DOI] [PMC free article] [PubMed] [Google Scholar]