Abstract
Psoriasis patients experience chronic systemic skin inflammation and develop cardiovascular comorbidities that shorten their lifespan. Whether cardiovascular disease is improved by treatment with current biologics that target disease-specific pathways is unclear. KC-Tie2 mice develop psoriasiform skin inflammation with increases in IL-23 and IL-17A and proinflammatory monocytosis and neutrophilia that precedes development of carotid artery thrombus formation. To examine whether targeted blockade of IL-23 or IL-17A in KC-Tie2 pso- riasis mice improves cardiovascular outcomes, mice were treated systemically for 6 weeks with antibodies targeting IL-17A, IL-17RA, IL-12/23p40, or IL-23p19. Skin inflammation; thrombosis clotting times; and percentage of splenic monocytes, neutrophils, and CD4 T cells were examined. Skin inflammation significantly improved in KC-Tie2 mice treated with each of the antibodies targeting IL-23, IL-17A, or IL-17RA, consistent with clinical efficacy observed in psoriasis patients. The time to occlusive thrombus formation lengthened in these mice and correlated with attenuated acanthosis. This decrease in skin inflammation paralleled decreases in splenic neutrophils (CD11b+Ly6G+) but not monocytes (CD11b+Ly6Chigh) or T cells (CD4+). Our data show that targeted inhibition of IL-23 or IL-17A improves psoriasis-like skin disease and also improves cardiovascular disease in mice.
INTRODUCTION
Chronic autoimmune diseases can affect specific organs including the skin (e.g., psoriasis) and joints (e.g., rheumatoid arthritis and spondyloarthritis). Epidemiological and clinical evidence suggests that patients with poorly controlled in- flammatory disease within peripheral tissues develop persis- tent systemic inflammation and have an increased risk of developing and dying from cardiovascular disease (CVD). This increased risk cannot be fully explained by traditional cardiovascular risk factors (for reviews Szekanecz et al., 2016; Yim and Armstrong, 2017), and the underlying mechanisms remain unclear. It is likely that tissue-derived soluble factors drive systemic inflammation and directly affect distant vessels and the development of athero- thrombosis. Treatment of the primary disease (i.e., psoriasis or rheumatoid arthritis) may improve CVD risk and reduce cardiovascular events.
Clinical studies examining a reduction in the number of cardiovascular events and/or increased lifespan after treat- ment of the primary disease are ongoing but currently limited because of the time required to observe a longitudinal result. Attempts to circumvent this limitation include shorter dura- tion studies that examine surrogate measures of CVD. These include coronary artery calcium scores (Santilli et al., 2016), vascular inflammation (18-fluorodeoxyglucose positron emission tomography-computed tomography [FDG-PET-CT]) (Naik et al., 2015), flow-mediated dilation, intima media thickness measures (Di Minno et al., 2015; Fang et al., 2016), and traditional serum risk factors (i.e., plasma lipids, C-reactive protein, IL-6, resistin, myeloperoxidase) (Cao et al., 2013) in patient populations, both before and after biologic treatment (Mazzoccoli et al., 2010; Piaserico et al., 2016; Pina et al., 2016; Ramonda et al., 2014).
A preclinical animal model that mimics human disease and develops similar CVD comorbidities could provide rapid insight into these clinically important questions. The KC-Tie2 mouse is a keratinocyte-specific, Tie2-overexpressing psori- asis model that recapitulates key aspects of human psoriasis including elevated systemic and cutaneous expression of IL-23 and IL-17A (Wolfram et al., 2009). These mice respond to treatment strategies that are successful in psoriasis patients (Wang et al., 2016; Wolfram et al., 2009) and similarly do not respond to unsuccessful human psoriasis treatment ap- proaches (Wang et al., 2016; Ward et al., 2015). KC-Tie2 mice develop CVD comorbidities subsequent to systemic inflammation, including monocytosis and neutrophilia, and are susceptible to thrombosis in an experimental occlusive thrombosis bioassay (Wang et al., 2012, 2016). Clinical studies have shown that targeting the critical psoriasis signature cytokines IL-23 or IL-17A using biologics leads to remarkable skin disease resolution (for review, see Ritchlin and Krueger, 2016). However, it still remains unclear how the inhibition of these cytokines affects cardiovascular events and/or risk in these patients.
RESULTS AND DISCUSSION
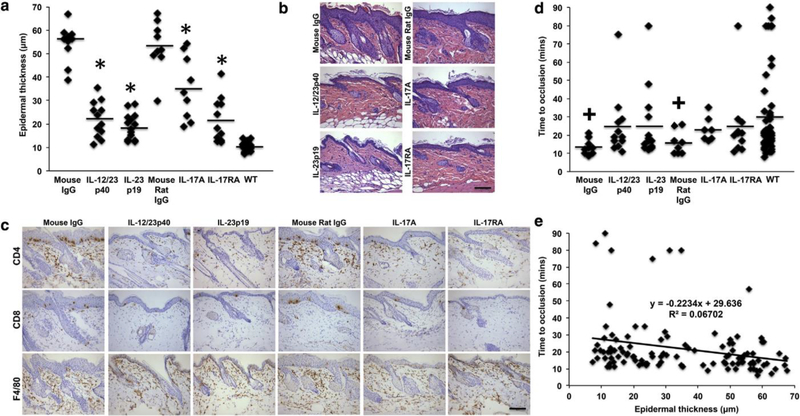
KC-Tie2 animals with established skin disease were treated weekly (for 6 weeks) with antibodies targeting IL-12/23p40, IL-23p19, IL-17A, or IL-17RA (or isotype IgG) to determine whether targeted blockade of IL-23 or IL-17A improved skin phenotype and lengthened thrombosis clotting times. Acan- thosis (epidermal thickness) and cutaneous CD4+ T cell numbers significantly decreased in KC-Tie2 mice treated with antibodies versus isotype IgG (acanthosis: F(5) = 32.99, P < 0.0001; CD4+ T cell number: F(5) = 9.588, P < 0.0001). Post hoc analyses showed that treatment with antibodies targeting IL-12/23p40, IL-23p19, and IL-17RA resulted in the greatest improvement in acanthosis (P < 0.0001 vs. isotype IgG and P < 0.002 vs. anti-IL-17A), followed by IL-17A in- hibition (P < 0.01 vs. isotype IgG) (Figure 1a and b). Simi- larly, cutaneous infiltration of CD4+ T cells decreased across all treatment groups (Figure 1c) (all P < 0.01 vs. isotype IgG), whereas infiltration of CD8+ T cells decreased in all groups except IL-17RA (Figure 1c) (P < 0.05). F4/80+ macrophages did not decrease significantly in any group relative to isotype IgG-treated mice (Figure 1c). The decrease in acanthosis with concomitant improvement in skin inflammation mirrors psoriasis patient clinical responsiveness to current biologics, including ustekinumab (IL-12/23p40 [Lebwohl et al., 2010]); guselkumab, tildrakizumab, and risankizumab (IL-23p19 [Amin et al., 2017; Blauvelt et al., 2017a; Papp et al., 2017; Reich et al., 2017]); ixekizumab and secukinumab (IL-17A [Gordon et al., 2016; Langley et al., 2014]); and brodalumab (IL-17RA [Blauvelt et al., 2017b]), and validates the KC-Tie2 mouse as a predictive preclinical model that mimics clinical patient response to psoriasis treatment.
Figure 1.
Skin inflammation and thrombosis clotting times improve in KC-Tie2 mice treated with antibodies targeting IL-12, IL-23, IL-17A, and IL-17RA.
(a) Quantification of epidermal thickness (μm) of hematoxylin and eosin-stained dorsal skin sections of KC-Tie2 mice treated with isotype IgG (n = 10, mouse; n = 9, mouse/rat) or antibodies targeting IL-12p40 (n = 12), IL-23p19 (n = 14), IL-17A (n = 9), or IL-17RA (n = 11) and control littermates (WT, n = 17). Representative images of (b) dorsal skin from each group stained with hematoxylin and eosin and (c) CD4 (T cell), CD8 (T cell), and F4/80 (macrophage).
(d) Occlusion times (minutes) after rose bengal induced photochemical injury of the carotid artery in KC-Tie2 mice treated with isotype IgG (n = 10, mouse; n = 8, mouse/rat) or antibodies targeting IL-12p40 (n = 12), IL-23p19 (n = 14), IL-17A (n = 7), or IL-17RA (n = 11) and control littermates (WT, n = 50).
(e) Correlation between acanthosis and clotting times for all animals. Each symbol represents an individual mouse. Solid straight lines indicate the mean for each group. Data were analyzed using a Fisher least significant difference test. *P < 0.05 versus isotype IgG in a, +P < 0.05 versus control littermates (WT) in d. Scale bars in b and c = 100 μm. WT, wild type.
We previously showed that repression of skin inflammation in the KC-Tie2 model using a tetracycline-repressible genetic approach resulted in decreases in acanthosis, IL-12, IL-23, and IL-17A (Wolfram et al., 2009) and lengthened thrombosis clotting times (Wang et al., 2012). Thus, we predicted that targeted blockade of IL-12/23 or IL-17A would also improve cardiovascular outcomes. KC-Tie2 mice treated with non- blocking isotype IgGs clotted more quickly than control an- imals (P < 0.05 vs. mouse IgG; P = 0.059 vs. mouse/rat IgG), confirming prior findings (Wang et al., 2012, 2016). KC-Tie2 mice treated with antibodies targeting IL-23 or IL-17A/IL- 17RA (Figure 1d) showed lengthened times to occlusive thrombus formation and no longer differed from wild-type controls. Furthermore, correlation analyses showed an in- verse correlation between acanthosis and clotting times (Figure 1e) (R2 = 0.067), suggesting that skin inflammation, using acanthosis as a surrogate measure, correlates with shortened clotting times. These findings show that improving psoriasis-like skin inflammation in mice by directly targeting IL-23 or IL-17A eliminates the susceptibility to thrombosis. These findings suggest that current biologic approaches targeting IL-23 or IL-17A used to treat cutaneous inflamma- tion in psoriasis patients may lower the risk of future car- diovascular events.
Although treatment of psoriasis patients with biologics is on the rise, these measures are generally reserved for pa- tients with moderate to severe disease. Therefore, a large subset of patients may not have optimal control of their skin inflammation. The impact of unrestrained skin inflammation versus treatment with biologics and the risk of cardiovas- cular events, vascular inflammation, and atherogenesis are topics of high interest in the fields of dermatology and cardiology.
Prospective studies are critical to ascertain major adverse cardiac event outcomes for patients currently taking biologics that target IL-23, IL-12/23, IL-17A, and IL-17RA. Thus far, there are no short- or long-term major adverse cardiac event signals for ustekinumab (Papp et al., 2015), and there are no short-term major adverse cardiac event signals for either anti- IL-17A drugs, brodalumab, or anti-p19 drugs. Ongoing studies are examining effects of secukinumab on surrogate biomarkers of CVD (ClinicalTrials.gov NCT02559622) and vascular inflammation using FDG-PET-CT (ClinicalTrials.gov NCT0269070). Furthermore, current randomized clinical trials are prospectively studying the impact of IL-12/23 blockade on vascular inflammation using FDG-PET-CT (ClinicalTrials.gov identifier NCT02187172). Additional studies of extended duration are needed to further assess the impact, either negative or positive, of these drugs on cardiovascular comorbidities.
Preclinical data are also unclear regarding the pro- versus anti-atherogenic effects of IL-17A (for review, see Taleb et al., 2015). One report showed that IL-17A inhibition destabilizes established atherosclerotic plaque, potentially leading to thrombotic events (Gistera et al., 2013). Another showed that low serum IL-17A levels were associated with an increased risk of cardiovascular events in patients with acute myocar- dial infarction (Simon et al., 2013). Others have described distant vessel endothelial dysfunction, increases in systolic blood pressure, and a shorter lifespan in mice overexpressing IL-17A under the keratin 14 promoter (Karbach et al., 2014). In our study, mice do not have existent atherosclerosis, sug- gesting that inhibition of the IL-17 pathway (directly or indirectly) may improve risk, likely as a result of controlling persistent skin-derived inflammation. These studies suggest that aggressive treatment of the primary disease (i.e., psori- asis) will decrease circulating IL-17A levels and confer pro- tection in cases where atherosclerosis is absent. Translating these findings may require psoriasis patient stratification based on atherosclerosis burden before initiating treatment with IL-17A inhibitors.
Tumor necrosis factor (TNF)-α inhibition is the most common biologic approach and has historically served as the standard-of-care treatment for psoriasis patients. The literature has suggested that TNF-α inhibition decreases the relative risk of CVD in patients (Mazzoccoli et al., 2010; Piaserico et al., 2016; Pina et al., 2016). However, a recent double-blind, placebo-controlled psoriasis trial of adalimu- mab reported a lack of effect on vascular inflammation in the ascending aorta and carotid arteries as assessed using FDG- PET-CT in psoriasis patients (Bissonnette et al., 2017); whether this reflects sensitivity or variability in the method- ology for measuring vascular inflammation remains unclear (Lensen et al., 2017). Other studies provide additional sup- port that TNF-α inhibition significantly reduces myocardial infarct risk and incidence (Wu and Poon, 2014; Wu et al., 2012). Because of a lack of murine-specific anti-TNF-α an- tibodies, we did not examine thrombosis susceptibility after TNF-α inhibition in KC-Tie2 mice; however, cutaneous TNF- α transcript levels decreased in the skin of all treated groups, except mice treated with anti-IL-17A (data not shown).
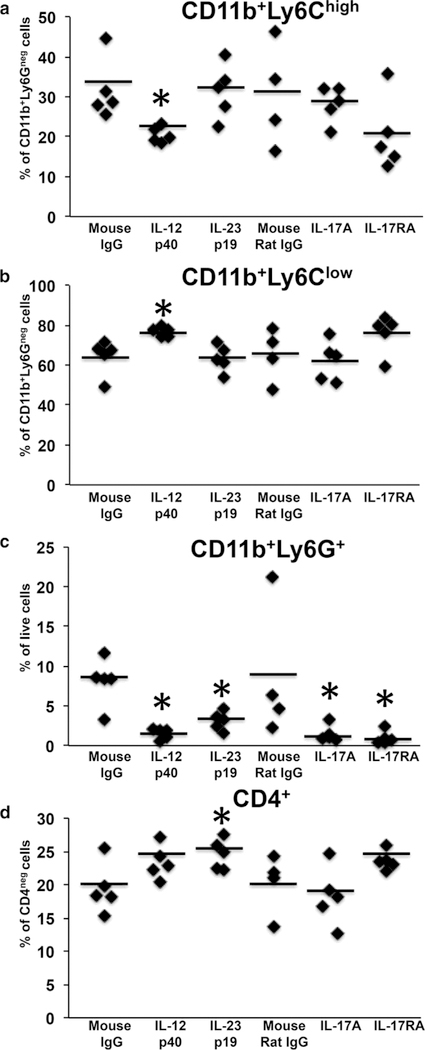
We previously showed that elevated circulating proin- flammatory monocytes and neutrophils precede shortened times to occlusive thrombus formation in KC-Tie2 mice (Wang et al., 2012). In the current study, splenic immune cell populations were examined for expression of CD11b, Ly6C, Ly6G, and CD4 after blockade of IL-12/23, IL-17A, or IL-17RA (Figure 2). CD11b+Ly6G+ neutrophils decreased significantly in each antibody treated KC-Tie2 group versus isotype IgG (Figure 2c) (F(5) = 4.75, P < 0.005). Anti-IL-12p40 was the only treatment to have a significant effect on the monocyte populations, such that CD11b+Ly6Chigh monocytes decreased concomitantly with a reciprocal increase in CD11b+Ly6Clow monocytes (Figure 2a and b), suggesting a role for IL-12p40 in both monocyte and neutrophil regulation. CD4+ T cells increased significantly only in mice treated with anti-IL- 23p19 antibodies (Figure 2d) (P < 0.05). These results suggest that decreases in circulating neutrophils correspond better than monocytes or T cells with lengthening of carotid artery thrombosis clotting times and confirm recent reports showing similar decreases in neutrophils in mice protected from thrombosis (Wang et al., 2016). These findings are also consistent with reports showing that psoriasis patients have elevated levels of circulating neutrophils that correlate with vascular inflammation (Naik et al., 2015).
Figure 2.
Neutrophils decrease significantly in KC-Tie2 mice after IL-12/23, IL- 17A, and IL-17RA blockade. (a) CD11b+Ly6Chigh monocytes, (b) CD11b+Ly6Clow monocytes, (c) CD11b+Ly6G+ neutrophils, and (d) CD4+ T cells from spleens of KC-Tie2 mice treated with isotype IgG (n = 5, mouse; n = 4, mouse rat), anti-IL- 12p40 (n = 5), anti-IL-23p19 (n = 5), anti-IL-17A (n = 5), or anti-IL-17RA (n = 5). Each symbol represents an individual mouse. Solid straight lines indicate the mean for each group. Data were analyzed using a Fishers least significant difference test. *P < 0.05 versus isotype IgG.
The role of neutrophils in thrombosis may reflect neutrophil interactions with platelets and potentially with neutrophil extracellular traps (NETs) (Mocsai, 2013). Chronic exposure to inflammation can extend the normal lifespan of a neutro- phil in circulation, which can in turn promote inflammatory events at distant sites (Mantovani et al., 2011). During thrombus formation, neutrophils are one of the first cell types to adhere to the site of vessel wall injury, which precedes platelet activation (Darbousset et al., 2012). Neutrophil- derived NETs also promote coagulation during thrombosis (Massberg et al., 2010), likely triggered via platelet activation (Fuchs et al., 2010). Our prior work showed parallel decreases in neutrophils and platelets in mice protected from thrombosis (Wang et al., 2016), and others have shown that neutrophil depletion reduces thrombosis (Darbousset et al., 2012). IL-17A has previously been shown to induce NETosis (Khandpur et al., 2013), activate platelets (Maione et al., 2011), and contribute to thrombus formation (de Boer et al., 2013). Thus, functional inhibition of the neutrophil activa- tion pathway, either directly by blocking IL-17A/17RA or indirectly by suppression of IL-23, may lead to decreases in NETosis-specific initiators and/or neutrophil-platelet interactions. These interactions are critical for thrombus formation and, as a result, lengthen the time required to form a clot in the experimental bioassay used in this study.
In summary, we show that functional blockade of IL-23 or IL-17A in an established mouse model of psoriasis improves skin inflammation, decreases the number of circulating neutrophils, and lengthens thrombosis clotting times, sug- gesting that targeting cytokines that mediate psoriatic inflammation may improve cardiovascular comorbidities.
METHODS
Mice
K5tTA and TetosTie2 mouse engineering, genotyping, and generation of double transgenic KC-Tie2 mice have been previously described (Wolfram et al., 2009). Transgenic mice inheriting a single, non- expressing gene (K5tTA or TetosTie2) or wild-type littermates served as controls.
Six-week-old male and female KC-Tie2 mice with established skin disease were treated systemically (intraperitoneally) once per week for a 6-week period with antibodies targeting IL-12/23p40 (10 mg/ kg), IL-23p19 (2 mg/kg), or mouse IgG isotype (10 mg/kg) for these two antibodies or with antibodies targeting either IL-17A (10 mg/kg) or IL-17RA (20 mg/kg) or mouse/rat IgG isotype control for these two antibodies (at 20 mg/kg). Dosing was determined and antibodies were generously provided by Kristine Kikly (Eli Lilly, Indianapolis, IN). Antibody specificity has been extensively characterized inter- nally at Eli Lilly and previously shown in a murine spondyloarthritis model (Benham et al., 2014).
Murine model of carotid arterial thrombosis
The carotid arterial thrombosis assay was completed as previously described (Wang et al., 2012). Time to occlusion was identified as the time point at which blood flow ceased for 10 minutes.
Tissue collection, histology, and immunostaining analyses
Skin was collected and processed for hematoxylin and eosin his- tology and CD4+, CD8+, and F4/80+ immunohistochemistry, with epidermal thickness and immune cell quantification performed as previously described (Wolfram et al., 2009).
Flow cytometry
Spleen cells from a separate cohort of mice that did not undergo the thrombosis assay were isolated and stained for analysis by flow cytometry as described previously (Wang et al., 2016) (see Supplementary Figure S1 online). Monocytes were classified as expressing CD11b+Ly6GlowLy6Chigh or CD11b+Ly6GlowLy6Clow, whereas neutrophils expressed CD11b+Ly6GhighLy6Clow. T cells were classified as expressing CD4+.
Statistics
Comparisons between groups for all outcomes were performed using a one-way analysis of variance. Simple effects were probed with a Fisher least significant difference test, with a focus on planned comparisons of interest (i.e., antibodies vs. control isotype IgG). Probability values less than 0.05 were considered significant.
Study approval
Animal protocols were consistent with guidelines issued by the American Association for Accreditation of Laboratory Animal Care and were approved by the Case Western Reserve University Insti- tutional Animal Care and Use Committee.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by The Lozick Discovery Grant and the National Psoriasis Foundation, the Murdough Family Center for Psoriasis, the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (P30 AR39750, P50 AR055508, R01 AR063437, R01 AR062546, R21 AR063852, R01 AR069071, T32AR007569), and the National Institute for Heart, Lung, and Blood of the National Institutes of Health (R01 HL057506).
Abbreviations
- CVD
cardiovascular disease
- FDG-PET-CT
18-fluorodeoxyglucose positron emission tomography-computed -tomography
- NET
neutrophil extracellular trap
- TNF
tumor necrosis factor
Footnotes
CONFLICT OF INTEREST
Kristy Kikly is an employee of Eli Lilly. The other authors state no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at https://doi.org/10.1016/j.jid.2017.09.021.
REFERENCES
- Amin M, Darji K, No DJ, Wu JJ. Review of phase III trial data on IL-23 In- hibitors Tildrakizumab and Guselkumab for psoriasis [e-pub ahead of print]. J Eur Acad Dermatol Venereol 2017;31:1627–32. [DOI] [PubMed] [Google Scholar]
- Benham H, Rehaume LM, Hasnain SZ, Velasco J, Baillet AC, Ruutu M, et al. Interleukin-23 mediates the intestinal response to microbial beta-1,3- glucan and the development of spondyloarthritis pathology in SKG mice. Arthritis Rheumatol 2014;66:1755–67. [DOI] [PubMed] [Google Scholar]
- Bissonnette R, Harel F, Krueger JG, Guertin MC, Chabot-Blanchet M, Gonzalez J, et al. TNF-alpha antagonist and vascular inflammation in patients with psoriasis vulgaris: a randomized placebo-controlled study. J Invest Dermatol 2017;137:1638–45. [DOI] [PubMed] [Google Scholar]
- Blauvelt A, Papp KA, Griffiths CE, Randazzo B, Wasfi Y, Shen YK, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol 2017a;76:405–17. [DOI] [PubMed] [Google Scholar]
- Blauvelt A, Papp KA, Lebwohl MG, Green LJ, Hsu SC, Bhatt V, et al. Rapid onset of action in patients with moderate-to-severe psoriasis treated with brodalumab: a pooled analysis of data from two phase 3 randomized clinical trials (AMAGINE-2 and AMAGINE-3). J Am Acad Dermatol 2017b;77:372–4. [DOI] [PubMed] [Google Scholar]
- Cao LY, Soler DC, Debanne SM, Grozdev I, Rodriguez ME, Feig RL, et al. Psoriasis and cardiovascular risk factors: increased serum myeloperoxidase and corresponding immunocellular overexpression by Cd11b(+) CD68(+) macrophages in skin lesions. Am J Transl Res 2013;6:16–27. [PMC free article] [PubMed] [Google Scholar]
- Darbousset R, Thomas GM, Mezouar S, Frere C, Bonier R, Mackman N, et al. Tissue factor-positive neutrophils bind to injured endothelial wall and initiate thrombus formation. Blood 2012;120:2133–43. [DOI] [PubMed] [Google Scholar]
- de Boer OJ, Li X, Teeling P, Mackaay C, Ploegmakers HJ, van der Loos CM, et al. Neutrophils, neutrophil extracellular traps and interleukin-17 asso- ciate with the organisation of thrombi in acute myocardial infarction. Thromb Haemost 2013;109:290–7. [DOI] [PubMed] [Google Scholar]
- Di Minno MN, Ambrosino P, Lupoli R, Di Minno A, Tasso M, Peluso R, et al. Cardiovascular risk markers in patients with psoriatic arthritis: A meta- analysis of literature studies. Ann Med 2015;47:346–53. [DOI] [PubMed] [Google Scholar]
- Fang N, Jiang M, Fan Y. Association between psoriasis and subclinical atherosclerosis: a meta-analysis. Medicine (Baltimore) 2016;95(20):e3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA 2010;107:15880–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gistera A, Robertson AK, Andersson J, Ketelhuth DF, Ovchinnikova O, Nilsson SK, et al. Transforming growth factor-beta signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin- 17-dependent pathway. Sci Transl Med 2013;5(196):196ra00. [DOI] [PubMed] [Google Scholar]
- Gordon KB, Blauvelt A, Papp KA, Langley RG, Luger T, Ohtsuki M, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med 2016;375:345–56. [DOI] [PubMed] [Google Scholar]
- Karbach S, Croxford AL, Oelze M, Schuler R, Minwegen D, Wegner J, et al. Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler Thromb Vasc Biol 2014;34:2658–68. [DOI] [PubMed] [Google Scholar]
- Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med 2013;5(178):178ra40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med 2014;371:326–38. [DOI] [PubMed] [Google Scholar]
- Lebwohl M, Papp K, Han C, Schenkel B, Yeilding N, Wang Y, et al. Ustekinumab improves health-related quality of life in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial. Br J Dermatol 2010;162:137–46. [DOI] [PubMed] [Google Scholar]
- Lensen KDF, van Sijl AM, Voskuyl AE, van der Laken CJ, Heymans MW, Comans EFI, et al. Variability in quantitative analysis of atherosclerotic pla- que inflammation using 18F-FDG PET/CT. PLoS One 2017;12(8):e0181847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione F, Cicala C, Liverani E, Mascolo N, Perretti M, D’Acquisto F. IL-17A increases ADP-induced platelet aggregation. Biophys Biochem Res Comm 2011;408:658–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 2011;11:519–31. [DOI] [PubMed] [Google Scholar]
- Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med 2010;16:887–96. [DOI] [PubMed] [Google Scholar]
- Mazzoccoli G, Notarsanto I, de Pinto GD, Dagostino MP, De Cata A, D’Alessandro G, et al. Anti-tumor necrosis factor-alpha therapy and changes of flow-mediated vasodilatation in psoriatic and rheumatoid arthritis patients. Intern Emerg Med 2010;5:495–500. [DOI] [PubMed] [Google Scholar]
- Mocsai A Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med 2013;210:1283–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik HB, Natarajan B, Stansky E, Ahlman MA, Teague H, Salahuddin T, et al. Severity of Psoriasis Associates With Aortic Vascular Inflammation Detec- ted by FDG PET/CT and Neutrophil Activation in a Prospective Observa- tional Study. Arterioscler Thromb Vasc Biol 2015;35:2667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp K, Gottlieb AB, Naldi L, Pariser D, Ho V, Goyal K, et al. Safety aur- veillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol 2015;14:706–14. [PubMed] [Google Scholar]
- Papp KA, Blauvelt A, Bukhalo M, Gooderham M, Krueger JG, Lacour JP, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med 2017;376:1551–60. [DOI] [PubMed] [Google Scholar]
- Piaserico S, Osto E, Famoso G, Zanetti I, Gregori D, Poretto A, et al. Treatment with tumor necrosis factor inhibitors restores coronary microvascular function in young patients with severe psoriasis. Atherosclerosis 2016;251:25–30. [DOI] [PubMed] [Google Scholar]
- Pina T, Corrales A, Lopez-Mejias R, Armesto S, Gonzalez-Lopez MA, Gomez- Acebo I, et al. Anti-tumor necrosis factor-alpha therapy improves endo- thelial function and arterial stiffness in patients with moderate to severe psoriasis: A 6-month prospective study. J Dermatol 2016;43:1267–72. [DOI] [PubMed] [Google Scholar]
- Ramonda R, Puato M, Punzi L, Rattazzi M, Zanon M, Balbi G, et al. Atherosclerosis progression in psoriatic arthritis patients despite the treat- ment with tumor necrosis factor-alpha blockers: a two-year prospective observational study. Joint Bone Spine 2014;81:421–5. [DOI] [PubMed] [Google Scholar]
- Reich K, Papp KA, Blauvelt A, Tyring SK, Sinclair R, Thaci D, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet 2017;390(10091):276–88. [DOI] [PubMed] [Google Scholar]
- Ritchlin CT, Krueger JG. New therapies for psoriasis and psoriatic arthritis. Curr Opin Rheumatol 2016;28:204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santilli S, Kast DR, Grozdev I, Cao L, Feig RL, Golden JB, et al. Visualization of atherosclerosis as detected by coronary artery calcium and carotid intima-media thickness reveals significant atherosclerosis in a cross- sectional study of psoriasis patients in a tertiary care center. J Transl Med 2016;14:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon T, Taleb S, Danchin N, Laurans L, Rousseau B, Cattan S, et al. Circulating levels of interleukin-17 and cardiovascular outcomes in patients with acute myocardial infarction. Eur Heart J 2013;34: 570–7. [DOI] [PubMed] [Google Scholar]
- Szekanecz Z, Kerekes G, Vegh E, Kardos Z, Barath Z, Tamasi L, et al. Autoimmune atherosclerosis in 3D: How it develops, how to diagnose and what to do. Autoimmun Rev 2016;15:756–69. [DOI] [PubMed] [Google Scholar]
- Taleb S, Tedgui A, Mallat Z. IL-17 and Th17 cells in atherosclerosis: subtle and contextual roles. Arterioscler Thromb Vasc Biol 2015;35:258–64. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gao H, Loyd CM, Fu W, Diaconu D, Liu S, et al. Chronic skin- specific inflammation promotes vascular inflammation and thrombosis. J Invest Dermatol 2012;132:2067–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Golden JB, Fritz Y, Zhang X, Diaconu D, Camhi MI, et al. Interleukin 6 regulates psoriasiform inflammation-associated thrombosis. JCI Insight 2016;1(20):e89384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NL, Bhagathavula N, Johnston A, Dawes SM, Fu W, Lambert S, et al. Erlotinib-induced skin inflammation is IL-1 mediated in KC-Tie2 mice and human skin organ culture. J Invest Dermatol 2015;135:910–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfram JA, Diaconu D, Hatala DA, Rastegar J, Knutsen DA, Lowther A, et al. Keratinocyte but not endothelial cell specific overexpression of Tie2 leads to the development of psoriasis. Am J Pathol 2009;174:1443–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JJ, Poon KY. Tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis, psoriatic arthritis, or both. J Drugs Dermatol 2014;13:932–4. [PubMed] [Google Scholar]
- Wu JJ, Poon KY, Channual JC, Shen AY. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol 2012;148:1244–50. [DOI] [PubMed] [Google Scholar]
- Yim KM, Armstrong AW. Updates on cardiovascular comorbidities associated with psoriatic diseases: epidemiology and mechanisms. Rheumatol Int 2017;37:97–105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.