Abstract
One of the major classes of pesticides is that of the organophosphates (OPs). Initial developments date back almost 2 centuries but it was only in the mid-1940s that OPs reached a prominent status as insecticides, a status that, albeit declining, is still ongoing. OPs are highly toxic to nontarget species including humans, the primary effects being an acute cholinergic toxicity (responsible for thousands of poisoning each year) and a delayed polyneuropathy. Several issues of current debate and investigation on the toxicology of OPs are discussed in this brief review. These include (1) possible additional targets of OPs, (2) OPs as developmental neurotoxicants, (3) OPs and neurodegenerative diseases, (4) OPs and the “aerotoxic syndrome,” (5) OPs and the microbiome, and (6) OPs and cancer. Some of these issues have been debated and studied for some time, while others are newer, suggesting that the study of the toxicology of OPs will remain an important scientific and public health issue for years to come.
Keywords: organophosphates, agents, acetylcholinesterase, neurotoxicity, pesticides, neurotoxicology
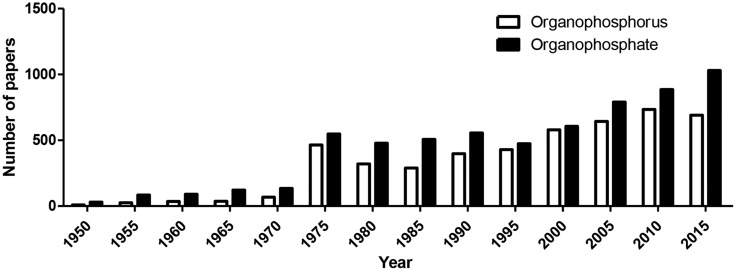
Both John Doull (see article by Eaton et al. in this issue) and my own mentor Sheldon D. Murphy started their career in the Toxicity Laboratory at the University of Chicago (the Tox Lab), where they both received a PhD degree in Pharmacology and worked mostly on the toxicology of organophosphates (OPs). As magnificently recounted by Doull (2001), the laboratory was created in 1941 to evaluate potential chemical warfare agents, and was closed in 1973, after it had changed its name to the U.S. Air Force Radiation Laboratory. John joined the Tox Lab in 1946 and graduated with a PhD in Pharmacology 5 years later, while Sheldon arrived at the Tox Lab in 1955, fresh from a degree in Pharmacy at South Dakota University. Ken Du Bois, who served as lab director for 20 years (1953–1973), was mentor to John and Sheldon, and both worked on various aspects of the toxicology of OPs. John’s work was on the acute and chronic toxicity of OPs, while Sheldon focused on the biochemical basis for the potentiation of malathion. For decades research in the laboratory focused on Du Bois’s interests in the toxicology of OPs; all the many students and postdocs who passed through his lab thus worked on OPs, and include many luminaries in the field of toxicology (eg, Bob Neal, Jules Brodeur, Gary Carlson, Bob Tardiff), and many continued to work on OPs for all their career (eg, Marion Ehrich, Sheldon Murphy). I got my feet wet in OP toxicology research when I joined Sheldon’s laboratory at the University of Texas in Houston as a postdoctoral fellow. The project I was given dealt with the study of the biochemical mechanisms involved in the tolerance to OP toxicity that was associated with repeated exposures. Together with graduate student Bradley Schwab, we identified the down-regulation of cholinergic muscarinic receptors as a main adaptive mechanism (Costa et al., 1982). My interest in OP toxicology continued, and over the years I have worked on various mechanisms of OP neurotoxicity (eg, Costa 1988; 2006), on the role of paraoxonase-1 (PON1) in modulating OP toxicity (eg, Costa et al., 2013), and on the developmental toxicity of OPs (eg, Cole et al., 2012). Interest in OPs by the research community has been strong for decades, and there is no sign of a decrease (Figure 1).
Figure 1.
Number of papers listed in PubMed in selected years (1950–2015) using the search terms “organophosphorus” or “organophosphate.”
This brief review is not intended to provide a comprehensive review of the toxicology of OPs. For that, a number of excellent books and book chapters should be consulted (eg, Hayes, 1982; Krieger 2001; 2010: Gupta, 2006; Satoh and Gupta, 2010; Lotti, 2010; Vale and Lotti, 2015). Here, together with some basic considerations on the history and toxicology of OPs, I will highlight some aspects that represent, in my view, recurrent or novel issues of discussion on these insecticides.
A BIT OF HISTORY
In an often quoted article, Swedish pharmacologist Bo Holmstedt ascribed the synthesis of the first OP (tetraetylpyrophosphate—TEPP) to the French chemist Philippe de Clermont in 1854 (Holmstedt, 1963). Others, however, have indicated that some OPs may have been synthesized even earlier. In 1820, Jean Louis Lassaigne reacted ethanol with phosphoric acid to obtain triethylphosphate (TEP) (Chambers, 1992), though this synthesis was later ascribed to Franz Anton Voegeli in 1848 (Petroianu, 2009). Even earlier, in 1801, another Frenchman, Jean Pierre Boudet is believed to have synthesized an OP from alcohol and phosphoric acid (Petroianu, 2010a). TEPP was nevertheless the first OP cholinesterase inhibitor, and in addition to de Clermont (with the help of Russian chemist Wladimir Moschnin, both working in the laboratory of Adolphe Wurtz in Paris), its synthesis was also accomplished by several other chemists (Petroianu, 2015). Neither the toxicity nor the mode of action of TEPP were known at the time, and indeed de Clermont also tasted the compound, and described it as a sticky liquid with a burning taste and a peculiar odor (Petroianu, 2010b). In 1932, Willy Lange at the University of Berlin synthesized some compounds containing the P-F bond. During the synthesis of dimethyl- and diethyl phosphofluoridate, he and graduate student Gerda von Krueger noticed the toxic effects of the vapors on themselves. They wrote “the vapours of these compounds have a pleasant and strongly aromatic odor, but a few minutes after inhaling a marked pressure develops in the larynx combined with breathlessness. Then, mild disturbances of consciousness set in, and also a feeling of being dazzled and painful hypersensitivity of the eyes to light. The symptoms decrease only after several hours… Very small quantities produce the effects” (Costa, 1987). Lange seemed to be aware of the potential of OP compounds to be developed as insecticides, but he soon left Germany, moved to the United States, where he was employed by the University of Cincinnati and by Procter & Gamble, and did not continue to work in the OP field (Holmstedt, 1963; Costa, 1987; Petroianu, 2010b).
Notwithstanding all these earlier efforts and accomplishments, the father of modern OP insecticide toxicology is considered Gerhard Schrader, a chemist at the I.G. Farbenindustrie in Germany. While working on the synthesis of organic fluorine and sulfur compounds, one day in December 1936 Schrader noticed “.that, on my way home my visual acuity was somewhat reduced. By the following day vision had practically returned to normal and I resumed my work. When other visual disturbances occurred, it became quite obvious that they were caused by a new synthetic substance” (Costa, 1987). The compound, 0-ethyl N, N-dimethyl-phosphoroamido-fluoridate, was found to be too toxic to warm-blooded animals to be used in agriculture. Schrader is credited for a new simple method to synthesize TEPP, which was the first commercialized OP insecticide under the trade name Bladan in a mixture with other hexa- compounds, though it was not sufficiently stable for plant protection. It is said that Schrader synthesized thousands of OP compounds (Holmsted, 1963; Costa, 1987). OMPA (octamethyl-pyrophosphoramide) was synthesized in 1942, but the true “breakthrough” came in 1944, when a new substance (code name E605) was synthesized, which had optimal stability and insecticidal activity. At the end of World War II, the methods for its synthesis were taken over by the Allies, and later E605 was introduced into the agricultural market under the trade name parathion, which became the most widely used insecticide of this class. In parallel with Schrader, British scientists McCombie and Saunders were also working on OPs, and subsequently patented dimefox and diisopropyl fluorophosphate (DFP). During those years some of the OPs synthesized by Schrader turned out to be extremely toxic to mammals. In 1938 the German Government declared all research on OPs to be “secret,” and the development of OPs followed 2 parallel strategies: one was to synthesize chemicals that were less toxic to mammals and effective as insecticides; the other was to develop compounds of high human toxicity and high volatility, to be used as poison gases instead of chlorine, mustard gas or phosgene. Compounds like Tabun, Sarin, and Soman were developed in that period for potential use as chemical warfare agents (Holmstedt, 1963; Delfino et al., 2009), though they were not used during World War II. Since the late 1930s, hundreds of OP compounds have been made and commercialized worldwide as insecticides in a variety of formulations. Their use peaked in the 1970s when most widely used organochlorine insecticides where phased out or banned. Until as recently as 2000, OPs constituted ∼70% of all insecticide used in the U.S., but that value has been halved in the following years (eg, 33% in 2012; Atwood and Paisley-Jones, 2017). However, use of OPs is still high in most developing countries, particularly because of the low cost of these chemicals compared to newer insecticides.
Concurrently with their synthesis, the mechanism of action of OPs, ie, inhibition of acetylcholinesterase (AChE), was also discovered. German scientists had noted the parasympathomimetic (cholinergic) effects of OPs, and found that atropine could serve as an antidote. These findings were certainly facilitated by the knowledge of the effects of physostigmine, an alkaloid isolated in 1864, whose miotic activity and antagonism by atropine was recognized at the same time, and whose mode of action as an AChE inhibitor was elucidated by Loewi and Navratil in 1926 (Casida, 1964). Indeed, the mechanism of action of OPs was suggested as early as 1939. A decade later Ken Du Bois (with John Doull) firmly established that parathion toxicity was due to inhibition of AChE (Du Bois et al., 1949). Other important milestones in the early history of OPs are the discovery of the reactivation and “aging” of the phosphorylated AChE. In 1951, Irwin Wilson at Columbia University in New York, showed that AChE inhibited by OPs could be reactivated by hydroxylamine (Petroianu, 2012). In a parallel effort during the next few years, Wilson (in the U.S.) and Albert Green and Dan Davies (in the U.K.), synthesized pralidoxime (2-PAM), which together with atropine remains a cardinal antidote for OP poisoning as of today (Davies and Green, 1959). This favorable development in the treatment of OP poisoning was somewhat counteracted by the discovery, also in the mid-1950s that the ability of oximes to reactivate phosphorylated (The more generic term phosphylate/phosphylation may also be used to describe the interaction of OPs with B-esterases.) AChE decreased with time, since “aging” (the nonenzymatic loss of an alkyl chain from the phosphate) would convert the inhibited enzyme into a nonreactivatable form (Hobbiger, 1963).
Inasmuch as insecticides such as pyrethroids and carbamates are known to derive from natural compounds, natural OPs have also been identified, though after synthetic OPs had been developed. Two OPs (named CGA 134735 and CGA 134736) were isolated from cultures of the soil microorganism Streptomyces antibioticus and found to be potent inhibitors of AChE activity (Neumann and Peter, 1987). Another natural compound, anatoxin-a, was isolated from the freshwater cyanobacterium anabaena flos-aquae strain NRC-525-17, and found to be an irreversible inhibitor of AChE (Mahmood and Carmichael, 1987). Thus, in the end, even for OPs, decades of chemical research have “reinvented” (and improved) what nature had already provided.
CHEMISTRY AND METABOLISM OF OPS
The chemistry of OPs has been thoroughly investigated, and their general structure, first indicated by Schrader in 1937, is shown in Figure 2. The pentavalent phosphorus is attached with a double bond to a sulfur (in this case the compound is defined as a phosphorothioate) or an oxygen; R1 and R2 are most commonly alkoxy groups (ie, OCH3 or OC2H5), though isopropyl substitutes are also possible, and X is the so-called “leaving group,” that is removed when the OP phosphorylates AChE, and is the most sensitive to hydrolysis. Several chemical subclasses of OPs also exist, eg, phosphonothioates, phosphoramidates, phosphonates, and others (Costa, 1988; Chambers et al., 2010a). Most OPs used as insecticides are phosphorothioates (ie, they have a P = S bond), and need to be bioactivated in vivo to their oxygen analogs to exert their toxic action, but some (eg, dichlorvos, methamidophos, or the nerve agents sarin or soman) have a P = O bond and do not require any bioactivation. This bioactivation is an oxidative desulfuration mediated by a variety of cytochrome P450 enzymes (CYPs; Chambers et al., 2010b). Other bioactivation reactions exist, for example formation of a sulfoxide, S = O, followed by the formation of a sulfone, O = S=O), also catalyzed by CYPs (eg, disulfoton; Costa, 1988). All other biochemical reactions, catalyzed by CYPs or by hydrolytic esterases (eg, carboxylesterase, paraoxonase-1) detoxify the OPs and lead to metabolites of lesser or no toxicity (Chambers et al., 2010b; Costa et al., 2013).
Figure 2.
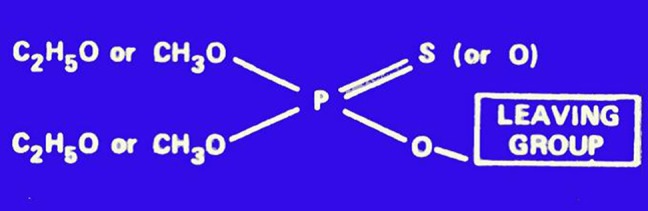
General structure of OP insecticides.
TOXICOLOGY OF OPS
As with most chemical insecticides in use today, OPs are primarily neurotoxicants, and act by poisoning the nervous systems of the target species (Casida, 2009). With few exceptions, their species-selectivity is low, hence mammals, including humans, are very sensitive to their toxicity. There are 2 main types of neurotoxicity associated with acute OP exposure: acute cholinergic toxicity and, in some cases, delayed neurotoxicity (Aldridge, 1981). Another type of toxicity, the intermediate syndrome, has been described in humans upon severe OP poisoning. It consists primarily of muscular weakness, and develops once acute cholinergic symptoms have subsided; its exact mechanism is not known (Costa, 2013).
Cholinergic Toxicity
As noted previously, the primary target for OPs is AChE, a B-esterase whose physiological role is that of hydrolyzing acetylcholine, a major neurotransmitter in the central and peripheral nervous systems. OPs with a P = O moiety phosphorylate a hydroxyl group on serine in the active site of the enzyme, thus impeding its action on the physiological substrate. Phosphorylated AChE is hydrolyzed by water at a very slow rate (several hours to days). While certain hydroxylamine derivatives can facilitate dephosphorylation of AChE and are utilized in the therapy of OP poisoning (see below), reactivation of phosphorylated AChE does not occur once the enzyme-inhibitor complex has “aged,” ie, there is the loss (by nonenzymatic hydrolysis) of one of the 2 alkyl (R) groups. When phosphorylated AChE has aged, the enzyme can be considered to be irreversibly inhibited, and the only means of replacing its activity is through synthesis of new enzyme, a process that may take days (Sultatos, 2006). Inhibition of AChE by OPs causes accumulation of acetylcholine at cholinergic synapses, with overstimulation of cholinergic receptors of the muscarinic and nicotinic type. As these receptors are localized in most organs of the body, a “cholinergic syndrome” ensues, which includes increased sweating and salivation, profound bronchial secretion, bronchoconstriction, miosis (constriction of the pupil), increased gastrointestinal motility, diarrhea, tremors, muscular twitching, and various CNS effects (dizziness, inhibition of central respiratory centers, convulsions, coma) (Holmstedt, 1959; Lotti, 2010). Death can occur as a result of respiratory failure. OPs are often the primary pesticides involved in acute human poisoning either because of accidental exposure or in suicidal attempts, as they have (with few exceptions, eg, malathion) relatively high acute toxicity by the oral and dermal routes (Colosio et al., 2010).
The muscarinic receptor antagonist atropine represents the cornerstone of the treatment for OP poisoning, as it prevents the action of accumulating acetylcholine on these receptors. Oximes such as 2-PAM are also used in the therapy of OP poisoning, as they can dephosphorylate AChE, thus restoring the catalytic site to its function (Davies et al., 1959). Controversies on the effectiveness of oximes have been ascribed to dosing and time interval after poisoning, as their therapeutic window is determined by the rate of aging (Lotti, 2010; Eddleston and Chowdhury, 2015; Worek et al., 2016). Recent efforts are also aimed at developing compounds (eg, N-methyl-2methoxypyridinium species) which could “resurrect” aged AChE, thus rendering it sensitive to the action of oximes (An et al., 2016).
Organophosphate-Induced Delayed Polyneuropathy
A few OPs may also cause another type of neurotoxicity, known as organophosphate-induced delayed polyneuropathy (OPIDP). Of much interest is that OPIDP, now very rare, was once the predominant type of toxicity associated with exposure to OP compounds, as several occurrences of mass human poisoning have occurred since the late 1800s, mostly involving tri-ortho-cresyl phosphate (TOCP) which is a poor AChE inhibitor (Davies, 1963). The largest outbreak (which may have involved >20 000 cases) occurred in the first part of 1930 in the Southeastern states of the United States (Smith et al., 1930a, 1930b) following consumption of a Jamaican Ginger extract contaminated with TOCP. Several other episodes of poisoning occurred in the next decades, mostly involving tricresylphosphate (TCP, a mixture of ortho, meta, and para isomers of tricresylphosphate). By the early 1960s Davies (1963) had tallied >40 000 cases of OPIDP, mostly from TCP or TOCP, though other OP used as insecticides (eg, leptophos) also have this capability. Incidence of this delayed neurotoxicity has then slowly declined, and only a few cases have been reported in the past decade (Colosio et al., 2010). Signs and symptoms of OPIDP include tingling of the hands and feet, followed by sensory loss, progressive muscle weakness and flaccidity of the distal skeletal muscles of the lower and upper extremities, and ataxia (Davies, 1963; Lotti, 1991; Lotti and Moretto, 2005). These effects are seen 2–3 weeks after exposure, when eventual signs of acute cholinergic toxicity have subsided. OPIDP can be classified as a distal sensorimotor axonopathy, in which the primary lesion is a bilateral degenerative change in distal levels of axons and their terminals, primarily affecting larger/longer myelinated central and peripheral nerve fibers, leading to breakdown of neuritic segments and the myelin sheaths (Ehrich and Jortner, 2010). Of utmost importance is that OPIDP is not related to AChE inhibition, and the putative target for initiation of OPIDP is thought to be another esterase, present in nerve tissues but also in other tissues (eg, lymphocytes, testis), named neuropathy target esterase (NTE) (Johnson and Glynn, 2001). A significant difference with the cholinergic toxicity deriving from inhibition of AChE by OPs is that inhibition of NTE’s catalytic activity is not sufficient for initiating OPIDP. Indeed, several OPs can inhibit NTE, as do some non-OPs (eg, carbamates and sulfonyl fluorides) but they do not cause delayed neurotoxicity. Only OPs whose chemical structure leads to aging of phosphorylated NTE (by a process analogous to that described for AChE) can cause OPIDP, suggesting that inhibition of NTE catalytic activity is not the mechanism of axonal degeneration, and that the aging of NTE is a key event in the initiation of OPIDP. In addition, for OPIDP to be initiated, phosphorylation and subsequent aging of at least 70% of NTE is necessary; this two-step process occurs within hours of poisoning, and NTE activity has fully recovered when the first clinical signs of OPIDP are evident some weeks later.
Despite progresses in molecular biology and protein chemistry, the exact physiological function(s) of NTE and the precise mechanisms of its involvement in OPIDP remain elusive. NTE is a large protein (1327 amino acids) with esterase and phospholipase activities (Glynn, 2006), and a member of a 9-protein family of patatin-like phospholipase domain-containing proteins (PNPLAs), of which NTE is PNPLA6 (Richardson et al., 2013). Mutations of the PNPLA6 gene have been associated with a number of congenital disorders involving alterations in the central and/or peripheral nervous systems (Hufnagel et al., 2015). Mammalian NTE is similar to the Swiss Cheese Protein (Sws) in Drosophila; Sws can regulate protein kinase A, and exposure to TOCP alters the Sws-PKA interactions, thereby inhibiting PKA activity and causing neurodegeneration (Wentzell et al., 2014), somewhat supporting the hypothesis that OPIDP may be the result of a toxic gain-of-function of phosphorylated and aged NTE (Lotti and Moretto, 2005). However, despite these and other recent advances, the exact chain of events occurring between phosphorylation and aging of NTE and axonal degeneration is still unknown. Before commercialization, OPs must undergo specific neurotoxicity testing in the hen (one of the most sensitive species) to determine whether OPIDP is produced, and this would exclude the compound from further development. Nevertheless, a few commercialized OPs (metamidophos, trichlorfon, and chlorpyrifos), which initially tested negative in the hen test, have caused OPIDP in humans, mostly as a result of extremely high exposures in suicide attempts (Lotti and Moretto, 2005; Kobayashi et al., 2017).
OP INTERACTIONS AND TOXICITY
Examining exposures to mixtures of insecticides is important for understanding the underlying mechanisms of multiple toxicity and for assessing aggregate risk (Casida, 2017a; Hernández et al., 2017), and indeed numerous studies have examined the effects of combinations of OPs. An example is represented by studies on the role of inhibition of carboxylesterase (CarE) by TOCP in the toxicity of malathion/malaoxon (DuBois, 1958; Murphy et al., 1959; Cohen and Murphy, 1971). CarE is actually a multigene family of enzymes that are widely distributed in the body; in rodents, but not in humans, it is also present in plasma and may contribute to higher detoxication of certain OPs (Li et al., 2005). CarEs can catalytically hydrolyze the carboxylic esters of malathion and malaoxon. Exposure to malathion or malaoxon, in combination with compounds that inhibit CarE, leads to a potentiation of malaoxon cholinergic toxicity (DuBois, 1969; Table 1). In addition to TOCP, several other OPs (eg, chlorpyrifos oxon, diazoxon, or paraoxon), and malathion impurities (eg, isomalathion) can inhibit CArE, thereby potentiating malathion and malaoxon toxicity (Baker et al., 1978; Cole et al., 2010). The low CarE activity in insects is believed to determine their high sensitivity to malathion toxicity compared to mammals (Costa, 2013).
Table 1.
Potentiation of Malaoxon (MO) Toxicity by Tri-Ortho-Cresyl Phosphate (TOCP)
Male mice were treated dermally with MO (60 mg/kg) alone or 24 h after dermal administration of TOCP (10 mg/kg). Liver and plasma carboxylesterase activities were inhibited 65 and 40% by TOCP.
p < .001. Adapted from Jansen et al. (2009).
Interactions of interest can also be found with regard to OPIDP. As said earlier, several compounds can inhibit NTE but if no aging occurs, these compounds are not neuropathic. When given before a neuropathic OP, these chemicals exert a protective role, by occupying the NTE active site. However, when given after a neuropathic OP, these compounds have been shown to “promote” OPIDP, ie, to potentiate the delayed neurotoxicity caused by initiators (Lotti and Moretto, 2005). The mechanisms underlying such “promotion” are still unknown, and the phenomenon may be less specific than initially thought, ie, promoters are effective even towards other types of neuropathies (Lotti and Moretto, 2005). The issue of promotion of chemical-induced neuropathies may have a bearing on the risk assessment of potential insecticide mixtures, as exposure to an initiator at a dose lower than that required to cause OPIDP, would nevertheless result in OPIDP if followed by exposure to a promoter of neuropathy (Costa, 2013).
SOME OLD AND NEW ISSUES OF DEBATE ON OP TOXICITY
The following sections are brief discussions on some issues on the toxicology of OPs. Some are “old” in the sense that they have been debated for some time, though they are still relevant and no definitive conclusion can be drawn (eg, additional targets, developmental neurotoxicity). Various others fall in this category (eg, effects of low chronic exposure, genetic susceptibility) and are not discussed here, but have been the subjects of several reviews. Instead, some potential novel avenues of OP toxicology are introduced, such as a possible link between OP exposure and neurodegeneration, the debated role of OPs in the “aerotoxic syndrome” and the interactions of OPs with the microbiome.
Are There Other Targets for OPs, and What Is Their Significance?
The possibility that OPs may act on targets other than the “traditional” ones, and the relevance that such interactions may have in mediating some of the effects of OPs, has been debated for some time (Casida and Quistad, 2004; Costa, 2006; Terry, 2012). For example, it has been pointed out that the pattern of symptoms may differ with different OPs, and cannot be explained by inhibition of AChE alone (Moser, 1995; Pope, 1999; Burke et al., 2017). The existence, and relevance, of additional targets has been widely used to discuss issues such as of common mechanism of action of OPs and the proposed cumulative risk assessment, possible CNS effects of long-term chronic exposure, as well as developmental neurotoxicity, particularly when effects are seen at OP doses that cause minimal or no AChE inhibition (Voorhees et al., 2017). OPs do indeed affect hundreds of enzymes, receptors, and other proteins. Thus, it is of utmost relevance to compare the relative potency of OPs toward these targets and AChE, to investigate eventual interaction of the parent compound and of cholinergically active/inactive metabolites, to verify findings in vivo upon acute/chronic exposures, and to define the relevance of the target by pharmacological or genetic means (Costa, 2006). Some examples of effects of OPs different from inhibition of AChE/NTE are briefly discussed below.
Various other components of the cholinergic system have been shown to be directly affected by some OPs, and muscarinic M2 receptors in particular have been shown to be a potential target (Costa, 2006). Several enzymes involved in the metabolism of peptides are inhibited by OPs. For example, acylpeptide hydrolase (APH), responsible for the removal of N-acetylated amino acids from the N-terminus of short peptides, such as beta-endorphin, is inhibited by various oxons (eg, dichlorvos, diazoxon) at low concentrations, and inhibition was also observed after in vivo exposure (Richards et al., 2000; Li et al., unpublished). Adducts of OPs to red blood cell APH may serve as biomarkers for monitoring OP exposure (Marsillach et al., 2013). Indirect evidence of inhibition of encephalin metabolism by DFP (a neuropathic OP, never used as insecticide) has also been reported (Costa and Murphy, 1986). OPs can also target the cannabinoid system; the endocannabinoid anandamide (which binds to brain cannabinoid receptor-1, also the target for the principal psychoactive ingredient of marijuana, Δ9-tetrahydocannabinol) is hydrolyzed by fatty acid amide hydrolase (FAAH), of which various OPs are potent inhibitors (Quistad et al., 2001; Buntyn et al., 2017), though its toxicological significance remains unclear (Quistad et al., 2002). Several OPs have also been shown to inhibit a variety of lipases, which may alter lipid metabolism, particularly in the nervous system (Quistad et al., 2006).
Two potentially important noncholinergic effects of OPs that have been investigated are oxidative stress and neuroinflammation. Both may be of high relevance as they are known to be involved in the pathogenesis of several neurodevelopmental and neurodegenerative diseases. OP-induced oxidative stress has been observed in experimental animal studies in vivo, in in vitro preparations, as well as in humans (Kovacic, 2003; Milatovic et al., 2006; Giordano et al., 2007). Of relevance is the fact that both the parent compounds as well the oxygen analogs were able to induce oxidative stress, and the fact that this effect did not appear to be related to AChE inhibition. Acute and chronic exposures to OPs have also been shown to cause neuroinflammation, with microglia activation and increase in pro-inflammatory cytokine levels in mice (Banks and Lein, 2012; Viviani et al., 2014), as well as in 3-dimensional brain cell cultures (Monnet-Tschudi et al., 2007). The exact molecular mechanisms involved in OP-induced oxidative stress and neuroinflammation are still elusive, though they may be secondary to mitochondrial toxicity (Terry, 2012). Overall, though the possibility that novel additional noncholinergic targets or effects may be involved in some adverse effects of OPs is of much interest, the evidence so far linking one or more of the observed effects to significant in vivo end-points is not fully convincing, and more rigorous investigations are needed. Importantly, the dose/concentration of OP capable to affect these alternative targets needs to be compared with that causing AChE inhibition.
OPs as Developmental Toxicants and Neurotoxicants
The Food Quality Protection Act (FQPA) of 1996 directed attention on the potential higher susceptibility of infants and children to the toxicity of pesticides (Abreu-Villaça and Levin, 2017). With regard to OPs, findings in animals clearly show that the young are more sensitive to the acute cholinergic toxicity, likely because of lower detoxication abilities (Benke and Murphy, 1975; Mortensen et al., 1996; Costa, 2006; Pope, 2010). In contrast, the young appear to be more resistant to OPIDP (Lotti and Moretto, 2005). In recent years, accumulating evidence suggests that perinatal exposure to OPs may cause developmental neurotoxicity. Several epidemiological studies have found associations between in utero or early childhood exposure to OPs and behavioral abnormalities, particularly deficits in learning and memory (reviewed in Eskenazi et al., 1999; Eaton et al., 2008; Muñoz-Quezada et al., 2013; González-Alzaga et al., 2014; Reiss et al., 2015). It has been pointed out that exposure to OPs, as shown by biological monitoring in children, though somewhat elevated in inner cities or farming communities, is still at levels below those causing any AChE inhibition. Several animal studies also showed developmental neurotoxicity effects of OPs, however, with few exceptions, effects were observed at levels causing significant AChE inhibition (see details in Eaton et al., 2008; Timofeeva and Levin, 2010). As indicated earlier, OPs can interact with targets other than AChE, and affect various cellular processes in vitro or ex-vivo, often at dose levels that produced no clear cholinergic signs of toxicity and at times that produce no AChE inhibition (reviewed in Eaton et al., 2008; Burke et al., 2017) For example, a recent in vitro study reported that chlorpyrifos and its oxon could inhibit axonal transport at concentrations below those required for inhibiting AChE activity (Gao et al., 2017a; Table 2). It should be noted that most of the human, animal and in vitro studies have focused on chlorpyrifos, a widely used OP, and to a minor extent on diazinon, leading to regulatory restrictions on their use. However, there is no strong reason to believe that other OP compound may not share similar novel mechanisms of action, and further research in this area is certainly warranted.
Table 2.
Inhibition of Axonal Transport by the OP Chlorpyrifos
| End-point |
Chlorpyrifos |
Chlorpyrifos Oxon |
|---|---|---|
| Lowest effective concentration | ||
| Anterograde transport | 100 nM | 0.1 nM |
| Retrograde transport | 10 μM | 10 nM |
| AChE activity | 10 μM | 10 nM |
Axonal transport was measured in rat cortical neurons. Adapted from Gao et al. (2017a).
OPs and Neurodegenerative Diseases: Is There a Link?
Aging is often associated with a wide variety of clinical and pathological conditions which can be classified as neurodegenerative diseases. Typical examples of such diseases are Parkinson’s disease (PD), Alzheimer’s disease (AD), or amyotrophic lateral sclerosis (ALS). The etiology of these diseases is unknown and both environmental and genetic factors may play a role; oxidative stress and neuroinflammatory processes are prominent in most neurodegenerative diseases.
Parkinson’s disease is a neurodegenerative disorder characterized by a slow and progressive degeneration of dopaminergic neurons in the substantia nigra, with degeneration of nerve terminals in the striatum. Once loss of dopaminergic neurons has reached about 80%, clinical signs appear which include resting tremor, rigidity, bradykinesia, and gait disturbances. Though genetic forms of PD have been associated with specific mutations in a number of genes, the great majority of PD cases is sporadic, and may be due to environmental factors or to gene-environment interactions, ie, exposure of genetically susceptible individuals to neurotoxic substances. Among the environmental factors believed to be associated with PD there are certain pesticides such as the herbicide paraquat and the insecticide rotenone. Acute exposure to OPs has been reported to cause Parkinsonism, but this was a pharmacological, reversible phenomenon (Müller-Vahl et al., 1999). However, Chuang et al. (2017) recently reported an increased risk of PD after OP (and carbamate) poisoning. In addition, positive association between chronic exposure to OPs and PD were found (eg, Wang et al., 2014), particularly in individuals with certain paraoxonase-1 genotypes (Paul et al., 2017). The mechanism(s) by which OPs may cause degeneration of dopaminergic neurons remains elusive, though oxidative stress and neuroinflammation have been suggested (Wani et al., 2017). The emerging role of gut microbiota in PD (Parashar and Udayabanu, 2017) and the effects of OPs on the microbiome (see below) may represent another fruitful avenue for mechanistic investigations.
Alzheimer’s disease is by far the most common cause of dementia, followed by dementia with Lewy bodies. Its most common symptom is memory loss for recent events, with diffuse cortical and hippocampal atrophy, and accumulation of abnormally folded amyloid beta and of tau proteins in amyloid plaques and neuronal tangles (Khan and Bloom, 2016; Selkoe and Hardy, 2016). Though anticholinesterase agents (eg, rivastigmine) are used, with limited success, to mitigate cognitive deficits of AD, acute and/or chronic exposures to OPs have been investigated as potential etiological factors in this disease. A review of the literature shows that evidence for such association in human studies is weak, and suggested mechanisms are again oxidative stress and neuroinflammation (Zaganas et al., 2013; Sanchez-Santed et al., 2016; Hernández et al., 2016), though the gut and oral microbiomes (both affected by OPs) may be worth investigating (Tremlett et al., 2017).
Amyotrophic lateral sclerosis, also known as motor neuron disease or Lou Gehrig’s disease, is a progressive neurodegenerative disorder of the motor neuron system, characterized by progressive weakness and wasting of striated muscle due to motor cortical and spinal neurodegeneration. Environmental factors potentially involved in the etiopathogenesis of ALS have been investigated to a very limited degree. Recently it has been proposed that OPIDP may represent a good model for ALS, suggesting that “the resemblances between OPIDP and ALS are striking at the clinical, etiological, neuropathological, cellular, and molecular levels” (Merwin et al., 2017). However, such arguments and conclusions have been rebutted, largely based on the significant differences between ALS and OPIDP (Lotti and Moretto, 2017). Overall, while investigations on possible associations between environmental factors and neurodegenerative diseases remain of much relevance, evidence that OPs may play an etiological role in such diseases remains weak.
Are OPs Involved in the “Aerotoxic Syndrome”?
Over the past 2 decades, aircrew and some passengers on various airlines have complained of ill health following exposures to toxic fumes in airplane cabins (Harrison and Mackenzie Ross, 2016). Air drawn from outside is circulated around the engine and then pumped into the aircraft; such bleed air may be contaminated with engine oil fumes which contain a number of toxic substances (Shehadi et al., 2016). The varied and complex arrays of signs and symptoms reported following these episodes (often referred to as “fume events”) have been given the name of “Aerotoxic Syndrome” (Harrison and Mackenzie Ross, 2016; Michaelis et al., 2017). Reported symptoms range from respiratory tract irritation, to gastrointestinal effects, to CNS problems of various types (tremors, disorientation, memory loss, and cognitive dysfunction) of short-and long-term nature. Several air monitoring studies have identified various toxic substances (eg, toluene, carbon monoxide, and N-phenyl-L-naphthylamine), but interest has focused on TCP and TOCP. As indicated in an earlier section, TOCP has been shown to cause OPIDP in humans. However, evidence available so far for the etiological involvement of TCP and/or TOCP in the Aerotoxic Syndrome is still unconvincing (de Boer et al., 2015; Duarte et al., 2017). These compounds can be detected in cabin air, but levels are very low (de Ree et al., 2014). Human exposure, determined by urine metabolite levels and by butyrylcholinesterase adducts, is also very low (Schindler et al., 2013; Liyasova et al., 2011). No significant inhibition of lymphocytic NTE activity was reported, and erythrocyte AChE activity was also, not surprisingly, unaffected (Heutelbeck et al., 2016). Nevertheless, any potential role of TCPs would need further, more thorough investigations. For example, the FAAH inhibitor BIA 10-2474 (not an OP) recently caused severe neurotoxicity in a Phase-1 clinical trial (Kerbrat et al., 2016) which was attributed to its off-target effects rather than by inhibition of FAAH (Van Esbroeck et al., 2017) (which is also inhibited by OPs; Quistad et al., 2001, 2002). Such off-target effects may be related to the ability of BIA 10-2474 to inhibit NTE, CarE, and various lipases, which are also targeted by OPs, including TCPs.
A few decades ago attention had focused on the formation of the bicyclophosphate esters upon pyrolysis of trimethylpropane polyesters, which, like the triarylphosphates, were also used as aircraft engine lubricants (Kalman et al., 1985). Bicyclic phosphorus esters do not inhibit AChE, but they are potent antagonists of the gamma-aminobutyic acid-A receptors, and hence potent convulsants (Bellet and Casida, 1973; Bowery et al., 1976). Issues related to the eventual roles of OPs in the aerotoxic syndrome remain emotionally charged and scientifically weak; perhaps a way forward would be that of developing bleedless aircrafts, as already done with some newer models.
OPs and the Microbiome
The fact that the human gut contains various organisms such as fungi, parasites, viruses and bacteria has been known for decades. There are >100 million bacteria which reside in the gastrointestinal tract, and many more can be found in essentially every part of the human body, from the skin to the nasal and auditory cavities, from the mouth to the urogenital system. These microbes whose genome, the “microbiome,” is believed to be 100-fold the size of the human genome, live in symbiosis with the smaller population of eukaryotic cells in the body, and play important roles in development and in general metabolic homeostasis. In recent years, attention has focused on the role that perturbation of human microbiota may have on disease (Mahana et al., 2016; Felice and O’Mahony, 2017). Significant associations have been found for example between alterations in the gut microbiome and diabetes, inflammatory diseases, liver damage, and also neurodevelopmental (eg, autism) and neurodegenerative (eg, PD) diseases, in line with the important role of the gut-brain axis (ZHu et al., 2017). Several chemicals have been shown to perturb the gut microbiota, and a handful of studies have focused on OPs. The OP diazinon, given in drinking water (4 mg/L for 13 weeks) to mice has been shown to alter the gut microbiome, the functional metagenome, and the associated metabolic profiles (Gao et al., 2017b). Interestingly, the effects were more pronounced in male than in female mice. Examples of observed effects include significant changes in bacterial genera, alterations in bile acids abundance, and a drastic decrease in taurine levels (Gao et al., 2017b). In a follow up study, Gao et al. (2017c) identified specific changes in the gut microbiome caused by diazinon involving oxidative stress pathways, fatty acids and carbohydrate metabolism, and quorum sensing systems. Of note is that prolonged exposure to diazinon was reported not to cause any AChE inhibition (Gao et al., 2017b, 2017c). Developmental exposure of rats to the OP chlorpyrifos (1 mg/kg/day during pregnancy until postnatal day 60) also caused significant alterations in gut microbiota, which mimicked those observed in the Simulator of the Human Intestinal Microbial Ecosystem (SHIME®) (Joly et al., 2013). Two similar studies, which utilized this and higher doses of chlorpyrifos (3.5 and 5.0 mg/kg) also found perturbations in the gut microbiome and related metabolic abnormalities (Joly Condette et al., 2015; Reygner et al., 2016). Similar findings were also reported in adult mice upon a 30 day exposure (1 mg/kg/day; Zhao et al., 2016). Unfortunately, none of these studies with chlorpyrifos indicates whether AChE activity was affected. A human study by Stanaway et al. (2017) examined oral buccal microbiomes in farmworkers using pesticides and found an association between exposure to azinphosmethyl and perturbations in 7 common bacterial taxa including significant reductions of Streptococcus. As research on the role of microbiota in human disease is rather recent, it would be expected that research on the interactions of OPs with the microbiome will increase, and perhaps provide some novel mechanism for some of the still unexplained effects of these insecticides, particularly as it relates to effects from prolonged low level exposures.
Are OPs a Cancer Risk?
Innumerable epidemiological studies have been carried out with the purpose of determining possible associations between exposure to pesticides and an increased risk of tumors. For some compounds, particularly some fungicides and herbicides, associations were found and were at times supported by animal and/or mechanistic data. As a class OPs have not been considered to pose a significant cancer risk (Woo et al., 1996), and very few were listed by the USEPA as possible human carcinogens (group C, limited evidence in animals). Two widely used OPs, chlorpyrifos and diazinon, are classified as unlikely to be carcinogens in humans; yet epidemiological studies have revealed associations between exposure to these 2 OPs and certain cancers (Weichenthal et al., 2012; Hu et al., 2017). Recently, the International Agency for Cancer Research (IARC) concluded that diazinon and malathion (one of the most widely used OPs; Hoppin et al., 2012) are probable human carcinogens (Group 2 A), while parathion and tetrachlorvinphos are classified as possible human carcinogens (Group 2B) (Guyton et al., 2015; IARC, 2017). For diazinon and malathion, the classification was based on limited to sufficient animal evidence, limited human evidence and some mechanistic evidence (genotoxicity, oxidative stress). What, however, created the most debate was the assignment to Group 2 A of another OP, which is not neither an insecticide nor an AChE/NTE inhibitor, ie, the herbicide glyphosate. For this compound, which is the most widely used pesticide worldwide, particularly because it is used in glyphosate-resistant crops, evidence by IARC was deemed sufficient for animal studies, limited for humans, and supported by evidence of genotoxicity and oxidative stress (Guyton et al., 2015). At about the same time the European Food Safety Authority concluded instead that glyphosate is unlikely to pose any carcinogenicity risk for humans (EFSA, 2015), starting a fiery debate which is still ongoing (eg, Williams et al., 2016; Portier et al., 2016), and will certainly continue.
WHAT’S NEXT FOR OPS?
As noted previously, the use of OPs as insecticides has been declining in recent years; however, this is not yet the time of their demise (Casida and Durkin, 2013). Features that keep OPs as major chemicals for pest control include decades of experience on their use, high effectiveness on many pests, availability of generic products of low cost, excellent overall environmental profile (Casida and Durkin, 2013), while high toxicity to mammalian species remains a major concern. The lack of new OPs in the industry R&D pipeline is mainly due to the development of resistance of multiple species of pests to OPs (Casida and Durkin, 2013; Casida, 2017a). Nevertheless, as recently stated, “OP toxicology is not just AChE inhibition” (Casida, 2017b). Indeed several OP compounds have and are being developed as herbicides (eg, glyphosate, glufosinate), fungicides (eg, iprobenfos, edifenphos), pharmaceutical drugs (eg, the antihypertensive fosinopril or the antineoplastic fotemustine), or flame retardants [eg, tris (1, 3 dichloro-2-propyl) phosphate—TDCPP, or tris (2-chloroethyl) phosphate—TCEP] (Casida, 2017b). These chemicals share some of the structural characteristics of OP insecticides (the P = O moiety) but act on target and nontarget organism by different mechanisms and present different sets of toxicological issues. For example, production and use of OP flame retardants have increased significantly in the past 2 decades, particularly since the more popular PBDEs (polybrominated diphenylethers) have been phased out or banned due to concerns on their potential developmental neurotoxicity (Costa and Giordano, 2007). However, like PBDEs, these OPs are also used as “additive” flame retardants, ie, they are not chemically bound to the product, and hence they leach in the environment and have become widespread environmental pollutants (van der Veen and de Boer, 2012; Greaves and Letcher, 2017). In addition, several OP flame retardants have been associated with a number of adverse health effects, as they are suspected carcinogens, developmental neurotoxicants, reproductive toxicants, and endocrine disruptors (van der Veen and de Boer, 2012; Greaves and Letcher, 2017). As for insecticidal OPs and for nerve agent OPs (ie, primarily AChE inhibitors) several toxicological issues still linger and need further investigations, as in part outlined in this review.
FUNDING
National Institute of Environmental Health Sciences (ES07033, ES04696).
ACKNOWLEDGMENTS
I would like to dedicate this article to my late mentor Sheldon D. Murphy (1933–1990) who introduced me to the field of OP toxicology and shared his knowledge and wisdom in this area. I also acknowledge my early Italian mentor Corrado L. Galli, who introduced me to the field of toxicology (and to Sheldon), and my colleague at the University of Washington, Clement E. Furlong, who kept our OP research on paraoxonases exciting for several decades. I thank Dr Larry Sheets and Dr David Eaton for helpful comments and suggestions. Support from NIEHS (ES04696, ES07033) is also acknowledged. Apologies are due to all colleagues whose important research contributions on OPs could not be cited because of space limitations.
REFERENCES
- Abreu-Villaça Y., Levin E. D. (2017). Developmental neurotoxicity of succeeding generations of insecticides. Environ. Int. 99, 55–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge W. N. (1981). Organophosphorus compounds: Molecular basis for their biological properties. Sci. Prog. 67, 131–147. [PubMed] [Google Scholar]
- An Y., Zhu Y., Yao Y., Liu J. (2016). Is it possible to reverse aged acetylcholinesterase inhibited by organophosphorus compounds. Insight from a theoretical study. Phys. Chem. Chem. Phys. 18, 9838–9846. 10.1039/C5CP07991H [DOI] [PubMed] [Google Scholar]
- Atwood D., Paisley-Jones C. (2017). Pesticides Industry Sales and Usage: 2008–2012 Market Estimates, pp. 32 US Environmental Protection Agency, Washington, DC. [Google Scholar]
- Baker E. L., Warren M., Zack M., Dobbin R. D., Miles J. W., Miller S., Alderman L., Teeters W. R. (1978). Epidemic malathion poisoning in Pakistan malaria workers. Lancet 311, 31–34. [DOI] [PubMed] [Google Scholar]
- Banks C. N., Lein P. J. (2012). A review of experimental evidence linking neurotoxic organophosphorus compounds and inflammation. Neurotoxicology 33, 575–584. 10.1016/j.neuro.2012.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellet E. M., Casida J. E. (1973). Bicyclic phosphorus esters: High toxicity without cholinesterase inhibition. Science 182, 1135–1136. 10.1126/science.182.4117.1135 [DOI] [PubMed] [Google Scholar]
- Benke G., Murphy S. D. (1975). The influence of age on the toxicity and metabolism of methylparathion and parathion in male and female rats. Toxicol. Appl. Pharmacol. 31, 254–269. 10.1016/0041-008X(75)90161-1 [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Collins J. F., Hill R. G. (1976). Bicyclic phosphorus esters that are potent convulsants and GABA antagonists. Nature 261, 601–603. 10.1038/261601a0 [DOI] [PubMed] [Google Scholar]
- Buntyn R. W., Alugubelly N., Hybart R. L., Mohammed A. N., Nail C. A., Parker G. C., Ross M. K., Carr R. L. (2017). Inhibition of endocannabinoid-metabolizing enzymes in peripheral tissues following developmental chlorpyrifos exposure in rats. Int. J. Toxicol.36, 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. D., Todd S. W., Lumsden E., Mullins R. J., Mamczarz J., Fawcett W. P., Gullapalli R. P., Randall W. R., Pereira E. F. R., Albuquerque E. X. (2017). Developmental neurotoxicity of the organophosphorus insecticide chlorpyrifos: From clinical findings to preclinical models and potential mechanisms. J. Neurochem. 142, 162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida J. E. (1964). Esterase inhibitors as pesticides. Science 146, 1011–1017. 10.1126/science.146.3647.1011 [DOI] [PubMed] [Google Scholar]
- Casida J. E. (2009). Pest toxicology: The primary mechanisms of pesticide actions. Chem. Res. Toxicol. 22, 609–619. 10.1021/tx8004949 [DOI] [PubMed] [Google Scholar]
- Casida J. E. (2017a). Pesticide interactions: Mechanisms, benefits, and risks. J. Agric. Food Chem. 65, 4553–4561. [DOI] [PubMed] [Google Scholar]
- Casida J. E. (2017b). Organophosphorus xenobiotic toxicology. Annu. Rev. Toxicol. Pharmacol. 57, 309–327. [DOI] [PubMed] [Google Scholar]
- Casida J. E., Quistad G. B. (2004). Organophosphate toxicology: Safety aspects of nonacetylcholinesterase secondary targets. Chem. Rev. Toxicol. 17, 983–988. 10.1021/tx0499259 [DOI] [PubMed] [Google Scholar]
- Casida J. E., Durkin K. A. (2013). Anticholinesterase insecticide retrospective. Chem. Biol. Interact. 203, 221–225. 10.1016/j.cbi.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers H. W. (1992). Organophosphorus compounds: An overview. In Organophosphates: Chemistry, Fate, and Effects (Chambers J. E., Levi P. E., Eds.), pp. 3–17. Academic Press, San Diego. [Google Scholar]
- Chambers H. W., Meek E. C., Chambers J. E. (2010a). Chemistry of organophosphorus insecticides In Hayes’ Handbook of Pesticide Toxicology (Krieger R., Ed.), pp. 1395–1398, Academic Press, San Diego. [Google Scholar]
- Chambers J. E., Meek E. C., Chambers H. W. (2010b). The metabolism of organophosphorus insecticides, In Hayes’ Handbook of Pesticide Toxicology (Krieger R., Ed.), pp 1399–1407, Academic Press, San Diego. [Google Scholar]
- Chuang C. S., Su H. L., Lin C. L., Kao C. H. (2017). Risk of Parkinson disease after organophosphate or carbamate poisoning. Acta Neurol. Scand. 136, 129–137. 10.1111/ane.12707 [DOI] [PubMed] [Google Scholar]
- Cohen S. D., Murphy S. D. (1971). Carboxylesterase inhibition as an indicator of malathion potentiation in mice. J. Pharmacol. Exp. Ther. 176, 733–742. [PubMed] [Google Scholar]
- Cole T. B., Jansen K., Park S., Li W. F., Furlong C. E., Costa L. G. (2010). The toxicity of mixtures of specific organophosphorus compounds is modulated by paraoxonase 1 status. Adv. Exp. Med. Biol. 660, 47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole T. B., Fisher J. C., Burbacher T. M., Costa L. G., Furlong C. E. (2012). Neurobehavioral assessment of mice following repeated postnatal exposures to chlorpyrifos oxon. Neurotoxicol. Teratol. 34, 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colosio C., Vellere F., Moretto A. (2010) Epidemiological studies of anticholinesterase pesticide poisoning: Global impact In Anticholinesterase Pesticides: Metabolism, Neurotoxicity and Epidemiology (Satoh T., Gupta R.C., Eds.), pp. 343–355. Wiley and Sons, Hoboken, NJ. [Google Scholar]
- Costa L. G. (1987). Toxicology of pesticides: A brief history In Toxicology of Pesticides: Experimental, Clinical, and Regulatory Perspectives (Costa L. G., Galli C. L., Murphy S. D., Eds.), NATO ASI Series, Vol. 113, pp. 1–9. Springer-Verlag, Berlin. [Google Scholar]
- Costa L. G. (1988). Organophosphorus compounds. In Recent Advances in Nervous System Toxicology (Galli C. L., Manzo L., Spencer P. S., Eds.), pp. 203–246. Plenum Press, New York, NY. [Google Scholar]
- Costa L. G. (2006). Current issues in organophosphate toxicology. Clin. Chim. Acta 366, 1–13. 10.1016/j.cca.2005.10.008 [DOI] [PubMed] [Google Scholar]
- Costa L. G. (2013). Toxic effects of pesticides In Casarett and Doull’s Toxicology. The Basic Science of Poisons, 8th ed (Klaassen C. D., Ed.), pp. 933–980. McGraw-Hill, New York, NY. [Google Scholar]
- Costa L. G., Murphy S. D. (1986). Cholinergic and opiate involvement in the antinociceptive effect of diisopropylfluorophosphate. Pharmacol. Biochem. Behav. 24, 723–736. [DOI] [PubMed] [Google Scholar]
- Costa L. G., Giordano G. (2007). Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology 28, 1047–1067. 10.1016/j.neuro.2007.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa L. G., Schwab B. W., Murphy S. D. (1982). Tolerance to anticholinesterase compounds in mammals. Toxicology 25, 79–97. 10.1016/0300-483X(82)90021-X [DOI] [PubMed] [Google Scholar]
- Costa L. G., Giordano G., Cole T. B., Marsillach J., Furlong C. E. (2013). Paraoxonase 1 (PON1) as a genetic determinant of susceptibility to organophosphate toxicity. Toxicology 307, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. R. (1963). Neurotoxicity of organophosphorus compounds In Cholinesterases and Anticholinesterase Agents, (Koelle G. B., Ed.), pp. 860–882. Springer-Verlag, Berlin. [Google Scholar]
- Davies D. R., Green A. L. (1959). The chemotherapy of poisoning by organophosphate anticholinesterase. Br. J. Industr. Med. 16, 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer J., Antelo A., van der Veen I., Brandsma S., Lammertse N. (2015). Tricresyl phosphate and the aerotoxic syndrome of flight crew members: Current gaps in knowledge. Chemosphere 119, S58–S61. [DOI] [PubMed] [Google Scholar]
- Delfino R. T., Ribeiro T. S., Figueroa-Villar J. D. (2009). Organophosphorus compounds as chemical warfare agents: A review. J. Braz. Chem. Soc. 20, 407–428. [Google Scholar]
- De Ree H., van den Berg M., Brand T., Mulder G. J., Simons R., van Zanten B. V., Westerink R. H. S. (2014). Health risk assessment of exposure to tricresyl phosphates (TCPs) in aircraft: A commentary. Neurotoxicology 45, 2019–2215. [DOI] [PubMed] [Google Scholar]
- Doull J. (2001). Toxicology comes of age. Annu. Rev. Pharmacol. Toxicol. 41, 1–21. 10.1146/annurev.pharmtox.41.1.1 [DOI] [PubMed] [Google Scholar]
- Duarte D. J., Rutten J. M. M., van den Berg M., Westerink R. H. S. (2017). In vitro neurotoxic hazard characterization of different tricresyl phosphate (TCP) isomers and mixtures. Neurotoxicology 59, 222–230. [DOI] [PubMed] [Google Scholar]
- DuBois K. P. (1958). Potentiation of toxicity of insecticidal organophosphates. Arch. Ind. Health 18, 488–496. [PubMed] [Google Scholar]
- DuBois K. P. (1969). Combined effects of pesticides. Can. Med. Assoc. J. 100, 173–179. [PMC free article] [PubMed] [Google Scholar]
- Du Bois K. P., Doull J., Salerno P. R., Coon J. M. (1949). Studies on the toxicity and mechanism of action of p-nitrophenyl diethyl thionophosphate (parathion). J. Pharmacol. Exp. Ther. 95, 79–91. [PubMed] [Google Scholar]
- Eaton D. L., Daroff R. B., Autrup H., Bridges J., Buffler P., Costa L. G., Coyle J., McKhann G., Mobley W. C., Nadel L., et al. (2008). Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit. Rev. Toxicol. 38, 1–125. [DOI] [PubMed] [Google Scholar]
- Eddleston M., Chowdhury F. R. (2016). Pharmacological treatment of organophosphorus insecticide poisoning: The old and the (possible) new. Br. J. Clin. Pharmacol. 81, 462–470. 10.1111/bcp.12784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) (2015). Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA J. 13, 4302. 10.2903/j.efsa.2015.4302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrich M., Jortner B. S. (2010). Organophosphorus-induced delayed neuropathy In Hayes’ Handbook of Pesticide Toxicology (Krieger R., Ed.), pp. 1479–1504. Academic Press, San Diego. [Google Scholar]
- Eskenazi B., Bradman A., Castorina R. (1999). Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ. Health Perspect. 107, 409–419. 10.1289/ehp.99107s3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felice V. D., O'Mahony S. M. (2017). The microbiome and disorders of the central nervous system. Pharmacol. Biochem. Behav. 160, 1–13. (in press). 10.1016/j.pbb.2017.06.016 [DOI] [PubMed] [Google Scholar]
- Gao J., Naughton S. X., Beck W. D., Hernandez C. M., Wu G., Wei Z., Yang X., Bartlett M. G., Terry A. V. (2017a). Chlorpyrifos and chlorpyrifos oxon impair the transport of membrane bound organelles in rat cortical axons. Neurotoxicology 62, 11–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B., Bian X., Mahbub R., Lu K. (2017b). Sex-specific effects of organophosphate diazinon on the gut microbiome and its metabolic functions. Environ. Health Perspect 125, 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B., Bian X., Chi L., Tu P., Ru H., Lu K. (2017c). Organophosphate diazinon altered quorum sensing, cell motility, stress response, and carbohydrate metabolism of gut microbiome. Toxicol. Sci. 157, 354–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano G., Afsharinejad Z., Guizzetti M., Vitalone A., Kavanagh T. J., Costa L. G. (2007). Organophosphorus insecticides chlorpyrifos and diazinon and oxidative stress in neuronal cells in a genetic model of glutathione deficiency. Toxicol. Appl. Pharmacol. 219, 181–189. [DOI] [PubMed] [Google Scholar]
- Glynn P. (2006). A mechanism for organophosphate-induced delayed neuropathy. Toxicol. Lett. 162, 94–97. 10.1016/j.toxlet.2005.10.012 [DOI] [PubMed] [Google Scholar]
- González-Alzaga B., Lacasaña M., Aguilar-Garduño C., Rodríguez-Barranco M., Ballester F., Rebagliato M., Hernández A. F. (2014). A systematic review of developmental effects of prenatal and postnatal organophosphate pesticide exposure. Toxicol. Lett. 230, 104–121. [DOI] [PubMed] [Google Scholar]
- Greaves A. K., Letcher R. J. (2017). A review of organophosphate esters in the environment from biological effects to distribution and fate. Bull. Environ. Contam. Toxicol. 98, 2–7. 10.1007/s00128-016-1898-0 [DOI] [PubMed] [Google Scholar]
- Gupta R.C. (Ed.) (2006) Toxicology of Organophosphate and Carbamate Compounds, pp. 763 Elsevier, Amsterdam. [Google Scholar]
- Guyton K. Z., Loomis D., Grosse Y., El Ghissassi F., Benbrahim-Tallaa L., Guha N., Scoccianti C., Mattock H., Straif K. (2015). Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 16, 490–491. [DOI] [PubMed] [Google Scholar]
- Harrison V., Mackenzie Ross S. J. (2016). An emerging concern: Toxic fumes in airplane cabins. Cortex 74, 297–302. 10.1016/j.cortex.2015.11.014 [DOI] [PubMed] [Google Scholar]
- Hayes W. J. (1982). Pesticides Studied in Man, pp. 672 Williams & Wilkins, Baltimore. [Google Scholar]
- Hernández A. F., González-Alzaga B., López-Flores I., Lacasaña M. (2016). Systematic review on neurodevelopmental and neurodegenerative disorders linked to pesticide exposure: Methodological features and impact on risk assessment. Environ. Int. 92–93, 657–679. [DOI] [PubMed] [Google Scholar]
- Hernandez A. F., Gil F., Lacasaña M. (2017). Toxicological interactions of pesticide mixtures: An update. Arch. Toxicol. 91, 3211–3223. [DOI] [PubMed] [Google Scholar]
- Heutelbeck A. R. R., Bornemann C., Lange M., Seeckts A., Müller M. M. (2016). Acetylcholinesterase and neuropathy target esterase activities in 11 cases of symptomatic flight crew members after fume events. J. Toxicol. Environ. Health A 79, 1050–1056. [DOI] [PubMed] [Google Scholar]
- Hobbiger F. (1963). Reactivation of phosphorylated acetylcholinesterase In: Cholinesterases and Anticholinesterase Agents, (Koelle G. B., Ed.), pp. 922–988. Springer-Verlag, Berlin. [Google Scholar]
- Holmstedt B. (1959). Pharmacology of organophosphorus cholinesterase inhibitors. Pharmacol. Rev. 11, 5676–5688. [PubMed] [Google Scholar]
- Holmstedt B. (1963). Structure-activity relathionships of the organophosphorus anticholinesterase agents In Cholinesterases and Anticholinesterase Agents, (Koelle G. B., Ed.), pp. 428–485. Springer-Verlag, Berlin. [Google Scholar]
- Hoppin J. A., Long S., Umbach D. M., Lubin J. H., Starks S. E., Gerr F., Thomas K., Hines C. J., Weichenthal S., Kamel F., et al. (2012). Lifetime organophosphorus insecticide use among private pesticide applicators in the Agricultural Health study. J. Expos. Sci. Environ. Epidemiol. 22, 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Luo D., Zhou T., Tao Y., Feng J., Mei S. (2017). The association between non-Hodgkin lymphoma and organophosphate pesticides exposure: A meta-analysis. Environ. Pollut. 231, 319–328. 10.1016/j.envpol.2017.08.028 [DOI] [PubMed] [Google Scholar]
- Hufnagel R. B., Arno G., Hein N. D., Hersheson J., Prasad M., Anderson Y., Krueger L. A., Gregory L. C., Stoetzel C., Jaworek T. J., et al. (2015). Neuropathy target esterase impairments cause Oliver-McFarlane and Laurence-Moon syndromes. J. Med. Genet. 52, 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) (2017). Some Organophosphate Insecticides and Herbicides. IARC Monographs, Vol. 12, pp. 464 WHO Press, Geneva. [PubMed] [Google Scholar]
- Jansen K. L., Cole T. B., Park S. S., Furlong C. E., Costa L. G. (2009). Paraoxonase 1 (PON1) modulates the toxicity of mixed organophosphorus compounds. Toxicol. Appl. Pharmacol. 236, 142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. K., Glynn P. (2001). Neuropathy target esterase In Handbook of Pesticide Toxicology, (Krieger R., Ed.), pp. 953–965. Academic Press, San Diego. [Google Scholar]
- Joly C., Gay-Queheillard J., Leke A., Chardon K., Delanaud S., Bach V., Khorsi-Cauet H. (2013). Impact of chronic exposure to low doses of chlorpyrifos on the intestinal microbiota in the Simulator of the Human Intestinal Microbial Ecosystem (SHIME®) and in the rat. Environ. Sci. Pollut. Res. 20, 2726–2734. [DOI] [PubMed] [Google Scholar]
- Joly Condette C., Bach V., Mayeur C., Gay-Queheillard J., Khorsi-Cauet H. (2015). Chlorpyrifos exposure during perinatal period affects intestinal microbiota associated with delay of maturation of digestive tract in rats. J. Pediatr. Gastroenterol. Nutr. 61, 30–40. [DOI] [PubMed] [Google Scholar]
- Kalman D. A., Voorhees K. J., Osborne D., Einhorn I. N. (1985). Production of a bicyclophosphate neurotoxic agent during pyrolysis f synthetic lubricant oil. J. Fire Sci. 3, 322–329. [Google Scholar]
- Kerbrat A., Ferré J.-C., Fillatre P., Ronzière T., Vannier S., Carsin-Nicol B., Lavoué S., Vérin M., Gauvrit J.-Y., Le Tulzo Y., et al. (2016). Acute neurologic disorder from an inhibitor of fatty acid amide hydrolase. New Eng. J. Med. 375, 1717–1725. [DOI] [PubMed] [Google Scholar]
- Khan S. S., Bloom G. S. (2016). Tau: the center of a signaling nexus in Alzheimer's disease. Front. neurosci. 10, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Okubo R., Ugawa Y. (2017). Delayed neuropathy induced by organophosphate poisoning. Intern. Med. 56, 1903–1905. 10.2169/internalmedicine.56.7921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacic P. (2003). Mechanism of organophosphates (nerve gases and pesticides) and antidotes: Electron transfer and oxidative stress. Curr. Medic. Chem. 10, 2705–2709. 10.2174/0929867033456314 [DOI] [PubMed] [Google Scholar]
- Krieger R. (Ed.) (2001). Handbook of Pesticide Toxicology, pp. 1908 Academic Press, San Diego. [Google Scholar]
- Krieger R. (Ed.) (2010). Hayes’ Handbook of Pesticide Toxicology, pp. 2342 Academic Press, San Diego. [Google Scholar]
- Li B., Sedlacek M., Manoharan I., Boopathy R., Duysen E. G., Masson P., Lockridge O. (2005). Butyrylcholinesterase, paraoxonase, and albumin esterase, but not carboxylesterase, are present in human plasma. Biochem. Pharmacol. 70, 1673–1684. 10.1016/j.bcp.2005.09.002 [DOI] [PubMed] [Google Scholar]
- Liyasova M., Li B., Schopfer L. M., Nachon F., Masson P., Furlong C. E., Lockridge O. (2011). Exposure to tri-o-cresyl phosphate detected in jet airplane passengers. Toxicol. Appl. Pharmacol. 256, 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotti M. (1991). The pathogenesis of organophosphate neuropathy. Crit. Rev. Toxicol. 21, 465–487. 10.3109/10408449209089884 [DOI] [PubMed] [Google Scholar]
- Lotti M. (2010). Clinical toxicology of anticholinesterases in humans In Hayes’ Handbook of Pesticide Toxicology (Krieger R., Ed.), pp. 1543–1589. Academic Press, San Diego. [Google Scholar]
- Lotti M., Moretto A. (2005). Organophosphate-induced delayed polyneuropathy. Toxicol. Rev. 24, 37–49. 10.2165/00139709-200524010-00003 [DOI] [PubMed] [Google Scholar]
- Lotti M., Moretto A. (2017). Commentary to Merwin SJ, Obis T, Nunez Y, Re DB (2017). Organophosphate neurotoxicity to the voluntary motor system on the trail of environment-caused amyotrophic lateral sclerosis: The known, the misknown, and the unknown. Arch. Toxicol. doi: 10.1007/s00204-016-1926-1. [DOI] [PMC free article] [PubMed]
- Mahana D., Trent C. M., Kurtz Z. D., Bokulich N. A., Battaglia T., Chung J., Müller C. L., Li H., Bonneau R. A., Blaser M. J. (2016). Antibiotic perturbation of the murine gut microbiome anhnces the adiposity, insulin resistance, and liver disease associated with high-fat diet. Genome Med. 8, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood N. A., Carmichael W. W. (1987). Anatoxin-a(s), an anticholinesterase from the cyanobacterium anabaena flos-aquae NRC-525-17. Toxicon 25, 1221–1227. 10.1016/0041-0101(87)90140-1 [DOI] [PubMed] [Google Scholar]
- Marsillach J., Costa L. G., Furlong C. E. (2013). Protein adducts as biomarkers of exposure to organophosphorus compounds. Toxicology 307, 46–54. 10.1016/j.tox.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merwin S. J., Obis T., Nunez Y., Re D. B. (2017). Organophosphate neurotoxicity to the voluntary motor system on the trail of environment-caused amyotrophic lateral sclerosis: The known, the misknown, and the unknown. Arch. Toxicol. 91, 2939–2952. 10.1007/s00204-016-1926-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis S., Burdon J., Howard C. V. (2017). Aerotoxic syndrome: A new occupational disease?. Public Health Panor. 3, 198–211. [Google Scholar]
- Milatovic D., Gupta R. C., Aschner M. (2006). Anticholinesterase toxicity and oxidative stress. Sci. World J. 6, 295–310. 10.1100/tsw.2006.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnet-Tschudi F., Zurich M. G., Honegger P. (2007). Neurotoxicant-induced inflammatory response in three-dimensional brain cell cultures. Human Exp. Toxicol. 26, 339–346. [DOI] [PubMed] [Google Scholar]
- Mortensen S., Chanda S., Hooper M., Padilla S. (1996). Maturational differences in chlorpyrifos-oxonase activity may contribute to age-related sensitivity to chlorpyrifos. J. Biochem. Toxicol. 11, 279–287. [DOI] [PubMed] [Google Scholar]
- Moser V. C. (1995). Comparisons of the acute effects of cholinesterase inhibitors using a neurobehavioral screening battery in rats. Neurotoxicol. Teratol. 17, 617–625. 10.1016/0892-0362(95)02002-0 [DOI] [PubMed] [Google Scholar]
- Muñoz-Quezada M. T., Lucero B. A., Barr D. B., Steenland K., Levy K., Ryan P. B., Iglesias V., Alvarado S., Concha C., Rojas E., et al. (2013). Neurodevelopmental effects in children associated with exposure to organophosphorus pesticides: A systematic review. Neurotoxicology 39, 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Vahl K. R., Kolbe H., Dengler R. (1999). Transient severe parkinsonism after acute organophosphate poisoning. J. Neurol. Neurosurg. Psychiatry 66, 253–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S. D., Anderson R. L., DuBois K. P. (1959). Potentiation of toxicity of malathion by triorthotolyl phosphate. Proc. Soc. Exp. Biol. Med. 100, 483–487. 10.3181/00379727-100-24668 [DOI] [PubMed] [Google Scholar]
- Neumann R., Peter H. H. (1987). Insecticidal organophosphates: Nature made them first. Experientia 43, 1235–1237. 10.1007/BF01945541 [DOI] [Google Scholar]
- Parashar A., Udayabanu M. (2017). Gut microbiota: Implications in Parkinson’s disease. Parkinsonism Relat. Disord. 38, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K. C., Sinsheimer J. S., Cockburn M., Bronstein J. M., Bordelon Y., Ritz B. (2017). Organophosphate pesticides and PON1 L55M in Parkinson’s disease progression. Environ. Int. 107, 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroianu G. A. (2009). The synthesis of phosphor ethers; who was Franz Anton Voegeli?. Pharmazie 64, 269–275. [PubMed] [Google Scholar]
- Petroianu G. A. (2010a). History of organophosphate synthesis: The very early days. Pharmazie 65, 306–311. [PubMed] [Google Scholar]
- Petroianu G. A. (2010b). Toxicity of phosphor esters; Willy Lange (1900–1976) and Gerda von Krueger (1907-after 1970). Pharmazie 65, 776–780. [PubMed] [Google Scholar]
- Petroianu G. A. (2012). The history of cholinesterase reactivation: Hydroxylamine and pyridium aldoximes. Pharmazie 67, 874–879. [PubMed] [Google Scholar]
- Petroianu G. A. (2015). Synthesis of tetraethyl pyrophosphate (TEPP): From physician Abbot and pharmacist Riegel to Chemist Nylen. Pharmazie 70, 427–434. [PubMed] [Google Scholar]
- Pope C. N. (1999). Organophosphorus pesticides: Do they all have the same mechanism of action? J. Toxicol. Environ. Health B. 2, 161–181. 10.1080/109374099281205 [DOI] [PubMed] [Google Scholar]
- Pope C. N. (2010). The influence of age on pesticide toxicity In Hayes’ Handbook of Pesticide Toxicology (Krieger R., Ed.), pp. 819–835. Academic Press, San Diego. [Google Scholar]
- Portier C. J., Armstrong B. K., Baguley B. C., Baur X., Belyaev I., Bellé R., Belpoggi F., Biggeri A., Bosland M. C., Bruzzi P., et al. (2016). Differences in the carcinogenic evaluation of glyphosate between the International Agency for Research on Cancer (IARC) and the European Food Safety Authority (EFSA). J. Epidemiol. Commun. Health 70, 741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quistad G. B., Sparks S. E., Casida J. E. (2001). Fatty acid amide hydrolase inhibition by neurotoxic organophosphorus pesticides. Toxicol. Appl. Pharmacol. 173, 48–55. 10.1006/taap.2001.9175 [DOI] [PubMed] [Google Scholar]
- Quistad G. B., Sparks S. E., Segall Y., Nomura D. K., Casida J. E. (2002). Selective inhibitors of fatty acid amide hydrolase relative to neuropathy target esterase and acetylcholinesterase: Toxicological implications. Toxicol. Sci. 179, 57–63. [DOI] [PubMed] [Google Scholar]
- Quistad G. B., Liang S. N., Fisher K. J., Nomura D. K., Casida J. E. (2006). Each lipase has a unique sensitivity profile for organophosphorus inhibitors. Toxicol. Sci. 91, 166–172. [DOI] [PubMed] [Google Scholar]
- Reygner J., Lichtenberger L., Elmhiri G., Dou S., Bahi-Jaber N., Rhazi L., Depeint F., Bach V., Khorsi-Cauet H., Abdennebi-Najar L., et al. (2016). Inulin supplementation lowered the metabolic defects of prolonged exposure to chlorpyrifos from gestation to young adult stage in offspring rats. PLoS One 11, e0164614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss R., Chang E. T., Richardson R. J., Goodman M. (2015). A review of epidemiologic studies of low-level exposures to organophosphorus insecticides in non-occupational populations. Crit. Rev. Toxicol. 45, 531–641. 10.3109/10408444.2015.1043976 [DOI] [PubMed] [Google Scholar]
- Richards P. G., Johnson M. K., Ray D. E. (2000). Identification of acylpeptide hydrolase as a sensitive site for reaction with organophosphorus compounds and a potential target for cognitive enhancing drugs. Mol. Pharmacol. 58, 577–583. [DOI] [PubMed] [Google Scholar]
- Richardson R. J., Hein N. D., Wijeyesakere S. J., Fink J. K., Makhaeva G. F. (2013). Neuropathy target esterase (NTE): Overview and future. Chem. Biol. Interact. 203, 238–244. [DOI] [PubMed] [Google Scholar]
- Sanchez-Santed F., Colomina M. T., Hernandez E. H. (2016). Organophosphate pesticide exposure and neurodegeneration. Cortex 74, 417–426. [DOI] [PubMed] [Google Scholar]
- Satoh T., Gupta R.C. (Eds.) (2010). Anticholinesterase Pesticides: Metabolism, Neurotoxicity and Epidemiology, pp. 625 John Wiley and Sons, Hoboken, NJ. [Google Scholar]
- Schindler B. K., Weiss T., Schütze A., Koslitz S., Broding H. C., Bünger J., Brüning T. (2013). Occupational exposure of air crews to tricresyl phosphate isomers and organophosphate flame retardants after fume events. Arch. Toxicol. 87, 645–648. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Hardy J. (2016). The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol. Med. 8, 595–608. 10.15252/emmm.201606210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehadi M., Jones B., Hosni M. (2016). Characterization of the frequency and nature of bleed air contamination events in commercial aircrafts. Indoor Air 26, 478–488. 10.1111/ina.12211 [DOI] [PubMed] [Google Scholar]
- Smith M. I., Elvove E., Valaer P. J., Frazier W. H., Mallory G. E. (1930a). Pharmacological and chemical studies of the cause of the so called Ginger paralysis. Public Health Rep. 45, 1703–1716.19315253 [Google Scholar]
- Smith M. I., Elvove E., Frazier W. H. (1930b). The pharmacological action of certain phenol esters, with special reference to the etiology of so-called Ginger paralysis. Public Health Rep. 45, 2509–2524.19315265 [Google Scholar]
- Stanaway I. B., Wallace J. C., Shojaie A., Griffith W. C., Hong S., Wilder C. S., Green F. H., Tsai J., Knight M., Workman T., et al. (2017). Human oral buccal microbiomes are associated with farmworker status and azynphos-methyl agricultural pesticide exposure. Appl. Environ. Microbiol. 83, e02149–e02116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultatos L. G. (2006). Interactions of organophosphorus and carbamate compounds with cholinesterases In Toxicology of Organophosphate and Carbamate Compounds (Gupta R.C., Ed.), pp. 209–218. Elsevier, Amsterdam. [Google Scholar]
- Terry A. V., Jr. (2012). Functional consequences of repeated organophosphate exposure: Potential non-cholinergic mechanisms. Pharmacol. Ther. 134, 355–365. 10.1016/j.pharmthera.2012.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva O. A., Levin E. D. (2010). Lasting behavioral consequences of organophosphate pesticide exposure during development In Hayes’ Handbook of Pesticide Toxicology (Krieger R., Ed.), pp 837–846. Academic Press, San Diego. [Google Scholar]
- Tremlett H., Bauer K. C., Appel-Cresswell S., Finlay B. B., Waubant E. (2017). The gust microbiome in human neurological disease: A review. Ann. Neurol. 81, 369–382. [DOI] [PubMed] [Google Scholar]
- Vale A., Lotti M. (2015) Organophosphorus and carbamate insecticide poisoning In Occupational Neurology-Handbook of Clinical Neurology (Lotti M., Bleecker M. L., Eds.), Vol. 131, pp 149–168. Elsevier, Amsterdam. [DOI] [PubMed] [Google Scholar]
- van Esbroeck A. C. M., Janssen A. P. A., Cognetta A. B., Ogasawara D., Shpak G., van der Kroeg M., Kantae V., Baggelaar M. P., de Vrij F. M. S., Deng H., et al. (2017). Activity-based protein profiling reveals off-target proteins of the FAAH inhibitor BIA 10-2474. Science 356, 1084–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen I., de Boer J. (2012). Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88, 1119–1153. 10.1016/j.chemosphere.2012.03.067 [DOI] [PubMed] [Google Scholar]
- Viviani B., Boraso M., Marchetti N., Marinovich M. (2014). Perspectives on neuroinflammation and excitotoxicity: A neurotoxic conspiracy?. Neurotoxicology 43, 10–20. [DOI] [PubMed] [Google Scholar]
- Voorhees J. R., Rohlman D. S., Lein P. J., Pieper A. A. (2017). Neurotoxicity in preclinical models of occupational exposure to oganophosphorus compounds. Front. Neurosci. 10, 590.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Cockburn M., Ly T. T., Bronstein J. M., Ritz B. (2014). The association between ambient exposure to organophosphates and Parkinson’s disease risk. Occup. Environ. Med. 71, 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani W. Y., Kandimalla R. J. L., Sharma D. R., Kaushal A., Ruban A., Sunkaria A., Vallamkondu J., Chiarugi A., Reddy P. H., Gill K. D. (2017). Cell cycle activation in p21 dependent pathway: An alternative mechanism of organophosphate induced dopaminergic neurodegeneration. Biochim. Biophys. Acta 1863, 1858–1866. [DOI] [PubMed] [Google Scholar]
- Weichenthal S., Moase C., Chan P. (2012). A review of pesticide exposure and cancer incidence in the Agricultural Health Study Cohort. Ciencia Suade Colet. 17, 255–270. 10.1590/S1413-81232012000100028 [DOI] [PubMed] [Google Scholar]
- Wentzell J. S., Cassar M., Kretzschmar D., Iijima K. M. (2014). Organophosphate-induced changes in the PKA regulatory function of swisscheese/NTE lead to behavioral deficits and neurodegeneration. PLoS One 9, e87526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. M., Aardema M., Acquavella J., Berry S. C., Brusick D., Burns M. M., de Camargo J. L. V., Garabrant D., Greim H. A., Kier L. D., et al. (2016). A review of the carcinogenic potential of glyphosate by four independent expert panels and comparison to the IARC assessment. Crit. Rev. Toxicol. 46, 3–20. [DOI] [PubMed] [Google Scholar]
- Woo Y. T., lai D. Y., Argus M. F., Arcos J. C. (1996). Carcinogenicity of organophosphorus pesticides/compounds: An analysis of their structure-activity relationships. Environ. Carcino. Ecotoxicol. Revs. C. 14, 1–42. 10.1080/10590509609373479 [DOI] [Google Scholar]
- Worek F., Thiermann H., Wille T. (2016). Oximes in organophosphate poisoning: 60 years of hope and despair. Chem. Biol. Interact. 259, 93–98. 10.1016/j.cbi.2016.04.032 [DOI] [PubMed] [Google Scholar]
- Zaganas I., Kapetanaki S., Mastorodemos V., Kanavouras K., Colosio C., Wilks M. F., Tsatsakis A. M. (2013). Linking pesticide exposure and dementia: What is the evidence?. Toxicology 307, 3–11. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhang Y., Wang G., Han R., Xie X. (2016). Effects of chlorpyrifos on the gut microbiome and urine metabolome in mouse (Mus musculus). Chemosphere 153, 287–293. 10.1016/j.chemosphere.2016.03.055 [DOI] [PubMed] [Google Scholar]
- Zhu X., Han Y., Du J., Liu R., Jin K., Yi W. (2017). Microbiota-gut-brain axis and the central nervous system. Oncotarget 8, 53829–53838. [DOI] [PMC free article] [PubMed] [Google Scholar]