ABSTRACT
Background
It has been suggested that the prognosis of immunoglobulin (IgA) nephropathy (IgAN) is adversely affected if there is codeposition of IgG in the glomeruli or if immune deposits are present in the glomerular capillary walls. We sought to understand how these variables affect clinical outcome.
Methods
A total of 80 IgAN biopsies were retrospectively divided into groups: (i) IgA without IgG deposition versus IgA + IgG and (ii) immune deposits restricted to the mesangium versus mesangium and peripheral capillary walls (PCWs). The association of these groups with the composite primary outcome of renal replacement therapy, renal transplant, death or doubling of serum creatinine (SCr) concentration was determined. The change in estimated glomerular filtration rate (eGFR) was also assessed. Covariates examined were age, sex, race, SCr and proteinuria level at biopsy and at follow-up, duration of follow-up, treatment, Oxford score and presence of crescents.
Results
IgG codeposition showed a trend toward endocapillary hypercellularity (P = 0.082); there were no other baseline differences between the IgA (n = 55) and IgA + IgG (n = 25) groups. At a median follow-up time of 29 months, the combined primary outcome was reached in 24 patients, 16 with IgA and 8 with IgA + IgG (P = 0.82). Patients with immune deposits in the PCWs (n = 21) presented with higher baseline proteinuria than those with deposits limited to the mesangium (n = 59; P = 0.025), were more likely to have crescents/segmental glomerular necrosis on biopsy (P = 0.047) and were more likely to reach the combined primary outcome (P = 0.026). Biopsies with crescents/segmental glomerular necrosis were associated with endocapillary hypercellularity (P < 0.001).
Conclusions
In this multicenter IgAN cohort, IgG co-deposition and the location of glomerular immune deposits in the PCWs were both associated with greater histologic activity on renal biopsy, but only the location of glomerular immune deposits in the PCWs was associated with a significantly increased risk for end-stage renal disease, transplant, death and/or doubling of SCr.
Keywords: clinical outcome, IgA nephropathy, IgG co-deposition, immune deposit location, Oxford score
INTRODUCTION
The development of immunoglobulin A (IgA) nephropathy (IgAN) appears to require an autoantibody (IgG or IgA) against the autoantigen galactose-deficient IgA1 [1–5]. Pathologically, IgAN is an IgA dominant or codominant immune complex–mediated glomerulonephritis that may have codeposition of IgG by immunofluorescence microscopy (IF). The Oxford classification system for IgAN is based on the demonstration that mesangial hypercellularity (M1), segmental sclerosis (S1) and tubulointerstitial fibrosis (T) are associated with poor long-term renal outcome and that endocapillary hypercellularity (E1) is associated with positive response to anti-inflammatory/immunosuppressive treatments [6–10]. Validation studies have confirmed the clinical relevance of the Oxford classification and some have suggested that other histologic lesions, including crescent formation, may portend a negative renal prognosis [7, 11, 12] or be related to biopsy timing [13].
Codeposition of IgG with IgA and the location of immune deposits [mesangium versus both mesangium and peripheral capillary wall (PCW)] were not specifically examined in the Oxford classification, but some studies have suggested that the prognosis of IgAN may be adversely affected by either codeposition of IgG within the glomerulus [1, 14–20] or the presence of immune deposits within the glomerular capillary walls [18, 21–26]. In a follow-up immunohistochemical study of the Oxford cohort, the presence of immune deposits within PCWs or the presence of IgG codeposition both correlated with higher mesangial cellularity and E1 scores [18] and there was a trend toward worse renal survival in patients with glomerular IgG deposits. We conducted this investigation to assess the association of glomerular IgG codeposition or the location of glomerular immune deposits in IgAN on clinical renal outcomes.
MATERIALS AND METHODS
This study was approved by the Institutional Review Boards at Ohio State University Wexner Medical Center (OSU) and University of Washington Medical Center (UW) and adheres to the Declaration of Helsinki. Native kidney biopsy reports and clinical data of patients with IgAN were retrospectively reviewed. Patients were diagnosed at OSU and UW between January 2001 and December 2013. Patients with IgAN associated with bacterial infections (i.e. Staphylococcus), advanced liver disease, hepatitis B or C or human immunodeficiency virus were excluded. The Oxford classification scores for M1, E1, S1, T presence of crescents/segmental glomerular necrosis and location and intensity of IF staining (on a scale of 0–4) for IgA, IgG, IgM, C3, C1q and kappa and lambda light chains were extracted from the pathology reports. Biopsies completed before 2009 were reevaluated and scored according to the Oxford classification. A total of 175 (UW = 128, OSU = 47) patients had renal biopsy–proven IgAN, of which 80 patients had available clinical follow-up data. These 80 patients were divided into groups showing IgA without IgG codeposition and patients who had glomerular IgA + IgG codeposition by IF evaluation (Figure 1). To be considered as codeposited, IgG intensity had to be read as greater than trace on the original biopsy report. These 80 patients were also divided into a group with immune deposits confined to the mesangium and a group with deposits in the mesangium and glomerular PCWs. The location of immune deposits was determined by IF due to the generally greater number of glomeruli evaluated by IF microscopy than electron microscopy. Groups were assessed for the combined primary outcome of requiring permanent renal replacement therapy (dialysis dependent or renal transplant), death or doubling of serum creatinine (SCr). The secondary outcome was the change (slope) in estimated glomerular filtration rate (eGFR), which was calculated using the Modification of Diet in Renal Disease equation [27]. The slope of eGFR was calculated as eGFR at follow-up minus eGFR at biopsy, divided by time in months. Proteinuria was measured either as urine protein:creatinine ratio (uPCR; in g/g) in spot urine samples (63 baseline and 55 follow-up samples) or protein excretion in 24-h urine collections (53 baseline and 18 follow-up samples). Based on the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines and studies demonstrating a relatively linear relationship between 24-h urine protein excretion and spot uPCR at the low cutoff range most pertinent to our study [28–31], we grouped proteinuria data as follows: high proteinuria, 24-h urine ≥1 g/day or uPRC ≥1 g/g; low proteinuria, 24-h urine <1g/day or uPCR <1 g/g.
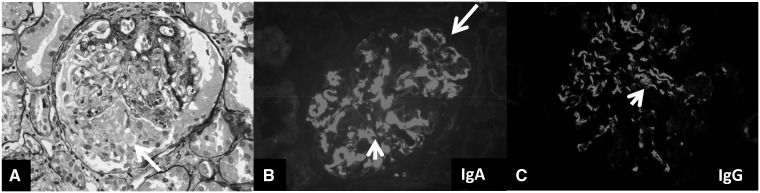
FIGURE 1.
IgAN with (A) glomerulus with segmental necrosis and cellular crescent (arrow) (×400, Jones silver stain); (B) granular mesangium (arrowhead) and PCW (arrow) IF staining for IgA (4+) (×400) and (C) mesangium staining for IgG (2+) (×400).
Statistical analyses were performed in Stata 13 (StataCorp, College Station, TX, USA); univariate differences in deposit type were evaluated using Wilcoxon rank-sum tests and Fisher’s exact tests for continuous and categorical variables, respectively. Continuous data are summarized as medians and interquartile ranges while categorical data are expressed as frequencies and percentages. Multivariate logistic and linear regressions were used to model the combined primary outcome and secondary outcome of eGFR slope. Covariates were determined through forward stepwise selection where age, sex, race, SCr, proteinuria at biopsy and at follow-up, Oxford score, presence of crescents/segmental glomerular necrosis and treatment [angiotensin-converting enzyme inhibitors (ACEis)/angiotensin receptor blockers (ARBs), corticosteroids and/or cytotoxic agent and fish oil] were considered for inclusion. As a secondary analysis, differences in time to achieve the combined primary outcome were evaluated with log-rank tests. P-values <0.05 were considered statistically significant.
RESULTS
Patients (n = 80) in whom follow-up was available were mostly male (64%) and Caucasian (69%); 14% were of Asian ancestry and 11% were African American. The median follow-up time was 29 months (range 1–361); 24 (30%) patients reached the combined primary outcome of renal replacement therapy, death or doubling SCr. Four patients lost to follow-up and four patients with end-stage renal disease (ESRD) at diagnosis were not included in the primary outcome analysis. Another four patients had no eGFR data available at the end of follow-up and were not included in the secondary outcome analysis. There was no significant difference in patient outcomes or racial distribution between the institutions. Data points were missing for the following variables: Oxford score (two biopsies with mostly sclerotic glomeruli for light microscopy insufficient for accurate Oxford classification), use of fish oil, prednisone or cytotoxic therapy (four patients), use of an ACEi or ARB (one patient), eGFR at follow-up (five patients), and proteinuria at follow-up (either spot uPCR or 24-h; 12 patients).
When the pathology biopsy data from all 175 IgAN biopsies from OSU and UW were compared, 42 (24%) had IgG codeposition and 75 (43%) had immune deposits in the PCWs; 16 cases (9%) had both IgG codeposition and mesangium + PCW immune deposits. Cases with mesangium + PCW immune deposits were more likely to have crescents or segmental glomerular necrosis compared with those with mesangial deposits only (51% versus 27%; P = 0.002). Approximately 76% of cases with PCW deposits by IF had subendothelial and/or intramembranous deposits by electron microscopy and 7% had subepithelial deposits; in 24%, no deposits were seen in the peripheral capillary loops by EM. There was no significant difference between the IgA and IgA + IgG groups regarding immune deposit location nor any of the other biopsy findings. Biopsies with crescents and/or segmental glomerular necrosis (n = 65) were associated with E (P < 0.001). There were no significant differences in any of the biopsy findings between cases with [n = 132 (75%)] versus without C3 deposition (data not shown). There were no significant differences in Oxford scores between the two institutions. UW biopsies showed more cases with crescents and/or segmental glomerular necrosis (OSU 23% versus UW 42%; P = 0.023) and more cases of mesangium + PCW immune deposits (OSU 15% versus UW 53%; P < 0.001).
Renal outcome and IgG codeposition
In all, 55 patients had biopsies demonstrating glomerular IgA deposits without IgG codeposition (IgA); and 25 had codeposition of glomerular IgG (IgA + IgG). The two groups were comparable with regard to sex, race, initial and follow-up eGFR, initial and follow-up proteinuria and treatment (Table 1). Patients with IgG codeposition showed a nonsignificant trend toward younger age (33 versus 38 years; P = 0.129) and E1 (58% versus 35%; P = 0.082) compared with patients without IgG codeposition. C3 was detected in 23 (92%) cases with IgG codeposition versus 44 cases (80%) without (statistically not significant).
Table 1.
Baseline characteristics and clinical outcome, IgAN by IgG codeposition
| IgA | IgA + IgG | P-value | |
|---|---|---|---|
| [n = 55 (69%)] | [n = 25 (31%)] | ||
| Clinical characteristics | |||
| Age (years) | 38 (25–47) | 33 (24–40) | 0.129 |
| Gender | 0.802 | ||
| Female | 35% (19) | 40% (10) | |
| Male | 65% (36) | 60% (15) | |
| Race | 0.709 | ||
| Asian | 15% (8) | 12% (3) | |
| Caucasian | 65% (36) | 76% (19) | |
| Other | 20% (11) | 12% (3) | |
| SCr at biopsy (mg/dL) | 1.3 (0.99–2.7) | 1.3 (0.93–2.0) | 0.604 |
| eGFR at biopsya (mL/min/1.73 m2) | 61.4 (27.7–92.1) | 56.0 (28.3–90.5) | 0.944 |
| uPCR at biopsy (g/g) | 1.33 (0.7–4.07) | 0.85 (0.44–2.52) | 0.192 |
| uPCR at biopsyb | 0.425 | ||
| High | 25% (14) | 36% (9) | |
| Low | 75% (41) | 64% (16) | |
| Length of follow-up (months) | 27 (9.0–55.3) | 32 (18.6–48.2) | 0.495 |
| Biopsy characteristics | |||
| Mesangial hypercellularity (M1) | 67% (36) | 79% (19) | 0.297 |
| Endocapillary hypercellularity (E1) | 35% (19) | 58% (14) | 0.082 |
| Segmental sclerosis (S1) | 74% (40) | 75% (18) | 1.000 |
| Interstitial fibrosis (T) | 0.553 | ||
| T0 | 52% (28) | 50% (12) | |
| T1 | 22% (12) | 33% (8) | |
| T2 | 26% (14) | 17% (4) | |
| Presence of crescents/segmental glomerular necrosis | 25% (14) | 36% (9) | 0.425 |
| Immune deposit localization | 0.584 | ||
| Mesangium + PCWs | 29% (16) | 20% (5) | |
| Mesangium only | 71% (39) | 80% (20) | |
| Treatment | |||
| Use of ACEi or ARB | 83% (45) | 96% (23) | 0.162 |
| Use of prednisone and/or cytotoxic agents | 40% (21) | 46% (11) | 0.803 |
| Use of fish oil | 38% (20) | 42% (10) | 0.806 |
| Outcome | |||
| Number of patients reaching combined outcomec | 29% (16) | 32% (8) | 0.823 |
| eGFR at follow-upa (mL/min/1.73 m2) | 69.0 (23.5–94.6) | 49.7 (25.0–97.0) | 0.768 |
| uPCR at follow-up (g/g) | 0.37 (0.1–0.6) | 0.54 (0.2–1.27) | 0.145 |
| uPCR at follow-upb | 0.346 | ||
| High | 24% (9) | 39% (7) | |
| Low | 76% (28) | 61% (11) | |
| Slope of eGFRa (mL/min/1.73 m2)/month | −0.05 ± 1.9 | −0.03 ± 0.82 | 0.980 |
Values are presented as median (interquartile range) and percentage and absolute numbers.
eGFR based of a total of 65 patients with follow-up and removal of outliers; slope of eGFR: change of eGFR over the observation period.
High is defined as a uPCR ≥1; low is defined as a uPCR <1.
Combined outcome: doubling of SCr concentration, renal replacement therapy, kidney transplantation or death.
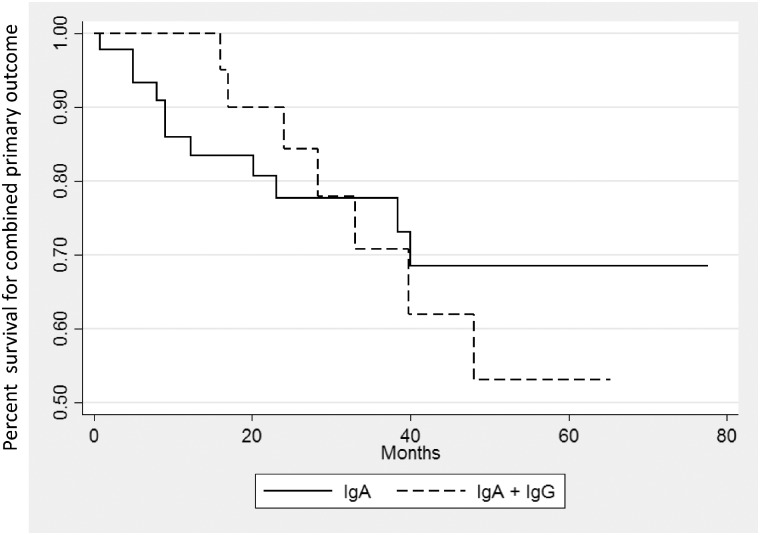
Of 24 patients reaching the combined primary outcome, 16 (67%) had only IgA deposits and the rest had IgA + IgG deposits (Table 2). There was no statistical difference between the IgA and IgA + IgG groups with regard to experiencing the primary outcome, with or without adjusting for SCr at biopsy, nor the time to achieve the combined primary outcome by survival analysis (Figure 2). After removal of outliers, 65 patients who did not reach the primary outcome had available eGFR information. No significant difference in the slope of eGFR between patients with only IgA deposits and patients with IgA + IgG deposits was found {P = 0.980 (95% confidence interval (CI) −0.87–0.89]; Table 1]}. Multivariate analysis showed that treatment with prednisone or a cytotoxic agent (coefficient 1.45; P = 0.001), treatment with fish oil (coefficient 1.24; P = 0.002), presence of segmental glomerular sclerosis (coefficient −1.67; P = 0.001) and mesangial deposition (coefficient 1.15; P = 0.013) were significant independent predictors of the slope of eGFR (positive coefficient indicates an association with increased eGFR; negative coefficient indicates an association with decreased eGFR). No significant difference in the slope of eGFR between patients with only IgA deposits and patients with IgA + IgG deposits was found [P = 0.686 (95% CI −0.634–0.956)]. Removal of 16 patients with <6 months follow-up did not produce a significant change in the results.
Table 2.
Primary outcome in IgAN
| Biopsy group | n (%) | Death | Dialysis dependent | Transplant | Double SCr | Total | P-value |
|---|---|---|---|---|---|---|---|
| IgA + IgG | 25 (31) | 1 (12) | 3 (38) | 2 (25) | 2 (25) | 8 (33) | 0.82 |
| IgA | 55 (69) | 1 (6) | 10 (63) | 4 (25) | 1 (6) | 16 (67) | |
| Total | 80 | 2 (8) | 13 (54) | 6 (25) | 3 (13) | 24 | |
| Mesangium + PCW | 21 (26) | 1 (10) | 4 (40) | 3 (30) | 2 (20) | 10 (42) | 0.047 |
| Mesangium | 59 (74) | 1 (7) | 9 (64) | 3 (22) | 1 (7) | 14 (58) | |
| Total | 2 (8) | 13 (54) | 6 (25) | 3 (13) | 24 |
Values are presented as n (%).
FIGURE 2.
There was no difference between the IgA and IgA + IgG groups in time to achieve the combined primary outcome by survival analysis (P = 0.74).
Renal outcome and PCW deposits
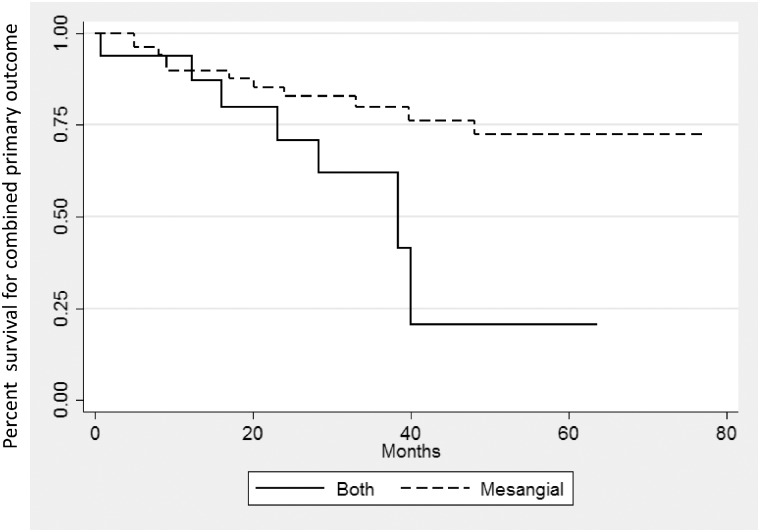
Of the 80 patients, 21 (26%) had immune deposits in the mesangium + PCW and the rest had immune deposits in the mesangium only (Table 3). Patients with immune deposits in the mesangium + PCW had greater proteinuria at biopsy (median uPCR = 3.35 versus 1.05 g/g; P = 0.025), were more likely to have crescents and/or segmental glomerular necrosis (48% versus 22% of biopsies; P = 0.047) and had a trend toward shorter follow-up time and/or time to reach the primary endpoint (P = 0.059; Figure 3) compared with those with deposits in the mesangium only. There were no significant differences between the two groups with regard to age, sex, race, initial eGFR, Oxford scores, follow-up proteinuria or treatment.
Table 3.
Baseline characteristics and clinical outcome for IgAN by glomerular immune deposit location
| Mesangium + PCWs | Mesangium only | P-value | |
|---|---|---|---|
| [n = 21 (26%)] | [n = 59 (74%)] | ||
| Clinical characteristics | |||
| Age (years) | 36 (25–43) | 37 (25–45) | 0.793 |
| Gender | 0.598 | ||
| Female | 43% (9) | 34% (20) | |
| Male | 57% (12) | 66% (39) | |
| Race | 0.929 | ||
| Asian | 14% (3) | 13% (8) | |
| Caucasian | 71% (15) | 68% (40) | |
| Other | 14% (3) | 19% (11) | |
| SCr at biopsy (mg/dL) | 1.3 (0.99–3.35) | 1.3 (0.95–2.50) | 0.768 |
| eGFR at biopsya (mL/min/1.73 m2) | 67.4 (29.7–79.3) | 52.8 (27.6–92.1) | 0.756 |
| uPCR at biopsy (g/g) | 3.35 (0.93–5.5) | 1.05 (0.6–2.01) | 0.025 |
| uPCR at biopsyb | 0.780 | ||
| High | 24% (5) | 31% (18) | |
| Low | 76% (16) | 69% (41) | |
| Length of follow-up (months) | 21 (12.2–35.3) | 33 (14.7–57.5) | 0.059 |
| Biopsy characteristics | |||
| Mesangial hypercellularity (M1) | 65% (13) | 72% (42) | 0.576 |
| Endocapillary hypercellularity (E1) | 55% (11) | 38% (22) | 0.201 |
| Segmental sclerosis (S1) | 75% (15) | 74% (43) | 1.000 |
| Interstitial fibrosis (T) | 0.409 | ||
| T0 | 55% (11) | 50% (29) | |
| T1 | 15% (3) | 29% (17) | |
| T2 | 30% (6) | 21% (12) | |
| Presence of crescents/segmental glomerular necrosis | 48% (10) | 22% (13) | 0.047 |
| Treatment | |||
| Use of ACEi or ARB | 80% (16) | 90% (52) | 0.267 |
| Use of prednisone and/or cytotoxic agents | 45% (9) | 41% (23) | 0.796 |
| Use of fish oil | 40% (8) | 39% (22) | 1.000 |
| Outcome | |||
| Number of patients reaching combined outcomec | 48% (10) | 24% (14) | 0.026 |
| eGFR at follow-upa (mL/min/1.73 m2) | 65.9 (9.0–93.9) | 64.9 (29.4–96.4) | 0.767 |
| uPCR at follow up (g/g) | 0.50 (0.16–1.60) | 0.45 (0.1–1.0) | 0.433 |
| uPCR at follow upb | 1.000 | ||
| High | 31% (4) | 29% (12) | |
| Low | 69% (9) | 71% (30) | |
| Slope of eGFRa (mL/min/1.73 m2)/month | −0.50 ± 2.66 | 0.09 ± 1.20 | 0.225 |
Values are presented as median (interquartile range) and percentage and absolute numbers.
eGFR based of a total of 65 patients with follow-up and removal of outliers; slope of eGFR: change of eGFR over the observation period.
High is defined as a uPCR ≥1; low is defined as a uPCR <1.
Combined outcome: doubling of SCr concentration, renal replacement therapy, kidney transplantation or death.
FIGURE 3.
Patients with immune deposits in the PCWs were significantly more likely to reach the primary outcome than those with deposits in the mesangium alone (P = 0.02). At ≥40 months of follow-up, 26 patients remained in the cohort. In all, 24 of these patients had immune deposits limited to the mesangium, 2 of whom reached the primary outcome. Two patients had immune deposits in the PCWs in addition to the mesangium, one of whom reached the primary outcome.
Of 24 patients reaching the primary outcome, 10 (42%) had mesangium + PCW deposits (Table 2; P = 0.026). The odds of experiencing the combined primary outcome for patients with deposits in the mesangium + PCW was 3.4-fold higher than for patients with mesangial deposits only [P = 0.026 (95% CI 1.15–10.09)]. The odds ratio increased to 4.93 after adjusting for SCr at diagnosis [P = 0.037 (95% CI 1.10–21.98)]. After removal of outliers, 65 patients who did not reach the primary outcome had available eGFR information. There was no significant difference in the slope of eGFR between patients with mesangium + PCW deposits versus patients with only mesangial deposits [P = 0.225 (95% CI −1.56–0.37); Table 3]. After adjusting for treatment with prednisone or a cytotoxic agent (coefficient 1.43; P = 0.001), treatment with fish oil (coefficient 1.23; P = 0.002), the presence of segmental glomerular sclerosis (coefficient −1.67; P < 0.001) and mesangial deposition (coefficient 1.15; P = 0.013) were significant independent predictors of the slope of eGFR. Removal of 16 patients with <6 months follow-up did not produce a significant change in the results.
DISCUSSION
In this study we retrospectively examined definitive patient and renal outcomes in two institutional IgAN cohorts with respect to variables not considered in the Oxford scoring system, including (i) the presence of IgG in glomerular immune deposits and (ii) the location of immune deposits in the glomerulus. Compared to IgAN without glomerular IgG codeposition, we found a trend toward greater histologic activity in the form of E1, but no association of IgG codeposition with the combined primary renal outcome of renal replacement therapy, death or doubling of SCr, nor for the secondary outcome of change in eGFR over time. Compared to biopsies with immune deposits limited to the mesangium, the presence of immune deposits in the glomerular capillary walls was associated with increased histologic activity in the form of crescents/segmental glomerular necrosis, greater proteinuria at presentation and a significantly greater likelihood of reaching the combined primary outcome. As a secondary outcome, when treatment and presence of S1 were added to a multivariate model for slope of eGFR, the rate of eGFR change was significantly higher in patients with immune deposits limited to the mesangium than those with deposits in the capillary loops; this raises the possibility of an interaction between immune deposit location and treatment and/or other histologic lesions. In the overall cohort we found that treatment with fish oil and/or prednisone or a cytotoxic agent were both significantly associated with an increase in eGFR over time. There was an association between S1 and impaired eGFR, consistent with observations from the Oxford classification studies [6].
Whether the presence of IgG codeposition in glomeruli plays a pathogenic role in injury or serves as a biomarker of prognosis or evidence of chronicity in IgAN [16, 17] has yet to be fully understood. Previous studies have suggested that IgG antibodies against the abnormal (aberrantly glycosylated in the hinge region) IgA1 may be formed in IgAN patients, making IgG–IgA immune complexes that can be found in the serum [4, 32–34, 35] and/or in the glomeruli. It was postulated that IgG–IgA immune complexes might predispose IgAN patients to worse renal outcomes than IgA–IgA complexes [36]. IgA is considered a poor activator of classical complement pathway [16], although it may activate complement through the alternative and mannose-binding lectin (MBL) pathways [2, 34], whereas IgG may trigger complement activation through the classical pathway [19], possibly increasing the likelihood of complement-mediated kidney injury. In a rat model [19], the addition of IgG to polymeric IgA resulted in significantly greater proteinuria, which was hypothesized to be the result of enhanced complement activation [37]. In our series, there were no significant differences in the presence of C3 or C1q deposition in IgAN biopsies with or without IgG codeposition. Other investigators have proposed that IgG codeposits could be a marker of chronicity [16, 17], not greater activity. However, in the current study, lesions of chronicity were not significantly different between patients with IgA or IgA + IgG codeposition.
Clinical studies have assessed the role of IgG deposition in IgAN. In a Caucasian cohort, IgG deposition in IgAN was associated with the development of hypertension at long-term follow-up and was an independent determinant of progressive renal failure [16]. In a Japanese cohort, IgG deposits correlated with a reduced likelihood for complete remission, greater proteinuria at diagnosis, more frequent PCW deposits and persistent urinary abnormalities [17]. A recent Korean study found glomerular IgG deposition to be independently associated with poor renal outcome in IgAN [20]. Finally, a follow-up study of the Oxford cohort by Bellur et al. [18] showed that IgG codeposition was associated with M1 and endocapillary proliferation, but not with worse renal survival. Our study confirms the finding of Bellur et al. of an association between IgG codeposition and E1, and not a primary renal outcome. It differs from their study in that their biopsies were evaluated by immunohistochemistry rather than immunofluorescence microscopy, and they had a lower percentage of patients with immune deposits in the peripheral capillary loops (15% versus 26% by IF in our study). Perhaps related to these or other differences, they found no significant association between the location of immune deposits and any of the outcome measures, which contrasts with the findings of our study. In the context of these studies, our investigation also differed by examining a predominantly Caucasian population and hard renal endpoints of ESRD, doubling of SCr or death. These differences may have contributed to not finding an association of IgG codeposition with renal outcomes.
The second question our study addressed was the clinical significance of the location of glomerular immune deposits, with particular attention to those within the PCWs by IF. Patients with immune deposits in the mesangium + PCW had a significantly greater likelihood of worse long-term renal outcome than those with deposits limited to the mesangium. The odds of experiencing the combined primary outcome for patients with deposits in the mesangium + PCW were 3.4-fold higher than for patients with mesangial deposits only, and increased to 4.93 after adjusting for SCr at diagnosis. The clinical implications of immune deposit location have also been noted in other studies. A Japanese study found that the degree of immune deposits in the peripheral capillary loop was associated with lower (<70 mL/min) baseline and/or follow-up creatinine clearance [22]. Similar to these findings, one Italian study observed that having mesangium + PCW immune deposition increased the relative risk for renal death or replacement therapy [21]. In another Japanese cohort, patients with immune deposits in the peripheral capillary loops had greater baseline proteinuria and were more likely to develop persistent renal functional abnormalities at 5 years [25]. In an American pediatric cohort, children with IgA deposits in the PCWs versus mesangium had more proteinuria at diagnosis, more glomerular crescents and more chronic changes, including segmental or global glomerular sclerosis as well as tubular atrophy and interstitial fibrosis; these children were also more likely to have persistent proteinuria and progressive kidney disease [24]. Finally, D’Amico et al. [21]showed that extension of IgA deposits into the peripheral capillary loops predicted progressive renal failure in IgAN [21].
Animal models of IgAN have also illustrated the impact of immune deposit location in determining disease activity [38, 39]. Mechanistically, subendothelial immune deposits may accumulate in the PCWs due to size and electric charge [37] and may persist for longer periods of time [39, 40]. These subendothelial capillary wall deposits may stimulate the release of chemotaxins to the circulation, leading to recruitment of inflammatory cells to the kidney [38], which may be partially independent of complement activation [40]. PCW deposits by IF often correlated with a subendothelial deposit location by electron microscopy in this study of IgAN. Perhaps the subendothelial location allows greater accessibility to circulating leukocytes and facilitates leukoctye recruitment through engagement of Fc receptors and/or greater release of chemotactic peptides such as C3a and C5a, contributing to a higher initial proteinuria, glomerular crescents/segmental necrosis and worse overall renal outcome seen in this study.
Limitations of this study include its retrospective nature, and therefore the inability to control for treatment, duration of follow-up, completeness and timing of data collection and other demographic and clinical differences. Our findings may differ from prior clinical studies due in part to the cutoff level for IgG codeposition we used, a mixed but predominantly Caucasian population, inclusion of patients with <6 months of follow-up or who had progressed within 6 months, lack of a specifically focused assessment of blood pressure or histologic vascular parameters and our use of definitive renal outcome as the combined primary endpoint. The somewhat limited number of patients reaching the combined primary endpoint also limits our ability to fully assess potential associations and risk factors influencing the outcome. As IgAN is typically a chronic disease and our study has a comparatively shorter median follow-up time, our results may be biased toward and more reflective of biopsy findings in patients whose disease progresses more rapidly. Biopsy findings between the two institutions were mostly comparable, and differences may reflect variation in local clinical practice with respect to the threshold for performing a kidney biopsy in suspected IgAN.
In conclusion, in this multicenter IgAN cohort, IgG co-deposition and location of glomerular immune deposits in the PCWs were both associated with greater histologic activity on renal biopsy, but only the location of glomerular immune deposits was significantly associated with definitive renal outcomes. Patients with immune deposits in the PCWs in addition to the mesangium had greater proteinuria at diagnosis and were at increased risk for ESRD, transplant, death and/or doubling of SCr. The generalizability of this observation needs to be validated in a larger, prospective cohort.
ACKNOWLEDGEMENTS
This work was presented in part in poster form at the American Society of Nephrology Kidney Week (2014) and the United States and Canadian Academy of Pathology Annual Meeting (2016).
FUNDING
Source of support: DK096927.
CONFLICT OF INTEREST STATEMENT
None declared. The results of this paper have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Barratt J, Feehally J.. IgA nephropathy. J Am Soc Nephrol 2005; 16: 2088–2097 [DOI] [PubMed] [Google Scholar]
- 2. Wyatt RJ, Julian BA.. IgA nephropathy. N Engl J Med 2013; 368: 2402–2414 [DOI] [PubMed] [Google Scholar]
- 3. Coppo R, Amore A.. Aberrant glycosylation in IgA nephropathy (IgAN). Kidney Int 2004; 65: 1544–1547 [DOI] [PubMed] [Google Scholar]
- 4. Suzuki H, Moldoveanu Z, Hall S. et al. IgA nephropathy: characterization of IgG antibodies specific for galactose-deficient IgA1. Contrib Nephrol 2007; 157: 129–133 [DOI] [PubMed] [Google Scholar]
- 5. Allen AC, Harper SJ, Feehally J.. Galactosylation of N- and O-linked carbohydrate moieties of IgA1 and IgG in IgA nephropathy. Clin Exp Immunol 1995; 100: 470–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int 2009; 76: 534–545 [DOI] [PubMed] [Google Scholar]
- 7. Lv J, Shi S, Xu D. et al. Evaluation of the Oxford classification of IgA nephropathy: a systematic review and meta-analysis. Am J Kidney Dis 2013; 62: 891–899 [DOI] [PubMed] [Google Scholar]
- 8. Yau T, Korbet SM, Schwartz MM. et al. The Oxford classification of IgA nephropathy: a retrospective analysis. Am J Nephrol 2011; 34: 435–444 [DOI] [PubMed] [Google Scholar]
- 9. Herzenberg AM, Fogo AB, Reich HN. et al. Validation of the Oxford classification of IgA nephropathy. Kidney Int 2011; 80: 310–317 [DOI] [PubMed] [Google Scholar]
- 10. Coppo R, Troyanov S, Bellur S. et al. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int 2014; 86: 828–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang Z, Wu Y, Wang QW. et al. Idiopathic IgA nephropathy with diffuse crescent formation. Am J Nephrol 2002; 22: 480–486 [DOI] [PubMed] [Google Scholar]
- 12. Haas M, Verhave JC, Liu ZH. et al. A multicenter study of the predictive value of crescents in IgA nephropathy. J Am Soc Nephrol 2017; 28: 691–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shima Y, Nakanishi K, Hama T. et al. Validity of the Oxford classification of IgA nephropathy in children. Pediatr Nephrol 2012; 27: 783–792 [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi Y, Hiki Y, Fujii K. et al. Moderately proteinuric IgA nephropathy: prognostic prediction of individual clinical courses and steroid therapy in progressive cases. Nephron 1989; 53: 250–256 [DOI] [PubMed] [Google Scholar]
- 15. Rekola S, Bergstrand A, Bucht H.. IgA nephropathy: a retrospective evaluation of prognostic indices in 176 patients. Scand J Urol Nephrol 1989; 23: 37–50 [DOI] [PubMed] [Google Scholar]
- 16. Nieuwhof C, Kruytzer M, Frederiks P. et al. Chronicity index and mesangial IgG deposition are risk factors for hypertension and renal failure in early IgA nephropathy. Am J Kidney Dis 1998; 31: 962–970 [DOI] [PubMed] [Google Scholar]
- 17. Wada Y, Ogata H, Takeshige Y. et al. Clinical significance of IgG deposition in the glomerular mesangial area in patients with IgA nephropathy. Clin Exp Nephrol 2013; 17: 73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bellur SS, Troyanov S, Cook HT. et al. Immunostaining findings in IgA nephropathy: correlation with histology and clinical outcome in the Oxford classification patient cohort. Nephrol Dial Transplant 2011; 26: 2533–2536 [DOI] [PubMed] [Google Scholar]
- 19. Van Dixhoorn MG, Sato T, Muizert Y. et al. Combined glomerular deposition of polymeric rat IgA and IgG aggravates renal inflammation. Kidney Int 2000; 58: 90–99 [DOI] [PubMed] [Google Scholar]
- 20. Shin DH, Lim BJ, Han IM. et al. IgG deposition predicts renal outcome in patients with IgA nephropathy. Mod Pathol 2016; 29: 743–752 [DOI] [PubMed] [Google Scholar]
- 21. D'Amico G, Minetti L, Ponticelli C. et al. Prognostic indicators in idiopathic IgA mesangial nephropathy. Q J Med 1986; 59: 363–378 [PubMed] [Google Scholar]
- 22. Kobayashi Y, Tateno S, Hiki Y. et al. IgA nephropathy: prognostic significance of proteinuria and histological alterations. Nephron 1983; 34: 146–153 [DOI] [PubMed] [Google Scholar]
- 23. Freese P, Nordén G, Nyberg G.. Morphologic high-risk factors in IgA nephropathy. Nephron 1998; 79: 420–425 [DOI] [PubMed] [Google Scholar]
- 24. Andreoli SP, Yum MN, Bergstein JM.. IgA nephropathy in children: significance of glomerular basement membrane deposition of IgA. Am J Nephrol 1986; 6: 28–33 [DOI] [PubMed] [Google Scholar]
- 25. Yoshimura M, Kida H, Abe T. et al. Significance of IgA deposits on the glomerular capillary walls in IgA nephropathy. Am J Kidney Dis 1987. May; 9: 404–409 [DOI] [PubMed] [Google Scholar]
- 26. Roberts IS. Pathology of IgA nephropathy. Nat Rev Nephrol 2014; 10: 445–454 [DOI] [PubMed] [Google Scholar]
- 27. Stevens LA, Coresh J, Greene T. et al. Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med 2006; 354: 2473–2483 [DOI] [PubMed] [Google Scholar]
- 28. Ginsberg JM, Chang BS, Matarese RA. et al. Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med 1983; 309: 1543–1546 [DOI] [PubMed] [Google Scholar]
- 29. Chitalia VC, Kothari J, Wells EJ. et al. Cost-benefit analysis and prediction of 24-hour proteinuria from the spot urine protein-creatinine ratio. Clin Nephrol 2001; 55: 436–447 [PubMed] [Google Scholar]
- 30. Wahbeh AM. Spot urine protein-to-creatinine ratio compared with 24-hour urinary protein in patients with kidney transplant. Exp Clin Transplant 2014; 12: 300–303 [PubMed] [Google Scholar]
- 31. Price CP, Newall RG, Boyd JC.. Use of protein: creatinine ratio measurements on random urine samples for prediction of significant proteinuria: a systematic review. Clin Chem 2005; 51: 1577–1586 [DOI] [PubMed] [Google Scholar]
- 32. Tomana M, Matousovic K, Julian BA. et al. Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int 1997; 52: 509–516 [DOI] [PubMed] [Google Scholar]
- 33. Tomana M, Novak J, Julian BA. et al. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest 1999. Jul; 104: 73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barratt J, Smith AC, Molyneux K. et al. Immunopathogenesis of IgAN. Semin Immunopathol 2007; 29: 427–443 [DOI] [PubMed] [Google Scholar]
- 35. Suzuki H, Fan R, Zhang Z. et al. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 2009; 119: 1668–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Berthoux F, Suzuki H, Thibaudin L. et al. Autoantibodies targeting galatose-deficient IgA1 associate with progression of IgA nephropathy. J Am Soc Nephrol 2012; 23: 1579–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Waldo FB, Cochran AM.. Mixed IgA-IgG aggregates as a model of immune complexes in IgA nephropathy. J Immunol 1989; 142: 3841–3846 [PubMed] [Google Scholar]
- 38. Salant DJ, Adler S, Darby C. et al. Influence of antigen distribution on the mediation of immunological glomerular injury. Kidney Int 1985; 27: 938–950 [DOI] [PubMed] [Google Scholar]
- 39. Fries JW, Mendrick DL, Rennke HG.. Determinants of immune complex-mediated glomerulonephritis. Kidney Int 1988; 34: 333–345 [DOI] [PubMed] [Google Scholar]
- 40. Thaiss F, Batsford S, Mihatsch MJ. et al. Mediator systems in a passive model of in situ immune complex glomerulonephritis. Role for complement, polymorphonuclear granulocytes and monocytes. Lab Invest 1986; 54: 624–635 [PubMed] [Google Scholar]