Abstract
Objective
To test the hypothesis that acute elevations in serum inflammatory markers predict symptom recovery after sport-related concussion (SRC).
Methods
High school and collegiate football players (n = 857) were prospectively enrolled. Forty-one athletes with concussion and 43 matched control athletes met inclusion criteria. Serum levels of interleukin (IL)–6, IL-1β, IL-10, tumor necrosis factor, C-reactive protein, interferon-γ, and IL-1 receptor antagonist and Sport Concussion Assessment Tool, 3rd edition (SCAT3) symptom severity scores were collected at a preinjury baseline, 6 and 24–48 hours postinjury, and approximately 8, 15, and 45 days following concussion. The number of days that athletes were symptomatic following SRC (i.e., duration of symptoms) was the primary outcome variable.
Results
IL-6 and IL-1RA were significantly elevated in athletes with concussion at 6 hours relative to preinjury and other postinjury visits, as well as compared to controls (ps ≤ 0.001). IL-6 and IL-1RA significantly discriminated concussed from control athletes at 6 hours postconcussion (IL-6 area under receiver operating characteristic curve 0.79 [95% confidence interval (CI) 0.65–0.92], IL-1RA AUC 0.79 [95% CI 0.67–0.90]). Further, IL-6 levels at 6 hours postconcussion were significantly associated with the duration of symptoms (hazard ratio for symptom recovery = 0.61 [95% CI 0.38–0.96], p = 0.031).
Conclusions
Results support the potential utility of IL-6 and IL-1RA as serum biomarkers of SRC and demonstrate the potential of these markers in identifying athletes at risk for prolonged recovery after SRC.
Classification of evidence
This study provides Class III evidence that serum levels of IL-6 and IL-1RA 6 hours postconcussion significantly discriminated concussed from control athletes.
Sport-related concussion (SRC) occurs frequently in the United States, with rates cited as high as 3.8 million per year.1 Clinical recovery is commonly based on the resolution of symptoms and is typically observed within 1 to 2 weeks of injury; however, a sizeable subset of athletes report more prolonged symptoms and recovery.2 Thus, there is great interest in identifying objective biomarkers to not only aid in the diagnosis of SRC but also to predict which patients may be at risk for medical morbidity or delayed recovery. To date, most research has focused on identifying blood biomarkers that are relatively specific to brain injury. However, blood biomarkers capturing other aspects of the known neurometabolic cascade of concussion may hold promise as prognostic markers for poor recovery following mild traumatic brain injury (mTBI) including SRC.
Inflammation is one of the known physiologic responses to brain injury of all severities.3,4 Numerous studies have demonstrated that the inflammatory effects of brain injury can be measured in the periphery (i.e., blood), with elevated cytokine profiles being documented in the blood of patients with moderate to severe traumatic brain injury (TBI). For example, increases in serum levels of inflammatory cytokines interleukin (IL)–1β, IL-6, IL-10, and tumor necrosis factor (TNF) have been documented in emergency department (ED) patients within 24 hours following injury.5–11 Importantly, additional research has reported associations between inflammatory cytokines measured within 24 hours in ED patients with worse outcomes on the Glasgow Outcome Score,9,11,12 as well as risk for mortality in intensive care unit patients.13
The acute inflammatory response following mTBI in humans has not been well-documented. Lagerstedt et al.14 reported IL-10 levels at 6 hours following mTBI differentiated between CT-positive and CT-negative patients, though there was no comparison to a control group without mTBI. Gill and colleagues15 reported acute and transient increases in IL-6 and TNF following moderate blast exposures in military personnel without diagnoses of mTBI. Two studies in overlapping samples of athletes observed downregulated mRNA expression of genes involved in the inflammatory response in peripheral blood mononuclear cells within 6 hours of SRC.16,17 Finally, another study reported that elevated C-reactive protein (CRP) levels, which are typically increased in response to IL-6, in mTBI patients at hospital admission predicted persistent symptoms 3 months postinjury.18
In the current study, we investigated acute (i.e., hours) and subacute (i.e., days to weeks) effects of SRC on circulating concentrations of inflammatory markers in high school and collegiate football players. Importantly, all athletes participated in baseline, preseason visits to allow for the comparison of postinjury to preinjury biomarker levels. Inflammatory markers were selected based on previous work and included IL-6, IL-1β, IL-10, TNF, CRP, and interferon-γ (IFN-γ). In addition, IL-1 receptor antagonist (IL-1RA) was investigated as a surrogate for IL-1β activity due to typically difficult to detect lower levels of IL-1β in serum.3 We hypothesized that football players with SRC would have elevated inflammatory markers at the acute phase relative to preinjury levels and relative to noninjured football players, with subsequent recovery at the subacute time points. In addition, we hypothesized that acute increases in inflammatory markers would predict longer duration of symptoms recovery following SRC.
Methods
The primary research questions were the following: What are the acute effects of SRC on serum inflammatory markers? Can acute serum inflammatory markers discriminate concussed from control athletes (Class III level of evidence)? Are elevated inflammatory markers associated with symptom duration following SRC?
Standard protocol approvals, registrations, and patient consents
The Medical College of Wisconsin institutional review board approved all aspects of this study. Minors provided written assent and parents of minors and adult participants provided written informed consent.
Participants
A subset of high school and collegiate football players enrolled as part of a prospective study of SRC in athletes from southeastern Wisconsin (Project Head to Head II) were included in the current work. Football players (n = 857) were recruited prior to the 2015 (n = 545) or 2016 (n = 312) football seasons (figure 1). A total of 90 concussions were documented in the time frame of this study; 67 participated in follow-up visits as part of Project Head to Head II based on the following exclusion criteria: history or suspicion of conditions known to cause cognitive dysfunction (e.g., moderate to severe TBI, epilepsy), injury that would preclude participation in the study protocol, current psychotic disorder or narcotic use, or any contraindication to study procedures. Athletes with concussion completed follow-up visits at approximately 6 hours and 24–48 hours postinjury, as well as approximately 8, 15, and 45 days postinjury. Identification and diagnosis of SRC was determined by team physicians or certified athletic trainers at participating institutions. Study investigators screened and triaged all injuries to confirm they met study requirements and definition. The study definition of concussion was based on the Centers for Disease Control and Prevention HEADS UP educational initiative, as previously described.19
Figure 1. Flowchart of athlete recruitment.
Athlete enrollment and selection from the parent project cohort (Head to Head II) and the current study. SRC = sport-related concussions. *Excluded participants could meet more than one exclusion criterion. **One control participant was not pulled for further analysis in error.
Control athletes for the parent project were selected using a prospective plan. Research assistants screened enrolled athletes who met criteria for the follow-up visits (described above) and attempted to identify individually matched controls for each injured athlete using the following matching criteria: level of play (i.e., college or high school), institution, team, age, estimated intellectual functioning (word reading performance at baseline), race, handedness, concussion history, and position. None of the control athletes was otherwise injured; there were no cases where a suspected concussion was determined to be a noninjury. The search for matched controls began as soon as possible following each injury given restrictions associated with scheduling and availability of athletes and staff. Given these restrictions, the selection of the matched control and start of the follow-up visits for controls did not always occur at the same time as the concussed athletes' visits, though they did occur within the same season. Following these procedures, a total of 64 contact controls were enrolled in the parent project.
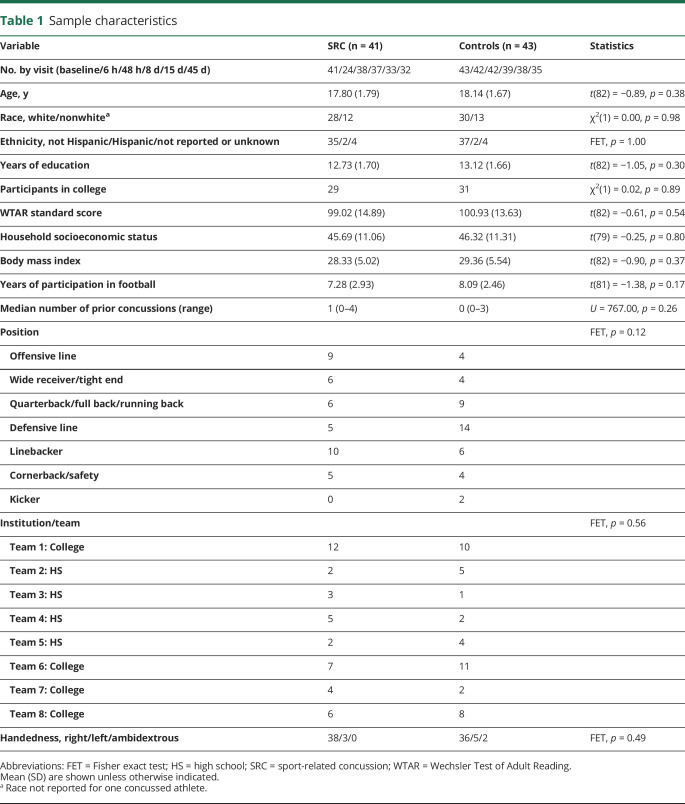
For the current study, additional exclusion criteria included history or current psychopathology (i.e., mood disorders), migraines or recurrent headaches, attention-deficit/hyperactivity disorder or memory difficulties, structural findings on MRI that required clinical follow-up as part of the parent study, history of potentially confounding illness/disease (e.g., meningitis), or declining to allow their data to be banked for future analyses beyond the scope of the Project Head to Head II. A total of 43 concussed football players and 47 noninjured control football players met the additional criteria for inclusion in the current study. This included 4 athletes who completed the control protocol and then subsequently the postinjury protocol and 2 athletes who enrolled in the postinjury protocol for repeat concussions. For these participants, only data from the first postinjury protocol were included in analyses, leaving a final total of 41 athletes with concussion and 43 controls. The final number of athletes with demographic information is reported in table 1.
Table 1.
Sample characteristics
Clinical battery
Health history, sport and concussion history, and demographic information was collected from all participants at the preseason visit. The Wechsler Test of Adult Reading was administered at the preseason visit to provide an estimate of intellectual functioning.20 A comprehensive clinical battery that included interviews, questionnaires, and cognitive testing was conducted at preseason and follow-up visits. For the current work, concussion severity was assessed using the Sport Concussion Assessment Tool, 3rd Edition, symptom severity score.21 Injury and recovery information was collected at follow-up visits. The number of days that athletes were symptomatic following SRC (i.e., duration of symptoms) was used as the primary outcome variable.
Serum biomarkers
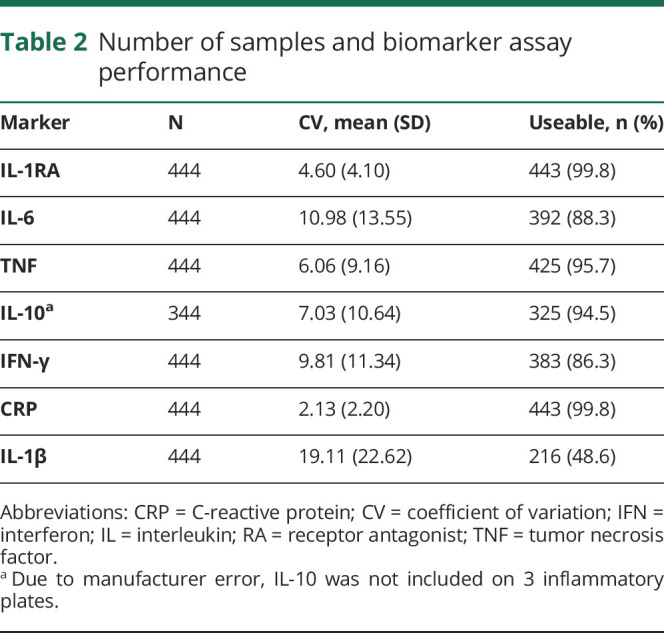
Venous blood was collected using Red Top BD (East Rutherford, NJ) Vacutainer tubes, left to clot at room temperature for 30 minutes, and centrifuged at 1,500 relative centrifugal force for 15 minutes. Serum concentrations of IL-6, IL-1β, IL-1RA, IL-10, TNF, CRP, and IFN-γ were measured in duplicate, blinded to concussion status, using a Meso Scale Discovery (MSD) (Rockville, MD) QuickPlex SQ 120 instrument and MSD V-PLEX assays following manufacturer's instructions. The average coefficient of variation (CV) for each marker and the final number of samples included in analyses is found in table 2.
Table 2.
Number of samples and biomarker assay performance
Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics version 21 (Armonk, NY) and visualized using Prism 7 (La Jolla, CA). Independent samples t tests, Mann-Whitney U tests, and χ2 tests were used to compare baseline variables between groups. Inflammatory markers were natural log-transformed to better approximate a normal distribution. Samples that were below the detection limit or with CV greater than 25% were excluded from analyses. Statistical analysis on IL-1β levels was not performed due to the small number of useable data points (<50% of total sample). Linear mixed-effects (LME) modeling was used to model changes in biomarkers and Sport Concussion Assessment Tool, 3rd edition (SCAT3) symptom severity scores within participants over time as a function of study group, with visit (i.e., baseline, 6 hours, 48 hours, 8 days, 15 days, 45 days), group (i.e., SRC vs control), and the group by visit interaction. A random intercept was modeled for each participant to allow inclusion of participants who did not complete all visits. Various models that included body mass index (BMI) or time since last physical exertion as covariates were compared using Bayesian information criterion (BIC) due to the known effects of exercise and adiposity on inflammatory markers.22,23 Of all available samples, 31 (22 SRC) had missing data for the time since last exertion due to lack of response; these data points were imputed using multiple imputation under the missing at random assumption. Group, visit, BMI, and log-transformed biomarker levels (excluding IL-1β) were included as predictors in the imputation process. The basic LME model including group, visit, and their interaction had the lowest BIC for all markers except IL-1RA and CRP, for which the model including BMI as a covariate had the lowest BIC. For consistency and simplicity, the basic interactive model with no covariates is reported for all biomarkers. Sensitivity analyses confirmed the inclusion of BMI did not affect CRP or IL-1RA results (see Results). Interactions or main effects of group or visit were considered significant at p < 0.05 with Bonferroni correction for 6 markers, corresponding to p < 0.008. Significant effects were followed up with simple effects testing, and if indicated, post hoc testing using Bonferroni correction at p < 0.05. Area under the receiver operating characteristic curve (AUC) was used to quantify the ability of markers to discriminate concussed from control athletes. Finally, time to recovery was analyzed using Cox proportional hazard model to determine if inflammatory markers were predictors of symptom duration in athletes with concussion after confirming the proportional hazard assumption was met. AUC and time to recovery analyses were limited to inflammatory markers that showed acute elevation following concussion (see Results).
Data availability statement
Anonymized data used in preparation of the figures and tables will be shared upon request from qualified investigators for purposes of replicating procedures and results.
Results
Demographic comparisons and injury information
Postinjury visits occurred at an average of 4.43 hours (SD 4.13), 40.20 hours (SD 47.13), 8.17 days (SD 1.53), 16.31 days (SD 4.63), and 43.99 days (SD 7.59). Athletes with concussion and control athletes did not differ in demographic information, confirming the detailed matching criteria (table 1). Athletes with concussion reported an average duration of symptoms of 8.86 days (SD 7.24). Recovery data for 5 athletes with concussion were lost to follow-up. No athletes with concussion reported loss of consciousness, 2 reported post-traumatic amnesia, and 1 reported retrograde amnesia.
SCAT3 symptom severity scores and inflammatory markers
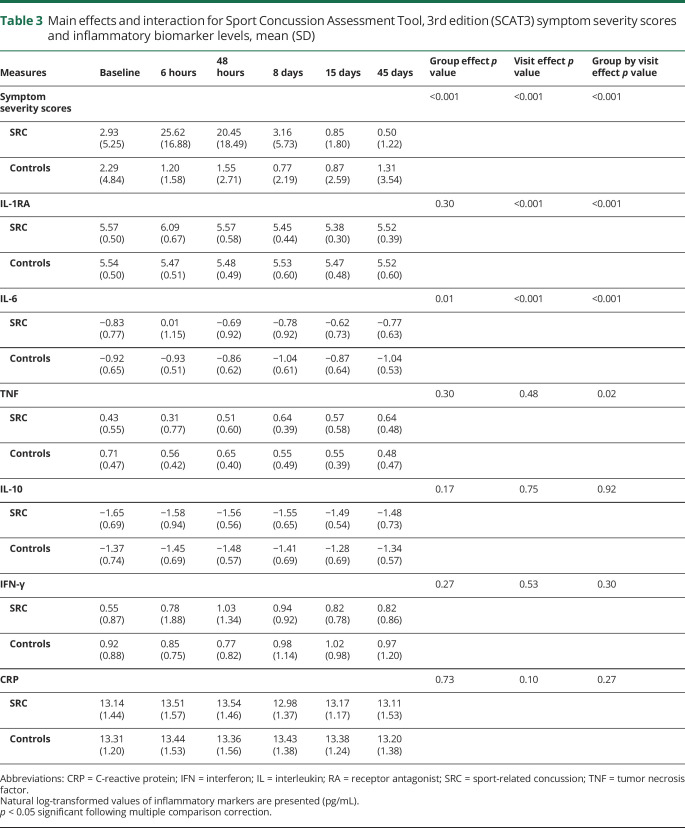
Means and SDs for the main effects and interactions are reported in table 3. Post hoc testing showed that SCAT3 symptom severity scores were significantly higher at the 6- and 24- to 48-hour visits relative to all other visits in athletes with concussion (ps ≤ 0.001) (figure 2). Athletes with concussion also had significantly higher symptom severity scores relative to control athletes at the 6-hour and 24- to 48-hour visits (ps < 0.001).
Table 3.
Main effects and interaction for Sport Concussion Assessment Tool, 3rd edition (SCAT3) symptom severity scores and inflammatory biomarker levels, mean (SD)
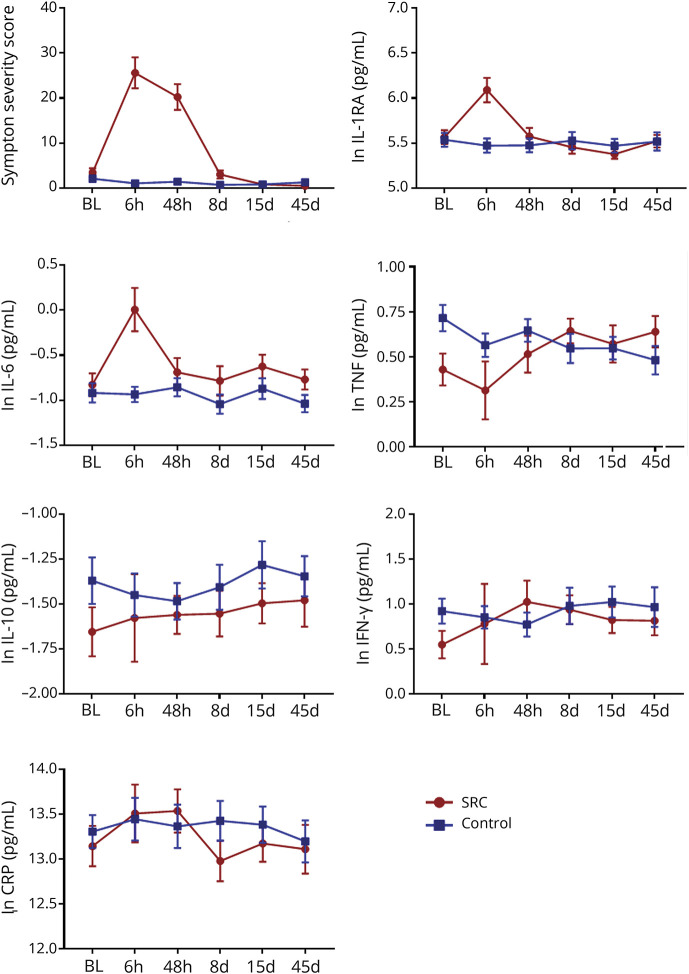
Figure 2. Symptom severity scores and inflammatory markers.
Shown are the Sport Concussion Assessment Tool, 3rd edition (SCAT3) mean symptom severity scores and the natural log-transformed (ln) levels of inflammatory markers for athletes with sport-related concussion (SRC) and controls at preseason baseline (BL), 6-hour (6 h), 24- to 48-hour (48 h), 8-day (8 d), 15-day (15 d), and 45-day (45 d) visits. Error bars are standard error of the mean. CRP = C-reactive protein; IFN = interferon; IL = interleukin; RA = receptor antagonist; TNF = tumor necrosis factor.
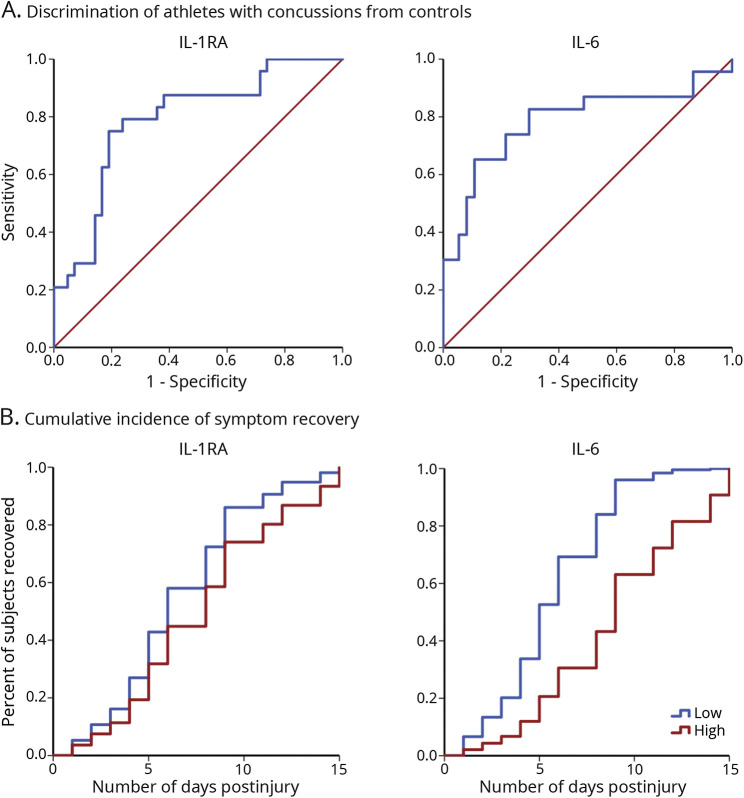
For inflammatory markers, significant effects were observed for IL-1RA and IL-6 (table 3; figure 2). Post hoc testing indicated that both IL-1RA and IL-6 were significantly elevated in athletes with concussion at the 6-hour visit relative to all other visits (ps ≤ 0.001). Of the injured athletes with measurable baseline and 6-hour data, 19 of 22 had elevated IL-6 and 21 of 24 had elevated IL-1RA at the 6-hour visit relative to their own baseline. In addition, both IL-1RA and IL-6 levels at the 6-hour visit were significantly higher in athletes with concussion compared to controls (ps < 0.001). The AUC for discriminating concussed from control athletes at the 6-hour visit was 0.79 (95% CI 0.67–0.90) for IL-1RA and 0.79 (95% CI 0.65–0.92) for IL-6 (figure 3).
Figure 3. Receiver operating characteristic (ROC) curves and cumulative incidence of symptom recovery.
(A) ROC curve for interleukin (IL)-6 and IL-1 receptor antagonist (RA) at the 6-hour visit. Red line represents reference line. (B) Cumulative incidence of concussion symptom recovery by IL-6 and IL-1RA at the 6-hour visit in athletes with concussion. For visualization purposes, IL-6 and IL-1RA levels are represented as low or high based on median split.
There were no significant main effects or interactions for TNF, IFN-γ, CRP, or IL-10 following multiple comparison correction (table 3; figure 2).
Sensitivity analyses for IL-1RA and CRP showed that the inclusion of BMI as a covariate did not affect results. There were no significant main effects or interactions for CRP (ps > 0.10). As in the primary analyses, there was a significant group by visit interaction for IL-1RA (p < 0.001), driven by increased IL-1RA levels in athletes with concussion at the 6-hour visit relative to all other visits as well as relative to controls (ps < 0.001).
Prediction of symptom recovery
Testing for associations between inflammatory markers and outcome in athletes with concussion (i.e., the duration of postconcussion symptoms in days) was limited to IL-1RA and IL-6 at the 6-hour visit. Eight of the 17 (47%) athletes with concussion who had elevated IL-6 levels at 6 hours postconcussion relative to their own baseline and complete data regarding symptom recovery were still symptomatic at the 8-day visit, while 8 of the 18 (44%) athletes with concussion with elevated IL-1RA were still symptomatic at the 8-day visit. All injured athletes with elevated IL-6 or IL-1RA were symptom free by the 15-day visit. Time-to-event analyses were conducted to determine if acute IL-6 or IL-1RA levels predicted the number of days that athletes were symptomatic. IL-6 levels at 6 hours postconcussion were significantly associated with symptom duration (mean natural log-transformed IL-6 −0.08 [SD 1.12], mean duration of symptoms 7.10 [SD 3.82] days, HR 0.61 [95% CI 0.38–0.96], p = 0.03), with a 1-unit increase in natural log-transformed IL-6 associated with 39% lower hazard of recovery (i.e., higher IL-6 levels were associated with slower recovery rates; figure 3). IL-1RA levels at 6 hours postconcussion were not significantly associated with speed of recovery (mean natural log-transformed IL-1RA 6.03 [SD 0.66], mean duration of symptoms 7.14 [3.73] days, HR 0.62 [95% CI 0.31–1.23], p = 0.17).
Discussion
This prospective study longitudinally measured serum levels of inflammatory markers from preseason through multiple postinjury visits after SRC. Consistent with previous large-scale studies,24 athletes with concussion had elevated symptom severity scores during the acute postinjury phase after SRC (i.e., 6 and 24–48 hours), with athletes with concussion reporting symptom recovery 8.79 days following injury, on average. Athletes with concussion also had elevated levels of IL-6 and IL-1RA at the early acute time point (i.e., 6 hours postinjury) relative to preinjury levels and relative to carefully selected noninjured football athletes who served as matched controls. Importantly, elevated IL-6 levels at 6 hours postconcussion were associated with longer symptom recovery in athletes with concussion. These results demonstrate the sensitivity of serum inflammatory markers to SRC and highlight the potential of these markers in identifying athletes at risk for prolonged recovery following SRC.
IL-6 is a pleiotropic cytokine that exerts both pro- and anti-inflammatory effects as part of the innate immune response and is also involved in adaptive immunity.25 The effects of brain injury on IL-6 have been extensively studied, with acute elevations documented in the brain following experimental brain injury and in CSF of patients following severe TBI.3,26 Previous studies have also documented acute elevations in IL-6 in serum or plasma following moderate to severe TBI in pediatric ED patients5,9 as well as in adult TBI patients compared to controls.6,8,10 Prior work in severe head injury has also demonstrated the prognostic potential of IL-6, with reported associations between acute IL-6 levels and negative outcomes,9,13,27 though null and even opposite results have been reported as well.5,12
Alterations in IL-6 have also been documented in a limited number of studies of less severe brain injury. Gill et al.15 reported increased serum levels of IL-6 in military personnel following moderate blast exposure with no diagnosis of mTBI relative to military personnel with low/no blast exposure. Moreover, they found that IL-6 levels were positively correlated with average peak pressure of the moderate blast exposure. Other recent work investigated changes in the gene expression of peripheral blood mononuclear cells within 6 hours following SRC and reported that IL-6 was a main hub of a network of differentially expressed genes following SRC that was associated with inflammatory response.17 The current study extends these findings, and the findings in moderate and severe TBI, to demonstrate that acute elevations of IL-6 in serum are associated with prolonged symptom duration following SRC.
The effects of brain injury on IL-1 activity have also been extensively studied. Preclinical models and work in moderate and severe TBI patients have repeatedly shown an increase in IL-1β expression acutely following injury.3 Few studies have specifically measured IL-1RA, an endogenous inhibitor of IL-1 activity that can act as an acute phase protein.28 One study reported significant elevations of IL-1RA levels in brain microdialysates that were inversely correlated with intracranial pressure and associated with more favorable outcome in mostly severe TBI patients.29 The current findings of elevated IL-1RA levels provide the first indirect evidence of increased IL-1 activity in the early acute phase following SRC or mTBI.
Substantial recent efforts have been made to identify blood biomarkers based on the known neurophysiologic consequences of brain injury (e.g., cell injury, gliosis) to aid in the diagnosis of mTBI and SRC, as well as in the prediction of patients at risk for prolonged recovery following mTBI and SRC. As previously mentioned, much of this work has focused on identifying markers that are, ideally, relatively specific to brain injury. For example, we have previously reported in an overlapping sample an increase in ubiquitin C-terminal hydrolase-L1 and S100 calcium-binding protein β at 6 hours postconcussion, with AUCs of 0.72 and 0.74, respectively.19 Inflammatory markers are not specific to brain injury (i.e., they are elevated in other conditions and diseases), and thus their clinical potential in diagnosing SRC in insolation is limited. However, inflammation may still play a causal role in injury severity and recovery. Moreover, current results suggest that they may hold promise as complementary diagnostic markers of concussion (AUCs 0.78) and prognostic markers of prolonged symptom recovery. Clinically, a biomarker panel that includes markers covering multiple aspects of the neurometabolic cascade of concussion is likely to have the greatest diagnostic and prognostic value. Follow-up studies with larger sample sizes are needed to sufficiently determine the sensitivity and specificity of inflammatory markers alone and in combination with other, more specific brain injury markers.
This prospective cohort study represents a crucial first step in determining the potential utility of inflammatory markers in the clinical management of concussion. Inflammatory markers were chosen based on prior brain injury literature. There are, however, numerous other inflammatory markers that could be investigated. Analyses of alternative inflammatory markers as well as markers capturing other potentially relevant biological pathways that are related to inflammation (e.g., kynurenine metabolites) or more specific to brain injury are ongoing.19,30 Future work in this cohort will investigate the combined diagnostic and prognostic potential of blood markers capturing multiple aspects of the neurometabolic cascade, as well as their relationship with other potential biomarkers (i.e., neuroimaging metrics). Additional work comparing inflammatory and other blood markers to non-football athletes as a secondary control group is also ongoing to account for the potential effects of repetitive head impacts that occur during football without diagnosed concussion.
The current work cannot determine whether measured IL-6 and IL-1RA originated from the brain or the periphery. Prior work has demonstrated that mTBI and even football participation without frank concussion can lead to compromised blood–brain barrier, highlighting the possibility that these markers reflect neuroinflammation.31,32 Alternatively, recent work has demonstrated that brain injury can result in changes to peripheral immune function likely via the sympathetic nervous system. Ritzel et al.33 found that controlled cortical impact in mice resulted in an acute increase in myeloid cells, specifically neutrophils and monocytes, in bone marrow as well as mobilization of these cells into circulation. Additional work is needed to determine the response of peripheral markers of inflammation to mTBI and SRC, their relationship with neuroinflammation, and their physiologic consequences following SRC. In addition, this work focused on male football athletes in high school and college. Thus, the extent to which the results generalize to female or youth athletes is unclear. The majority of athletes in the current study showed fairly typical recovery following their injury, which is consistent with the prospective nature of the study. The extent to which the current results extend to athletes who develop postconcussion syndrome is unclear. Finally, the number of athletes with SRC in this study may not be large enough to adequately determine the precision, accuracy, sensitivity, and specificity of inflammatory markers as clinical biomarkers. Large-scale follow-up studies are warranted.
Circulating levels of IL-6 and IL-1RA are increased in the early acute phase following SRC in high school and college football athletes, with IL-6 levels predicting the duration of symptoms reported by athletes with concussion. These markers may have promise as diagnostic markers of SRC as well as prognostic markers for recovery after SRC. Moreover, these findings highlight the IL-6 and IL-1 pathways as possible targets for treatment development, consistent with recent attempts to determine the efficacy of recombinant human IL-1RA as treatment for severe TBI in humans.34
Acknowledgment
The authors thank Ashlee Taylor and Brenda Davis from University of Oklahoma Integrative Immunology Center for analysis of serum cytokines; Ashley LaRoche and Alexa Wild from the Department of Neurosurgery at the Medical College of Wisconsin for study coordination; and Dr. Aniko Szabo from the Department of Biostatistics at the Medical College of Wisconsin for statistical consultation.
Glossary
- AUC
area under the receiver operating characteristic curve
- BIC
Bayesian information criterion
- BMI
body mass index
- CI
confidence interval
- CRP
C-reactive protein
- CV
coefficient of variation
- ED
emergency department
- HR
hazard ratio
- IFN
interferon
- IL
interleukin
- IL-1RA
interleukin-1 receptor antagonist
- LME
linear mixed effects
- MSD
Meso Scale Discovery
- mTBI
mild traumatic brain injury
- SCAT3
Sport Concussion Assessment Tool, 3rd edition
- SRC
sport-related concussion
- TBI
traumatic brain injury
- TNF
tumor necrosis factor
Appendix. Authors
Footnotes
Study funding
Supported by the Department of Defense Broad Agency Announcement for Extramural Medical Research through award number W81XWH-14-1-0561, National Institute of Neurological Disorders and Stroke of the NIH under award number R21NS099789, and National Institute of Neurological Disorders and Stroke under award number R01NS102225. The National Institute of General Medical Sciences (P20GM121312) and the National Institute of Mental Health (R21MH113871) also provided support for this project. The REDCap electronic database and the Adult Translational Research Unit used for this project were supported by the National Center for Advancing Translational Sciences, NIH, award number UL1TR001436.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 2006;21:375–378. [DOI] [PubMed] [Google Scholar]
- 2.McCrea M, Guskiewicz K, Randolph C, et al. Incidence, clinical course, and predictors of prolonged recovery time following sport-related concussion in high school and college athletes. J Int Neuropsychol Soc 2013;19:22–33. [DOI] [PubMed] [Google Scholar]
- 3.Woodcock T, Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Front Neurol 2013;4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery 2014;75(suppl 4):S24–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalabalikis P, Papazoglou K, Gouriotis D, et al. Correlation between serum IL-6 and CRP levels and severity of head injury in children. Intens Care Med 1999;25:288–292. [DOI] [PubMed] [Google Scholar]
- 6.Maier B, Schwerdtfeger K, Mautes A, et al. Differential release of interleukins 6, 8 and 10 in cerebral spinal fluid and plasma after traumatic brain injury. Shock 2001;15:421–426. [DOI] [PubMed] [Google Scholar]
- 7.Tasci A, Okay O, Gezici AR, Ergun R, Ergungor F. Prognostic value of interleukin-1 beta levels after acute brain injury. Neurol Res 2003;25:871–874. [DOI] [PubMed] [Google Scholar]
- 8.Minambres E, Cemborain A, Sanchez-Velasco P, et al. Correlation between transcranial interleukin-6 gradient and outcome in patients with acute brain injury. Crit Care Med 2003;31:933–938. [DOI] [PubMed] [Google Scholar]
- 9.Chiaretti A, Genovese O, Aloe L, et al. Interleukin 1B and interleukin 6 relationship with paediatric head trauma severity and outcome. Child's Nervous Syst 2005;21:185–193. [DOI] [PubMed] [Google Scholar]
- 10.Venetsanou K, Vlachos K, Moles A, Fragakis G, Fildissis G, Baltopoulos G. Hypolipoproteinemia and hyperinflammatory cytokines in serum of severe and moderate traumatic brain injury (TBI) patients. Eur Cytokine Netw 2007;18:206–209. [DOI] [PubMed] [Google Scholar]
- 11.Di Battista AP, Rhind SG, Hutchison MG, et al. Inflammatory cytokine and chemokine profiles are associated with patient outcome and the hyperadrenergic state following acute brain injury. J Neuroinflammation 2016;13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raheja A, Sinha S, Samson N, et al. Serum biomarkers as predictors of long-term outcome in severe traumatic brain injury: analysis from a randomized placebo-controlled phase II clinical trial. J Neurosurg 2016;125:631–641. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira LC, Regner A, Miotto KD, et al. Increased levels of interleukin-6, -8 and -10 are associated with fatal outcome following severe traumatic brain injury. Brain Inj 2014;28:1311–1316. [DOI] [PubMed] [Google Scholar]
- 14.Lagerstedt L, Egea-Guerrero JJ, Rodriguez-Rodriguez A, et al. Early measurement of interleukin-10 predicts the absence of CT scan lesions in mild traumatic brain injury. PLoS One 2018;13:e0193278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill J, Motamedi V, Osier N, et al. Moderate blast exposure results in increased IL-6 and TNFalpha in peripheral blood. Brain Behav Immun 2017;65:90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gill J, Merchant-Borna K, Lee H, et al. Sports-related concussion results in differential expression of nuclear factor-kappaB pathway genes in peripheral blood during the acute and subacute periods. J Head Trauma Rehabil 2016;31:269–276. [DOI] [PubMed] [Google Scholar]
- 17.Merchant-Borna K, Lee H, Wang D, et al. Genome-wide changes in peripheral gene expression following sports-related concussion. J Neurotrauma 2016;33:1576–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su SH, Xu W, Li M, et al. Elevated C-reactive protein levels may be a predictor of persistent unfavourable symptoms in patients with mild traumatic brain injury: a preliminary study. Brain Behav Immun 2014;38:111–117. [DOI] [PubMed] [Google Scholar]
- 19.Meier TB, Nelson LD, Huber DL, Bazarian JJ, Hayes RL, McCrea MA. Prospective assessment of acute blood markers of brain injury in sport-related concussion. J Neurotrauma 2017;34:3134–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wechsler D. Wechsler Test of Adult Reading: WTAR. San Antonio: Psychological Corporation; 2001. [Google Scholar]
- 21.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med 2013;47:250. [DOI] [PubMed] [Google Scholar]
- 22.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers. J Am Coll Cardiol 2005;45:1563. [DOI] [PubMed] [Google Scholar]
- 23.Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci 2017;13:851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCrea M, Guskiewicz KM, Marshall SW, et al. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA 2003;290:2556–2563. [DOI] [PubMed] [Google Scholar]
- 25.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol 2015;16:448–457. [DOI] [PubMed] [Google Scholar]
- 26.Zeiler FA, Thelin EP, Czosnyka M, Hutchinson PJ, Menon DK, Helmy A. Cerebrospinal fluid and microdialysis cytokines in severe traumatic brain injury: a scoping systematic review. Front Neurol 2017;8:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arand M, Melzner H, Kinzl L, Bruckner UB, Gebhard F. Early inflammatory mediator response following isolated traumatic brain injury and other major trauma in humans. Langenbecks Arch Surg 2001;386:241–248. [DOI] [PubMed] [Google Scholar]
- 28.Palomo J, Dietrich D, Martin P, Palmer G, Gabay C. The interleukin (IL)-1 cytokine family--Balance between agonists and antagonists in inflammatory diseases. Cytokine 2015;76:25–37. [DOI] [PubMed] [Google Scholar]
- 29.Hutchinson PJ, O'Connell MT, Rothwell NJ, et al. Inflammation in human brain injury: intracerebral concentrations of IL-1alpha, IL-1beta, and their endogenous inhibitor IL-1ra. J Neurotrauma 2007;24:1545–1557. [DOI] [PubMed] [Google Scholar]
- 30.Singh R, Savitz J, Teague TK, et al. Mood symptoms correlate with kynurenine pathway metabolites following sports-related concussion. J Neurol Neurosurg Psychiatry 2016;87:670–675. [DOI] [PubMed] [Google Scholar]
- 31.Marchi N, Bazarian JJ, Puvenna V, et al. Consequences of repeated blood-brain barrier disruption in football players. PLoS One 2013;8:e56805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weissberg I, Veksler R, Kamintsky L, et al. Imaging blood-brain barrier dysfunction in football players. JAMA Neurol 2014;71:1453–1455. [DOI] [PubMed] [Google Scholar]
- 33.Ritzel RM, Doran SJ, Barrett JP, et al. Chronic alterations in systemic immune function after traumatic brain injury. J Neurotrauma 2018;35:1419–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helmy A, Guilfoyle MR, Carpenter KL, Pickard JD, Menon DK, Hutchinson PJ. Recombinant human interleukin-1 receptor antagonist in severe traumatic brain injury: a phase II randomized control trial. J Cereb Blood Flow Metab 2014;34:845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data used in preparation of the figures and tables will be shared upon request from qualified investigators for purposes of replicating procedures and results.