ABSTRACT
The macroautophagy/autophagy pathway includes successive steps of phagophore assembly structure formation, phagophore expansion, autophagosome (AP) closure and AP fusion with the lysosome/vacuole. Although information about regulators, factors and molecular mechanisms important for early and late steps of autophagy is abundant, information about AP closure is scarce. In 2017, we reported that the Vps21/RAB5 GTPase module regulates AP closure in yeast. In a recent paper, we show that Vps21 regulates the recruitment of ESCRT to APs to catalyze their closure by controlling an Atg17-Snf7 interaction. Thus, we identify a regulator, a factor, and a molecular mechanism important for AP closure.
Abbreviations: AP: Autohagosome; Atg: autophagy-related gene; ESCRT: the endosomal sorting complex required for transport; ILVs: intralumenal vescicles
KEYWORDS: Atg proteins, autophagosome closure, ESCRT, phagophore, Rab5, Vps21
In autophagy, damgaged proteins and organelles are engulfed by the cup-shaped double-membrane phagophores, which then close, mature and fuse with the lysosome (vacuole in yeast). Because this process is relevant to physiology and pathology in eukaryotes, much attention was drawn to autophagy research. However, there are still multiple unanswered questions about this process. For example, it is unclear which proteins regulate and mediate AP closure and how they function.
Our previous studies showed that the endocytic Vps21/RAB5 GTPases module intersects with autophagy as depletion of proteins of this module results in accumulation of AP clusters near the vacuole. When analyzing these eye-catching AP clusters using a protease-protection assay, we found that they are unclosed APs.
However, a remaining question was why depletion of the Vps21 GTPase module results in an AP closure defect. In the endocytic pathway, Vps21 functions upstream of the endosomal sorting complex required for transport (ESCRT), which can seal or repair cellular membrane structures, e.g., pinching off of intralumenal vescicles (ILVs) to form multivesicular bodies or repairing of plasma membrane ruptures. Considering the similar topology of open APs to ILVs in endosomes before they are pinched off, ESCRT was proposed to function in AP closure. Therefore, we asked whether the absence of ESCRT results in accumulation of unclosed APs and whether the Vps21/RAB5 GTPase module regulates AP closure through ESCRT.
To elucidate the role of ESCRT in autophagy, we deleted each ESCRT subunit individually and found that most mutants showed autophagy phenotypes similar to those of vps21Δ mutant cells, including accumulation of GFP-Atg8 clusters near their vacuoles, and suggesting that ESCRT functions in AP closure [1]. We then focused on the autophagy phenotypes caused by the absence of 2 ESCRT subunits, Snf7 and Vps4. We found that under nitrogen starvation, snf7Δ and vps4Δ mutant cells exhibit autophagy phenotypes similar to those of vps21Δ mutant cells. Further analysis using the conventional protease-protection assay combined with an immunoblot readout to detect AP cargos, showed that the APs that accumulate in ESCRT mutant cells are unclosed. To support these results, we developed 2 new assays for distinguishing between open and closed APs. One new assay is still based on protease-protection analysis, but uses fluorescence of GFP-Atg8 as a readout (instead of immunoblot analysis). The second new assay is based on accessibility of antibodies to cargos inside open, but not closed, APs, in combination with immunofluorescence microscopy. Using the 2 new assays further supports our conclusion that open APs accumulate in ESCRT mutant cells. In addition, we show that APs that accumulate in ESCRT mutants are decorated with Atg proteins, as expected for immature APs. Most importantly, in vitro suppression of AP closure defects of ESCRT mutants with the cognate recombinant proteins provids evidence for a direct role of ESCRT in AP closure. Our conclusion that ESCRT mediates AP closure in yeast is consistent with a recent report by others proposing a role for ESCRT in AP closure in mammalian cells.
We then asked what is the relationship between Vps21/RAB5 and ESCRT in autophagy. We found that the localization of the ESCRT subunits Snf7 and Vps4 to APs is dependent on Vps21. Furthermore, in vps21Δ mutant cells, Snf7 does not interact with Atg17 and is not recruited to APs, suggesting that this is why ESCRT does not mediate AP closure. We reasoned that if the Atg17-Snf7 interaction and colocalization is important for ESCRT’s ability to close open APs, a forced colocalization of Atg17 and Snf7 in vps21Δ mutant cells might rescue the autophagic defect of these mutant cells. We used NanoTrap to artificially colocalize Snf7 and Atg17 in vps21Δ cells, and showed that the autophagy defect of prApe1 maturation in vps21Δ mutant cells was rescued. These results indicate that Vps21 controls the recruitment of ESCRT to APs to catalyze their closure through a key Atg17-Snf7 interaction and colocalization. Deletion of the genes encoding either Vps21 or ESCRT subunits results in an AP closure defect due to the absence of ESCRT at the right location (Figure 1). To our knowledge, this is the first report about a molecular mechanism of AP closure. Remaining future questions include the role of other ESCRT subunits in AP closure, the reason why some ESCRT subunits are more important than others in autophagy, and why mutant cells depeleted for Vps21 or ESCRT exhibit only partial autophagic defects under starvation conditions.
Figure 1.
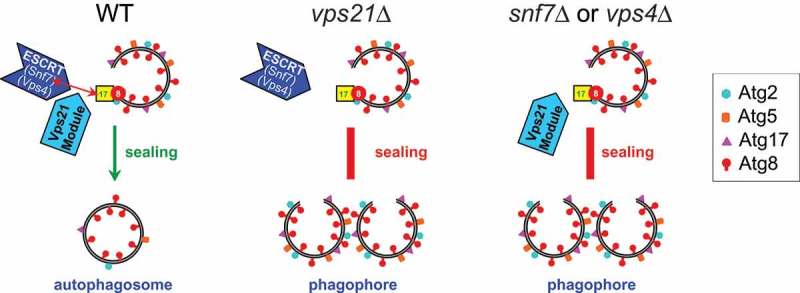
A schematic model for the molecular mechanism of Vps21/RAB5-controlled AP closure by ESCRT. In wild-type cells, the Vps21 GTPase module controls the interaction and colocalization of Atg17 with the ESCRT subunit Snf7 on APs, which results in localization of ESCRT to APs, where it catalyzes their closure (left). This closure is followed by Atg protein release and generation of mature APs that can fuse with the lysosome. Two types of mutant cells are defective in AP closure: 1) In vps21∆ cells, the interaction and colocalization between Snf7 and Atg17 is abolished so that ESCRT can not be recruited to APs (middle); 2) In ESCRT mutant cells snf7∆ or vps4∆, even though the Vps21 GTPase module is present, functional ESCRT is not present (right). Both types of mutants accumulate unclosed APs decorated with Atg proteins (such as Atg2, Atg5, Atg18, and Atg17), which cannot fuse with the vacuole.
Funding Statement
We are grateful to the Natural Science Foundation of China (31871428, 31671479 to YL) and the National Institutes of Health (GM-45444 to NS) for funding.
Disclosure statement
No potential conflict of interest was reported by the authors.
Reference
- [1].Zhou F, Wu Z, Zhao M, et al. Rab5-dependent autophagosome closure by ESCRT. J Cell Biol. 2019;218:1908–1927. PMID: 31010855. [DOI] [PMC free article] [PubMed] [Google Scholar]


