Abstract
The food-entrainable oscillator (FEO) is a mysterious circadian clock because its anatomical location(s) and molecular timekeeping mechanism are unknown. Food anticipatory activity (FAA), which is defined as the output of the FEO, emerges during restricted feeding. FAA disappears immediately during ad libitum feeding and re-appears during subsequent fasting. A free-running FAA rhythm has been observed only in rare circumstances when food was provided with a period outside the range of entrainment. Therefore, it is difficult to study the circadian properties of the FEO. Numerous studies have attempted to identify the critical molecular components of the FEO using mutant and genetically engineered mouse models. Herein we critically review the experimental protocols and findings of these studies in mouse models. Several themes emerge from these studies. First, there is little consistency in restricted feeding protocols between studies. Moreover, the protocols were sometimes not optimal, resulting in erroneous conclusions that FAA was absent in some mouse models. Second, circadian genes are not necessary for FEO timekeeping. Thus, another non-canonical timekeeping mechanism must exist in the FEO. Third, studies of mouse models have shown that signaling pathways involved in circadian timekeeping, reward (dopaminergic), and feeding and energy homeostasis can modulate, but are not necessary for, the expression of FAA. In sum, the approaches to date have been largely unsuccessful in discovering the timekeeping mechanism of the FEO. Moving forward, we propose the use of standardized and optimized experimental protocols that focus on identifying genes that alter the period of FAA in mutant and engineered mouse models. This approach is likely to permit discovery of molecular components of the FEO timekeeping mechanism.
Keywords: circadian, food anticipatory activity, FEO, restricted feeding, food entrainment
Introduction
In 1922, Curt Richter described that the “spontaneous activity of the rat is very intimately related to the food habits of the animal.” When Richter fed rats every day at noon (for 25 min), he found that “activity increased rapidly… right up to the time of the next feeding period (Richter, 1922).” Thus was born the study of food anticipatory activity (FAA). Beginning in the 1970’s, Frederick Stephan intensively studied this FAA and showed that it was controlled by an autonomous circadian oscillator, the food-entrainable oscillator (FEO). It was so-named because FAA persisted in constant conditions (i.e. during fasting) and entrained to a circadian range of feeding intervals [for review see (Boulos and Terman, 1980; Mistlberger, 1994)].
After Stephan established that anticipation of daily restricted meals (called FAA) was an output of the circadian FEO, Mistlberger and others spent the next 2 decades searching for the anatomical locus of the FEO. The FEO is not in the suprachiasmatic nucleus (SCN), nor in dozens of other brain regions that Mistlberger and others, and later, Davidson and Stephan, explored [for review see (Davidson, 2009)]. Thus, the elusive anatomical location(s) of the FEO remains one of the unsolved mysteries in the field of circadian rhythms.
In 2003, another mystery surrounding the FEO began to emerge, namely that canonical circadian clock genes may not be required by the FEO for circadian timekeeping. In the SCN, the canonical circadian timekeeping mechanism is well-characterized. Heterodimers of Brain and Muscle ARNT-Like 1 (BMAL1) and Circadian Locomotor Output Cycles Kaput (CLOCK) activate the transcription of Period (Per1, 2) and Cryptochrome (Cry 1, 2) genes. As CRY and PER proteins accumulate, they form complexes and inhibit the transcription factor activity of BMAL1/CLOCK, thereby inhibiting their own transcription. However, Rae Silver and colleagues were the first to report that mice lacking the functional canonical circadian timekeeping mechanism had normal FAA, when they showed that ClockΔ19 mutant mice had robust FAA that persisted during fasting in the light-dark cycle (Pitts et al., 2003). Subsequently, studies performed in Cry1−/−/Cry1−/− double mutant and Per1−/−/Per1−/−/Per1−/− triple mutant mice, definitively showed that canonical timekeeping genes were not required for FAA, because FAA persisted during restricted feeding and subsequent fasting in constant darkness (when the SCN was disabled) (Iijima et al., 2005; Pendergast et al., 2012). Several other studies showed that the FEO was intact in most circadian gene mutant mice, but compromised in a few mutants. Herein we will review the studies of mutant and engineered mouse models (circadian gene mutants as well as other molecular pathways) that have been examined for their effects on FAA [for earlier reviews of FAA neurogenetics and methodological issues, see (Challet et al., 2009; Mistlberger, 2009)].
Measuring FAA that is controlled by an autonomous circadian clock
The system for food entrainment is organized similarly to the light entrainment pathway (Fig. 1A, Fig. 2). Each system is comprised of inputs, a pacemaker, and outputs. Light is the most salient input to the light-entrainable oscillator in the SCN. The SCN pacemaker then controls outputs, such as the nocturnal activity rhythm. Changes in the nocturnal activity rhythm could result from alterations in the input, pacemaker, or output pathways. Since we know the anatomical locus of the light-entrainable oscillator, experiments can be designed to determine which component of the pathway contributes to a phenotype. Food is the most salient (known) input to the FEO, which then controls FAA. Studying the autonomous FEO is challenging. Since its anatomical location(s) is unknown, the FEO cannot be cultured in a dish, or lesioned, or manipulated with targeted injections of siRNA. Additionally, omics methods cannot be used to measure gene, protein, or metabolic oscillations in the FEO. Thus, when the magnitude of FAA is altered, it is difficult, or impossible, to conclude whether the input pathway, pacemaker, or output pathway is responsible. On the other hand, when the period of FAA is altered in a mutant mouse, it is more likely that the FEO pacemaker has been altered. However, measuring the period of FAA is challenging and has not been widely studied.
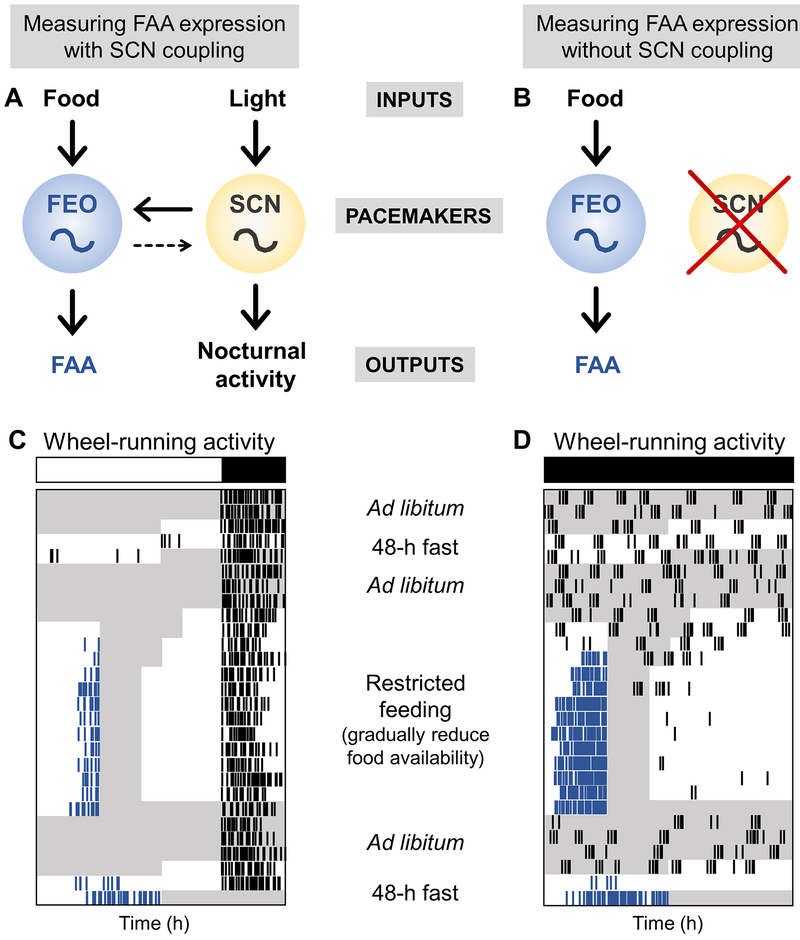
Figure 1. Experimental protocols to measure FAA expression.
A. The inputs, food and light, entrain the pacemakers, the FEO and SCN, which thereby regulate the outputs, FAA and nocturnal activity, respectively. The FEO is strongly coupled to the SCN, while the SCN is weakly coupled to the FEO. B. When the SCN is disabled, either by SCN lesion, or by housing arrhythmic mutant mice in constant darkness, FAA expression is measured without the influence of the SCN on the FEO. C. To measure robust FAA when the FEO is coupled to the SCN, mice are housed in 18L:6D and wheel-running activity is recorded. D. To measure FAA expression without the influence of the SCN, mice (SCN-lesioned or arrhythmic mice) are housed in constant darkness. In both protocols, mice are fasted for 48-h before restricted feeding and then returned to ad libitum feeding. Food availability is then gradually reduced (8h/day to 6h/day to 4h/day). Food is manually added and removed 4h later. The mice are allowed to eat as much as possible during the restricted feeding window. Food may be placed on the bottom of the cage during restricted feeding. Mice are then fed for 4h/day for 10 days. Mice are then fed ad libitum for several days and then fasted for 48h. Fasting should be timed so the hours 40 to 48 of fasting occur during the predicted time of FAA expression. White and black bars represent light and dark, respectively. Gray shading shows when food is available. Blue ticks show wheel-running activity controlled by the FEO and black ticks show other wheel-running activity. The x-axis is time (in hours). Mice may be fed on non-24h cycles (in D) to determine the period of the FEO. The y-axis is days.
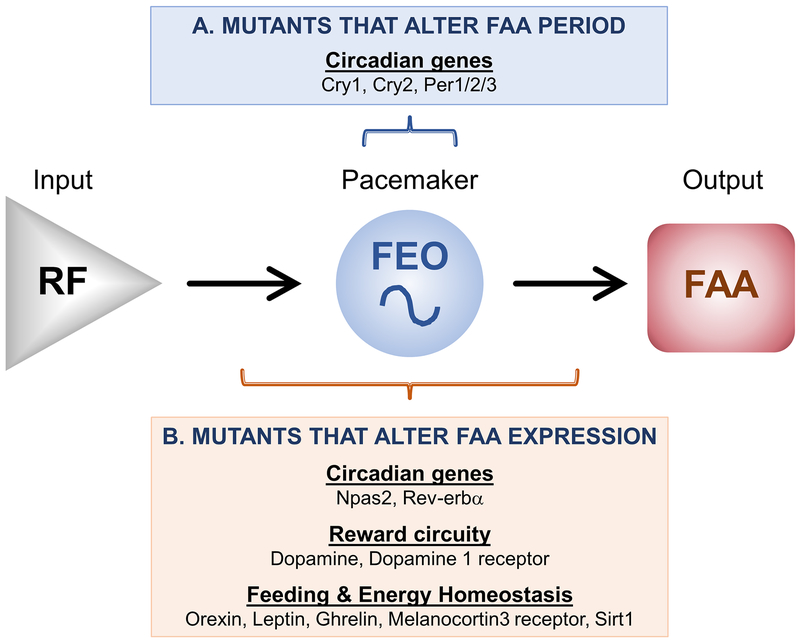
Figure 2. Genes that modulate FAA expression.
A. Several circadian mutant mice have altered periods of FAA expression, suggesting that these genes participate in the timekeeping mechanism of the FEO. B. Genes involved in circadian rhythmicity, reward circuitry, and feeding and energy homeostasis have altered FAA expression levels. These genes could modulate the input pathway, the FEO pacemaker, and/or the output pathway.
Interpreting restricted feeding studies of mutant mice is also difficult because the SCN and FEO are coupled (Fig. 1A) (Rosenwasser et al., 1984; Stephan, 1986; Ottenweller et al., 1990; Pendergast and Yamazaki, 2014). If the SCN is functional in a mutant mouse, then the SCN could be used as phase reference and FAA would appear normal. In addition, the SCN could moderate the period of the mutant FEO, resulting in apparently normal FAA. It is only when the SCN is disabled, ideally by lesioning the SCN, but also in circadian mutants that are arrhythmic in constant darkness (e.g. Bmal1−/−;Per1−/−/Per1−/−), that we can study the output of the FEO alone (and not in a complex with the SCN). Therefore, to study the genes that are required for, or participate in, FEO timekeeping, the SCN must be disabled (Fig. 2B). Most of the studies of the FEO/FAA to date have not eliminated the SCN rhythm. Thus, our discussion will focus on how mutation of a specific gene affects FAA, noting that this could be a defect in input, timekeeping, or output of the FEO. We will also highlight the studies that have disabled the SCN, since these provide information about the genes that may regulate the timekeeping mechanism of the FEO.
Some studies combine temporal restricted feeding with caloric restriction. For example, a mouse will be given ~60% of its typically daily food intake at mid-day. The mice typically eat all of the food provided in several hours. Thus, this protocol is easier technically because food does not have to be removed from cages daily. However, this protocol could be problematic because caloric restriction changes the phase of the SCN circadian rhythm (Challet, 2010). Since the FEO is coupled to the SCN, then changes in the phase of FAA could result from the effects of caloric restriction on the SCN, rather than the FEO. We have reviewed the studies of FAA that used a caloric restriction protocol herein, but they should be interpreted with caution.
It is also critical to assess the endogenous, self-sustained nature of the FEO. This means that FAA must be assessed both during restricted feeding and in the absence of a feeding time cue. Since FAA disappears during ad libitum feeding, the endogenous nature of the FEO is assessed during fasting (Fig. 1C, D). Notably, 2 days of fasting are required. The appearance of FAA on the second day of fasting shows that it is the output of a self-sustained oscillator (rather than an hourglass timer which would still drive FAA on the first day of fasting). In this review, we will highlight studies that assessed FAA during both restricted feeding and fasting.
Circadian gene mutant mice and FAA
The SCN and peripheral tissue clocks use the canonical circadian timekeeping mechanism (Takahashi, 2016). However, mounting evidence strongly suggests that the FEO oscillation does not require the canonical circadian transcription translation feedback loop(s) (Table 1). Two independent studies showed that ClockΔ19 mice had FAA during restricted feeding and subsequent fasting in the light-dark cycle (Pitts et al., 2003; Horikawa et al., 2005). However, these experiments alone were not sufficient to demonstrate that the FEO used a non-canonical timekeeping mechanism. This is because the locomotor activity of ClockΔ19 mice entrained to the light-dark cycle, thus the light-driven SCN oscillation could have controlled food anticipation. SCN-controlled locomotor activity becomes arrhythmic in constant darkness in homozygous ClockΔ19 mutant mice (i.e. the SCN is disabled in constant darkness) (Vitaterna et al., 1994). Pitts et al. also showed that FAA was present during restricted feeding in ClockΔ19 mice in constant darkness (2003). However, since they did not fast the ClockΔ19 mutant mice after restricted feeding in constant darkness, the possibility remained that the SCN participated in food entrainment (Pitts et al., 2003). Two later studies of other circadian mutant mice did demonstrate that the canonical circadian loop is not required for timekeeping by the FEO. First, Cryl−/−/Cryl−/− double mutants had FAA during restricted feeding and fasting in the light-dark cycle (Iijima et al., 2005). Importantly, when the SCN was disabled in Cryl−/−/Cryl−/− mice (in either SCN-lesioned mice or in mice housed in constant darkness), FAA was still present during restricted feeding and fasting (Iijima et al., 2005). Similarly, we showed that FAA was present in Per1−/−/Per2−/−/Per3−/− triple mutants during restricted feeding and fasting in constant darkness when the SCN was disabled (Pendergast et al., 2012). Storch et al. also showed that Per1−/−/Per2−/− double mutants in constant darkness had FAA during restricted feeding, but the mice were not subsequently fasted (2009). Together these studies show that, under ideal conditions to assess the timekeeping mechanism, the FEO keeps time in the absence of canonical clock genes.
Table 1.
Food anticipatory activity in circadian gene mutant and engineered mice.
| Gene disrupted | Line | Strain | RF phase (ZT) | Output | FAA during RF | FAA during fasting | References |
|---|---|---|---|---|---|---|---|
| Clock | Δ19 | C57BL/6J x BALB/cj | 6-10 | Wheel | Present1 | Present | (Pitts et al., 2003) |
| Δ19 | C57BL/6J | 5-9 | Thermal radiation detector | Present | Present | (Horikawa et al., 2005) | |
| Npas2 | Global | C57BL/6J (N9) | 6-10 | Wheel | Attenuated2 | ND | (Dudley et al., 2003) |
| Cry1/Cry2 | Global | 129 x C57BL/6 x ICR | 5-9 | Infrared sensor | Present3 | Present3 | (Iijima et al., 2005) |
| Cryl | Global | C57BL/6 | SCN-X4 | Infrared sensor | Present4 | Present4 | (Takasu et al., 2012) |
| Cryl | Over-express C414A mutation | BDF1 | 5-9 | Wheel | Present1 | Present | (Okano et al., 2016) |
| Cry2 | Global | C57BL/6 | SCN-X4 | Infrared sensor | Present4 | Present4 | (Takasu et al., 2012) |
| Bmal1 | Global | N5 | 4-8 | Telemetry | Absent | ND | (Fuller et al., 2008) |
| Global | C57BL/6J | 6-10 | Infrared sensor | Present | Present | (Mistlberger et al., 2008) | |
| Global | C57BL/6J | 8-125 | Wheel | Present1 | Present6 | (Pendergast et al., 2009) | |
| Global | N5 | 6-97 | Wheel | Present1 | ND | (Storch and Weitz, 2009) | |
| Global | C57BL/6J | DD | Infrared sensor | Present | Absent | (Takasu et al., 2012) | |
| Nestin-Cre floxed | N5-6 | 5-8 | Infrared sensor | Present | Present | (Mieda and Sakurai, 2011) | |
| Camk2a::iCre B floxed | Not reported | 6-10 | Wheel | Present | ND | (Izumo et al., 2014) | |
| Atoh1-Cre floxed | C57BI/6 x 129 | 6-12 | Telemetry | Present | ND | (Bering et al., 2017) | |
| Period1 | Brdm1 | C57BL/6×129S5/SvEvBrd | 4-12 & 4: 66% CR | Wheel | Present | Present8 | (Feillet et al., 2006) |
| Brdm1 | C57BL/6×129S5/SvEvBrd | 6-12 | Wheel | Present | ND | (Mendoza et al., 2010) | |
| Brdm1 | C57BL/6J | 4-8 | Wheel | Present | ND | (Li et al., 2015) | |
| Idc | C57BL/6J | 6-10 | Wheel | Present | Present | (Pendergast et al., 2017) | |
| Period2 | Brdm1 | C57BL/6×129S5/SvEvBrd | 4-12 | Wheel | Absent | Obscured by free-running SCN-controlled rhythm | (Feillet et al., 2006) |
| Brdm1 | C57BL/6×129S5/SvEvBrd | 6-12 | Wheel | Absent | ND | (Mendoza et al., 2010) | |
| Brdm1 | C57BL/6J | 4-8 | Wheel | Weak or absent | ND | (Li et al., 2015) | |
| Brdm1 | 129/C57BL/6-Tyrc-Brd | 6-10 | Wheel | Present | Present | (Pendergast et al., 2017) | |
| Idc | 129xC57BL/6 | 6-9 | Wheel | Present | ND | (Storch and Weitz, 2009) | |
| Idc | C57BL/6J | 6-10 | Wheel | Present | Present | (Pendergast et al., 2017) | |
| CMV-Cre floxed (global) | C57BL/6 | 4-12; 70%-80% CR | Wheel & telemetry | Attenuated | ND | (Chavan et al., 2016) | |
| Nestin-Cre floxed (brain) | C57BL/6 | 4-12; 70%-80% CR | Wheel & telemetry | Present9 | ND | (Chavan et al., 2016) | |
| Albumin-Cre floxed (liver) | C57BL/6 | 4-12; 70%-80% CR | Wheel & telemetry | Attenuated9 | ND | (Chavan et al., 2016) | |
| Period3 | C57BL/6J | 6-10 | Wheel | Present | Present | (Pendergast et al., 2017) | |
| Per1/Per2 | Idc | 129 | 6-9 | Wheel | Present1 | ND | (Storch and Weitz, 2009) |
| Brdm1 | C57BL/6×129S5/SvEvBrd | DD | Wheel | Present | ND | (Mendoza et al., 2010) | |
| Per1/Per2/Per3 | Idc | C57BL/6J | 8-145 | Wheel | Present | Present10 | (Pendergast et al., 2012) |
| Nr1d1−/− | Global | C57BL/6J | 6-12 | Telemetry | Attenuated | ND | (Delezie et al., 2016) |
| Nestin-Cre floxed | C57Bl/6J | 6-12 | Telemetry | Absent or attenuated9 | Absent | (Delezie et al., 2016) |
All experiments were performed in 12L:12D unless otherwise indicated. All mice had intact (not lesioned) SCN unless otherwise indicated. RF: restricted feeding; FAA: food anticipatory activity; ND: not determined; CR: caloric restriction
FAA was also present in constant darkness
FAA was attenuated and had delayed onset in constant darkness
FAA was also present during restricted feeding and fasting in constant darkness. Additionally, FAA was present in SCN-lesioned Cry1/Cry2 double knockout mice during restricted feeding and fasting in LD
SCN-X: These mice were SCN-lesioned
These experiments were performed in 18L:6D and restricted feeding began 8h after lights on
In constant darkness, the mice are hyperactive and FAA could not be resolved
These experiments were performed in 16L:8D and feeding began 6h after lights on
Fasting was performed in constant darkness
Identical actograms of body temperature were incidentally presented for both Nestin-Cre-Per2flox and Albumin-Cre-Per2flox mice
In constant darkness, Per1/Per2/Per3 knockout mice exhibited FAA during restricted feeding and fasting when food was provided on a 21-h cycle (but not 24-h cycle)
Anticipation of food availability has also been examined in single Period mutant mice (Table 1). Several studies have shown that Perl−/− and Per3−/− mice have normal FAA (Feillet et al., 2006; Mendoza et al., 2010a; Li et al., 2015; Pendergast et al., 2017). However, the results from Per2−/− mice were discrepant. Per2 was implicated as essential for FEO timekeeping when Feillet et al. reported that Per2−/− (Brdm1 line) mice lacked FAA during restricted feeding and fasting (Feillet et al., 2006). This finding was subsequently repeated by the same lab (in the same line and in a Per2flox line) and by another lab (after backcrossing with the C57BL/6J strain), although FAA during fasting was not examined in these studies (Mendoza et al., 2010a; Li et al., 2015; Chavan et al., 2016). In contrast, Storch and Weitz (2009) found that Per2−/− (Brdm1 line) mice had FAA during restricted feeding. We also recently showed that both the Idc and Brdm1 lines of Period2−/− mice had robust FAA during restricted feeding and fasting (Pendergast et al., 2017). It is likely that differences in experimental protocols were responsible for the discrepant results. Nonetheless, several studies have now observed robust FAA in Per2−/− mice, demonstrating the Per2 is not essential for FAA.
Bmal1−/− mice are widely studied because Bmal1 has been targeted to generate tissue-specific knockout or rescue of the circadian clock (Bunger et al., 2000). However, FAA has been difficult to study in Bmal1−/− mice, in part because they are unhealthy (Table 1) (Rudic et al., 2004; Bunger et al., 2005; Kondratov et al., 2006). The first study of FAA in Bmal1−/− mice showed that these mice had no FAA, but that FAA was rescued by injecting virus expressing Bmal1 into the dorsomedial nucleus of the hypothalamus (Fuller et al., 2008). However, several independent laboratories have since demonstrated that Bmal1−/− mice had FAA (Mistlberger et al., 2008; Pendergast et al., 2009; Storch and Weitz, 2009; Mieda and Sakurai, 2011; Takasu et al., 2012; Izumo et al., 2014). The discrepancy in results could be attributed, in part, to the fact that Bmal1−/− mice may have been ill during restricted feeding in the Fuller et al. study because food availability was not gradually reduced (for further discussion of FAA in Bmal1−/− mice, see (Fuller et al., 2009; Mistlberger et al., 2009b; Mistlberger et al., 2009a). In sum, numerous studies, performed in different labs have demonstrated that BMAL1 is not necessary for FAA.
Although circadian genes are not necessary for FEO timekeeping, several studies have shown that they modulate FAA. Dudley et al. showed that mice lacking functional Neuronal PAS domain protein 2 (Npas2), the paralog of Clock, had delayed expression of FAA, but after several days of restricted feeding displayed FAA that was indistinguishable from wild-types (2003). Our lab also showed that the period of the FEO rhythm was shortened in Per1−/−/Per2−/−/Per3−/− triple mutant mice (Pendergast et al., 2012). Likewise, Takasu et al. showed that the FEO period was shortened in SCN-lesioned Cry1−/− mice and lengthened in SCN-lesioned Cry2−/− mice (2012). Global and nervous system-specific Nr1d1−/− (Rev-erbα mutant mice) mice also had attenuated FAA (Delezie et al.,2016).
In sum, Cryl, Cry2, and Per1/2/3 alter the period of FAA and therefore likely participate in the FEO timekeeping mechanism (although they are not necessary for FEO timekeeping; Fig. 2A). On the other hand, Npas2 and Rev-erbα (Nr1d1) modulate FAA via the input or output pathways, or FEO timekeeping itself (Fig. 2B).
FAA in neurotransmitter/neuromodulator mutant mice
Most neurotransmitter mutant mice examined to date expressed normal FAA, except mice with disrupted dopamine signaling (Table 2). Dopamine signaling is involved in reward-seeking behaviors (Baik, 2013). Rodents anticipate not only timed chow diet, but also rewarding timed palatable meals and stimulant drugs (Mistlberger and Rusak, 1987; Jansen et al., 2012; Keith et al., 2013; Flores et al., 2016b). In addition, dopamine-deficient mice do not forage for food (Szczypka et al., 1999). Thus, it has been postulated that the FEO may require (or be located in) the dopaminergic circuitry. Several studies have examined the effects of pharmacological activation or inhibition of dopamine signaling on FAA. Mice co-treated with D1 and D2 receptor antagonists had attenuated FAA (Liu et al., 2012). In addition, mice developed anticipatory activity to timed treatment with a D1 receptor agonist even without restricted feeding (Gallardo et al., 2014). Consistent with these pharmacological studies, mice lacking functional dopamine D1 receptor (Drd1−/−) had attenuated FAA during restricted feeding (Gallardo et al., 2014; Michalik et al., 2015). Restricted feeding with caloric restriction (which could alter the phase of the SCN rhythm) showed that FAA was normal in dopamine D2 receptor mutant (Drd2−/−) mice (Gallardo et al., 2014). Moreover, virus-mediated rescue of dopamine expression in the dorsal striatum of dopamine-deficient mice (that do not express FAA because they do not forage at all) permitted the development of FAA during restricted feeding (Gallardo et al., 2014). Together these studies implicate the dopaminergic brain circuitry, and the dopamine D1 receptor, as a regulator of FAA. There may be functional redundancy in the circuitry since FAA was not abolished in dopamine receptor mutant mice.
Table 2.
Food anticipatory activity in neurotransmitter/neuromodulator mutant mice
| Gene disrupted | Line | Strain | RF phase (ZT) | Output | FAA during RF | FAA during fasting | References |
|---|---|---|---|---|---|---|---|
| Drd1 | Global | C57BL/6J (N8) | 6-10 | Disc | Attenuated | ND | (Gallardo et al., 2014) |
| Global | C57BL/6J (N8) | 8; 60% CR1 | Video | Present | ND | (Gallardo et al., 2014) | |
| Global | C57BL/6J (>N10) | 6-10 | Disc | Attenuated | ND | (Michalik et al., 2015) | |
| Drd2 | Global | C57BL/6J | 8; 60% CR1 | Video | Present | ND | (Gallardo et al., 2014) |
| μ-opioid receptor | Global | C57BL/6 | 12-15 | Wheel | Present | ND | (Kas et al., 2004) |
| 5-HT2CR | Global | C57BL/6J (N9) | 4-8 | Photobeam | Present2 | Present | (Hsu et al., 2010) |
All experiments were performed in 12L:12D unless otherwise indicated. All mice had intact (not lesioned) SCN unless otherwise indicated. RF: restricted feeding; FAA: food anticipatory activity; ND: not determined; CR: caloric restriction
Experiments were performed in 13L:11D
FAA was also present in constant dim red light
Studies have shown that FAA is normal in other neurotransmitter and neuromodulator mutant mice (Table 2). μ opioid receptor signaling modulates dopaminergic signaling before anticipation of reward in rodents, and therefore could regulate FAA via dopaminergic or other signaling pathways (Spruijt et al., 2001). However, mice with global knock out of the μ-opioid receptor had FAA during restricted feeding (Kas et al., 2004). Likewise, although serotonin signaling via the serotonin 2C receptor (5-HT2CR) regulates food intake (Tecott et al., 1995), 5-HT2CR−/− mice had FAA during both restricted feeding and subsequent fasting (Hsu et al., 2010).
In summary, disruption of reward-related pathways modulates the development and robustness of FAA. However, it is not clear if the components of the reward circuitry participate in the input pathway, the pacemaker, and/or the output pathway (Fig. 2B).
FAA in hormone and hormone signaling mutant mice
Several hormones in the peripheral circulation and brain regulate food intake and energy balance and therefore are poised to regulate FAA. However, contrary to their roles in homeostatic regulation of food intake, it appears that they modulate, but are not necessary for, FAA (Table 3).
Table 3.
Food anticipatory activity in hormone mutant mice.
| Gene disrupted | Line | Strain | RF phase (ZT) | Output | FAA during RF | FAA during fasting | References |
|---|---|---|---|---|---|---|---|
| ob/ob | Global | C57BL/6J | 12-16, then 4-8 | Wheel & home-cage activity | Enhanced | Present | (Ribeiro et al., 2011) |
| ob/ob | Global | C57BL/6J | 10; 60% CR1 | Behavior recorded by video | Present, but some behaviors reduced | ND | (Gunapala et al., 2011) |
| SGhsr | Global (Regeneron) | C57BL/6 | 6-14 | Wheel | Attenuated | Attenuated | (LeSauter et al., 2009) |
| C57BL/6J x DBA | 4-8 | Wheel; infrared beam | Attenuated2 | Attenuated | (Blum et al., 2009) | ||
| Global (AstraZeneca) | C57BL/6 | 12-14 | Wheel | Attenuated | ND | (Verhagen et al., 2011) | |
| Global (Zigman & Elmquist) | C57BL/6J | 12:00-16:00 (no ZT given) | Photobeam breaks | Absent | ND | (Davis et al., 2011) | |
| Preproghrelin | Global | C57BL/6J | 4-8 | Telemetry | Present | ND | (Szentirmai et al., 2010) |
| Ghrelin | Global | C57BL/6J x SJL/J | 10; 60% CR1 | Behavior recorded by video | Present | ND | (Gunapala et al., 2011) |
| Mc3r | Global | C57BL/6 | 7-11; 50% CR | Wheel & spontaneous activity (CLAMS) | Attenuated | ND | (Sutton et al., 2008) |
| Mc3r & ob/ob | Global | C57BL/6J | 4-8 | Wheel | Enhanced | ND | (Ribeiro et al., 2011) |
| Mc4r | Global | C57BL/6 | 7-11; 50% CR | Wheel | Present | ND | (Sutton et al., 2008) |
| Mchr1 | Global | F3 C57BL/6J x 129SvEv | 5-9 | Wheel | Present | ND | (Zhou et al., 2005) |
All experiments were performed in 12L:12D unless otherwise indicated. All mice had intact (not lesioned) SCN unless otherwise indicated. RF: restricted feeding; FAA: food anticipatory activity; ND: not determined; CR: caloric restriction
Note that data collected under conditions of restricted feeding with purposeful caloric restriction are not presented herein
Experiments were performed in 13L:11D
FAA was also present in constant darkness (Lamont et al., 2014)
Leptin is released from fat stores and suppresses food intake (Zhang et al., 1994). In rodents, the peak of the daily rhythm of plasma leptin coincides with meal timing (Ahima et al., 1998; Bodosi et al., 2004; Arble et al., 2011), suggesting that it could regulate FAA. On the other hand, the leptin rhythm, unlike FAA, does not persist during fasting, suggesting that leptin may not contribute to FAA (Martinez-Merlos et al., 2004). Indeed, ob/ob mice, which have mutated leptin, had enhanced or normal FAA during restricted feeding and normal FAA during subsequent fasting compared to control animals (Gunapala et al., 2011; Ribeiro et al., 2011). These studies demonstrate that leptin is not necessary for FAA, but can regulate its robustness.
Another hormone that could regulate FAA is ghrelin. Plasma ghrelin increases before habitual mealtime and it activates dopaminergic signaling in reward brain circuits in rodents (Bodosi et al., 2004; Abizaid et al., 2006). Several studies in transgenic mice have demonstrated that ghrelin modulates the expression of FAA. Mice with non-functional ghrelin receptors (Ghsr−/− from Regeneron) had attenuated FAA during restricted feeding and fasting (Blum et al., 2009; LeSauter et al., 2009). Ghsr1a−/− (from Astrozeneca) mice also had attenuated FAA during restricted feeding. Davis et al. reported that FAA was absent during restricted feeding in Ghsr−/− mice (from Zigman & Elmquist) (Zigman et al., 2005; Davis et al., 2011). However, in this study, locomotor activity, measured as photobeam breaks, was measured only for 2h before scheduled feeding (Davis et al., 2011). We have shown that wheel-running activity anticipates food availability while general (non-wheel) locomotor activity occurs during scheduled feeding (Flores et al., 2016a). Therefore, because general (non-wheel) activity was measured during a brief time window prior to restricted feeding, it is unknown whether the Ghsr−/− mice expressed FAA during food availability. Mice lacking functional preproghrelin, which is the precursor to ghrelin, or mice that lacked the ghrelin ligand, had normal FAA during restricted feeding (Szentirmai et al., 2010; Gunapala et al., 2011). Together, these studies show that ghrelin signaling modulates the expression of FAA, but is not necessary for FAA.
Leptin and ghrelin signaling pathways converge on the melanocortin system in the hypothalamus (Ellacott and Cone, 2006). Thus, while leptin and ghrelin are not necessary for FAA, it is possible that the central melanocortin mechanisms regulating food intake could mediate FAA. The hypothalamic melanocortin system regulates energy homeostasis by coordinating food intake and energy expenditure (Ellacott and Cone, 2006). Of the five melanocortin G-protein coupled receptors, melanocortin receptors 3 and 4 (Mc3r and Mc4r), regulate energy homeostasis. MCR agonists, such as the proopiomelanocortin (POMC)-derived α-melanocyte stimulating hormone (α-MSH), inhibit food intake, while MCR antagonists, such as agouti-related protein (AgRP), stimulate feeding (Fan et al., 1997; Anderson et al., 2016). Mc4r is widely expressed throughout the brain, while Mc3r expression is relatively limited and was detected at high levels in the hypothalamus and limbic/reward circuitry (e.g. ventral tegmental area) (Roselli-Rehfuss et al., 1993; Liu et al., 2003). Reward brain circuits have been implicated in regulation of FAA (see discussion above), suggesting that Mc3r could underlie FAA. Mc4r−/− mice had normal FAA, while Mc3r−/− mice had attenuated FAA, during restricted feeding, but FAA was not measured during subsequent fasting (Sutton et al.,2008). Since central melanocortin signaling is downstream of leptin, Ribeiro et al. determined whether mice lacking both leptin and Mc3r [ob/obMc3r-/-) would have impaired FAA (2011). Instead, ob/obMc3r−/− double mutant mice had enhanced FAA during restricted feeding compared to ob/ob mice (Ribeiro et al., 2011). Therefore, the melanocortin system is not necessary for FAA.
Melanin-concentrating hormone (MCH) regulates energy homeostasis and mice lacking functional MCH receptor (Mch1r−/−) are hyperphagic (Chen et al., 2002; Marsh et al., 2002). Despite its role in regulating food intake, FAA in Mch1r−/− mice was indistinguishable from wild-type controls (Zhou et al., 2005).
Together these data of mutant mice with non-functional hormone signaling pathways suggest that the well-characterized brain circuitries that regulate food intake and energy homeostasis are not necessary for FAA. Instead, ghrelin, leptin, and the melanocortin signaling pathways appear to regulate the magnitude of FAA during restricted feeding. This regulation could result from altering the input pathway, the amplitude of the FEO, and/or the output pathway (Fig. 2B).
FAA in neuropeptide mutant mice
Neuropeptide signaling pathways that are related to food intake and arousal could regulate FAA (Table 4). Orexin A and B are neuropeptides that signal via OX1R and OX2R and regulate arousal (Alexandre et al., 2013). Infusion of orexin A can stimulate food intake while OX1R antagonists reduce food intake, suggesting that the orexin signaling pathway could constitute the FEO (Lubkin and Stricker-Krongrad, 1998; Sakurai et al., 1998; Smart et al., 2002). Postnatal ablation of orexin neurons attenuated FAA in orexin/ataxia-3 mice (Akiyama et al., 2004; Mieda et al., 2004). However, the ablated orexin neurons in this study also expressed dynorphin, an endogenous opioid, and galanin, a neuropeptide that has been implicated in feeding. A subsequent study of orexin mutant mice (Hcrt−/−) in which only orexin was non-functional, showed that FAA was attenuated, but the anticipatory rise in body temperature prior to restricted feeding was normal (Kaur et al., 2008). FAA (activity and body temperature) persisted during fasting after restricted feeding in Hcrt−/− mice (Kaur et al., 2008). Two other studies measured behavior prior to food availability using video cameras and found that Hcrt−/− mice had either attenuated (Clark et al., 2009) or normal (Gunapala et al., 2011) FAA using a caloric restriction restricted feeding protocol (neither study measured FAA during subsequent fasting). Finally, disabling the high-frequency firing of orexin neurons, in mice lacking functional TWIK-related acid-sensitive potassium channels (TASK1/3), did not affect FAA during restricted feeding or subsequent fasting (Gonzalez et al., 2009).
Table 4.
Food anticipatory activity in neuropeptide mutant mice.
| Gene disrupted | Line | Strain | RF phase (ZT) | Output | FAA during RF | FAA during fasting | References |
|---|---|---|---|---|---|---|---|
| Hcrt (orexin) | Orexin neurons1; post-natal | C57BL/6J | 5-9 | Area sensor & thermal radiation detector | Attenuated | Attenuated | (Akiyama et al., 2004) |
| Orexin neurons1, post-natal | C57BL/6J (N4-N7) x 129Svev F1 | 4-8 | EEG-EMG & open field activity | Attenuated | Attenuated | (Mieda et al., 2004) | |
| Global | C57BL/6J | 4-8 | Telemetry-activity | Attenuated | Attenuated | (Kaur et al., 2008) | |
| Global | C57BL/6J | 4-8 | Telemetry-core body temperature | Present | Present | (Kaur et al., 2008) | |
| Global | C57BL/6J (N8) | 15; 60% CR | Video and EEG-EMG | Attenuated | ND | (Clark et al., 2009) | |
| Global | C57BL/6J | 10; 60% CR2 | Video | Present | ND | (Gunapala et al., 2011) | |
| K2p3.1 & K2p 9.1 (TASK1/3) | Global; impairs high frequency firing in orexin neurons | C57BL/6J x R1 | 3-7 | Wheel | Present | Present | (Gonzalez et al., 2009) |
| Npy | Global | 129S1 | 10; 60% CR2 | Video | Present | ND | (Gunapala et al., 2011) |
All experiments were performed in 12L:12D unless otherwise indicated. All mice had intact (not lesioned) SCN unless otherwise indicated. RF: restricted feeding; FAA: food anticipatory activity; CR: caloric restriction; ND: not determined
These neurons also co-express dynorphin and galanin
Experiments were performed in 13L:11D
Together these studies show that orexin is not necessary FAA, but it modulates the expression of FAA (Fig. 2B).
Neuropeptide Y (NPY) released from neurons in the arcuate nucleus of the hypothalamus potently stimulates food intake (Loh et al., 2015). In addition, NPY expression increases in the hypothalamus prior to restricted feeding, suggesting that NPY could underlie or regulate FAA (Yoshihara et al., 1996). However, NPY mutant mice had normal FAA during calorically-restricted restricted feeding, demonstrating that NPY is not necessary for FAA (Gunapala et al., 2011).
FAA in other mutant and engineered mice
FAA has also been investigated in various other mutant and engineered mice (Table 5). Prokineticin2 (PK2) is a secreted protein that has been postulated to act as an SCN output factor (Cheng et al., 2002). This is, in part, because PK2 is highly expressed in the SCN, and PK2−/− mice have low-amplitude activity and body temperature rhythms (but normal SCN circadian gene rhythms) (Li et al., 2006). If PK2 is an output of the SCN, then perhaps it is not surprising that PK2−/− mice had normal FAA, since FAA persists in SCN-lesioned mice (Li et al., 2006).
Table 5.
Food anticipatory activity in other mutant and engineered mice.
| Gene disrupted | Line | Strain | RF phase (ZT) | Output | FAA during RF | FAA during fasting | References |
|---|---|---|---|---|---|---|---|
| Prokineticin2 | Global | C57BL/6 × 129/Ola | 3-7 | Infrared beam breaks | Present | Present | (Li et al., 2006) |
| Vipr2 | Global | C57BL/6J | 4-8 | Wheel | Present | ND | (Sheward et al., 2007) |
| PAC1 receptor | Global | 129 | 4-8 | Wheel | Present1 | ND | (Hannibal et al., 2016) |
| Poly(ADP-Ribose) Polymerase 1 | Global | C57BL/6J | 3-9 | Wheel | Present | ND | (Asher et al., 2010) |
| Sirt1 | Global | FVB (N6) | 6-10 | Wheel | Attenuated | ND | (Satoh et al., 2010) |
| Global | CD1 | 7; 60% CR | Video | Present | ND | (Assali et al., 2018) | |
| Y/Y mutant, Global, no catalytic activity | CD1 | 7; 60% CR | Video | Present | ND | (Assali et al., 2018) | |
| CAGG-Cre-ER floxed (global in adulthood) | C57BL/6 | 9; 60% CR | Video | Present | ND | (Assali et al., 2018) | |
| Tyrosine hydroxylase-Cre floxed | C57BL/6 | 9; 60% CR | Video | Present | ND | (Assali et al., 2018) | |
| Pomc-Cre floxed | FVB/N x C57BL/6J x 129 | 9; 60% CR | Video | Present | ND | (Assali et al., 2018) | |
| Nestin-Cre floxed | C57BL/6J | 9; 60% CR | Video | Present | ND | (Assali et al., 2018) | |
| CamKII-alpha-Cre floxed | C57BL/6 | 9; 60% CR | Video | Present | ND | (Assali et al., 2018) | |
| Sirt1 over-expression | Brain (BRASTO) | C57BL/7J | 6-10 | Wheel | Enhanced | ND | (Satoh et al., 2010) |
| Grid2 ho/ho | Hotfoot mutation (ho/ho) | C57BL/6J | 6-12 | Wheel and telemetry | Attenuated | ND | (Mendoza et al., 2010) |
| Human Huntington Disease gene with CAG repeats | R6/2 | C57BL/6 x CBA F1 | 6-11 | Infrared sensors | Present | ND | (Maywood et al., 2010) |
| Rgs16 | Global | C57BL/6J | 6-10 | Area sensor | Attenuated | ND | (Hayasaka et al., 2011) |
| PKCγ | Global | C57BL/6 × 129SvJ | 6-10 | Wheel | Present | ND | (Zhang et al., 2012) |
| Wolfram syndrome 1 | Global | 129 Sv/EvTac | 4-8 | Wheel | Present | ND | (Luuk et al., 2012) |
| Tissue-type plasminogen activator | Global | C57BL/6J | 6-10 | Wheel | Present | ND | (Krizo et al., 2018) |
All experiments were performed in 12L:12D unless otherwise indicated. All mice had intact (not lesioned) SCN unless otherwise indicated. RF: restricted feeding; FAA: food anticipatory activity; ND: not determined; CR: caloric restriction
FAA was also present in constant dim red light
Vasoactive intestinal peptide (VIP) signals through the VIP receptor2 to synchronize the rhythms of SCN neurons (Aton et al., 2005; Maywood et al., 2006). Mice lacking VIP receptor 2 (Vipr2−/−) had impaired SCN and locomotor activity rhythms, but normal FAA during restricted feeding (Sheward et al., 2007).
Pituitary adenylate cyclase activating polypeptide (PACAP), together with glutamate, transmits light signals from the intrinsically photosensitive retinal ganglion cells in the retina to the SCN using the PACAP receptor 1 (PAC1). PAC1−/− mice had normal FAA during restricted feeding (Hannibal et al., 2016).
The expression of FAA may rely on detection of the metabolic state of cells. Cellular NAD+/NADH levels are a readout of metabolism. Several studies have examined FAA in mice with mutations in the molecular machinery that participates in sensing and responding to cellular NAD+/NADH levels. Poly(ADP-Ribose) Polymerase 1 (PARP1) is an NAD+-dependent ADP-ribosyltransferase that regulates the activity of transcriptional regulatory proteins(Schreiber et al., 2006). Thus, PARP1 is poised to sense changes in cellular metabolism and enact transcriptional responses. However, PARP1 is not necessary for FAA since PARP1−/− mice had normal FAA during restricted feeding (Asher et al., 2010). Sirtuins are another family of proteins that require NAD, but not NADH, for their deacetylase activity (Imai and Guarente, 2010). Therefore, they also act as cellular metabolism sensors. Sirtuin 1 (SIRT1) has been shown to regulate physiological responses to diet restriction (Imai and Guarente, 2010). In addition, SIRT1 is required for increased locomotor activity during fasting in mice (Chen et al., 2005). Therefore, SIRT1 could regulate FAA since it both senses cellular metabolism and can regulate locomotor activity. One study showed that Sirt1−/− mice had attenuated FAA, while brain-specific overexpression of SIRT1 increased FAA (Satoh et al., 2010). Therefore, while SIRT1 regulates FAA, it is not necessary for its expression (Fig. 2B).
One study investigated the role of the cerebellum in FAA (Mendoza et al., 2010b). The cerebellum coordinates locomotor activity and motivational processes (Caston et al., 1998). Moreover, the molecular circadian Period1-luciferase rhythm advanced in cerebellar explants in response to daytime restricted feeding (Mendoza et al., 2010b), suggesting that the cerebellum could harbor the FEO. When the function of the cerebellar circuitry was impaired in Nancy hotfoot (Gridho/ho) mutant mice, which lack the coding sequence for the ionotropic glutamate receptor δ2 gene in Purkinje cells, FAA was attenuated, but still present, during restricted feeding (Mendoza et al., 2010b).
The neurodegenerative Huntington’s disease is characterized by progressive motor symptoms and sleep and circadian disruption (Morton et al., 2005). The R6/2 mouse is a model of Huntington’s disease that has the 5’ end of the human Huntington gene with about 120 CAG repeat expansions (Mangiarini et al., 1996). R6/2 mutant mice have progressive disruption of circadian rhythms of locomotor activity and circadian gene expression in the SCN (Morton et al., 2005). Consistent with the numerous studies that have shown that the SCN and FEO are distinct, FAA during restricted feeding was intact in R6/2 mutant mice (Maywood et al., 2010) (Table 5). Importantly, restricted feeding restored daily rhythms of locomotor activity in late-stage R6/2 mice that previously had arrhythmic activity (Maywood et al., 2010). These data support the hypothesis that the FEO is an oscillator that can operate at the top of the circadian hierarchy when the SCN is disabled (Pendergast and Yamazaki, 2017). Restricted feeding could serve as a therapeutic to restore rhythms via the FEO in Huntington’s disease patients.
The role of signaling molecules in regulating FAA have also been investigated (Table 5). RGS16 regulates G-protein coupled receptor signaling and is rhythmically expressed in the SCN and liver. Since Rgsl6 expression in the liver was altered by fasting and re-feeding, Hayasaka et al. (2011) examined FAA in mice with transgenic knockdown of Rgs16 (Huang et al., 2006). Rgsl6 knockdown mice had attenuated FAA during restricted feeding (Huang et al., 2006). Protein kinase Cγ (PKCγ) also regulates molecular pathways, is expressed almost exclusively in neurons, and in brain regions that entrain to restricted feeding (Saito and Shirai, 2002; Verwey and Amir, 2009). However, PKCγ−/− mice had normal FAA during restricted feeding that was indistinguishable from wild-type mice (Zhang et al., 2012).
The Wolfram syndrome 1 (WSF1) gene is expressed in the DMH (and widely expressed in the brain), but only weakly expressed in the SCN (Luuk et al., 2008). The DMH was initially implicated as the locus of the FEO, although subsequent studies demonstrated that the DMH modulated FAA, but was not the FEO (Gooley et al., 2006; Landry et al., 2006; Mieda et al., 2006; Fuller et al., 2008; Moriya et al., 2009; Acosta-Galvan et al., 2011). Because of the DMH’s putative role in food entrainment, and the expression of Wsf1 in the DMH, one study examined FAA in Wsf1−/− mice (Table 5). They found that FAA was normal in Wsf1−/− mice (Luuk et al., 2012).
Brain-derived neurotrophic factor (BDNF) signals via TrkB receptors to suppress food intake and regulate energy homeostasis (Marosi and Mattson, 2014). The production of BDNF is, in part, regulated by tissue-type plasminogen activator (tPA) (Pang et al., 2004). Therefore, tPA could play a role in regulating FAA. However, FAA was normal in tPA−/− mice, suggesting that mature BDNF signaling is not necessary for FAA (Krizo et al., 2018).
Conclusions
Despite decades of research, the molecular and anatomical substrates of circadian food entrainment remain a mystery. While mutant and engineered mouse models have been key to the discovery of many physiological processes and behaviors in the field of circadian rhythms, the use of these models for studying the FEO have been largely unsuccessful. One conclusion that is clear from studies of mouse models is that canonical circadian genes are not required for the expression of FAA or for FEO timekeeping. Although this conclusion may fall into the category of ‘negative results,’ the implications of these studies are exciting and far-reaching. That the FEO can keep circadian time in the absence of the canonical clock genes means that there is (at least) one other robust circadian timekeeping mechanism that can function independently from the SCN. This timekeeping mechanism remains to be discovered. The only other non-canonical circadian oscillators discovered to date are the reduction-oxidation cycles of peroxiredoxin proteins (Reddy, 2016). Thus, it is possible that the FEO uses a circadian timekeeping mechanism akin to the redox and metabolic oscillators.
Another overarching conclusion from the study of mouse models is that signaling pathways that regulate food intake and energy homeostasis, or respond to changes in cellular metabolism, regulate the magnitude of FAA, but are not necessary for FAA. Experiments have not been performed to examine the role of these hormones in FEO timekeeping (i.e. in SCN-lesioned mice during fasting subsequent to restricted feeding). Energy homeostasis signals converge on the dopaminergic brain circuitry, which has also been shown to be important for food entrainment (Gallardo et al., 2014; Michalik et al., 2015). However, it is impossible to determine if the FEO is functional in mice with abolished dopamine signaling. This is because dopamine-deficient mice do not forage (or have normal locomotion) and thus they die without intervention (Zhou and Palmiter, 1995).
Critical review of studies of FAA in mutant and engineered mouse models also reveals the technical challenges of studying the FEO. First, there is little consistency among protocols for studying FAA. Studies use different outcome measures (e.g. wheel vs. general activity), different phases and durations of restricted feeding (e.g. 3h or 4h window, or caloric restriction), and different quantifications of FAA (e.g. 3h vs. 1h before food presentation or normalized vs. not normalized to total activity). And, importantly, the majority of studies did not examine whether FAA persisted during fasting after restricted feeding to test the fundamental circadian property of persistence in constant conditions. These inconsistencies across studies make it difficult to systematically compare the factors that modulate FAA.
We have presented 2 protocols for studying FAA that we have optimized for detecting FAA in mutant and engineered mouse models. In the first protocol (Fig. 1A), coupling between the SCN and FEO is intact. In the second protocol (Fig. 1B), the SCN is disabled so FAA is measured without FEO-SCN coupling. Because genetically altered mice sometimes have known or unknown health problems, we have found that it is critical to gradually reduce food availability (from 8h- to 6h- and finally to 4h/day). In addition, it is sometimes necessary to provide food on the bottom of the cage, even if the mutant mice are healthy when given food in the hopper during ad libitum feeding (as with Bmal1−/−and Drd1−/−mice) (Pendergast et al., 2009; Gallardo et al., 2014). FAA is also more robust when wheel-running activity (compared to general activity) is measured. It is also important to note that the phase of FAA may be altered depending on the mode of activity. Wheel-running FAA typically occurs during the hours immediately before food availability and stops abruptly when food is presented. In contrast, general locomotor FAA (e.g. measured by a passive infrared sensor) typically occurs during food availability. Finally, the timing and duration of fasting should be carefully considered. We have found that 48 hours of fasting is ideal to reveal the expression of robust FAA. Fasting should be timed so that hours 40 to 48 of fasting occur during the predicted time of FAA. FAA may be weak or absent on the first day of fasting, but is typically robustly expressed on the second day of fasting. Moreover, fasting is necessary to determine if FAA is the output of an hourglass timer or a self-sustained oscillator. FAA on the first day of fasting could be the output of an hourglass timer, while FAA on the second day of fasting is the output of a self-sustained oscillator.
Finally, studies of FAA expression must be interpreted with caution. Changes in the robustness, or magnitude, of FAA could result from changes in the input or output, and not necessarily from changes in the FEO amplitude. The best evidence that a gene is important for FEO timekeeping is if mutating that gene causes a change in the period of FAA. However, measuring the period of the FEO is challenging (because it doesn’t free-run in ad libitum feeding conditions). Only a few studies have reported free-running FAA under a feeding cycle that is outside the range of entrainment (Stephan, 1992; Pendergast et al., 2012; Takasu et al., 2012). The period of the FEO can be estimated in mutant and engineered mice by measuring the range of entrainment using different T cycles of food availability. However, this approach is costly, time-consuming, and difficult.
Acknowledgments
We believe we have reviewed all FAA studies in mutant and genetically engineered mice herein. If we have overlooked a study, we apologize and would like to be notified of said study. J.S.P is supported by National Institutes of HeaIth (NIH) grants DK098321, P20 GM103527 (Junior Investigator), DK107851, and the University of Kentucky. S.Y. is supported by NIH grant R21 NS099809.
References
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB, and Horvath TL (2006) Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest 116:3229–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Galvan G, Yi CX, van der Vliet J, Jhamandas JH, Panula P, Angeles-Castellanos M, Del Carmen Basualdo M, Escobar C, and Buijs RM (2011) Interaction between hypothalamic dorsomedial nucleus and the suprachiasmatic nucleus determines intensity of food anticipatory behavior. Proceedings of the National Academy of Sciences of the United States of America 108:5813–5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS, Prabakaran D, and Flier JS (1998) Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest 101:1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama M, Yuasa T, Hayasaka N, Horikawa K, Sakurai T, and Shibata S (2004) Reduced food anticipatory activity in genetically orexin (hypocretin) neuron-ablated mice. The European journal of neuroscience 20:3054–3062. [DOI] [PubMed] [Google Scholar]
- Alexandre C, Andermann ML, and Scammell TE (2013) Control of arousal by the orexin neurons. Curr Opin Neurobiol 23:752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Cakir I, Carrington SJ, Cone RD, Ghamari-Langroudi M, Gillyard T, Gimenez LE, and Litt MJ (2016) 60 YEARS OF POMC: Regulation of feeding and energy homeostasis by alpha-MSH. J Mol Endocrinol 56:T157–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arble DM, Vitaterna MH, and Turek FW (2011) Rhythmic leptin is required for weight gain from circadian desynchronized feeding in the mouse. PloS one 6:e25079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher G, Reinke H, Altmeyer M, Gutierrez-Arcelus M, Hottiger MO, and Schibler U (2010) Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell 142:943–953. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, and Herzog ED (2005) Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci 8:476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik JH (2013) Dopamine signaling in reward-related behaviors. Front Neural Circuits 7:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum ID, Patterson Z, Khazall R, Lamont EW, Sleeman MW, Horvath TL, and Abizaid A (2009) Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor deficient mice. Neuroscience 164:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodosi B, Gardi J, Hajdu I, Szentirmai E, Obal F, Jr., and Krueger JM (2004) Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol Regul Integr Comp Physiol 287:R1071–1079. [DOI] [PubMed] [Google Scholar]
- Boulos Z, and Terman M (1980) Food availability and daily biological rhythms. Neurosci Biobehav Rev 4:119–131. [DOI] [PubMed] [Google Scholar]
- Bunger MK, Walisser JA, Sullivan R, Manley PA, Moran SM, Kalscheur VL, Colman RJ, and Bradfield CA (2005) Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus. Genesis 41:122–132. [DOI] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, and Bradfield CA (2000) Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103:1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caston J, Chianale C, Delhaye-Bouchaud N, and Mariani J (1998) Role of the cerebellum in exploration behavior. Brain research 808:232–237. [DOI] [PubMed] [Google Scholar]
- Challet E (2010) Interactions between light, mealtime and calorie restriction to control daily timing in mammals. J Comp Physiol B 180:631–644. [DOI] [PubMed] [Google Scholar]
- Challet E, Mendoza J, Dardente H, and Pevet P (2009) Neurogenetics of food anticipation. The European journal of neuroscience 30:1676–1687. [DOI] [PubMed] [Google Scholar]
- Chavan R, Feillet C, Costa SS, Delorme JE, Okabe T, Ripperger JA, and Albrecht U (2016) Liver-derived ketone bodies are necessary for food anticipation. Nat Commun 7:10580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Steele AD, Lindquist S, and Guarente L (2005) Increase in activity during calorie restriction requires Sirt1. Science 310:1641. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hu C, Hsu CK, Zhang Q, Bi C, Asnicar M, Hsiung HM, Fox N, Slieker LJ, Yang DD, Heiman ML, and Shi Y (2002) Targeted disruption of the melanin-concentrating hormone receptor-1 results in hyperphagia and resistance to diet-induced obesity. Endocrinology 143:2469–2477. [DOI] [PubMed] [Google Scholar]
- Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, and Zhou QY (2002) Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature 417:405–410. [DOI] [PubMed] [Google Scholar]
- Clark EL, Baumann CR, Cano G, Scammell TE, and Mochizuki T (2009) Feeding-elicited cataplexy in orexin knockout mice. Neuroscience 161:970–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ (2009) Lesion studies targeting food-anticipatory activity. The European journal of neuroscience 30:1658–1664. [DOI] [PubMed] [Google Scholar]
- Davis JF, Choi DL, Clegg DJ, and Benoit SC (2011) Signaling through the ghrelin receptor modulates hippocampal function and meal anticipation in mice. Physiol Behav 103:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delezie J, Dumont S, Sandu C, Reibel S, Pevet P, and Challet E (2016) Rev-erbalpha in the brain is essential for circadian food entrainment. Scientific reports 6:29386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellacott KL, and Cone RD (2006) The role of the central melanocortin system in the regulation of food intake and energy homeostasis: lessons from mouse models. Philos Trans R Soc Lond B Biol Sci 361:1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, and Cone RD (1997) Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385:165–168. [DOI] [PubMed] [Google Scholar]
- Feillet CA, Ripperger JA, Magnone MC, Dulloo A, Albrecht U, and Challet E (2006) Lack of food anticipation in Per2 mutant mice. Current biology : CB 16:2016–2022. [DOI] [PubMed] [Google Scholar]
- Flores DE, Bettilyon CN, Jia L, and Yamazaki S (2016a) The Running Wheel Enhances Food Anticipatory Activity: An Exploratory Study. Front Behav Neurosci 10:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores DE, Bettilyon CN, and Yamazaki S (2016b) Period-independent novel circadian oscillators revealed by timed exercise and palatable meals. Scientific reports 6:21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller PM, Lu J, and Saper CB (2008) Differential rescue of light- and food-entrainable circadian rhythms. Science 320:1074–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller PM, Lu J, and Saper CB (2009) Standards of evidence in chronobiology: A response. J Circadian Rhythms 7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo CM, Darvas M, Oviatt M, Chang CH, Michalik M, Huddy TF, Meyer EE, Shuster SA, Aguayo A, Hill EM, Kiani K, Ikpeazu J, Martinez JS, Purpura M, Smit AN, Patton DF, Mistlberger RE, Palmiter RD, and Steele AD (2014) Dopamine receptor 1 neurons in the dorsal striatum regulate food anticipatory circadian activity rhythms in mice. Elife 3:e03781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JA, Jensen LT, Doyle SE, Miranda-Anaya M, Menaker M, Fugger L, Bayliss DA, and Burdakov D (2009) Deletion of TASK1 and TASK3 channels disrupts intrinsic excitability but does not abolish glucose or pH responses of orexin/hypocretin neurons. The European journal of neuroscience 30:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Schomer A, and Saper CB (2006) The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci 9:398–407. [DOI] [PubMed] [Google Scholar]
- Gunapala KM, Gallardo CM, Hsu CT, and Steele AD (2011) Single gene deletions of orexin, leptin, neuropeptide Y, and ghrelin do not appreciably alter food anticipatory activity in mice. PloS one 6:e18377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Georg B, and Fahrenkrug J (2016) Altered Circadian Food Anticipatory Activity Rhythms in PACAP Receptor 1 (PAC1) Deficient Mice. PloS one 11:e0146981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa K, Minami Y, Iijima M, Akiyama M, and Shibata S (2005) Rapid damping of food-entrained circadian rhythm of clock gene expression in clock-defective peripheral tissues under fasting conditions. Neuroscience 134:335–343. [DOI] [PubMed] [Google Scholar]
- Hsu JL, Yu L, Sullivan E, Bowman M, Mistlberger RE, and Tecott LH (2010) Enhanced food anticipatory activity associated with enhanced activation of extrahypothalamic neural pathways in serotonin2C receptor null mutant mice. PloS one 5:e11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Pashkov V, Kurrasch DM, Yu K, Gold SJ, and Wilkie TM (2006) Feeding and fasting controls liver expression of a regulator of G protein signaling (Rgs16) in periportal hepatocytes. Comp Hepatol 5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M, Yamaguchi S, van der Horst GT, Bonnefont X, Okamura H, and Shibata S (2005) Altered food-anticipatory activity rhythm in Cryptochrome-deficient mice. Neurosci Res 52:166–173. [DOI] [PubMed] [Google Scholar]
- Imai S, and Guarente L (2010) Ten years of NAD-dependent SIR2 family deacetylases: implications for metabolic diseases. Trends Pharmacol Sci 31:212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumo M, Pejchal M, Schook AC, Lange RP, Walisser JA, Sato TR, Wang X, Bradfield CA, and Takahashi JS (2014) Differential effects of light and feeding on circadian organization of peripheral clocks in a forebrain Bmal1 mutant. Elife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen HT, Sergeeva A, Stark G, and Sorg BA (2012) Circadian discrimination of reward: evidence for simultaneous yet separable food- and drug-entrained rhythms in the rat. Chronobiology international 29:454–468. [DOI] [PubMed] [Google Scholar]
- Kas MJ, van den Bos R, Baars AM, Lubbers M, Lesscher HM, Hillebrand JJ, Schuller AG, Pintar JE, and Spruijt BM (2004) Mu-opioid receptor knockout mice show diminished food-anticipatory activity. The European journal of neuroscience 20:1624–1632. [DOI] [PubMed] [Google Scholar]
- Kaur S, Thankachan S, Begum S, Blanco-Centurion C, Sakurai T, Yanagisawa M, and Shiromani PJ (2008) Entrainment of temperature and activity rhythms to restricted feeding in orexin knock out mice. Brain research 1205:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith DR, Hart CL, Robotham M, Tariq M, Le Sauter J, and Silver R (2013) Time of day influences the voluntary intake and behavioral response to methamphetamine and food reward. Pharmacol Biochem Behav 110:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, and Antoch MP (2006) Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev 20:1868–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizo JA, Moreland LE, Rastogi A, Mou X, Prosser RA, and Mintz EM (2018) Regulation of Locomotor activity in fed, fasted, and food-restricted mice lacking tissue-type plasminogen activator. BMC Physiol 18:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry GJ, Simon MM, Webb IC, and Mistlberger RE (2006) Persistence of a behavioral food-anticipatory circadian rhythm following dorsomedial hypothalamic ablation in rats. Am J Physiol Regul Integr Comp Physiol 290:R1527–1534. [DOI] [PubMed] [Google Scholar]
- LeSauter J, Hoque N, Weintraub M, Pfaff DW, and Silver R (2009) Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proceedings of the National Academy of Sciences of the United States of America 106:13582–13587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JD, Hu WP, Boehmer L, Cheng MY, Lee AG, Jilek A, Siegel JM, and Zhou QY (2006) Attenuated circadian rhythms in mice lacking the prokineticin 2 gene. The Journal of neuroscience : the official journal of the Society for Neuroscience 26:11615–11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wang Y, Sun KK, Wang K, Sun ZS, Zhao M, and Wang J (2015) Sex-related difference in food-anticipatory activity of mice. Horm Behav 70:38–46. [DOI] [PubMed] [Google Scholar]
- Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, and Elmquist JK (2003) Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. The Journal of neuroscience : the official journal of the Society for Neuroscience 23:7143–7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Liu TY, Qu WM, Hong ZY, Urade Y, and Huang ZL (2012) Dopamine is involved in food-anticipatory activity in mice. J Biol Rhythms 27:398–409. [DOI] [PubMed] [Google Scholar]
- Loh K, Herzog H, and Shi YC (2015) Regulation of energy homeostasis by the NPY system. Trends Endocrinol Metab 26:125–135. [DOI] [PubMed] [Google Scholar]
- Lubkin M, and Stricker-Krongrad A (1998) Independent feeding and metabolic actions of orexins in mice. Biochemical and biophysical research communications 253:241–245. [DOI] [PubMed] [Google Scholar]
- Luuk H, Fahrenkrug J, and Hannibal J (2012) Circadian rhythms and food anticipatory behavior in Wfs1-deficient mice. Biochemical and biophysical research communications 424:717–723. [DOI] [PubMed] [Google Scholar]
- Luuk H, Koks S, Plaas M, Hannibal J, Rehfeld JF, and Vasar E (2008) Distribution of Wfs1 protein in the central nervous system of the mouse and its relation to clinical symptoms of the Wolfram syndrome. J Comp Neurol 509:642–660. [DOI] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, and Bates GP (1996) Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87:493–506. [DOI] [PubMed] [Google Scholar]
- Marosi K, and Mattson MP (2014) BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab 25:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh DJ, Weingarth DT, Novi DE, Chen HY, Trumbauer ME, Chen AS, Guan XM, Jiang MM, Feng Y, Camacho RE, Shen Z, Frazier EG, Yu H, Metzger JM, Kuca SJ, Shearman LP, Gopal-Truter S, MacNeil DJ, Strack AM, MacIntyre DE, Van der Ploeg LH, and Qian S (2002) Melaninconcentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proceedings of the National Academy of Sciences of the United States of America 99:3240–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Merlos MT, Angeles-Castellanos M, Diaz-Munoz M, Aguilar-Roblero R, Mendoza J, and Escobar C (2004) Dissociation between adipose tissue signals, behavior and the food-entrained oscillator. J Endocrinol 181:53–63. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Fraenkel E, McAllister CJ, Wood N, Reddy AB, Hastings MH, and Morton AJ (2010) Disruption of peripheral circadian timekeeping in a mouse model of Huntington’s disease and its restoration by temporally scheduled feeding. The Journal of neuroscience : the official journal of the Society for Neuroscience 30:10199–10204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Reddy AB, Wong GK, O’Neill JS, O’Brien JA, McMahon DG, Harmar AJ, Okamura H, and Hastings MH (2006) Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Current biology : CB 16:599–605. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Albrecht U, and Challet E (2010a) Behavioural food anticipation in clock genes deficient mice: confirming old phenotypes, describing new phenotypes. Genes Brain Behav 9:467–477. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Pevet P, Felder-Schmittbuhl MP, Bailly Y, and Challet E (2010b) The cerebellum harbors a circadian oscillator involved in food anticipation. The Journal of neuroscience : the official journal of the Society for Neuroscience 30:1894–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalik M, Steele AD, and Mistlberger RE (2015) A sex difference in circadian food-anticipatory rhythms in mice: Interaction with dopamine D1 receptor knockout. Behav Neurosci 129:351–360. [DOI] [PubMed] [Google Scholar]
- Mieda M, and Sakurai T (2011) Bmal1 in the nervous system is essential for normal adaptation of circadian locomotor activity and food intake to periodic feeding. The Journal of neuroscience : the official journal of the Society for Neuroscience 31:15391–15396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M, Williams SC, Richardson JA, Tanaka K, and Yanagisawa M (2006) The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proceedings of the National Academy of Sciences of the United States of America 103:12150–12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M, Williams SC, Sinton CM, Richardson JA, Sakurai T, and Yanagisawa M (2004) Orexin neurons function in an efferent pathway of a food-entrainable circadian oscillator in eliciting food-anticipatory activity and wakefulness. The Journal of neuroscience : the official journal of the Society for Neuroscience 24:10493–10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger R, and Rusak B (1987) Palatable daily meals entrain anticipatory activity rhythms in free-feeding rats: dependence on meal size and nutrient content. Physiol Behav 41:219–226. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE (1994) Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev 18:171–195. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE (2009) Food-anticipatory circadian rhythms: concepts and methods. The European journal of neuroscience 30:1718–1729. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Buijs RM, Challet E, Escobar C, Landry GJ, Kalsbeek A, Pevet P, and Shibata S (2009a) Food anticipation in Bmal1−/− and AAV-Bmal1 rescued mice: a reply to Fuller et al. J Circadian Rhythms 7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE, Buijs RM, Challet E, Escobar C, Landry GJ, Kalsbeek A, Pevet P, and Shibata S (2009b) Standards of evidence in chronobiology: critical review of a report that restoration of Bmal1 expression in the dorsomedial hypothalamus is sufficient to restore circadian food anticipatory rhythms in Bmal1−/− mice. J Circadian Rhythms 7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE, Yamazaki S, Pendergast JS, Landry GJ, Takumi T, and Nakamura W (2008) Comment on “Differential rescue of light- and food-entrainable circadian rhythms”. Science 322:675; author reply 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya T, Aida R, Kudo T, Akiyama M, Doi M, Hayasaka N, Nakahata N, Mistlberger R, Okamura H, and Shibata S (2009) The dorsomedial hypothalamic nucleus is not necessary for food-anticipatory circadian rhythms of behavior, temperature or clock gene expression in mice. The European journal of neuroscience 29:1447–1460. [DOI] [PubMed] [Google Scholar]
- Morton AJ, Wood NI, Hastings MH, Hurelbrink C, Barker RA, and Maywood ES (2005) Disintegration of the sleep-wake cycle and circadian timing in Huntington’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience 25:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenweller JE, Tapp WN, and Natelson BH (1990) Phase-shifting the light-dark cycle resets the food-entrainable circadian pacemaker. Am J Physiol 258:R994–1000. [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, and Lu B (2004) Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science 306:487–491. [DOI] [PubMed] [Google Scholar]
- Pendergast JS, Nakamura W, Friday RC, Hatanaka F, Takumi T, and Yamazaki S (2009) Robust food anticipatory activity in BMAL1-deficient mice. PloS one 4:e4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergast JS, Oda GA, Niswender KD, and Yamazaki S (2012) Period determination in the food-entrainable and methamphetamine-sensitive circadian oscillator(s). Proceedings of the National Academy of Sciences of the United States of America 109:14218–14223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergast JS, Wendroth RH, Stenner RC, Keil CD, and Yamazaki S (2017) mPeriod2 (Brdm1) and other single Period mutant mice have normal food anticipatory activity. Scientific reports 7:15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergast JS, and Yamazaki S (2014) Effects of light, food, and methamphetamine on the circadian activity rhythm in mice. Physiol Behav 128:92–98. [DOI] [PubMed] [Google Scholar]
- Pendergast JS, and Yamazaki S (2017) Extra-SCN circadian pacemakers In Biological Clocks, Honma K, and Honma S, eds, pp 141–152, Hokkaido Univ. Press, Sapporo. [Google Scholar]
- Pitts S, Perone E, and Silver R (2003) Food-entrained circadian rhythms are sustained in arrhythmic Clk/Clk mutant mice. Am J Physiol Regul Integr Comp Physiol 285:R57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AB (2016) Redox and Metabolic Oscillations in the Clockwork In A Time for Metabolism and Hormones, Sassone-Corsi P, and Christen Y, eds, pp 51–61, Cham (CH). [PubMed] [Google Scholar]
- Ribeiro AC, Ceccarini G, Dupre C, Friedman JM, Pfaff DW, and Mark AL (2011) Contrasting effects of leptin on food anticipatory and total locomotor activity. PloS one 6:e23364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CP (1922) A behavioristic study of the activity of the rat. Comparative Psychology Monographs 1:1–54. [Google Scholar]
- Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB, and Cone RD (1993) Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proceedings of the National Academy of Sciences of the United States of America 90:8856–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser AM, Pelchat RJ, and Adler NT (1984) Memory for feeding time: possible dependence on coupled circadian oscillators. Physiol Behav 32:25–30. [DOI] [PubMed] [Google Scholar]
- Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, and Fitzgerald GA (2004) BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol 2:e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito N, and Shirai Y (2002) Protein kinase C gamma (PKC gamma): function of neuron specific isotype. J Biochem 132:683–687. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, and Yanagisawa M (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:573–585. [DOI] [PubMed] [Google Scholar]
- Satoh A, Brace CS, Ben-Josef G, West T, Wozniak DF, Holtzman DM, Herzog ED, and Imai S (2010) SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. The Journal of neuroscience : the official journal of the Society for Neuroscience 30:10220–10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber V, Dantzer F, Ame JC, and de Murcia G (2006) Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol 7:517–528. [DOI] [PubMed] [Google Scholar]
- Sheward WJ, Maywood ES, French KL, Horn JM, Hastings MH, Seckl JR, Holmes MC, and Harmar AJ (2007) Entrainment to feeding but not to light: circadian phenotype of VPAC2 receptor-null mice. The Journal of neuroscience : the official journal of the Society for Neuroscience 27:4351–4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart D, Haynes AC, Williams G, and Arch JR (2002) Orexins and the treatment of obesity. Eur J Pharmacol 440:199–212. [DOI] [PubMed] [Google Scholar]
- Spruijt BM, van den Bos R, and Pijlman FT (2001) A concept of welfare based on reward evaluating mechanisms in the brain: anticipatory behaviour as an indicator for the state of reward systems. Appl Anim Behav Sci 72:145–171. [DOI] [PubMed] [Google Scholar]
- Stephan FK (1986) Coupling between feeding- and light-entrainable circadian pacemakers in the rat. Physiol Behav 38:537–544. [DOI] [PubMed] [Google Scholar]
- Stephan FK (1992) Resetting of a feeding-entrainable circadian clock in the rat. Physiol Behav 52:985–995. [DOI] [PubMed] [Google Scholar]
- Storch KF, and Weitz CJ (2009) Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proceedings of the National Academy of Sciences of the United States of America 106:6808–6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton GM, Perez-Tilve D, Nogueiras R, Fang J, Kim JK, Cone RD, Gimble JM, Tschop MH, and Butler AA (2008) The melanocortin-3 receptor is required for entrainment to meal intake. The Journal of neuroscience : the official journal of the Society for Neuroscience 28:12946–12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczypka MS, Rainey MA, Kim DS, Alaynick WA, Marck BT, Matsumoto AM, and Palmiter RD (1999) Feeding behavior in dopamine-deficient mice. Proceedings of the National Academy of Sciences of the United States of America 96:12138–12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai E, Kapas L, Sun Y, Smith RG, and Krueger JM (2010) Restricted feeding-induced sleep, activity, and body temperature changes in normal and preproghrelin-deficient mice. Am J Physiol Regul Integr Comp Physiol 298:R467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS (2016) Molecular Architecture of the Circadian Clock in Mammals In A Time for Metabolism and Hormones, Sassone-Corsi P, and Christen Y, eds, pp 13–24, Cham (CH). [PubMed] [Google Scholar]
- Takasu NN, Kurosawa G, Tokuda IT, Mochizuki A, Todo T, and Nakamura W (2012) Circadian regulation of food-anticipatory activity in molecular clock-deficient mice. PloS one 7:e48892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, and Julius D (1995) Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature 374:542–546. [DOI] [PubMed] [Google Scholar]
- Verwey M, and Amir S (2009) Food-entrainable circadian oscillators in the brain. The European journal of neuroscience 30:1650–1657. [DOI] [PubMed] [Google Scholar]
- Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, and Takahashi JS (1994) Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 264:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara T, Honma S, and Honma K (1996) Effects of restricted daily feeding on neuropeptide Y release in the rat paraventricular nucleus. Am J Physiol 270:E589–595. [DOI] [PubMed] [Google Scholar]
- Zhang L, Abraham D, Lin ST, Oster H, Eichele G, Fu YH, and Ptacek LJ (2012) PKCgamma participates in food entrainment by regulating BMAL1. Proceedings of the National Academy of Sciences of the United States of America 109:20679–20684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, and Friedman JM (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432. [DOI] [PubMed] [Google Scholar]
- Zhou D, Shen Z, Strack AM, Marsh DJ, and Shearman LP (2005) Enhanced running wheel activity of both Mch1r- and Pmch-deficient mice. Regul Pept 124:53–63. [DOI] [PubMed] [Google Scholar]
- Zhou QY, and Palmiter RD (1995) Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 83:1197–1209. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, and Elmquist JK (2005) Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest 115:3564–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]