Abstract
In 2018, the American Heart Association (AHA) published a Scientific Statement on resistant hypertension. We compared the prevalence of apparent treatment resistant hypertension (aTRH) among US adults as defined in the 2018 and 2008 AHA Scientific Statements using data from 4,158 participants with hypertension, taking antihypertensive medication in the 2009–2014 National Health and Nutrition Examination Survey. Blood pressure (BP) was measured three times and antihypertensive medication classes were identified through a pill bottle review. In both Scientific Statements, aTRH was defined as uncontrolled BP while taking ≥3 classes of antihypertensive medication or taking ≥4 classes of antihypertensive medication regardless of BP level. Uncontrolled BP was defined as systolic/diastolic BP ≥140/90 mmHg (≥130/80 mmHg for those with diabetes or chronic kidney disease) in the 2008 Scientific Statement and systolic/diastolic BP ≥130/80 mmHg (systolic BP ≥130 mmHg only for low-risk adults ≥65 years of age) in the 2018 Scientific Statement. The prevalence of aTRH was 17.7% and 19.7% according to the 2008 and 2018 Scientific Statement definitions, respectively (Δ=2.0%; 95%CI 1.5%, 2.7%). Overall, 10.3 million US adults had aTRH according to the 2018 Scientific Statement. The most common three drug combination taken included an angiotensin-converting enzyme inhibitor, β-blocker, and thiazide diuretic. Using the 2018 definition, 3.2% of US adults with aTRH were taking a thiazide-like diuretic (chlorthalidone or indapamide) and 9.0% were taking a mineralocorticoid receptor blocker (spironolactone or eplerenone). In conclusion, the prevalence of aTRH is only modestly higher using the definition in the 2018 versus 2008 resistant hypertension Scientific Statement.
Keywords: Resistant hypertension, hypertension, blood pressure control, antihypertensive medication, scientific statements
Resistant hypertension is defined as having a blood pressure (BP) that remains above the recommended goal despite treatment with three classes of antihypertensive medication or treatment with four or more classes of antihypertensive medication with any level of BP.1, 2 In 2018, the American Heart Association (AHA) published an update to its 2008 Scientific Statement on Resistant Hypertension. The major difference in the definitions of resistant hypertension between these Scientific Statements is the BP goal. The 2008 AHA Scientific Statement defined BP control as systolic BP (SBP) < 140 mm Hg and diastolic BP (DBP) < 90 mm Hg for most adults, with SBP < 130 mm Hg and DBP < 80 mm Hg for adults with diabetes or chronic kidney disease (CKD).1 The 2018 AHA Scientific Statement on Resistant Hypertension was consistent with the 2017 American College of Cardiology (ACC)/AHA BP clinical practice guideline, in defining BP control as SBP < 130 mm Hg and DBP < 80 mm Hg for all adults < 65 years of age and for adults ≥ 65 years of age with diabetes, CKD, history of cardiovascular disease (CVD) or 10-year predicted atherosclerotic CVD (ASCVD) risk ≥ 10% on the Pooled Cohort risk equations.2, 3 Only SBP < 130 mm Hg was used to define BP control for adults ≥ 65 years of age without diabetes, CKD, history of CVD or 10-year predicted ASCVD risk ≥ 10%. In the 2017 ACC/AHA BP guideline, it was estimated that the prevalence of apparent treatment resistant hypertension (aTRH) would be 4% higher when SBP <130 mm Hg and DBP < 80 mm Hg was used to define control, but the guideline writing committee noted that this estimate needed to be validated in future studies.3 The term aTRH is used for population-based studies in which it is not possible to exclude pseudoresistance (e.g., inaccurate measurement of BP, medication non-adherence or white coat effect).
The primary purpose of the present study was to compare the prevalence and characteristics of adults with aTRH using the definitions in the 2018 versus the 2008 AHA Scientific Statement. A secondary goal was to determine the most frequent antihypertensive medication combinations and the corresponding BP control rates for US adults taking three or more classes of antihypertensive medication. Also, we examined the use of thiazide-like diuretics and mineralocorticoid receptor blockers among US adults with aTRH according to the 2018 AHA Scientific Statement. To accomplish these goals, we analyzed data from the 2009–2014 US National Health and Nutrition Examination Survey (NHANES).
METHODS
Data used in the current study are available on the National Center for Health Statistics of the Center for Disease Control and Prevention website: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx. Other study material is available from the corresponding author. The NHANES is a cross-sectional survey of the US population designed and conducted by National Center for Health Statistics of the Center for Disease Control and Prevention.4 Since 1999–2000, NHANES has been conducted in two-year cycles. For each two-year cycle, a stratified, multistage, probability sampling method was employed to identify a non-institutionalized US population for enrollment. The current analysis included data from the 2009–2010, 2011–2012, 2013–2014 cycles, pooled together. The analysis was restricted to participants ≥ 20 years of age who self-reported taking antihypertensive medication and had at least one class of antihypertensive medication identified during a pill bottle review conducted as part of the NHANES examination (n=4,520). We excluded 362 participants without three BP measurements obtained during their study exam resulting in a final sample size of 4,158 participants for all analyses. The protocols for NHANES 2009–2011, 2011–2012 and 2013–2014 were approved by the National Center for Health Statistics of the Center for Disease Control and Prevention Institutional Review Board. All participants provided written informed consent.
Data collection
NHANES data were collected through questionnaires and a medical examination conducted at a mobile clinic. Using questionnaires, data on age, race/ethnicity, sex, a previous diagnosis of diabetes, coronary heart disease, myocardial infarction, stroke and heart failure were collected. Self-reported use of antihypertensive and glucose-lowering medication was also assessed.
Blood pressure measurement and antihypertensive medication use
Trained physicians measured BP three times using a mercury sphygmomanometer and an appropriately-sized BP cuff. The first measurement was performed after participants had rested, seated for five minutes with a 30-second interval between readings. The average of the three BP measurements was used to define SBP and DBP. Quality control procedures included re-certification of all physicians every three months and re-training every 12 months. There was no digit preference for either SBP or DBP (Table S1).
Antihypertensive medication classes and aTRH
Participants were asked to bring all prescription medications taken in the prior two weeks to their NHANES medical evaluation. Pill bottles were reviewed, and medication names were recorded and coded into drug classes based on their generic equivalents. These classes included angiotensin-converting enzyme (ACE) inhibitors, α-blockers, angiotensin receptor blockers (ARBs), β-blockers, dihydropyridine and non-dihydropyridine calcium channel blockers (CCBs), central acting α2-agonists, mineralocorticoid receptor antagonists, thiazide or thiazide-like diuretics, potassium-sparing diuretics, loop diuretics, and vasodilators. Single-pill combinations were categorized into their component classes. aTRH was defined using the BP goals for adults taking antihypertensive medication in the 2008 and 2018 AHA Scientific Statements as described in the introduction and presented in Table 1.1–3
Table 1.
Definition of apparent treatment resistant hypertension from the 2008 and 2018 American Heart Association Scientific Statements used in the analysis.
| Blood pressure measure | American Heart Association definition of apparent treatment resistant hypertension | |
|---|---|---|
| 2008 Scientific Statement | 2018 Scientific Statement | |
| Taking three classes of antihypertensive medication | ||
| Systolic blood pressure, mm Hg | ||
| General population | ≥140 | ≥130 |
| Adults with diabetes | ≥130 | ≥130 |
| Adults with chronic kidney disease | ≥130 | ≥130 |
| Diastolic blood pressure, mm Hg | ||
| General population | ≥ 90 | ≥ 80* |
| Adults with diabetes | ≥ 80 | ≥ 80 |
| Adults with chronic kidney disease | ≥ 80 | ≥ 80 |
| Taking four or more classes of antihypertensive medication | ||
| Systolic blood pressure, mm Hg | Any | Any |
| Diastolic blood pressure, mm Hg | Any | Any |
CKD: Chronic kidney disease.
CVD: Cardiovascular disease.
Except for adults ≥ 65 years of age without diabetes, chronic kidney disease, history of cardiovascular disease or 10-year predicted risk ≥ 10% on the Pooled Cohort risk equations. Only systolic blood pressure is used for adults ≥ 65 years of age without diabetes, chronic kidney disease, history of cardiovascular disease or 10-year predicted risk ≥ 10% on the Pooled Cohort risk equations.
Covariates
Diabetes was defined as a fasting serum glucose ≥ 126 mg/dL, non-fasting serum glucose ≥ 200 mg/dL, hemoglobin A1c ≥ 6.5% or self-report of a history of diabetes with concurrent glucose-lowering medication use. Estimated glomerular filtration rate (eGFR) was calculated using serum creatinine levels and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.5 An albumin to creatinine ratio (ACR) was calculated using the spot urine samples. CKD was defined as an eGFR < 60 ml/min/1.73 m2 or ACR > 30 mg/dL. History of CVD was defined by self-reported prior diagnosis of myocardial infarction, coronary heart disease, stroke or heart failure. The Pooled Cohort risk equations were used to calculate 10-year predicted risk for ASCVD among participants without a history of CVD.6 Participants were categorized into five mutually exclusive groups including history of CVD and no history of CVD with10-year predicted ASCVD risk < 5%, 5% to < 10%, 10% to < 20% and ≥ 20%.
Statistical analysis
The distribution of US adults by number of antihypertensive medication classes being taken cross-classified by SBP/DBP categories (<120/80, 120–129/<80, 130–139/80–89 and ≥140/90 mm Hg) was calculated. Additionally, we calculated the proportion of US adults taking antihypertensive medication with uncontrolled BP, defined using the 2008 and 2018 Scientific Statement definitions separately, overall and by number of classes being taken. Characteristics of US adults taking antihypertensive medication were computed for three mutually exclusive groups defined by not having aTRH using either the 2008 or 2018 Scientific Statement definitions, having aTRH using the 2018 definition but not the 2008 definition, and having aTRH using both definitions. Among US adults taking antihypertensive medication, the number and percentage with aTRH based on the 2008 and 2018 Scientific Statement definitions, separately, were calculated for the overall population and in sub-groups defined by age group (20–49, 50–59, 60–69 and ≥ 70 years), sex, race/ethnicity, diabetes status, CKD status, ACR ≤ 30 mg/dL or > 30 mg/dL, eGFR ≥ 60 or < 60 ml/min/1.73 m2, 10-year ASCVD risk category (<5%, 5% - <10%, 10% - <20%, ≥20% and history of CVD) and SBP/DBP category (<130/80, 130–139/80–89 and ≥140/90 mm Hg). The number and percentage of US adults with aTRH according to the 2018 but not the 2008 definition was also calculated. The NHANES 2009–2014 sample sizes used for these calculations are presented in Table S2. In a secondary analysis, we calculated the prevalence of aTRH using a modification of the 2008 and 2018 definitions in which use of a thiazide diuretic was required to be considered as having aTRH. Among US adults taking three or more classes of antihypertensive medication, we calculated the frequency for which the most common three antihypertensive medication combinations were being taken. Additionally, the percentage of US adults taking these medication combinations with an SBP < 140 mm Hg and DBP < 90 mm Hg and with an SBP < 130 mm Hg and DBP < 80 mm Hg, separately, were computed. In a final analysis, we calculated the proportion of US adults with aTRH using the definition in the 2018 Scientific Statement taking either chlorthalidone or indapamide and spironolactone or eplerenone.
The multi-stage sampling approach used in NHANES was taken into account by weighting the calculations to obtain nationally representative estimates. These weights were recalibrated based on the proportion of participants missing data by age, sex, and race-ethnicity within each NHANES cycle, assuming that data within strata were missing at random. Data management was conducted using SAS version 9.4 (SAS Institute, Cary, NC) and data analysis was conducted using Stata V15 (Stata Corporation, College Station, TX).
RESULTS
Overall, 36.7%, 36.1%, 17.6% and 9.7% of US adults taking antihypertensive medication were taking 1, 2, 3 or ≥4 classes of antihypertensive medication, respectively (Figure 1). Among US adults taking antihypertensive medication, 4.3% and 5.7% were taking three classes of antihypertensive medication and had SBP between 130 to 139 mm Hg or DBP between 80 to 89 mm Hg and SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg, respectively. Also, 2.2% and 2.8% were taking four or more classes of antihypertensive medication and had SBP between 130 to 139 mm Hg or DBP between 80 to 89 mm Hg and SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg, respectively. The proportion of US adults taking antihypertensive medication with uncontrolled BP, overall and by number of drug classes being taken, is presented in Table S3.
Figure 1.
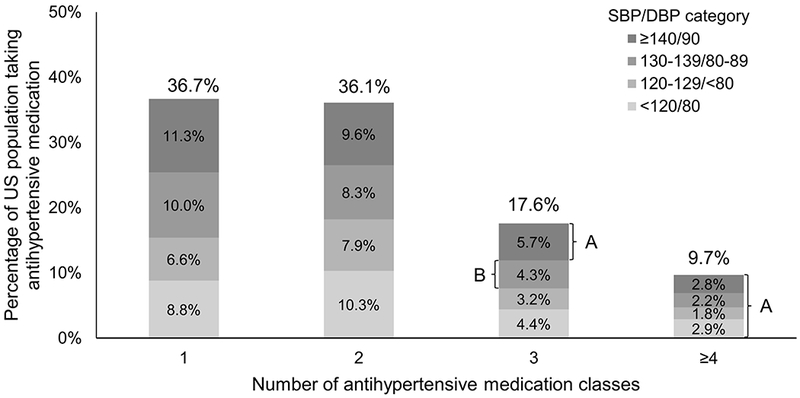
Distribution of US adults taking antihypertensive medication by number of antihypertensive drug classes they were taking and blood pressure levels.
SBP: Systolic blood pressure, DBP: Diastolic blood pressure.
Due to rounding, the numbers in the figure sum to 100.1% instead of 100%.
A: These individuals have apparent treatment resistant hypertension according to the 2008 and 2018 American Heart Association Scientific Statement definitions.
B: All of these individuals have apparent treatment resistant hypertension according to the 2018 American Heart Association’s Scientific Statement definition. Also, 2.3% of these individuals (i.e., those in this group with diabetes or chronic kidney disease) have apparent treatment resistant hypertension according to the 2008 American Heart Association’s Scientific Statement definition.
Adults with aTRH according to both the 2008 and 2018 definitions of BP control were older, more likely to be non-Hispanic black and have diabetes, CKD, ACR > 30 mg/g, eGFR < 60 ml/min/1.73 m2, a 10-year predicted ASCVD risk ≥ 20% or a history of CVD compared to their counterparts without aTRH (Table 2). Those with aTRH according to the 2018 but not the 2008 Scientific Statement were older and more likely to be non-Hispanic black compared with those who did not meet the definition for aTRH according to either the 2008 or 2018 Scientific Statements. The prevalence of 10-year predicted ASCVD risk ≥ 20% and history of CVD were similar among US adults with aTRH according to the 2018 but not the 2008 Scientific Statement and those without aTRH according to either Scientific Statement.
Table 2.
Characteristics of US adults with apparent treatment resistant hypertension according to the 2008 and 2018 American Heart Association Scientific Statements.
| Characteristic | aTRH by 2008 Scientific Statement | ||
|---|---|---|---|
| No | Yes | ||
| aTRH by 2018 Scientific Statement | |||
| No* (n=3226) | Yes (n=88) | Yes (n=844) | |
| Age group, years | |||
| 20 to 49 | 20.0% | 24.8% | 8.3% |
| 50 to 59 | 25.3% | 18.7% | 16.5% |
| 60 to 69 | 26.5% | 21.3% | 27.4% |
| ≥70 | 28.2% | 35.1% | 47.8% |
| Female sex | 55.1% | 52.2% | 55.7% |
| Race/ethnicity | |||
| Non-Hispanic white | 72.5% | 70.0% | 69.0% |
| Non-Hispanic black | 13.3% | 17.2% | 20.7% |
| Hispanic | 8.4% | 8.4% | 7.2% |
| Other | 5.8% | 4.4% | 3.1% |
| Diabetes | 23.5% | 0% | 48.1% |
| Chronic kidney disease | 26.5% | 0% | 50.8% |
| ACR >30 mg/dL | 14.6% | 0% | 30.2% |
| eGFR <60 ml/min/1.73 m2 | 16.3% | 0% | 33.8% |
| 10-year risk categories | |||
| <5% | 25.6% | 24.9% | 7.3% |
| 5% to <10% | 17.9% | 19.5% | 7.3% |
| 10% to <20% | 19.3% | 24.0% | 13.6% |
| ≥20% | 18.7% | 14.6% | 31.1% |
| History of CVD† | 18.4% | 17.0% | 40.8% |
| SBP/DBP category, mm Hg | |||
| <130/80 | 51.2% | 0% | 26.2% |
| 130-139/80-89 | 22.8% | 100% | 25.3% |
| ≥ 140/90 | 26.0% | 0% | 48.5% |
Numbers in the table are column percentage.
aTRH – apparent treatment resistant hypertension, ACR - albumin-to-creatinine ratio, eGFR – estimated glomerular filtration rate, CVD - cardiovascular disease, SBP – systolic blood pressure, DBP – diastolic blood pressure.
Restricted to US adults taking antihypertensive medication.
All participants with a history of CVD are in this category regardless of their 10-year predicted risk.
Overall, 17.7% of US adults taking antihypertensive medication had aTRH according to the 2008 Scientific Statement compared with 19.7% according to the 2018 Scientific Statement resulting in a net difference of 2.0% (95% CI 1.5%, 2.7%) having aTRH by the 2018 but not the 2008 Scientific Statement (Table 3). There was no change in the prevalence of aTRH for those with diabetes, CKD, ACR >30 mg/dL, eGFR <60 ml/min/1.73m2, and SBP/DBP <130/80 mm Hg or SBP/DBP ≥ 140/90 mm Hg. With the exception of those with SBP/DBP of 130–139/80–89 mm Hg, the prevalence of aTRH was 1% to 3% higher in every other sub-group when defined using the 2018 versus 2008 Scientific Statement. The prevalence of aTRH was 8.0% higher among adults with SBP/DBP of 130–139/80–89 mm Hg according to the 2018 versus 2008 Scientific Statement. Among US adults with aTRH, 31.3% and 23.6% were taking ≥ 4 classes of antihypertensive medication and had controlled BP according to the 2008 and 2018 definitions, respectively. Overall, 9.2 million US adults met the 2008 Scientific Statement definition for aTRH compared with 10.3 million US adults meeting the definition in the 2018 Scientific Statement (Table S4). There were 1.0 (95% CI 0.7, 1.3) million US adults with aTRH when defined by the 2018 Scientific Statement but not the 2008 Scientific Statement.
Table 3.
Percentage of US adults taking antihypertensive medication with apparent treatment resistant hypertension according to definitions in the 2008 and 2018 American Heart Association’s Scientific Statements.
| Sub-group | American Heart Association definition of apparent treatment resistant hypertension | ||
|---|---|---|---|
| 2008 Scientific Statement | 2018 Scientific Statement | 2018 but not 2008 | |
| Overall | 17.7% (16.0%, 19.5%) | 19.7% (17.9%, 21.6%) | 2.0% (1.5%, 2.7%) |
| Age group, years | |||
| 20 to 49 | 8.1% (5.7%, 11.5%) | 10.8% (7.8%, 14.8%) | 2.7% (1.7%, 4.7%) |
| 50 to 59 | 12.4% (9.8%, 15.6%) | 14.0% (11.1%, 17.4%) | 1.6% (0.8%, 3.1%) |
| 60 to 69 | 18.2% (15.3%, 21.6%) | 19.9% (16.6%, 23.6%) | 1.6% (0.8%, 3.1%) |
| ≥70 | 26.6% (23.1%, 30.4%) | 28.8% (25.3%, 32.6%) | 2.2% (1.5%, 3.2%) |
| Male sex | 17.5% (15.2%, 20.0%) | 19.6% (17.2%, 22.2%) | 2.1% (1.5%, 3.1%) |
| Female sex | 17.9% (15.6%, 20.4%) | 19.8% (17.5%, 22.3%) | 1.9% (1.3%, 2.8%) |
| Race/ethnicity, % | |||
| Non-Hispanic white | 17.0% (14.9%, 19.2%) | 18.9% (16.8%, 21.2%) | 1.9% (1.3%, 2.8%) |
| Non-Hispanic black | 25.0% (22.3%, 27.8%) | 27.3% (24.4%, 30.4%) | 2.3% (1.6%, 3.3%) |
| Hispanic | 15.6% (12.2%, 19.8%) | 17.7% (14.3%, 21.6%) | 2.0% (1.0%, 5.8%) |
| Other | 10.4% (7.3%, 14.5%) | 12.0% (8.2%, 17.4%) | 1.7% (0.5%, 6.5%) |
| No diabetes | 12.6% (11.2%, 14.3%) | 15.4% (13.7%, 17.2%) | 2.7% (2.0%, 3.7%) |
| Diabetes | 31.1% (27.4%, 35.1%) | 31.1% (27.4%, 35.1%) | 0%† |
| No chronic kidney disease | 12.5% (10.7%, 14.5%) | 15.3% (13.3%, 17.6%) | 2.9% (2.1%, 3.8%) |
| Chronic kidney disease | 29.7% (26.6%, 33.1%) | 29.7% (26.6%, 33.1%) | 0%† |
| ACR ≤30 mg/dL | 14.9% (13.2%, 16.7%) | 17.3% (15.4%, 19.3%) | 2.4% (1.8%, 3.2%) |
| ACR >30 mg/dL | 31.3% (27.4%, 35.6%) | 31.3% (27.4%, 35.6%) | 0%† |
| eGFR ≥60 ml/min/1.73 m2 | 14.5% (12.8%, 16.4%) | 17.0% (15.0%, 19.0%) | 2.5% (1.8%, 3.3%) |
| eGFR <60 ml/min/1.73 m2 | 31.3% (27.4%, 35.5%) | 31.3% (27.4%, 35.5%) | 0%† |
| 10-year risk categories, % | |||
| < 5% | 5.7% (3.6%, 8.8%) | 7.8% (5.4%, 11.3%) | 2.2% (1.1%, 4.0%) |
| 5% to < 10% | 7.9% (5.4%, 11.4%) | 10.3% (7.3%, 14.3%) | 2.3% (1.3%, 4.2%) |
| 10% to < 20% | 12.9% (10.2%, 16.1%) | 15.4% (12.6%, 18.7%) | 2.5% (1.3%, 4.9%) |
| ≥ 20% | 26.2% (22.1%, 30.6%) | 27.5% (23.5%, 32.0%) | 1.4% (0.7%, 2.6%) |
| History of CVD* | 32.0% (28.2%, 36.0%) | 33.4% (29.6%, 37.5%) | 1.5% (0.9%, 2.4%) |
| SBP/DBP category, mm Hg | |||
| < 130/80 | 10.1% (8.3%, 12.4%) | 10.1% (8.3%, 12.4%) | 0%† |
| 130–139/80–89 | 18.1% (15.2%, 21.5%) | 26.2% (23.1%, 29.5%) | 8.0% (6.2%, 10.4%) |
| ≥ 140/90 | 29.1% (25.1%, 33.5%) | 29.1% (25.1%, 33.5%) | 0% † |
Numbers in table represent percent (95% confidence interval) with apparent treatment resistant hypertension.
Due to rounding, the numbers in the column “2018 but not 2008” do not always equal the estimate in the “2018 Scientific Statement” minus “2008 Scientific Statement” columns.
ACR - albumin-to-creatinine ratio, eGFR – estimated glomerular filtration rate, CVD - cardiovascular disease, SBP –systolic blood pressure, DBP – diastolic blood pressure.
All participants with a history of CVD are in this category regardless of their 10-year predicted risk.
There is no confidence interval as, by definition, no participants with SBP/DBP < 130/80 mm Hg or with ≥ 140/90 mm Hg have apparent treatment resistant hypertension by one Scientific Statement but not the other.
When requiring the use of a thiazide or thiazide-like diuretic to be considered as having aTRH, the prevalence of aTRH was 10.3% according to the 2008 definition and 11.4% using the 2018 definition (Table S5). In this latter analysis, the difference in the prevalence of aTRH between the 2008 and 2018 Scientific Statements was 1.1% (95% CI 0.8%, 1.5%).
Among US adults taking three or more classes of antihypertensive medication, the most common three drug combinations included an (1) ACE, β-blocker, thiazide diuretic, (2) ACE, dihydropyridine calcium channel blocker, β-blocker or (3) ARB, β-blocker and thiazide diuretic (Table 4). The proportion with SBP/DBP < 140/90 mm Hg ranged from 54.4% among those taking an ARB, dihydropyridine calcium channel blocker and β-blocker to 76.0% among their counterparts taking a ACE inhibitor, dihydropyridine CCB, and thiazide diuretic. The proportion with SBP/DBP <130/80 mm Hg was less than 50% in each of the sub-groups except for those taking an ACE inhibitor, β-blocker and loop diuretic, where it was 54.6%.
Table 4.
Percentage of US adults taking three or more classes of antihypertensive medications using the ten most common three antihypertensive medication drug class regimens and, among those taking these regiments, the percentage with systolic and diastolic blood pressure <140 mm Hg and 90 mm Hg, respectively, and <130 mm Hg and 80 mm Hg, respectively.
| Antihypertensive medication combinations* | % taking combination† | % (95% CI) with SBP/DBP < 140/90 mm Hg | % (95% CI) with SBP/DBP < 130/80 mm Hg |
|---|---|---|---|
| ACE, beta blocker, thiazide diuretic | 15.7% | 69.5% (61.2%, 76.7%) | 49.7% (39.6%, 59.8%) |
| ACE, dhCCB, beta blocker | 14.4% | 64.3% (51.8%, 75.2%) | 44.3% (34.5%, 54.6%) |
| ARB, beta blocker, thiazide diuretic | 12.4% | 65.6% (53.9%, 75.7%) | 48.2% (39.3%, 57.3%) |
| ARB, dhCCB, beta blocker | 10.7% | 54.4% (45.0%, 63.5%) | 27.8% (20.8%, 36.1%) |
| ACE, dhCCB, thiazide diuretic | 10.6% | 76.0% (65.2%, 84.3%) | 49.9% (41.7%, 58.1%) |
| dhCCB, beta blocker, thiazide diuretic | 9.8% | 75.4% (65.1%, 83.5%) | 47.5% (36.5%, 58.7%) |
| ACE, beta blocker, loop diuretic | 7.9% | 75.7% (64.2%, 84.4%) | 54.6% (45.4%, 63.4%) |
| ARB, dhCCB, thiazide diuretic | 7.6% | 74.9% (65.3%, 82.6%) | 48.4% (37.9%, 59.1%) |
| ARB, beta blocker, loop diuretic | 5.9% | 63.4% (44.4%, 79.0%) | 44.7% (29.6%, 60.9%) |
| dhCCB, beta blocker, loop diuretic | 4.7% | 55.7% (37.0%, 72.9%) | 26.0% (14.4%, 42.3%) |
Numbers in table represent population percent (95% confidence interval).
Participants may have been taking a fourth, fifth or more medications.
Percentage calculated among US adults taking three or more classes of antihypertensive medication.
CI: Confidence interval, aTRH – apparent treatment resistant hypertension, ACE – angiotensin converting enzyme inhibitors, dhCCB – dihydropyridine calcium channel blockers, ARB – angiotensin receptor blockers, SBP – systolic blood pressure, DBP – diastolic blood pressure.
Overall, 3.2% of US adults with aTRH according to the 2018 Scientific Statement were taking recommended long-acting thiazide-like diuretics (chlorthalidone or indapamide) and 9.0% were taking mineralocorticoid receptor blockers (spironolactone or eplerenone) (Figure 2). Whereas the proportion of US adults with aTRH taking a thiazide-like diuretic was similar for those with uncontrolled and controlled BP (2.8% and 4.4%), the proportion taking a mineralocorticoid receptor blocker was lower among those with uncontrolled versus controlled BP (4.7% versus 22.8%).
Figure 2.
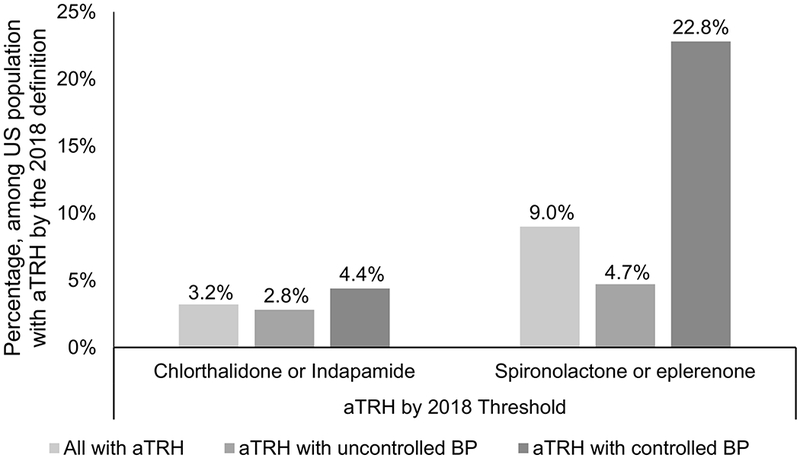
Proportion of US adults with apparent treatment resistant hypertension according to the 2018 American Heart Association Scientific Statement on resistant hypertension taking chlorthalidone or indapamide (left panel) and spironolactone or eplerenone (right panel).
aTRH: apparent treatment resistant hypertension, BP: blood pressure
According to the 2018 American Heart Association Scientific Statement on resistant hypertension, uncontrolled blood pressure was defined as systolic blood pressure ≥ 130 mm Hg and diastolic blood pressure ≥ 80 mm Hg. For adults ≥ 65 years of age without diabetes, chronic kidney disease, history of cardiovascular disease or 10-year predicted risk ≥ 10% on the Pooled Cohort risk equations only systolic blood pressure ≥ 130 mm Hg is used to defined uncontrolled blood pressure.
According to the 2018 American Heart Association Scientific Statement on resistant hypertension, controlled blood pressure was defined as systolic blood pressure <130 mm Hg and diastolic blood pressure < 80 mm Hg. For adults ≥ 65 years of age without diabetes, chronic kidney disease, history of cardiovascular disease or 10-year predicted risk ≥ 10% on the Pooled Cohort risk equations, controlled blood pressure was defined as systolic blood pressure < 130 mm Hg.
DISCUSSION
This analysis of the 2009–2014 NHANES data demonstrates an increase in the prevalence of aTRH of 2.0% when defined according to the 2018 compared with the 2008 definition of resistant hypertension recommended in the respective AHA Scientific Statements.1, 2 Among US adults taking antihypertensive medication, the prevalence of aTRH was 17.7% (9.2 million persons) when applying the definition in the 2008 Scientific Statement whereas it was 19.7% (10.3 million persons) using the 2018 Scientific Statement definition. In a secondary analysis, in which we required adults to be taking a thiazide or thiazide-like diuretic to be considered as having aTRH, the prevalence of aTRH was 10.3% using the 2008 definition and 11.4% using the 2018 definition. Overall, using the 2018 Scientific Statement definition instead of the 2008 Scientific Statement definition for resistant hypertension increased the prevalence of aTRH among US adults by only 1.1% to 2.0%. This marginal change in the prevalence of aTRH was the result of lower BP control thresholds used to define aTRH by AHA in 2018 compared with 2008.
The current estimates are consistent with previous population-based studies in which the prevalence of aTRH among adults with hypertension has been reported as approximately 12% to 15%.2 The prevalence of aTRH was higher in the current study when compared to a previous analysis of US adults taking antihypertensive medication in NHANES 2003–2008 (12.8%).7 This is possibly due to the fact that the latter study defined BP control as <140/90 mm Hg for all adults, whereas we used the same definition for the general population but BP <130/80 mm Hg for adults with diabetes or CKD.7 The prevalence of aTRH among those with diabetes and CKD was the same when using the 2008 and 2018 definitions.
The term aTRH is used when at least one of the following elements of information is missing: medication doses, assessment of medication adherence or out-of-office BP measurements by home BP monitoring or ambulatory BP monitoring. None of this information was available in the current study. Therefore, we cannot be certain that medications were being taken at maximal or maximally tolerated doses and on a regular basis in accordance with caregiver instructions. In addition, it is impossible to determine whether white coat effect, office BP above goal but out-of-office BP below goal, might have influenced the prevalence of resistant hypertension reported here. It has been established that a substantial proportion of adults taking three or more classes of antihypertensive medication have one or more of these factors.8, 9 For this reason, both the 2018 AHA Scientific Statement and the 2018 European Society of Cardiology (ESC)/European Society of Hypertension (ESH) hypertension guidelines now require exclusion of medication non-adherence and white coat effect to make the diagnosis of resistant hypertension.2, 10 Therefore, the prevalence of resistant hypertension may be lower than the estimates from the current study. In contrast, we cannot exclude the possibility that some US adults with uncontrolled BP on 1 or 2 classes of antihypertensive medication might still have uncontrolled BP if they had been taking 3 or more classes of antihypertensive medications.
The characteristics of US adults with aTRH according to the 2008 and the 2018 AHA Scientific Statement definitions are consistent with prior studies.7, 11–13 Individuals with aTRH are more likely to have CVD risk factors including diabetes, CKD and a high 10-year predicted ASCVD risk. In the current study, US adults with aTRH according to the 2018 but not the 2008 Scientific Statement on resistant hypertension had a 10-year predicted ASCVD risk similar to those without aTRH according to either Scientific Statement. The implication of this finding is not clear but its impact may be small since most people with aTRH according to the 2018 Scientific Statement definition also had aTRH according to the 2008 Scientific Statement.
We analyzed the combinations of antihypertensive medications among US adults taking three or more drug classes. JNC-7 recommended a diuretic as first step drug therapy with agents from other major drug classes (including β-blockers) to be added in a stepped care therapy approach. 14 Consequently, β-blockers were included among the drug classes recommended by the 2008 Scientific Statement. 1 The 2017 ACC/AHA and the 2018 ESC/ESH clinical practice guidelines recommend combination therapy from 4 drug classes (diuretics, CCBs, ACEIs and ARBs) but allow for β-blockers as second line agents 3,10 or when a compelling indication exists for their use in managing a co-morbidity. In the 2018 Scientific Statement, β-blockers are recommended in the management of resistant hypertension as a fifth add-on drug under specific clinical circumstances. 2 In the current analysis, β-blockers were employed as a component of the most frequently used three drug regimens among US adults. However, this is likely to change as guideline recommendations are incorporated into clinical practice over time.
The frequency with which the drug combinations were employed among US adults did not correlate with the degree of BP control to < 140/90 mm Hg or <130/80 mm Hg. As expected, fewer individuals achieved BP control below 130/80 mm Hg compared with below 140/90 mm Hg. Prospective assessment of BP control rates comparing different combinations of antihypertensive agents in resistant hypertension are not currently available and would be helpful to facilitate evidence-based therapeutic decision-making in the future.
Both the 2017 ACC/AHA and the 2018 ESC/ESH hypertension guidelines recommend initiation of antihypertensive drug therapy with 2 drugs in combination for most adults with hypertension.3, 10 According to the current study, over one-third of US adults taking antihypertensive medication were taking a single drug class and in over half of these individuals, BP was uncontrolled by the 2017 ACC/AHA guideline criteria. A total of 17.6% of adults taking antihypertensive drugs were taking three antihypertensive medication classes with only 7.6% having their BP controlled to <130/80 mm Hg.
Diuretic administration is critically important in resistant hypertension because the majority of patients have extracellular fluid volume expansion.2, 15, 16 Thiazide-like diuretics (i.e., chlorthalidone and indapamide) have a substantial evidence base for BP control and reduction in CVD events in resistant hypertension.17–20 According to the 2018 AHA Scientific Statement, the first step in the management of resistant hypertension is to substitute a thiazide-like diuretic for a thiazide diuretic to achieve BP control.2 In the present study, we documented very infrequent use of thiazide-like diuretics among US adults with aTRH. Similarly, there was little use of mineralocorticoid receptor antagonists (spironolactone or eplerenone) in US adults with aTRH. As prior studies strongly support the efficacy of mineralocorticoid receptor blockers to reduce BP in resistant hypertension, the 2018 AHA Scientific Statement recommended these agents as the primary (fourth) add-on drug in the management of resistant hypertension.2, 21–25 Adults with resistant hypertension have an increased risk for CVD events and death compared to their counterparts without treatment resistance.2 In view of the documented poor prognosis in uncontrolled resistant hypertension, increased attention to substitution of a thiazide-like diuretic and addition of a mineralocorticoid receptor blocker should be of major critical importance in the management of resistant hypertension.1, 2
The current study has several strengths. NHANES provides nationally representative estimates for the non-institutionalized US population and the results of this analysis have broad generalizability. NHANES enrolled a large sample size and over-sampled population groups that facilitated the conduct of subgroup analysis. BP was measured following a standardized protocol. The classes of antihypertensive medication being taken were verified by a pill bottle review. However, the results of this study should be interpreted in the context of known and potential limitations. The data we present applied the 2018 definition of apparent treatment resistant hypertension and BP control to data collected prior to the publication of these recommendations. Data were not collected on medication dose and frequency and medication adherence. Additionally, out-of-office BP monitoring was not performed. Therefore, some participants were likely misclassified as having resistant hypertension when they in fact had pseudo-resistant hypertension. This may have led to over-estimation of the prevalence of resistant hypertension. Also, BP was measured at a single visit in NHANES. The 2017 ACC/AHA hypertension guideline recommends basing the diagnosis of hypertension on the average of multiple BP measurements obtained at two or more visits. Also, the NHANES BP measurement protocol included the use of a mercury sphygmomanometer, which likely differs from the usual approach in most settings. However, high agreement for SBP and DBP levels was present in a study comparing the BP measurement methods in NHANES to a protocol using automated office BP measurements.26
Perspectives
In conclusion, analysis of 2009–2014 NHANES data demonstrates the prevalence of aTRH of 19.7% when defined according to the 2018 AHA Scientific Statement definition. Compared with prior recommendations, using the most recently recommended SBP/DBP cut-points for defining uncontrolled BP resulted in only a modest increase in the prevalence of aTRH. However, the vast majority of US adults with aTRH were not taking thiazide-like diuretics and mineralocorticoid receptor blockers as recommended by the 2018 Scientific Statement. Increasing the use of thiazide-like diuretics and mineralocorticoid receptor blockers has the potential to substantially improve BP control and reduce the prevalence of resistant hypertension in the future.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
Analysis of data from the 2009–2014 National Health and Nutrition Examination Survey (NHANES) indicates that the prevalence of apparent treatment resistant hypertension (aTRH) increases by only 1.1%−2.0% when applying the definition of apparent resistant hypertension in the 2018 as compared with the 2008 American Heart Association Scientific Statement on resistant hypertension. This increase is not overly burdensome and does not signify a major increase in the frequency of resistant hypertension using lower blood pressure targets. There is marked underutilization of recommended drugs including thiazide-like diuretics (chlorthalidone and indapamide) and mineralocorticoid receptor blockers (spironolactone and eplerenone), in managing resistant hypertension. This presents a substantial opportunity to lower BP to goal and reduce the prevalence of resistant hypertension in the future.
What is relevant?
The 2018 American Heart Association Scientific Statement on resistant hypertension redefined resistant hypertension using thresholds from the 2017 American College of Cardiology/American Heart Association guideline to define uncontrolled blood pressure.
Summary
Analyzing data from NHANES 2009–2014, we calculated that the most recently recommended blood pressure thresholds increase the prevalence of aTRH by only 1.1% to 2.0%.
Acknowledgements:
Sources of funding: Dr. Robert M. Carey received support from grants R01-HL128189 and P01-HL074940 from the National Heart, Lung and Blood Institute. Dr. Paul K Whelton received support from grant P20GM109036 from the National Institute for General Medical Sciences. Dr. Paul Muntner received support from American Heart Association grant 15SFRN2390002.
Footnotes
Conflicts of Interest/Disclosure: Drs. Carey and Calhoun were Chair and Vice Chair of the 2018 American Heart Association Scientific Statement on Resistant Hypertension. Drs. Carey, Whelton and Muntner were Vice Chair, Chair and a member of the 2017 American College of Cardiology / American Heart Association Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults. Ms. Sakhuja has no disclosures.
References
- 1.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: Diagnosis, evaluation, and treatment: A scientific statement from the American Heart Association professional education committee of the council for high blood pressure research. Circulation. 2008;117:e510–526 [DOI] [PubMed] [Google Scholar]
- 2.Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, et al. ;on behalf of the American Heart Association professional/public education and publications committee of the council on hypertension; council on cardiovascular and stroke nursing; council on clinical cardiology; council on genomic and precision medicine; council on peripheral vascular disease; council on quality of care and outcomes research; and stroke council. Resistant hypertension: Detection, evaluation, and management: A scientific statement from the American Heart Association. Hypertension. 2018;72:e000–e000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHAA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71:126–1324 [DOI] [PubMed] [Google Scholar]
- 4.Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National Health and Nutrition Examination Survey: Sample design, 2011-2014. National Center For Health Statistics. Vital Health Stat 2. 2014; 162:1–33. [PubMed] [Google Scholar]
- 5.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB Sr., Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2935–2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57:1076–1080 [DOI] [PubMed] [Google Scholar]
- 8.Elliott WJ. What factors contribute to the inadequate control of elevated blood pressure? Journal of clinical hypertension (Greenwich, Conn.). 2008;10:20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de la Sierra A, Segura J, Banegas JR, Gorostidi M, de la Cruz JJ, Armario P, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898–902 [DOI] [PubMed] [Google Scholar]
- 10.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104 [DOI] [PubMed] [Google Scholar]
- 11.Hyman DJ, Pavlik VN. Characteristics of patients with uncontrolled hypertension in the United States. N Engl J Med. 2001;345:479–486 [DOI] [PubMed] [Google Scholar]
- 12.Sim JJ, Bhandari SK, Shi J, Liu IL, Calhoun DA, McGlynn EA, et al. Characteristics of resistant hypertension in a large, ethnically diverse hypertension population of an integrated health system. Mayo Clinic proceedings. 2013;88:1099–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acharya T, Tringali S, Singh M, Huang J. Resistant hypertension and associated comorbidities in a veterans affairs population. Journal of clinical hypertension (Greenwich, Conn.). 2014;16:741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252 [DOI] [PubMed] [Google Scholar]
- 15.Gaddam KK, Nishizaka MK, Pratt-Ubunama MN, Pimenta E, Aban I, Oparil S, et al. Characterization of resistant hypertension: Association between resistant hypertension, aldosterone, and persistent intravascular volume expansion. Archives of internal medicine. 2008;168:1159–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams B, MacDonald TM, Morant SV, Webb DJ, Sever P, McInnes GT, et al. Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: The pathway-2 mechanisms substudies. The lancet. Diabetes & endocrinology. 2018;6:464–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roush GC, Sica DA. Diuretics for hypertension: A review and update. American journal of hypertension. 2016;29:1130–1137 [DOI] [PubMed] [Google Scholar]
- 18.DiNicolantonio JJ, Bhutani J, Lavie CJ, O’Keefe JH. Evidence-based diuretics: Focus on chlorthalidone and indapamide. Future Cardiol. 2015;11:203–217 [DOI] [PubMed] [Google Scholar]
- 19.Ernst ME, Carter BL, Goerdt CJ, Steffensmeier JJ, Phillips BB, Zimmerman MB, et al. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension. 2006;47:352–358 [DOI] [PubMed] [Google Scholar]
- 20.Khosla N, Chua DY, Elliott WJ, Bakris GL. Are chlorthalidone and hydrochlorothiazide equivalent blood-pressure-lowering medications? Journal of clinical hypertension (Greenwich, Conn.). 2005;7:354–356 [DOI] [PubMed] [Google Scholar]
- 21.Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (pathway-2): A randomised, double-blind, crossover trial. Lancet (London, England). 2015;386:2059–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao D, Liu H, Dong P, Zhao J. A meta-analysis of add-on use of spironolactone in patients with resistant hypertension. International journal of cardiology. 2017;233:113–117 [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Xiong B, Huang J. Efficacy and safety of spironolactone in patients with resistant hypertension: A meta-analysis of randomised controlled trials. Heart Lung Circ. 2016;25:1021–1030 [DOI] [PubMed] [Google Scholar]
- 24.Rosa J, Widimsky P, Waldauf P, Lambert L, Zelinka T, Taborsky M, et al. Role of adding spironolactone and renal denervation in true resistant hypertension: One-year outcomes of randomized prague-15 study. Hypertension. 2016;67:397–403 [DOI] [PubMed] [Google Scholar]
- 25.de Souza F, Muxfeldt E, Fiszman R, Salles G. Efficacy of spironolactone therapy in patients with true resistant hypertension. Hypertension. 2010;55:147–152 [DOI] [PubMed] [Google Scholar]
- 26.Ostchega Y, Zhang G, Sorlie P, Hughes JP, Reed-Gillette DS, Nwankwo T, et al. Blood pressure randomized methodology study comparing automatic oscillometric and mercury sphygmomanometer devices: National health and nutrition examination survey, 2009–2010. National health statistics reports. 2012:1–15 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


