Abstract
Purpose
This article reviews research on executive function (EF) skills in children with autism spectrum disorder (ASD) and the relation between EF and language abilities. The current study assessed EF using nonverbal tasks of inhibition, shifting, and updating of working memory (WM) in school-age children with ASD. It also evaluated the association between children's receptive and expressive language abilities and EF performance.
Method
In this study, we sought to address variables that have contributed to inconsistencies in this area of research—including task issues, group comparisons, and participant heterogeneity. EF abilities in children with ASD (n = 48) were compared to typically developing controls (n = 71) matched on age, as well as when statistically controlling for group differences in nonverbal cognition, socioeconomic status, and social communication abilities. Six nonverbal EF tasks were administered—2 each to evaluate inhibition, shifting, and WM. Language abilities were assessed via a standardized language measure. Language–EF associations were examined for the ASD group as a whole and subdivided by language status.
Results
Children with ASD exhibited significant deficits in all components of EF compared to age-mates and showed particular difficulty with shifting after accounting for group differences in nonverbal cognition. Controlling for social communication—a core deficit in ASD—eliminated group differences in EF performance. A modest association was observed between language (especially comprehension) and EF skills, with some evidence of different patterns between children on the autism spectrum with and without language impairment.
Conclusions
There is a need for future research to examine the direction of influence between EF and language. It would be beneficial for EF interventions with children with ASD to consider language outcomes and, conversely, to examine whether specific language training facilitates aspects of executive control in children on the autism spectrum.
Presentation Video
This research forum contains papers from the 2017 Research Symposium at the ASHA Convention held in Los Angeles, California.
Executive function (EF) refers to a set of cognitive processes that underlie goal-directed behavior. That is, EF is an umbrella term for multiple cognitive processes that are necessary for managing thought and behavior (Diamond, 2013; Miyake et al., 2000). There is no uniform agreement about the specific cognitive processes that comprise EF, but large-scale empirical studies with adults using latent variable analysis indicate that EF consists of related but separable subcomponents (Friedman et al., 2008; Miyake et al., 2000, Vaughan & Giovanello, 2010). According to these investigations, core components of EF include inhibition, task shifting, and updating of working memory (WM). Inhibition refers to the ability to suppress attention to irrelevant information, task shifting is the ability to flexibly switch between operations and mental states, and updating involves incorporating new information into WM. This same structure of core EF components has been found in typically developing (TD) children ranging from 7 to 13 years of age (Duan, Wei, Wang, & Shi, 2010; Lehto, Juujärvi, Kooistra, & Pulkkinen, 2003; Rose, Feldman, & Jankowski, 2011; Wu et al., 2011). Core EF components combine in various ways to constitute higher-level EF functions such as planning, problem solving, organization, and reasoning (Diamond, 2013).
EF is fundamental for learning and academic achievement, emotional regulation, and social competence and has a profound impact on overall quality of life issues across the life span (Best, Miller, & Naglieri, 2011; Blair & Razza, 2007; Broidy et al., 2003; Ferrier, Bassett, & Denham, 2014; Moffitt et al., 2011). Moffitt and colleagues (2011) followed 1,000 children into adulthood and found that a gradient of childhood self-control (inhibition) predicted physical health, finances, and criminal offenses at 32 years of age. With respect to individuals with autism spectrum disorder (ASD), recent research has found that EF predicts school readiness in preschool children (Pellicano et al., 2017) and has revealed a link between EF abilities and mental health outcomes for adults on the autism spectrum (Zimmerman, Ownsorth, O'Donovan, Roberts, & Gullo, 2017). Individual differences in children's EF, particularly in inhibitory control and WM, were uniquely related to variation in school readiness skills composed of basic concepts, social competence, and phonological awareness (Pellicano et al., 2017). Findings for adults with ASD suggest that impaired EF skills are associated with anxiety, whereas better nonverbal reasoning, cognitive flexibility, and social cognition are associated with negative self-concept (Zimmerman et al., 2017).
The broad aims of this review article are to (a) examine EF as a multidimensional construct, (b) review empirical findings regarding EF abilities in children with ASD and the association between language and EF, (c) describe a theoretical framework for positing links between EF and language, (d) explore potential reasons for contradictory findings in the literature, and (e) present a study that attempts to resolve current contradictions.
EF Abilities in ASD
Children with ASD have been reported to have deficits in core components of EF and in higher-order EF functions across numerous studies. Studies have found that children with ASD exhibit significant difficulties on tasks measuring inhibitory control (Christ, Holt, White, & Green, 2007; Christ, Kester, Bodner, & Miles, 2011; Corbett, Constantine, Hendren, Rocke, & Ozonoff, 2009; Joseph, McGrath, & Tager-Flusberg, 2005; Pellicano et al., 2017; Van Eylen, Boets, Steyaert, Wagemans, & Noens, 2015), task shifting (cognitive flexibility; Pellicano, 2010; Russo et al., 2007; Semrud-Clikeman, Fine, & Bledsoe, 2014), and updating of WM (Joseph et al., 2005; Pellicano et al., 2017; review by Kercood, Grskovic, Banda, & Begeske, 2014). Although Durrleman and Franck (2015) did not find significant differences in inhibition or shifting performance for children with ASD compared to TD controls matched on nonverbal IQ, the children with ASD were, on average, 2 years older than the controls. Studies that have examined higher-order EF skills in children with ASD have found deficits in planning, organization, reasoning, and problem solving (Diamond, 2013; Joseph et al., 2005; Pellicano, 2010; Unterrainer et al., 2016).
Although the bulk of evidence indicates that, as a group, children with ASD display EF deficits, there are some contradictory findings within the literature. For instance, Robinson, Goddard, Dritschel, Wisley, and Howlin (2009) found no significant deficits in cognitive flexibility (shifting) or generativity for children with ASD but did report difficulties with inhibition, planning, and self-monitoring. Inconsistencies in these research findings are likely due to the array of different tasks used to measure EF constructs and task impurity (see discussion by Van Eylen et al., 2015), differing group matching criteria, and participant heterogeneity within the ASD group (e.g., cognitive level, language abilities, autism severity). For example, the lack of shifting difficulties observed in Robinson et al. (2009) was likely influenced by the fact that they matched their samples on age, IQ, gender, and vocabulary—resulting in a select, more capable sample of children on the autism spectrum. Matching groups on nonverbal cognitive abilities is quite common across studies examining EF skills in ASD, but language abilities are often allowed to vary. Kercood et al. (2014) noted that none of the 24 studies examining WM in individuals with ASD, which they included in their review, considered the implications of linguistic abilities. Yet some studies have reported that children with ASD no longer showed EF deficits compared to controls when differences in language abilities were covaried (Joseph et al., 2005; Liss et al., 2001). The specific group matching criteria that are most appropriate for a given study primarily depend on the particular research question being addressed. If the question is whether children with ASD have delays in their development of EF abilities, an age-matched comparison is needed. Because intellectual disability can co-occur with ASD and because of the interrelationship between IQ and EF, nonverbal cognition is often equated across groups in an attempt to specifically isolate differences in components of EF. Language matches are appropriate when task demands would confound EF task performance or when there is a question about the role of verbal mediation in EF.
Whereas a considerable amount of research has demonstrated EF deficits in children with ASD at the group level, there is evidence for a good deal of individual variation. Pellicano (2010) reported that 62% of very young children with ASD in her sample had an EF impairment, and in the three studies examined by Geurts, Sinzig, Booth, and Happé (2014), 30%–70% of children with ASD showed deficits in EF. Based on a meta-analysis of shifting/cognitive flexibility studies, Leung and Zakzanis (2014) concluded that the Shift subscale of the Behavior Rating Inventory of Executive Functions (BRIEF) is a promising clinical marker for ASD, yet their results also highlighted the fact that cognitive flexibility impairments were not a uniform characteristic of individuals with ASD. Overall, the evidence does not support an executive dysfunction (single-deficit) cognitive theory of autism (see review by Pellicano, 2011) in that EF deficits are not sufficient to account for the various symptoms of social communication deficits and restricted and repetitive behaviors that comprise the ASD phenotype. Nevertheless, it is important to understand how difficulties in executive control affect those children who do display EF deficits and how these difficulties are related to other areas in which considerable variation is observed in children with ASD, such as language.
Association Between Language and EF
There are both theoretical and empirical reasons for examining the link between EF and language. The theoretical motivation for examining the relationship between language and EF stems from a leading developmental theory of executive control by Zelazo and colleagues—the hierarchical competing systems model (HCSM; Marcovitch & Zelazo, 2009; Zelazo, 2004). According to the HCSM, goal-directed behavior entails two hierarchical systems—a habit system that is dependent on prior experience and a representational system involving conscious reflection on behavior. This model purports that language is used to manage executive control, such that it facilitates retention of information in WM and enables reflection and conscious consideration. For instance, labeling increases the influence of the conscious representational system, allowing the habit system to be overridden, exerting top-down control over behavior. Within this view, developmental changes in reflection during childhood are due to increases in self-initiated labeling strategies, and higher-ability learners will engage in conscious reflection and override prepotent responses more effectively than lower-ability learners. Findings from typical development supporting this claim include reductions in EF performance under articulatory suppression conditions and increases in performance (for younger children, but not older) when prompts are provided to use labels (Fatzer & Roebers, 2012; Kray, Eber, & Lindenberger, 2004; Kray, Gaspard, Karbach, & Blaye, 2013). As part of our larger research project focused on language and EF, we have directly examined claims of the HCSM for TD monolingual and bilingual children (Gangopadhyay, MacDonald, Ellis Weismer, & Kaushanskaya, 2018) and children with ASD (Gangopadhyay et al., 2015) using a dual-task paradigm involving articulatory suppression or motor suppression compared to no competing task. This theoretical framework has influenced the current study in several ways. It highlights the need to minimize linguistic stimuli in EF tasks to mitigate this confound, provides motivation for exploring the link between language abilities and EF, and leads to the expectation that children with poorer language abilities will also perform more poorly on EF tasks.
In addition to the theoretical rationale for studying language–EF relationships, empirical findings from a number of studies point to a connection between these areas of functioning in typical development (Ibbotson & Kearvell-White, 2015; Kaushanskaya, Park, Gangopadhyay, Davidson, & Ellis Weismer, 2017; Khanna & Boland, 2010; Kuhn, Willoughby, Vernon-Feagans, Blair, & The Family Life Project Key Investigators, 2016; Mazuka, Jincho, & Onishi, 2009; Minai, Jincho, Yamane, & Mazuka, 2012; Pellicano et al., 2017; Weiland, Barata, & Yoshikawa, 2013; Woodard, Pozzan, & Trueswell, 2016). Inhibition/shifting has also been linked to both lexical–semantic processing (Khanna & Boland, 2010) and syntactic processing (Mazuka et al., 2009; Woodard et al., 2016). For example, inhibition skills have been associated with children's ability to resolve lexical and syntactic ambiguity (Khanna & Boland, 2010) and to avoid overgeneralization errors on a past tense task (Ibbotson & Kearvell-White, 2015). Similarly, Kaushanskaya et al. (2017) found that inhibition (but not shifting or updating of WM) accounted for unique variance in school-age children's syntactic abilities as measured by a standardized language task even after accounting for age, socioeconomic status (SES), and nonverbal IQ. Research has demonstrated an association between updating of WM and lexical–semantic processing (Khanna & Boland, 2010; Weiland et al., 2013) as well as the ability to detect morphosyntactic errors within sentences (Gangopadhyay, Davidson, Ellis Weismer, & Kaushanskaya, 2016).
Research focused directly on the interplay between EF and language in children with ASD has yielded mixed findings (Akbar, Loomis, & Paul, 2013; Joseph et al., 2005; Liss et al., 2001). Liss and colleagues (2001) compared EF abilities in children with “high-functioning autism” to children with developmental language disorder (specific language impairment [SLI]); groups were matched on age, Full Scale IQ, and performance IQ but differed on verbal IQ (the autism group scored lower). EF tasks measured shifting (Wisconsin Card Sort Task; Grant & Berg, 1948), a combination of planning/inhibition/shifting (Wechsler Intelligence Scale for Children–Revised Mazes; Wechsler, 1974), and sustained attention (visual search underlining test and rapid automatized naming). The only significant group difference between the children with language disorder and those with autism was in perseveration errors on the Wisconsin Card Sort Task, but that difference was eliminated when verbal IQ was partialed out. All of the EF tasks, except for visual search underlining, were significantly correlated (rs ranging from .46 to .67) to verbal IQ in children with autism. The association between EF and language ability in verbal school-age children with autism was also investigated by Joseph et al. (2005); the EF abilities examined included WM, inhibitory control plus WM, and planning. Correlational analyses revealed significant associations between EF and language for the control group, but EF performance was not related to language ability in children with autism. Joseph and colleagues (2005) interpreted these results as indicating that difficulties in EF in children with autism are not related to language impairment per se but arise due to a failure to use language in the service of executive control. Akbar et al. (2013) examined four components of EF—WM, organization, shifting, and inhibition—in children with ASD using standardized neuropsychological measures, parent/teacher surveys, and standardized language measures. Stepwise regression analyses revealed that language, nonverbal cognition, and autism severity were significant concurrent predictors of several EF domains. Organization was predicted by nonverbal cognition, and shifting was predicted by nonverbal cognition and autism severity. Findings further indicated that WM was predicted by structural and pragmatic language ability. Thus, Akbar et al. (2013) suggested that language is associated with the WM component of EF.
Prior research using experimental language processing tasks has shown a link between certain aspects of language and specific EF components in children with ASD (Ellis Weismer et al., 2017; Haebig, Kaushanskaya, & Ellis Weismer, 2015). Haebig et al. (2015) examined the role of EF abilities in lexical processing for school-age children with ASD, SLI, and typical development. When matched on vocabulary level, children with ASD did not display EF deficits on measures of shifting or updating WM. Furthermore, shifting and WM each explained unique variance in performance on the lexical decision task (accuracy and reaction time) for the ASD, SLI, and TD groups, suggesting similar associations between language and executive control for all groups. Ellis Weismer and colleagues (2017) investigated the association between nonverbal WM and grammatical processing. Children with ASD who were matched on age, nonverbal cognition, and SES to TD and SLI groups did not show a deficit in nonverbal WM. However, individual differences in nonverbal WM significantly predicted sensitivity to morphosyntactic errors occurring early and late in the sentence for the ASD group and late in the sentence for the TD group.
Current Study
When we began the larger research project examining the association between EF and language, we were interested in addressing issues that have contributed to inconsistency across the findings with respect to EF abilities in ASD. There are many different tasks that have been used to measure various EF constructs (Hill, 2004; Kenworthy, Yerys, Anthony, & Wallace, 2008; Van Eylen et al., 2015), and the issue of “task impurity” confounds the problem further; that is, no task requires only the one specific cognitive process of interest (Jurado & Rosselli, 2007; Miyake & Friedman, 2012). Therefore, we started with a detailed survey of prior EF research to identify the tasks and specific indexes from those tasks that were used to measure a given construct. Then we selected two different tasks that had previously been used to assess each of the three core EF components identified by Miyake et al. (2000). In this way, we sought to obtain converging evidence through the use of more than a single task across multiple EF components. We then used an empirical, statistical approach involving latent variable analysis to establish which tasks and particular indexes of behavioral responses best measured the constructs of interest (Kaushanskaya et al., 2017). There are multiple outcome measures that might be used from each task, and rather than searching for the ones that display significant group differences, we used a principled, a priori method based on latent variable analysis with a TD sample of children.
Another possible task-related cause of inconsistency in findings across the ASD literature pertains to the fact that some EF tasks consist of verbal stimuli and others do not. We removed verbal elements from all tasks to the extent possible given our interest in the relation between EF and language. Therefore, we refer to the EF tasks in this study as “nonverbal” as a shorthand to mean that no verbal stimuli were presented, no verbal responses were required, and that orientation to the tasks minimized verbal instruction to the extent possible and included visual demonstrations and nonverbal feedback (happy/sad faces after practice trials). Nevertheless, we acknowledge the potential impact of the verbal instructions and possibility that individuals may draw on verbal abilities when completing these visual tasks. Finally, inconsistent findings across the literature are also due to the use of different matching criteria and to participant heterogeneity. Researchers have pointed out the pitfalls of different types of participant matching for comparing children with language disorder to controls (Plante, Swisher, Kiernan, & Restrepo, 1993), as well as concerns about statistical control of participant characteristics (Miller & Chapman, 2001) particularly in children with neurodevelopmental disorders (Dennis et al., 2009). However, it is impossible to interpret findings without some basis of comparison across groups so we employed a hybrid approach in the current study that matched on chronological age but then allowed for separate statistical control of additional child characteristics. It should be noted that we do not mean to imply that this hybrid method is the optimal approach, but rather for the purposes of this review, we thought it was more instructive to break down the impact of using one method or the other to consider this important point about how to assess group differences in EF.
The research questions addressed by this study were as follows: (a) How well do the various components of EF (inhibition, shifting, and WM), as reflected by our nonverbal tasks, measure a higher-order latent EF construct in a population of children with and without ASD? (b) Do children with ASD exhibit deficits on nonverbal EF tasks (1) compared to age-matched controls and (2) when additional child characteristics are taken into account? (c) Is there an association between language and EF (1) for the ASD and TD comparison and (2) when breaking down the ASD group by language status?
Method
Participants
Children were recruited through area schools, clinics, and community centers by website postings and flyers. In addition, a research registry at the Waisman Center consisting of families who indicated an interest in enrolling their child in research projects was used to recruit children with ASD. This study was approved by the Education/Social-Behavioral Institutional Review Board at the University of Wisconsin–Madison; parents provided written consent, and children gave oral assent for participation. Participants in the current study were part of a larger project examining the association between EF and language abilities in TD monolingual and bilingual school-age children, children with SLI, and children with ASD. TD monolingual and ASD groups are the focus of this report. Some of these children participated in studies addressing other research questions in prior publications by our research team (e.g., Ellis Weismer et al., 2017; Haebig et al., 2015; Kaushanskaya et al., 2017).
Children in this study were monolingual English speakers who had not been exposed to other languages in the home. Demographic information regarding participants' race and ethnicity was obtained via parent survey. With respect to the racial/ethnic background of the TD group, 78% were White, 11% were Black, 10% were multiracial, 1% were Asian, and 1% were Hispanic/Latino. The racial/ethnic composition of the ASD group was 85% White, 13% multiracial, 2% Asian, and 10% Hispanic/Latino. Children had normal or corrected-to-normal vision based on parental report and passed a hearing screening at the time of assessment using pure-tone audiometry at 20 dB HL at the frequencies 1000, 2000, and 4000 Hz (per the American Speech-Language-Hearing Association guidelines). Children in the control group had typical developmental histories with no reported variations that were deemed relevant to the focus of the current study and were in regular education classrooms according to parental report.
A total of 119 school-age children participated in this study, including 71 TD controls (38 boys, 33 girls) and 48 children with ASD (42 boys, six girls). Children in both groups met the following inclusionary criteria: 8–12 years of age, monolingual English speakers, normal hearing, and normal (corrected) visual acuity. Exclusionary criteria for the ASD group included intellectual disability or comorbid neurodevelopmental disorders other than autism (e.g., fragile X syndrome). The TD and ASD groups were matched on chronological age, t(117) = −0.74, p = .46, but differed significantly on nonverbal IQ, t(116) = 3.03, p < .01, maternal education, t(115) = 2.03, p = .04, social communication, t(116) = −17.10, p < .01, and core language abilities, t(114) = 8.18, p < .01. See Table 1 for a summary of the TD and ASD group characteristics. The Perceptual Reasoning Index of the Wechsler Intelligence Scale for Children–Fourth Edition (WISC-IV; Wechsler, 2003) was used to assess nonverbal IQ; this index is composed of the Block Design, Matrix Reasoning, and Picture Concepts subtests. The average nonverbal IQ for both groups was within normal range, but there was considerable variability in cognitive level for the TD group and the ASD group. Number of years of maternal education (1 = first grade, 12 = high school, > 12 = postsecondary) served as a proxy for SES. Although the mean educational level of mothers was higher for the TD group than the ASD group, there was again wide variability with 8% of both groups' mothers having at most a high school education. As expected, the TD group obtained significantly lower (better) scores on the Social Communication Questionnaire (SCQ; Rutter, Bailey, & Lord, 2003), a parent report autism screening measure. That is, the TD group did not display difficulties in social communication that were suggestive of ASD. The recommended guideline of 15 or below on the SCQ was used as indicative of typical social communication abilities. One child with an SCQ score of 16 who had a typical developmental history, no special services, normal language abilities, and no concerns regarding ASD was included in the TD group. Children with a community diagnosis of ASD who scored below the suggested cutoff score of 15 on this screening measure were included in the ASD group if they met criteria on the autism diagnostic measure described below. Language abilities were evaluated by administering the Receptive, Expressive, and Core Language (combination of Receptive and Expressive Language scales) of the Clinical Evaluation of Language Fundamentals–Fourth Edition (CELF-4; Semel, Wiig, & Secord, 2003). As a group, the children with ASD displayed significantly poorer language abilities than the TD controls (see Table 1); however, language skills varied widely within the ASD group, as described below.
Table 1.
Participants' demographic information and performance on standardized tests, for the typically developing (TD) group and the group with autism spectrum disorder (ASD) as a whole, as well as subdivided according to language status.
| Participant characteristics | TD (n = 71) | ASD–All (n = 48) | ASD LI (n = 20) | ASD LN (n = 28) | p |
|---|---|---|---|---|---|
| Age in years | |||||
| Mean (SD) | 9.3 (1.0) | 9.5 (1.2) | 9.4 (1.0) | 9.6 (1.4) |
ns
(p = .6) |
| Range | 8.0–11.9 | 8.0–12.5 | 8.0–11.0 | 8.0–12.5 | |
| Skewness | 0.5 | 0.4 | 0.3 | −0.4 | |
| Kurtosis | −0.6 | −1.0 | −1.3 | −1.2 | |
| Maternal education in years (SES) | |||||
| Mean (SD) | 17.2 (3.1) | 16.1 (2.7) | 15.9 (2.0) | 16.3 (3.0) |
ns
(p = .1) |
| Range | 10.0–24.0 | 12.0–24.0 | 12.0–19.0 | 12.0–24.0 | |
| Skewness | −0.1 | 0.5 | −0.2 | 0.5 | |
| Kurtosis | −0.4 | 0.3 | −0.8 | −0.1 | |
| Nonverbal cognition (WISC-IV) | |||||
| Mean (SD) | 111.5 (12.7) | 103.1 (17.5) | 92.3 (14.1) | 110.8 (15.5) | TD > ASD LI**
ASD LN > ASD LI** |
| Range | 84.0–141.0 | 69.0–137.0 | 69.0–115.0 | 79.0–137.0 | |
| Skewness | 0.1 | −0.1 | −0.2 | −0.3 | |
| Kurtosis | −0.5 | −0.7 | −1.3 | −0.7 | |
| Social communication (SCQ) | |||||
| Mean (SD) | 3.8 (3.6) | 19.6 (6.4) | 21.2 (5.8) | 18.4 (6.6) | TD > ASD LI & ASD LN** |
| Range | 0.0–16.0 | 4.0–35.0 | 9.0–31.0 | 4.0–35.0 | |
| Skewness | 1.4 | 0.1 | −0.2 | 0.4 | |
| Kurtosis | 1.7 | −0.1 | −0.7 | 0.7 | |
| Core language (CELF-4) | |||||
| Mean (SD) | 109.1 (12.0) | 84.3 (20.6) | 64.0 (12.2) | 98.6 (10.9) | TD > ASD LI & ASD LN**
ASD LN > ASD LI** |
| Range | 87.0–134.0 | 40.0–129.0 | 40.0–81.0 | 84.0–129.0 | |
| Skewness | 0.1 | −0.3 | −0.5 | 1.0 | |
| Kurtosis | −0.9 | −0.4 | −0.6 | 1.3 |
Note. Standard scores were used for the WISC-IV, CELF-4, and SCQ. ASD LI = autism spectrum disorder, language impairment (scored ≤ 81.25 on CELF-4 Core Language standard score, −1.25 SD); ASD LN = autism spectrum disorder, language normal (scored > 81.25 on CELF-4 Core Language standard score); SES = socioeconomic status; WISC-IV = Wechsler Intelligence Scale for Children–Fourth Edition; SCQ = Social Communication Questionnaire; CELF-4 = Clinical Evaluation of Language Fundamentals–Fourth Edition.
Subgroup difference at p < .01.
All of the school-age children in the ASD group had a prior clinical or educational community diagnosis from a pediatrician, psychologist, or interdisciplinary team. At the time of assessment, the ASD diagnosis was confirmed by an experienced, licensed psychologist based on the Childhood Autism Rating Scale–Second Edition for high-functioning individuals (CARS2-HF; Schopler, Van Bourgondien, Wellman, & Love, 2010). A cutoff total raw score of 25 was selected, which corresponds to the 10th percentile of CARS2-HF scores for individuals with ASD in the standardization sample. The ASD group in this study obtained a mean raw score of 31.85 (SD = 4.60). Forty-five of the 48 children in the ASD group met the cutoff score on the CARS2-HF. The remaining three children scored just slightly below this cutoff (indicating fewer symptoms). They were nevertheless included in the sample because of their community diagnoses along with the fact that two children had previously been diagnosed with ASD by our research team using a comprehensive assessment approach including the Autism Diagnostic Observation Schedule (Lord, Rutter, DiLavore, & Risi, 2001) and the Autism Diagnostic Interview–Revised (Rutter, LeCouteur, & Lord, 2003). The community ASD diagnosis was confirmed for the third child based on the totality of testing and expert clinical judgment.
Language abilities were assessed using the CELF-4 (Semel et al., 2003). Core Language standard scores from the CELF-4 were used to characterize group differences in language level, whereas standard scores on the Receptive Language and Expressive Language scales were used in analyses of the association between EF and language abilities. As noted above, structural language abilities (vocabulary/grammar) varied widely in children with ASD. For certain analyses, the ASD group was subdivided into two groups based on language status. Children in the Language Impairment (ASD LI) group (n = 20) scored at least 1.25 SDs below the mean for Core Language, whereas the Language Normal (ASD LN) group (n = 28) scored within normal range (1.25 SDs from the mean or above). Participant characteristics for the two ASD groups are compared to the TD controls in Table 1. Groups were matched on chronological age, F(2, 116) = 0.48, p = .62, and did not differ significantly with respect to maternal education, F(2, 114) = 2.17, p = .12. Groups did differ, however, in terms of nonverbal cognition, F(2, 115) = 16.19, p < .01. Least significant difference post hoc tests indicated that TD > ASD LI, p < .01, and ASD LN > ASD LI, p < .01. Groups also differed significantly in social communication skills, F(2, 115) = 151.34, p < .01, with pairwise comparisons revealing that TD > ASD LI, p < .01, and ASD LN, p < .01. Finally, groups were significantly different with respect to their language abilities, F(2, 113) = 109.83, p < .01. Post hoc analyses indicated that TD > ASD LI and ASD LN, p < .01, and ASD LN > ASD LI, p < .01.
Procedure
Testing occurred in child-friendly assessment suites in a research laboratory at the Waisman Center. Assessments were conducted in two sessions that lasted approximately 2 hr each. Children were evaluated by trained examiners, including a certified speech-language clinician and licensed psychologist with expertise in diagnosis of ASD.
EF Tasks
Two tasks were used to tap into each of three core components of EF—inhibition, task shifting, and updating WM. Measures were selected that had been used in prior research to assess that specific construct. Tasks were adapted to ensure that they were appropriate for school-age children (confirmed through pilot testing). Stimuli within each task were nonlinguistic in nature and required no verbal response. In addition, verbal instructions were simplified to the extent possible and supplemented with visual supports and ample practice with nonverbal feedback. The EF tasks were administered using E-Prime 2.0 software, which recorded accuracy and response time from button presses on a response box; accuracy data were used for the current study. Children in both groups had adequate motor skills to respond to the computerized tasks as evidenced by the lack of missing data. A large four-button response box (left/right, top/bottom) was used for five of the six EF tasks (rather than a keyboard button press). The Corsi Blocks tasks used a two-button mouse to select the appropriate block on the screen. Detailed descriptions and sample stimuli for the EF tasks are available as Supplemental Material S1, accompanying the report by Kaushanskaya et al. (2017).
Inhibition was assessed using a flanker task and a go/no-go task. Numerous studies have examined inhibition using the flanker task (Diamond, Barnett, Thomas, & Munro, 2007; Mullane, Corkum, Klein, & McLaughlin, 2009; Salthouse, 2010) and the go/no-go task (Brocki & Bohlin, 2004; Cragg & Nation, 2008). For the flanker, the children's task was to push buttons on a response box to indicate the direction that the center stimulus was facing (fish facing left or right) while ignoring the surrounding stimuli. Three types of items comprised the flanker task: neutral (fish surrounded by seaweed), congruent (center fish surrounded by four other fish facing the same direction), and incongruent (center fish surrounded by four other fish facing the opposite direction). In the go/no-go task, children were trained to push a response box button when they saw target stimuli (rectangular boxes with diagonal lines or a vertical line within the box) but not to respond to the nontarget stimulus (rectangle with a vertical line extending beyond the box). The ratio of target to nontarget stimuli in the go/no-go task was 3:1. Based on findings from Kaushanskaya et al. (2017), the specific index used to assess performance on the flanker was accuracy on incongruent items; for the go/no-go task, the dependent variable of interest was no-go accuracy.
Task shifting was examined using the local/global task and the card sort task. Previous research has used the local/global task (Miyake et al., 2000; Vaughan & Giovanello, 2010) as well as the Dimensional Change Card Sort task (Diamond & Kirkham, 2005; Zelazo et al., 2013) to measure the ability to flexibly shift between rules/mental states. The local/global task required children to shift between identifying shapes at the local and global levels. Stimuli consisted of large shapes constructed from smaller shapes. This task was composed of congruent trials (e.g., large circle composed of small circles), incongruent trials (e.g., large square composed of small circles), and neutral trials (e.g., large triangle composed of small circles). For each trial, a visual cue (arrow pointing to a large or small tree) appeared on the screen to indicate whether the child should identify the large (global) or small (local) shape. Children were trained to push a response button associated with the square (left button) or circle (right button). The card sort task involved sorting colored shapes on one dimension (color) and then switching to the other dimension (shape). During one condition within the task, mixed switching was required. We adapted Zelazo's version of this card sort task in the NIH Tool Box Cognitive Battery to replace the linguistic cues for sorting (“color” or “shape”) with visual cues (colored abstract shape “blobs” or grayed out circles and squares). Children were trained to press the right or left response button to correspond with the appropriate dimension (color/shape) depicted by the object on the right or left of the screen (e.g., a red square would be sorted with a red circle if the cue was color but with a blue square if the cue was shape). The index used to assess performance on the local/global task was incongruent global accuracy, and the index for the card sort task was mixed-switch accuracy (Kaushanskaya et al., 2017).
Updating of WM was assessed by n-back and Corsi blocks tasks. The n-back task has been used by numerous researchers to evaluate the ability to manipulate and maintain information for a brief period of time (Owen, McMillan, Laird, & Bullmore, 2005; Vaughan & Giovanello, 2010; Wilhelm, Hildebrandt, & Oberauer, 2013). In the n-back task, children were required to push response buttons to indicate if they had seen the shape that appeared on the computer screen n positions previously. There were three conditions of increasing difficulty: 0-back, 1-back, and 2-back trials. The n-back task visual stimuli were abstract shapes for which it was difficult to provide a verbal label (Attneave & Arnoult, 1956). The Corsi blocks task is a well-established measure for assessing spatial memory (Pagulayan, Bush, Medina, Bartok, & Krikorian, 2006; Pellicano et al., 2017). For the Corsi blocks task, children were asked to recall a sequence of spatial locations on the screen. A total of nine blocks were arranged in a random spatial pattern on the screen, and children watched as individual blocks turned black. They responded by tapping a touch-screen computer to imitate the sequence of spatial locations. The task began with a span length of two blocks and ended with a span of nine. A capacity score was computed for the Corsi blocks task to reflect the highest span length at which two out of three items were recalled in the correct order. Performance on the n-back task was indexed by 1-back accuracy, and performance on the Corsi blocks task was indexed by capacity scores (per Kaushanskaya et al., 2017).
Analyses
A second-order factor model was fit to the data to determine the extent of each of the three components—inhibition, shifting, and updating WM—loaded on a secondary factor of EF. Independent samples t tests were conducted for each nonverbal EF task to assess group differences between the age-matched TD and ASD groups. To address the issue of the contribution of additional child characteristics, a series of regression models was run in which each child variable (individually or combined) was entered as a predictor of accuracy on each EF measure and the residuals were saved. We then tested for differences in the mean residuals using independent samples t tests to determine whether group differences were still present after controlling for child characteristics.
In order to evaluate the association between language and EF, we conducted linear regression analyses predicting EF task performance from scores on the Receptive Language or Expressive Language Scale of the CELF-4. Separate regressions were applied for the TD group and the ASD group as a whole, as well as for the ASD group broken down by language status. From a clinical perspective, there is an argument to be made for considering a threshold of language ability below which children are typically diagnosed as having a language disorder (we used −1.25 SDs, which has been used in prior research for children with developmental language disorder). The subdivision of children on the autism spectrum into those with and without structural language deficits is one that is common in prior literature, facilitating comparisons. With regard to assumptions underlying the use of parametric tests, some measures were found to show departures from normality (see skewness and kurtosis statistics for the language and EF measures in Tables 1 and 2). At the same time, the t tests and other parametric tests are known to show some robustness to violations of normality. Checks using nonparametric tests did not, on the whole, reveal noticeable differences so we report the results of the parametric tests.
Table 2.
Participants' performance on executive function (EF) tasks for the typically developing (TD) group and the group with autism spectrum disorder (ASD) as a whole, as well as subdivided according to language status.
| EF component | EF task | TD (n = 71) | ASD (n = 48) | ASD LI (n = 20) | ASD LN (n = 28) |
|---|---|---|---|---|---|
| Inhibition | Flanker | ||||
| Mean (SD) | 0.9 (0.1) | 0.9 (0.2) | 0.8 (0.2) | 0.9 (0.1) | |
| Range | 0.6–1.0 | 0.3–1.0 | 0.3–1.0 | 0.5–1.0 | |
| Skewness | −2.2 | −1.6 | −0.8 | −2.4 | |
| Kurtosis | 6.0 | 2.0 | −0.1 | 7.1 | |
| Go/no-go | |||||
| Mean (SD) | 0.8 (0.1) | 0.7 (0.2) | 0.7 (0.3) | 0.8 (0.2) | |
| Range | 0.4–1.0 | 0.0–1.0 | 0.0–1.0 | 0.1–1.0 | |
| Skewness | −0.8 | −1.5 | −1.2 | −1.8 | |
| Kurtosis | 0.4 | 2.2 | 2.1 | 3.2 | |
| Shifting | Card sort | ||||
| Mean (SD) | 0.7 (0.1) | 0.7 (0.1) | 0.6 (0.2) | 0.7 (0.1) | |
| Range | 0.4–1.0 | 0.3–1.0 | 0.3–1.0 | 0.5–0.9 | |
| Skewness | −0.1 | 0.1 | −0.9 | 0.2 | |
| Kurtosis | −0.3 | −0.3 | 0.5 | −0.9 | |
| Local/global | |||||
| Mean (SD) | 0.9 (0.2) | 0.7 (0.2) | 0.6 (0.2) | 0.8 (0.2) | |
| Range | 0.3–1.0 | 0.3–1.0 | 0.3–1.0 | 0.4–1.0 | |
| Skewness | −2.0 | 0.4 | 0.1 | −1.0 | |
| Kurtosis | 4.5 | −1.3 | −0.7 | −0.6 | |
| Updating working memory | Corsi blocks | ||||
| Mean (SD) | 4.8 (0.8) | 4.2 (1.2) | 3.9 (1.2) | 4.4 (1.2) | |
| Range | 3.0–7.0 | 1.0–6.0 | 1.0–6.0 | 1.0–6.0 | |
| Skewness | −0.4 | −0.5 | −0.1 | −0.9 | |
| Kurtosis | 0.7 | 0.2 | 0.3 | 1.2 | |
| n-Back | |||||
| Mean (SD) | 0.8 (0.2) | 0.7 (0.2) | 0.6 (0.3) | 0.8 (0.2) | |
| Range | 0.1–1.0 | 0.1 | 0.2 | 0.1 | |
| Skewness | −1.4 | −0.9 | 0.3 | −2.1 | |
| Kurtosis | 2.4 | 0.0 | −0.9 | 6.5 |
Note. ASD LI = autism spectrum disorder, language impairment (scored ≤ 81.25 on CELF-4 Core Language standard score, −1.25 SD); ASD LN = autism spectrum disorder, language normal (scored > 81.25 on CELF-4 Core Language standard score); CELF-4 = Clinical Evaluation of Language Fundamentals–Fourth Edition.
Results
Assessment of EF Components/Tasks
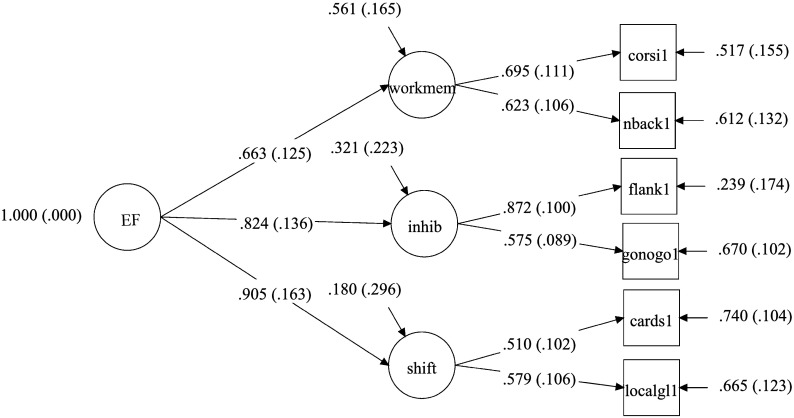
To address our first research question, a second-order factor analysis (Mplus Version 7.4; Muthen & Muthen, 2015) was conducted using the entire sample (N = 119), with six continuous dependent variables (EF task scores) and four continuous latent variables representing the first- and second-order factors. The first-order factors consisted of Factor 1: “working memory” (n-back and Corsi), Factor 2: “inhibition” (flanker and go/no-go), and Factor 3: “shifting” (card sort and local/global); the second-order factor consisted of “executive function.” Results revealed that “shifting” (Factor 3) had the highest loading on the second-order factor EF, with a standardized loading of .905, standard error of .163, t value of 5.543, and two-tailed p value of < .01. This was followed by “inhibition” (Factor 2), with a standardized loading of .824 on the second-order factor EF, standard error of .136, t value of 6.083, and p value of < .01, and then by “working memory,” with a standardized loading of .663, standard error of .125, t value of 5.313, and p value of < .01. Figure 1 presents a path diagram for the second-order factor analysis.
Figure 1.
Path diagram and completely standardized second-order factor model estimates for the executive function (EF) components of working memory (workmem), inhibition (inhib), and shifting (shift). Tasks include Corsi blocks (corsi), n-back (nback), flanker (flank), go/no-go (gonogo), card sort (cards), and local global (localgl).
Group Differences in EF
We conducted a preliminary analysis to assess sex/gender differences in EF performance because of the imbalance of the gender ratios across groups and prior findings in the literature suggesting that there are some differences in EF skills for boys/girls at certain stages of development (e.g., Klenberg, Korkman, & Lahti-Nuuttila, 2001; Wiebe, Espy, & Charak, 2008). Given that the ASD group in this study was predominately boys whereas the TD group was more evenly divided with respect to boys and girls (as they occur in the population), gender could be a confounding factor. To examine this possibility, we conducted both independent sample t tests and Mann–Whitney U tests to assess scores of boys versus girls, broken down by group, for the six EF measures. The results were statistically similar so only the t-test findings are reported. Gender differences in performance were not significantly different (p < .05) for any of the EF tasks for either the ASD group or the TD group. Specifically, t-test comparisons of male/female performance on the EF tasks for children with ASD ranged from t values of −2.66 to 0.57, with p values of .10–.97 (two-tailed). For the TD group, t values ranged from −1.90 to 0.94, with p values of .07–.42 (two-tailed). Given the lack of significant differences in EF performance based on gender, this variable was not included in further analyses. Despite this lack of significant group differences, we acknowledge that the imbalance of boys/girls across the groups is a limitation of the study.
Table 2 summarizes descriptive data for the six EF tasks for the TD and ASD groups as a whole and subdivided by language status. Independent samples t tests were applied (using SPSS) to assess group differences between the age-matched ASD and TD groups' performance on the EF tasks. Group differences were statistically significant for the flanker task, t(116) = 2.73, p = .01, d = .49; this is considered to be a medium effect size. This finding indicates that the ASD group scored more poorly on this inhibition measure (M = .87, SD = .18) than TD age-mates (M = .94, SD = .09). However, group differences on the other inhibition measure, the go/no-go task, did not reach statistical significance, t(116) = 1.91, p = .06, though the TD group did score higher (M = .81, SD = .14) than the ASD group (M = .74, SD = .23). Significant group differences were found for both of the task-shifting measures: local/global, t(117) = 3.40, p < .01, d = .61, and card sort, t(117) = 3.34, p < .01, d = .62; results yielded medium effect sizes for both tasks. The TD group scored higher (M = .87, SD = .22) than the ASD group (M = .70, SD = .33) on the local/global task; similarly, the TD group outperformed (M = .74, SD = .14) the ASD group (M = .65, SD = .15) on the card sort task. This same pattern was observed for the tasks that measured updating of WM. There was a significant difference between age-matched TD and ASD groups on the n-back task, t(115) = 2.27, p = .03, d = .41 (small to medium effect size), such that the TD group scored higher (M = .80, SD = .19) than the ASD group (M = .71, SD = .25). Likewise, there was a significant group effect for the Corsi blocks task, t(117) = 3.37, p < .01, d = .60 (medium effect size), with the TD group performing better (M = 4.77, SD = .81) than the ASD group (M = 4.15, SD = 1.22). Examination of the data for the one participant with a community ASD diagnosis who just missed the cutoff on the CARS2-HF revealed that his EF data were in line with the ASD group as a whole and did not influence the patterns of findings.
A series of linear regression analyses were conducted in which selected child variables were entered individually or in combination as predictors of EF task accuracy. Residuals from these regression models were then compared using independent samples t tests to assess whether differences remained between the TD and ASD groups after accounting for the effects associated with the corresponding child characteristics. The child characteristics examined in these analyses were nonverbal cognition (WISC-IV), SES (maternal education), and social communication (SCQ), and residuals were determined for each control variable separately and in combination. Table 3 provides a summary of t-test results for the analysis of group differences based on residuals from the regressions. Controlling for differences between the groups in nonverbal cognition eliminated significant group effects (ps = .09–.27) on three of the six EF tasks—the flanker, go/no-go, and n-back. Accounting for SES differences across the groups did not influence the original findings for the age-matched groups. That is, the TD group scored significantly better than the ASD group on all EF measures, except for the go/no-go task, for which there was no significant group effect without controlling for SES. On the other hand, when social communication differences between groups were controlled (or all three child variables were added), the TD and ASD groups did not differ significantly on their performance for any of the EF tasks (ps = .32–.92).
Table 3.
Summary of t test and p values for the analysis of group differences of residualized executive function (EF) variables controlling for child characteristics.
| EF component | EF task | Nonverbal cognition (WISC-IV) | SES (maternal education in years) | Social communication (SCQ score) | All |
|---|---|---|---|---|---|
| Inhibition | Flanker | t(115) = 1.71 | t(114) = 2.69 | t(115) = −0.87 | t(112) = −0.69 |
| p = .09 | p = .01** | p = .38 | p = .50 | ||
| d = .49 | |||||
| Go/no-go | t(115) = 1.23 | t(114) = 1.73 | t(115) = −0.87 | t(112) = −0.80 | |
| p = .22 | p = .09 | p = .39 | p = .43 | ||
| Shifting | Card sort | t(116) = 2.35 | t(115) = 2.93 | t(116) = −0.26 | t(113) = −0.13 |
| p = .02* | p = .00** | p = .79 | p = .90 | ||
| d = .43 | d = .57 | ||||
| Local/global | t(116) = 2.49 | t(115) = 3.08 | t(116) = 0.83 | t(113) = 1.01 | |
| p = .01* | p = .00** | p = .41 | p = .32 | ||
| d = .44 | d = .53 | ||||
| Updating working memory | Corsi blocks | t(116) = 2.17 | t(115) = 2.99 | t(116) = 0.11 | t(113) = 0.34 |
| p = .03* | p = .00** | p = .92 | p = .74 | ||
| d = .39 | d = .54 | ||||
| n-Back | t(114) = 1.11 | t(113) = 2.04 | t(114) = −0.14 | t(111) = 0.11 | |
| p = .27 | p = .04* | p = .89 | p = .91 | ||
| d = .37 |
Note. Standard scores were used for the WISC-IV and SCQ scores. SES = socioeconomic status; WISC-IV = Wechsler Intelligence Scale for Children–Fourth Edition; SCQ = Social Communication Questionnaire; d = Cohen's d effect size.
Group difference at p < .05.
Group difference at p < .01.
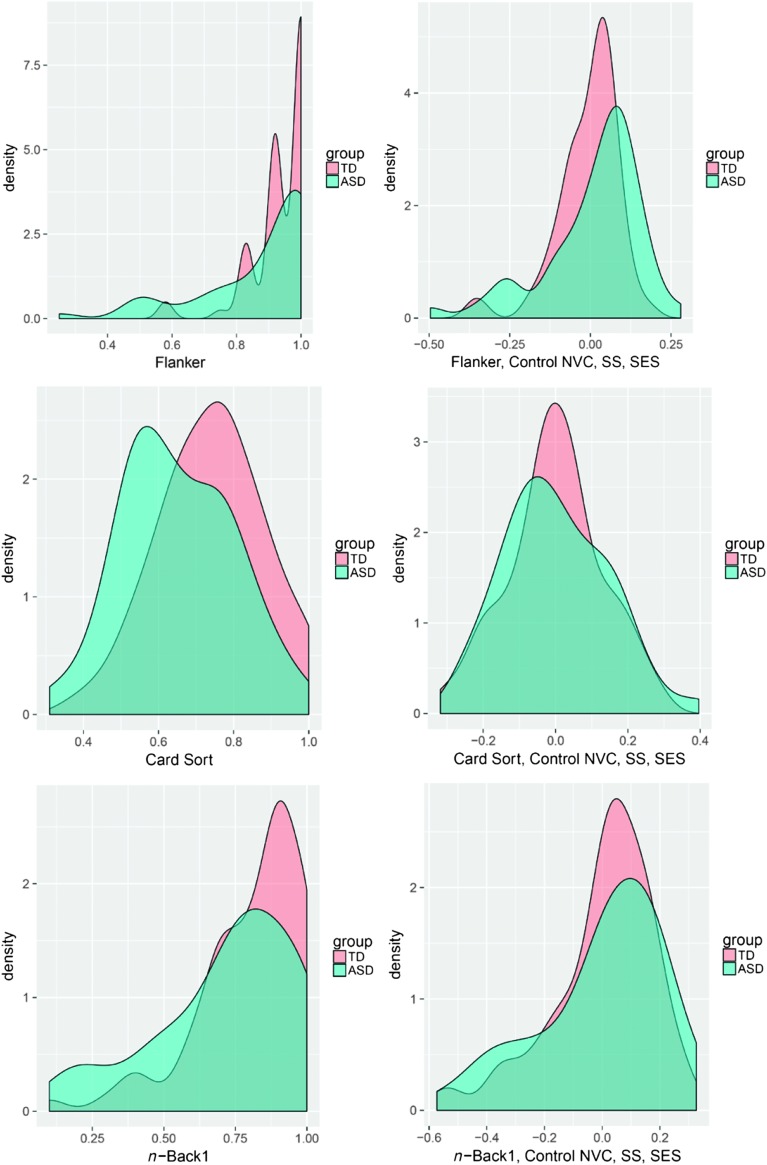
Figure 2 presents density plots produced using the R function ggplot2 (Wickham, 2009) for the TD–ASD age group comparison (left-hand graph) for a given task and the density plot based on residuals after controlling for all three child variables (right-hand graph). Density plots are used to visualize the distribution of data over a continuous interval. The peaks of a density plot display where values are concentrated over the interval of scores. As can be seen in Figure 2, the shape of the distribution of scores on the EF tasks are quite distinct for the age-matched comparison (left-hand graph), with the peak (or multiple peaks) for the TD group shifted toward higher scores compared to the ASD group. In contrast, it is possible to observe the increased similarities in distributions of EF scores (right-hand graph) when the additional child characteristics are controlled. The density plots underscore the discussion regarding matching/covarying variables on which groups differ and show how the distribution of EF scores for the TD and ASD groups shifts to become more similar as we account for more variables.
Figure 2.
This six-panel figure presents density plots for one inhibition task (top row), shifting task (middle row), and working memory task (bottom row) to illustrate the shape of the distributions for the typically developing (TD) group (pink) and autism spectrum disorder (ASD) group (green). The left column displays age group comparisons, and the right column shows density plots based on residuals after controlling for nonverbal cognition (NVC), social communication skills (SS), and socioeconomic status (SES).
Association Between Language and EF
To examine the association between language and EF, linear regression analyses were conducted predicting EF abilities from Receptive Language or Expressive Language Scale scores of the CELF-4. It is important to note that the predictor and criterion variables for the regression analyses were collected at the same point in time; therefore, they do not indicate temporal precedence. Separate regression analyses were run for the TD and ASD groups and for the ASD group subdivided by language status. Results (coefficients and t tests) from the regression analyses for the ASD group, which showed the greatest association between language abilities and EF, are summarized in Table 4. Findings indicated significant concurrent prediction of performance on the flanker, card sort, local/global, and n-back tasks from both Receptive Language and Expressive Language (CELF-4) for the ASD group (but not the TD group). Receptive language and expressive language explained a significant portion of variance in flanker scores (R 2 = .13, F(1, 46) = 6.78, p = .01 and R 2 = .14, F(1, 44) = 7.36, p = .01, respectively). A significant portion of variance in card sort scores was also accounted for by receptive language and expressive language abilities (R 2 = .08, F(1, 46) = 4.15, p < .05 and R 2 = .12, F(1, 44) = 6.11, p = .02, respectively). Similarly, receptive and expressive language explained significant variance in local/global scores (R 2 = .21, F(1, 46) = 12.40, p < .01 and R 2 = .11, F(1, 44) = 5.40, p = .03, respectively). n-Back task performance was significantly predicted by receptive language, R 2 = .27, F(1, 44) = 16.56, p < .01, and expressive language, R 2 = .24, F(1, 43) = 13.40, p < .01, for the ASD group and by receptive language for the TD group, R 2 = .11, F(1, 69) = 8.56, p < .01. Corsi blocks performance was predicted only by CELF-4 Receptive Language scores for both the ASD group, R 2 = .11, F(1, 47) = 5.69, p = .02, and TD group, R 2 = .07, F(1, 70) = 4.82, p = .03. The amount of significant variance in EF task performance accounted for by language scores ranged from 8% to 27% for the ASD group as a whole and from 7% to 11% for the TD group.
Table 4.
Language predictors of executive function (EF) task performance for the autism spectrum disorder group.
| EF task | Language measure | B | β | SE | t | p |
|---|---|---|---|---|---|---|
| Flanker | Expressive | .003 | .379 | .001 | 2.713 | .009** |
| Receptive | .003 | .358 | .001 | 2.603 | .012* | |
| Go/no-go | Expressive | .000 | .029 | .002 | 0.194 | .847 |
| Receptive | .002 | .215 | .002 | 1.490 | .143 | |
| Card sort | Expressive | .003 | .349 | .001 | 2.472 | .017* |
| Receptive | .002 | .288 | .001 | 2.038 | .047* | |
| Local/global | Expressive | .005 | .331 | .002 | 2.324 | .025* |
| Receptive | .008 | .461 | .002 | 3.522 | .001** | |
| Corsi blocks | Expressive | .013 | .208 | .009 | 1.408 | .166 |
| Receptive | .020 | .332 | .008 | 2.385 | .021* | |
| n-Back | Expressive | .006 | .488 | .002 | 3.661 | .001** |
| Receptive | .006 | .523 | .002 | 4.069 | .000** |
Note. Standard scores were used for the Clinical Evaluation of Language Fundamentals–Fourth Edition Expressive and Receptive subscales.
p < .05.
p < .01.
Another set of linear regression analyses were conducted for the ASD sample subdivided by language status (as described above). Table 5 summarizes the coefficients and t tests for these regression analyses; only results from the CELF-4 Receptive Language Scale are reported because no significant results were observed for the Expressive Language Scale. Go/no-go performance was significantly predicted by receptive language scores for the ASD LN group, R 2 = .16, F(1, 26) = 5.03, p = .03, but not the ASD LI group. Conversely, local/global task performance was significantly predicted by receptive language scores for the ASD LI group, R 2 = .24, F(1, 18) = 5.59, p = .03, but not the ASD LN group.
Table 5.
Receptive language as a predictor of executive function (EF) task performance for the autism spectrum disorder group, divided according to language status.
| EF task | Language group | B | β | SE | t | p |
|---|---|---|---|---|---|---|
| Flanker | ASD LI | −.001 | −.080 | .004 | −0.343 | .736 |
| ASD LN | .002 | .258 | .002 | 1.362 | .185 | |
| Go/no-go | ASD LI | .000 | .002 | .005 | 0.007 | .994 |
| ASD LN | .006 | .403 | .003 | 2.243 | .034* | |
| Card sort | ASD LI | −.003 | −.223 | .003 | −0.968 | .346 |
| ASD LN | .002 | .269 | .001 | 1.422 | .167 | |
| Local/global | ASD LI | .013 | .487 | .004 | 2.365 | .029* |
| ASD LN | .000 | .014 | .004 | 0.071 | .944 | |
| Corsi blocks | ASD LI | .017 | .170 | .023 | 0.730 | .475 |
| ASD LN | .029 | .343 | .016 | 1.862 | .074 | |
| n-Back | ASD LI | .010 | .378 | .006 | 1.632 | .122 |
| ASD LN | .003 | .220 | .003 | 1.151 | .260 |
Note. ASD LI = autism spectrum disorder, language impairment (scored ≤ 81.25 on CELF-4 Core Language standard score, −1.25 SD); ASD LN = autism spectrum disorder, language normal (scored > 81.25 on CELF-4 Core Language standard score); CELF-4 = Clinical Evaluation of Language Fundamentals–Fourth Edition.
p < .05.
Discussion
Components of EF
Results confirmed that, in a sample that included children with ASD, each of the three components examined—inhibition, shifting, and updating WM—appeared representative of the broader construct of EF as measured by these specific experimental tasks. Shifting (card sort and local/global tasks) was most closely aligned with EF, followed by inhibition (flanker and go/no-go) and then by updating WM (n-back and Corsi blocks). According to an integrative framework of EF, cognitive processes form a hierarchy in which lower-level components subserve higher-order abilities (Garon, Bryson, & Smith, 2008; Kapa, Plante, & Doubleday, 2017; Miyake et al., 2000). For example, superordinate EF skills such as planning are assumed to involve coordination of lower-level EF components such as WM and inhibition. Furthermore, viewing core EF components as a hierarchy, such that updating WM is subordinate to inhibition and inhibition is subordinate to shifting, might be supported given the age and overall ability level of our participants. Specifically, we might question whether some of the contribution of the shifting tasks is strengthened by a potential involvement of inhibition in these tasks. Some have argued (see Mayr & Keele, 2000) that shifting between tasks, as in the card sort task, involves the inhibition of the previous task set, known as “backward inhibition.” If it were the case that our shifting tasks (card sort and local/global) also tap into inhibition, this may have strengthened the contribution of shifting to broader EF while weakening the contribution of the inhibition tasks.
As an alternative to the integrative hierarchy explanation, we might question whether the individual tasks that comprised the updating WM component contributed to the finding that this component was less representative of the construct of EF than task shifting and inhibition. Both the n-back task and Corsi blocks task have been used in prior studies to examine updating of WM (de Paula, Malloy-Diniz, & Romano-Silva, 2016; Smith & Jonides, 1999; Szmalec, Verbruggen, Vandierendonck, & Kemps, 2011; Vandierendonck, Kemps, Fastame, & Szmalec, 2004), but other research has used Corsi blocks as a measure of short-term memory (Ang & Lee, 2008; Park et al., 2002). Thus, it could be argued that the Corsi blocks task was tapping into different cognitive processes than the n-back task (short-term memory as opposed to WM). However, short-term memory and WM have been proposed to be aspects of the same construct within some theoretical frameworks (Nairne & Neath, 2013; Unsworth & Engle, 2007), and correlational findings have revealed substantial to complete overlap for short-term and WM measures (Colom, Shih, Flores-Mendoza, & Quiroga, 2006; Hornung, Brunner, Reuter, & Martin, 2011; Miyake, Friedman, Rettinger, Shah, & Hegarty, 2001), particularly within the visual–spatial domain (Miyake et al., 2001). In addition, findings from the second-order factor analysis in the current study indicated that first-order standardized loadings of the Corsi blocks and n-back scores on the second-order factor of EF were very similar (.695 and .623, respectively). Therefore, our findings provide no evidence that differences in the tasks selected to measure updating of WM influenced the relatively lower weighting of this component of EF.
EF in Children With ASD
Findings from this study indicated that school-age children with ASD displayed deficits on nonverbal EF tasks tapping inhibition, task shifting, and updating of WM compared to TD age-mates. This was a robust result—with the ASD group performing significantly poorer on five out of six EF measures tapping the three different components of EF abilities. Although group differences approached but did not reach statistical significance on one of the inhibition measures, the go/no-go task (p = .058), the mean score for the TD controls was better than for children with ASD (81% vs. 74% accuracy). Medium effect sizes were found for the age group differences across the EF tasks, with Cohen's d values ranging from .41 to .62. It should be noted that the largest effect sizes were found for the two shifting measures, which is interesting in light of the finding that task shifting was the component most closely aligned with the broad construct of EF in this study. Results from this study are consistent with those of Corbett et al. (2009), who also compared school-age children with ASD with normal range cognitive abilities to age-matched TD controls on various measures of EF. They reported that children with ASD displayed significant deficits in response inhibition, cognitive flexibility/switching, and WM (as well as vigilance) relative to age-mates.
When additional child characteristics—nonverbal cognition, SES, and social communication—were included in the analyses, fewer significant differences in EF performance between the ASD and TD groups were observed in the current study. Accounting for group differences in nonverbal cognition resulted in nonsignificant findings for half of the EF tasks. Specifically, when we adjusted for nonverbal cognitive abilities, the performance of children with ASD and TD children was roughly equivalent on both of the inhibition measures (flanker and go/no-go) and one of the WM measures (n-back). A number of prior studies with children with ASD have found that nonverbal cognition is related to EF abilities (Blijd-Hoogewys, Bezemer, & Van Geert, 2014; Liss et al., 2001; Memari et al., 2013; Van Eylen et al., 2015; but see Robinson et al. 2009). While controlling for nonverbal cognition resulted in increased similarity in the EF performance of the TD children and the children with ASD in this study, we continued to observe deficits for the ASD group on both shifting measures and one WM task. These findings are partially consistent with results by Pellicano et al. (2017), who reported deficits on set shifting and WM, as well as inhibition when preschool children with ASD were matched to controls on age and nonverbal IQ. Although the TD and ASD groups in the current study differed significantly in SES as measured by maternal education, controlling for this difference had no impact on group differences on EF task performance. With a couple of exceptions (e.g., Liss et al., 2001), few studies examining EF in children with ASD have included SES as a matching variable or controlled for it statistically.
In contrast to the lack of impact of SES, when differences in social communication were controlled in this study, group differences in EF performance were eliminated across all three components of inhibition, shifting, and updating of WM. Recall that our measure of social communication was the SCQ (Rutter, Bailey, et al., 2003), a parent report screening measure for autism. This measure is composed of items pertaining to the domains of social relating, communication, and range of interests. Thus, our measure of social communication tapped into core symptoms of ASD. Prior research has found a significant relationship between EF abilities and autism symptoms/severity, including restricted and repetitive behaviors (Akbar et al., 2013; Pellicano, 2013; Van Eylen et al., 2015) and social/communication skills (Happé, Booth, Charlton, & Hughes, 2006). Our finding that group differences in shifting were only eliminated when controlling for differences in autism symptoms (SCQ scores) is consistent with the results of Akbar et al. (2013) demonstrating that shifting was predicted by level of autistic severity. Thus, the social communication variable that was investigated in this study encompasses more than language or communication alone. We interpret these findings to indicate that deficits observed in the EF components studied here are related to autism symptomatology more broadly.
There was no evidence of significant group differences in core EF abilities in this study after accounting for age, nonverbal IQ, and social communication (or social communication individually). This result is not entirely surprising given that most studies have not attempted to control for social communication differences, which gets at the heart of the distinction between the TD and ASD groups as discussed above. The lack of group differences in EF abilities may also relate to the fact that participants in this study were composed of verbal children with ASD whose nonverbal cognitive abilities fell within the normal range. Finally, this result might reflect the use of nonverbal EF tasks and/or the computerized administration of all EF tasks, which reduced social task demands (see Blijd-Hoogewys et al., 2014; Robinson et al., 2009) and may have attenuated EF deficits in children with ASD (Kenworthy et al., 2008; but see Landry & Al-Taie, 2016).
Several cautions are in order when drawing conclusions about EF abilities in children with ASD based on group comparisons. First, we need to keep in mind that there is considerable individual variation such that EF deficits are not uniformly observed in ASD (Geurts et al., 2014; Pellicano, 2010), regardless of the basis of comparison used (age, nonverbal cognition, etc.). The range of EF scores in this study confirms this point. In addition, the density plots for the EF data from the current study illustrate the distinct shapes of the distribution of EF scores for the TD and ASD groups; thus, when additional child characteristics are partialed out, it is possible that different portions of the distribution are being affected so that removing differences in nonverbal cognition, for example, only influences scores at the low end of performance.
EF–Language Associations
Results indicated a clear, but modest, association between EF and language abilities for school-age children with ASD. That is, language scores from a standardized, omnibus measure of language comprehension and production (CELF-4) predicted concurrent performance on nonverbal EF tasks. This finding held across the three EF components examined (though one of the two inhibition tasks, the go/no-go task, did not show significant associations). In the cases of significant EF–language associations for children with ASD, both receptive and expressive language accounted for unique variance in EF performance with the exception of the Corsi blocks task, where receptive language, but not expressive language, was associated with EF scores. When the ASD group was subdivided into two groups based on structural language status, an interesting pattern emerged such that different components of EF abilities were related to language abilities in children with and without language disorders. Receptive language abilities were associated with shifting (tapped by the local/global task) for the ASD LI group, whereas receptive language was associated with inhibition (as measured by the go/no-go task) for the ASD LN group. The TD group displayed a significant association between language and WM, with a trend for a relation between language and shifting.
The findings from the current study stand in contrast to those of Joseph et al. (2005) in several ways. Our results suggest a relation between EF and language abilities for both children with ASD and the controls, whereas Joseph and colleagues (2005) did not find an association for their ASD group. Further, in this study, there was evidence of a stronger EF–language link in the ASD group than in the TD group. The absence of a significant association between EF skills and language for the autism group in the Joseph et al. (2005) study was likely due to the fact that nonverbal cognitive skills were partialed out of the correlations between EF task performance and language measures. In this study, we examined the impact of including (or not including) nonverbal cognition in evaluating group differences in EF performance but did not use it as a covariate in assessing links between language and EF abilities. When we explored the use of partial correlations (accounting for nonverbal IQ scores), we found that shifting (local/global task) performance in the ASD group was significantly related to receptive language (partial correlation = .304, p = .04) and that EF–receptive language links for the TD group approached but did not reach significance for shifting (local/global, partial correlation = .225, p = .07) and inhibition (go/no-go, partial correlation = .233, p = .06). It is generally agreed that EF and IQ are separate constructs, yet measures of nonverbal IQ often draw on EF processes such as WM (note that the significant association between WM and receptive language observed in the TD group disappeared when we accounted for nonverbal cognitive abilities with the partial correlations). Thus, our findings—with or without accounting for nonverbal cognition—do not appear to support the assertion that, unlike TD children, children with ASD do not employ language in the service of executive control (Joseph et al., 2005; Russell, 1997).
It is notable that this study revealed EF–language associations given the lack of similarity in task demands between the standardized language measure and EF tasks. Prior positive findings in the literature have often involved language tasks that draw heavily on the same cognitive processes tapped by the EF task, for example, use of inhibition during conflict resolution in the case of garden path sentences or inhibition of past tense overgeneralizations (Ibbotson & Kearvell-White 2015; Mazuka et al., 2009; Woodard et al., 2016). Our finding in the current study that receptive language was more closely related to EF performance than expressive language may be attributable to greater overlap in task demands for the EF tasks and comprehension; compared to comprehension, language production involves additional EF skills such as planning and organization (that were not tapped by these core EF tasks) as well as additional linguistic processes such as lexical retrieval and sentence formulation.
Broadly, our findings align with the HCSM such that a relationship exists between language ability and EF in this sample of school-age TD children and children with ASD. That this relationship may change depending on the language status of the ASD group is also consistent with the HCSM. Our findings indicate that social communication was the covariate that eliminated group differences across all EF measures. As indexed in this study, social communication is a broad construct that includes nonverbal communication and communication accomplished through language as well as other behavioral symptoms associated with autism. Within the HCSM, it is structural language that is implicated in verbal mediation and assumed to play a role in managing nonverbal executive control.
It is important to acknowledge that there are “third variable” issues to consider when interpreting EF–language associations. For instance, it could be argued that both the standardized tests and experimental tasks involved a test-like paradigm that may similarly draw on anxiety, motivation, attention and other constructs that impact performance on these domains of functioning. The EF tasks were presented as computer games and the children appeared to perceive them as more fun and engaging than the language testing; however, both the language and EF measures did entail some degree of social interaction with the experimenter and required attention. It is possible that these factors, which were not independently assessed in the current study, contributed to the observed link between language and EF.
Summary and Future Directions
This study provided a global assessment of core EF skills in children with ASD and an evaluation of the overall relationship between executive control and structural language abilities as assessed by an off-line measure of language comprehension and production that is widely used in clinical settings. Results indicated that children with ASD had significant deficits in inhibition, shifting, and updating of WM relative to age-matched controls and displayed particular difficulties in shifting even after accounting for differences in nonverbal IQ. It was only after controlling for social communication abilities that group differences in EF abilities were no longer found. These findings are interpreted to suggest that the EF deficits observed in these school-age children with ASD were tied to core autism symptoms characterized by social communication rather than other factors. EF skills are thought to underlie social function according to a developmental framework of social skills proposed by Beauchamp and Anderson (2010). Our findings suggest that additional research is warranted to examine the impact of social skills intervention on EF abilities (e.g., Strichter et al., 2010) and the potential of EF intervention to impact social development (e.g., Diamond & Lee, 2011; Parsons & Mitchell, 2002) in children with ASD.
A modest association was revealed between EF skills and language (especially receptive language) in this sample of children with ASD. There was some evidence to suggest that there are different patterns of relationships between language and specific components of EF for children on the autism spectrum with and without language impairment. One of the limitations of the current study, like other prior investigations, is that it does not provide insight into the direction of the relationship between EF and language abilities. Ongoing work by our research team is delving into this issue. One way of elucidating the directionality question is through longitudinal investigation. We have administered language and EF tasks to children at two time points separated by approximately 1 year in order to analyze direction of influence. Preliminary findings suggest that certain EF skills predict subsequent receptive language abilities (indexed by a standardized test) for children without language disorder (TD and ASD), but there was no evidence that earlier language, as measured by this omnibus measure, predicts later EF. However, these tentative results will need to be confirmed.
Establishing directionality is an important issue for future research from the perspective of understanding the underlying mechanisms involved in this association as well as for the purpose of intervention. The importance and effectiveness of EF interventions for children on the autism spectrum has been demonstrated for improving problem solving, flexibility, and planning/organization (e.g., Kenworthy et al., 2014). Given research findings suggesting a link between EF and language abilities, it would be beneficial for future EF intervention studies to also examine outcomes related to language and communication. Conversely, contrastive language training in TD preschoolers has been shown to boost performance on “conflict” EF tasks (Doebel & Zelazo, 2016). The use of this type of language training with children on the autism spectrum who have difficulties with executive control might yield promising results.
Supplementary Material
Acknowledgments
This article stems from the 2017 Research Symposium at ASHA Convention, which was supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under Award R13DC003383. Research reported in this publication was also supported by Award R01 DC011750 (Ellis Weismer & Kaushanskaya, MPIs), as well as by a core grant to the Waisman Center from the National Institute of Child Health and Human Development (U54 HD090256). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to thank Tristan Mahr for his help with initial visualization of the data. We want to acknowledge the members of the Language Processes Lab and the Language Acquisition and Bilingualism Lab for their contributions to this work. Finally, we are sincerely grateful to the children and families who participated in the research.
Funding Statement
This article stems from the 2017 Research Symposium at ASHA Convention, which was supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under Award R13DC003383. Research reported in this publication was also supported by Award R01 DC011750 (Ellis Weismer & Kaushanskaya, MPIs), as well as by a core grant to the Waisman Center from the National Institute of Child Health and Human Development (U54 HD090256).
References
- Akbar M., Loomis R., & Paul R. (2013). The interplay of language on executive functions in children with ASD. Research in Autism Spectrum Disorders, 7(3), 494–501. [Google Scholar]
- Ang S. Y., & Lee K. (2008). Central executive involvement in children's spatial memory. Memory, 16, 918–933. [DOI] [PubMed] [Google Scholar]
- Attneave F., & Arnoult M. D. (1956). The quantitative study of shape and pattern perception. Psychological Bulletin, 53, 452–471. [DOI] [PubMed] [Google Scholar]
- Beauchamp M., & Anderson H. (2010). SOCIAL: An integrative framework for the development of social skills. Psychological Bulletin, 136, 39–64. [DOI] [PubMed] [Google Scholar]
- Best J. R., Miller P. H., & Naglieri J. A. (2011). Relations between executive function and academic achievement from ages 5 to 17 in a large, representative national sample. Learning and Individual Differences, 21, 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C., & Razza R. P. (2007). Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten, Child Development, 78, 647–663. [DOI] [PubMed] [Google Scholar]
- Blijd-Hoogewys E. M. A., Bezemer M. L., & van Geert P. L. C. (2014). Executive functioning in children with ASD: An analysis of the BRIEF. Journal of Autism and Developmental Disorders, 44, 3089–3100. [DOI] [PubMed] [Google Scholar]
- Brocki K. C., & Bohlin G. (2004). Executive functions in children aged 6 to 13: A dimensional and developmental study. Developmental Neuropsychology, 26, 571–593. [DOI] [PubMed] [Google Scholar]
- Broidy L. M., Nagin D. S., Tremblay R. E., Brame B., Dodge K. A., & Fergusson D. E. (2003). Developmental trajectories of childhood disruptive behaviors and adolescent delinquency: A six-site cross-national study. Developmental Psychology, 30, 222–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ S. E., Holt D. D., White D. A., & Green L. (2007). Inhibitory control in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 37, 1155–1165. [DOI] [PubMed] [Google Scholar]
- Christ S. E., Kester L. E., Bodner K. E., & Miles J. H. (2011). Evidence for selective inhibitory impairment in individuals with autism spectrum disorder. Neuropsychology, 25, 690–701. [DOI] [PubMed] [Google Scholar]
- Colom R., Shih P. C., Flores-Mendoza C., & Quiroga M. A. (2006). The real relationship between short-term memory and working memory. Memory, 14, 804–813. [DOI] [PubMed] [Google Scholar]
- Corbett B. A., Constantine L. J., Hendren R., Rocke D., & Ozonoff S. (2009). Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Research, 166, 210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg L., & Nation K. (2008). Go or no-go? Developmental improvements in the efficiency of response inhibition in mid-childhood. Developmental Science, 11, 819–827. [DOI] [PubMed] [Google Scholar]
- Dennis M., Francis D., Cirino P., Schachar P., Barnes M., & Fletcher J. (2009). Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society, 15, 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paula J. J., Malloy-Diniz L. F., & Romano-Silva M. A. (2016). Reliability of working memory assessment in neurocognitive disorders: A study of the Digit Span and Corsi Block-Tapping tasks. Revista Brasileira de Psiquiatria, 38, 262–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. (2013). Executive functions. Annual Review of Psychology, 64, 135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A., Barnett W. S., Thomas J., & Munro S. (2007). Preschool program improves cognitive control. Science, 318, 1387–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A., & Kirkham N. (2005). Not quite as grown-up as we like to think. Psychological Science, 16, 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A., & Lee K. (2011). Interventions shown to aid executive function development in children 4 to 12 years old. Science, 333, 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebel S., & Zelazo P. D. (2016). Seeing conflict and engaging control: Experience with contrastive language benefits executive function in preschoolers. Cognition, 157, 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X., Wei S., Wang G., & Shi J. (2010). The relationship between executive functions and intelligence on 11- to 12-year-old children. Psychological Test and Assessment Modeling, 52, 419–431. [Google Scholar]
- Durrleman S., & Franck J. (2015). Exploring links between language and cognition in autism spectrum disorders: Complement sentences, false belief, and executive functioning. Journal of Communication Disorders, 54, 15–31. [DOI] [PubMed] [Google Scholar]
- Ellis Weismer S., Davidson M. M., Gangopadhyay I., Sindberg H., Roebuck H., & Kaushanskaya M. (2017). The role of nonverbal working memory in morphosyntactic processing by children with specific language impairment and autism spectrum disorders. Journal of Neurodevelopmental Disorders, 9, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatzer S. T., & Roebers C. M. (2012). Language and executive functions: The effect of articulatory suppression on executive functioning in children. Journal of Cognition and Development, 13, 454–472. [Google Scholar]
- Ferrier D. E., Bassett H. H., & Denham S. A. (2014). Relations between executive function and emotionality in preschoolers: Exploring a transitive cognition–emotion linkage. Frontiers in Psychology, 5, 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman N. P., Miyake A., Young S. E., DeFries J. C., Corley R. P., & Hewitt J. K. (2008). Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General, 137, 201–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangopadhyay I., Buac M., Haebig E., Davidson M. M., Kaushanskaya M., & Ellis Weismer S. (2015, May). Exploring the role of verbal mediation in executive functioning in children with autism. Poster presented at the International Meeting for Autism Research, Salt Lake City, UT. [Google Scholar]
- Gangopadhyay I., Davidson M. M., Ellis Weismer S., & Kaushanskaya M. (2016). The role of nonverbal working memory in morphosyntactic processing by school-aged monolingual and bilingual children. Journal of Experimental Child Psychology, 142, 171–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangopadhyay I., MacDonald M., Ellis Weismer S., & Kaushanskaya M. (2018). Planning abilities in bilingual and monolingual children: Role of verbal mediation. Frontiers in Psychology. https://doi.org/10.3389/fpsyg.2018.00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon N., Bryson S., & Smith I. M. (2008). Executive function in preschoolers: A review using an integrative framework. Psychological Bulletin, 134, 31–60. [DOI] [PubMed] [Google Scholar]
- Geurts H., Sinzig J., Booth R., & Happé F. (2014). Neuropsychological heterogeneity in executive functioning in autism spectrum disorders. International Journal of Developmental Disabilities, 60, 155–162. [Google Scholar]
- Grant D. A., & Berg E. (1948). A behavioral analysis of degree of reinforcement and ease of shifting to new responses in Weigl-type card-sorting problem. Journal of Experimental Psychology, 38, 404–411. [DOI] [PubMed] [Google Scholar]
- Haebig E., Kaushanskaya M., & Ellis Weismer S. (2015). Lexical processing in school-age children with autism spectrum disorder and children with specific language impairment: The role of semantics. Journal of Autism and Developmental Disorders, 45, 4109–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happé F., Booth R., Charlton R., & Hughes C. (2006). Executive function deficits in autism spectrum disorders and attention-deficit/hyperactivity disorder: Examining profiles across domains and ages. Brain and Cognition, 61, 25–39. [DOI] [PubMed] [Google Scholar]
- Hill E. L. (2004). Evaluating the theory of executive dysfunction in autism. Developmental Review, 24, 189–233. [Google Scholar]
- Hornung C., Brunner M., Reuter R., & Martin R. (2011). Children's working memory: Its structure and relationship to fluid intelligence. Intelligence, 39, 210–221. [Google Scholar]
- Ibbotson P., & Kearvell-White J. (2015). Inhibitory control predicts grammatical ability. PLoS One, 10, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R. M., McGrath L. M., & Tager-Flusberg H. (2005). Executive dysfunction and its relation to language ability in verbal school-age children with autism. Developmental Neuropsychology, 27, 361–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado M. B., & Rosselli M. (2007). The elusive nature of executive functions: A review. Neuropsychology Review, 17, 213–233. [DOI] [PubMed] [Google Scholar]
- Kapa L. L., Plante E., & Doubleday K. (2017). Applying an integrative framework of executive function to preschoolers with specific language impairment. Journal of Speech, Language, and Hearing Research, 60, 2170–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushanskaya M., Park J., Gangopadhyay I., Davidson M., & Ellis Weismer S. (2017). The relationship between executive functions and language abilities in children: A latent variables approach. Journal of Speech, Language, and Hearing Research, 60, 912–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy L., Anthony L. G., Naiman D. Q., Cannon L., Wills M. C., Luong-Tran C., & Wallace G. L. (2014). Randomized controlled effectiveness trial of executive function intervention for children on the autism spectrum. Journal of Child Psychology and Psychiatry and Allied Disciplines, 55, 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy L., Yerys B. E., Anthony L. G., & Wallace G. L. (2008). Understanding executive control in autism spectrum disorders in the lab and in the real world. Neuropsychology Review, 18, 320–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kercood S., Grskovic J. A., Banda D., & Begeske J. (2014). Working memory and autism: A review of literature. Research in Autism Spectrum Disorders, 8, 1316–1332. [Google Scholar]
- Khanna M. M., & Boland J. E. (2010). Children's use of language context in lexical ambiguity resolution. The Quarterly Journal of Experimental Psychology, 63, 160–193. [DOI] [PubMed] [Google Scholar]
- Klenberg L., Korkman M., & Lahti-Nuuttila P. (2001). Differential development of attention and executive functions in 3- to 12-year-old Finnish children. Developmental Neuropsychology, 20, 407–428. [DOI] [PubMed] [Google Scholar]
- Kray J., Eber J., & Lindenberger U. (2004). Age differences in executive functioning across the lifespan: The role of verbalization in task preparation. Acta Psychologica, 115, 143–165. [DOI] [PubMed] [Google Scholar]
- Kray J., Gaspard H., Karbach J., & Blaye A. (2013). Developmental changes in using verbal self-cueing in task-switching situations: The impact of task practice and task-sequencing demands. Frontiers in Psychology, 4, 940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn L. J., Willoughby M. T., Vernon-Feagans L., Blair C. B., & The Family Life Project Key Investigators. (2016). The contribution of children's time-specific and longitudinal expressive language skills on developmental trajectories of executive function. Journal of Experimental Child Psychology, 148, 20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry O., & Al-Taie S. (2016). A meta-analysis of the Wisconsin Card Sort Task in autism. Journal of Autism and Developmental Disorders, 46, 1220–1235. [DOI] [PubMed] [Google Scholar]
- Lehto J. E., Juujärvi P., Kooistra L., & Pulkkinen L. (2003). Dimensions of executive functioning: Evidence from children. British Journal of Developmental Psychology, 21, 59–80. [Google Scholar]
- Leung R. C., & Zakzanis K. K. (2014). Brief report: Cognitive flexibility in autism spectrum disorders: A quantitative review. Journal of Autism and Developmental Disorders, 44, 2628–2645. [DOI] [PubMed] [Google Scholar]
- Liss M., Fein D., Allen D., Dunn M., Feinstein C., Morris R., & Rapin I. (2001). Executive functioning in high-functioning children with autism. The Journal of Child Psychology and Psychiatry, 42, 261–270. [PubMed] [Google Scholar]
- Lord C., Rutter M., DiLavore P. C., & Risi S. (2001). Autism Diagnostic Observation Schedule (ADOS). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Marcovitch S., & Zelazo P. D. (2009). A hierarchical competing systems model of the emergence and early development of executive function. Developmental Science, 12, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr U., & Keele S. W. (2000). Changing internal constraints on action: The role of backward inhibition. Journal of Experimental Psychology: General, 129, 4–26. [DOI] [PubMed] [Google Scholar]
- Mazuka R., Jincho N., & Onishi H. (2009). Development of executive control and language processing. Language and Linguistics Compass, 3, 59–89. [Google Scholar]
- Memari A. H., Ziaee V., Shayestehfar M., Ghanouni P., Mansournia M. A., & Moshayedi P. (2013). Cognitive flexibility impairments in children with autism spectrum disorders: Links to age, gender and child outcomes. Research in Developmental Disabilities, 34, 3218–3225. [DOI] [PubMed] [Google Scholar]
- Miller G. A., & Chapman J. P. (2001). Misunderstanding analysis of covariance. Journal of Abnormal Psychology, 110, 40–48. [DOI] [PubMed] [Google Scholar]
- Minai U., Jincho N., Yamane N., & Mazuka R. (2012). What hinders child semantic computation: Children's universal quantification and the development of cognitive control. Journal of Child Language, 39, 919–956. [DOI] [PubMed] [Google Scholar]
- Miyake A., & Friedman N. P. (2012). The nature and organization of individual differences in executive functions: Four general conclusions. Current Directions in Psychological Science, 21, 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A., Friedman N. P., Emerson M. J., Witzki A. H., Howerter A., & Wager T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. [DOI] [PubMed] [Google Scholar]
- Miyake A., Friedman N. P., Rettinger D. A., Shah P., & Hegarty M. (2001). How are visuospatial working memory, executive functioning, and spatial abilities related? A latent-variable analysis. Journal of Experimental Psychology: General, 130, 621–640. [DOI] [PubMed] [Google Scholar]
- Moffitt T. E., Arseneault L., Belsky D., Dickson N., Hancox R. J., Harrington H., & Caspi A. (2011). A gradient of childhood self-control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences, 108, 2693–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullane J. C., Corkum P. V., Klein R. M., & McLaughlin E. (2009). Interference control in children with and without ADHD: A systematic review of flanker and Simon task performance. Child Neuropsychology, 15, 321–342. [DOI] [PubMed] [Google Scholar]
- Muthen L. K., & Muthen B. O. (2015). Mplus user's guide (7th ed.). Los Angeles, CA: Muthen & Muthen. [Google Scholar]
- Nairne J. S., & Neath I. (2013). Sensory and working memory. In Healy A. F. & Proctor R. W. (Eds.), Comprehensive handbook of psychology, Vol. 4: Experimental psychology (2nd ed., pp. 419–445). New York, NY: Wiley. [Google Scholar]
- Owen A., McMillan K., Laird A., & Bullmore E. (2005). N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping, 25, 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagulayan K. F., Busch R. M., Medina K. L., Bartok J. A., & Krikorian R. (2006). Developmental normative data for the Corsi block-tapping task. Journal of Clinical and Experimental Neuropsychology, 28, 1043–1052. [DOI] [PubMed] [Google Scholar]
- Park D. C., Lautenschlager G., Hedden T., Davidson N. S., Smith A. D., & Smith P. K. (2002). Models of visuospatial and verbal memory across the adult life span. Psychology and Aging, 17, 299–320. [PubMed] [Google Scholar]
- Parsons S., & Mitchell P. (2002). The potential of virtual reality in social skills training for people with autistic spectrum disorders. Journal of Intellectual Disability Research, 46, 430–443. [DOI] [PubMed] [Google Scholar]
- Pellicano E. (2010). Individual differences in executive function and central coherence predict developmental changes in theory of mind in autism. Developmental Psychology, 46, 530–544. [DOI] [PubMed] [Google Scholar]
- Pellicano E. (2011). Psychological models of autism: An overview. In Roth I. & Rezaie P. (Eds.), Researching the autism spectrum: Contemporary perspectives (pp. 219–265). Cambridge, United Kingdom: Cambridge University Press. [Google Scholar]
- Pellicano E. (2013). Testing the predictive power of cognitive atypicalities in autistic children: Evidence from a 3-year follow-up study. Autism Research, 6, 258–267. [DOI] [PubMed] [Google Scholar]
- Pellicano E., Kenny L., Brede J., Klaric E., Lichwa H., & McMillin R. (2017). Executive function predicts school readiness in autistic and typical preschool children. Cognitive Development, 43, 1–13. [Google Scholar]
- Plante E., Swisher L., Kiernan B., & Restrepo M. A. (1993). Language matches: Illuminating or confounding? Journal of Speech and Hearing Research, 36, 772–776. [PubMed] [Google Scholar]
- Robinson S., Goddard L., Dritschel B., Wisley M., & Howlin P. (2009). Executive functions in children with autism spectrum disorders. Brain and Cognition, 71, 362–368. [DOI] [PubMed] [Google Scholar]
- Rose S. A., Feldman J. F., & Jankowski J. J. (2011). Modeling a cascade of effects: The role of speed and executive function- ing in preterm/full-term differences in academic achievement. Developmental Science, 14, 1161–1175. [DOI] [PubMed] [Google Scholar]
- Russell J. (1997). How executive disorders can bring about an inadequate “theory of mind.” In Russell J. (Ed.), Autism as an executive disorder (pp. 256–304). New York, NY: Oxford University Press. [Google Scholar]
- Russo N., Flanagan T., Iarocci G., Berringer D., Zelazo P. D., & Burack J. A. (2007). Deconstructing executive deficits among persons with autism: Implications for cognitive neuroscience. Brain and Cognition, 65, 77–86. [DOI] [PubMed] [Google Scholar]
- Rutter M., Bailey A., & Lord C. (2003). The Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Rutter M., Le Couteur A., & Lord C. (2003). Autism Diagnostic Interview–Revised (ADI-R) manual. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Salthouse T. A. (2010). Is flanker-based inhibition related to age? Identifying specific influences of individual differences on neurocognitive variables. Brain and Cognition, 73, 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopler E., Van Bourgondien M. E., Wellman G. J., & Love S. R. (2010). Childhood Autism Rating Scale–Second Edition. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Semel E., Wiig E. H., & Secord W. A. (2003). Clinical Evaluation of Language Fundamentals–Fourth Edition. San Antonio, TX: Pearson. [Google Scholar]
- Semrud-Clikeman M., Fine J. G., & Bledsoe J. (2014). Comparison among children with children with autism spectrum disorder, nonverbal learning disorder and typically developing children on measures of executive functioning. Journal of Autism and Developmental Disorders, 44, 331–342. [DOI] [PubMed] [Google Scholar]
- Smith E. E., & Jonides J. (1999). Storage and executive processes in the frontal lobes. Science, 283, 1657–1661. [DOI] [PubMed] [Google Scholar]
- Strichter J., Herzog M., Visovsky K., Schmidt C., Randolph J., Schultz T., & Gage N. (2010). Social competence intervention for youth with Asperger syndrome and high-functioning autism: An initial investigation. Journal of Autism and Developmental Disorders, 40, 1067–1079. [DOI] [PubMed] [Google Scholar]
- Szmalec A., Verbruggen F., Vandierendonck A., & Kemps E. (2011). Control of interference during working memory updating. Journal of Experimental Psychology: Human Perception and Performance, 37, 137–151. [DOI] [PubMed] [Google Scholar]
- Unsworth N., & Engle R. W. (2007). The nature of individual differences in working memory capacity: Active maintenance in primary memory and controlled search from secondary memory. Psychological Review, 114, 104–132. [DOI] [PubMed] [Google Scholar]
- Unterrainer J. M., Rauh R., Rahm B., Hardt J., Kaller C. P., Klein C., … Biscaldi M. (2016). Development of planning in children with high-functioning autism spectrum disorders and/or attention deficit/hyperactivity disorder. Autism Research, 9, 739–751. [DOI] [PubMed] [Google Scholar]
- Vandierendonck A., Kemps E., Fastame M., & Szmalec A. (2004). Working memory components of the Corsi blocks task. British Journal of Psychology, 95, 57–79. [DOI] [PubMed] [Google Scholar]
- Van Eylen L., Boets B., Steyaert J., Wagemans J., & Noens I. (2015). Executive functioning in autism spectrum disorders: Influence of task and sample characteristics and relation to symptom severity. European Child & Adolescent Psychiatry, 24, 1399–1417. [DOI] [PubMed] [Google Scholar]
- Vaughan L., & Giovanello K. (2010). Executive function in daily life: Age-related influences of executive processes on instrumental activities of daily living. Psychology and Aging, 25, 343–355. [DOI] [PubMed] [Google Scholar]
- Wechsler D. (1974). Wechsler Intelligence Scale for Children–Revised. New York: Psychological Corporation. [Google Scholar]
- Wechsler D. (2003). Wechsler Intelligence Scale for Children–Fourth Edition. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Weiland C., Barata M. C., & Yoshikawa H. (2013). The co-occurring development of executive function skills and receptive vocabulary in preschool-aged children: A look at the direction of the developmental pathways. Infant and Child Development, 23, 4–21. [Google Scholar]
- Wickham H. (2009). ggplot2: Elegant graphics for data analysis. New York, NY: Springer-Verlag. [Google Scholar]
- Wiebe S. A., Espy K. A., & Charak D. (2008). Using confirmatory factor analysis to understand executive control in preschool children: I. Latent structure. Developmental Psychology, 44, 575–585. [DOI] [PubMed] [Google Scholar]
- Wilhelm O., Hildebrandt A., & Oberauer K. (2013). What is working memory capacity, and how can we measure it? Frontiers in Psychology, 4, 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard K., Pozzan L., & Trueswell J. C. (2016). Taking your own path: Individual differences in executive function and language processing skills in child learners. Journal of Experimental Child Psychology, 141, 187–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K. K., Chan S. K., Leung P. W. L., Liu W. S., Leung F. L., & Ng R. (2011). Components and developmental differences of executive functioning for school-aged children. Developmental Neuropsychology, 36, 319–337. [DOI] [PubMed] [Google Scholar]
- Zelazo P. D. (2004). The development of conscious control in childhood. Trends in Cognitive Sciences, 8, 12–17. [DOI] [PubMed] [Google Scholar]
- Zelazo P. D., Anderson J. E., Richler J., Wallner-Allen K., Beaumont J. L. & Weintraub S. (2013). II. NIH toolbox cognitive battery (CB): Measuring executive function and attention. Monographs of the Society for Research on Child Development, 78, 16–33. [DOI] [PubMed] [Google Scholar]
- Zimmerman D., Ownsworth T., O'Donovan A., Roberts J., & Gullo M. J. (2017). Associations between executive functions and mental health outcomes for adults with autism spectrum disorder. Psychiatry Research, 253, 360–363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.