Abstract
OBJECTIVES
The prevalence, profile and prognosis of severe obesity in a large contemporary acute HF (AHF) population was evaluated.
BACKGROUND
Better prognosis has been reported for obese compared to non-obese heart failure (HF) patients, but in other cardiovascular populations, this effect has not been demonstrated for severely obese patients.
METHODS
A cohort of 795 participants with body mass index (BMI) measured at time of admission and complete follow-up were identified from enrollment in 3 contemporary AHF trials (DOSE, CARRESS-HF and ROSE). Patients were stratified into 4 BMI categories according to standard World Health Organization criteria (normal weight, 18.5–25kg/m2 [n=128]; overweight, 25–29.9kg/m2 [n=209]; mild-moderate obese, 30–39.9kg/m2 [n=301]; severe obese, ≥40kg/m2 [n=157]). The relationship between BMI and 60-day composite outcome (death, rehospitalization or unscheduled provider visit) was investigated.
RESULTS
Patients with severe obesity (19.7%) were younger, more often female, hypertensive and diabetic, and more likely to have higher blood pressures and left ventricular ejection fraction, and lower N-terminal pro-B-type natriuretic peptide and troponin I levels than other BMI category patients. Following admission for AHF, patients with normal weight showed the highest risk of the 60-day composite outcome, followed by patients who were severely obese. Overweight and mild-moderately obese patients showed lowest risk.
CONCLUSIONS
Nearly one-fifth of AHF patients enrolled in contemporary randomized clinical trials are severely obese. A U-shaped curve for short-term prognosis according to BMI is seen in AHF. These findings may help to better inform both HF clinical care and future clinical trial planning.
Keywords: Obesity, Severe obesity, Acute decompensated heart failure, Prognosis
CONDENSED ABSTRACT
This study aimed to assess the prevalence, profile, and prognosis of severe obesity in a large, contemporary population derived from 3 randomized clinical trials (n=795 eligible participants) of decongestion strategies in AHF. Severe obesity was identified in 19.7% of participants who showed distinct clinical profiles compared to other BMI category patients. A U-shaped curve for short-term prognosis in AHF according to BMI was demonstrated, with the highest event rates occurring in normal weight and severely obese (BMIs>40kg/m2) patients. These findings may help to better inform both HF clinical care and future trial planning.
Obesity has emerged as a global epidemic, including in the United States, where 69% of adults are now listed as overweight or obese, compared to approximately 25% 40 years ago.(1–3) Importantly, the distribution of obesity according to BMI has also shifted, to the extent that the prevalence of severe obesity (defined by BMI >40kg/m2) has shown an incremental increase compared to that of overweight (BMI 25–29.9kg/m2) or mild-moderate (BMI 30–40kg/m2) degrees of obesity.(1,4) The age-adjusted prevalence of severe obesity in 2013–2014, based on a recently published analysis of data from the National Health and Nutrition Examination Survey, was 7.7% (5.5% for men and 9.9% for women).(5)
Obesity is a well-established risk factor for the development of incident heart failure (HF) often mediated through its strong association with other major cardiovascular risk factors, including atherosclerosis, hypertension, diabetes, dyslipidemia, sleep-disordered breathing and metabolic syndrome.(4) However, obesity is itself independently associated with adverse structural, functional and hemodynamic cardiovascular alterations, mediated primarily through excessive adipose tissue accumulation and related downstream effects on blood volume and adverse cardiac chamber remodeling.(6) This hostile pathophysiological milieu occurring in the setting of higher BMI is in turn most pronounced in those with severe obesity,(4,6) with one classic study of 74 severely obese patients documenting clinical HF in up to one-third of the cohort.(7)
Meanwhile, despite the relationship between obesity and risk of incident HF, once HF is manifest, multiple chronic HF studies have demonstrated a better prognosis for overweight and obese patients with HF compared to normal or underweight HF patients, a phenomenon known as the obesity paradox.(8) This paradox has also been borne out in the acute HF (AHF) setting, both in an earlier large registry study (9) and a more recent combined analysis of 12 global prospective observational cohorts.(10)
However, in other cardiovascular populations, a differential effect of obesity on cardiac outcomes according to the severity of obesity have been reported, with beneficial effects limited to overweight and mild-moderately obese patients.(4,11) Two contemporary large registry studies in acute coronary syndrome (ACS) cohorts have confirmed this “U-shaped” relationship between BMI and mortality, with significant adverse risk also seen in severely obese in addition to normal and underweight patients.(12,13) In chronic HF, a large meta-analysis of 6 studies also found that increasing degree of obesity was not linearly related to cardiovascular outcomes.(14) To date, whether or not a BMI-based U-shaped curve for prognosis exists in AHF patients, and whether it extends to additional outcomes such as readmission risk, has not been systematically studied.
Accordingly, we sought to explore the prevalence, clinical and biomarker profiles, and prognosis of severe obesity in a large, contemporary, well characterized AHF population derived from 3 prospective randomized clinical trials.
METHODS
Study Population
This analysis used data from three prospective, double-blinded, randomized trials (Diuretic Strategies Optimization Evaluation [DOSE], Cardiorenal Rescue Study in Acute Decompensated Heart Failure [CARRESS-HF] and Renal Optimization Strategies Evaluation in Acute Heart Failure [ROSE]) conducted by the National Heart, Lung and Blood Institute-funded Heart Failure Clinical Research Network.(15–17) Each study protocol was approved by the Steering, Protocol Review and Data Safety Monitoring Committees of the Heart Failure Network in addition to individual institutional review boards at each participating site. All study participants provided written informed consent prior to randomization.
Briefly, all three studies were designed to test the efficacy of specific decongestion strategies in patients hospitalized with AHF with objective evidence of congestion, irrespective of left ventricular ejection fraction (LVEF). DOSE-HF randomized 308 patients with AHF to receive intravenous furosemide by either intermittent bolus or continuous infusion administration and using either a low or high-dose strategy.(15) CARRESS-HF randomly assigned 188 patients with both AHF and worsened renal function to either stepped pharmacological therapy or ultrafiltration.(16) Finally, ROSE-HF randomized a total of 360 AHF patients with renal dysfunction (defined by estimated glomerular filtration rate 15–60mL/min/1.73m2) to either low-dose dopamine or low-dose nesiritide, compared with a pooled placebo group, in addition to high-dose intravenous loop diuretics.(17) All trials excluded patients with end-stage chronic kidney disease. For both DOSE-HF and CARRESS-HF, patients requiring intravenous inotropes or vasodilators were excluded.
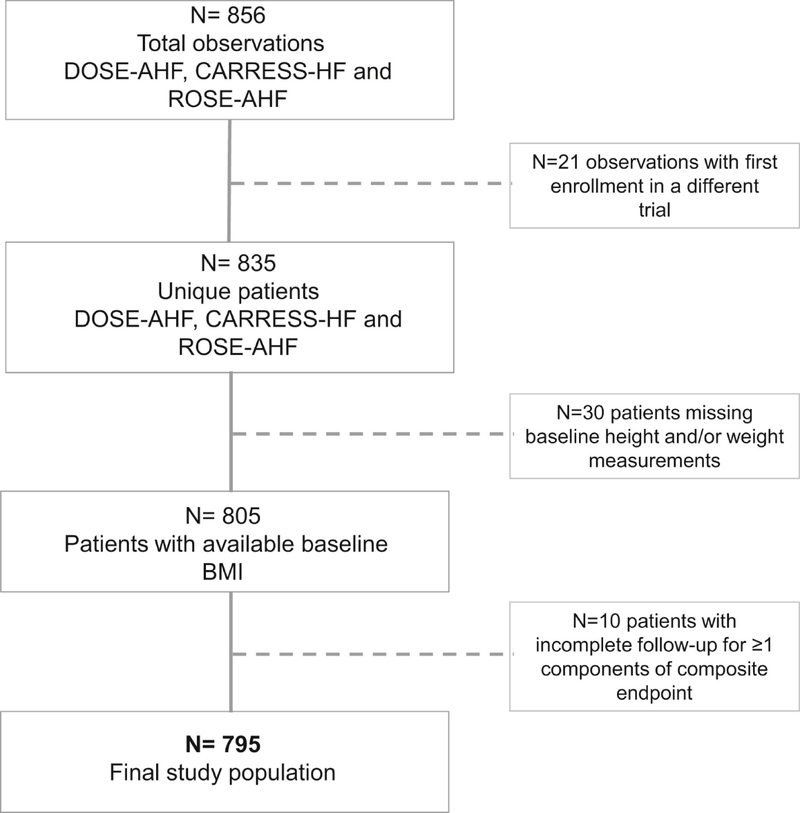
For the present analysis, all non-duplicate participants in each trial with both baseline height and weight measurements available at the time of admission and complete follow-up for outcome assessment, were included (Figure 1). BMI was calculated as weight (kg) divided by height squared (m2).
Figure 1.
The CONSORT Diagram Illustrating Study Cohort Selection
BMI = body mass index; CARRESS-AHF = Cardiorenal Rescue Study in Acute Heart Failure; CONSORT = Consolidated Standards of Reporting Trials; DOSE-AHF = Diuretic Strategies Optimization Evaluation in Acute Heart Failure; ROSE-AHF = Renal Optimization Strategies in Evaluation in Acute Heart Failure.
Biomarkers (serum creatinine, serum sodium, blood urea nitrogen, cystatin C, serum albumin, N-terminal pro-B-type natriuretic peptide [NT-proBNP] and cardiac troponin I) were measured at baseline and analyzed at a core laboratory at the University of Vermont, Burlington, VT.
Follow-up and Study Endpoints
Patients were followed after discharge at pre-specified intervals according to trial design. Clinical outcomes were assessed at 60-days for all patients. The primary outcome analyzed was a composite of death, rehospitalization, and unscheduled provider visit (clinic or emergency room) at 60-days.
Statistical Analysis
The study cohort was stratified into 4 BMI categories based on admission BMI according to standard World Health Organisation (WHO) criteria (normal, 18.5–25kg/m2 [n=128]; overweight, 25–29.9kg/m2 [n=209]; mild-moderate obese, 30–39.9kg/m2 [n=301]; severe obese, ≥40kg/m2 [n=157]). Of note, just 3 patients (0.4%) met WHO criteria for underweight status (<18.5kg/m2); for the purposes of the present analysis these patients were included in the <25kg/m2 category.
Continuous variables were expressed as median (25th, 75th percentile). Categorical variables were expressed as frequencies and percentages. Demographic, clinical and biochemical characteristics at time of AHF admission recorded per each trial protocol were compared across BMI groups using Pearson’s chi-square (categorical variables) or Kruskal-Wallis (continuous variables) statistical tests as appropriate. In the case of a significant p-value across categories, pairwise Wilcoxon rank sum and chi-square tests were performed for each category versus the severely obese group.
After testing whether the proportional hazard assumption was met, the association between 60-day composite outcome (death, rehospitalization or unscheduled provider visit) and BMI was investigated using Cox proportional hazards regression models that adjusted for trial, hypertension, diabetes, HF with preserved (≥50%) LVEF (HFpEF) subtype and diuretic dose in the 24 hours prior to randomization. Linear splines were used to test whether the relationship between BMI and composite endpoint was linear. With a violation of the linearity assumption, predicted probability rates of composite endpoint at 60 days were plotted across BMI values.
Finally, the normal weight and overweight categories were combined and the association of BMI category (<30, 30–40, >40) with the composite endpoint was tested in relevant subgroups and shown with a forest plot. Hazard ratios associated with BMI occurring within the category of 30–40 or >40, compared to <30, were computed. Hazard ratios <1 indicate that particular BMI category has a lower risk of the composite endpoint compared to those with BMI <30. Cox proportional hazards regression models that included the three-category BMI, the subgroup, and BMI category-by-subgroup interaction were used to test for differential associations between BMI and endpoint in various subgroups.
P-values <0.05 were considered statistically significant and there were no adjustments for multiple comparisons. SAS version 9.4 (Cary, NC) was used for all analyses.
RESULTS
Study Population
A total of 795 participants met study inclusion criteria (Figure 1). Baseline characteristics of the patient population according to BMI are shown in Table 1. Discharge or day 7 BMI was available for 94% of enrolled patients; correlation between admission BMI and discharge/day 7 BMI was 0.98.
Table 1.
Baseline characteristics of the total cohort by body mass index category (N=795)
| All patients (N=795) | BMI <25mg/kg2 (N=128) | BMI 25–29.9mg/kg2 (N=209) | BMI 30–39.9mg/kg2 (N=301) | BMI ≥40 (N=157) | p-value* | |
|---|---|---|---|---|---|---|
| Demographic | ||||||
| Age, years | 69 (60, 78) | 76 (66, 83) | 72 (65, 82) | 68 (59, 76) | 63 (54, 73) | <0.0001 abc |
| Male sex | 587/795 (73.8) | 91/128 (71.1) | 172/209 (82.3) | 228/301 (75.7) | 96/157 (61.1) | 0.0001 bc |
| White race | 601/795 (75.6) | 102/128 (79.7) | 165/209 (78.9) | 226/301 (75.1) | 108/157 (68.8) | 0.0929 |
| BMI, kg/m2 | 31.4 (26.7, 37.8) | 23.3 (21.5, 24.3) | 27.5 (26.3, 28.5) | 34.2 (31.8, 36.4) | 46.0 (41.7, 50.4) | <0.0001 abc |
| Ischemic etiology | 473/795 (59.5) | 78/128 (60.9) | 135/209 (64.6) | 177/301 (58.8) | 83/157 (52.9) | 0.1519 |
| Ejection fraction, % | 32 (20, 54) | 25 (19, 42) | 30 (20, 50) | 32 (22, 55) | 43 (25, 55) | <0.0001 abc |
| LVEF ≥50% | 242/788 (30.7) | 30/127 (23.6) | 56/209 (26.8) | 93/297 (31.3) | 63/155 (40.6) | 0.0083 abc |
| Diabetes | 444/795 (55.8) | 38/128 (29.7) | 96/209 (45.9) | 200/301 (66.4) | 110/157 (70.1) | <0.0001ab |
| Atrial fibrillation / flutter | 446/795 (56.1) | 76/128 (59.4) | 124/209 (59.3) | 170/301 (56.5) | 76/157 (48.4) | 0.1555 |
| Hypertension | 651/795 (81.9) | 88/128 (68.8) | 170/209 (81.3) | 249/301 (82.7) | 144/157 (91.7) | <0.0001 abc |
| Clinical | ||||||
| HF-hospitalization in past year | 559/787 (71.0) | 83/128 (64.8) | 147/206 (71.4) | 219/299 (73.2) | 110/154 (71.4) | 0.3741 |
| NYHA class | 0.5541 | |||||
| I/II | 26/744 (3.5) | 8/122 (6.6) | 8/198 (4.0) | 9/277 (3.2) | 1/147 (0.7) | |
| III | 476/744 (64.0) | 78/122 (63.9) | 126/198 (63.6) | 176/277 (63.5) | 96/147 (65.3) | |
| IV | 242/744 (32.5) | 36/122 (29.5) | 64/198 (32.3) | 92/277 (33.2) | 50/147 (34.0) | |
| Heart rate, beats/min | 75 (66, 85) | 74 (69, 87) | 75 (65, 82) | 75.5 (64.5, 85) | 75 (69, 86) | 0.3369 |
| Systolic blood pressure, mmHg | 114 (103, 128) | 109 (100, 121) | 112 (101, 124) | 117 (104, 128) | 118 (109, 137) | <0.0001 ab |
| Edema ≥2+ | 614/793 (77.4) | 83/127 (65.4) | 164/209 (78.5) | 230/301 (76.4) | 137/156 (87.8) | 0.0001 abc |
| Orthopnea | 691/760 (90.9) | 110/121 (90.9) | 183/204 (89.7) | 260/288 (90.3) | 138/147 (93.9) | 0.5588 |
| JVP ≥8cm | 716/759 (94.3) | 118/123 (95.9) | 198/208 (95.2) | 265/288 (92.0) | 135/140 (96.4) | 0.1772 |
| Rales | 446/790 (56.5) | 76/127 (59.8) | 127/207 (61.4) | 175/300 (58.3) | 68/156 (43.6) | 0.0036 abc |
| Medications | ||||||
| ACE inhibitor or ARB | 422/795 (53.1) | 66/128 (51.6) | 101/209 (48.3) | 170/301 (56.5) | 85/157 (54.1) | 0.3231 |
| Hydralazine | 139/795 (17.5) | 13/128 (10.2) | 35/209 (16.7) | 59/301 (19.6) | 32/157 (20.4) | 0.0824 |
| Nitrates | 219/795 (27.5) | 32/128 (25.0) | 60/209 (28.7) | 87/301 (28.9) | 40/157 (25.5) | 0.7598 |
| Beta-blocker | 654/795 (82.3) | 102/128 (79.7) | 175/209 (83.7) | 244/301 (81.1) | 133/157 (84.7) | 0.6075 |
| Aldosterone antagonist | 218/795 (27.4) | 39/128 (30.5) | 56/209 (26.8) | 86/301 (28.6) | 37/157 (23.6) | 0.5702 |
| Digoxin | 190/795 (23.9) | 40/128 (31.3) | 56/209 (26.8) | 66/301 (21.9) | 28/157 (17.8) | 0.0354 ab |
| IV furosemide equivalent diuretic dose in the 24h before randomization | 100 (60, 170) | 100 (60, 160) | 100 (60, 160) | 80 (60, 160) | 120 (80, 222) | 0.0293abc |
| Biochemical | ||||||
| Albumin, g/dL*** | 3.50 (3.10, 3.90) | 3.60 (3.10, 3.99) | 3.50 (3.10, 3.90) | 3.50 (3.12, 3.80) | 3.50 (3.20, 3.90) | 0.6854 |
| Creatinine, mg/dl | 1.63 (1.27, 2.05) | 1.56 (1.23, 1.90) | 1.69 (1.29, 2.16) | 1.60 (1.27, 1.98) | 1.66 (1.28, 2.25) | 0.1794 |
| Cystatin C, mg/L | 1.72 (1.37, 2.20) | 1.61 (1.33, 2.19) | 1.81 (1.40, 2.25) | 1.69 (1.37, 2.13) | 1.84 (1.45, 2.32) | 0.1432 |
| Sodium, mg/L | 138 (136, 141) | 137 (135, 140) | 138 (135, 141) | 139 (136, 141) | 139 (136, 141) | 0.0001 ab |
| GFR, mL/min/1.73m2 | 41.2 (30.8, 55.1) | 41.0 (30.7, 57.2) | 39.8 (30.2, 54.0) | 42.7 (32.9, 54.4) | 40.4 (29.5, 57.9) | 0.5461 |
| BUN, mg/dl | 38 (26, 55) | 38 (25, 57) | 41 (28, 56) | 38 (26, 53) | 33 (24, 50) | 0.1585 |
| NT-proBNP, pg/ml | 4519 (2301, 10064) | 8447 (5057, 18339) | 7352 (3410, 12323) | 3865 (2060, 7951) | 2106 (1145, 4357) | <0.0001 abc |
| Troponin I (pg/mL)** | 23.9 (13.8, 47.6) | 25.1 (15.7, 52.9) | 29.7 (14.9, 57.2) | 24.9 (14.6, 45.6) | 17.1 (11.1, 30.1) | 0.0015 abc |
Represents differences across groups using X2 or Kruskal-Wallis as appropriate.
Available for n=430 patients.
Available for 539 patients.
indicates a significant difference in the normal weight and severe obese categories.
indicates a significant difference in the overweight and severe obese categories.
indicates a significant difference in the mild-moderate obese and severe obese categories.
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; BUN, blood urea nitrogen; CARRESS, Cardiorenal Rescue Study in Acute Decompensated Heart Failure; DOSE, Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure; GFR, glomerular filtration rate; HF, heart failure; JVP, jugular venous pressure; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid-receptor antagonist; ROSE, Renal Optimization Strategies Evaluation; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association.
Prevalence and Profile of Severe Obesity
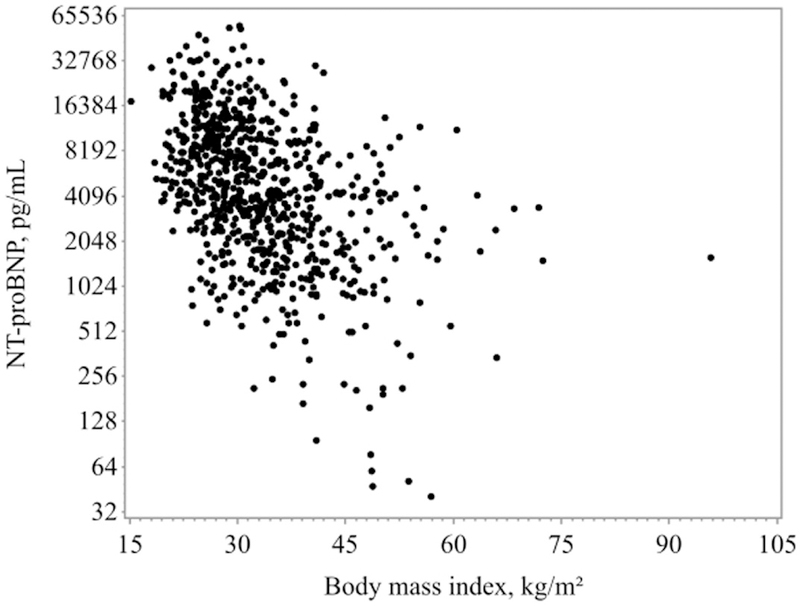
Almost 20% (n=157, 19.7%) of the total cohort were classified as severely obese. These patients were younger, more likely female, and were more likely to be hypertensive and diabetic than their lower BMI counterparts. They also had higher LVEF and a greater proportion of HFpEF patients compared to the other BMI categories. These patients were also significantly more likely to be taking a higher diuretic dose in the 24 hours prior to trial randomization. Severely obese patients also differed by admission examination profile: systolic blood pressures were higher, edema ≥2+ was more likely and rales less likely than for normal weight, overweight or mild-moderately obese patients. Biochemical testing showed that those with a BMI ≥40kg/m2 also had significantly lower NT-proBNP (p<0.0001) and cardiac troponin I (p=0.0015) versus the lower BMI patients. Figure 2 illustrates the relationship between BMI and NT-proBNP (log-transformed). These same relationships were significant in pairwise tests of mild or moderately obese versus severe obese categories with the exception of systolic blood pressure and diabetes prevalence which did not differ significantly when specifically comparing the different levels of obesity groups.
Figure 2.
Relationship Between BMI and NT-proBNP in Acute Heart Failure
Graph of the log2 transformed NT-proBNP versus BMI showing an approximately linear relationship. Spearman’s correlation coefficient = −0.47 (p < 0.0001). NT-proBNP = N-terminal pro–B-type natriuretic peptide; other abbreviation as in Figure 1.
Post-hospitalization Outcome and Severe Obesity
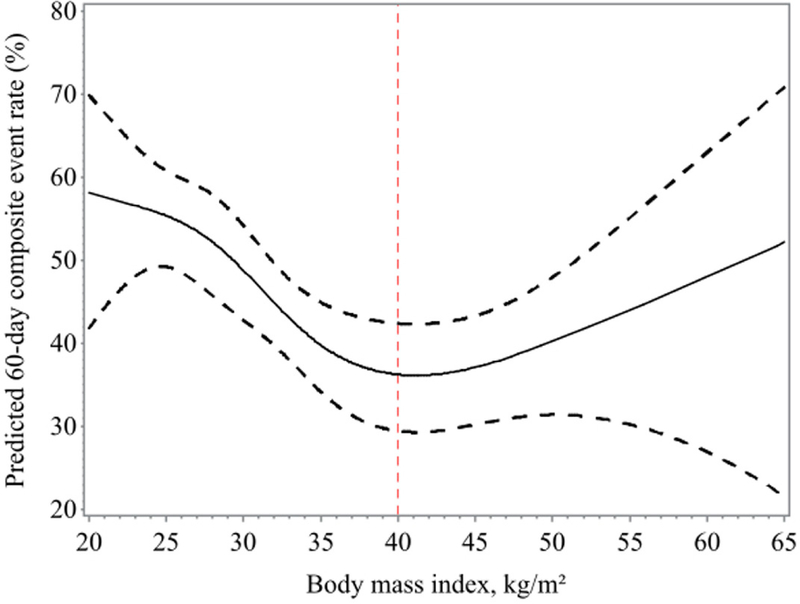
At 60-days, 353 patients developed ≥1 adverse event, (12.7% [n=45] deaths without rehospitalization, 76.8% [n=271] rehospitalizations or unscheduled provider visits without death, and 10.5% [n=37] hospitalizations or unscheduled provider visit followed by death). As shown in Figure 3, following an admission for AHF, the lowest BMI patients had the highest risk of 60-day adverse events (BMI 25: 55.4%, 95%CI 49.2–60.8). Risk then declined as BMI progressively increased and reached a nadir in those with a BMI of 40kg/m2 (36.2%, 95%CI 29.4–42.4). Above a BMI of 40kg/m2, the risk of the composite event rate rose again and continued to increase linearly in patients with BMIs of >40kg/m2 (BMI 50: 40.3%, 95%CI 31.4–48.0), confirming a U-shaped relationship between BMI and short-term adverse events in the AHF population.
Figure 3.
Predicted 60-Day Primary Composite Event Rates
Predicted rates for the primary composite endpoint of death, rehospitalization, or unscheduled provider visit at 60 days (N = 353 events). A U-shaped relationship is demonstrated between BMI and short-term adverse events in the AHF population. AHF = acute heart failure; other abbreviation as in Figure 1.
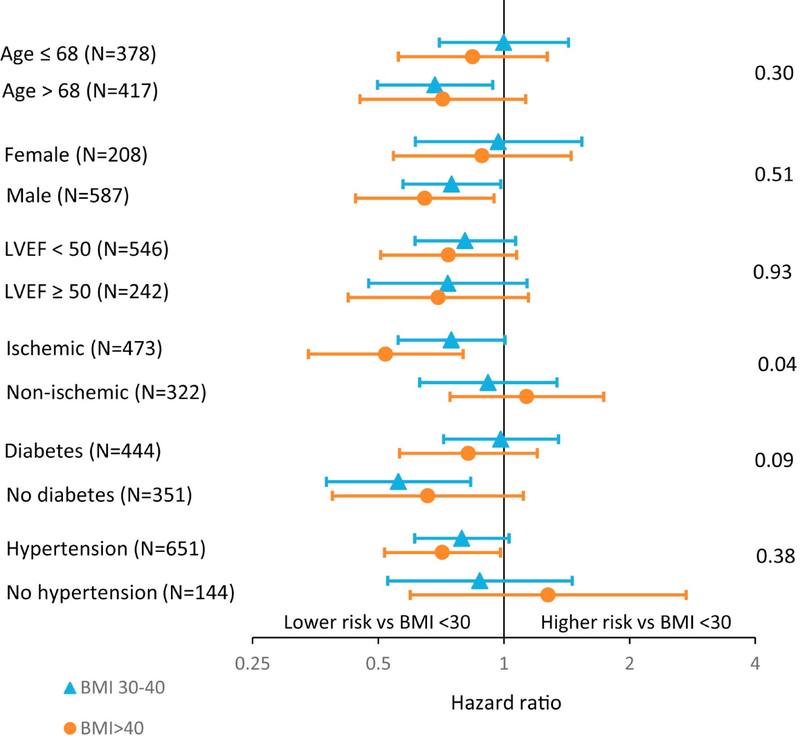
Forest plots depicting the relationship between important subgroups (age, gender, LVEF <50 versus ≥50%, ischemic etiology of HF, presence of diabetes or hypertension) and the composite endpoint according to BMI category (<30, 30–40, >40) are shown in Figure 4. The only evidence of a significant interaction (indicating that the relationship between the subgroup and the composite endpoint is different according to BMI category) occurred for ischemic versus non-ischemic etiology. In patients with ischemic heart disease, BMI>40kg/m2 conferred a lower risk of the composite endpoint compared to having a BMI <30kg/m2. For patients without ischemic heart disease, there was no association between BMI category and the composite endpoint. No other subgroup interaction terms including whether LVEF was preserved or reduced reached significance (Figure 4).
Figure 4.
Forest Plots Show Relationship Between Subgroups and Composite Endpoint According to BMI category
Forest plots show relationship between relevant subgroups (age, sex, LVEF <50% vs ≥50%, ischemia cause, presence of diabetes or hypertension) and the composite endpoint according to BMI category (<30, 30–40, >40 kg/m2), with BMI <30 kg/m2 serving as the reference group. Markers to the left of 1 indicate whether patients in that particular BMI category have a lower risk of composite endpoint than those with BMI <30 kg/m2. Interaction p values are shown. The only subgroup that showed a relationship different from that of the composite endpoint according to BMI category was ischemia versus nonischemia cause. LVEF = left ventricular ejection fraction; other abbreviation as in Figure 1.
DISCUSSION
The present analysis confirms a high prevalence of severe obesity in contemporary AHF clinical trial populations. Almost one-fifth of patients enrolled in these recent trials of decongestion strategies in hospitalized HF patients were severely obese with a BMI>40kg/m2. Severely obese patients admitted with AHF had distinctive clinical (more likely younger, female, diabetic, hypertensive and more edema on presentation) and biochemical (lower NT-proBNP and troponin I) profiles. Post-discharge, a U-shaped relationship was confirmed between BMI and short-term adverse outcomes. This relationship was maintained in almost all relevant subgroups, including preserved or reduced LVEF groups.
Prevalence of Severe Obesity in AHF
The increasing prevalence of both obesity and heart failure recently observed has led to growing recognition of the inextricable links between the two conditions, which are not entirely explained by mutually associated risk factors including diabetes and hypertension.(18) The impact of severe obesity on the prevalence of HF is becoming especially relevant given the shift in distribution in the profile of obesity, with disproportionate rise in severe obesity compared to milder degrees.(1–3) Despite this, very little is known about the prevalence of severe obesity in contemporary heart failure populations, including AHF. The Acute Decompensated Heart Failure National (ADHERE) registry analyzed 108,927 patients admitted with AHF from 2001–2004 according to quartiles of BMI and found an overall obesity prevalence of 38%. While no specific breakdown of severe obesity was provided, 25% of the population had a BMI ranging from 33.4–60kg/m2.(9) The present analysis, derived from a more recent randomized clinical trial cohort (patients enrolled between 2008–2013) found an overall obesity prevalence of 58%, with over one-third of these - 20% of the total population –fulfilling criteria for severely obese status. Notably, only 16% of patients were classified as “normal” weight based on BMI. The striking emergence of severe obesity in contemporary AHF highlights the need to better define clinical profiles and prognosis for these patients, so that both clinical care and research efforts can be better focused to improve their outcomes.
Clinical Profile of Severe Obesity in AHF
Prior studies in obese populations including those in AHF have demonstrated increased prevalence of both hypertension and diabetes in obese compared to non-obese participants. (9,10,12) In the current study, severely obese patients had the highest overall prevalence of these cardiovascular risk factors. The increased incidence of hypertension in severely obese compared to mild-moderately obese patients has been previously documented in epidemiological studies and associated with higher systemic vascular resistance, compensatory concentric LV hypertrophy, and ultimately LV diastolic dysfunction and HF.(11) Not surprisingly, in our study population, severely obese patients were more likely to have HFpEF than the other BMI categories, an observation also reported in the ADHERE registry (9) and, as suggested by recent data (19), severe obesity itself is likely to be a contributor to the HFpEF syndrome.
A distinct profile for severely obese HF patients compared to less obese or non-obese patients is also suggested by their different biomarker profiles. The reduced expression of B-type natriuretic peptides has been consistently shown in obese HF populations, both in chronic stable outpatients and in AHF patients.(9,10,20) In the current analysis severely obese patients had significantly lower levels of NT-proBNP compared to mild-moderately obese patients. Expressed graphically, an approximate linear relationship was confirmed between BMI and NT-proBNP in this sizeable cohort of AHF patients. This relationship was seen despite the fact that severely obese patients were significantly more likely to be taking a higher diuretic dose in the 24 hours prior to trial randomization, underlining the difficulties associated with using this biomarker to guide clinical congestion in this patient group. Notably, none of the enrollment criteria for any of the 3 clinical trials included NT-proBNP; given the variation in levels according to BMI category, with overall similar clinical AHF syndromes, these findings suggest use of this biomarker to define contemporary HF populations for research or clinical therapy may result in unintended skewing of patient populations by weight. Cardiac troponins are well established as prognostic biomarkers in AHF (21,22); therefore it is noteworthy that severely obese patients, who had poorer outcomes, had significantly lower detected levels of cardiac troponin I to all other BMI groups, including mild-moderately obese patients. Other studies in AHF have also found decreased levels of troponin I in an all-comer obese (BMI >30) population compared to overweight and normal weight patients.(10)
Relationship between Severe Obesity and Outcome after AHF
The present study extends the findings of large ACS registries indicating a U-shaped rather than linear relationship between BMI and outcome.(12,13) It is important to point out that in contrast to these large ACS registries and other cardiovascular-centered obesity paradox studies, the endpoint used in the present study was a composite one which incorporated readmission and unscheduled provider visit rates as well as mortality rates. Given the short-term post-hospitalization setting, the majority of events (87%) were driven by this component of the endpoint. Readmission rates after AHF remain a critical target for clinical outcomes improvement in HF and given the significant prevalence of severe obesity in this cohort, focusing on this composite endpoint with respect to BMI is particularly relevant.
Although lowest risk for this composite endpoint was seen in overweight and mild-moderately obese patients, event rates rose sharply again in patients with BMIs >40, illustrating the presence of this U-shaped relationship for short-term prognosis according to BMI in modern-era AHF populations. A potential shift in this curve to the right over time is suggested when compared to previous clinical trial data both in chronic HF as well as ACS registry data, both of which found mortality increasing as BMI rose >35.(13,23) Notably, the global registry of AHF patients (n=6,142) also found that when grades of obesity were looked at separately, those with BMI >40 did not have an equivalent or lower hazard of all-cause mortality relative to normal weight patients.(10) The reasons for this perceived shift are outside the scope of the present analysis, but it is notable that it is occurring in parallel with the shift in the distribution of obesity in the overall population, with greater proportion of severely obese patients. It may also reflect unique pathophysiological differences between AHF and other cardiovascular disease states; perhaps a mildly higher BMI continues to be ‘beneficial’ in these patients up to a threshold, which appears to be occur between 40–45. Notably the relationship between BMI and early post-discharge outcome in AHF was maintained across most relevant subgroups, including whether LVEF was preserved or reduced. Only in patients with HF of ischemic etiology was a lower risk of adverse outcome seen in those with BMI >40 compared to BMI<30 patients.
These findings may have clinical implications not only for AHF care planning and outcomes but also for future advanced therapy utilization and outcomes. Severely obese patients were found to be significantly younger than the other groups, with a median age of 63 years. Again this is a consistent finding for all-comer obese patients in HF studies (9,10); however it is a particularly notable finding alongside the prevalence of this population in contemporary AHF trials given the potential consequences for the future profile of candidates for advanced therapies, including mechanical circulatory support (MCS) and cardiac transplantation. Presently, severe obesity remains a relative contraindication for listing for cardiac transplantation (24). MCS devices may be required to support these patients while weight loss is attempted or ultimately as destination therapy; however, clinical trials of MCS devices have typically excluded those with a BMI ≥40kg/m2.(25)
Limitations
Several limitations should be acknowledged. This was a post hoc analysis of 3 randomized clinical trials in AHF patients testing differential hypotheses using different interventions. However, all studies required systematic phenotyping of enrolled patients at time of admission for AHF including BMI measurement and subsequent systematic follow-up of identical outcomes for equivalent time periods. Moreover, heterogeneity in clinical profiles and comorbidity burdens across trial populations - specifically, CARRESS-HF and ROSE patients were a “sicker” AHF population than DOSE patients based mainly on renal function – could also have influenced event rates across individual trials. However, the regression models used for outcome analysis were adjusted for individual trial as well as for relevant clinical variables that differed between BMI groups. Given that the study population was exclusively derived from trial populations with inherent exclusion criteria, results may not be generalizable to an all-comer AHF population, including those with hemodynamic instability or more severe degrees of renal impairment. Notably, only 3 of the 795 participants (0.38%) were underweight as defined by WHO criteria (BMI <18.5); these patients were included in the <25 category. It is unclear whether the under-representation of underweight patients across these 3 contemporary randomized clinical AHF trials represents a selection bias inherent in the original trial designs (such as exclusion of patients with cancer and other severe comorbidities leading to exclusion of these patients by default) and/or whether it simply represents a reflection of the dominant prevalence of patients with higher ranges of BMI in contemporary HF populations. BMI is a surrogate rather than a direct measure of total body fat, which may be more accurately reflected by anthropometric measures such as waist circumference or direct body fat measuring modalities. However, BMI and alternative measures of fat mass have been shown to be highly correlated;(26) furthermore the obesity paradox has also been demonstrated in HF populations using both waist circumference and percent body fat.(27,28) Finally, the outcomes analyses and related conclusions are limited by the shorter-term follow-up (60 days) pre-specified for these clinical trials; future prospective studies investigating longer-term outcomes according to BMI in contemporary HF cohorts are needed.
Conclusions
Almost one-fifth of AHF patients enrolled in contemporary clinical trials are severely obese, and represent a phenotypically different cohort to AHF patients with lower BMIs. The U-shaped curve for short-term outcome according to BMI shown in other cardiovascular populations is also demonstrated in AHF patients, but has shifted to the right, with the lowest event rates occurring in patients with a BMI around 40kg/m2, and highest rates in normal weight patients followed by those with BMIs>40kg/m2. These findings have implications for both future clinical trial planning in HF populations as well as for real-world HF clinical care.
CLINICAL PERSPECTIVES
Almost one-fifth of patients enrolled in contemporary clinical trials of decongestion strategies in AHF are severely obese, as defined by a BMI ≥40kg/m2. Severely obese HF patients have distinctive clinical and biochemical profiles on admission with AHF. A U-shaped curve for short-term prognosis according to BMI is seen in AHF, with the lowest risk seen in overweight and mild-moderate obese patients compared to normal weight patients not maintained in severe obese patients.
TRANSLATIONAL OUTLOOK
Improved profiling of contemporary HF patients enrolling in randomized clinical trials may help to better inform future clinical trial planning in this important population as well as help to better direct real-world HF clinical care. The increasing prevalence of severe obesity in HF patients has major clinical implications for assessment, biomarker profile interpretation and management strategies in these patients, including downstream candidacy and utilization of mechanical circulatory support and heart transplantation.
ABBREVIATIONS AND ACRONYMS
- ACS
acute coronary syndrome
- ADHERE
Acute Decompensated Heart Failure National registry
- AHF
acute heart failure
- BMI
body mass index
- CARRESS-HF
Cardiorenal Rescue Study in Acute Decompensated Heart Failure
- CI
confidence interval
- DOSE
Diuretic Strategies Optimization Evaluation
- HF
heart failure
- HFpEF
heart failure with preserved left ventricular ejection fraction
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- MCS
mechanical circulatory support
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- ROSE
Renal Optimization Strategies Evaluation
- WHO
World Health Organisation
Footnotes
Conflicts of Interest/Disclosures: The authors have no conflicts of interest relevant to the current manuscript to disclose. Drs Joyce, Groarke and Stevenson were supported from Grant U10HL110337. Dr Cooper was supported by grant T32HL069749-11A1 from the NIH.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA 2002;288:1723–7. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014;311:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol 2009;53:1925–32. [DOI] [PubMed] [Google Scholar]
- 5.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA 2016;315:2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiological reviews 2008;88:389–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alpert MA, Terry BE, Mulekar M et al. Cardiac morphology and left ventricular function in normotensive morbidly obese patients with and without congestive heart failure, and effect of weight loss. Am J Cardiol 1997;80:736–40. [DOI] [PubMed] [Google Scholar]
- 8.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol 2001;38:789–95. [DOI] [PubMed] [Google Scholar]
- 9.Fonarow GC, Srikanthan P, Costanzo MR et al. An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108,927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J 2007;153:74–81. [DOI] [PubMed] [Google Scholar]
- 10.Shah R, Gayat E, Januzzi JL Jr., et al. Body mass index and mortality in acutely decompensated heart failure across the world: a global obesity paradox. J Am Coll Cardiol 2014;63:778–85. [DOI] [PubMed] [Google Scholar]
- 11.Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail 2013;1:93–102. [DOI] [PubMed] [Google Scholar]
- 12.Das SR, Alexander KP, Chen AY et al. Impact of body weight and extreme obesity on the presentation, treatment, and in-hospital outcomes of 50,149 patients with ST-Segment elevation myocardial infarction results from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol 2011;58:2642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angeras O, Albertsson P, Karason K et al. Evidence for obesity paradox in patients with acute coronary syndromes: a report from the Swedish Coronary Angiography and Angioplasty Registry. Eur Heart J 2013;34:345–53. [DOI] [PubMed] [Google Scholar]
- 14.Sharma A, Lavie CJ, Borer JS et al. Meta-analysis of the relation of body mass index to all-cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol 2015;115:1428–34. [DOI] [PubMed] [Google Scholar]
- 15.Felker GM, Lee KL, Bull DA et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011;364:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bart BA, Goldsmith SR, Lee KL et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367:2296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen HH, Anstrom KJ, Givertz MM et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013;310:2533–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenchaiah S, Evans JC, Levy D et al. Obesity and the risk of heart failure. N Engl J Med 2002;347:305–13. [DOI] [PubMed] [Google Scholar]
- 19.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nature reviews Cardiology 2014;11:507–15. [DOI] [PubMed] [Google Scholar]
- 20.Mehra MR, Uber PA, Park MH et al. Obesity and suppressed B-type natriuretic peptide levels in heart failure. J Am Coll Cardiol 2004;43:1590–5. [DOI] [PubMed] [Google Scholar]
- 21.Peacock WFt, De Marco T, Fonarow GC et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med 2008;358:2117–26. [DOI] [PubMed] [Google Scholar]
- 22.Felker GM, Hasselblad V, Tang WH et al. Troponin I in acute decompensated heart failure: insights from the ASCEND-HF study. Eur J Heart Fail 2012;14:1257–64. [DOI] [PubMed] [Google Scholar]
- 23.Kenchaiah S, Pocock SJ, Wang D et al. Body mass index and prognosis in patients with chronic heart failure: insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 2007;116:627–36. [DOI] [PubMed] [Google Scholar]
- 24.Mehra MR, Kobashigawa J, Starling R et al. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates−−2006. J Heart Lung Transplant 2006;25:1024–42. [DOI] [PubMed] [Google Scholar]
- 25.Slaughter MS, Pagani FD, Rogers JG et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant 2010;29:S1–39. [DOI] [PubMed] [Google Scholar]
- 26.Turkbey EB, McClelland RL, Kronmal RA et al. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA). JACC Cardiovasc Imaging 2010;3:266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavie CJ, Osman AF, Milani RV, Mehra MR. Body composition and prognosis in chronic systolic heart failure: the obesity paradox. Am J Cardiol 2003;91:891–4. [DOI] [PubMed] [Google Scholar]
- 28.Clark AL, Fonarow GC, Horwich TB. Waist circumference, body mass index, and survival in systolic heart failure: the obesity paradox revisited. J Card Fail 2011;17:374–80. [DOI] [PubMed] [Google Scholar]