Abstract
Previous research has demonstrated long-term deficits in neurocognitive function in individuals with a history of sport-related concussion. The purpose of this study was to examine the relationship between a history of concussion and behavioral and event-related potential (ERP) indices of pre- and post-response conflict and error monitoring. A secondary aim was to determine whether years of high risk sport participation were related to impairments in these cognitive control processes. Forty-seven former athletes (age = 20.8 ± 2.2 years) with (n = 25; 5 females) and without (n = 22; 9 females) a history of concussion completed a modified flanker task while behavioral performance, N2, error-related negativity (ERN), and error positivity (Pe) components were assessed. An increase in pre-response conflict (N2) and post-response error-related (ERN) brain activity was observed in individuals with a prior sport-related concussion relative to non-concussed controls; however, no behavioral performance differences were found between groups. No significant associations were found between ERP and behavioral measures and the number of years of high-risk sport participation; however, time since last injury was associated with shorter N2 latency. Together, these findings suggest a persistent impairment in cognitive control and error-related processing in individuals with a history of concussion. These findings are interpreted within the framework of the compensatory error-monitoring hypothesis.
Keywords: concussion, cognitive control, ERN, event-related potential, sport
1. Introduction
Sport-related concussions have received increasing media attention, in part due to their high prevalence rates and potential for long-term consequences. Although an estimated 1.6 to 3.8 million sport-related concussions occur annually in the United States (Langlois et al., 2006), many of these mild traumatic brain injuries (mTBIs) go unreported (Meehan et al., 2013). Despite this underreporting, concussion incidence rates have increased over the past two decades in part due to the increased awareness and improved diagnostic criteria surrounding these injuries (Clark and Guskiewicz, 2016). A recent meta-analysis of 57 studies demonstrated that a history of TBI, including concussions, is associated with increased risk for Alzheimer’s disease, Parkinson’s disease, mild cognitive impairment, depression, mixed affective disorders, and bipolar disorder (Perry et al., 2016). Therefore, advancing understanding of the dynamic process of brain recovery and the potential for intervention following injury remains paramount.
Concussions are often associated with a diverse range of neuropathological symptoms that affect normal, healthy functioning. Many of these symptoms (e.g., headache, balance problems, feeling “in a fog”) appear immediately, while others may not be observable for days or even months following injury (Centers for Disease Control and Prevention [CDC], 2016). However, most of these symptoms gradually resolve and observable neurological status typically returns to baseline levels within 7–10 days following injury (Harmon et al., 2013; Pontifex et al., 2009). Although there is rapid restoration of symptomatology following an acute injury, there are growing concerns about the potential long-term effects of sport-related concussions on brain and cognitive function. Indeed, evidence suggests that cognitive impairment may persist much longer than subjective symptoms following a concussion (e.g., Harmon et al., 2013; McCrea et al., 2003). Unfortunately, the majority of studies examining the relationship between a history of concussion and cognitive function have relied solely on behavioral performance measures of reaction time and response accuracy and standard neuropsychological tests, such as the Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT). This is problematic since these behavioral measures may not have the requisite sensitivity and specificity to reveal subtle, persistent impairments in cognition after symptoms have resolved (Broglio et al., 2006; Guskiewicz et al., 2002; Iverson, 2005). Thus, enhancing the reliability and precision of neurocognitive testing following concussion remains a priority.
To advance clinical practice, experts have recommended combining sensitive neuroscientific techniques with neuropsychological and symptom-based assessments (Slobounov et al., 2012). Numerous neuroscientific techniques, including electroencephalography (EEG), functional magnetic resonance imaging (fMRI), diffusion tensor imaging (DTI), positron emission tomography (PET), and magnetic resonance spectroscopy (MRS) offer promise for advancing the clinical management of concussions. Event-related potentials (ERPs) represent one particularly useful approach to document subtle neurocognitive deficits following concussion (see Broglio et al., 2011 for a review). ERPs reflect voltage fluctuations in the ongoing EEG that are time-locked to an event, such as the presentation of a visual stimulus or execution of a manual response (Kappenman and Luck, 2012). Importantly, the millisecond-level resolution of the ERP technique allows for the detection of subtle changes in the stream of information processing to be isolated and quantified (Kappenman et al., 2016; Moore et al., 2017). ERPs have allowed for the identification of select alterations in sensory, motor, and cognitive functions following concussive injuries (Broglio et al., 2011).
Numerous studies examining different ERP components have been conducted to enhance our understanding of the immediate and potential delayed consequences of sport-related concussions (Broglio et al., 2011; Ellemberg et al., 2009; Moore et al., 2015; Pontifex et al., 2009). The majority of studies in this area have focused on the P3 (P300 or P3b) component, suggesting that it may serve as an ERP index of chronic cognitive impairments associated with a history of concussion (Broglio et al., 2011). Recently, the stimulus-locked N2 and response-locked error-related negativity (ERN) components have also received considerable research attention due to their relevance to cognitive control. Cognitive control is a broad term used to describe the set of mental functions or operations involved in guiding thoughts and actions in the service of goal-directed behaviors, and importantly, may be particularly affected by concussion history (Pontifex et al., 2009). The N2 and ERN components are most often elicited during cognitive control tasks involving inhibition (e.g., the flanker task), where individuals must override and control a strong internal disposition or external lure (i.e., stimuli) to successfully complete a particular goal (Folstein and Van Petten, 2008). The N2 is a negative deflection in the stimulus-locked waveform with a frontocentral scalp distribution that peaks approximately 250–350 ms after stimulus presentation (Botvinick et al., 2004; Clawson et al., 2013; Folstein and Van Petten, 2008) while the ERN is a negative deflection in the response-locked waveform that occurs within 100 ms after the commission of an error (Gehring et al., 2012; Holroyd et al., 1998). Previous evidence suggests that the N2 represents pre-response conflict generated by activation of competing response options, such as the target stimulus and flanking stimuli during a typical flanker task (Larson et al., 2014; Olvet and Hajcak, 2008). Thus, the N2 component relates to the process of conflict monitoring immediately prior to task completion and is typically more negative for trials of higher conflict (i.e., incongruent relative to congruent flanker trials; Folstein and Van Petten, 2008). The ERN, on the other hand, represents an index of post-response conflict generated by a competing mental representation of an error response and a subsequent corrective response prompted by the target stimulus (Larson et al., 2014). Previous research suggests that the ERN represents neural activity signaling the need to adjust behavior and upregulate cognitive control processes for subsequent performance (Falkenstein et al., 1991; Gehring et al., 1993; Holroyd and Coles, 2002). More specifically, the ERN is thought to function as an ‘alarm’ that signals from the anterior cingulate cortex (ACC) and supplementary motor regions of the medial prefrontal cortex (mPFC) to the lateral PFC that an error has occurred, in order to optimize subsequent performance (Moran et al., 2015; Shenhav et al., 2013). In addition, almost immediately following the ERN, a positive deflection is observed following error trials and is referred to as the error positivity (Pe). The Pe is maximal approximately 200–400 ms after error commission (Falkenstein et al., 2003; Nieuwenhuis et al., 2001) and has been suggested to reflect error awareness (Leuthold and Sommer, 1999; Nieuwenhuis et al., 2001), an affective or emotional response (Falkenstein et al., 2000), or a P3-like orienting response to errors (Hajcak, McDonald, & Simons, 2003). Relative to the N2 or ERN, the Pe component has received much less attention in ERP studies among individuals with a history of concussion.
In a recent study examining the potential long-term consequences of pediatric concussion, children who previously suffered a concussion exhibited increased amplitude and longer latency of the N2 during a modified flanker task (Moore et al., 2015). These findings were interpreted as impairments in monitoring and resolving stimulus conflict, indicating subtle but persistent deficits in attention and cognitive control processes. More recently, Ledwidge and Molfese (2016) found no between-group differences among 44 varsity football athletes with and without a history of concussion on neuropsychological tests or behavioral performance measures during an auditory oddball task. However, athletes with a concussion history exhibited significantly larger N2 amplitudes, suggesting increased recruitment of inhibitory control processes in order to successfully meet task demands. In contrast, Broglio et al. (2009) reported smaller N2 amplitudes elicited by a three-stimulus oddball task among a group of young athletes with a self-reported history of concussion relative to those athletes who reported no previous concussion history. Moreover, a number of previous studies have not found differences in N2 amplitude or latency to be associated with a history of concussion (e.g., Gaetz et al., 2000; Gosselin et al., 2012; Moore et al., 2016). Importantly, although the N2 is elicited during various tasks such as the go/no-go, stop signal, and oddball paradigm, the N2 elicited by these tasks may reflect different cognitive processes such as response inhibition, target probability, perceptual novelty, and mismatch (Folstein & Van Petten, 2008). This evidence led Larson et al. (2014) to conclude that “not all N2s are created equally” and to recommend caution when attempting to compare N2 findings across different cognitive tasks or paradigms. Collectively, the initial findings are mixed relative to the impact of a previous concussion history on long-term impairment of pre-response cognitive control processes, as indexed by N2.
Findings related to the post-response ability to detect errors and adaptively regulate behavior in a changing environment (reflected by the ERN) are also currently mixed. For instance, young adults with a history of concussion (average of 2.9 years since last injury) had a significantly smaller flanker ERN amplitude compared to non-concussed, otherwise healthy controls, even in the presence of normal functioning on the ImPACT test (Pontifex et al., 2009). Interestingly, a negative association was found between the number of previous concussions and ERN, such that an increased number of reported concussions was associated with lower ERN amplitudes. In a recent study of pediatric concussion, Moore et al. (2015) demonstrated decreased flanker ERN amplitudes in children with a history of concussion, with significantly larger ERN group differences for the more difficult incongruent flanker task condition, suggesting long-term impairments for tasks that require greater amounts of cognitive control. In contrast, Larson et al. (2012) used a modified color-naming version of the Stroop task and found no significant differences in ERN amplitudes between 36 individuals with a history of mTBI from sports-related incidents (n = 25; 69%), falls (n = 7; 19%), motor vehicle accidents (n = 2; 6%), and other accidents (n = 2; 6%) and 46 neurologically-healthy controls. Differences between these studies could be due to a number of moderating variables that have yet to be examined in the concussion and neurocognitive function relationship. For instance, individual differences due to age, gender, variability in concussion severity, and the relative influence of subconcussive “hits” may all influence the relationship between concussion and ERP component amplitude. Critically, no published study to date has examined both pre- and post-response conflict processes using N2 and ERN components, respectively, to advance understanding of whether or not a history of concussion results in lasting and meaningful impairment to cognitive control processes. These two components have been shown to be dissociated, and Larson and colleagues (2014) have recommended that both ERP components be studied in unison for a more comprehensive understanding of pre- and post-response cognitive control operations among psychiatric and/or neurologic patients.
Therefore, the aim of this study was to determine the relationship between a history of sport-related concussion on pre- and post-response conflict and error-related performance monitoring, as indexed by N2 and ERN and Pe, respectively. In line with previous research that used a flanker paradigm (Moore et al., 2015; Pontifex et al., 2009), individuals with a history of concussion were hypothesized to exhibit alterations in N2 amplitude and latency compared to controls. We also predicted differences in ERN amplitude among the concussed group, suggestive of impairments in error-related brain activity, while we expected no group differences in Pe amplitude, which is in line with previous findings (Larson et al., 2007; Pontifex et al., 2009). Additionally, in accordance with recent research addressing the potential influence of repetitive sub-concussive impacts on neurocognitive function (Moore et al., 2017), we examined the relationship between years of high risk sport participation on behavioral and ERP indices. Years of high risk sports participation were hypothesized to be negatively correlated with N2 and ERN amplitudes, suggesting that those with a greater number of years playing sports considered at high risk for accumulating subconcussive impacts (e.g., football, hockey, soccer) would demonstrate deficits in conflict and error-related performance monitoring processes. Given the high level of cognitive reserve inherent in the age of our sample, behavioral and ImPACT performance measures were not expected to differ between individuals with and without a history of sport-related concussion. However, we also evaluated post-error behavioral adjustments of accuracy and reaction time following error responses, as well as a measure of intra-individual variability of behavioral performance (coefficient of variation of reaction time [CVRT]). These measures have been used to reveal subtle, but important differences in the absence of differences in overall accuracy or latency measures (Moore et al., 2015; Pontifex et al., 2015).
2. Methods
2.1. Participants
Forty-seven individuals between the ages of 18 and 30 were recruited from Rutgers, The State University of New Jersey and the surrounding community through the use of advertisements and social media. All participants included in the study were native English speakers, reported normal or corrected-to-normal visual acuity, and played high school varsity sports. Exclusion criteria included a diagnosis of attention deficit hyperactivity disorder (ADHD), any brain injury unrelated to sport, or psychotropic medication use. Participants were separated into two groups (concussed, control) based on a previous history of a physician-diagnosed concussion. Participants in the concussed group (n = 25; 5 females) were screened to confirm that the diagnosed concussion resulted during participation in the sporting activity and that they were symptom-free at the time of testing. The control group (n = 22; 9 females) consisted of young adults with a similar sport participation background, but without a history of concussion or head injury resulting in a loss of consciousness. Similar to the Moore et al. (2014) study, all participants were asked two additional questions related to experiencing any previous head injury and concussion-like symptoms following any blow to their head to verify that athletes were appropriately grouped into previously concussed versus control groups. All participants provided written informed consent that was approved by the university Institutional Review Board. Participant demographic, injury and sport participation data can be found in Table 1, while the primary sport played for the concussed and control groups is indicated in Table 2. All of the athletes with a previous history of concussion reported being injured while participating in their primary sport.
Table 1.
Participant demographics by concussion status (M ± SD).
| Characteristic | Concussed | Control |
|---|---|---|
| Sample size | 25 (5 females) | 22 (9 females) |
| Age (years) | 21.0 ± 2.3 | 20.5 ± 2.0 |
| BMI (kg/m2) * | 25.4 ± 3.4 | 22.6 ± 2.5 |
| K-BIT 2 score | 107.1 ± 11.2 | 111.3 ± 14.3 |
| Education (years) | 14.0 ± 1.7 | 13.8 ± 1.3 |
| High risk sport participation (years) | 13.0 ± 10.1 | 11.0 ± 9.8 |
| Number of concussion(s) | 1.8 ± 1.0 [range = 1–4] | - |
| Loss of consciousness (#) | 0.3 ± 0.9 [range = 0–4] | - |
| Time since last concussion (months) | 30.3 ± 29.8 [range = 4–123] | - |
* Significant difference, unpaired t test between groups, p < .05.
Table 2.
Primary sport participation by concussion status.
| Sport | Concussed (n = 25) | Control (n = 22) |
|---|---|---|
| Basketball | 1 | 0 |
| Cheerleading | 0 | 1 |
| Crew | 0 | 1 |
| Cross-Country | 0 | 1 |
| Fencing | 1 | 0 |
| Field Hockey | 0 | 2 |
| Football | 4 | 3 |
| Ice Hockey | 3 | 0 |
| Lacrosse | 1 | 0 |
| Rugby | 3 | 0 |
| Downhill Skiing | 1 | 0 |
| Soccer | 3 | 6 |
| Softball | 1 | 0 |
| Swimming and Diving | 1 | 2 |
| Tennis | 0 | 1 |
| Track and Field | 2 | 3 |
| Volleyball | 3 | 1 |
| Wrestling | 1 | 1 |
2.2. Procedures
Upon arrival to the laboratory, participants completed a series of questionnaires, including a general medical and health history questionnaire, and an athletic participation and concussion history questionnaire. The Kaufman Brief Intelligence Test, Second Edition (K-BIT 2; Kaufman and Kaufman, 2004) was administered by a trained researcher to assess and control for intelligence quotient (IQ) scores. Following completion of the K-BIT 2, participants completed the ImPACT test to assess functional cognitive performance. Then, participants were fitted with a geodesic sensor net (Electrical Geodesics Inc. [EGI]; Eugene, OR, USA) to record the continuous EEG during a modified flanker task. Each participant completed a practice block of 30 trials before completing the experimental task. At the conclusion of the experiment, participants were compensated and briefed on the purpose of the study.
2.3. Measures
2.3.1. ImPACT
The ImPACT (ImPACT Applications, Inc., Pittsburgh, PA, USA) is the most widely-used concussion assessment tool used to guide concussion management decisions from youth to professional sports. The ImPACT is a computerized assessment that consists of a demographic questionnaire and concussion symptom report, followed by a battery of neuropsychological tasks assessing neurocognitive performance. ImPACT outcome variables include composite scores of verbal memory, visual memory, visuomotor processing speed, reaction time, and impulse control (see Schatz et al., 2006 for a detailed description).
2.3.2. Flanker task
Participants completed a modified arrow version of the Eriksen flanker task (see Figure 1; Eriksen and Eriksen, 1974). On each trial, participants were presented with five horizontal arrows presented at the center of the display approximately 70 cm from eye level, and were asked to respond to the direction of the center arrow. Congruent trials consisted of the central target arrow being flanked by arrows pointing in the same direction (i.e., < < < < < or > > > > >), whereas the central target arrow was flanked by arrows facing the opposite direction for incongruent trials (i.e., < < > < < or > > < > >). Arrows were 1.5 cm tall x 8 cm long, displayed in a black font on a light grey background and appeared at vertical and horizontal visual angles of 1.2° and 6.6°, respectively. All stimuli were presented on a 17 in (43.18 cm) Dell LCD computer monitor using E-Prime version 2.0 Professional software (Psychology Software Tools, Inc., Pittsburgh, PA, USA). Each trial began with a fixation cross (+) presented for 500 ms followed by stimulus presentation for 100 ms. Upon stimulus offset, there was a 1000 ms response window followed by a random intertrial interval (1100, 1300, and 1500 ms). Following 30 practice trials with performance feedback, participants completed two blocks of 110 trials consisting of equiprobable congruent and incongruent flanker stimuli. Participants were instructed to respond as quickly and accurately as possible by making a left or right button press corresponding to the directionality of the central target arrow. No feedback was provided during these two blocks of trials. The dependent measures included response accuracy (%), reaction time (in milliseconds), CVRT, calculated as standard deviation of reaction time ÷ mean reaction time, and measures of post-error behavioral adjustments, including post-error slowing (in milliseconds) and post-error accuracy (%).
Figure 1. Modified Eriksen flanker task.
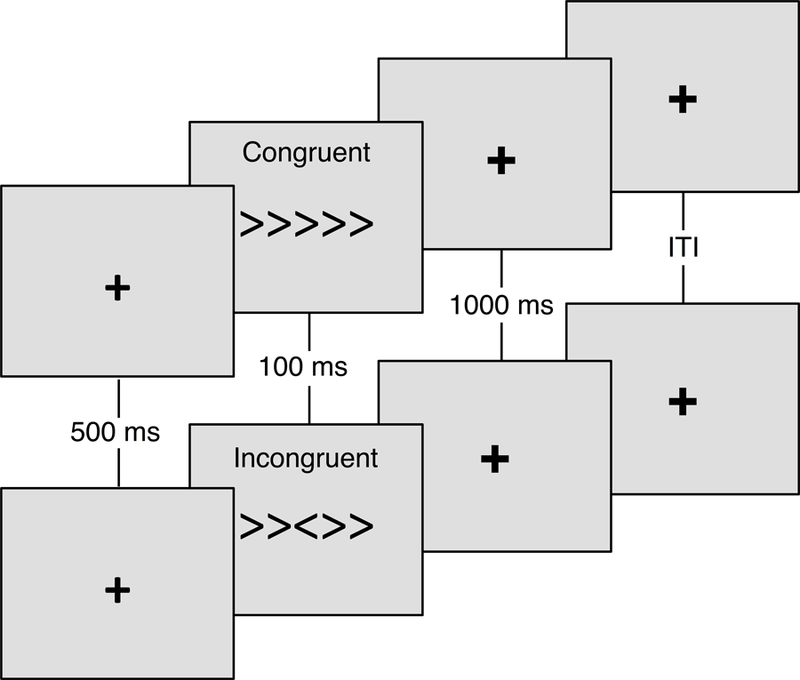
Randomly selected and equiprobable congruent (e.g., < < < < <) and incongruent (e.g., < < > < <) trials were displayed for 100 ms following a 500 ms fixation cross (+). Participants responded to the direction of the central arrow within the 1000 ms response window.
2.3.3. Event-related potentials
Continuous EEG activity, online referenced to the vertex electrode (Cz), was recorded from 64 scalp electrode sites using a geodesic sensor net that was arranged based on the International 10–10 system (Chatrian et al., 1988). The data were amplified and digitized by an EGI amplifier with a nominal gain of 20,000, bandpass of 0.10–100 Hz and a sampling rate of 250 Hz with a 24-bit analog-to-digital converter. Horizontal electrooculogram (EOG) activity was recorded from electrodes located lateral to the external canthi to measure saccadic eye movements, while vertical EOG activity was recorded from electrodes located above and below each eye. Continuous data were visualized in NetStation 4.0 and impedances were kept below 50 kΩ (Clayson and Larson, 2013).
All data were exported from NetStation 4.0 to EEGLAB toolbox (Delorme and Makeig, 2004) in Matlab to perform data preprocessing operations. Data were bandpass filtered using a 2nd order infinite impulse response (IIR) Butterworth filter of 0.1–30 Hz. Data were then adjusted for DC offset and visually inspected to identify and remove segments containing large muscle-related artifacts or extreme offsets. Stimulus-locked epochs were created for correct trials using a −100 to 1000 ms time window, while response-locked epochs were created for both correct and incorrect (i.e., error commission) trials using a −400 to 800 ms time window. Ocular artifacts and eye blinks were removed from the segmented waveforms using ICA blink templates that were provided by ERP PCA toolkit (Dien, 2010) and generated from the current dataset. ICA components that correlated 0.9 or higher with scalp topographies of the blink template were removed. Trials were also rejected if there was a voltage difference of 100 µV between minimum and maximum values in that trial or if channels differed by more than 30 µV from the neighboring 6 closest channels that were marked bad. Trials with >10% of channels marked as bad were also removed. Remaining bad channels were corrected through interpolation obtained from “good” channels of the scalp voltage field within each segment. Lastly, epochs were re-referenced to the mean of the mastoids (Bertrand et al., 1985; Tucker et al., 1994), averaged by flanker trial type (congruent, incongruent), and baseline corrected using a −100 to 0 ms and −400 to −200 ms pre-stimulus period for stimulus-locked and response-locked epochs, respectively.
Consistent with previous ERP research and due to the scalp distribution reflecting the components of interest, N2 and ERN amplitudes were assessed across a region of interest (ROI) at frontocentral electrode sites (Fz, FCz; Endrass et al., 2010; Folstein and Van Petten, 2008; Riesel et al., 2013). Pe amplitude was assessed across a ROI at centroparietal electrode sites (Cz, Pz; Larson et al., 2007). Correct trials for N2 amplitude were measured as the mean amplitude in an a priori time window of 200–350 ms post-stimulus onset (Alderman et al., 2015; Olson et al., 2016) while correct responses for CRN and incorrect responses for ERN were measured as the mean amplitude in an a priori time window of 0–100 ms around the response (Riesel et al., 2013). Correct and incorrect responses for Pe were measured as the mean amplitude in an a priori time window of 200–400 ms following the response (Larson et al., 2007). ERP difference waves (∆N2: incongruent minus congruent trial waveforms; ∆ERN = error minus correct trial waveforms; ∆Pe: error minus correct trial waveforms) were constructed to isolate activity associated with pre- and post-conflict and error-related brain activity. To analyze N2 and ERN latency, centroid latency measures were derived using the same time windows from above. The centroid measure of latency assesses the overall area under the curve in order to capture the central tendency of the waveform (Dien et al., 2004). Pe latency measurements were not conducted due to the component’s tonic nature, as suggested by previous research (Overbeek et al., 2005). Lastly, all processing procedures were performed by authors who were blinded to the group status of the participants (C.J.B. and P.J.E.).
2.4. Data analysis
Descriptive statistics were conducted on participant characteristics and presented as M ± SD (see Table 1). Repeated measures analyses of variance (RM-ANOVA) were conducted with a two-tailed family-wise error rate of 0.05 for behavioral performance and ERP measures. One-way ANOVAs were used to compare the composite outcome measures on ImPACT test performance between groups (concussed, control). Analyses of behavioral performance data (reaction time, accuracy) and N2 included a within-subjects factor of task congruency (congruent, incongruent) and a between-subjects factor of group (concussed, control). For ERN and Pe, analyses included a within-subjects factor of trial accuracy (correct, error) and a between-subjects factor of group (concussed, control). Greenhouse-Geisser epsilon corrections for nonsphericity were used to adjust the probability values in cases where the sphericity assumption was not met (Jennings and Wood, 1976). Effect sizes for the RM-ANOVAs were reported as eta-squared (η2) values. Correlation analyses were conducted to investigate the relationship between behavioral performance, ERP measures, and injury-related moderators, including number of concussions, number of concussions resulting in loss of consciousness, time (in months) since last concussion, age at first concussion, and years of high risk sport participation. All data analyses were performed using JASP version 0.8.0.0 software (JASP Team, http://www.jasp-stats.org/).
3. Results
Preliminary descriptive analyses revealed between group differences for body mass index (BMI), t(45) = 3.1, p < .05, while no other significant between group differences for demographic variables of age, t(45) = 0.8, p = .44, IQ, t(45) = −1.1, p = .28, and years of education, t(45) = 0.5, p = .44, were observed. No significant correlations were found between BMI and any of the behavioral or ERP outcome measures. In addition, no significant differences in behavioral performance or ERP measures were found between men and women; therefore, data were collapsed across male and female participants for all subsequent analyses.
3.1. Behavioral performance
ImPACT and flanker task behavioral performance variables are presented as M ± SD in Table 3.
Table 3.
Behavioral performance measures by concussion status (M ± SD).
| Concussed | Control | p-value | |
|---|---|---|---|
| ImPACT | |||
| Verbal memory (% correct) | 90.5 ± 8.5 | 93.0 ± 7.6 | .31 |
| Visual memory (% correct) | 76.6 ± 12.4 | 76.5 ± 12.7 | .97 |
| Motor speed (composite score) | 39.9 ± 5.3 | 40.1 ± 5.6 | .93 |
| Reaction time (ms) | 575.4 ± 71.9 | 620.3 ± 98.1 | .09 |
| Impulse control (composite score) | 4.6 ± 2.3 | 5.9 ± 6.4 | .39 |
| Flanker performance | |||
| Response accuracy (%) | |||
| Congruent | 97.8 ± 2.7 | 95.6 ± 7.3 | .43 |
| Incongruent | 85.2 ± 10.0 | 87.2 ± 10.5 | .49 |
| Reaction time (ms) | |||
| Congruent | 409.3 ± 47.4 | 428.8 ± 48.5 | .17 |
| Incongruent | 501.2 ± 63.8 | 514.6 ± 58.5 | .46 |
| Post-error accuracy (%) | 10.4 ± 12.7 | 9.3 ± 6.3 | .71 |
| Post-error slowing (ms) | 38.5 ± 47.8 | 39.7 ± 38.2 | .93 |
| CVRT (ms) | 0.53 ± 0.02 | 0.53 ± 0.01 | .87 |
Note. ImPACT = Immediate Post-Concussion Assessment and Cognitive Testing; CVRT = coefficient of variation of reaction time.
3.1.1. ImPACT
The analyses revealed no significant differences by group for composite scores of verbal memory, F(1,45) = 1.1, p = .31, η2 = .03, visual memory, F(1,45) = .01, p = .97, η2 < .01, motor speed, F(1,45) = .01, p = .93, η2 < .01, reaction time, F(1,45) = 3.0, p = .09, η2 = .07, and impulse control, F(1,45) = .8, p = .39, η2 = .02.
3.1.2. Flanker task
Analysis of response accuracy revealed a main effect of congruency, F(1,45) = 77.0, p < .001, η2 = .62, with greater accuracy for congruent (97.2 ± 5.4%) relative to incongruent (86.1 ± 10.2%) trials. Similarly for reaction time, there was a main effect of congruency, F(1,45) = 474.3, p < .001, η2 = .91, with faster responses on congruent (418.4 ± 48.4 ms) compared to incongruent (507.4 ± 61.1 ms) trials. No significant group differences or group by congruency interactions emerged for accuracy and reaction time measures.
Analysis of response accuracy following correct and error trials revealed a main effect of accuracy, F(1,45) = 42.4, p < .001, η2 = .50, indicating decreased accuracy following correct trials (84.6 ± 10.6%) compared to performance after error trials (94.5 ± 9.2%). For reaction time slowing, there was a main effect of accuracy, F(1,45) = 35.4, p < .001, η2 = .46, which confirmed a general slowing following errors (472.0 ± 67.4 ms) relative to correct trials (432.9 ± 55.4 ms). In terms of reaction time intra-individual variability (i.e., CVRT), the analysis revealed a marginally significant effect of congruency, F(1,45) = 3.3, p = .08, η2 = .07, such that there was greater variability on incongruent (0.527 ± 0.02 ms) relative to congruent (0.523 ± 0.01 ms) trials. No significant group differences or interactions by group were observed for post-error accuracy, post-error slowing, and CVRT measures.
3.2. ERP analyses
For number of trials used for the ERP analyses, no significant group x congruency interactions emerged, which indicated that the number of trials contributing to the stimulus- (N2) and response-locked (ERN; Pe) components were similar between concussed and control groups, ps > .05. For the response-locked analyses, two control participants were excluded from the analyses due to the commission of fewer than six errors (Meyer et al., 2013; Olvet and Hajcak, 2009). Stimulus- and response-locked ERPs are depicted in Figures 2 and 3, respectively. In addition, amplitude and latency measures for stimulus- and response-locked ERPs are reported in Table 4.
Figure 2. Stimulus-locked grand average N2 parent, difference waveforms, and topographic distributions.
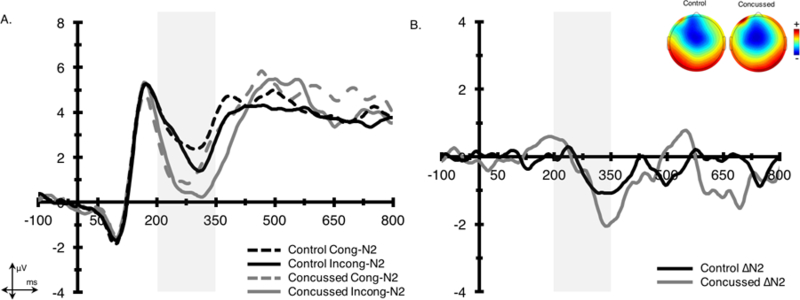
Waveforms were collapsed across frontocentral electrode sites (Fz and FCz) for (A.) parent and (B.) difference waveforms. Shading indicates the 200–350 ms stimulus-locked N2 time window. The topographic plots represent the average activity across the 200–350 ms time window for the N2 difference waveform.
Figure 3. Response-locked grand average ERN parent, difference waveforms, and topographic distributions.
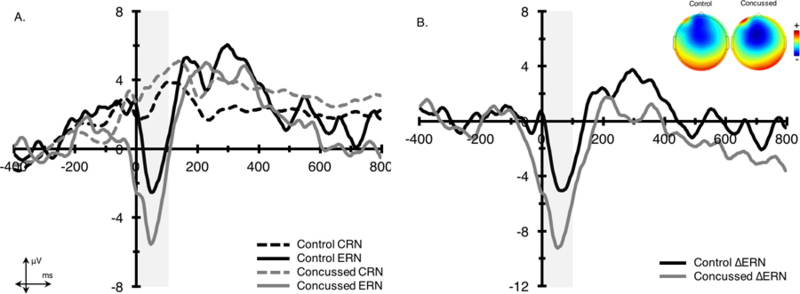
Waveforms were collapsed across frontocentral electrode sites (Fz and FCz) for (A.) parent and (B.) difference waveforms. Shading indicates the 0–100 ms response-locked ERN time window. The topographic plots represent the average activity across the 0–100 ms time window for the ERN difference waveform (ΔERN).
Table 4.
Amplitude and latency measures for N2, ERN, Pe, ΔN2, ∆ERN, and ΔPe (within-subjects standard errors in parentheses).
| Concussed | Control | F | p-value | |
|---|---|---|---|---|
| Mean amplitude (μV) | ||||
| N2 | ||||
| Congruent | 1.62 (0.66) | 2.94 (0.74) | 1.77 | .19 |
| Incongruent | 0.84 (0.62) | 2.42 (0.65) | 3.33 | .08 |
| ERN | ||||
| Correct | 3.98 (0.91) | 2.75 (0.91) | 0.90 | .35 |
| Error | −4.37 (1.15) | −1.34 (1.11) | 3.46 | .07 |
| Pe | ||||
| Correct | 3.37 (0.53) | 1.61 (0.51) | 5.48 | .02 |
| Error | 7.09 (1.07) | 8.00 (1.63) | 0.23 | .63 |
| Difference waves | ||||
| ΔN2 | −0.78 (0.40) | −0.53 (0.47) | 0.18 | .68 |
| ΔERN* | −8.35 (1.53) | −4.09 (1.19) | 4.46 | .04 |
| ΔPe | 3.72 (1.16) | 6.39 (1.59) | 1.93 | .17 |
| Centroid latency (ms) | ||||
| N2 | ||||
| Congruent | 273.2 (16.3) | 280.2 (19.2) | 1.83 | .20 |
| Incongruent | 282.5 (14.7) | 282.6 (19.5) | 0.01 | .98 |
| ERN | ||||
| Correct | 47.8 (4.2) | 51.4 (3.8) | 1.90 | .18 |
| Error | 45.7 (3.6) | 50.8 (4.9) | 1.73 | .19 |
Note. ΔN2 = incongruent – congruent difference wave; ∆ERN = error – correct difference wave; ΔPe = error – correct difference wave;
p < .05.
3.2.1. N2
The analyses for N2 amplitude revealed a main effect of congruency, F(1,45) = 4.6, p < .05, η2 = .09, indicating larger N2 amplitudes on incongruent (1.6 ± 3.0 µV) relative to congruent (2.2 ± 3.4 µV) trials. There was no group x congruency interaction observed for N2 amplitude; however, a group main effect was marginally significant, F(1,45) = 2.7, p = .10, η2 = .06, such that N2 amplitudes were more negative in individuals with a history of concussion (1.2 ± 3.0 µV) relative to controls (2.7 ± 3.0 µV). For latency, the analyses revealed a main effect of congruency, F(1,45) = 8.6, p < .01, η2 = .15, with individuals displaying longer latencies for incongruent (282.6 ± 16.9 ms) relative to congruent (276.5 ± 3.0 ms) trials. No additional between group differences or group x congruency interactions were observed.
3.2.2. ERN
For amplitude, a main effect of accuracy, F(1,43) = 38.1, p < .001, η2 = .45, revealed that the most negative amplitudes were observed for error (−3.0 ± 5.6 µV) compared to correct (3.4 ± 4.3 µV) trials. This main effect was superseded by a significant accuracy x group interaction, F(1,43) = 4.5, p < .05, η2 = .05, such that individuals with a history of concussion exhibited larger error-related brain activity (∆ERN = −8.4 ± 7.7 µV) relative to controls (∆ERN = −4.1 ± 5.3 µV). For latency, a main effect of accuracy approached significance, F(1,43) = 3.2, p = .08, η2 = .07, with individuals displaying a trend for faster latencies on correct (44.6 ± 10.5 ms) relative to error (48.0 ± 11.8 ms) trials. No further significant group differences or accuracy x group interactions were observed.
3.2.3. Pe
For Pe amplitude, a significant main effect of accuracy, F(1,43) = 27.7, p < .001, η2 = .38, indicated that the most positive amplitudes were observed following error (7.5 ± 6.2 µV) relative to correct (2.6 ± 2.6 µV) trials. There was no significant group x accuracy interaction for Pe amplitude.
3.3. Correlations
Pearson correlations indicated that time since last diagnosed concussion was associated with slower N2 latencies for congruent, r(23) = −.50, p < .05, and incongruent trials, r(23) = −.55, p < .05, which suggests that recent concussions may be related to longer latencies. Spearman rho correlations revealed a negative relationship between number of concussions resulting in a loss of consciousness and response accuracy on congruent, r(23) = −.54, p < .05, and incongruent trials, r(23) = −.54, p < .05, indicating that more severe concussions are related to impaired response accuracy. Bivariate correlations for the relationship between concussion-related variables and neurocognitive performance are reported in Table 4. Lastly, Pearson correlations were conducted on the whole sample to investigate relationships between high risk sports participation and primary outcome variables as well as relationships between the various ERP measures and behavioral task performance measures; however, all correlations were nonsignificant with behavioral and ERP measures, ps > .05.
4. Discussion
The purpose of the study was to examine the relationship between a history of sport-related concussion on pre-response and post-response conflict and error monitoring processes, as indexed by N2, ERN, and Pe components. A secondary aim was to investigate the relationship between years of high risk sport participation and performance monitoring as a proxy for the potential influence of repetitive subconcussive impacts on neurocognitive function. The current results revealed selective impairments in cognitive control and performance monitoring processes, such that increased error-related brain activity (i.e., larger ∆ERN) was observed in individuals with a history of sport-related concussion relative to their neurologically healthy peers. N2 amplitudes were also more negative among individuals with a history of concussion, an effect that approached significance. This ERP evidence suggests that both pre-response and post-response cognitive control processes may be impacted by a history of concussion. These ERP findings were observed despite no significant between group differences in behavioral performance (reaction time, response accuracy), post-error adjustments, and intra-individual behavioral performance measures (CVRT). These findings are interpreted below within the framework of the compensatory error-monitoring hypothesis (Moser et al., 2013).
Our finding of increased ∆ERN in individuals with a history of concussion is consistent with previous studies indicating alterations in error-related brain activity for both children and young adults with a history of sport-related concussion (De Beaumont et al., 2013; Moore et al., 2015; Pontifex et al., 2009); however, not all studies have found a relationship between ERN and concussion (Larson et al., 2012). In contrast to our findings of increased ∆ERN, the three previous studies of impaired error processing in concussion all reported reduced ERN amplitude in individuals with a concussion history. Moore et al. (2015) found smaller flanker ERN amplitude in children with a history of concussion while Pontifex et al. (2009) reported attenuated ERN among college-aged adults with a history of concussion, as well as a negative correlation between ERN amplitude and the number of concussive events. It is unclear why increased rather than decreased error-related brain activity was observed in the current study. It is possible that variability in the number or severity of concussions or methodological differences may account for the between-study differences. For instance, De Beaumont et al. included current athletes who reported between 2–7 previous concussions while Moore et al. examined the long-term consequences of concussion among 8–10 year old children, who may be at increased risk due to the rapid brain development characteristic of this developmental period. Our study sample is most similar to the Pontifex et al. study; however, they found between group differences on behavioral performance outcomes such that those with a history of concussion exhibited impaired response accuracy and slower interference RT during flanker task performance. Thus, although their data suggested a suppressed ERN and impaired behavioral performance outcomes associated with a history of concussion, our sample demonstrated increased error-related brain activity and no difference in behavioral performance measures relative to their non-concussed peers. Our findings suggest that individuals with a history of concussion may need to upregulate neural resources following error commission in order to attain similar behavioral performance on subsequent trials.
Schroeder and Moser (2014) previously outlined the utility in combining behavioral with ERP measures to better understand the functional significance of error-related brain activity. Their suggestions, combined with the compensatory error-monitoring hypothesis (Moser et al., 2013), may help to clarify the inconsistent findings. That is, the increased ∆ERN in our sample, and perhaps even the increase in N2 amplitude, may reflect a compensatory effort reflecting the need for more neural resources to achieve comparable performance, such that increased ACC activity and absence of deficits in behavioral performance might suggest a compensatory mechanism required for optimal performance. The altered ERN and impaired behavioral performance observed in the Pontifex et al. study might suggest greater impairment among their participants (Eysenck et al., 2007). The current findings of enhanced ∆ERN and N2 in individuals with a history of concussions may reflect inefficient pre- and post-response conflict, indicating the need for greater conflict and error monitoring resources to achieve the same level of behavioral performance as their non-concussed counterparts. The enhanced ∆ERN may be a neural signature of this compensatory effort or greater utilization of processing resources to optimize performance. Taken together, these findings suggest the persistence of overactive performance monitoring processes following a history of sport-related concussion may impair the ability to evaluate response conflict and flexibly adjust subsequent behavior. Therefore, persistent deficits in pre- and post-response conflict processes may be a persistent consequence of concussive injuries. Future studies are warranted to examine individual differences in concussions (severity, number, location of injury) that may be associated with variability in these two ERP components of conflict processing.
It is possible that the larger error-related brain activity observed in the present sample may reflect the presence of or increased risk for a mood disorder (e.g., anxiety; Olvet and Hajcak, 2008). Following a concussion, many individuals often experience post-concussion affective and neuropsychiatric symptoms (Kontos et al., 2016). However, many of these symptoms are subclinical in nature and often do not meet clinical criteria for a clinical disorder. Importantly, these mood disorders have been shown to differentially modulate error-related brain activity, such that Holmes and Pizzagalli (2010) observed enhanced ERN in major depressive disorder, while Ladouceur and colleagues (2012) found reductions in ERN associated with depression. Relative to healthy individuals, individuals with anxiety tend to exhibit increased ERN (Hajcak, 2012), which highlights the importance of considering the occurrence of mood disorders in individuals with a history of concussion. Although other studies examining the relationship between sport-related concussion and error-related brain activity excluded individuals with neurological disorders (e.g., cognitive or attention-related disorders; Moore et al., 2015; Pontifex et al., 2009), many of these studies have not accounted for the presence or history of affective disorders, which may help to explain the divergent findings. Given the heterogeneity in concussions as well as the accompanying sequelae, studies assessing pre-season neurocognitive functioning using ERPs may help to better understand the nature of injury and rehabilitation, as well as potential risk for post-concussion affective disorders. It is also important for future studies to account for presence or history of affective disorders, as well as any changes in other health risk behaviors (drug and alcohol use disorders) in the examination of concussions and neurocognitive function.
In line with previous studies, the current findings relative to N2 suggest persistent alterations in pre-response conflict processes related to concussion (Broglio et al., 2009; Moore et al., 2015). Although there were no between group differences for N2 latency measures in the current study, the time since last concussion was negatively associated with N2 latency. Moore et al. (2015) also reported increased N2 latency in children with a history of concussion and collectively, these findings suggest that pre-response conflict monitoring processes may be influenced by both a presence of a concussive event and the time since the injury occurred. The N2 is generally linked to target identification and discrimination and reflects conflict generated by competing responses from task-relevant and task-irrelevant information (e.g., flanker congruent and incongruent trials). Combined with the ERN findings, these data suggest that there are persistent deficits related to concussions that manifest as difficulties in conflict and error-monitoring processes of cognitive control. In agreement with several previous studies (Larson et al., 2007; Pontifex et al., 2009), no significant between group differences were found for the error positivity (Pe), which has been proposed to reflect additional processing that occurs after error detection, such as conscious error recognition (Falkenstein et al., 2000). Thus, the long-term impact of a concussive history may impact more immediate temporal processes surrounding the response, rather than a later stage of cognitive processing. Future studies using ERPs to disentangle the temporal aspects of cognitive processing may help to elucidate any possible general or selective neurocognitive impairments that accompany sport-related concussions, and how these are related to important individual difference variables.
Accumulating research indicates that repetitive subconcussive impacts can alter brain structure and function. Recently, Moore et al. (2017) compared neurophysiological and neuropsychological function of contact athletes with and without a history of concussion relative to a group of non-contact control athletes. Subconcussive impacts were defined as the number of soccer headers across a season and, importantly, soccer games were videotaped across the season to confirm the number of self-reported headers across a season. Athletes in the concussion and subconcussion groups demonstrated alterations in brain function related to attentional orienting (P3a) and attentional resource allocation (P3b) while only athletes in the concussion group showed reductions in the amplitude of an ERP measure of perceptual attention (N1). The precise measurement of the extent and severity of concussions has proven to be challenging to the field; however, the nature of subconcussive impacts on neurocognitive function is perhaps even more elusive due to the lack of specificity and standardization of assessment. In this study, the number of years of high risk sport participation were not correlated with behavioral and ERP outcome measures. Therefore, in the present sample, the incidence of concussion was related to alterations in conflict and error-related brain activity, while high risk sport participation alone was not related to any of the neurocognitive outcomes. However, it is possible that years of high risk sport participation alone is not the best proxy measure of accumulation of subconcussive impacts. Future studies incorporating a similar methodology as the Moore et al. study where videotape analysis was used to confirm self-reported headers in soccer may help to advance our understanding of the potential risk of cognitive impairment following subconcussive injuries.
5. Limitations
Due to the cross-sectional nature of this study, it is not possible to draw conclusions regarding a potential causal relation between concussions and N2 and ERN components of performance monitoring. It is possible that preexisting individual differences may have accounted for the observed increases in conflict and error-related brain activity. As mentioned, there is a large and growing literature of ERN in anxiety (Hajcak et al., 2003; Moser et al., 2016; Moser et al., 2013; Proudfit et al., 2013) and it is possible that participants in this study were dealing with elevated post-concussive anxiety. Future studies should account for any mental health or affective disorders in their samples, as well as the potential moderating effect of mental health status on the relationship between concussion history and persistent cognitive impairments. Participants in this study were allowed to self-report their concussion history, which may not be as accurate as prospectively assessing neurocognitive functioning following a clinical diagnosis of concussion. However, all individuals were asked to list the number of “physician or primary health care provider diagnosed concussion” and this was followed with a number of questions related to symptoms of acute injury to corroborate their reported history. Another potential limitation is the neuroscientific technique used to assess neurocognitive function in the present study. Although we used the ERP technique for its advantages in unveiling the temporal resolution of conflict and error-related processing, ERPs have poor spatial resolution, which limits our ability to make definitive conclusion about impairments in specific brain regions associated with a history of concussion. Furthermore, it is important to note that this study did not assess changes in ERPs from pre-concussion (baseline) values; therefore, it is essential that longitudinal cohort studies be conducted to determine change from baseline. A number of recent reviews have recommended that studies compare concussed individuals to their preinjury baseline values, particularly given the heterogeneous nature of concussive injuries (Broglio et al., 2011; Clark and Guskiewicz, 2016). Lastly, it should also be noted that our ERN effects were apparent only for the relative response to errors compared to correct responses (i.e., ∆ERN), which differs from previous work that has identified group differences in the magnitude of the ERN specifically (e.g., Moore et al., 2015; Pontifex et al., 2009). Although no significant group differences in ERN were observed in this study, it is important to analyze not only the parent waveforms, but also the difference wave (∆ERN) in order to isolate brain activity of interest that is uniquely related to error-related processing (Simons, 2010). Future research should evaluate the functional significance of ∆ERN compared to ERN in order to determine whether these distinct patterns of effects can further inform our understanding of psychopathology following sport-related concussions.
6. Conclusion
In sum, our data extend the growing literature on the influence of sport-related concussions on neurocognitive function by examining both pre-response and post-response cognitive control processes. In particular, the current study demonstrated increased pre- and post-response conflict-related brain activity in individuals with a history of sport-related concussion. These neuroelectric findings were found in the absence of behavioral performance differences, indicating that individuals with a history of concussion require more neural resources to achieve a similar of functioning as non-concussed counterparts (i.e., a compensation effect). In addition, injury-related moderators of time since last concussion and number of concussions resulting in a loss of consciousness were related to impaired N2 latencies and flanker response accuracy. Although subconcussive events during sport have been previously to influence neurocognitive function, years of high risk sport participation were unrelated to neurocognitive performance, as indicated by both behavioral and neuroelectric markers. Thus, our data suggest that the incidence of a sport-related concussion may explain the select alterations in conflict- and error-monitoring, while the number of years of playing high risk sports is not associated with any persistent changes in conflict-related performance monitoring. These findings extend a growing body of literature suggesting the potential for long-term cognitive impairments following a sport-related concussion and that it may be the injury per se rather than years of playing high-risk sport that may be most pressing.
Table 5.
Correlations between injury variables, flanker performance, and neuroelectric measures.
| Measure | Number of concussions | Age of first concussion | Time since last concussion | Loss of consciousness | High risk sport participation |
|---|---|---|---|---|---|
| Flanker performance | |||||
| Congruent accuracy | −0.19 | 0.04 | 0.30 | −0.54* | 0.05 |
| Incongruent accuracy | 0.38 | 0.29 | 0.06 | −0.54* | −0.08 |
| Congruent RT | −0.36 | 0.06 | −0.13 | −0.07 | −0.11 |
| Incongruent RT | −0.42 | −0.03 | −0.07 | −0.07 | −0.18 |
| Post-error accuracy | 0.11 | −0.14 | 0.03 | 0.22 | 0.21 |
| Post-error slowing | 0.01 | −0.05 | −0.11 | −0.20 | 0.01 |
| CVRT | −0.08 | 0.15 | −0.04 | −0.02 | −0.22 |
| N2 | |||||
| Congruent amplitude | −0.11 | 0.21 | 0.15 | −0.17 | 0.13 |
| Incongruent amplitude | 0.08 | 0.34 | −0.03 | −0.34 | 0.32 |
| Congruent latency | 0.27 | 0.10 | −0.50* | −0.01 | 0.35 |
| Incongruent latency | 0.01 | 0.30 | −0.55* | −0.24 | 0.11 |
| ERN | |||||
| Correct amplitude | 0.18 | 0.10 | −0.09 | −0.37 | −0.21 |
| Error amplitude | 0.01 | 0.03 | 0.37 | 0.03 | 0.15 |
| Correct latency | −0.21 | −0.08 | 0.31 | −0.29 | 0.05 |
| Error latency | −0.05 | −0.23 | −0.03 | 0.33 | −0.17 |
| Pe | |||||
| Correct amplitude | −0.17 | −0.36 | 0.24 | 0.13 | −0.14 |
| Error amplitude | −0.01 | −0.05 | −0.13 | 0.15 | 0.03 |
| ΔN2 | |||||
| Mean amplitude | 0.06 | 0.16 | −0.28 | −0.24 | 0.29 |
| ΔERN | |||||
| Mean amplitude | 0.27 | −0.04 | −0.29 | 0.26 | 0.24 |
| ΔPe | |||||
| Mean amplitude | 0.08 | 0.11 | −0.23 | 0.05 | 0.09 |
Note. RT = reaction time; CVRT = coefficient of variation of reaction time; ΔN2 = incongruent – congruent difference wave; ∆ERN = error – correct difference wave; ∆Pe = error – correct difference wave;
p < .05.
Footnotes
Disclosure statement: The authors declare that they have no conflict of interest.
References
- Alderman BL, Olson RL, Bates ME, Selby EA, Buckman JF, Brush CJ, Panza EA, Kranzler A, Eddie D, Shors TJ, 2015. Rumination in major depressive disorder is associated with impaired neural activation during conflict monitoring. Front Hum Neurosci 9, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand O, Perrin F, Pernier J, 1985. A theoretical justification of the average reference in topographic evoked potential studies. Electroencephalogr Clin Neurophysiol 62, 462–464. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS, 2004. Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn Sci 8, 539–546. [DOI] [PubMed] [Google Scholar]
- Broglio SP, Ferrara MS, Piland SG, Anderson RB, Collie A, 2006. Concussion history is not a predictor of computerised neurocognitive performance. Br J Sports Med 40, 802–805; discussion 802–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglio SP, Moore RD, Hillman CH, 2011. A history of sport-related concussion on event-related brain potential correlates of cognition. Int J Psychophysiol 82, 16–23. [DOI] [PubMed] [Google Scholar]
- Broglio SP, Pontifex MB, O’Connor P, Hillman CH, 2009. The persistent effects of concussion on neuroelectric indices of attention. J Neurotrauma 26, 1463–1470. [DOI] [PubMed] [Google Scholar]
- Broglio SP, Puetz TW, 2008. The effect of sport concussion on neurocognitive function, self-report symptoms and postural control : a meta-analysis. Sports Med 38, 53–67. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). 2016. TBI: Get the Facts Retrieved from https://www.cdc.gov/traumaticbraininjury/get_the_facts.html [Google Scholar]
- Chatrian GE, Lettich E, Nelson PL, 1988. Modified nomenclature for the “10%” electrode system. J Clin Neurophysiol 5, 183–186. [PubMed] [Google Scholar]
- Clark M, Guskiewicz K, 2016. Sport-Related Traumatic Brain Injury, in: Laskowitz D, Grant G (Eds.), Translational Research in Traumatic Brain Injury, Boca Raton (FL). [Google Scholar]
- Clawson A, Clayson PE, Larson MJ, 2013. Cognitive control adjustments and conflict adaptation in major depressive disorder. Psychophysiology 50, 711–721. [DOI] [PubMed] [Google Scholar]
- Clayson PE, Larson MJ, 2013. Psychometric properties of conflict monitoring and conflict adaptation indices: response time and conflict N2 event-related potentials. Psychophysiology 50, 1209–1219. [DOI] [PubMed] [Google Scholar]
- De Beaumont L, Beauchemin M, Beaulieu C, Jolicoeur P, 2013. Long-term attenuated electrophysiological response to errors following multiple sports concussions. J Clin Exp Neuropsychol 35, 596–607. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S, 2004. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134, 9–21. [DOI] [PubMed] [Google Scholar]
- Dien J, 2010. The ERP PCA Toolkit: an open source program for advanced statistical analysis of event-related potential data. J Neurosci Meth 187, 138–145. [DOI] [PubMed] [Google Scholar]
- Dien J, Spencer KM, Donchin E, 2004. Parsing the late positive complex: mental chronometry and the ERP components that inhabit the neighborhood of the P300. Psychophysiology 41, 665–678. [DOI] [PubMed] [Google Scholar]
- Ellemberg D, Henry LC, Macciocchi SN, Guskiewicz KM, Broglio SP, 2009. Advances in sport concussion assessment: from behavioral to brain imaging measures. J Neurotrauma 26, 2365–2382. [DOI] [PubMed] [Google Scholar]
- Endrass T, Schuermann B, Kaufmann C, Spielberg R, Kniesche R, Kathmann N, 2010. Performance monitoring and error significance in patients with obsessive-compulsive disorder. Biol Psychol 84, 257–263. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW, 1974. Effects of noise letters upon the identification of a target letter in a nonsearch task. Atten Percept Psychophys 16, 143–149. [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG, 2007. Anxiety and cognitive performance: attentional control theory. Emotion 7, 336–353. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L, 1991. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol 78, 447–455. [DOI] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C, 2008. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology 45, 152–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MG, Meyer DE, Donchin E, 1993. A neural system for error detection and compensation. Psychol Sci 4, 385–390. [Google Scholar]
- Gehring WJ, Liu Y, Orr JM, Carp J, 2012. The error-related negativity (ERN/Ne). Oxford handbook of event-related potential components, 231–291. [Google Scholar]
- Guskiewicz KM, Marshall SW, Broglio SP, Cantu RC, Kirkendall DT, 2002. No evidence of impaired neurocognitive performance in collegiate soccer players. Am J Sports Med 30, 157–162. [DOI] [PubMed] [Google Scholar]
- Hajcak G, 2012. What we’ve learned from mistakes insights from error-related brain activity. Curr Dir Psychol Sci 21, 101–106. [Google Scholar]
- Hajcak G, McDonald N, Simons RF, 2003. Anxiety and error-related brain activity. Biol Psychol 64, 77–90. [DOI] [PubMed] [Google Scholar]
- Harmon KG, Drezner JA, Gammons M, Guskiewicz KM, Halstead M, Herring SA, Kutcher JS, Pana A, Putukian M, Roberts WO, 2013. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med 47, 15–26. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Pizzagalli DA, 2010. Effects of task-relevant incentives on the electrophysiological correlates of error processing in major depressive disorder. Cogn Affect Behav Neurosci 10, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG, 2002. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev 109, 679–709. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Dien J, Coles MG, 1998. Error-related scalp potentials elicited by hand and foot movements: evidence for an output-independent error-processing system in humans. Neurosci Lett 242, 65–68. [DOI] [PubMed] [Google Scholar]
- Iverson GL, 2005. Outcome from mild traumatic brain injury. Curr Opin Psychiatry 18, 301–317. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Wood CC, 1976. Letter: The epsilon-adjustment procedure for repeated-measures analyses of variance. Psychophysiology 13, 277–278. [DOI] [PubMed] [Google Scholar]
- Kappenman ES, Luck SJ, 2012. ERP Components: The Ups and Downs of Brainwave Recordings, in: Luck SJ, Kappenman ES (Eds.), The Oxford Handbook of Event-Related Potential Components Oxford University Press, New York, NY, pp. 3–30. [Google Scholar]
- Kappenman ES, Luck SJ, Kring AM, Lesh TA, Mangun GR, Niendam T, Ragland JD, Ranganath C, Solomon M, Swaab TY, Carter CS, 2016. Electrophysiological Evidence for Impaired Control of Motor Output in Schizophrenia. Cereb Cortex 26, 1891–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL, 2004. Kaufman brief intelligence test. Wiley Online Library [Google Scholar]
- Kontos AP, Deitrick JM, Reynolds E, 2016. Mental health implications and consequences following sport-related concussion. Br J Sports Med 50, 139–140. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Slifka JS, Dahl RE, Birmaher B, Axelson DA, Ryan ND, 2012. Altered error-related brain activity in youth with major depression. Dev Cogn Neurosci 2, 351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM, 2006. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 21, 375–378. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Clayson PE, Farrer TJ, 2012. Performance monitoring and cognitive control in individuals with mild traumatic brain injury. J Int Neuropsychol Soc 18, 323–333. [DOI] [PubMed] [Google Scholar]
- Ledwidge PS, Molfese DL, 2016. Long-Term Effects of Concussion on Electrophysiological Indices of Attention in Varsity College Athletes: An Event-Related Potential and Standardized Low-Resolution Brain Electromagnetic Tomography Approach. J Neurotrauma 33, 2081–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, Onate JA, Yang J, Kelly JP, 2003. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA 290, 2556–2563. [DOI] [PubMed] [Google Scholar]
- Meehan WP, Mannix RC, O’Brien MJ, Collins MW, 2013. The prevalence of undiagnosed concussions in athletes. Clin J Sport Med 23, 339–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Riesel A, Hajcak Proudfit G, 2013. Reliability of the ERN across multiple tasks as a function of increasing errors. Psychophysiology 50, 1220–1225. [DOI] [PubMed] [Google Scholar]
- Moore DR, Pindus DM, Raine LB, Drollette ES, Scudder MR, Ellemberg D, Hillman CH, 2016. The persistent influence of concussion on attention, executive control and neuroelectric function in preadolescent children. Int J Psychophysiol 99, 85–95. [DOI] [PubMed] [Google Scholar]
- Moore RD, Lepine J, Ellemberg D, 2017. The independent influence of concussive and sub-concussive impacts on soccer players’ neurophysiological and neuropsychological function. Int J Psychophysiol 112, 22–30. [DOI] [PubMed] [Google Scholar]
- Moore RD, Pindus DM, Drolette ES, Scudder MR, Raine LB, Hillman CH, 2015. The persistent influence of pediatric concussion on attention and cognitive control during flanker performance. Biol Psychol 109, 93–102. [DOI] [PubMed] [Google Scholar]
- Moran TP, Bernat EM, Aviyente S, Schroder HS, Moser JS, 2015. Sending mixed signals: worry is associated with enhanced initial error processing but reduced call for subsequent cognitive control. Soc Cogn Affect Neurosci, nsv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JS, Moran TP, Kneip C, Schroder HS, Larson MJ, 2016. Sex moderates the association between symptoms of anxiety, but not obsessive compulsive disorder, and error-monitoring brain activity: A meta-analytic review. Psychophysiology 53, 21–29. [DOI] [PubMed] [Google Scholar]
- Moser JS, Moran TP, Schroder HS, Donnellan MB, Yeung N, 2013. On the relationship between anxiety and error monitoring: a meta-analysis and conceptual framework. Front Hum Neurosci 7, 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson RL, Chang YK, Brush CJ, Kwok AN, Gordon VX, Alderman BL, 2016. Neurophysiological and behavioral correlates of cognitive control during low and moderate intensity exercise. Neuroimage 131, 171–180. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G, 2008. The error-related negativity (ERN) and psychopathology: toward an endophenotype. Clin Psychol Rev 28, 1343–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G, 2009. The stability of error-related brain activity with increasing trials. Psychophysiology 46, 957–961. [DOI] [PubMed] [Google Scholar]
- Perry DC, Sturm VE, Peterson MJ, Pieper CF, Bullock T, Boeve BF, Miller BL, Guskiewicz KM, Berger MS, Kramer JH, Welsh-Bohmer KA, 2016. Association of traumatic brain injury with subsequent neurological and psychiatric disease: a meta-analysis. J Neurosurg 124, 511–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontifex MB, O’Connor PM, Broglio SP, Hillman CH, 2009. The association between mild traumatic brain injury history and cognitive control. Neuropsychologia 47, 3210–3216. [DOI] [PubMed] [Google Scholar]
- Proudfit GH, Inzlicht M, Mennin DS, 2013. Anxiety and error monitoring: the importance of motivation and emotion. Front Hum Neurosci 7, 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesel A, Weinberg A, Endrass T, Meyer A, Hajcak G, 2013. The ERN is the ERN is the ERN? Convergent validity of error-related brain activity across different tasks. Biol Psychol 93, 377–385. [DOI] [PubMed] [Google Scholar]
- Schatz P, Pardini JE, Lovell MR, Collins MW, Podell K, 2006. Sensitivity and specificity of the ImPACT Test Battery for concussion in athletes. Arch Clin Neuropsychol 21, 91–99. [DOI] [PubMed] [Google Scholar]
- Schroder HS, Moser JS, 2014. Improving the study of error monitoring with consideration of behavioral performance measures. Front Hum Neurosci 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD, 2013. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron 79, 217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RF, 2010. The way of our errors: theme and variations. Psychophysiology 47, 1–14. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Gay M, Johnson B, Zhang K, 2012. Concussion in athletics: ongoing clinical and brain imaging research controversies. Brain Imaging Behav 6, 224–243. [DOI] [PubMed] [Google Scholar]
- Tucker DM, Liotti M, Potts GF, Russell GS, Posner MI, 1994. Spatiotemporal analysis of brain electrical fields. Hum Brain Mapp 1, 134–152. [Google Scholar]


