Abstract
Biological systems use post-translational modifications (PTMs) to control the structure, location, and function of proteins after expression. Despite the ubiquity of PTMs in biology, their use to create genetically encoded recombinant biomaterials is limited. We have utilized a natural lipidation PTM (hedgehog-mediated cholesterol modification of proteins) to create a class of hybrid biomaterials called cholesterol-modified polypeptides (CHaMPs), that exhibit programmable self-assembly at the nanoscale. To demonstrate the biomedical utility of CHaMPs, we used this approach to append cholesterol to the biologically active peptide exendin-4 that is an approved drug for the treatment of type II diabetes. The exendin-cholesterol conjugate self-assembled into micelles and these micelles activate the glucagon-like peptide-1 receptor with a potency comparable to current gold standard treatments.
Keywords: Post-translational modifications, Hedgehog, recombinant therapeutics, Self-assembly, Cholesterol
Graphical Abstract
INTRODUCTION
Protein-based materials have received increased attention as sequence-defined polymers for diverse biomedical applications, such as tissue engineering scaffolds and therapeutics.1–7 Advances in gene synthesis and recombinant expression have accelerated our ability to create new genetically encoded sequence-defined peptide polymers faster and at lower cost.8,9 However, the precision and versatility of recombinant expression is limited by the available repertoire of canonical amino acids, which restricts the sequence space of peptide polymers. Expanding the genetic code to incorporate non-canonical amino acids is one solution for increasing the chemical diversity of protein-based materials, and significant efforts have been devoted to achieve this goal.10–13
An orthogonal approach, and one that Nature uses, leverages post-translational modifications (PTMs)—a class of chemical reactions that modify proteins after expression—to expand the repertoire of chemical building blocks in proteins and diversify the proteome.14 By appending non-proteinaceous moieties to polypeptides, PTMs can alter the structure and function of proteins. Despite the hundreds of PTMs identified in native proteins to date, the use of PTMs to create genetically encoded recombinant biomaterials has been largely limited to the hydroxylation of tyrosine and proline, typically to mimic materials such as mussel foot protein and collagen.15–17
Motivated by this lacuna and the opportunity it presents, we recently demonstrated the synthesis of a new class of hybrid materials by reprogramming an existing post-translational modification involving N-terminal myristoylation via the addition of C14:0 to an N-terminal glycine to create fatty-acid-modified polypeptides. These materials form either spherical micelles or macroscopic materials depending on the precise sequence used as the substrate for the myristoylation reaction, and exhibit temperature-triggered hierarchical self-assembly.18,19 In our efforts to continue to expand the toolkit of post-translationally modified polypeptides with useful structure and function, here we focus on the cholesterol PTM by exploiting the native process via which proteins in the hedgehog family are covalently appended with a single cholesterol moiety.
We were intrigued by the potential of this rare PTM for several reasons. First, cholesterolysis is the only sterol-based modification of proteins among naturally occurring lipidations20,21 and the physical properties of cholesterol are significantly different from other fatty acids, due to the presence of a series of fused rings.22,23 Second, in contrast to N-myristoylation, cholesterol modification occurs at the C-terminus of the protein through the formation of an ester bond. Consequently, this modification is complementary to N-myristoylation and is likely to be useful for the many peptides and proteins that require a free, unblocked N-terminus for their biological activity. Finally, based on reports that cholesterol modification increases the oligomerization propensity of fused signal proteins24, we reasoned that it should be possible to use this PTM for multivalent display of appended biologically active peptides by leveraging the hydrophobicity of a cholesterol molecule to drive self-assembly of a polypeptide that presents a bioactive peptide the opposite —N-terminal— end of the cholesterol moiety. We envisioned that this multivalent display is useful for designing molecules with enhanced avidity for their targets.
Seminal studies by Beachy and coworkers have demonstrated that the cholesterol PTM is carried out by members of the Hedgehog family.25,26 Hedgehog proteins (Hh) contain two domains, an N-terminal signaling domain that is fused to an auto-processing C-terminal domain (HhC). HhC is homologous to intein-like proteins and contains an additional sterol binding site.27 Recent studies have shown that the intein-like activity is gated in the absence of cholesterol.28 Upon binding to cholesterol, the auto-processing domain undergoes an N → S acyl shift to form an intermediate with a thioester bond connecting the N- and C-terminal domains. Subsequently, the 3β-hydroxyl of the bound cholesterol reacts with this intermediate, resulting in cleavage of the C-terminal domain and modification of the N-terminal signaling domain with a cholesterol moiety (Figure 1a).29,30
Figure 1.
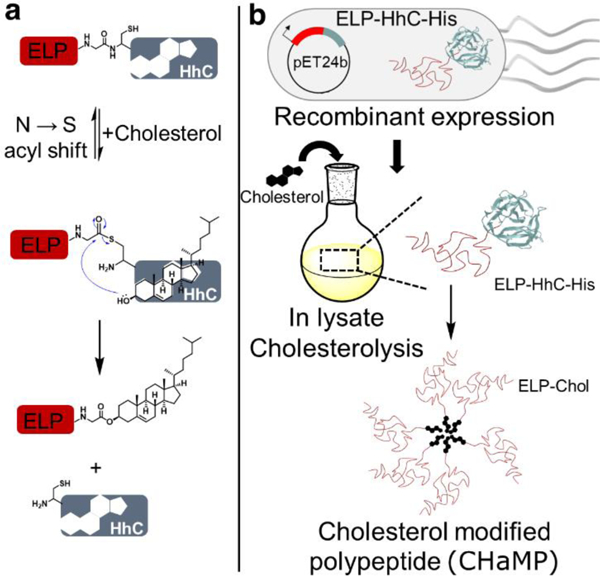
Hedgehog-mediated cholesterolysis can be used to create recombinant Cholesterol Modified Peptide polymers (CHaMPs). a) Hedgehog-mediated cholesterolysis involves binding of cholesterol to HhC, which triggers an intein-like N → S acyl shift to form a reactive thioester intermediate. The 3β-hydoxyl of the cholesterol molecule bound to HhC then reacts with the thioester intermediate, resulting in the cleavage of HhC and formation of the cholesterol-modified polypeptide. b) Our methodology involves recombinant expression of a fusion of an artificial peptide polymer with the C-terminal domain of HHc, followed by cholesterolysis in the cell lysate, and then purification of the resulting CHaMPs by leveraging the peptide-polymer reversible LCST phase-transition.
RESULTS AND DISCUSSION
Our strategy, summarized in Figure 1, involves using this PTM for the recombinant synthesis of self-assembling Cholesterol Modified Peptide-polymers (CHaMPs). We hypothesized that substitution of the N-terminal signaling domain with a peptide-polymer can be used to recreate this PTM in E. coli. For proof-of-concept, we used an elastin-like polypeptide (ELP) as an artificial peptide polymer. ELPs are a class of genetically encoded materials with the sequence (GXGVP), in which X can be any amino acid except proline. We chose ELPs, as they have several useful attributes: 1) they can be expressed recombinantly at high yields; 2) they undergo lower temperature-triggered phase transition, in which they form insoluble coacervates upon heating above a lower critical solubility temperature (LCST); and 3) their LCST behavior is sensitive to the modification and/or self-assembly state.31–33 These last two properties provide an opportunity to purify the resulting CHaMPs using inverse transition cycling (ITC), a facile, inexpensive, and scalable non-chromatographic method to purify ELP fusion by exploiting their LCST phase behavior (Figure 1b).34
To recombinantly append a cholesterol moiety to an ELP, we designed a plasmid to express a fusion containing the following two domains. The first, N-terminal domain was an ELP with the sequence [G(V8/A2) GVP]10GKG]8, in which the guest residue of the GXGVP pentapeptide (X) was 80% valine and 20% alanine. We chose this sequence with the following two considerations in mind: 1) it has a relatively high transition temperature (Tt), that should compensate for the hydrophobicity of the cholesterol molecule (logP = 7.1) 35 to ensure that the CHaMP exhibits LCST phase behavior in a biologically relevant temperature range. Introduction of alanine (A) at the guest residue (X) position, and distribution of lysine (K) residues along the backbone are expected to increase the hydrophilicity and Tt of this construct (vide infra for details); 2) Distributed lysine residues in the ELP sequence also allow easy visualization of protein bands using Coomassie blue staining (as canonical ELPs typically do not stain well by Coomassie blue) in addition to providing sites for the attachment of other molecules, such as fluorophores. The ELP was fused to the C-terminal domain of a Hedgehog protein from Drosophila melanogaster (second domain) using a short and flexible (GGS)2 linker, whose length was chosen to ensure that the ELP did not interfere with the autoprocessing function of the HhC. We also introduced an octa-His tag (His) at the C-terminus of the construct to allow visualization of protein expression by western blotting and provide an orthogonal method to ITC for purification of the fusion via immobilized metal affinity chromatography (IMAC). The amino acid sequence of the constructs and details of the cloning procedures are provided in the Supplementary Information. The plasmid containing the ELP-HhC-His gene was transformed into E. coli BL21(DE3) cells, and expression of the ELP-HHc-His construct was induced by addition of Isopropyl β-D-1-thiogalactopyranoside to the culture medium (See SI for details). After 18 h, the cells were harvested by centrifugation and resuspended in 20 mM Tris, 150 mM NaCl, pH = 7.1, and lysed by sonication. We then investigated whether cholesterolysis could be efficiently reconstituted in lysate by supplementing the media with cholesterol (Figure 2). To improve the solubility of cholesterol, we also added 1 mM Triton X-100 to the reaction. Additionally, the buffer contained 5 mM TCEP and 10 mM EDTA to ensure that the active site cysteine of HhC was in a reduced state to facilitate the N → S acyl shift (see SI for details).
Figure 2.
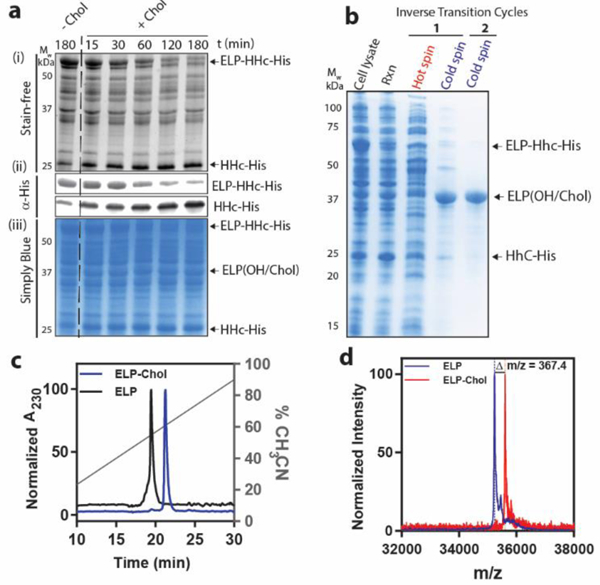
Reconstitution of cholesterolysis in E. coli cell lysate provides a facile approach to synthesize CHaMPs. a) Addition of cholesterol to cell lysate after expression of ELP-HhC-His fusion results in cleavage of the fusion protein and formation of ELP-Chol and HhC-His. Panel (i) is a SDS-PAGE gel visualized by Stain-Free technology, wherein proteins are visualized by the autofluorescence of their tryptophan residues. Panel (ii) is a western blot using anti-His primary antibody conjugated to DyLight® 550 for fluorescence visualization. Panel (iii) is a SDS PAGE gel visualized by staining with Simply Blue (a Coomassie-based dye). b) CHaMPs can be conveniently purified by exploiting their temperature-triggered LCST phase behavior using ITC. c) Reversed phase HPLC demonstrates the increased hydrophobicity of the ELP constructs after modification with cholesterol. The retention time of ELP-Chol is 19.4 min (black) while that of unmodified ELP is 21.2 min (blue). d) The MALDI-TOF MS spectrum of ELP-Chol (red) shows an increase in molecular weight compared to the ELP (blue), corresponding to the addition of a cholesterol moiety and the removal of water due to ester formation.
Given the large number of proteins present in E. coli lysate, we found that the reaction progress could be conveniently monitored by SDS-PAGE gels visualized using Stain-Free technology, which leverages the autofluorescence of tryptophan residues, of which HhC-His contains five. We also note that the ELP is not visible on this gel, as it does not contain any tryptophan residues.36 As shown in Figure 2a, the addition of cholesterol results in the consumption of ELP-HhC-His (Mw = 60.1 kDa) within 3 h, and the formation of HhC-His (Mw= 24.9 kDa)—a product of the auto-processing activity of HhC (see bands marked with arrows in panel (i) of Figure 2a). The concentration of ELP-HhC-His remained constant over the 3 h time frame in the negative control reaction that did not contain cholesterol (Figure 2a, lane 1). We then confirmed the extent of cholesterolysis by a western blot against the His-tag encoded at the C-terminus of the ELP-HhC-His construct (Figure 2a, panel ii). Finally, we stained the gels using Coomassie blue (Figure 2a, panel iii). Consistent with previous studies of HhC, we observed that some auto-processing occurred during protein expression or in the lysate reaction in the absence of cholesterol.37 This basal activity likely results in the formation of ELP with a free C-terminal carboxylic acid (labeled as ELP-OH in Figure 2b) and HhC-His (see below and SI for more details). However, When the constructs are first purified by IMAC and then reacted with cholesterol, quantitative conversion to cholesterol-modified product can be detected (Figure S2, yield > 97%). Together, these results indicate that it is possible to reconstitute the cholesterol modification in the complex environment of E. coli lysate.
After cholesterolysis, the CHaMP was purified to homogeneity with two rounds of ITC (Figure 2b, yield 5–10 mg/L of culture). Practically, adding cholesterol before ITC is advantageous because it allows the separation of the final product from Hhc-His and other endogenous E.coli proteins in the same step. We then used reversed phase high performance liquid chromatography (RP-HPLC) and matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) to characterize the purified construct and to confirm the C-terminal cholesterol modification. Using a gradient of water and acetonitrile in RP-HPLC, we observed an increase in the retention time of the cholesterol-modified construct compared to the parent ELP (Figure 2c). This increase in retention time is consistent with the increased hydrophobicity of ELP-Chol compared to the unmodified ELP control. Additionally, the MALDI-TOF MS spectrum of ELP-Chol exhibited an increase in molecular mass of 367.4 Da compared to the ELP, which corresponds to the addition of a cholesterol moiety (386.6 Da) and removal of a water molecule (−18 Da, due to ester formation). Proteolytic digestion of ELP-Chol with trypsin and subsequent mass spectrometry confirmed that the cholesterol moiety was added at the C-terminus (Figure S4, S8, and S9). Incubation of ELP-Chol with 50 mM potassium hydroxide solution, results in the hydrolysis of the C-terminal cholesterol ester and reduction in the molecular weight of the constructs (Figure S7 and S10). Taken together, these experiments show that HhC fusion can be used to recombinantly introduce a single cholesterol moiety into the C-terminus of a protein polymer.
After molecular characterization, we next examined the self-assembly of ELP-Chol using a combination of spectroscopy, light scattering, and imaging techniques. We first investigated the temperature-triggered LCST phase transition of ELP-Chol and compared it with the unmodified ELP by turbidimetry. Introduction of a cholesterol moiety influences the phase transition of the ELP in two ways (Figure 3a). First, the Tt of ELP-Chol, defined as the inflection point in the turbidity curve, is lower than the ELP (Figure 3a, solid versus dashed lines), consistent with the increased hydrophobicity of the cholesterol-modified constructs.32 For example, at 50 μM, the transition temperature of ELP-Chol is 20 °C lower than that of the ELP. Second, the Tt of the ELP-Chol construct shows little dependence on the bulk concentration of the construct as opposed to the ELP, whose Tt exhibits a sharp inverse dependence on concentration. For example, lowering the concentration of ELP-Chol from 100 to 25 μM, resulted in an increase in its Tt by less than 1 °C. Meanwhile the same decrease in ELP concentration increased the Tt by more than 10 °C. The lack of concentration dependence of an ELP is typically indicative of its self-assembly32 and suggested that the addition of a single cholesterol is enough to drive the self-assembly of ELP-Chol into micelles.
Figure 3.
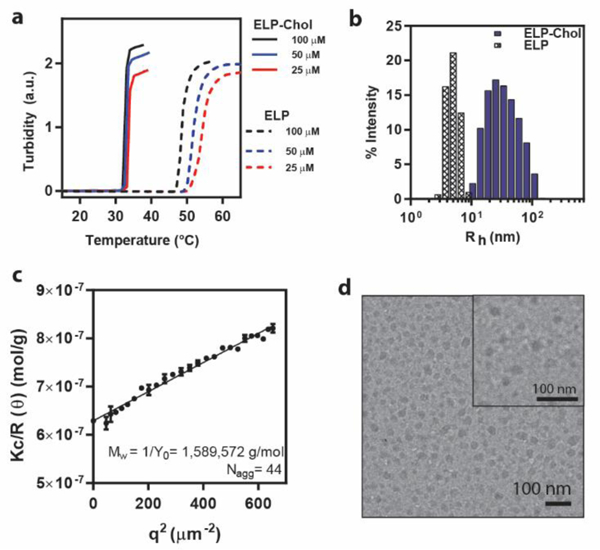
Characterization of the self-assembly behavior of the ELP-Chol as a representative CHaMP using different spectroscopic, scattering, and imaging techniques. a) Turbidimetry analysis of the temperature-triggered LCST phase transition shows that the cholesterol modification decreases the Tt of the ELP-Chol and reduces its concentration dependence. b and c) DLS and SLS indicate that the ELP-Chol self-assemble into micelles with a hydrodynamic radius (Rh) of 24 nm, which is consistent with the observed changes in the phase-behavior. d) Cryo-TEM imaging of ELP-Chol micellar nanoaggregates.
To confirm this hypothesis, we used dynamic and static light scattering (DLS and SLS) to characterize the hydrodynamic size and aggregation of ELP-Chol constructs. As shown in Figure 3b, we observed that ELP-Chol forms micelles with a hydrodynamic radius (Rh) of ~24 nm, while the ELP molecules exist as unimers with a Rh of ~4 nm. Given the hydrophilicity, charge, and size of the ELP, it is remarkable that the addition of a single cholesterol moiety (<1% of the total molecular weight) is enough to drive the self-assembly of the construct into micelles.
Using SLS (Figure 3c), we calculated that 44 chains of the ELP-Chol are self-assembled into ellipsoid micelles. Finally, to visualize these aggregates in their native hydrated state, we used cryo-transmission electron microscopy (cryo-TEM) to image the ELP-Chol micelles (Figure 3d). Due to the hydrophobicity of the cholesterol, the core of these micelles exhibited high contrast and were easily visualized in the ice layer. Consistent with the scattering results, we observed approximately spherical micelles with an average diameter of 28.3 ± 4.5 nm. Given the hydrophobicity of the core, we believe it should be possible to encapsulate hydrophobic therapeutics in these recombinant carriers. These studies will be reported elsewhere.
As proof-of-concept to demonstrate the utility of our methodology, we synthesized a therapeutically active CHaMP. Specifically, we leveraged the site-specific incorporation of cholesterol at the C-terminus to decorate ELP-Chol micelles with exendin-4 (Exe) —a peptide drug for the treatment of type II diabetes— at the opposite N-terminal end as Chol.38 Exendin-4 activates the glucagon-like peptide-1 receptor (GLP-1R) upon binding, leading to an increased concentration of the secondary messenger intracellular cyclic adenosine monophosphate (cAMP) and downstream secretion of insulin in response to the blood glucose level. Previous studies have shown that the N-terminus of Exe is crucial for the activation of GLP-1R39, making it a good choice for the demonstration of the functional activity of a CHaMP.
We used recombinant expression of Exe-ELP-HhC followed by in lysate cholesterolysis to create Exe-ELP-Chol, followed by two rounds of ITC to purify the construct (see SI for details). After verifying the molecular weight and the site-selective cholesterol modification at the c-terminal by SDS-PAGE, RP-HPLC, and MALDI-TOF MS (Figure S1, S3, S5, and S6), we characterized the self-assembly of Exe-ELP-Chol by DLS and cryo-TEM. As shown in Figure 4a and 4b, Exe-ELP-Chol formed micelles with an average size of 29.9 ± 5.9 nm.
Figure 4.
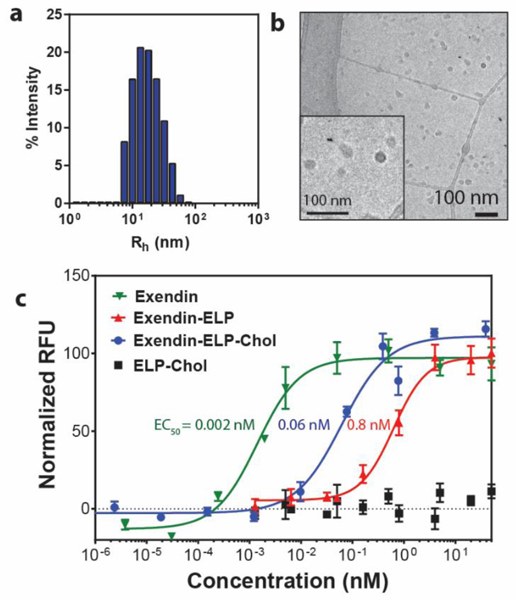
Recombinant expression can be used to conveniently modify CHaMPs with biologically active peptides. Fusion of exendin-4 to the N-terminus of ELP-Chol does not affect the self-assembly of micelles, as seen by (a) DLS and (b) cryo-TEM. c) Exendin-4 requires its free N-terminus to bind its target, GLP-1R. Cholesterol modification is compatible with the expression of exendin biological activity, resulting in a post-translationally modified recombinant drug with comparable potency to current gold standard GLP-1R agonist treatment options, such as Trulicity® and Liraglutide.
We then investigated the activity of Exe-ELP-Chol using a human embryonic kidney 293 cell line engineered to stably express GLP-1R. These cells also contained a cAMP reporter element coupled to the expression of luciferase.39 This last feature provides a convenient and facile readout of the intracellular cAMP levels (and GLP-1R activation) by quantifying the chemiluminescence of luciferin. As shown in Figure 4c, Exe-ELP-Chol is a potent agonist of GLP-1R, with a half-effective maximum concentration (EC50) of ~0.06 nM, demonstrating that the cholesterol modification does not interfere with the biological activity of exendin-4. Interestingly, Exe-ELP-Chol is more than an order of magnitude more active than Exe-ELP, with an EC50 of 0.8 nM. The 400-fold lower potency of Exe-ELP compared to Exe, which has an EC50 of 0.002 nM40, is consistent with previous reports of exendin fused to polymers such as PEG and POEGMA with similar length as the ELP used here, presumably because of steric hindrance to receptor engagement imposed by the polymer.41–43 Notably, presenting Exe on an ELP-Chol micelle partially rescues this loss of potency by an order of magnitude, which can help address the loss of potency seen by conjugates of Exe and other peptide drugs with linear polymers and polypeptides. The increased potency, we believe, can be attributed to the ability of CHaMP micelles to engage multiple receptors on the cell surface.44 Although the activity of the Exe-decorated micelles is lower than that of the native peptide, this loss is an acceptable trade-off as the micelle is likely to significantly improve the pharmacokinetics of exendin, which has a short, 0.7 h half-life and is rapidly cleared from the blood. This assertion is based on previous work by our group, where we showed that fusion of GLP1 —an analog of exendin— that also has a short plasma half-life, with a soluble ELP led to a 20-fold loss in the EC50 for GLP-1R binding, but a significantly longer —48 h— duration of glucose control as compared to the ~6 h of glucose control for GLP1.42
While peptide-polymers such as diblock ELPs can be programmed to form micelles, the presentation of peptide drugs on the corona of the micelle can have unpredictable effects on the self-assembly of these structures. From a theoretical standpoint, fusion of the peptide domain to a diblock ELP results in the formation of a triblock, with a significantly larger number of possible phases and hence a far more complex phase diagram than diblock copolymers.45 This problem is exacerbated when the fused peptide contains charged residues (as is the case for GLP-1 analogues, such as exendin-4) as the Coulombic repulsion can destabilize the micellar structure. We have not been able to design self-assembling ELP-diblock copolymers that can reliably display charged peptides, such as GLP-1 analogues, as micelles. As one representative example, we picked an ELP amphiphile with the hydrophobic trailer containing phenylalanine, (FGG)8.33 Turbidimetry and DLS confirmed that this asymmetric amphiphile self-assembles into micelle with the hydrodynamic radius of ~40 nm (Figure S11a,b). However, incorporation of glucagon-like peptides at the N-terminus of these amphiphiles perturbed the self-assembly of the micelles and resulted in formation of a large fraction of unimers (Figure S11c,d). Cholesterol modification provides a facile, and genetically encodable approach to drive the self-assembly of peptide polymers even when the sequences contain a large number of charged amino acids. Since cholesterol is significantly more hydrophobic than individual amino acids (i.e., has a much higher interaction parameter with water than any amino acid), our methodology provides a simple and robust route to the synthesize of micelles without the complicated process of screening diblocks with different lengths and hydrophobicities.
CONCLUSION AND OUTLOOK
In summary, we have demonstrated a facile strategy to create cholesterol-modified peptide-polymers using a genetically encoded PTM. We have demonstrated that Hh fusions can be used to site-specifically incorporate a cholesterol molecule at the C-terminus of an ELP. The resulting conjugate can be easily purified by exploiting its temperature-triggered LCST phase behavior. Introduction of a single cholesterol moiety is enough to drive the self-assembly of the conjugate into micelles, which can be decorated with biologically active peptides.
This method has several useful attributes. First, it provides a modular and facile approach to recombinantly synthesize biologically active post-translationally modified polymers. In this communication, we focused on a single ELP, but the peptide polymer sequence and chain length can be easily altered as needed. Second, this method provides a useful alternative to chemical derivatization of peptides with cholesterol, which typically involves reactions of nucleophilic amino acids with cholesteroyl-chloroflormate—a chemical approach that suffers from a lack of selectivity in the site of modification and can abolish peptide activity if the nucleophilic amino acid is necessary for binding to a target receptor. Even though elegant strategies have been developed to introduce cholesterol (and other lipids) using a linker during or after peptide synthesis,46–48 it is difficult to apply these strategies to longer peptides due to the limitations of solid phase synthesis, such as the reduced coupling efficiency as the peptide chain grows. Our method offers a new strategy with the distinct advantage of site-selectivity and ease of recombinant expression and should be applicable to different peptides and proteins.
Third, with this demonstration of a genetically encoded approach to cholesterol modification, we have further expanded the toolkit of PTM hybrid biopolymers. The two methods that we have demonstrated to date—N-terminal myristoylation and C-terminal cholesterolysis demonstrated herein—provide distinct advantages, such as regio- and site-selectivity, as well the type of the attached lipid (saturated fatty acid versus sterol). It should be possible to combine these methods to create constructs bearing tandem PTMs. We also envision that cholesterol-modified polypeptides can be combined with other systems to create hybrid materials with unique properties. For example, it should be possible to combine ChaMPs with other lipids and create protein-decorated liposomes, which to date has not been possible to achieve with ELPs. These studies are underway in our laboratories and will be reported in due course.
Supplementary Material
ACKNOWLEDGMENT
The cell line used in this study was a generous gift from Dr. Timothy Kieffer (University of British Columbia). Duke University Shared Materials Instrumentation Facility (SMIF) is a member of the North Carolina Research Triangle Nanotechnology Network (RTNN), which is supported by the National Science Foundation (Grant ECCS-1542015) as part of the National Nanotechnology Coordinated Infrastructure (NNCI).
Funding Sources
We acknowledge the financial support of NSF through (NSF MRSEC; DMR-1121107) and NIH through (R01 GM61232 and R35 GM127042).
Footnotes
ASSOCIATED CONTENT
Experimental details, including cloning, expression, modification, purification, and characterization of CHaMPs, are provided in the Supporting Information.
REFERENCES
- (1).Lutz J-F; Ouchi M; Liu DR; Sawamoto M Sequence-Controlled Polymers. Science 2013, 341 (6146), 1238149. [DOI] [PubMed] [Google Scholar]
- (2).Yang YJ; Holmberg AL; Olsen BD Artificially Engineered Protein Polymers. Annu. Rev. Chem. Biomol. Eng. 2017, 8 (1), 549–575. [DOI] [PubMed] [Google Scholar]
- (3).Kim M; Chen WG; Kang JW; Glassman MJ; Ribbeck K; Olsen BD Artificially Engineered Protein Hydrogels Adapted from the Nucleoporin Nsp1 for Selective Biomolecular Transport. Adv. Mater. 2015, 27 (28), 4207–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Park WM; Champion JA Two-Step Protein Self-Assembly in the Extracellular Matrix. Angew. Chem. Int. Ed. 2013, 52 (31), 8098–8101. [DOI] [PubMed] [Google Scholar]
- (5).Wang H; Paul A; Nguyen D; Enejder A; Heilshorn SC Tunable Control of Hydrogel Microstructure by Kinetic Competition between Self-Assembly and Crosslinking of Elastin-like Proteins. ACS Appl. Mater. Interfaces 2018, 10 (26), 21808–21815. [DOI] [PubMed] [Google Scholar]
- (6).Dooling LJ; Buck ME; Zhang W-B; Tirrell DA Programming Molecular Association and Viscoelastic Behavior in Protein Networks. Adv. Mater. 2016, 28 (23), 4651–4657. [DOI] [PubMed] [Google Scholar]
- (7).Li L; Mahara A; Tong Z; Levenson EA; McGann CL; Jia X; Yamaoka T; Kiick KL Recombinant Resilin-Based Bioelastomers for Regenerative Medicine Applications. Adv. Healthc. Mater. 2016, 5 (2), 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Payne SC; Patterson M; Conticello VP Bioengineering of Sequence-Repetitive Polypeptides: Synthetic Routes to Protein-Based Materials of Novel Structure and Function In Protein Engineering Handbook Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp 915–938. [Google Scholar]
- (9).Mozhdehi D; Luginbuhl KM; Roberts S; Chilkoti A Design of Sequence-Specific Polymers by Genetic Engineering. Sequence‐Controlled Polymers. December 4, 2017, pp 91–115. [Google Scholar]
- (10).Young TS; Schultz PG Beyond the Canonical 20 Amino Acids: Expanding the Genetic Lexicon. J. Biol. Chem. 2010, 285 (15), 11039–11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Johnson JA; Lu YY; Van Deventer JA; Tirrell DA Residue-Specific Incorporation of Non-Canonical Amino Acids into Proteins: Recent Developments and Applications. Curr. Opin. Chem. Biol. 2010, 14 (6), 774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Amiram M; Haimovich AD; Fan C; Wang Y-S; Aerni H-R; Ntai I; Moonan DW; Ma NJ; Rovner AJ; Hong SH; Kelleher NL; Goodman AL; Jewett MC; Söll D; Rinehart J; Isaacs FJ Evolution of Translation Machinery in Recoded Bacteria Enables Multi-Site Incorporation of Nonstandard Amino Acids. Nat. Biotechnol. 2015, 33 (12), 1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Costa SA; Simon JR; Amiram M; Tang L; Zauscher S; Brustad EM; Isaacs FJ; Chilkoti A Photo-Crosslinkable Unnatural Amino Acids Enable Facile Synthesis of Thermoresponsive Nano- to Microgels of Intrinsically Disordered Polypeptides. Adv. Mater. 2018, 30 (5), 1704878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Walsh CT; Garneau-Tsodikova S; Gatto GJ Protein Posttranslational Modifications: The Chemistry of Proteome Diversifications. Angew. Chem. Int. Ed. 2005, 44 (45), 7342–7372. [DOI] [PubMed] [Google Scholar]
- (15).Brennan MJ; Kilbride BF; Wilker JJ; Liu JC A Bioinspired Elastin-Based Protein for a Cytocompatible Underwater Adhesive. Biomaterials 2017, 124, 116–125. [DOI] [PubMed] [Google Scholar]
- (16).Wang X; Pu J; An B; Li Y; Shang Y; Ning Z; Liu Y; Ba F; Zhang J; Zhong C Programming Cells for Dynamic Assembly of Inorganic Nano-Objects with Spatiotemporal Control. Adv. Mater. 2018, 30 (16), 1705968. [DOI] [PubMed] [Google Scholar]
- (17).Pinkas DM; Ding S; Raines RT; Barron AE Tunable, Post-Translational Hydroxylation of Collagen Domains in Escherichia Coli. ACS Chem. Biol. 2011, 6 (4), 320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Luginbuhl KM; Mozhdehi D; Dzuricky M; Yousefpour P; Huang FC; Mayne NR; Buehne KL; Chilkoti A Recombinant Synthesis of Hybrid Lipid-Peptide Polymer Fusions That Self-Assemble and Encapsulate Hydrophobic Drugs. Angew. Chem. Int. Ed. 2017, 56 (45), 13979–13984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Mozhdehi D; Luginbuhl KM; Simon JR; Dzuricky M; Berger R; Varol HS; Huang FC; Buehne KL; Mayne NR; Weitzhandler I; Bonn M; Parekh SH; Chilkoti A Genetically Encoded Lipid–polypeptide Hybrid Biomaterials That Exhibit Temperature-Triggered Hierarchical Self-Assembly. Nat. Chem. 2018, 10 (5), 496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Jiang H; Zhang X; Chen X; Aramsangtienchai P; Tong Z; Lin H Protein Lipidation: Occurrence, Mechanisms, Biological Functions, and Enabling Technologies. Chem. Rev. 2018, 118 (3), 919–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Mejuch T; Waldmann H Synthesis of Lipidated Proteins. Bioconjug. Chem. 2016, 27 (8), 1771–1783. [DOI] [PubMed] [Google Scholar]
- (22).Simons K; Vaz WLC Model Systems, Lipid Rafts, and Cell Membranes. Annu. Rev. Biophys. Biomol. Struct. 2004, 33 (1), 269–295. [DOI] [PubMed] [Google Scholar]
- (23).Maxfield FR; Tabas I Role of Cholesterol and Lipid Organization in Disease. Nature 2005, 438 (7068), 612–621. [DOI] [PubMed] [Google Scholar]
- (24).Vyas N; Goswami D; Manonmani A; Sharma P; Ranganath HA; VijayRaghavan K; Shashidhara LS; Sowdhamini R; Mayor S Nanoscale Organization of Hedgehog Is Essential for Long-Range Signaling. Cell 2008, 133 (7), 1214–1227. [DOI] [PubMed] [Google Scholar]
- (25).Porter JA; Young KE; Beachy PA Cholesterol Modification of Hedgehog Signaling Proteins in Animal Development. Science 1996, 274 (5285), 255–259. [DOI] [PubMed] [Google Scholar]
- (26).Mann R Cholesterol Modification of Proteins. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 2000, 1529 (1–3), 188–202. [DOI] [PubMed] [Google Scholar]
- (27).Hall TMT; Porter JA; Young KE; Koonin EV; Beachy PA; Leahy DJ Crystal Structure of a Hedgehog Autoprocessing Domain: Homology between Hedgehog and Self-Splicing Proteins. Cell 1997, 91 (1), 85–97. [DOI] [PubMed] [Google Scholar]
- (28).Xie J; Owen T; Xia K; Callahan B; Wang C A Single Aspartate Coordinates Two Catalytic Steps in Hedgehog Autoprocessing. J. Am. Chem. Soc. 2016, 138 (34), 10806–10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Ciulla DA; Jorgensen MT; Giner J-L; Callahan BP Chemical Bypass of General Base Catalysis in Hedgehog Protein Cholesterolysis Using a Hyper-Nucleophilic Substrate. J. Am. Chem. Soc. 2018, 140 (3), 916–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Vincent S; Thomas A; Brasher B; Benson JD Targeting of Proteins to Membranes through Hedgehog Auto-Processing. Nat. Biotechnol. 2003, 21 (8), 936–940. [DOI] [PubMed] [Google Scholar]
- (31).Urry DW Physical Chemistry of Biological Free Energy Transduction as Demonstrated by Elastic Protein-Based Polymers. J. Phys. Chem. B 1997, 101 (51), 11007–11028. [Google Scholar]
- (32).McDaniel JR; Bhattacharyya J; Vargo KB; Hassouneh W; Hammer D a.; Chilkoti, A. Self-Assembly of Thermally Responsive Nanoparticles of a Genetically Encoded Peptide Polymer by Drug Conjugation. Angew. Chem. Int. Ed. 2013, 52 (6), 1683–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).McDaniel JR; Weitzhandler I; Prevost S; Vargo KB; Appavou MS; Hammer DA; Gradzielski M; Chilkoti A Noncanonical Self-Assembly of Highly Asymmetric Genetically Encoded Polypeptide Amphiphiles into Cylindrical Micelles. Nano Lett. 2014, 14 (11), 6590–6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Meyer DE; Chilkoti A Purification of Recombinant Proteins by Fusion with Thermally-Responsive Polypeptides. Nat. Biotechnol. 1999, 17 (11), 1112–1115. [DOI] [PubMed] [Google Scholar]
- (35).Wenz JJ; Barrantes FJ Steroid Structural Requirements for Stabilizing or Disrupting Lipid Domains †. Biochemistry 2003, 42 (48), 14267–14276. [DOI] [PubMed] [Google Scholar]
- (36).Gürtler A; Kunz N; Gomolka M; Hornhardt S; Friedl AA; McDonald K; Kohn JE; Posch A Stain-Free Technology as a Normalization Tool in Western Blot Analysis. Anal. Biochem. 2013, 433 (2), 105–111. [DOI] [PubMed] [Google Scholar]
- (37).Porter JA; Ekker SC; Park W-J; von Kessler DP; Young KE; Chen C-H; Ma Y; Woods AS; Cotter RJ; Koonin EV; Beachy PA Hedgehog Patterning Activity: Role of a Lipophilic Modification Mediated by the Carboxy-Terminal Autoprocessing Domain. Cell 1996, 86 (1), 21–34. [DOI] [PubMed] [Google Scholar]
- (38).Gilroy CA; Luginbuhl KM; Chilkoti A Controlled Release of Biologics for the Treatment of Type 2 Diabetes. J. Control. Release 2016, 240, 151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Göke R; Fehmann HC; Linn T; Schmidt H; Krause M; Eng J; Göke B Exendin-4 Is a High Potency Agonist and Truncated Exendin-(9–39)-Amide an Antagonist at the Glucagon-like Peptide 1-(7–36)-Amide Receptor of Insulin-Secreting Beta-Cells. J. Biol. Chem. 1993, 268 (26), 19650–19655. [PubMed] [Google Scholar]
- (40).Finan B; Ma T; Ottaway N; Muller TD; Habegger KM; Heppner KM; Kirchner H; Holland J; Hembree J; Raver C; Lockie SH; Smiley DL; Gelfanov V; Yang B; Hofmann S; Bruemmer D; Drucker DJ; Pfluger PT; Perez-Tilve D; et al. Unimolecular Dual Incretins Maximize Metabolic Benefits in Rodents, Monkeys, and Humans. Sci. Transl. Med. 2013, 5 (209), 209ra151. [DOI] [PubMed] [Google Scholar]
- (41).Pang Y; Liu J; Qi Y; Li X; Chilkoti A A Modular Method for the High-Yield Synthesis of Site-Specific Protein–Polymer Therapeutics. Angew. Chem. Int. Ed. 2016, 55 (35), 10296–10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Luginbuhl KM; Schaal JL; Umstead B; Mastria EM; Li X; Banskota S; Arnold S; Feinglos M; D’Alessio D; Chilkoti A One-Week Glucose Control via Zero-Order Release Kinetics from an Injectable Depot of Glucagon-like Peptide-1 Fused to a Thermosensitive Biopolymer. Nat. Biomed. Eng. 2017, 1 (6), 0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Qi Y; Simakova A; Ganson NJ; Li X; Luginbuhl KM; Ozer I; Liu W; Hershfield MS; Matyjaszewski K; Chilkoti A A Brush-Polymer/Exendin-4 Conjugate Reduces Blood Glucose Levels for up to Five Days and Eliminates Poly(Ethylene Glycol) Antigenicity. Nat. Biomed. Eng. 2017, 1 (1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Zhang Y; Zou H; Wang Y; Caballero D; Gonzalez J; Chao E; Welzel G; Shen W; Wang D; Schultz PG; Wang F Rational Design of a Humanized Glucagon-Like Peptide-1 Receptor Agonist Antibody. Angew. Chem. Int. Ed. 2015, 54 (7), 2126–2130. [DOI] [PubMed] [Google Scholar]
- (45).Bates FS; Hillmyer MA; Lodge TP; Bates CM; Delaney KT; Fredrickson GH Multiblock Polymers: Panacea or Pandora’s Box? Science 2012, 336 (6080), 434–440. [DOI] [PubMed] [Google Scholar]
- (46).Knerr PJ; Finan B; Gelfanov V; Perez-Tilve D; Tschöp MH; DiMarchi RD Optimization of Peptide-Based Polyagonists for Treatment of Diabetes and Obesity. Bioorg. Med. Chem. 2018, 26 (10), 2873–2881. [DOI] [PubMed] [Google Scholar]
- (47).Pessi A; Langella A; Capitò E; Ghezzi S; Vicenzi E; Poli G; Ketas T; Mathieu C; Cortese R; Horvat B; Moscona A; Porotto M A General Strategy to Endow Natural Fusion-Protein-Derived Peptides with Potent Antiviral Activity. PLoS One 2012, 7 (5), e36833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Ward BP; Ottaway NL; Perez-Tilve D; Ma D; Gelfanov VM; Tschöp MH; DiMarchi RD Peptide Lipidation Stabilizes Structure to Enhance Biological Function. Mol. Metab. 2013, 2 (4), 468–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


